Abstract
Fresh onions (Allium cepa L.) emit volatile organic compounds (VOCs) naturally in very low concentrations. The aim of the present study was to determine the emission rate of low-boiling VOCs from healthy and naturally infected onion bulbs at 4, 15, and 25 °C and to evaluate the applicability of the VOC method to monitor quality changes during 12 weeks of storage of two cultivars (‘Hystand’ and ‘Hoza’) of yellow onions. VOCs were extracted from the headspace of bulbs by solid phase micro-extraction (SPME) up to 5 times during storage and analyzed by gas chromatography–mass spectrometry (GC–MS). A total of twenty-nine compounds were measured and twenty-seven of these were identified while thirteen were reported for the first time from yellow onion bulbs. Acetone (0.10–18.0 nmol kg−1 day−1), dimethyl disulfide (0.12–18.9 nmol kg−1 day−1) and hexanal (0.05–4.40 nmol kg−1 day−1) were among the most abundant volatiles emitted from healthy bulbs. The concentration of these compounds as well as the total volatiles decreased with time in storage. However, microbial infection resulted in higher emission of propene, carbon disulfide, isoprene, pentane, 2-methylfuran, 3-methylfuran, 1-propenethiol, hexane, and methyl propyl sulfide, indicating that VOC emission may be used as an indicator to monitor natural senescence and decay of stored onion bulbs.
Keywords: Solid-phase microextraction, Gas chromatography–mass spectrometry, Headspace analysis, Senescence, Quality, Disease
Introduction
Onion (Allium cepa L.) is one of the most important vegetables cultivated around the world and the bulbs are stored between seasons to supply consumers with fresh produce year-round (Opara and Geyer 1999). Quality of onions changes as a result of natural senescence and decay. The characteristic pungent aroma and flavor decrease due to enzymatic degradation of S-alk(en)yl cysteine sulfoxides and formation of sulfurous compounds such as sulfenic acids, monosulfides, disulfides, trisulfides, and tetrasulfides (Block et al. 1992). Other quality changes are loss of firmness, top and root sprouting, and development of storage rots caused by organisms like Botrytis allii and Botrytis aclada (neck rot), Burkholderia cepacia, formerly Pseudomonas cepacia (sour skin), and Fusarium oxysporum (basal rot) (Opara and Geyer 1999; Snowdon 1990).
Recently, attempts have been made to monitor the early quality changes in fresh produce by use of analysis of volatiles (Luca et al. 2017). Many factors influence the volatile organic compound (VOC) profile extracted from fresh produce: the raw material, the extraction method, and the method of detection (Li et al. 2011; Løkke et al. 2012; Prithiviraj et al. 2004; Sinha et al. 2018; Vikram et al. 2005). Usually, fresh produce is macerated to increase the release of volatiles, but maceration introduces damages and wounding and mixing of enzymes and substrates (Christensen et al. 2007) and this changes the VOC profile (Løkke et al. 2012). Therefore, VOCs must be sampled from undamaged or naturally damaged onion bulbs to yield information on senescence and decay.
Fresh vegetables emit a wealth of VOCs but the release is low in undamaged tissue, usually in the parts-per-trillion (ppt) range (Luca et al. 2017), making it challenging to sample VOCs for the monitoring of fresh produce quality. Several attempts to detect microbial infections in onion bulbs have been made with variable results (Li et al. 2011; Prithiviraj et al. 2004; Sinha et al. 2018; Vikram et al. 2005). Higher intensity of dimethyl disulfide, dipropyl disulfide, methyl propyl disulfide, undecane, and 2-undecane were found in sour skin (B. cepacia) infected bulbs 16 days after inoculation at 4 °C and 3 days after inoculation at 25 °C by use of field asymmetric ion mobility spectrometry analysis (Sinha et al., 2018). Li et al. (2011) also found higher levels of dimethyl disulfide, dipropyl disulfide, and undecane in bulbs infected with B. cepacia. Recently, it has been shown that solid-phase microextraction (SPME) on a Carboxen/polydimethylsiloxane (CAR/PDMS) fiber and separation on a HP-PLOT/Q column is suitable for giving detailed information on senescence and microbial decay of packaged wild rocket (Diplotaxis tenuifolia L.) (Luca et al. 2017) and stored onion bulbs (Wang et al. 2016).
The aim of the present study was to determine the emission rate of low-boiling VOCs from healthy and naturally infected fresh onion bulbs and to evaluate the applicability of the VOC method to monitor quality changes in onion bulbs during storage. Two cultivars of yellow onions (‘Hystand’ and ‘Hoza’) were stored for 12 weeks in glass jars at 4, 15, and 25 °C in darkness, and VOCs were extracted up to 5 times during storage by SPME and analyzed by GC–MS. Following storage, bulb quality (weight loss, sprouting, rooting, decay) was evaluated.
Materials and methods
Reagents and chemicals
Authentic reference compounds of acetaldehyde, acetic acid, acetone, 2-butanone, carbon disulfide, dimethyl disulfide, 2,5-dimethylfuran, 3,4-dimethylthiophene, heptane, hexanal, hexane, isoprene, methanethiol, methyl formate, 2-methylfuran, 3-methyl-2-pentanone, 2-methyl-2-propanol, methyl propyl disulfide, pentanal, pentane, 2-pentanone, isopropyl alcohol, propanal, 1-propanethiol, 1-propanol, and propene were obtained from Sigma-Aldrich Chemie GmbH (Stenheim, Germany), methyl propyl sulfide from Alfa Aesar (Haverhill, USA), and 3-methylfuran from Acros Organics (Geel, Belgium). Ultrapure water (resistivity 18.2 MΩ cm) was produced by a SG Ultra Clear UV Water Purification System (SG Wasseraufbereitung und Regenerierstation GmbH, Barsbüttel, Germany).
