Abstract
In this work, supercritical fluid extraction (SFE) for the recovery of phenolic antioxidants from papaya agroindustrial waste (seeds) was explored, making use of neat supercritical CO2 and CO2 added with ethanol (CO2-EtOH). A full factorial design played on in order to evaluate the effect of CO2 extraction parameters (temperature between 40 and 60 °C, and pressure between 10 and 30 MPa) on yield and total phenols content (TPC), then ethanol was applied as a co-solvent and its effect on the recovery of phenolics was analyzed. The SFE was compared to the conventional extraction using ethanol. The antioxidant activity of all extracts was evaluated, and the phenolic composition in selected extracts was assessed by HPLC-ESI-MS. The highest extraction yields (21.02–26.46%) and TPC (15.34–34.23 mgGAE/g) were found in extracts obtained with CO2-EtOH and ethanol. Good and selective phenolic recovery was obtained by using CO2-EtOH, (44.81% of TPC recovered). The CO2-EtOH extracts showed high radical scavenging activity and higher antioxidant effect against lipid oxidation. Some phenolic acids and flavonoids were observed in the extracts with better antioxidants properties. The results showed that SFE is a suitable green technology for the phenolic recovery from papaya agroindustrial waste, and also an alternative for its valorization.
Keywords: Agroindustrial by-products, Papaya seeds, Green extraction, Phenolic antioxidants, Food preservatives
Introduction
The industrial processing of agricultural food products produces high amounts of waste. On average, it is estimated that 30–50% of processed food is discarded as agroindustrial waste (Dávila et al. 2014; Lin et al. 2013). The industrial consumption of fruits produces large amounts of waste such as pomace, peels, seeds, and kernels. Worldwide, fruit processing generates more than 0.6 billion tons of waste/year, both the wine and beverage industries are the main waste producers (Lin et al. 2013). According to the Food and Agriculture Organization (FAO) of the United Nations, the global production of papaya was 13.1 million tons in 2016. India and Brazil were the major producers with 5.7 and 1.4 million tons, respectively, whereas Colombia produced about 188,305 tons (FAO 2016). Industrial papaya consumption is mainly for the production of papain and the manufacturing of food products. These uses dispose approximately 25%w/w of processed fruit as waste, particularly seeds (Desai and Wagh 1995). Nowadays, the global production of agroindustrial papaya waste is estimated around three million tons/year, 70% are seeds (FAO 2016).
In the last two decades, the consumers’ interest in healthy food has increased, meaning that a modern trend to supplement foods with polyphenols, carotenoids, or tocopherols obtained from natural sources can be seen. Agroindustrial waste from processing fruits is well known as a source of high-value compounds with wide variety of bioactive properties and promising uses. Seeds and kernels are rich in mono or polyunsaturated fatty acids, whereas pomace, peels, and some seeds are rich in antioxidants (Kao and Chen 2013). Polyphenols, carotenoids or tocopherols from apple and grape pomace (Herrero et al. 2015), cocoa bean hulls (Mazzutti et al. 2018; Valadez-Carmona et al. 2018), mango and banana peels (Martins and Ferreira 2017), and berries waste (Lorenzo et al. 2018) have been studied as preservatives and nutraceutical ingredients in food products.
Papaya agroindustrial waste, particularly papaya seeds, have been reported as a source of antioxidant compounds. A good content of phenolic compounds has been reported in papaya seed extracts and correlated with their antioxidant properties: previous studies reported the free radical scavenging activity of various fractions obtained from papaya seeds; methanol and ethyl acetate fractions showed the better antioxidant properties against 2,2-diphenyl-1-picrylhydrazyl radical (DPPH·) (Gogna et al. 2015; Faisal et al. 2016; Khor and Wong 2016; Salla et al. 2016). In vitro studies have shown that both methanol and hexane fractions from papaya seeds have high protective effect against induced oxidative stress in cells (Gogna et al. 2015; Salla et al. 2016). Elseways, polyphenols obtained from papaya seeds were added to mackerel (Rastrelliger kanagurta) as preservatives, and as a result, these compounds reduced the lipid and protein oxidation in fish during its storage on ice (Faisal et al. 2016). Different fractions (polar and non-polar) obtained from papaya seeds showed high antioxidant properties against lipid oxidation in vegetable edible oils (Castro-Vargas et al. 2016).
Nowadays, the recovery of antioxidants from agroindustrial waste is an attractive option for its exploitation and valorization, for this purpose, different extraction methods are available (Oroian and Escriche 2015). The supercritical fluid extraction (SFE) is considered as an appropriate technique for antioxidants recovery from agroindustrial waste (Herrero et al. 2015; Oroian and Escriche 2015). This method is characterized by to use green solvents as carbon dioxide (CO2), low thermal degradation of antioxidants compounds, obtaining free solvent extracts, and variable selectivity on the target compounds recovery. Neat CO2, and CO2 added with ethanol as co-solvent (CO2-EtOH) have been used for the recovery of antioxidants from residues generated by the industrial processing of fruits (Herrero et al. 2015; Oroian and Escriche 2015; Banerjee et al. 2017).
