Abstract
Background:
Exosomes are membrane-enclosed extracellular vesicles implicated in cell–cell communication. Exosomes contain proteins, mRNAs, non-coding RNAs (miRNAs and lncRNAs) and lipids that are derived from producing cells. These nano-sized vesicles are present in biofluids including blood, urine, saliva, amniotic fluid, semen and conditioned media of cultured cells.
Methods:
This review summarizes current progress on the strategies of development of diagnostic biomarkers and drug loading onto exosomes for overcoming cancer progression.
Results:
A number of studies indicate that the exosome appears to be a key player in tissue repair and regeneration of in a number of animal disease models. In addition, alterations of the molecular profiles in exosomes are known to be correlated with the disease progression including cancer, suggesting their usefulness in disease diagnosis and prognosis. Studies utilizing engineered exosomes either by chemical or biological methods have demonstrated promising results in a number of animal models with cancer.
Conclusion:
Understanding the molecular and cellular properties of exosomes offer benefits for cancer diagnosis by liquid biopsy and for their application in therapeutic drug delivery systems. Studies have shown that genetic or molecular engineering of exosomes augmented their target specificity and anticancer activity with less toxicity. Thus, deeper understanding of exosome biology will facilitate their therapeutic potential as an innovative drug delivery system for cancer.
Keywords: Exosomes, Drug delivery, Cancer, Extracellular vesicles, Target specificity
Introduction
The key requirements for ideal drug delivery system (DDS) are safety, nontoxicity, efficiency, non-immunogenicity, bioavailability, and targeting ability. While a number of DDS, such as liposomes, micelles, nanoparticles and hydrogels, have been developed for the purpose of effective drug delivery [1], most of them faced two critical issues: high systemic toxicity and low bioavailability. The most recent addition to the fields of DDS is nano-sized extracellular vesicles, such as exosomes and microvesicles, that possess organotropism [2], good bioavailability with little toxicity and immunogenicity [3].
The exosomes and microvesicles are a relatively new addition to the complex avenue of the intercellular communication. Their existence was known for decades by electron microscopy from pellets from plasma ultracentrifugation [4]. The presence of exosomes in in vitro cultured cells and in vivo tumor ascites was first reported by Dvorak et al. [5] in 1981. Later, exosomes found to be produced by nearly all types of cells including immune cells [6, 7], epithelial cells [8], neurons [9], mesenchymal stem cells [10] and red blood cells [11]. In addition, they were identified in most bodily fluids including blood plasma [12], cerebrospinal fluid [13], urine and amniotic fluids [14], breast milk [15] and saliva [16].
Exosomes are membrane-enclosed nanosphere of 30–150 nm in diameter originated from a subset of late endosomes (also known as multivesicular bodies, MVBs) during ceramide-dependent initiation phase. Fusion of outer membrane of MVBs with plasma membrane releases the exosomes [17] that deliver cargo including soluble and membrane bound proteins, lipids, mRNAs, microRNAs and chemical messengers into extracellular space (Fig. 1). Reflecting their subcellular origin, exosomes contain a number of endosomal membrane proteins, proteins involved in exosome biogenesis [18], vesicle trafficking proteins and plasma membrane-associated proteins [19, 20]. Similar to lipid rafts, they are rich in cholesterol, glycosphingolipids, and phosphatidylserine [21] accounting for their higher stability and rigidity. In addition to these, exosomes cargo large amounts of nucleic acids, including mRNAs, microRNAs (miRNAs) and long non-coding RNAs (lncRNAs) [22].
Fig. 1.
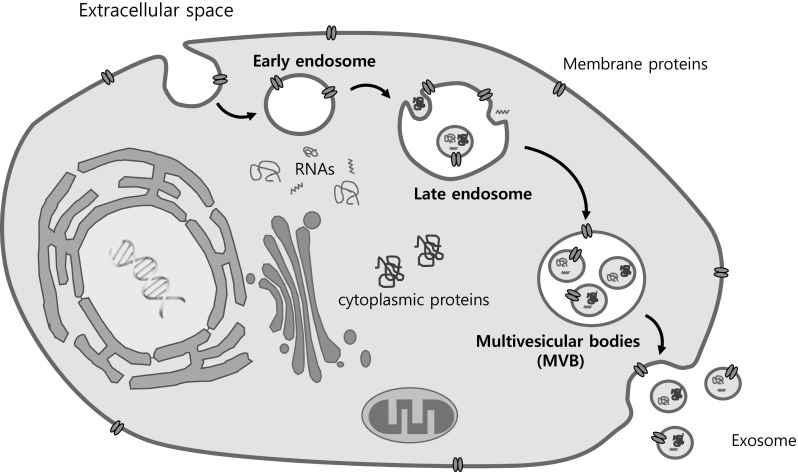
Biogenesis of exosomes from multivesicular endosome. Exosomes formed by invagination of endosomal membrane are secreted vesicles derived from intraluminal vesicles within multivesicular body (MVB). Upon fusion of MVB with plasma membrane, exosomes carrying proteins, RNAs and lipids that are derived from donor cells are released the in the extracellular space
Physiological function of exosomes
These subcellular particles are ubiquitous and secreted by virtually all types of cells in our body. Upon fusion with by local or distant target cells, exosomes deliver complex sets of biological information to recipient cells thereby modulating their behaviors by their molecular cargo. While nomenclatures of exosomes, microvesicles and apoptotic bodies were based on their sources and sizes, the terms of exosome and microvesicle are confusing and often used interchangeably due to their overlapping sizes as well as sharing common markers. A number of different strategies have been developed to isolate and/or purify these extracellular vesicles from biofluids, including ultracentrifugation, size exclusion chromatography, density gradient centrifugation, immunoaffinity-mediated sorting, microfluidics and filtration [23–25]. Since these technologies cannot distinguish exosomes and microvesicles, the functional assignment and physiological significance of these particles is a challenging project. Due to the complexity of molecular cargo including proteins, nucleic acids and lipids, exosomes can deliver multiple biological information at once. To date, large number of transcriptome and proteome analyses have been performed to elucidate the physiological function of exosomal components (many of them are cataloged in the ExoCarta database, www.exocarta.org) [26].
Exosomes are secreted from cells under change of physiological conditions by cell growth, cell injury, inflammation, hypoxia, oxidative stress and carcinogenesis [27]. For instances, exosomes is a potential mediator for development process through WNT and Hedgehog signaling that regulate cell proliferation and differentiation during embryonic development [28]. In addition, immune cells-derived exosomes accounts for a large population of circulating exosomes within blood [29]. Dendritic cells, a pivotal player of innate and adaptive immunity, are known to modulate T cell responses [30, 31] and natural killer (NK) cell function [32] via secreted exosomes. Exosomes derived from proinflammatory (M1-polarized) macrophages exhibited a trophism toward lymph nodes upon in vivo administration and induced strong cytotoxic T cell response [33] suggesting their potential use as an immunoadjuvant for cancer therapy.
Recently, exosomes are considered as novel biomarkers for diagnosis of early detection, chemoresistance, therapy response and poor prognosis leading to relapse in cancer research. Cancer-derived exosomes appears to play important roles in cancer initiation, promotion and progression by cell–cell communication [34] and thus exosomal biomarkers can serve as a potential indicator of real-time status of the disease progression. Based on the possibility to isolate exosomes from human body fluid, exosomal biomarkers have been widely investigated to screen progression of cancers with low-cost, short time, reduced pain by liquid biopsy [35]. Here, we will highlight and discuss the more recent issues related to molecular mechanisms of exosome in cancer development and the utilization of exosomes as DDS for their potential clinical applications.