Plant material and storage conditions
Bulbs of two cultivars (‘Hystand’ and ‘Hoza’) of yellow onions (Allium cepa L.) were taken from commercial storage house facilities in March 2015 after approximately 6 months in storage. After harvest, bulbs were cured to dry the outer scales, and then stored at 0.5 °C and approximately 85% relative humidity (RH). None of the cultivars was treated with sprout inhibitors in the field or in storage (Benkeblia et al. 2002). A batch of 20 kg of each cultivar was taken and transported to Aarhus University and manually sorted for the experiment. Only firm bulbs of similar size that were free of skin cracks, top sprouts, secondary roots, and external diseases were selected. The weight of three onions was combined into one sample to give a total of 320 ± 10 g and samples were stored in triplicate in 1-L glass jars inside storage chambers (Binder KB400, Binder, Tuttlingen, Germany) for 12 weeks at 4, 15, and 25 °C.
Sampling of VOCs
Every 2–3 weeks, jars were made airtight with lids having inlet and outlet fittings for gas purging, and a septum for O2 and CO2 measurements and VOC sampling. Jars were flushed with clean, compressed, dry air (AGA Gas AB, Sundbyberg, Sweden) for 10 min at 800 mL min−1 to have the same background level of volatiles at beginning, and then kept airtight for 24 h to allow VOCs to build up. VOCs were sampled for 5 min with a 85-μm CAR/PDMS SPME fiber (Supelco, Bellefonte, PA) through the septum attached to the lid. The fiber was then transferred to another 1-L glass jar for 5 min at 18 °C for external standard addition (0.447 nmol L−1 3-methyl-2-pentanone in N2). This method enabled extraction of VOCs emitted naturally from onion bulbs during storage (Wang et al. 2016) without handling the bulbs. Following VOC sampling, the gas composition inside the jars was monitored by use of a CheckMate 9900 Headspace Gas Analyzer (PBI Dansensor, Ringsted, Denmark) and from these values the respiration rate of bulbs was determined (Seefeldt et al. 2012).
Analysis and quantification of VOCs
The VOCs on the SPME fiber were thermally desorbed at 200 °C in splitless mode in the inlet of an Agilent 5975C GC (Agilent Technologies, Palo Alto, CA) equipped with a SPME liner (0.75 mm i.d., Supelco, Bellefonte, PA). Compounds were separated on an HP-PLOT/Q (Agilent Technologies, Palo Alto, CA) column (30 m × 0.32 mm, film thickness 20 μm) (Luca et al. 2015b), and detected on an Agilent 5975C inertXL MS detector (Agilent Technologies, Palo Alto, CA). The GC oven temperature program was the following: 30 °C (1 min hold time), a temperature ramp of 20 °C min−1 to 250 °C and 18 min hold time at 250 °C. The carrier gas was helium with a constant flow rate of 1 mL min−1. The MS operated in positive ion mode with an electron ionization energy of 70 eV. The instrument acquired mass spectra in total ion current mode over a mass-to-charge ratio (m/z) of 20–200 amu. Compounds identified were verified by comparing the retention times and mass spectral data of the NIST database (ver. 2.1.2, NIST, Gaithersburg, MD) with those of authentic reference compounds. Compounds were quantified as shown in Eq. 1 relative to the external standard (ES) 3-methyl-2-pentanone and by use of a correction factor (F):
| 1 |
where [VOC] is the VOC emission rate in nmol kg−1 day−1, AVOC is the peak area of a VOC in a sample and AES is the peak area of the external standard in the same sample, ES is the concentration of the external standard (3-methyl-2-pentanone) in nmol L−1, Vf is the free volume in L of a jar containing three onion bulbs, W is the fresh weight of the onion bulbs in kg, and t is the total emission time in days. Isoprene was selected as the compound for determination of the correction factor (F) as it eluted in the middle of the chromatogram. F was determined as the ratio between the peak area of isoprene extracted from air at 4, 15, and 25 °C using the same procedure as with the onions.
Quality assessment of onions
The weight loss, sprouting, rooting, and the levels of decay were assessed at the end of storage. The weight loss was determined as the difference between the initial and final weight of the bulbs in percentage of the initial weight. Sprouting and rooting were determined on each bulb on a 4-point scale from 0 to 3 as the length of the longest leaf emerging from the neck (Pak et al. 1995): 0 = no green leaf; 1 = leaf < 2 cm; 2 = leaf 2–5 cm; and 3 = leaf > 5 cm. Rooting was monitored similarly, as the length of the white secondary roots emerging from the base plate (Miedema 1994); 0 = no roots; 1 = roots < 1 cm; 2 = roots 1–2 cm; and 3 = roots > 2 cm. The amount of decayed tissue was evaluated on a 4-point scale from 0 to 3 after the onions were cut longitudinally with a sterilized knife, and the base plate, bulb neck and internal and external scales were inspected; 0 = no visual decay; 1 = < 10% decay; 2 = 10–20% decay; and 3 = > 20% decay. From these data, a mean sprouting, rooting and decay index for each of the 18 jars was calculated. To identify the specific diseases, 54 bulbs from the 18 jars (3 bulbs in each jar) were incubated separately in darkness at 20 °C at > 85% RH. Tissue from infected bulbs was cut and spread on PDA and LB agar plates (Sigma-Aldrich Chemie GmbH, Stenheim, Germany), and incubated at 25 and 30 °C, respectively, for 2–4 days to identify the morphological characteristics of the fungi or bacteria (Cranshaw et al. 2007). Diseases were identified by the visual symptoms on the onions and phenotypic identification of microorganisms (Cranshaw et al. (2007).
Data analysis
Parallel Factor Analysis 2 (PARAFAC2) (Amigo et al. 2010) was applied to overcome baseline drifts, peak shifts, low signal-to-noise ratios, and co-elution in the chromatograms. The chromatograms were divided into 21 local intervals in MATLAB® (The Mathworks Inc., Natick, MA) and PARAFAC2 models were developed for each interval using the PLS-Toolbox (PLS-Toolbox v. 8.0.2, Eigenvector Research Inc., Wenatchee, WA). From this analysis, peak areas were calculated. Before statistical analysis, all data were carefully inspected to avoid misinterpretations in the data analysis. Two-way analysis of variance (ANOVA) was applied to the VOC data at beginning to identify whether there were significant differences between cultivar, temperature, and cultivar * temperature and to the quality data measured at the end of storage. If one factor was nonsignificant, the statistical model was reduced to a two-way model without interaction or a one-way ANOVA. For multiple comparisons, Tukey’s honest significance difference (HSD) test at P = 0.05 was applied. Statistical analysis was performed in R (v. 3.2.3, R Development Core Team, 2015).
