Abstract
Aflatoxins (AFs) are secondary metabolites produced by aflatoxigenic strains of Aspergillus flavus and A. parasiticus, the most toxic being aflatoxin B1 (AFB1). The purpose of the present work was to investigate the effects of industrial-grade packaging materials (low-density polyethylene, polypropylene, polyethylene-laminated aluminium); temperatures (25 °C, 30 °C); and water activities (0.74 aw, 0.85 aw) on AFB1 production by A. flavus and A. parasiticus in stored peanut kernels. Commercially-obtained samples were segregated into packaging materials, separately inoculated with the aflatoxigenic Aspergillus spp., and stored for 1 month under various °C + aw regimes. AFB1 production was quantified by high performance liquid chromatography with fluorescence detector (HPLC–FLD). For A. flavus in PELA, no AFB1 was detected (100% reduction) at 25 °C for both aw tested. For A. parasiticus in PELA, no AFB1 was detected at 25 °C (0.85 aw) and 30 °C (0.74 aw). Highest concentration of AFB1 was detected in LDPE for both A. flavus (46.41 ppb) and A. parasiticus (414.42 ppb), followed by PP (A. flavus 24.29 ppb; A. parasiticus 386.73 ppb). In conclusion, storing peanut kernels in PELA in a dry place at room temperature has been demonstrated as an adequate and inexpensive method in inhibiting growth of Aspergillus spp. and lowering AFB1 contamination in peanuts.
Keywords: Peanuts, Aspergillus flavus, Aspergillus parasiticus, Packaging, Storage conditions, Aflatoxin
Introduction
Aflatoxins (AFs) are a group of difuranocoumarin metabolites produced by aflatoxigenic strains of Aspergillus flavus and A. parasiticus (Chiou et al. 2002; Juan et al. 2008) during metabolism (Abriba et al. 2013), with the most potent and widely studied being AFB1. Favourable growth conditions for causal fungi include substrate moisture content (≈ 15–30%), ambient temperature (≈ 25–30 °C) and relative humidity (≈ 85%) (Sulaiman et al. 2007). Therefore, by manipulating these ecophysiological parameters, fungal contamination and toxin production could be prevented.
In controlling the AFB1 contamination in stored peanuts, the type of packaging materials also plays important role especially in developing countries where food handling and proper storage technology is less advanced and should have a low water vapour transmission rate (WVTR) to avoid moisture being absorbed from the environment (Leong et al. 2010). The objective of the present work was therefore to determine the effects of different packaging materials, temperatures and water activities on AFB1 contamination in stored peanut kernels artificially inoculated with aflatoxigenic A. flavus and A. parasiticus.
Materials and methods
Chemicals
The AFB1 standard at a concentration of 300 ng/mL was purchased from Supelco (PA, USA). All solvents used in the experiments were of HPLC-grade, and supplied by Merck (Darmstadt, Germany). AflatestWB immunoaffinity columns (IAC) were purchased from Vicam (MA, USA).
Fungal strains
Aflatoxigenic strains of Aspergillus flavus NRRL 3357 and A. parasiticus FRR 2999 were used (CSIRO; North Ryde, N.S.W., Australia). Both strains were maintained in 0.05% Tween-80 spore suspension. A haemocytometer (Neubauer, Germany) was used to adjust the concentration of fungal spores to 103 spores/mL (Gunterus et al. 2007).
Packaging materials
Packaging materials tested were low-density polyethylene (LDPE), polypropylene (PP), and polyethylene-laminated aluminium (PELA) which were locally purchased from an industrial-grade packaging supplier (Good and Well Trading; Seri Kembangan, Malaysia).
Experimental design
The experimental design used was full factorial with the two factors being temperature (25 and 30 °C) and water activity (0.74 and 0.85 aw). The treatments were carried out in triplicate.