Exosomes in cancer progression and diagnostics
Unlike normal physiology, the onset of cancer may alter the molecular cargo of exosomes, i.e., lipids, proteins, mRNAs, miRNAs and lncRNAs. Recently, exosomal RNAs have been widely reported in cancer progression with aspects of cell proliferation [36], migration [37], apoptosis, metastasis [38], angiogenesis [39], and chemoresistance [40]. The lncRNAs in cancer cells-derived exosomes regulates survival rates of the cells by transferring genetic information via cell-to-cell communication for mediating cancer microenvironment. In colon cancer, lncRNA H19 in cancerous cell-derived exosomes activates β-catenin signaling leading to cancer development and chemoresistance [41]. In addition, first identified lncRNA MALAT-1 related in lung cancer progression isolated from serum exosome stimulates cell proliferation and migration whereas it suppresses cell death of lung cancer cells [42]. Also, lncRNAs functions as an oncogene by increasing non-controlled proliferation of cancerous cells originated from liver, thyroid, bladder, lung and ovary [43–45]. For example, lncRNA FAL1 was upregulated in tumor tissues and serum exosomes from hepatocellular carcinoma (HCC) patients and the transfer of exosomal FAL1 to HCC cells increased their proliferative and migratory capacity which was mediated by competitive binding to miR-1236 [46]. Taken together, these studies suggest that exosomal lncRNAs regulate key cellular events, via epigenetic and transcriptional regulation, in tumor progression and metastasis.
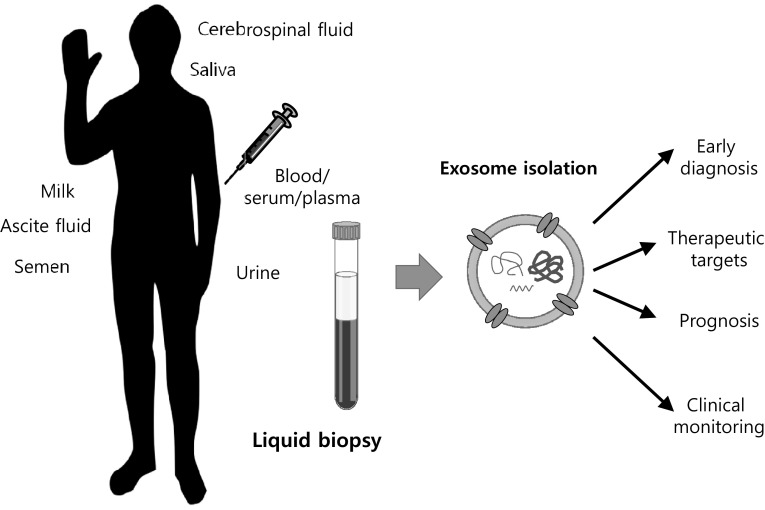
For diagnosis of early stage cancers, it is important to develop an optimal biomarker. Although tissue biopsy has been commonly used for histopathological analysis, it is hard to use in cancer screening and determination of heterogeneous characteristics of cancers for therapy. In addition, the tissue biopsy has limitations in cost burden, invasive tissue collection, pains, and identification of genetic changes for monitoring therapy response and prognosis of cancers [47]. In order to overcome these limits, new approaches for detection of early diagnosis, chemoresistance, relapse and microenvironment of cancers are required in these days. Liquid biopsy has a variety of advantages of diagnosis, treatment and therapy response in cancer research based on genetic materials of exosomes and circulating tumor cells (Fig. 2) [35, 48]. Especially, analytical methods of exosomal genomic materials have been developed for cancer diagnosis and prevention (Table 1). For examples, serum exosomal expression of prostate cancer associated transcript 1 (PCAT-1), upregulated in bladder cancer 1 (UBC1), small nucleolar RNA host gene 16 (SNHG16), and H19 belonging to lncRNAs show high diagnostic accuracy for bladder cancer [49, 50]. In addition, exosomal miR-21, miR-105, miR-155, miR-301, and miR-1246 derived from the blood of breast cancer patients can be used as biomarkers to predict the progression of malignancy and metastasis [51–53]. In colorectal cancer patients resistant to treatment with cetuximab, the expression of urothelial carcinoma-associated 1 (UCA1), the lncRNAs, is high in serum exosome [54]. Diagnosis of esophageal cancer can also be performed through the exosome of saliva [55]. The expression of GOLM1-NAA35 chimeric RNA can be used to predict the response to chemoradiation. Exosomal miRNAs derived from cancerous cells are important for metastatic procedure and drug resistance. MiR-210, abundantly detected in serum exosome and tissues from hepatocellular carcinoma patients and exosome-derived from hepatocellular carcinoma cells, is transmitted into adjacent endothelial cells leading to angiogenesis by suppressing SMAD4 and STAT6 activities [56]. Furthermore, prostate cancer derived exosomes overexpressing miR-100-5p, miR-21-5p and miR-139-5p increased the expression of receptor activator of nuclear factor kappa Β ligand (RANKL) and metalloproteinases-2, -9 and -13 in cancer-associated fibroblasts that can lead to cancer progression and metastasis [57]. The expression of alpha-2-HS-glycoprotein (AHSG), extracellular matrix protein 1 (ECM1), and miR-21-5p, miR-126-3p, and miR-140-5p is higher in exosome in patients with lung cancer than in healthy group, so it could be used as a good diagnostic marker for lung cancer [58, 59]. Also, serum exosomal miR-99 is highly upregulated in ovarian cancer patients as compared to benign tumor patients or healthy women. However, the expression of miR-99 significantly decreases after surgical management of cancers. In addition, neighboring human peritoneal mesothelial cells transfected miR-99 promotes invasive properties by an increase in fibronectin and vitronetin leading to cancer growth [60]. The mRNA of a specific gene also shows clinical utility in the diagnosis of cancer through exosomes. Expression of WASF2 mRNA in exosomes isolated in serum from patients with pancreatic cancer is strongly correlated with risk of disease [61]. Highly expressed miR-30d-5p in response to hypoxia, which contributes to the high risk of locally advanced rectal cancer, helps to predict metastatic progression using plasma exosomes in rectal cancer patients [62]. Likewise, exosomal miRNAs are important for diagnostics and therapy response in cancers.
Fig. 2.
Exosomes present in a number of biological fluids, including cerebrospinal fluid, milk, saliva, blood (serum/plasma) and semen can be obtained using a liquid biopsy that can be useful for early diagnosis, targeted therapy, prognosis and clinical monitoring
Table 1.
Examples of cancer diagnosis using exosome
| Disease target | Clinical sample | Measured biomarker | Clinical application | References |
|---|---|---|---|---|
| Bladder cancer | Serum | PCAT, UBC1, and SNHG16 | Malignant progression | [49] |
| H19 | [50] | |||
| Breast cancer | Serum | miR-21 and miR-105 | Metastatic progression | [51] |
| Plasma | miR-155 and miR-301 | Malignant progression | [52] | |
| miR-1246 | [53] | |||
| Colorectal cancer | Serum | UCA1 | Drug resistance | [54] |
| Esophageal carcinoma | Saliva | GOLM1-NAA35 chimeric RNA | Therapeutic response | [55] |
| Hepatocellular carcinoma | Serum | miR-210 | Microvessel density | [56] |
| Lung cancer | Serum | AHSG and ECM1 | Malignant progression | [58] |
| miR-21-5p, miR-126-3p, and miR-140-5p | [59] | |||
| Ovarian cancer | Serum | miR-99a-5p | Malignant progression | [60] |
| Pancreatic cancer | Serum | WASF2 mRNA | Malignant progression | [61] |
| Rectal cancer | Plasma | miR-30d-5p | Metastatic progression | [62] |
Exosomes as drug delivery system for oncotherapy
The most common example of drug delivery systems (DDS) for oncotherapy include synthetic polymers, liposomes, micelles, super magnetic particles, protein and recombinant viral vectors have been developed [63–67] and some of them are currently in clinical testing [68]. A more recent addition includes smart or intelligent polymeric hydrogels that respond to external environmental changes and encapsulate or release its cargo [69]. While some of these innovative drug delivery systems have been exploited and may lead to clinical benefits in cancer patients, there are many potential barriers hinder their efficient drug delivery for their toxicity, bioavailability, stability, and target delivery. Although chemical modification, their systemic bioavailability and stability can be pursued [70–72], these strategies are associated with stronger immunogenicity against the carriers thereby leading to their quicker clearance in vivo [73, 74]. In this regards, the use of exosome provides an attractive alternative for targeted drug delivery.