Results and discussions
The weight loss ranged between 2 and 6% after 12 weeks of storage (Table 1) and it was thus below 10%, which is the limit for marketability of onions (Robinson et al. 1975). The highest weigh loss was observed at 25 °C and the lowest at 4 °C and 15 °C. These differences most likely were attributed to the observed higher respiration rate at 25 °C than at 4 °C (data not shown) and lower RH at 25 °C than at 4 °C which influence the water vapor pressure deficit and the loss of water during storage from fresh produce (Paull 1999). No top sprouting was observed during the first 4 weeks of storage (data not shown) but after 12 weeks, sprouting was higher at 15 °C than at 4 °C and 25 °C (Table 1). This is in agreement with Benkeblia et al. (2002) who reported more top sprouting in onion bulbs stored at 7.5–15 °C than at 2–4 °C and 20–25 °C. Secondary roots emerged slightly earlier than top sprouts from the base plates of the bulbs. After 12 weeks, onions stored at 4 and 15 °C had more rooting than those stored at 25 °C (Table 1). High humidity stimulates rooting of onions in storage if bulbs are prone to secondary root growth (Islam et al. 2015). The RH in storage could have been higher at 4 and 15 °C than at 25 °C as air can hold more water vapor at 25 °C than at 4 and 15 °C at the same RH level. Overall, the decay index was low (Table 1) and there was no significant difference between treatments after 12 weeks of storage. However, it was possible to acquire important information about VOC emission from diseased and healthy onion bulbs, as 28% of the jars (5 out of 18) had one diseased bulb (Table 1), and the remaining jars had only healthy bulbs. Three onion bulbs had sour skin (B. cepacia); it was ‘Hystand’ stored at 15 °C, ‘Hoza’ stored at 15 °C, and ‘Hoza’ stored at 25 °C (Table 1). One ‘Hystand’ bulb stored at 15 °C had basal rot (F. oxysporum) and one ‘Hoza’ bulb stored at 4 °C had neck rot (Botrytis spp.) (Table 1). Overall, onion bulbs stored at 15 °C had more top sprouting and more bulbs were infected stored at 4 and 25 °C.
Table 1.
Quality of onions after 12 weeks of storage at 4, 15, and 25 °C
| Temperature (°C) | Weight loss (%) | Sprouting index | Rooting index | Decay index | Diseasesa |
|---|---|---|---|---|---|
| 4 | 2.29 ± 0.20bAc | 0.06 ± 0.06 B | 1.95 ± 0.23 A | 0.17 ± 0.17 A | Botrytis spp. (1) |
| 15 | 2.56 ± 0.41 A | 2.33 ± 0.26 A | 2.53 ± 0.22 A | 0.33 ± 0.17 A |
B. cepacia (2) and F. oxysporum (1) |
| 25 | 6.02 ± 0.41 B | 0.72 ± 0.26 B | 0.55 ± 0.36 B | 0.11 ± 0.11 A | B. cepacia (1) |
aThe number of jars with a diseased bulb is given in parenthesis. Only one bulb was diseased in each jar
bData are the mean values ± standard errors over cultivars (n = 6)
cMean values followed by different letters within column indicate significant differences at P = 0.05
Volatile organic compounds emitted from fresh onions during storage
Twenty-nine VOCs were naturally emitted from onion bulbs during storage (Table 2). These compounds included 1 acid, 3 alkanes, 3 alkenes, 2 alcohols, 4 aldehydes, 1 ester, 3 furans, 3 ketones, 7 sulfides, and 2 thiols. Most of the compounds (23 of 29) were VOCs with boiling points below 110 °C, in agreement with the selected method, which was selective and sensitive to extraction and separation of low-boiling volatile compounds (Luca et al. 2015b). Thirteen compounds were reported for the first time from ‘Hystand’ and ‘Hoza’ onions, including methyl formate, isopropyl alcohol, isoprene, pentane, 2-methyl-2-propanol, 2-methylfuran, 3-methylfuran, 2-butanone, acetic acid, hexane, 2,5-dimethylfuran, pentanal, heptane, and 2-pentanone while the remaining compounds had been reported earlier in onions or in other vegetables from the Allium genus. Those compounds were acetaldehyde (Kim and Kim 2014); propene and (Z)-methyl 1-propenyl sulfide from steam-distilled onion oil (Boelens et al. 1971); acetone from fresh-cut (Løkke et al. 2012) or diseased onion bulbs (Li et al. 2011); methanethiol and propanal from fresh-cut onions (Løkke et al. 2012) or distilled onion oil (Boelens et al. 1971); carbon disulfide, methyl propyl sulfide and 1-propanol from crushed onions (Kallio and Salorinne 1990); and 1-propanol from blanched (Kebede et al. 2014) or diseased onion bulbs (Vikram et al. 2005); 1-propanethiol from crushed (Kallio and Salorinne 1990), cut and high pressure processed or freeze dried (Colina-Coca et al. 2013), or blanched onions (Kebede et al. 2014); dimethyl disulfide, 3,4-dimethylthiophene, methyl 1-propenyl disulfide, and methyl propyl disulfide from onion oil, fresh-cut or diseased onion bulbs (Boelens et al. 1971; Løkke et al. 2012; Vikram et al. 2005), and hexanal from onion oil (Kuo and Ho 1992) or cut and high pressure processed or freeze dried onions (Colina-Coca et al. 2013; Kebede et al. 2014). The data reported here showed that onion bulbs emit many different low-boiling VOCs naturally which can be sampled by SPME without crushing the tissue.
Table 2.