The previous background showed that papaya seeds are a promising source of phenolic antioxidants, and that SFE can be an appropriate method for its recovery. The aim of the present study were to explore the uses of SFE for the recovery of phenolic antioxidants from papaya seeds and to evaluate their antioxidant properties. Neat CO2 and CO2-EtOH were used as solvents, and their efficiency for the recovery of phenolic compounds was compared with a conventional extraction method. A three-level full factorial design, together with a response surface analysis (RSA) were used in order to study the effects of the extraction parameters (temperature and pressure) on the extraction yield and total phenol contents (TPC). This work proposes an alternative for the exploitation and valorization of papaya agroindustrial waste as source of antioxidants that could find usefulness in the pharmaceutical and food industries.
Materials and methods
Papaya seed samples
The papaya seeds were supplied by Alimentos SAS S.A. (Bogota Colombia), which were obtained from the agroindustrial processing of papaya. 50 kg of papaya seeds were procured, dried and powdered. The sample with particle size between 0.300 and 0.850 mm (+ 50/− 18 US Standard size sieves) was used in SFE and conventional extraction method. The mean particle diameter was 0.496 ± 0.03 mm, calculation that was made based on the mean size distribution.
Supercritical fluid extraction
Supercritical fluid extraction experiments were performed using neat CO2 and CO2-EtOH in the dynamic extraction unit previously described (Zetzel et al. 2003). For extraction, a sample of papaya seeds 10.0 g used, and glass beads were placed inside the extraction column as a fixed bed of particles, and the extraction parameters (temperature, pressure, co-solvent percentage, and solvent flow rate) were adjusted. The extraction time was set up by kinetic assays performed at 50 °C, 20 MPa, and constant CO2 flow rate of 0.50 ± 0.05 kg/h. All extractions were performed for 180 min and the CO2 flow above mentioned. The mixture solvent/extract (e.g., CO2/extract or CO2-EtOH/extract) was separated to ambient pressure (101.3 kPa) and the extracts were collected in amber flasks. For the CO2-EtOH extracts, ethanol was removed under vacuum. Finally, the extracts were weighted in an analytical balance (SHIMADZU Model AY220, São Paulo-SP, Brazil), then flushed with a nitrogen stream, sealed, and stored at − 20 °C. The extractions with neat CO2 were performed using three different levels of temperature and pressure according to the experimental design shown in Table 1 (extract 1 to 9), and explained in further section. On the other hand, the extractions with CO2-EtOH were performed at 50 °C, 20 MPa, and three levels of co-solvent percentage (2, 5 and 8%w/w), temperature and pressure were established according to extraction yield and total phenol content results observed for the neat CO2 experiments. All extractions were performed by triplicate; the extraction yields were expressed as weight-to-weight percentage on dry basis (% w/w d.b.).
Table 1.
Extraction yield, total phenols content, total phenols recoveries and DPPH radical scavenging of the extracts obtained from papaya seeds
| Extract | Solvent | Coded variablesa | Temperature (°C) | Pressure (MPa) | Extraction yield (% w/w d.b.) | TPCb(mgGAE/g extract) | TPC recoveredb (%) | DPPH radical scavengingb (IC50 mg/mL) | |
|---|---|---|---|---|---|---|---|---|---|
| X 1 | X 2 | ||||||||
| 1 | CO2 | − 1.0 | − 1.0 | 40 | 10 | 4.59 | 1.40 ± 0.08i | 4.09 ± 0.29 h | 80.60 ± 1.61a |
| 2 | CO2 | − 1.0 | 0.0 | 40 | 20 | 19.40 | 1.65 ± 0.07 h | 4.82 ± 0.25 g | 79.24 ± 2.84a |
| 3 | CO2 | − 1.0 | 1.0 | 40 | 30 | 18.80 | 2.08 ± 0.09 g | 6.08 ± 0.33f | 73.70 ± 1.09b |
| 4 | CO2 | 0.0 | − 1.0 | 50 | 10 | 1.60 | 1.56 ± 0.10i | 4.56 ± 0.36 g,h | 67.50 ± 2.33c |
| 5.1c | CO2 | 0.0 | 0.0 | 50 | 20 | 20.75 | 3.78 ± 0.14e | 11.04 ± 0.51d | 20.95 ± 1.60e |
| 5.2 | CO2 | 0.0 | 0.0 | 50 | 20 | 20.42 | |||
| 5.3 | CO2 | 0.0 | 0.0 | 50 | 20 | 20.60 | |||
| 5.4 | CO2 | 0.0 | 0.0 | 50 | 20 | 20.68 | |||
| 5.5 | CO2 | 0.0 | 0.0 | 50 | 20 | 20.49 | |||
| 6 | CO2 | 0.0 | 1.0 | 50 | 30 | 19.55 | 3.20 ± 0.15f | 9.35 ± 0.55e | 27.77 ± 1.10d |
| 7 | CO2 | 1.0 | − 1.0 | 60 | 10 | 0.50 | 0.92 ± 0.04j | 2.69 ± 0.11i | 81.83 ± 0.48a |
| 8 | CO2 | 1.0 | 0.0 | 60 | 20 | 19.63 | 3.13 ± 0.11f | 9.14 ± 0.40e | 25.13 ± 0.64d |
| 9 | CO2 | 1.0 | 1.0 | 60 | 30 | 20.35 | 3.35 ± 0.09f | 9.79 ± 0.33e | 26.05 ± 0.19d |