Peanut sampling and adjustment of water activities
A total of 3 kg samples of packed raw peanut kernels were randomly purchased from different supermarkets in Serdang, Selangor, Malaysia. The initial aw of the peanuts were measured at 0.62. The samples were thoroughly mixed, surface-disinfected through immersion in a 0.4% solution of sodium hypochlorite (NaOCl) for 2 min, rinsed with sterile distilled water (dH2O), and dried overnight on paper towels in a laminar-flow hood (Pitt et al. 1993). Following drying, samples of the raw peanut kernels were halved, and each portion was separately rehydrated by addition of dH2O to achieve 0.74 and 0.85 aw (Malaysian humidity range) based on a peanut moisture absorption curve (Malaysian Meteorological Department, 2017; Zhang et al. 2017). The adjusted aw values were verified with an AquaLab model CX-2 water activity meter (Decagon Devices Inc.: WA, USA).
Treatments
The 0.74 and 0.85 aw peanut kernels were further divided into 36 sub-samples (3 packagings × 2 strains × 2 temperatures × 3 replicates) of 30 g each. Artificial inoculation of fungal strains was performed with 20 µL spore suspension (102 spores). Inoculated samples were sealed using a BTK-300 Balance Impulse Hand Sealer (Ban Hing Holdings; Kuala Lumpur, Malaysia). Sealed samples were separately stored for 1 month at 25 and 30 °C. Uninoculated peanut kernels (3 packagings × 2 water activities × 2 temperatures × 3 replicates) served as negative control.
Aflatoxin B1 extraction and clean-up
Extraction of AFB1 from incubated peanut kernels were performed following the AOAC official method 991.31 (Truckness 2000) with minor modification (Afsah-Hejri et al. 2011). Following the 1 month storage, mouldy peanut kernels were ground using a Waring blender (Vicam: Milford, MA, USA) for 3 min. Next, ground peanut samples (25 g) were homogenised with 5 g NaCl and 125 mL methanol/water (70:30, v/v) for 2 min. Homogenate were diluted with 30 mL dH2O, filtered through a 24 cm Ø fluted filter paper (Vicam: Milford, MA, USA), and again through an 11 cm Ø glass microfiber filter (Vicam, Milford MA, USA). Next, 15 mL filtrate was passed through the immunoaffinity column (Aflatest; Vicam, Milford, MA, USA) containing monoclonal antibody specific for AFB1 for purification at a flow rate of 1 mL/min (Jinap et al. 2012). The IAC was then washed with 10 mL dH2O twice following which the AFB1 was eluted with 1 mL absolute methanol. The eluent was diluted with 1 mL dH2O and stored in HPLC vials until analysis.
Aflatoxin B1 quantification by HPLC-FLD
The purified AFB1 were quantified using reverse-phase high performance liquid chromatography system (Waters 600: NY, USA) with fluorescence detector (Waters 2475: NY, USA) with a post-column photochemical reactor for enhanced detection (PHRED) (Aura Industries: NY, USA) and improve the HPLC column (C18: 4.6 mm × 25 cm; Waters: NY, USA) sensitivity. Excitation and emission wavelengths were 365 and 435 nm respectively. Injection volume was 20 µL with a isocratic mode solvent composition of H2O:MeOH:ACN (55:35:10 v/v) at a flow rate of 0.6 mL/min. AFB1 standard curve was constructed with seven concentrations of 2 ppb, 4 ppb, 6 ppb, 10 ppb, 25 ppb, 50 ppb and 100 ppb. The R2 obtained from the curve was 0.995. The limit of detection (LOD) and limit of quantification (LOQ) for the method was 0.03 ng/g and 0.1 ng/g, respectively. For data acquisition and processing, Empower 2 Chromatography Data Software (Waters: NY, USA) was used. Processing and acquisition of data was obtained by input of injection volume, run time, vial position, method set, processing method and standard curve to calibrate and quantitate the results.
Statistical analysis
Measurements from triplicates were averaged as mean ± SD. A two-way analysis of variance (ANOVA) was applied on normally-distributed datasets to analyse significant and synergistic effects of each of the tested parameters (packaging materials, temperatures, water activities) using the statistical software Minitab® version 16 (Minitab Inc.; Pennsylvania, USA). p < 0.05 was accepted as significant difference.