Volatile organic compounds detected in the headspace of ‘Hystand’ and ‘Hoza’ bulbs during 12 weeks of storage at 4, 15, and 25 °C
| No. | Volatile compounda | Retention time (min) | Chemical group | Boiling pointb (°C) | Top abundant ionsc |
|---|---|---|---|---|---|
| 1 | Propene | 5.89 | Alkene | − 48 | 41 (100), 39 (72), 42 (69) |
| 2 | Acetaldehyde | 7.29 | Aldehyde | 21 | 44 (100), 29 (99), 43 (54) |
| 3 | Methanethiol | 7.59 | Thiol | 6 | 47 (100), 48 (82), 45 (62) |
| 4 | Methyl formate | 7.95 | Ester | 32 | 31 (100), 60 (89), 29 (62) |
| 5 | Propanal | 9.31 | Aldehyde | 48 | 58 (100), 29 (91), 28 (64) |
| 6 | Acetone | 9.42 | Ketone | 56 | 43 (100), 58 (37), 42 (7) |
| 7 | Carbon disulfide | 9.45 | Sulfide | 46 | 76 (100), 78 (9), 49 (7) |
| 8 | Isopropyl alcohol | 9.87 | Alkene | 36–37 | 55 (100), 70 (52), 42 (51) |
| 9 | Isoprene | 9.98 | Alkene | 34 | 67 (100), 68 (68), 53 (57) |
| 10 | Pentane | 10.04 | Alkane | 36 | 43 (100), 42 (60), 41 (53) |
| 11 | 1-propanol | 10.40 | Alcohol | 97 | 31 (100), 59 (22), 42 (17) |
| 12 | 2-methyl-2-propanol | 10.70 | Alcohol | 82 | 59 (100), 31 (23), 41 (18) |
| 13 | 2-methylfuran | 10.83 | Furan | 63–66 | 82 (100), 81 (61), 53 (55) |
| 14 | 3-methylfuran | 10.96 | Furan | 67 | 82 (100), 53 (59), 81 (54) |
| 15 | 2-butanone | 11.02 | Ketone | 80 | 43 (100), 72 (20), 29 (10) |
| 16 | 1-propanethiol | 11.15 | Thiol | 68 | 76 (100), 47 (62), 41 (47) |
| 17 | Hexane | 11.53 | Alkane | 69 | 57 (100), 41 (65), 43 (57) |
| 18 | Acetic acid | 11.63 | Acid | 118 | 43 (100), 45 (96), 60 (77) |
| 19 | 2,5-dimethylfuran | 12.20 | Furan | 92–94 | 96 (100), 95 (86), 43 (72) |
| 20 | Pentanal | 12.46 | Aldehyde | 103 | 44 (100), 58 (44), 29 (43) |
| 21 | 2-pentanone | 12.35 | Ketone | 102 | 43 (100), 57 (74), 86 (41) |
| 22 | Methyl propyl sulfide | 12.56 | Sulfide | 96 | 61 (100), 90 (75), 48 (27) |
| 23 | (Z)-methyl 1-propenyl sulfided | 12.60 | Sulfide | 102 | 88 (100), 73 (97), 45 (69) |
| 24 | Dimethyl disulfide | 12.73 | Sulfide | 110 | 94 (100), 79 (49), 45 (34) |
| 25 | Heptane | 12.91 | Alkane | 98 | 43 (100), 71 (80), 57 (61) |
| 26 | Hexanal | 14.17 | Aldehyde | 131 | 44 (100), 43 (96), 41 (81) |
| 27 | 3,4-dimethylthiophene | 15.77 | Sulfide | 144–146 | 111 (100), 112 (69), 97 (44) |
| 28 | Methyl 1-propenyl disulfided | 17.04 | Sulfide | 140 | 120 (100), 45 (49), 72 (35) |
| 29 | Methyl propyl disulfide | 17.20 | Sulfide | 154 | 122 (100), 80 (98), 41 (36) |
aCompounds suggested by the NIST database (version 2.1.2, NIST, Gaithersburg, MD) were verified by comparing the retention times and mass spectral data with those of authentic reference compounds unless noted
bBoiling points are given at 760 mmHg and data are from https://pubchem.ncbi.nlm.nih.gov/compound
cThe values in parentheses are the percentage relative to the most abundant ion
dTentatively identified as no reference compounds were available
Effect of cultivar and storage temperature on volatile emission
The VOC emission rates from ‘Hystand’ and ‘Hoza’ bulbs stored at the three temperatures are shown in Table 3 in the first week of storage. At this time, bulbs had no top sprouts or secondary roots (data not shown). The VOC emission rates were quantified by use of an external standard and by use of a correction factor to compensate for differences in the extraction efficiency at the different temperatures. Many factors influence the extraction efficiency; temperature, relative humidity, and the gas matrix effects (Luca et al. 2015a; Nielsen and Jonsson 2002). In the present study, the O2 concentration ranged between 8 and 19% and the concentration of CO2 between 2 and 12%, and the gas composition was thus within the range of having a minor effect on the VOC extraction efficiency (Luca et al. 2015a).
Table 3.