Unlike synthetic drug delivery systems, exosomal membrane is derived from donor cell, they are non-immunogenic and thus may avoid rapid clearance from circulation and thereby increasing their bioavailability [75, 76]. Chemical drugs, proteins, RNAs, DNAs and lipids can be loaded with into exosomal cargo through different methods that can enhance their bioavailability while limiting toxicity. They are an ideal carriers for lipid-soluble drugs to the target cells. Immunogenic or toxic drugs can be encapsulated and be transmitted to target cells thereby reducing their systemic toxicity. In addition, they possess blood brain barrier passing ability [77], homing ability and cell/tissue tropism due to their surface proteins [78, 79] Exosomes can be further engineered endogenously or exogenously for the loading of therapeutic molecules (such as chemicals, nucleic acids, proteins or lipids) in order to enhance targeting efficiency and bioavailability.
Exogenous loading of therapeutic molecules to exosomes
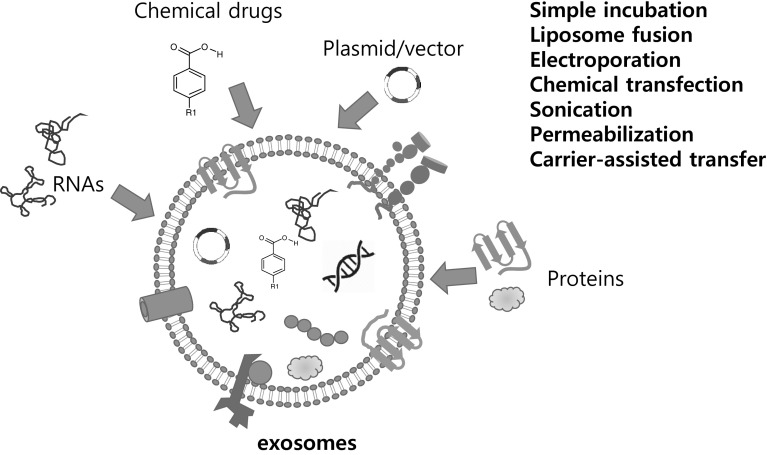
Exosomes can be loaded with drug of interests (chemicals, DNAs, RNAs, Proteins or lipids) upon purification from producing cells or biofluids. This can be achieved by passive diffusion of hydrophobic molecules, mechanical (such as sonication), electroporation or chemical-mediated transfer (lipofection) of hydrophilic molecules by (Fig. 3). Water-insoluble chemicals (such as anti-cancer drugs) can interact and cross hydrophobic exosomal membrane under ambient conditions thereby increasing in vivo drug bioavailability. Indeed, exosome loaded with chemotherapeutic drugs exhibited stronger cytotoxicity against drug resistant cancer cell lines in vitro [80], stronger anti-tumor activity in vivo than that of free drugs [81].
Fig. 3.
Ex vivo loading of therapeutic molecules into exosomes. Exosomes can be loaded with RNAs, chemical drugs/prodrugs, plasmid/vectors, and proteins ex vivo. Therapeutic molecules can be loaded into the purified exosomes from donor cells by simple incubation, liposomes, electroporation, freeze thawing, sonication and carrier-assisted delivery
Chemotherapeutics and large molecules, such as miRNAs and siRNAs, can also be incorporated into exosomes by electroporation [82]. Electric field creates transient pores into exosomal membrane allowing temporal movement of drugs into the exosomal lumen. Since exosomes are the natural delivery vehicles for RNAs [83, 84] which are unstable and extremely inefficient in target specificity in free form, exosomal loading of these molecules overcome these limitations. A number of studies validated successful delivery of exosome-loaded siRNAs and selective silencing of target genes [85–88]. Drug loading efficiency of chemical drugs and siRNA into the lumen of exosomes were as high as 25% for siRNA [82, 85] and 11.7% for chemical drugs [77], the accurate measurement of loading efficiency is not easy due to molecular complexity of exosomes. In addition, electroporation is known to induce vesicular aggregation [89] or siRNA aggregation [90] thereby affecting the integrity of exosome or therapeutic efficacy of RNA molecules.
In order to increase the loading efficiency and preserve the integrity of exosomes, various strategies were proposed. Pre-formed mixture of negative charged RNAs with cationic liposome can be fused with exosomes thereby incorporating RNA molecules [85, 91]. Alternatively, the hydrophobicity of siRNA or therapeutic molecules can be modified to increase its loading efficiency into exosomes. This strategy was validated in silencing of Huntington RNA by siRNA-loaded exosomes in vitro cultured neuronal cells as well as in vivo mouse striatum upon infusion [92]. Exosomal aggregation upon electroporation can be minimized by a use of trehalose pulse media (TPM) [89].
While exosomes are a natural vehicle of proteins [10], their innate chemical properties hinder their uptake into exosomes. Various strategies are developed to overcome this problem, such as simple incubation, physical insults (freeze-thawing, sonication, extrusion), permeabilization, liposome-mediate fusion and polymer-mediated transfer [93]. Haney et al. [94] reported various methods for loading exosome with catalase, 250 kDa complex and compared their loading efficiency and therapeutic efficacy in vivo. The study showed that permeabilization with saponin, sonication and extrusion among tested methods resulted in good loading efficiency of catalase while maintaining the exosomal integrity. Furthermore, intranasal administration of catalase-loaded exosome led to behavioral recovery in murine model of Parkinson’s disease demonstrating that exosome can cross the blood brain barrier for brain tumor therapy. However, mechanical or physical insults on exosomes can compromise membrane integrity as well as protein integrity [93] thereby significantly affecting their therapeutic activity.
Endogenous loading of therapeutic molecules to exosomes
Although exogenous loading of therapeutic molecules has been successfully demonstrated, clinical use requires scale production of exosome prior to in vitro drug packaging. In this regards, production and isolation of large quantity of exosome carrying therapeutic molecules from host cells can be an attractive alternative. Endogenous loading of therapeutic exosomes containing more therapeutic drugs (proteins and/or RNAs) can be accomplished by engineering host cells chemically or genetically. Pretreating or priming cells with chemical drugs (free or vehicle-mediated) may lead to an increase of cytoplasmic drug concentration and subsequently to their uptake into exosomal cargo.
Exosomes can be engineered to cargo chemotherapeutics at cellular level. For instance, exosomes isolated from paclitaxel-primed mesenchymal stem cells exhibited strong anti-proliferative activity to a pancreatic cancer cell line in vitro [95] suggesting that exosome producing cells can serve as a packaging factory chemical drugs. In another study, a synthetic fusogenic liposome-mediated transfer of chemical drugs to exosome-producing cells led to an efficient loading of the chemical drugs into exosomes [96]. Although these studies clearly demonstrated the feasibility of the endogenous drug loading to exosomes, the pitfall of these approaches is the low yield of exosomes from unmodified cells. This obstacle can be solved by one of the following 3 strategies. First, as Jang et al. [97] and Kalimuthu et al. [98] reported, mechanical extrusion of drug-primed cells generates large quantity of drug-loaded exosome-like (exosome-mimetic) nanovesicles with strong antitumor activity in vivo. Culturing cells in 3-dimension [99] or priming with cytokines, chemicals [100] or physical means including hypoxia [101] or hyperthermia are all known to significantly affect the yield of exosomes. Finally, exosome production from engineered cells can be enhanced by transducing some of the key genes that boost the exosome biogenesis. Indeed, Kojima et al. [102] demonstrated the feasibility of such engineered exosome for increased yield in vitro as well as their therapeutic usefulness in in vivo model of Parkinson’s disease and potentially for brain tumors.