| 10 | CO2-EtOH 2%w/w | – | – | 50 | 20 | 21.02 ± 0.05d | 7.79 ± 0.38d | 22.76 ± 1.38c | 14.35 ± 0.45f |
| 11 | CO2-EtOH 5%w/w | – | – | 50 | 20 | 23.75 ± 0.04c | 10.42 ± 0.69c | 30.44 ± 2.51b | 7.02 ± 0.13 g |
| 12 | CO2-EtOH 8%w/w | – | – | 50 | 20 | 24.52 ± 0.06b | 15.34 ± 0.86b | 44.81 ± 3.13a | 5.95 ± 0.32 h |
| 13 | Ethanol | – | – | – | – | 26.46 ± 2.66a | 34.23 ± 2.75a | – | 2.29 ± 0.09i |
aTemperature (X1); Pressure (X2)
bTPC, TPC recovered and DPPH radical scavenging columns shows the mean ± standard deviation (n = 3)
cCentral point was replicate five times (extract 5.1–5.5). TPC, TPC recovered and DPPH radical scavenging values for central point correspond to average (n = 5)
Means in columns followed by the same letter are not statistical different
Experimental design
For the SFE with neat CO2, three-level full factorial design coupled to response surface analysis (RSA) were used to evaluate the effect of temperature (X1) and pressure (X2) on the response variables on extraction yield (Y1) and TPC (Y2). The temperature and pressure levels and their coded variables are shown in Table 1 (extracts 1–9). The experimental design consisted of 9 experimental runs, and was performed in randomized in order to minimize the effects of unexpected variability on the responses. Central point was replicated five times (extract number 5.1–5.5). The second order models were obtained [according to Eq. (1)] to aim at explaining the Yn response variables as a function of the extraction parameters:
| 1 |
where β0 is the intercept, β1 and β2 are linear regression coefficients, β11 and β22 are quadratic regression coefficients, and β12 is the interaction coefficient. The response surface plots were generated to show the effect of the extraction parameters on the response variables. The fit of the model was evaluated by analysis of variance (ANOVA).
Conventional extraction
The conventional extraction with ethanol by Soxhlet method was developed as an exhaustive extraction process of phenolic antioxidants. The extractions were performed under reduced pressure in order to avoid the thermal degradation of target compounds. The pressure of the extraction system was adjusted to reduce the boiling point of the ethanol at 35 °C. The papaya seed samples (10.0 g) were extracted for 8 h. After the extraction, the ethanol was removed under vacuum. The extractions were performed in triplicate and the extraction yield was expressed as % w/w d.b.
Total phenols content
The Total Phenols Content (TPC) in all extracts was determined by the Folin-Ciocalteu method with the slight modifications proposed by Castro-Vargas et al. (2010). The dry extracts were resuspended in a mixture of ethyl acetate:ethanol (1:1) at final concentration range from 10 to 20 mg/mL. The 100 μL of each extract solution was mixed with 750 μL of the Folin-Ciocalteu reagent (10%w/w); after 5 min, 750 μL of sodium carbonate solution (6%w/w) were added. Finally, the mixture was stirred and left to react in the dark and room temperature for 90 min. Subsequently, the absorbance at 765 nm (Thermo Scientific Evolution 600 UV/Vis) was measured. The TPC was expressed as mg of gallic acid equivalents per g of dry extract (mgGAE/g extract), calculated using a standard curve of gallic acid (concentrations between 0 and 100 μg/mL). All measurements were carried out in triplicate.
Antioxidant activity
The antioxidant properties of papaya seed extracts were evaluated using two methods: the in vitro free radical-scavenging activity using 1,1-diphenyl-2-picrylhydrazyl (DPPH·), and the antioxidant activity against the lipid oxidation in vegetable edible oil (VEO).
DPPH· scavenging activity
The DPPH· scavenging activity was determined by following the procedure that has been previously described by Castro-Vargas et al. (2010), where 1 mL of DPPH· solution 0.1 M in ethanol, with initial absorbance measured at 517 nm (A0), was added to 50 μL of each extract (dissolved in a mixture of ethyl acetate:ethanol 1:1), and the final absorbance at 517 nm (Af) was measured after 60 min. The scavenging percentage was determined as % = (A0 − Af)/A0. The extract concentration (mg/mL) necessary to decrease the initial DPPH· concentration by 50% (IC50) was determined by interpolation in the standard curve. Gallic acid was used as reference phenolic antioxidant in order to compare its DPPH· scavenging activity versus the papaya seed extracts. All measurements were performed by triplicate.