The emission rate (nmol kg−1 day−1) of volatile organic compounds from ‘Hystand’ and ‘Hoza’ onions stored at 4, 15, and 25 °Ca
| Compound | Cultivar | Storage temperature | Significance levelb | |||||
|---|---|---|---|---|---|---|---|---|
| ‘Hystand’ | ‘Hoza’ | 4 °C | 15 °C | 25 °C | Cultivar | Temperature | Interaction | |
| Propene | 0.06 Ac | 0.07 A | 0.06 A | 0.09 A | 0.05 A | nsd | ns | * |
| Acetaldehyde | 0.65 A | 0.95 A | 0.20 B | 0.27 B | 1.84 A | ns | ** | ns |
| Methanethiol | 0.88 A | 0.02 A | 0.01 A | 1.4 A | 0.07 A | ns | ns | ns |
| Methyl formate | 0.65 A | 0.54 A | 0.20 B | 0.35 B | 1.22 A | ns | *** | ns |
| Propanal | 0.18 A | 0.51 A | 0.06 B | 0.06 B | 0.84 A | ns | ** | ns |
| Acetone | 3.25 A | 5.35 A | 0.61 B | 0.58 B | 11.0 A | ns | *** | ns |
| Carbon disulfide | 0.61 A | 0.74 A | 0.33 B | 0.70 A | 0.95 A | ns | *** | ns |
| Isopropyl alcohol | 0.05 A | 0.06 A | 0.04 A | 0.06 A | 0.07 A | ns | ns | * |
| Isoprene | 0.66 A | 0.30 A | 0.03 B | 1.10 A | 0.36 AB | ns | * | ns |
| Pentane | 0.63 A | 0.68 A | 0.57 A | 0.80 A | 0.59 A | ns | ns | * |
| 1-Propanol | 0.19 A | 0.33 A | 0.01 B | 0.03 B | 0.70 A | ns | ** | ns |
| 2-methyl-2-propanol | 0.25 A | 0.20 A | 0.04 B | 0.14 B | 0.49 A | ns | *** | ns |
| 2-methylfuran | 0.44 A | 0.23 B | 0.04 C | 0.26 B | 0.72 A | *** | *** | ** |
| 3-methylfuran | 0.45 A | 0.52 A | 0.16 C | 0.40 B | 0.86 A | ns | *** | ns |
| 2-butanone | 0.44 A | 0.42 A | 0.04 B | 0.17 B | 1.04 A | ns | *** | ns |
| 1-propanethiol | 2.06 A | 0.34 A | 0.12 A | 3.16 A | 0.57 A | ns | ns | ns |
| Hexane | 0.11 A | 0.13 A | 0.02 C | 0.10 B | 0.24 A | ns | *** | ns |
| Acetic acid | 4.91 A | 4.88 A | 0.12 B | 0.14 B | 13.9 A | ns | *** | ns |
| 2,5-dimethylfuran | 0.24 A | 0.17 A | 0.03 B | 0.10 B | 0.48 A | ns | *** | ns |
| Pentanal | 0.13 A | 0.21 A | 0.02 B | 0.03 B | 0.43 A | ns | *** | ns |
| 2-pentanone | 0.13 A | 0.13 A | 0.02 B | 0.02 B | 0.34 A | ns | *** | ns |
| Methyl propyl sulfide | 1.55 A | 0.43 A | 0.16 A | 2.61 A | 0.37 A | ns | ns | ns |
| (Z)-methyl 1-propenyl sulfide | 0.19 A | 0.12 A | 0.02 A | 0.24 A | 0.21 A | ns | ns | ns |
| Dimethyl disulfide | 5.06 A | 2.19 A | 1.05 A | 5.09 A | 5.03 A | ns | ns | ns |
| Heptane | 0.13 B | 0.47 A | 0.05 A | 0.34 A | 0.44 A | * | ns | ns |
| Hexanal | 0.70 A | 1.10 A | 0.21 B | 0.17 B | 2.18 A | ns | *** | ns |
| 3,4-dimethylthiophene | 0.07 A | 0.05 A | 0.01 B | 0.03 B | 0.13 A | ns | *** | ns |
| Methyl 1-propenyl disulfide | 0.40 A | 0.20 A | 0.03 B | 0.17 AB | 0.72 A | ns | * | ns |
| Methyl propyl disulfide | 3.17 A | 0.72 A | 0.30 A | 4.69 A | 1.20 A | ns | ns | ns |
| Total volatiles | 19.6 A | 22.1 A | 4.54 B | 8.57 B | 47.0 A | ns | *** | ns |
aThe volatiles were collected during the first week of storage when all bulbs were free of top sprouts and secondary roots
bThe results are from two-way ANOVA with interaction
cMeans with different letters within row indicate significant differences between cultivar or temperature
d*Significant at P ≤ 0.05, **Significant at P ≤ 0.01, ***Significant at P ≤ 0.001, and ns not significant (P > 0.05)
Overall, there were significant differences between cultivars for 2-methylfuran and heptane and significant differences between temperatures for most of the compounds (Table 3). A significant interaction was observed for propene, isopropyl alcohol, pentane, and 2-methylfuran showing that the VOC emission rates differed between cultivars and temperatures for these four compounds (Table 3). The total VOC emission rates were 4.54, 8.57, and 47.0 nmol kg−1 day−1 at 4, 15, and 25 °C, respectively. Overall, more VOCs were emitted at 25 °C than at 4 °C. This observation may be explained by a higher activity of alliinase at higher than at lower temperature (Hanum et al. 1995). This enzyme degrades S-alk(en)yl cysteine sulfoxides into acetone, acetic acid, dimethyl disulfide, and other sulfurous compounds such as sulfenic acids, monosulfides, disulfides, trisulfides, and tetrasulfides in onion bulbs and other vegetables of the Allium genus (Block et al. 1992). In the present study, more acetone and acetic acid were extracted at 25 °C than at 4 °C (Table 3), which corresponds with a higher enzyme activity at a higher than at a lower temperature.