Studies have shown that exosomes isolated from host cells transfected with miRNA-encoding vectors could deliver the target miRNAs in animal models [87, 103]. For example, miR-146b-overexpressing and miR-122-overexpressing exosomes from engineered mesenchymal stem cells inhibited the glioma growth in rat brain [103] and significantly increased the chemosensitivity of hepatocellular carcinoma to sorafenib [104], respectively. Exosome loaded with anti-miR-214, siRNA to GRP78, and siRNA to PLK-1 could reduce gastric tumor growth [105] and hepatocellular carcinoma [106], respectively, by reversing their chemoresistance. Recent studies showed that RNA packaging into exosomes during biogenesis are mediated by a sets of RNA binding proteins (RBPs) and overexpression of these RBPs led to higher levels of exosomal RNAs [107, 108] suggesting that engineering of host cells with these RNA and/or protein sorting machineries may provide efficient tools for the packaging of theses therapeutic molecules into exosomes and for their clinical applications. Thus, the delineation and exploitation of exosomal sorting machineries as well as ligand-receptor web of exosomal proteins will open new avenues for targeted drug delivery.
While exosomes contain proteins and RNA cargo in vivo and are difficult to incorporate these large molecules in vitro, the genetic modification of exosome-producing cells is one of the most preferred strategy for active packaging of therapeutic RNAs or proteins. Exosomal delivery of TRIM3, a potential tumor suppressor for gastric cancer cell proliferation and migration, inhibited gastric cancer progression and metastasis [109]. Exosomes from TRAIL- or suicide gene-transduced exosome producing cells induced a significant tumor inhibition in animal tumor models [110, 111]. Studies also demonstrated that exosomes from genetically modified host cells with transgenes encoding model proteins, including ovalbumin, catalase and glial cell-line derived neurotropic factor, successfully delivered the proteins to target tissues and exhibit therapeutic efficacy in the animal model of Parkinson’s disease. Utilizing of targeting ligands on the engineered exosome can further enhance the targeting efficiency [112]. Ohno et al. [87] demonstrated that targeted delivery of miRNA-loaded exosomes could be greatly enhanced by EGFR ligand expression on the surface of exosomes in a mouse model. The finding of Grapp et al. [113] that folate receptor-α on exosomal surface plays an important role in the blood–brain barrier (BBB) crossing and delivery of therapeutics into brain parenchyma suggesting that engineering host cells with this exosomal membrane protein can be used in cerebral drug targeting for the treatment of neurodegenerative disease or malignancy. Engineering exosomes to carry ligands for adhesion molecules or surface receptors on the exosomal membrane can facilitate the targeted delivery of their cargo to cells with corresponding receptors [96, 111–113]. These studies highlighted the therapeutic potential of engineered exosomes as DDS for the treatment of cancer. Translational applications of engineered exosomes for cancer therapy are summarized in Table 2.
Table 2.
Engineered exosomes for cancer therapy in preclinical studies
| Therapeutic molecules | Exosome origin | Disease target | Drug loading | References |
|---|---|---|---|---|
| Chemical | ||||
| Paclitaxel | MSC | Pancreatic cancer | Endogenous, incubation | [95] |
| MSC | Breast cancer | Exogenous, extrusion | [98] | |
| RAW264.7 | Drug-resistant lung cancer | Exogenous, sonication | [80] | |
| Doxorubicin | U937 RAW264.7 | Colon cancer | Exogenous, extrusion | [97] |
| DCs expressing iRGD | Breast cancer | Exogenous, electroporation | [112] | |
| miRNA/siRNA | ||||
| Let-7a | HEK293 expressing GE11 | Breast cancer with EGFR | Endogenous, transfection | [87] |
| miR-146b | MSC | Glioma | Endogenous, transfection | [103] |
| miR-122 | MSC | Hepatocellular carcinoma | Endogenous, transfection | [104] |
| Anti-miR-214 | HEK293T | Gastric cancer | Endogenous, transfection | [105] |
| PLK-1 siRNA | HEK293 + MSC | Bladder cancer | Exogenous, electroporation | [88] |
| GRP78 siRNA | MSC | Hepatocellular carcinoma | Endogenous, lipofection | [106] |
| Proteins/mRNA | ||||
| TRIM3 | Gastric cancer cells | Gastric cancer | Endogenous, transfection | [109] |
| CD-UPRT fusion protein | HEK293T | Schwannoma | Endogenous, transfection | [111] |
| TRAIL | K562 | Lymphoma | Endogenous, transduction | [110] |
Conclusions
Exosomes clearly play key roles in cancer development. Exosomal biomarkers can greatly improve theragnostics of cancer patients by liquid biopsy. In addition, the use of engineered exosomes may represent a new class of drug delivery system for their ability to cross biological barriers with little or no safety concerns associated with therapeutics, including drug toxicity, immune responses, biodistribution and targeted delivery. Low stability and/or transducibility of therapeutics (chemical drugs, proteins, and RNAs) in circulation can be solved by encapsulating them into exosomes. To increase the therapeutic efficacy of exosomes, a number of endogenous or exogenous loading strategies have been developed and validated in vitro as well as in vivo animal models. Promising results of this cell-free therapeutics were obtained from a number of relevant animal models for human diseases and clinical translation of exosomes has already initiated in cancer therapy and organ transplantation and their safety was validated. Although further studies are required to standardize methods involved in exosome purification and characterization for their application as a drug delivery system in clinical research, the engineered exosomes may serve as an ideal DDS for cancer therapy due to their high biocompatibility, minimal toxicity, low immunogenicity with high target specificity in future. However, successful clinical translation of exosome-based therapeutics critically depends on not only our understanding of the therapeutic mechanisms of exosomes, but also our ability to isolate as well as design exosomes for their optimal potency to cure or treat diseases.
Acknowledgements
This research was supported by grants from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health and Welfare, Republic of Korea (No. HI17C0929) and from the Bio & Medical Technology Development Program of the National Research Foundation (NRF), funded by Ministry of Science and ICT (MSIT) (No. 2017M3A9B4042583), Republic of Korea.
Author contributions
WL: manuscript writing, conception and design, HSK: manuscript writing and final approval of manuscript.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical statement
Ethical approval and consent to participate is not applicable to this article as no data were generated or analyzed during the current study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Whasun Lim, Email: wlim@kookmin.ac.kr.
Han-Soo Kim, Phone: +82-33-649-7237, Email: hankim63@gmail.com.