Results
Figure 1 shows the mean AFB1 levels (ppb) detected in peanut kernels inoculated with A. flavus at different temperatures and water activities on LDPE, PP, and PELA. Across the three types of packaging, peanut kernels incubated in PELA yielded the lowest amount of AFB1 as compared to LDPE and PP regardless of temperatures and water activities tested. For water activities, significantly higher amounts of AFB1 were observed at 0.85 aw across all packaging tested. For temperatures, incoherent pattern was observed in which both 25 and 30 °C yielded different amounts of AFB1.
Fig. 1.
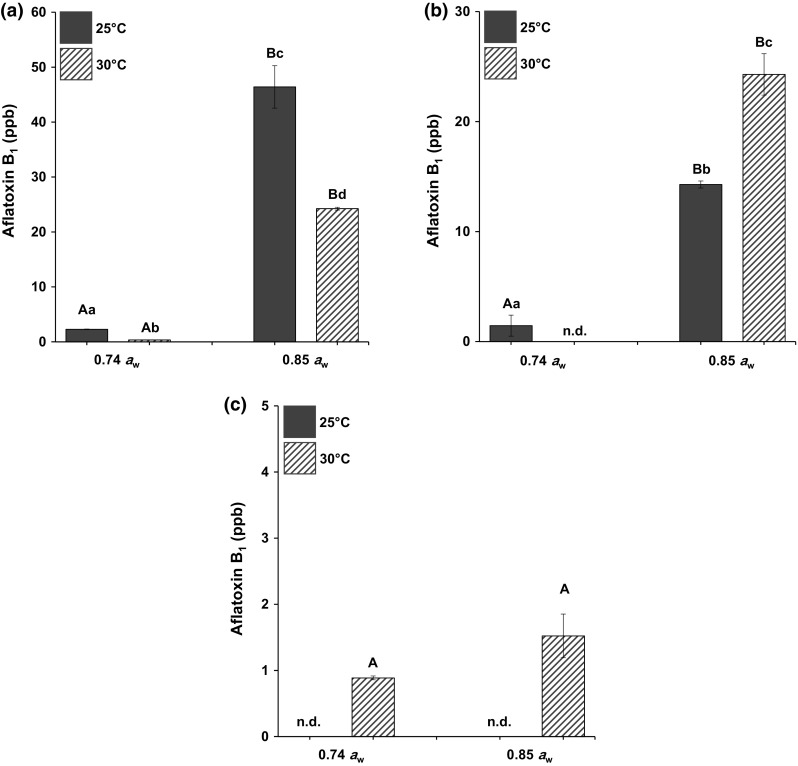
Aflatoxin B1 production (ppb) in peanut kernels inoculated with Aspergillus flavus NRRL 3357 on a low-density polyethylene; LDPE, b polypropylene; PP and c polyethylene-laminated aluminium; PELA, incubated at different temperatures (°C) and water activities (aw) for 1 month. Data are means of triplicates with bars indicating SD. Capital letters indicate significant difference (p < 0.05) between aw and small letters between °C. n.d.: not detected
Figure 2 depicts the mean AFB1 levels (ppb) detected in peanut kernels inoculated with A. parasiticus at different temperatures and water activities on LDPE, PP, and PELA. Similar to A. flavus, PELA yielded the lowest amount of AFB1 regardless of temperatures and water activities across the packaging tested. For temperatures, at 30 °C, AFB1 levels were observed to be significantly lower than 25 °C across the packaging tested except for PELA at 0.74 aw. For water activities, incoherent pattern was observed in which both 0.74 and 0.85 aw yielded different amounts of AFB1.
Fig. 2.