Changes in volatile emissions during storage of healthy onions
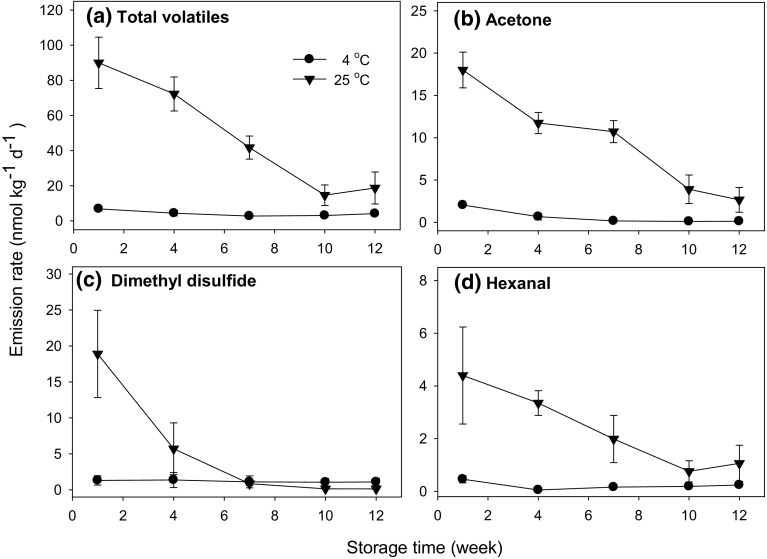
The volatile emission rates from healthy onion bulbs for selected VOCs are shown in Fig. 1 at 4 °C and 25 °C. The data showed that the emission rates decreased with storage time regardless of storage temperature for total volatiles, acetone, dimethyl disulfide, and hexanal (Fig. 1). The total emission rate dropped 40% from 6.86 to 4.13 nmol kg−1 day−1 at 4 °C and 79% from 90.00 to 18.76 nmol kg−1 day−1 at 25 °C (Fig. 1a). For acetone, the drop was 94% from 2.04 to 0.12 nmol kg−1 day−1 at 4 °C and 85% from 18.01 to 2.65 nmol kg−1 day−1 at 25 °C (Fig. 1b), and for dimethyl disulfide it was 17% from 1.31 to 1.09 nmol kg−1 day−1 at 4 °C and 99% from 18.89 to 0.12 nmol kg−1 day−1 at 25 °C (Fig. 1c). The observed decrease in acetone and dimethyl disulfide could be attributed to a drop in the methyl cysteine sulfoxide content of onions during storage (Kopsell et al. 1999), which is an important non-amino acid of vegetables belonging to the Allium genus. Hexanal also dropped during storage (Fig. 1d) from 0.46 to 0.24 nmol kg−1 day−1 at 4 °C (a 48% decrease) and from 4.40 to 1.06 nmol kg−1 day−1 at 25 °C (a 76% decrease). Hexanal is formed by oxidation of linoleic acid, which is one of the major fatty acid in onions (Tsiaganis et al. 2006). Overall, the emission rate of more than half of the VOCs decreased with time in storage (data not shown). Lower VOC emission rates with time in storage at a given temperature may thus be an indicator for advanced senescence and reduced quality and pungency of onion bulbs.
Fig. 1.
Emission rates of total volatiles (a), acetone (b), dimethyl disulfide (c) and hexanal (d) during 12 weeks storage of healthy onions at 4 °C and 25 °C. Data are presented as the mean values ± standard error (n = 5)
Increased volatile emission with diseased onions
Diseased onion bulbs had higher total VOC emission rates with time in storage than healthy bulbs at all temperatures except for ‘Hoza’ infected with B. cepacia at 15 °C (data not shown). This result is in accordance with the studies by Vikram et al. (2005) and Sinha et al. (2018) on onion bulbs stored at 4 and 25 °C. Overall, the VOC emission rate of propene, carbon disulfide, isoprene, pentane, 2-methylfuran, 3-methylfuran, 1-propanethiol, hexane, and methyl propyl disulfide increased during storage of ‘Hystand’ infected with B. cepacia (Fig. 2). Of these compounds, carbon disulfide, pentane, 2-methylfuran, 3-methylfuran, and methyl propyl disulfide have previously been related to senescence and microbial decay of wild rocket and onion bulbs (Banwart and Bremner 1975; Luca et al. 2017; Nielsen and Jonsson 2002; Sinha et al. 2018). Additionally, the total VOC emission rate increased during storage of ‘Hystand’ infected with F. oxysporum (data not shown), especially due to an increase in isoprene emission (Fig. 2c). Isoprene is an important antioxidant protecting membranes against oxidation (Loreto and Velikova 2001), but isoprene may also be related with growth of Erwinia herbicola and species of Bacillus and Pseudomonas (Effmert et al. 2012). Overall, microbial infections resulted in higher VOC emission rates in all samples except for ‘Hoza’ infected with B. cepacia at 15 °C, which had a VOC profile that was like the profile of healthy ‘Hoza’ and ‘Hystand’ bulbs stored at the same temperature (Fig. 2). These findings may indicate the bulbs of ‘Hoza’ had an early infection of B. cepacia at week 12. Wang et al. (2019) reported similar results for ‘Summit’ bulbs 1 week after artificial inoculation with pathogenic F. oxysporum f. sp. cepae. These bulbs had visual symptoms but no increase in the VOC emission rates.
Fig. 2.
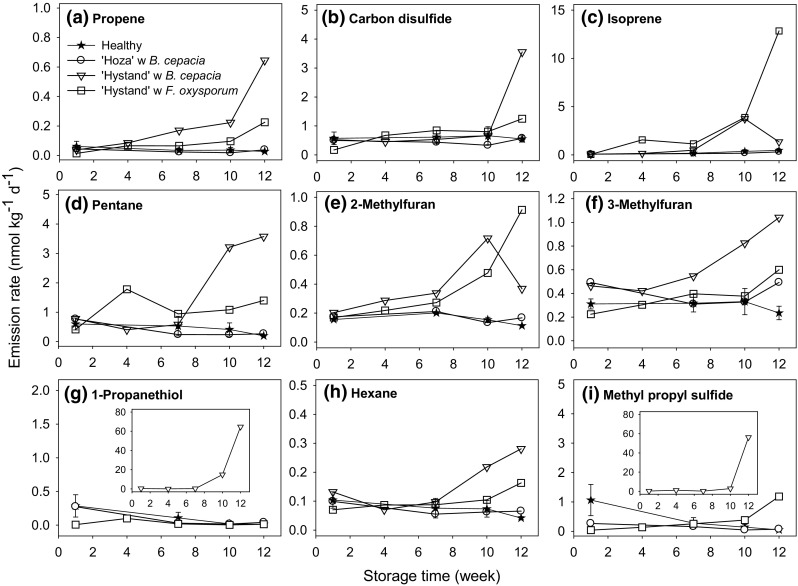
Emission rates of propene (a), carbon disulfide (b), isoprene (c), pentane (d), 2-methylfuran (e), 3-methylfuran (f), 1-propanethiol (g), hexane (h), and methyl propyl sulfide (i) during 12 weeks storage of onions at 15 °C. Data are presented as the mean values ± standard error (n = 3) for healthy onions, and as individual values for diseased onions
Conclusion
Onion bulbs emit many different low-boiling volatile organic compounds (VOCs) into the headspace during storage that can be sampled by SPME, separated on a HP-PLOT/Q column, and identified by GC–MS. Twenty-nine VOCs were extracted from fresh onion bulbs during storage, of which thirteen were reported for the first time from yellow onion bulbs. Temperature affected the VOC emission rate as more VOCs were emitted at 25 °C than at 4 °C and 15 °C. In contrast, cultivar had a minor effect on the VOC emission rates. The emission rates of total volatiles, acetone, dimethyl disulfide, and hexanal decreased during storage of healthy bulbs regardless of temperature. In the contrary, microbial infection resulted in higher emission rates of propene, carbon disulfide, isoprene, pentane, 2-methylfuran, 3-methylfuran, 1-propenethiol, hexane, and methyl propyl sulfide in all infected bulbs except for ‘Hoza’ infected with B. cepacia at 15 °C, which had similar VOC emission rates as those of the healthy control onions. This result indicates that VOCs emission may be used to monitor quality changes related to natural senescence and microbial decay of onion bulbs during storage.