Antioxidant activity in vegetable edible oil
The antioxidant properties of papaya seed extracts against the lipid oxidation in vegetable edible oil (VEO) were evaluated and compared with the synthetic antioxidant tert-butyhydroquinone (TBHQ). The VEO sample (without antioxidants) was a mixture of different kinds of edible oil, whose general composition was 70% of unsaturated fatty acids triglycerides (40% oleic, 55% linoleic, and 5% of others), and 30% of stearic acid triglycerides. Previously, we studied the VEO oxidation process and its variables, and an experimental procedure for antioxidant activity (AA) measures was establishing (Castro-Vargas et al. 2016). The AA experiments were performed following two steps: (1) accelerated oxidation of VEO samples added with ferrous ion as pro-oxidant, and each extract (dry) or TBHQ (liquid) as antioxidants at a final concentration of 300 mg/kg. One VEO sample without antioxidants was used as control. All VEO samples were kept in oxidation for 15 days at 60 °C. (2) The antioxidant properties of extracts and TBHQ were tested by measuring some lipid oxidation products; lipid hydroperoxides (LHP), thiobarbituric acid reactive substances (TBARS), and hexanal. The LHPs were measured by conjugated dienes method; the TBARS were analyzed using their absorbance at 532 nm, whereas the hexanal was measured by headspace-solid phase microextraction-gas chromatography. The oxidation products were quantified after 15 days of oxidation and the results were expressed as the percentage of inhibition of lipid oxidation products formation according to Eq. (2):
| 2 |
where [LOP]C and [LOP]A are the lipid oxidation products concentration in control and added with antioxidants samples, respectively.
Analysis of phenolic compounds
The extracts with the best qualities (extraction yield, TPC, and AA) were selected and analyzed by HPLC-ESI-MS in order to identify some phenolic compounds. Analyses were performed using an Agilent Technologies 1260 chromatograph (Palo Alto, CA, USA) equipped with a binary pump (G1312B), a degasser, an autosampler, and a thermostatted column compartment. Each extract was dissolved in acetonitrile at 20 mg/mL and the injected sample was 1 μL. Chromatographic separation was performed on a Bischoff ProntoSIL 300-5-C-18 column (150 mm × 4 mm × 5 μm) kept at 30 °C. The mobile phase was composed of 0.1% formic acid (A) and acetonitrile (B) at a flow rate of 0.3 mL/min. The gradient program was as follows: 0% B (0 min), 10% B (20 min), 50% B (28 min), 70% B (30 min), 90% B (50 min), 0% B (55 min), finally the initial conditions were maintained for 10 min. The choice of the mobile phase gradient was based on not reported experiments. The chromatographic system was coupled to a Quadrupole-Time-of-Flight MS detector (Agilent Technologies 6520 Q-TOF), equipped with an electrospray ionization (ESI) source operating at the negative ionization mode. The MS and MS/MS analysis settings were as follows: ESI capillary voltage, 3.5 kV; nitrogen as drying and nebulizing gas at flow rate 5 L/min, temperature 300 °C, and pressure 30 psi; collimator voltage 175 V; and octopole voltage 750 V. The spectra were acquired over a mass to charge ratio (m/z) ranging from 50 to 1000. For the MS/MS analysis, the selected ions were fragmented using nitrogen as collision gas at 350 °C and 20 eV. All results were analyzed using the MassHunter Workstation Agilent Technologies software. The phenolic compounds were identified using their mass spectra information, the molecular formula, and bibliographic data.
Statistical analysis
The statistical differences of extraction yield, TPC, LHP, hexanal, and TBARS were determined by means of ANOVA. All the statistical analyses were developed using the software Statgraphics Centurion XVII with a confidence level of 95%.
Results and discussion
Recovery of phenolic antioxidants from papaya seeds
In this work, the effects of pressure and temperature on the recovery of phenolic compounds with neat CO2 were studied by RSA. The suitable extraction pressure and temperature were selected; after that, ethanol was used as a co-solvent, and the effects on the recovery of target compounds was evaluated. Table 1 presents the extraction yields obtained from papaya seeds using SFE with CO2 and CO2-EtOH, and exhaustive extraction with ethanol. The yields show the efficiency of the extraction methods and solvents for the recovery of extractable solutes. From Table 1, it can be seen that higher yield was obtained when using ethanol (26.46%), followed by the extracts number 11 and 12 obtained with CO2-EtOH (23.75 and 22.52%, respectively), and the extract number 5 (20.59%) obtained with neat CO2. The high extraction yield observed in the ethanolic extract suggests that the extraction process was exhaustive. Khor and Wong (2016) previously reported on phenolics extraction from papaya seeds using exhaustive maceration with ethanol, obtaining lower extraction yield (5.2%). On the other hand, the SFE with neat CO2 at 20 and 30 MPa allowed to obtain low polarity extracts with yields between 18.80 and 20.59%. Previously, reports showed that papaya seeds are a source of low polarity extracts; da Silva and Jorge (2017) reported oil content in papaya seeds above 20%, while Li et al. (2015) recovered oil with yields between 22 and 26% by ultrasound assisted extraction. In these studies, a high content of polyunsaturated fatty acids in oils (particularly linoleic acid) were observed, however, phenolic compounds were not reported. In disagreement with the above, Kothari and Seshadri (2010) observed high content of phenolic compounds (mostly flavonoids) in papaya oil obtained using hexane. This background suggests that papaya seed extracts obtained by SFE with neat CO2 may contain polyunsaturated fatty acids and some phenolic compounds, which is in agreement with the TPC results observed in the present work and explained in further text.