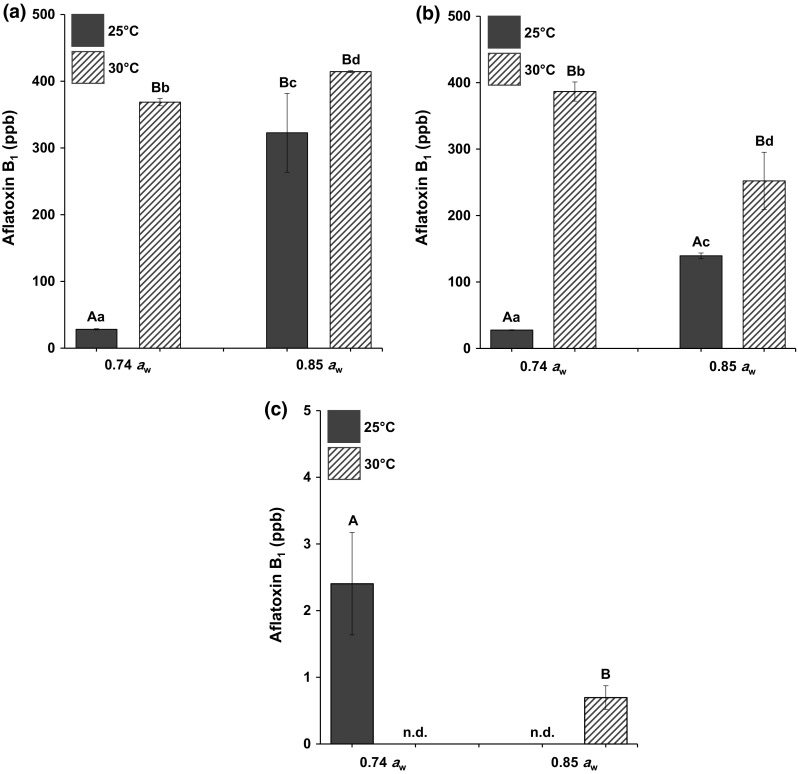
Aflatoxin B1 production (ppb) in peanut kernels inoculated with Aspergillus parasiticus FRR 2999 on a low-density polyethylene; LDPE, b polypropylene; PP and c polyethylene-laminated aluminium; PELA, incubated at different temperatures (°C) and water activities (aw) for 1 month. Data are means of triplicates with bars indicating SD. Capital letters indicate significant difference (p < 0.05) between aw and small letters between °C. n.d.: not detected
Based on the results obtained, it is also apparent that the AFB1 produced by A. parasiticus was significantly higher by many folds when compared to that of A. flavus in all the treatments tested.
Table 1 lists the p values of both parameters tested and their synergistic effects on AFB1 production by A. flavus and A. parasiticus across all packagings. For A. flavus; °C and aw had significant effects on AFB1 production in LDPE and PP. However, the same was not observed in PELA where only °C had significant effect on AFB1 production. For A. parasiticus; °C and aw had significant effects on AFB1 production in LDPE and PELA, while only °C had significant effect on AFB1 production in PP. All in all, across the packagings tested, temperature had undoubtedly significant effect on AFB1 production by both strains. As expected, no amount of AFB1 was detected in the negative control treatment from both Aspergillus spp.
Table 1.