Acknowledgements
We acknowledge the technical assistance of Jens Michael Madsen and Birgitte Foged, Aarhus University for sampling and GC–MS analysis, Jessica Perez and José Manuel Amigo, Copenhagen University with PARAFAC2 modelling, and the assistance of Heleen Bukman, Bejo Zaden B.V., The Netherland with disease identification.
Abbreviations
- CO2
Carbon dioxide
- LB
Lysogeny broth
- N2
Nitrogen
- O2
Oxygen
- PDA
Potato dextrose agar
Funding
This work was funded by the Innovation Fund Denmark in the project “Strategies and Technologies to Reduce Postharvest Losses of Potatoes and Vegetables” J. No. 1382-00057B.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Amigo JM, Popielarz MJ, Callejón RM, Morales ML, Troncoso AM, Petersen MA, Toldam-Andersen TB. Comprehensive analysis of chromatographic data by using PARAFAC2 and principal components analysis. J Chromatogr A. 2010;1217(26):4422–4429. doi: 10.1016/j.chroma.2010.04.042. [DOI] [PubMed] [Google Scholar]
- Banwart W, Bremner J. Formation of volatile sulfur compounds by microbial decomposition of sulfur-containing amino acids in soils. Soil Biol Biochem. 1975;7(6):359–364. doi: 10.1016/0038-0717(75)90050-4. [DOI] [Google Scholar]
- Benkeblia N, Varoquaux P, Shiomi N, Sakai H. Storage technology of onion bulbs cv Rouge Amposta: effects of irradiation, maleic hydrazide and carbamate isopropyl, N-phenyl (CIP) on respiration rate and carbohydrates. Int J Food Sci Technol. 2002;37(2):169–175. doi: 10.1046/j.1365-2621.2002.00554.x. [DOI] [Google Scholar]
- Block E, Putman D, Zhao SH. Allium chemistry: GC–MS analysis of thiosulfinates and related compounds from onion, leek, scallion, shallot, chive, and Chinese chive. J Agric Food Chem. 1992;40(12):2431–2438. doi: 10.1021/jf00024a018. [DOI] [Google Scholar]
- Boelens M, De Valois PJ, Wobben HJ, Van der Gen A. Volatile flavor compounds from onion. J Agric Food Chem. 1971;19(5):984–991. doi: 10.1021/jf60177a031. [DOI] [Google Scholar]
- Christensen LP, Edelenbos M, Kreutzmann S. Fruits and vegetables of moderate climate. In: Berger RG, editor. Flavours and fragrances: chemistry, bioprocessing and sustainability. Berlin: Springer; 2007. pp. 135–187. [Google Scholar]
- Colina-Coca C, Gonzalez-Pena D, Vega E, de Ancos B, Sanchez-Moreno C. Novel approach for the determination of volatile compounds in processed onion by headspace gas chromatography–mass spectrometry (HS GC–MS) Talanta. 2013;103:137–144. doi: 10.1016/j.talanta.2012.10.022. [DOI] [PubMed] [Google Scholar]
- Cranshaw WS, Crowe F, Davis RM, Dugan FM. Infections/biotic diseases. In: Schwartz HF, Mohan SK, editors. Compendium of onion and garlic diseases and pests. 2. St. Paul, Minnesota: The American Phytopothological Society; 2007. [Google Scholar]
- Effmert U, Kalderás J, Warnke R, Piechulla B. Volatile mediated interactions between bacteria and fungi in the soil. J Chem Ecol. 2012;38(6):665–703. doi: 10.1007/s10886-012-0135-5. [DOI] [PubMed] [Google Scholar]
- Hanum T, Sinha NK, Cash JN. Characteristics of gamma-glutamyl-transpeptidase and allinase of onion and their effects on the enhancement of pyruvate formation in onion macerates. J Food Biochem. 1995;19(1):51–65. doi: 10.1111/j.1745-4514.1995.tb00520.x. [DOI] [Google Scholar]
- Islam MN, Wang A, Pedersen JS, Edelenbos M. Microclimate tools to monitor quality changes in stored onions. Acta Hortic. 2015;1154:229–234. [Google Scholar]
- Kallio H, Salorinne L. Comparison of onion varieties by headspace gas chromatography–mass spectrometry. J Agric Food Chem. 1990;38(7):1560–1564. doi: 10.1021/jf00097a029. [DOI] [Google Scholar]
- Kebede BT, Grauwet T, Mutsokoti L, Palmers S, Vervoort L, Hendrickx M, Van Loey A. Comparing the impact of high pressure high temperature and thermal sterilization on the volatile fingerprint of onion, potato, pumpkin and red beet. Food Res Int. 2014;56:218–225. doi: 10.1016/j.foodres.2013.12.034. [DOI] [PubMed] [Google Scholar]
- Kim K-H, Kim Y-H. Composition of key offensive odorants released from fresh food materials. Atmos Environ. 2014;89:443–452. doi: 10.1016/j.atmosenv.2014.02.032. [DOI] [Google Scholar]
- Kopsell DE, Randle WM, Eiteman MA. Changes in the S-alk(en)ylcysteine sulfoxides and their biosynthetic intermediates during onion storage. J Am Soc Hortic Sci. 1999;124(2):177–183. doi: 10.21273/JASHS.124.2.177. [DOI] [Google Scholar]
- Kuo MC, Ho CT. Volatile constituents of the distilled oils of Welsh onions (Allium fistulosum L. variety maichuon) and scallions (Allium fistulosum L. variety caespitosum) J Agric Food Chem. 1992;40(1):111–117. doi: 10.1021/jf00013a021. [DOI] [Google Scholar]
- Li C, Schmidt NE, Gitaitis R. Detection of onion postharvest diseases by analyses of headspace volatiles using a gas sensor array and GC–MS. LWT Food Sci Technol. 2011;44(4):1019–1025. doi: 10.1016/j.lwt.2010.11.036. [DOI] [Google Scholar]