References
- 1.Seo JY, Lee B, Kang TW, Noh JH, Kim MJ, Ji YB, et al. Electrostatically interactive injectable hydrogels for drug delivery. Tissue Eng Regen Med. 2018;15:513–520. doi: 10.1007/s13770-018-0146-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Antimisiaris SG, Mourtas S, Marazioti A. Exosomes and exosome-inspired vesicles for targeted drug delivery. Pharmaceutics. 2018;10:218. doi: 10.3390/pharmaceutics10040218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee JY, Kim HS. Extracellular vesicles in neurodegenerative diseases: a double-edged sword. Tissue Eng Regen Med. 2017;14:667–678. doi: 10.1007/s13770-017-0090-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wolf P. The nature and significance of platelet products in human plasma. Br J Haematol. 1967;13:269–288. doi: 10.1111/j.1365-2141.1967.tb08741.x. [DOI] [PubMed] [Google Scholar]
- 5.Dvorak HF, Quay SC, Orenstein NS, Dvorak AM, Hahn P, Bitzer AM, et al. Tumor shedding and coagulation. Science. 1981;212:923–924. doi: 10.1126/science.7195067. [DOI] [PubMed] [Google Scholar]
- 6.Raposo G, Nijman HW, Stoorvogel W, Liejendekker R, Harding CV, Melief CJ, et al. B lymphocytes secrete antigen-presenting vesicles. J Exp Med. 1996;183:1161–1172. doi: 10.1084/jem.183.3.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Laulagnier K, Motta C, Hamdi S, Roy S, Fauvelle F, Pageaux JF, et al. Mast cell- and dendritic cell-derived exosomes display a specific lipid composition and an unusual membrane organization. Biochem J. 2004;380:161–171. doi: 10.1042/BJ20031594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Niel G, Raposo G, Candalh C, Boussac M, Hershberg R, Cerf-Bensussan N, et al. Intestinal epithelial cells secrete exosome-like vesicles. Gastroenterology. 2001;121:337–349. doi: 10.1053/gast.2001.26263. [DOI] [PubMed] [Google Scholar]
- 9.Faure J, Lachenal G, Court M, Hirrlinger J, Chatellard-Causse C, Blot B, et al. Exosomes are released by cultured cortical neurones. Mol Cell Neurosci. 2006;31:642–648. doi: 10.1016/j.mcn.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 10.Kim HS, Choi DY, Yun SJ, Choi SM, Kang JW, Jung JW, et al. Proteomic analysis of microvesicles derived from human mesenchymal stem cells. J Proteome Res. 2012;11:839–849. doi: 10.1021/pr200682z. [DOI] [PubMed] [Google Scholar]
- 11.Johnstone RM, Bianchini A, Teng K. Reticulocyte maturation and exosome release: transferrin receptor containing exosomes shows multiple plasma membrane functions. Blood. 1989;74:1844–1851. [PubMed] [Google Scholar]
- 12.Caby MP, Lankar D, Vincendeau-Scherrer C, Raposo G, Bonnerot C. Exosomal-like vesicles are present in human blood plasma. Int Immunol. 2005;17:879–887. doi: 10.1093/intimm/dxh267. [DOI] [PubMed] [Google Scholar]
- 13.Vella LJ, Sharples RA, Lawson VA, Masters CL, Cappai R, Hill AF. Packaging of prions into exosomes is associated with a novel pathway of PrP processing. J Pathol. 2007;211:582–590. doi: 10.1002/path.2145. [DOI] [PubMed] [Google Scholar]
- 14.Keller S, Rupp C, Stoeck A, Runz S, Fogel M, Lugert S, et al. CD24 is a marker of exosomes secreted into urine and amniotic fluid. Kidney Int. 2007;72:1095–1102. doi: 10.1038/sj.ki.5002486. [DOI] [PubMed] [Google Scholar]
- 15.Admyre C, Johansson SM, Qazi KR, Filén JJ, Lahesmaa R, Norman M, et al. Exosomes with immune modulatory features are present in human breast milk. J Immunol. 2007;179:1969–1978. doi: 10.4049/jimmunol.179.3.1969. [DOI] [PubMed] [Google Scholar]
- 16.Ogawa Y, Kanai-Azuma M, Akimoto Y, Kawakami H, Yanoshita R. Exosome-like vesicles with dipeptidyl peptidase IV in human saliva. Biol Pharm Bull. 2008;31:1059–1062. doi: 10.1248/bpb.31.1059. [DOI] [PubMed] [Google Scholar]
- 17.Trajkovic K, Hsu C, Chiantia S, Rajendran L, Wenzel D, Wieland F, et al. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science. 2008;319:1244–1247. doi: 10.1126/science.1153124. [DOI] [PubMed] [Google Scholar]
- 18.Conde-Vancells J, Rodriguez-Suarez E, Embade N, Gil D, Matthiesen R, Valle M, et al. Characterization and comprehensive proteome profiling of exosomes secreted by hepatocytes. J Proteome Res. 2008;7:5157–5166. doi: 10.1021/pr8004887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Escola JM, Kleijmeer MJ, Stoorvogel W, Griffith JM, Yoshie O, Geuze HJ. Selective enrichment of tetraspan proteins on the internal vesicles of multivesicular endosomes and on exosomes secreted by human B-lymphocytes. J Biol Chem. 1998;273:20121–20127. doi: 10.1074/jbc.273.32.20121. [DOI] [PubMed] [Google Scholar]
- 20.Théry C, Regnault A, Garin J, Wolfers J, Zitvogel L, Ricciardi-Castagnoli P, et al. Molecular characterization of dendritic cell-derived exosomes. Selective accumulation of the heat shock protein hsc73. J Cell Biol. 1999;147:599–610. doi: 10.1083/jcb.147.3.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Skotland T, Hessvik NP, Sandvig K, Llorente A. Exosomal lipid composition and the role of ether lipids and phosphoinositides in exosome biology. J Lipid Res. 2019;60:9–18. doi: 10.1194/jlr.R084343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ, Lötvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 23.Xu R, Greening DW, Zhu HJ, Takahashi N, Simpson RJ. Extracellular vesicle isolation and characterization: toward clinical application. J Clin Invest. 2016;126:1152–1162. doi: 10.1172/JCI81129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li P, Kaslan M, Lee SH, Yao J, Gao Z. Progress in exosome isolation techniques. Theranostics. 2017;7:789–804. doi: 10.7150/thno.18133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Konoshenko MY, Lekchnov EA, Vlassov AV, Laktionov PP. Isolation of extracellular vesicles: general methodologies and latest trends. Biomed Res Int. 2018;2018:8545347. doi: 10.1155/2018/8545347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mathivanan S, Simpson RJ. ExoCarta: a compendium of exosomal proteins and RNA. Proteomics. 2009;9:4997–5000. doi: 10.1002/pmic.200900351. [DOI] [PubMed] [Google Scholar]
- 27.Ståhl AL, Johansson K, Mossberg M, Kahn R, Karpman D. Exosomes and microvesicles in normal physiology, pathophysiology, and renal diseases. Pediatr Nephrol. 2019;34:11–30. doi: 10.1007/s00467-017-3816-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McGough IJ, Vincent JP. Exosomes in developmental signalling. Development. 2016;143:2482–2493. doi: 10.1242/dev.126516. [DOI] [PubMed] [Google Scholar]
- 29.Ismail N, Wang Y, Dakhlallah D, Moldovan L, Agarwal K, Batte K, et al. Macrophage microvesicles induce macrophage differentiation and miR-223 transfer. Blood. 2013;121:984–995. doi: 10.1182/blood-2011-08-374793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chaput N, Flament C, Viaud S, Taieb J, Roux S, Spatz A, et al. Dendritic cell derived-exosomes: biology and clinical implementations. J Leukoc Biol. 2006;80:471–478. doi: 10.1189/jlb.0206094. [DOI] [PubMed] [Google Scholar]
- 31.Näslund TI, Gehrmann U, Qazi KR, Karlsson MC, Gabrielsson S. Dendritic cell-derived exosomes need to activate both T and B cells to induce antitumor immunity. J Immunol. 2013;190:2712–2719. doi: 10.4049/jimmunol.1203082. [DOI] [PubMed] [Google Scholar]