Table 1 shows the TPC observed in papaya seed extracts, these results are present as mg of gallic acid equivalents per g of extract (mgGAE/g extract). In addition, the efficiency of the SFE to phenolic recovery is presented as recovered %TPC. These values were obtained by comparison between the TPC values for supercritical extracts and the TPC value for ethanolic extract. The highest TPC was observed in the ethanolic extract (34.23 ± 2.75 mgGAE/g extract), followed by CO2-EtOH extracts (from 7.79 ± 0.38 to 15.34 ± 0.86 mgGAE/g extract), and neat CO2 extract number 5 (3.78 ± 0.14 mgGAE/g extract). The high TPC observed in ethanolic extract is due to the ethanol polarity, which allows extracting medium to high polarity compounds such as polyphenols. Additionally, conventional extraction was performed in this work under low temperature in order to avoid the thermal degradation of the target compound. Phenolic extraction from papaya seeds with polar solvents by conventional extraction methods has been previously reported: the aqueous extracts showed TPC 0.98 mgGAE/mg extract (Oboh et al. 2013), methanolic extracts presented 118 mgGAE/g extract (Khor and Wong 2016), and ethanolic extracts 307 mgGAE/g extract (Kothari and Seshadri 2010). The TPC observed in the ethanolic extract obtained in the present work was lower than the one reported by Kothari and Seshadri (2010); difference that can be caused by the variations in the concentration of phenolic compounds in papaya seeds. Several reports have shown that the concentration and distribution of secondary metabolites in fruits (e.g., polyphenols) can be affected by parameters associated with their crop, post-harvest handling, and industrial processing (Patthamakanokporn et al. 2008).
The TPC recoveries between 2.69 and 11.04% were obtained by using neat CO2. The lower recovery was obtained at 60 °C and 10 MPa, and the higher recovery was observed at 50 °C and 20 MPa. The low recoveries observed are mainly due to the non-polar character of CO2, although some simple phenolic compounds and phenolic acids may be extracted using neat CO2, its solubility is rarely high (Bitencourt et al. 2016, 2018). Despite the low TPC recoveries observed, these were higher compared with the development of similar studies with agroindustrial wastes, such as passion fruit by-products (%TPC recovered between 4.9 and 10%) (Oliveira et al. 2016) and cocoa bean hulls (%TPC recovered between 3.5 and 7.8%) (Mazzutti et al. 2018).
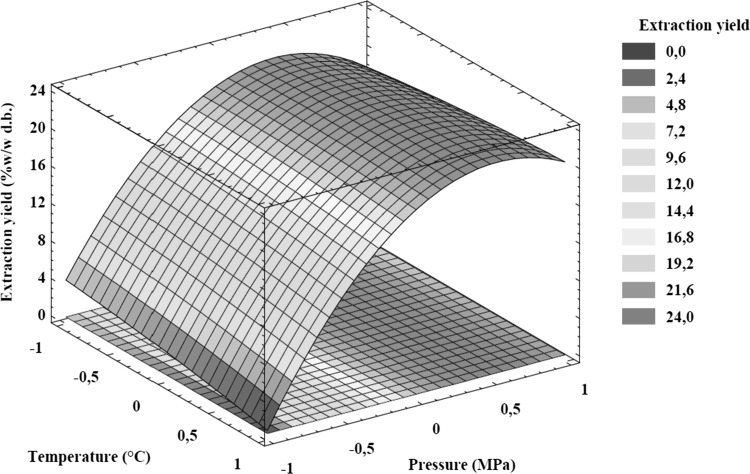
In the SFE with neat CO2, RSA was used to evaluate the effect of temperature (X1) and pressure (X2) on response variables such as extraction yield (Y1) and TPC (Y2). Table 2 presents the ANOVA results used to evaluate the fit of the second order model and the effect of extraction parameters. For the experimental region explored, the experimental data was fitted to the second order model. The Eq. (3) presents the resulting model equation for extraction yield with R2 (99.61%), adj-R2 (99.27%), standard error (0.691), and lack of fit (p = 0.873). The R2 and the lack of fit indicate that the regression model explains the process behavior in a satisfactory manner. On the other hand, the parameters with the highest effect on the extraction yield were the linear and quadratic coefficients of pressure (both with p < 0.0001), followed by the interaction between temperature and pressure (p = 0.0058), and the linear coefficient of temperature (p = 0.0249). Figure 1 shows the response surface graph of the second order model for extraction yield, this plot permitted to visualize the effect of the extraction parameters on the response variable. The response surface graph shows that the extraction yield improved with the increase of the extraction pressure between 10 and 20 MPa; for example, at 60 °C the yield increased 41 times (from 0.50 to 20.50%) when the pressure was changed from 10 to 20 MPa. This behavior is mainly due to the increment of the supercritical CO2 density and therefore its solvent power. High pressure favors the solutes extraction from the vegetable matrix, therefore the global yield can be enhanced. On the other hand, the negative effect of temperature on the extraction yield was observed at low and middle pressure level; for example, at 10 MPa the increase of temperature between 40 and 60 °C reduced the global yield from 4.59 to 0.50%. This result could be explained by the decrease of CO2 density with increased temperature.
| 3 |
Table 2.