Analysis of variance (ANOVA) for parameters tested (°C, aw) and their synergistic effects on aflatoxin B1 production (ppb) by Aspergillus flavus NRRL 3357 and Aspergillus parasiticus FRR 2999 incubated for 1 month in low-density polyethylene (LDPE), polypropylene (PP) and polyethylene-laminated aluminium (PELA). p < 0.05 indicates significant effect
| Factors | Aspergillus flavus | Aspergillus parasiticus |
|---|---|---|
| p value | ||
| Low-density polyethylene (LDPE) | ||
| Temperature (°C) | < 0.05 | < 0.05 |
| Water activity (aw) | < 0.05 | < 0.05 |
| °C × aw | < 0.05 | < 0.05 |
| Polypropylene (PP) | ||
| Temperature (°C) | < 0.05 | < 0.05 |
| Water activity (aw) | < 0.05 | 0.600 |
| °C × aw | < 0.05 | < 0.05 |
| Polyethylene-laminated aluminium (PELA) | ||
| Temperature (°C) | < 0.05 | < 0.05 |
| Water activity (aw) | 0.073 | < 0.05 |
| °C × aw | 0.073 | < 0.05 |
Discussion
Based on the obtained results, peanut kernels packed in PELA significantly yielded the lowest AFB1, followed by PP and LDPE. This might be explained by the fact that PELA has the best heat and oxygen barrier qualities among the three packaging materials tested due to its multi-layered structure (PE–aluminium–PE) and excellent heat sealing properties (TFO 2010). For PP, although it tends to hold heat within thus creating a slightly favourable condition for fungal growth and subsequently AFB1 contamination, lower AFB1 levels observed might be due to the fact that PP is also known to be an excellent moisture barrier and an adequate oxygen barrier (Kennedy and Devereau 1994). This moisture and oxygen blockage will further prevent fungal proliferation and the subsequent toxin production. For LDPE which has the lowest softening and melting points among the three packaging materials tested, it is highly suitable for heat sealing, but fares poorly as moisture and oxygen barrier (Shakerardekani and Karim 2013) thus providing a conducive micro-environment for fungal proliferation inside the packaging and the subsequent high toxin production as indicated in the results. The gas transmission rate of packaging materials used had a significant effect on fungal growth and AFB1 production with PELA having the lowest gas transmission rate, yielded the lowest fungal growth and AFB1 production, followed by PP and PE yielded the highest fungal growth and AFB1 production, having the highest gas transmission rate among the packaging materials used.
In terms of the effects of incubation water activities (aw), 0.85 aw yielded higher AFB1 when compared to 0.74 aw in A. flavus across all packaging materials tested. This agrees with a study by Abdel-Hadi et al. (2012) who found a positive correlation between decreasing aw and decreasing AFB1, and also in accordance with Good Agricultural Practice (GAP) and Good Manufacturing Practice (GMP) which in principle is to store food commodities in a dry and low humidity environment (Gordon 2016). As of 2017 in Malaysia, the mean humidity level is 0.76 ± 0.07 aw (Malaysian Meteorological Department 2017). However, the same pattern was not entirely observed in A. parasiticus where in certain treatments AFB1 levels were in fact higher at 0.74 aw as compared to 0.85 aw. This phenomenon might be explained by the fact that fungal infestation and the subsequent toxin production can also occur under ecophysiological stresses (e.g., decreased in humidity; Agag 2004).
In terms of the effects of incubation temperatures (°C), incoherent patterns of high and low AFB1 levels produced by A. flavus and A. parasiticus at both temperatures tested might be explained by the fact that aflatoxigenic Aspergillus spp. has a wide range of temperature tolerance (19–35 °C) with 28 °C being the optimal temperature for growth and 28–30 °C for AFs production (Sanchis and Magan 2004). In the present work, majority of the treatments (i.e., 8 of 12; Figs. 1 and 2) exhibited high levels of AFB1 at 30 °C. These findings concur with that of Saleemullah et al. (2006) who reported greater conidial development and AFs production by aflatoxigenic Aspergillus spp. at 30 °C. However, it is also noteworthy that higher levels of AFB1 at temperature (25 °C) lower than the optimal range as indicated in several treatments (i.e., in LDPE and PP for A. flavus, in PELA for A. parasiticus) observed in the present work might actually be a technical discrepancy rather than a theoretical one. After filling the packaging materials with peanut kernels, the packagings were sealed with a Balance Impulse Hand Sealer and it was noticed that tiny pores were formed at the edges of the sealing lines (Stehling and Meka 1994), hence making the packaging not airtight. Therefore, it is probable that the non-airtight condition has caused air to freely flow in and out of the packaging, which in turn promoted higher levels of AFB1 (Hotchkiss 1995). The findings are in agreement with Ellis et al. (1991, 1993) who stated that higher AFB1 was produced by A. flavus and A. parasiticus at a higher atmospheric gases quantity condition within a packaging.