- 32.Viaud S, Terme M, Flament C, Taieb J, André F, Novault S, et al. Dendritic cell-derived exosomes promote natural killer cell activation and proliferation: a role for NKG2D ligands and IL-15Ralpha. PLoS One. 2009;4:e4942. doi: 10.1371/journal.pone.0004942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cheng L, Wang Y, Huang L. Exosomes from M1-polarized macrophages potentiate the cancer vaccine by creating a pro-inflammatory microenvironment in the lymph node. Mol Ther. 2017;25:1665–1675. doi: 10.1016/j.ymthe.2017.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rajagopal C, Harikumar KB. The origin and functions of exosomes in cancer. Front Oncol. 2018;8:66. doi: 10.3389/fonc.2018.00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Palmirotta R, Lovero D, Cafforio P, Felici C, Mannavola F, Pellè E, et al. Liquid biopsy of cancer: a multimodal diagnostic tool in clinical oncology. Ther Adv Med Oncol. 2018;10:1758835918794630. doi: 10.1177/1758835918794630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Richards KE, Zeleniak AE, Fishel ML, Wu J, Littlepage LE, Hill R. Cancer-associated fibroblast exosomes regulate survival and proliferation of pancreatic cancer cells. Oncogene. 2017;36:1770–1778. doi: 10.1038/onc.2016.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liao J, Liu R, Shi YJ, Yin LH, Pu YP. Exosome-shuttling microRNA-21 promotes cell migration and invasion-targeting PDCD4 in esophageal cancer. Int J Oncol. 2016;48:2567–2579. doi: 10.3892/ijo.2016.3453. [DOI] [PubMed] [Google Scholar]
- 38.Zhang H, Deng T, Liu R, Bai M, Zhou L, Wang X, et al. Exosome-delivered EGFR regulates liver microenvironment to promote gastric cancer liver metastasis. Nat Commun. 2017;8:15016. doi: 10.1038/ncomms15016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maji S, Chaudhary P, Akopova I, Nguyen PM, Hare RJ, Gryczynski I, et al. Exosomal Annexin II promotes angiogenesis and breast cancer metastasis. Mol Cancer Res. 2017;15:93–105. doi: 10.1158/1541-7786.MCR-16-0163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Au Yeung CL, Co NN, Tsuruga T, Yeung TL, Kwan SY, Leung CS, et al. Exosomal transfer of stroma-derived miR21 confers paclitaxel resistance in ovarian cancer cells through targeting APAF1. Nat Commun. 2016;7:11150. doi: 10.1038/ncomms11150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ren J, Ding L, Zhang D, Shi G, Xu Q, Shen S, et al. Carcinoma-associated fibroblasts promote the stemness and chemoresistance of colorectal cancer by transferring exosomal lncRNA H19. Theranostics. 2018;8:3932–3948. doi: 10.7150/thno.25541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang R, Xia Y, Wang Z, Zheng J, Chen Y, Li X, et al. Serum long non coding RNA MALAT-1 protected by exosomes is up-regulated and promotes cell proliferation and migration in non-small cell lung cancer. Biochem Biophys Res Commun. 2017;490:406–414. doi: 10.1016/j.bbrc.2017.06.055. [DOI] [PubMed] [Google Scholar]
- 43.Hu X, Feng Y, Zhang D, Zhao SD, Hu Z, Greshock J, et al. A functional genomic approach identifies FAL1 as an oncogenic long noncoding RNA that associates with BMI1 and represses p21 expression in cancer. Cancer Cell. 2014;26:344–357. doi: 10.1016/j.ccr.2014.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pan C, Yao G, Liu B, Ma T, Xia Y, Wei K, et al. Long noncoding RNA FAL1 promotes cell proliferation, invasion and epithelial-mesenchymal transition through the PTEN/AKT signaling axis in non-small cell lung cancer. Cell Physiol Biochem. 2017;43:339–352. doi: 10.1159/000480414. [DOI] [PubMed] [Google Scholar]
- 45.Xue M, Chen W, Xiang A, Wang R, Chen H, Pan J, et al. Hypoxic exosomes facilitate bladder tumor growth and development through transferring long non-coding RNA-UCA1. Mol Cancer. 2017;16:143. doi: 10.1186/s12943-017-0714-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li B, Mao R, Liu C, Zhang W, Tang Y, Guo Z. LncRNA FAL1 promotes cell proliferation and migration by acting as a CeRNA of miR-1236 in hepatocellular carcinoma cells. Life Sci. 2018;197:122–129. doi: 10.1016/j.lfs.2018.02.006. [DOI] [PubMed] [Google Scholar]
- 47.Esposito A, Criscitiello C, Locatelli M, Milano M, Curigliano G. Liquid biopsies for solid tumors: understanding tumor heterogeneity and real time monitoring of early resistance to targeted therapies. Pharmacol Ther. 2016;157:120–124. doi: 10.1016/j.pharmthera.2015.11.007. [DOI] [PubMed] [Google Scholar]
- 48.Perakis S, Speicher MR. Emerging concepts in liquid biopsies. BMC Med. 2017;15:75. doi: 10.1186/s12916-017-0840-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang S, Du L, Wang L, Jiang X, Zhan Y, Li J, et al. Evaluation of serum exosomal LncRNA-based biomarker panel for diagnosis and recurrence prediction of bladder cancer. J Cell Mol Med. 2019;23:1396–1405. doi: 10.1111/jcmm.14042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang J, Yang K, Yuan W, Gao Z. Determination of serum exosomal H19 as a noninvasive biomarker for bladder cancer diagnosis and prognosis. Med Sci Monit. 2018;24:9307–9316. doi: 10.12659/MSM.912018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rodríguez-Martínez A, de Miguel-Pérez D, Ortega FG, García-Puche JL, Robles-Fernández I, Exposito J, et al. Exosomal miRNA profile as complementary tool in the diagnostic and prediction of treatment response in localized breast cancer under neoadjuvant chemotherapy. Breast Cancer Res. 2019;21:21. doi: 10.1186/s13058-019-1109-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stevic I, Müller V, Weber K, Fasching PA, Karn T, Marmé F, et al. Specific microRNA signatures in exosomes of triple-negative and HER2-positive breast cancer patients undergoing neoadjuvant therapy within the GeparSixto trial. BMC Med. 2018;16:179. doi: 10.1186/s12916-018-1163-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhai LY, Li MX, Pan WL, Chen Y, Li MM, Pang JX, et al. In situ detection of plasma exosomal MicroRNA-1246 for breast cancer diagnostics by a Au nanoflare probe. ACS Appl Mater Interfaces. 2018;10:39478–39486. doi: 10.1021/acsami.8b12725. [DOI] [PubMed] [Google Scholar]
- 54.Yang YN, Zhang R, Du JW, Yuan HH, Li YJ, Wei XL, et al. Predictive role of UCA1-containing exosomes in cetuximab-resistant colorectal cancer. Cancer Cell Int. 2018;18:164. doi: 10.1186/s12935-018-0660-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lin Y, Dong H, Deng W, Lin W, Li K, Xiong X, et al. Evaluation of salivary exosomal chimeric GOLM1-NAA35 RNA as a potential biomarker in esophageal carcinoma. Clin Cancer Res. 2019. 10.1158/078-0432.CCR-18-3169. [DOI] [PubMed]
- 56.Lin XJ, Fang JH, Yang XJ, Zhang C, Yuan Y, Zheng L, et al. Hepatocellular carcinoma cell-secreted exosomal MicroRNA-210 promotes angiogenesis in vitro and in vivo. Mol Ther Nucleic Acids. 2018;11:243–252. doi: 10.1016/j.omtn.2018.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sánchez CA, Andahur EI, Valenzuela R, Castellon EA, Fulla JA, Ramos CG, et al. Exosomes from bulk and stem cells from human prostate cancer have a differential microRNA content that contributes cooperatively over local and pre-metastatic niche. Oncotarget. 2016;7:3993–4008. doi: 10.18632/oncotarget.6540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Niu L, Song X, Wang N, Xue L, Song X, Xie L. Tumor-derived exosomal proteins as diagnostic biomarkers in non-small cell lung cancer. Cancer Sci. 2019;110:433–442. doi: 10.1111/cas.13862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Feng M, Zhao J, Wang L, Liu J. Upregulated expression of serum exosomal microRNAs as diagnostic biomarkers of lung adenocarcinoma. Ann Clin Lab Sci. 2018;48:712–718. [PubMed] [Google Scholar]