- Løkke MM, Edelenbos M, Larsen E, Feilberg A. Investigation of volatiles emitted from freshly cut onions (Allium cepa L.) by real time proton-transfer reaction–mass spectrometry (PTR–MS) Sensors. 2012;12(12):16060–16076. doi: 10.3390/s121216060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loreto F, Velikova V. Isoprene produced by leaves protects the photosynthetic apparatus against ozone damage, quenches ozone products, and reduces lipid peroxidation of cellular membranes. Plant Physiol. 2001;127(4):1781–1787. doi: 10.1104/pp.010497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luca A, Bach V, Edelenbos M. Effect of water vapor content and gas composition on quantification of volatile organic compounds from wild rocket by SPME. Food Anal Method. 2015;8(10):2626–2634. doi: 10.1007/s12161-015-0155-1. [DOI] [Google Scholar]
- Luca A, Bach V, Edelenbos M. Optimization of headspace solid-phase microextraction and static headspace sampling of low-boiling volatiles emitted from wild rocket (Diplotaxis tenuifolia L.) Food Anal Methods. 2015;8(5):1185–1196. doi: 10.1007/s12161-014-9993-5. [DOI] [Google Scholar]
- Luca A, Kjær A, Edelenbos M. Volatile organic compounds as markers of quality changes during the storage of wild rocket. Food Chem. 2017;232:579–586. doi: 10.1016/j.foodchem.2017.04.035. [DOI] [PubMed] [Google Scholar]
- Miedema P. Bulb dormancy in onion. I. The effects of temperature and cultivar on sprouting and rooting. J Hortic Sci. 1994;69:29–39. doi: 10.1080/14620316.1994.11515245. [DOI] [Google Scholar]
- Nielsen AT, Jonsson S. Quantification of volatile sulfur compounds in complex gaseous matrices by solid-phase microextraction. J Chromatogr A. 2002;963(1):57–64. doi: 10.1016/S0021-9673(02)00556-3. [DOI] [PubMed] [Google Scholar]
- Opara LU, Geyer M. 2.3 Onion storage. In: Bakker-Arkema FW, DeBaerdemaeker J, Amirante P, Ruiz-Altisent M, Studman CJ, editors. CIGR handbook of agricultural engineering. St. Joseph: American Society of Agricultural Engineers; 1999. pp. 125–156. [Google Scholar]
- Pak C, van der Plas LHW, de Boer AD. Importance of dormancy and sink strength in sprouting of onions (Allium cepa) during storage. Physiol Plant. 1995;94(2):277–283. doi: 10.1111/j.1399-3054.1995.tb05312.x. [DOI] [Google Scholar]
- Paull R. Effect of temperature and relative humidity on fresh commodity quality. Postharvest Biol Technol. 1999;15(3):263–277. doi: 10.1016/S0925-5214(98)00090-8. [DOI] [Google Scholar]
- Prithiviraj B, Vikram A, Kushalappa AC, Yaylayan V. Volatile metabolite profiling for the discrimination of onion bulbs infected by Erwinia carotovora ssp. carotovora, Fusarium oxysporum and Botrytis allii. Eur J Plant Pathol. 2004;110(4):371–377. doi: 10.1023/B:EJPP.0000021058.81491.f8. [DOI] [Google Scholar]
- Robinson J, Browne K, Burton W. Storage characteristics of some vegetables and soft fruits. An Appl Biol. 1975;81(3):399–408. doi: 10.1111/j.1744-7348.1975.tb01656.x. [DOI] [PubMed] [Google Scholar]
- Seefeldt HF, Løkke MM, Edelenbos M. Effect of variety and harvest time on respiration rate of broccoli florets and wild rocket salad using a novel O2 sensor. Postharvest Biol Technol. 2012;69:7–14. doi: 10.1016/j.postharvbio.2012.01.010. [DOI] [Google Scholar]
- Sinha R, Khot LR, Schroeder BK, Sankaran S. FAIMS based volatile fingerprinting for real-time postharvest storage infections detection in stored potatoes and onions. Postharvest Biol Technol. 2018;135:83–92. doi: 10.1016/j.postharvbio.2017.09.003. [DOI] [Google Scholar]
- Snowdon A. A color atlas of post-harvest diseases of fruits and vegetables. Boca Raton: CRC Press; 1990. [Google Scholar]
- Tsiaganis MC, Laskari K, Melissari E. Fatty acid composition of Allium species lipids. J Food Compos Anal. 2006;19(6–7):620–627. doi: 10.1016/j.jfca.2005.06.003. [DOI] [Google Scholar]
- Vikram A, Hamzehzarghani H, Kushalappa AC. Volatile metabolites from the headspace of onion bulbs inoculated with postharvest pathogens as a tool for disease discrimination. Can J Plant Pathol. 2005;27(2):194–203. doi: 10.1080/07060660509507216. [DOI] [Google Scholar]
- Wang A, Casadei F, Johansen A, Bukman H, Edelenbos M. Emission of volatile organic compounds from healthy and diseased onions. Acta Hortic. 2016;1144:333–339. doi: 10.17660/ActaHortic.2016.1144.49. [DOI] [Google Scholar]
- Wang A, Islam MN, Haapalainen M, Latvala S, Johansen A, Edelenbos M. Pathogenic Fusarium oxysporum f. sp. cepae growing inside onion bulbs emits volatile organic compounds that correlate with the extent of infection. Postharvest Biol Technol. 2019;152:19–28. doi: 10.1016/j.postharvbio.2019.02.010. [DOI] [Google Scholar]