Analysis of variance of regression coefficients and statistical indicators of second order model for extraction yield (Y1) and total phenols content (Y2)
| Y 1 | Y 2 | |||
|---|---|---|---|---|
| Coefficient | p value | Coefficient | p value | |
| X a1 | − 0.360 | 0.0249* | 0.378 | 0.0636 |
| X a2 | 8.693 | < 0.0001** | 0.791 | 0.0032* |
| X 1 X 2 | 1.447 | 0.0058** | 0.437 | 0.0757 |
| − 0.3 | 0.5052 | − 0.916 | 0.0105* | |
| − 9.240 | < 0.0001** | − 0.926 | 0.0100* | |
| Lack of fit | (p = 0.873) | (p = 0.778) | ||
| R2 | 99.61% | 92.41% | ||
| Adj-R2 | 99.27% | 86.09% | ||
| Standard errorb | 0.691 | 0.408 | ||
a Temperature (X1); pressure (X2)
b Standard error relative to Y1 and Y1 values
**highly significant (p < 0.01)
*significant (0.01 < p < 0.05)
Fig. 1.
Response surface plot for the effect of SFE parameters on extraction yield from papaya seeds. Temperature and pressure coded levels (− 1, 0, + 1) are described in the Table 1
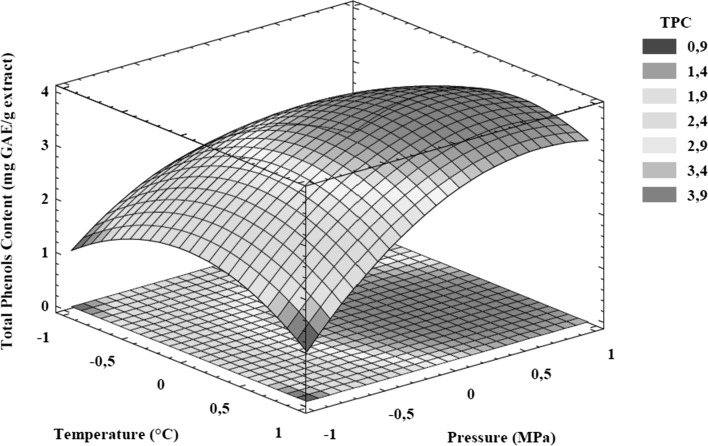
For the phenolic extraction using neat CO2, TPC data was fitted to the second order model and Eq. (4) was obtained. The R2 and the lack of fit values showed that the regression model satisfactorily explains the effect of pressure and temperature on phenolic compound extraction (Table 2). The RSA indicates that the extraction parameter with the higher effect was the linear coefficient of pressure, followed by the quadratic coefficients of pressure and temperature. The response surface graph of the second order model for TPC is presented in Fig. 2. The positive effect of pressure was observed at all temperatures, the pressure increased improving the phenolic compounds extraction from papaya seeds, this effect was more notorious when the pressure was enhanced from 10 to 20 MPa; above 20 MPa, the change in TPC is little and the surface showed a plateau zone. The pressure effect that was observed may be due to the little enhance in the dielectric constant of CO2 generated with the increase of pressure, however, highest pressures are necessary to obtain more appreciable changes (Mukhopadhyay 2000). Noteworthy, Pérez et al. (2015) observed a similar effect in the extraction of grape seed oil using neat supercritical CO2; at a constant temperature, the increase of extraction pressure from 10 to 30 MPa enhanced the TPC in the extracts from 3 to 16 mgGAE/g.
| 4 |
Fig. 2.
Response surface plot for the effect of SFE parameters on total phenols content in papaya seeds extracts. Temperature and pressure coded levels (− 1, 0, + 1) are described in the Table 1
Table 1 shows that by the SFE the higher extraction yield and TPC recovered values were obtained with neat CO2 at 50 °C and 20 MPa. Moreover, Figs 1 and 2 showed their maximum response values at pressures above 20 MPa, and temperatures between 50 and 60 °C. Considering these results, 50 °C and 20 MPa were the selected extraction conditions for the SFE experiments with CO2 added with ethanol as co-solvent. It is worth mentioning that these conditions correspond to middle temperature and pressure levels, which from the industrial-scale view can be suitable to reduce both energetic and technical requirements. Ethanol was employed as co-solvent in order to improve the phenolic compounds extraction from papaya seeds. Furthermore, ethanol is a GRAS solvent, which allows its safe usage in food, and pharmaceutical and cosmetic products. Three levels of ethanol were used (2, 5, and 8%), and its effect on extraction yields and TPC recoveries was observed (Table 1). The addition of 5 and 8% of co-solvent improved the extraction yields compared to the neat CO2; an increase from 20.59% (extract number 5) to 23.75 and 24.52%, (extracts number 11 and 12, respectively), was observed. As expected, the TPC recoveries were also enhanced; the ethanol addition to CO2 augmented the TPC recoveries between 2 and 4 times compared to the neat CO2. These results could be explained considering that ethanol can increase the polarity of the supercritical solvent, improving the solubilization and extraction of medium polarity solutes (Manna et al. 2015). Additionally, the solute-matrix bindings could be broken due to the fact that the hydroxyl group from ethanol forms a hydrogen bond with the matrix, so the solutes that are released from matrix increase. The improvement of the extraction yields and TPC recoveries on function of the ethanol percentage increase is due to the increase of CO2 solvation power, however, this behavior is affected by the behavior of the mixture phase under the extraction conditions (Valadez-Carmona et al. 2018).