- 60.Yoshimura A, Sawada K, Nakamura K, Kinose Y, Nakatsuka E, Kobayashi M, et al. Exosomal miR-99a-5p is elevated in sera of ovarian cancer patients and promotes cancer cell invasion by increasing fibronectin and vitronectin expression in neighboring peritoneal mesothelial cells. BMC Cancer. 2018;18:1065. doi: 10.1186/s12885-018-4974-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kitagawa T, Taniuchi K, Tsuboi M, Sakaguchi M, Kohsaki T, Okabayashi T, et al. Circulating pancreatic cancer exosomal RNAs for detection of pancreatic cancer. Mol Oncol. 2019;13:212–227. doi: 10.1002/1878-0261.12398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bjørnetrø T, Redalen KR, Meltzer S, Thusyanthan NS, Samiappan R, Jegerschöld C, et al. An experimental strategy unveiling exosomal microRNAs 486-5p, 181a-5p and 30d-5p from hypoxic tumour cells as circulating indicators of high-risk rectal cancer. J Extracell Vesicles. 2019;8:1567219. doi: 10.1080/20013078.2019.1567219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tesauro D, Accardo A, Diaferia C, Milano V, Guillon J, Ronga L, et al. Peptide-based drug-delivery systems in biotechnological applications: recent advances and perspectives. Molecules. 2019;24:E351. doi: 10.3390/molecules24020351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hoffman AS. Hydrogels for biomedical applications. Adv Drug Deliv Rev. 2002;54:3–12. doi: 10.1016/s0169-409x(01)00239-3. [DOI] [PubMed] [Google Scholar]
- 65.Malam Y, Loizidou M, Seifalian AM. Liposomes and nanoparticles: nanosized vehicles for drug delivery in cancer. Trends Pharmacol Sci. 2009;30:592–599. doi: 10.1016/j.tips.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 66.Duncan R. The dawning era of polymer therapeutics. Nat Rev Drug Discov. 2003;2:347–360. doi: 10.1038/nrd1088. [DOI] [PubMed] [Google Scholar]
- 67.Gang J, Park SB, Hyung W, Choi EH, Wen J, Kim HS, et al. Magnetic poly epsilon-caprolactone nanoparticles containing Fe3O4 and gemcitabine enhance anti-tumor effect in pancreatic cancer xenograft mouse model. J Drug Target. 2007;15:445–453. doi: 10.1080/10611860701453901. [DOI] [PubMed] [Google Scholar]
- 68.Bobo D, Robinson KJ, Islam J, Thurecht KJ, Corrie SR. Nanoparticle-based medicines: a review of FDA-approved materials and clinical trials to date. Pharm Res. 2016;33:2373–2387. doi: 10.1007/s11095-016-1958-5. [DOI] [PubMed] [Google Scholar]
- 69.Basso J, Miranda A, Nunes S, Cova T, Sousa J, Vitorino C. Hydrogel-based drug delivery nanosystems for the treatment of brain tumors. Gels. 2018;4:E62. doi: 10.3390/gels4030062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Suk JS, Xu Q, Kim N, Hanes J, Ensign LM. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv Drug Deliv Rev. 2016;99:28–51. doi: 10.1016/j.addr.2015.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kraft JC, Freeling JP, Wang Z, Ho RJ. Emerging research and clinical development trends of liposome and lipid nanoparticle drug delivery systems. J Pharm Sci. 2014;103:29–52. doi: 10.1002/jps.23773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ramalingam P, Ko YT. Enhanced oral delivery of curcumin from N-trimethyl chitosan surface-modified solid lipid nanoparticles: pharmacokinetic and brain distribution evaluations. Pharm Res. 2015;32:389–402. doi: 10.1007/s11095-014-1469-1. [DOI] [PubMed] [Google Scholar]
- 73.Dams ET, Laverman P, Oyen WJ, Storm G, Scherphof GL, van Der Meer JW, et al. Accelerated blood clearance and altered biodistribution of repeated injections of sterically stabilized liposomes. J Pharmacol Exp Ther. 2000;292:1071–1079. [PubMed] [Google Scholar]
- 74.Ishida T, Kashima S, Kiwada H. The contribution of phagocytic activity of liver macrophages to the accelerated blood clearance (ABC) phenomenon of PEGylated liposomes in rats. J Control Release. 2008;126:162–165. doi: 10.1016/j.jconrel.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 75.Johnsen KB, Gudbergsson JM, Skov MN, Pilgaard L, Moos T, Duroux M. A comprehensive overview of exosomes as drug delivery vehicles—endogenous nanocarriers for targeted cancer therapy. Biochim Biophys Acta. 2014;1846:75–87. doi: 10.1016/j.bbcan.2014.04.005. [DOI] [PubMed] [Google Scholar]
- 76.Wiklander OP, Nordin JZ, O’Loughlin A, Gustafsson Y, Corso G, Mager I, et al. Extracellular vesicle in vivo biodistribution is determined by cell source, route of administration and targeting. J Extracell Vesicles. 2015;4:26316. doi: 10.3402/jev.v4.26316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yang T, Martin P, Fogarty B, Brown A, Schurman K, Phipps R, et al. Exosome delivered anticancer drugs across the blood-brain barrier for brain cancer therapy in Danio rerio. Pharm Res. 2015;32:2003–2014. doi: 10.1007/s11095-014-1593-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lai RC, Yeo RW, Tan KH, Lim SK. Exosomes for drug delivery—a novel application for the mesenchymal stem cell. Biotechnol Adv. 2013;31:543–551. doi: 10.1016/j.biotechadv.2012.08.008. [DOI] [PubMed] [Google Scholar]
- 79.Kooijmans SAA, Schiffelers RM, Zarovni N, Vago R. Modulation of tissue tropism and biological activity of exosomes and other extracellular vesicles: new nanotools for cancer treatment. Pharmacol Res. 2016;111:487–500. doi: 10.1016/j.phrs.2016.07.006. [DOI] [PubMed] [Google Scholar]
- 80.Kim MS, Haney MJ, Zhao Y, Mahajan V, Deygen I, Klyachko NL, et al. Development of exosome-encapsulated paclitaxel to overcome MDR in cancer cells. Nanomedicine. 2016;12:655–664. doi: 10.1016/j.nano.2015.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Munagala R, Aqil F, Jeyabalan J, Gupta RC. Bovine milk-derived exosomes for drug delivery. Cancer Lett. 2016;371:48–61. doi: 10.1016/j.canlet.2015.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Alvarez-Erviti L, Seow Y, Yin H, Betts C, Lakhal S, Wood MJ. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol. 2011;29:341–345. doi: 10.1038/nbt.1807. [DOI] [PubMed] [Google Scholar]
- 83.Wang K, Zhang S, Weber J, Baxter D, Galas DJ. Export of microRNAs and microRNA-protective protein by mammalian cells. Nucleic Acids Res. 2010;38:7248–7259. doi: 10.1093/nar/gkq601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lee Y, El Andaloussi S, Wood MJ. Exosomes and microvesicles: extracellular vesicles for genetic information transfer and gene therapy. Hum Mol Genet. 2012;21:R125–R134. doi: 10.1093/hmg/dds317. [DOI] [PubMed] [Google Scholar]
- 85.Wahlgren J, De L Karlson T, Brisslert M, Vaziri Sani F, Telemo E, Sunnerhagen P, et al. Plasma exosomes can deliver exogenous short interfering RNA to monocytes and lymphocytes. Nucleic Acids Res. 2012;40:e130. doi: 10.1093/nar/gks463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pan Q, Ramakrishnaiah V, Henry S, Fouraschen S, de Ruiter PE, Kwekkeboom J, et al. Hepatic cell-to-cell transmission of small silencing RNA can extend the therapeutic reach of RNA interference (RNAi) Gut. 2012;61:1330–1339. doi: 10.1136/gutjnl-2011-300449. [DOI] [PubMed] [Google Scholar]