Higher AFB1 production by A. parasiticus when compared to that of A. flavus by approximately tenfold observed in the present work agreed with the findings of Fani (2013) who found that A. parasiticus produced higher AFB1 than A. flavus. This might be explained by the difference in genetics between both strains (genotype) which in turn influences the difference in their toxin production capacity (phenotype).
Conclusion
The present work demonstrates that polyethylene-laminated aluminium (PELA) when used as packaging yielded the lowest concentration of AFB1 by both strains. Of the two temperatures tested, 25 °C has been shown to significantly reduce AFB1 production by both strains. In terms of water activity, A. flavus has been shown to produce lower AFB1 at drier condition (0.74 aw) in stored peanut kernels, but not exactly in A. parasiticus. More knowledge and understanding are therefore needed on proper storage practices and choosing the right packaging material in the context of raw peanut kernels and its handling methods against common fungal contaminants. As peanuts are mainly contaminated during storage, storing them in PELA at a dry place and around room temperature can be adopted by the peanut-based food industries as an adequate and inexpensive method in ensuring reduction of AFs in the peanuts as evidenced in the present work.
Acknowledgements
The authors would like to acknowledge Universiti Putra Malaysia for financial support through the UPM Grant, GP-IPB/2013/9425401, HICOE grant from the Ministry of Education Malaysia and the assistance given by the Ministry of Health Malaysia.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abdel-Hadi A, Schmidt-Heydt M, Parra R, Geisen R, Magan N. A systems approach to model the relationship between aflatoxin gene cluster expression, environmental factors, growth and toxin production by Aspergillus flavus. J R Soc Interface. 2012;9:757–767. doi: 10.1098/rsif.2011.0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abriba C, Lennox JA, Asikong BE, Asitok A, Ikpoh IS, Henshaw EE, Eja ME. Isolation of aflatoxin producing species of Aspergillus from foodstuffs sold in Calabar markets, Cross River state, Nigeria. J Microbiol Biotechnol Res. 2013;3:8–13. [Google Scholar]
- Afsah-Hejri L, Jinap S, Arzandeh S, Mirhosseini H. Optimization of HPLC conditions for quantitative analysis of aflatoxins in contaminated peanut. Food Control. 2011;22:381–388. doi: 10.1016/j.foodcont.2010.09.007. [DOI] [Google Scholar]
- Agag BI. Mycotoxins in food and feeds 1: aflatoxins. Assiut University BES. 2004;7:173–206. [Google Scholar]
- Chiou CH, Miller M, Wilson DL, Trail F, Linz JE. Chromosomal location plays a role in regulation of aflatoxin gene expression in Aspergillus parasiticus. Appl Environ Microbiol. 2002;68:306–315. doi: 10.1128/AEM.68.1.306-315.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis WO, Smith JP, Simpson BK, Oldham JH. Aflatoxins in food: occurrence, biosynthesis, effects on organisms, detection, and methods of control. Crit Rev Food Sci Nutr. 1991;30:403–439. doi: 10.1080/10408399109527551. [DOI] [PubMed] [Google Scholar]
- Ellis WO, Smith JP, Simpson BK, Khanizadeh S, Oldham JH. Control of growth and aflatoxin production of Aspergillus flavus under modified atmosphere packaging (MAP) conditions. Food Microbiol. 1993;10:9–21. doi: 10.1006/fmic.1993.1002. [DOI] [PubMed] [Google Scholar]
- Fani O. Comparison of aflatoxin B1 production by Aspergillus flavus and Aspergillus parasiticus under various conditions of temperature, light and pH. Armaghane Danesh J. 2013;18:210–218. [Google Scholar]