In this study, 44.81% of phenolic compounds were recovered using CO2-EtOH at 50 °C, 20 MPa and 8% of co-solvent (extract number 12). Compared with other kinds of waste generated in the industrial processing of fruits, which have been explored as sources of phenolic compounds using SFE with CO2-EtOH, extract number 12 showed a TPC (15.34 mgGAE/g extract) that is higher than in the extracts obtained from grape skins (3 mgGAE/g, at 40 °C, 20 MPa, and 5% EtOH) (Manna et al. 2015), blackberry bagasse (12.73 mgGAE/g at 60 °C, 15 MPa, and 10% EtOH) (Pasquel et al. 2014), cacao pod husk (13.63 mgGAE/g at 50 °C, 30 MPa, and 15% EtOH) (Valadez-Carmona et al. 2018), and passion fruit seeds cake (15 mgGAE/g at 40 °C, 30 MPa, and 5% EtOH) (Oliveira et al. 2016). It is worth mentioning that grape and blackberry waste are recognized as good sources of phenolic compounds.
Antioxidant activity
DPPH scavenging activity
Table 1 presents the results of the DPPH· scavenging expressed as IC50; the low IC50 values mean high AA against DPPH·. The higher DPPH· scavenging activity was exhibited by ethanolic extract (IC50 = 2.29 mg/mL), followed by the extracts obtained by using SFE with CO2-EtOH (IC50 between 5.95 and 14.35 mg/mL). Gallic acid was used as reference phenolic antioxidant in order to compare its DPPH· scavenging activity versus the papaya seed extracts, this compound showed IC50 = 6.09 ± 0.25 mg/mL. Extracts number 12 and 13 showed higher AA compared to the gallic acid, furthermore, the ANOVA results indicate that extract number 11 and gallic acid have similar AA. Table 1 shows that the addition of ethanol as a co-solvent in SFE improved the DPPH· scavenging activity of papaya seed extracts; this result is due to the increase of phenolic compound concentration in extracts. A Pearson correlation analysis between TPC and DPPH· scavenging activity indicates a high positive correlation (R2 = 0.9785), thus, extracts with high TPC have large DPPH· scavenging activity. The results observed suggest that papaya seeds are a source of phenolic antioxidants with free radical-scavenging activity. Previous reports showed that papaya seed extracts have high DPPH· scavenging activity. Khor and Wong (2016) reported IC50 = 0.078 mg/mL for methanol extract, whereas Zunjar et al. (2015) observed IC50 = 3.11 mg/mL for aqueous extract.
Antioxidant activity in vegetable edible oil
Papaya seed extracts and TBHQ as reference antioxidant compound were added to VEO, and their antioxidant properties against lipid oxidation were evaluated. The antioxidant effectiveness was evaluated as %inhibition of HPL, TBARS, and hexanal formation [calculated according Eq. (2)]. Figure 3 shows the AA results, all extracts and TBHQ showed antioxidant effect against VEO oxidation (although the extracts number 1 and 4 did not show inhibitory effect on the hexanal formation). In general, it can be observed that the LHP formation was inhibited between 67.9% and 93.0% (extracts number 13 and 11, respectively), while the production of TBARS was delayed between 41.5% and 90.9% (extracts number 1 and 11, respectively), and the hexanal formation was reduced between 1.6% and 58.7% (extracts number 7 and 11, respectively). The higher AA was observed for the extract number 11, obtained using CO2-EtOH at 50 °C, 20 MPa, and 5% of co-solvent. Other extracts with high AA were number 10 and 12, obtained with CO2-EtOH, and number 5 and 9, recovered with neat CO2. Table 1 shows that the mentioned extracts also presented high TPC values (particularly those obtained by using CO2-EtOH), these results suggest that the phenolic compounds in extracts are related with their antioxidant properties. The Pearson correlation analysis between TPC and %inhibition of HPL, TBARS, and hexanal indicate a good positive correlation between TPC and TBARS inhibition (R2 = 0.9201), and TPC and hexanal inhibition (R2 = 0.9314), however, a poor correlation between TPC and LHP inhibition was observed (R2 = 0.5119).
Fig. 3.