- 87.Ohno S, Takanashi M, Sudo K, Ueda S, Ishikawa A, Matsuyama N, et al. Systemically injected exosomes targeted to EGFR deliver antitumor microRNA to breast cancer cells. Mol Ther. 2013;21:185–191. doi: 10.1038/mt.2012.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Greco KA, Franzen CA, Foreman KE, Flanigan RC, Kuo PC, Gupta GN. PLK-1 silencing in bladder cancer by siRNA delivered with exosomes. Urology. 2016;91:241.e1–7. doi: 10.1016/j.urology.2016.01.028. [DOI] [PubMed] [Google Scholar]
- 89.Hood JL, Scott MJ, Wickline SA. Maximizing exosome colloidal stability following electroporation. Anal Biochem. 2014;448:41–49. doi: 10.1016/j.ab.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kooijmans SAA, Stremersch S, Braeckmans K, de Smedt SC, Hendrix A, Wood MJA, et al. Electroporation-induced siRNA precipitation obscures the efficiency of siRNA loading into extracellular vesicles. J Control Release. 2013;172:229–238. doi: 10.1016/j.jconrel.2013.08.014. [DOI] [PubMed] [Google Scholar]
- 91.Shtam TA, Kovalev RA, Varfolomeeva EY, Makarov EM, Kil YV, Filatov MV. Exosomes are natural carriers of exogenous siRNA to human cells in vitro. Cell Commun Signal. 2013;11:88. doi: 10.1186/1478-811X-11-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Didiot MC, Hall LM, Coles AH, Haraszti RA, Godinho BM, Chase K, et al. Exosome-mediated delivery of hydrophobically modified siRNA for Huntingtin mRNA silencing. Mol Ther. 2016;24:1836–1847. doi: 10.1038/mt.2016.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Luan X, Sansanaphongpricha K, Myers I, Chen H, Yuan H, Sun D. Engineering exosomes as refined biological nanoplatforms for drug delivery. Acta Pharmacol Sin. 2017;38:754–763. doi: 10.1038/aps.2017.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Haney MJ, Klyachko NL, Zhao Y, Gupta R, Plotnikova EG, He Z, et al. Exosomes as drug delivery vehicles for Parkinson’s disease therapy. J Control Release. 2015;207:18–30. doi: 10.1016/j.jconrel.2015.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Pascucci L, Coccè V, Bonomi A, Ami D, Ceccarelli P, Ciusani E, et al. Paclitaxel is incorporated by mesenchymal stromal cells and released in exosomes that inhibit in vitro tumor growth: a new approach for drug delivery. J Control Release. 2014;192:262–270. doi: 10.1016/j.jconrel.2014.07.042. [DOI] [PubMed] [Google Scholar]
- 96.Lee J, Kim J, Jeong M, Lee H, Goh U, Kim H, et al. Liposome-based engineering of cells to package hydrophobic compounds in membrane vesicles for tumor penetration. Nano Lett. 2015;15:2938–2944. doi: 10.1021/nl5047494. [DOI] [PubMed] [Google Scholar]
- 97.Jang SC, Kim OY, Yoon CM, Choi DS, Roh TY, Park J, et al. Bioinspired exosome-mimetic nanovesicles for targeted delivery of chemotherapeutics to malignant tumors. ACS Nano. 2013;7:7698–7710. doi: 10.1021/nn402232g. [DOI] [PubMed] [Google Scholar]
- 98.Kalimuthu S, Gangadaran P, Rajendran RL, Zhu L, Oh JM, Lee HW, et al. A new approach for loading anticancer drugs into mesenchymal stem cell-derived exosome mimetics for cancer therapy. Front Pharmacol. 2018;9:1116. doi: 10.3389/fphar.2018.01116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kim M, Yun HW, Park DY, Choi BH, Min BH. Three-dimensional spheroid culture increases exosome secretion from mesenchymal stem cells. Tissue Eng Regen Med. 2018;15:427–436. doi: 10.1007/s13770-018-0139-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gudbergsson JM, Johnsen KB, Skov MN, Duroux M. Systematic review of factors influencing extracellular vesicle yield from cell cultures. Cytotechnology. 2016;68:579–592. doi: 10.1007/s10616-015-9913-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.King HW, Michael MZ, Gleadle JM. Hypoxic enhancement of exosome release by breast cancer cells. BMC Cancer. 2012;12:421. doi: 10.1186/1471-2407-12-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kojima R, Bojar D, Rizzi G, Hamri GC, El-Baba MD, Saxena P, et al. Designer exosomes produced by implanted cells intracerebrally deliver therapeutic cargo for Parkinson’s disease treatment. Nat Commun. 2018;9:1305. doi: 10.1038/s41467-018-03733-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Katakowski M, Buller B, Zheng X, Lu Y, Rogers T, Osobamiro O, et al. Exosomes from marrow stromal cells expressing miR-146b inhibit glioma growth. Cancer Lett. 2013;335:201–204. doi: 10.1016/j.canlet.2013.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lou G, Song X, Yang F, Wu S, Wang J, Chen Z, et al. Exosomes derived from miR-122-modified adipose tissue-derived MSCs increase chemosensitivity of hepatocellular carcinoma. J Hematol Oncol. 2015;8:122. doi: 10.1186/s13045-015-0220-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wang X, Zhang H, Bai M, Ning T, Ge S, Deng T, et al. Exosomes serve as nanoparticles to deliver anti-miR-214 to reverse chemoresistance to cisplatin in gastric cancer. Mol Ther. 2018;26:774–783. doi: 10.1016/j.ymthe.2018.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Li H, Yang C, Shi Y, Zhao L. Exosomes derived from siRNA against GRP78 modified bone-marrow-derived mesenchymal stem cells suppress Sorafenib resistance in hepatocellular carcinoma. J Nanobiotechnology. 2018;16:103. doi: 10.1186/s12951-018-0429-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Villarroya-Beltri C, Gutiérrez-Vázquez C, Sánchez-Cabo F, Pérez-Hernández D, Vázquez J, Martin-Cofreces N, et al. Sumoylated hnRNPA2B1 controls the sorting of miRNAs into exosomes through binding to specific motifs. Nat Commun. 2013;4:2980. doi: 10.1038/ncomms3980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Statello L, Maugeri M, Garre E, Nawaz M, Wahlgren J, Papadimitriou A, et al. Identification of RNA-binding proteins in exosomes capable of interacting with different types of RNA: RBP-facilitated transport of RNAs into exosomes. PLoS One. 2018;13:e0195969. doi: 10.1371/journal.pone.0195969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Fu H, Yang H, Zhang X, Wang B, Mao J, Li X, et al. Exosomal TRIM3 is a novel marker and therapy target for gastric cancer. J Exp Clin Cancer Res. 2018;37:162. doi: 10.1186/s13046-018-0825-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Rivoltini L, Chiodoni C, Squarcina P, Tortoreto M, Villa A, Vergani B, et al. TNF-related apoptosis-inducing ligand (TRAIL)-armed exosomes deliver proapoptotic signals to tumor site. Clin Cancer Res. 2016;22:3499–3512. doi: 10.1158/1078-0432.CCR-15-2170. [DOI] [PubMed] [Google Scholar]
- 111.Mizrak A, Bolukbasi MF, Ozdener GB, Brenner GJ, Madlener S, Erkan EP, et al. Genetically engineered microvesicles carrying suicide mRNA/protein inhibit schwannoma tumor growth. Mol Ther. 2013;21:101–108. doi: 10.1038/mt.2012.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Tian Y, Li S, Song J, Ji T, Zhu M, Anderson GJ, et al. A doxorubicin delivery platform using engineered natural membrane vesicle exosomes for targeted tumor therapy. Biomaterials. 2014;35:2383–2390. doi: 10.1016/j.biomaterials.2013.11.083. [DOI] [PubMed] [Google Scholar]
- 113.Grapp M, Wrede A, Schweizer M, Hüwel S, Galla HJ, Snaidero N, et al. Choroid plexus transcytosis and exosome shuttling deliver folate into brain parenchyma. Nat Commun. 2013;4:2123. doi: 10.1038/ncomms3123. [DOI] [PubMed] [Google Scholar]