- Gordon A. Introduction: effective implementation of food safety and quality systems: prerequisites and other considerations. In: Gordon A, editor. food safety and quality systems in developing countries. Massachusetts: Academic; 2016. pp. 1–19. [Google Scholar]
- Gunterus A, Roze LV, Beaudry R, Linz JE. Ethylene inhibits aflatoxin biosynthesis in Aspergillus parasiticus grown on peanuts. Food Microbiol. 2007;24:658–663. doi: 10.1016/j.fm.2006.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotchkiss JH. Chapter 11: safety considerations in active packaging. In: Rooney ML, editor. Active food packaging. USA: Springer; 1995. pp. 238–255. [Google Scholar]
- Jinap S, de Rijk TC, Arzandeh S, Kleijnen HCH, Zomer P, van der Weg G, Mol JGJ. Aflatoxin determination using in-line immunoaffinity chromatography in foods. Food Control. 2012;26:42–48. doi: 10.1016/j.foodcont.2011.12.007. [DOI] [Google Scholar]
- Juan C, Zinedine A, Moltó JC, Idrissi L, Mañes J. Aflatoxins levels in dried fruits and nuts from Rabat-Salé area, Morocco. Food Control. 2008;19:849–853. doi: 10.1016/j.foodcont.2007.08.010. [DOI] [Google Scholar]
- Kennedy L, Devereau A (1994) Observations on large-scale outdoor maize storage in jute and woven polypropylene sacks in Zimbabwe. In: 6th IWCSPP
- Leong YH, Ismail N, Latif AA, Ahmad R. Aflatoxin occurrence in nuts and commercial nutty products in Malaysia. Food Control. 2010;21:334–338. doi: 10.1016/j.foodcont.2009.06.002. [DOI] [Google Scholar]
- Malaysian Meteorological Department (2017) Daily report on relative humidity for meteorological stations in Malaysia. Malaysian Ministry of Science, Technology and Innovations. www.met.gov.my/relativehumidity. Accessed Jan 2017
- Pitt JI, Hocking AD, Bhudhasamai K, Miscamble BF, Wheeler KA, Tanboon-Ek P. The normal mycoflora of commodities from Thailand. 1. nuts and oilseeds. Int J Food Microbiol. 1993;20:211–226. doi: 10.1016/0168-1605(93)90166-E. [DOI] [PubMed] [Google Scholar]
- Saleemullah Iqbal A, Khalil IA, Shah H. Aflatoxin contents of stored and artificially inoculated cereals and nuts. Food Chem. 2006;98:699–703. doi: 10.1016/j.foodchem.2005.06.034. [DOI] [Google Scholar]
- Sanchis V, Magan N. Environmental profiles for growth and mycotoxin production. In: Magan N, Olsen M, editors. Mycotoxins in food: detection and control. Cambridge: Woodhead Publishing Ltd.; 2004. pp. 174–189. [Google Scholar]
- Shakerardekani A, Karim R. Effect of different types of plastic packaging films on the moisture and aflatoxin contents of pistachio nuts during storage. J Food Sci Technol. 2013;50:409–411. doi: 10.1007/s13197-012-0624-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stehling FC, Meka P. Heat sealing of semicrystalline polyer films. II. Effect of melting distribution on heat sealing behavior of polyolefins. J Appl Polym Sci. 1994;51:105–119. doi: 10.1002/app.1994.070510112. [DOI] [Google Scholar]
- Sulaiman MR, Chye FY, Hamid A, Yatim AM. The occurrence of aflatoxins in raw shelled peanut samples from three districts of Perak, Malaysia. Electron J Agric Food Chem. 2007;6:2045–2052. [Google Scholar]
- TFO (2010) Session 4: plastic packaging films and laminates; properties, specifications and purchasing. Presented during the TFO Canada workshop of procurement of packaging for exports. Guyana, pp 1–39
- Truckness MW. AOAC Official Method 991.31, Aflatoxin in corn, raw peanuts, and peanut butter. J AOAC Int. 2000;49:22–24. [Google Scholar]
- Zhang L, Sun DW, Zhang Z. Methods for measuring water activity (aw) of foods and its applications to moisture sorption isotherm studies. Crit Rev Food Sci Nutr. 2017;57:1052–1058. doi: 10.1080/10408398.2015.1108282. [DOI] [PubMed] [Google Scholar]