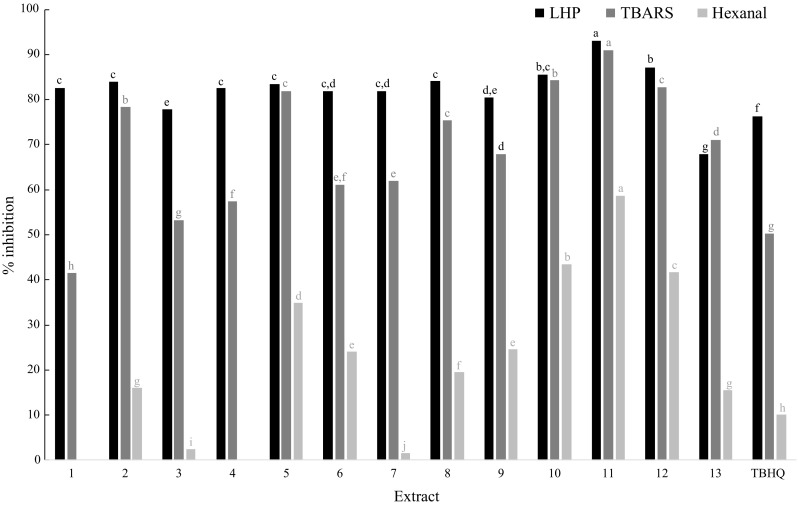
Antioxidant activity in vegetable edible oil of papaya seeds extracts and TBHQ presented as inhibition percentage of lipid hydroperoxides (LHP), thiobarbituric acid reactive substances (TBARS) and hexanal formation. For each lipid oxidation product, different letter means statistical differences
The SFE with neat CO2 allowed to obtain extracts with good AA in VEO (e.g., extracts number 5, 6, 8, and 9), moreover, the addition of ethanol as a co-solvent improved the antioxidant properties of extracts. Figure 3 shows that the extracts number 10, 11, and 12 (obtained with CO2-EtOH) presented high effectiveness to inhibit the TBARS and hexanal formation compared to extract number 5 (obtained using neat CO2). These results are due to the enhancement in the concentration of phenolic compounds in papaya seed extracts generated by the use of ethanol as a co-solvent (discussed in previous section).
Lipid oxidation in foods is a complex process, which can be developed by different ways and reaction mechanisms. The oxidation of polyunsaturated fatty acids can generate a wide range of products: hydroperoxides, aldehydes, ketones, esters, ethers, short-chain acids, alcohols, lactones, furans, and aliphatic and aromatic hydrocarbons (Kazutoshi and Takayuki 2004). Hydroperoxides are the initial lipid oxidation products, while some TBARS and the hexanal are considered as intermediate and final products (Guillén-Sans and Guzmán-Chozas 1998; Kazutoshi and Takayuki 2004). A suitable antioxidant for food products should reduce/inhibit the formation of oxidation products in its initial, intermediate, and final stages in order to avoid/minimize food spoilage and its hazardous effects on consumers. We have observed that papaya seed extracts obtained by SFE, particularly CO2-EtOH extracts, inhibits the VEO oxidation in its different stages; extract number 11 showed high efficacy to reduce the initial LHP, and secondary-final TBARS and hexanal. Additionally, Fig. 3 shows that various papaya seed extracts (number 5, 6, 8, 9, 10, 11, and 12) exhibited higher AA compared to the antioxidant reference TBHQ. Noteworthy, TBHQ is widely used by the edible oil industries in Colombia and other American countries due to its high antioxidant effectiveness and low price (between USD$20 and USD$50 per kg). However, this compound has been very questioned respect to its thermal instability and possible carcinogenic effects (Eskandani et al. 2014). Considering the facts mentioned above, papaya seeds could be considered a promising source of phenolic antioxidants (via SFE) with possible use in edible oils as preservatives, in addition, these extracts may be explored to replace TBHQ in food products.
Analysis of phenolic compounds
The results presented in the previous sections showed that papaya seeds could represent a valuable source of phenolic extracts, which have good free radical scavenging activity and high protective effect against lipid oxidation in edible oils. Two extracts were selected and analyzed by HPLC-ESI-MS/MS; the ethanolic extract was selected due to its high values of extraction yield, TPC, and DPPH· scavenging activity; on the other hand, CO2-EtOH extract number 11 was selected considering its good yield, TPC values, DPPH· scavenging activity, and high AA in VEO. Table 3 shows the chromatographic and mass spectra information of phenolic compounds tentatively identified in selected papaya seed extracts. Five phenolic acids, three flavonoids, and two flavonoid glycosides were identified in the ethanolic extract, whereas five phenolic acids were observed in CO2-EtOH extract. The phenolic compounds detected in the present work have been previously observed in different parts of papaya fruit; kaempferol-3-O-glycoside, caffeic acid, ferulic acid, p-hydroxybenzoic acid, and p-coumaric acid were identified in the seeds (Kadiri et al. 2017); caffeic acid, ferulic acid, and p-coumaric acid were observed in the peel (Nieto Calvache et al. 2016); while all the compounds cited in Table 3 were detected in the pulp (Zunjar et al. 2015). Kadiri et al. (2017) study reported concentrations of these substances between 0.07 and 0.38 mg/g dry extract, ferulic acid was observed as the most abundant compound with concentrations between 0.26 and 0.38 mg/g. The phenolic compounds observed in papaya seed extracts possess notable antioxidant properties. Some compounds as ferulic acid and quercetin have been approved in certain countries as food additives to prevent lipid oxidation and as color ingredients (Srinivasan et al. 2007).
Table 3.
Tentatively identified phenolic compounds in papaya seeds extracts by HPLC-ESI-MS/MS
| Rt (min) | Molecular formula | [M-H]− (m/z) | MS2 (m/z) | Tentative identification |
|---|---|---|---|---|
| 10.5 | C16H18O9 | 353 | 191 | Chlorogenic acida,b |
| 12.9 | C9H8O4 | 179 | 135 | Caffeic acida,b |
| 17.6 | C10H10O4 | 193 | 134, 117 | Ferulic acida,b |
| 20.7 | C7H6O3 | 137 | 93 | p-Hydroxybenzoic acida,b |
| 25.3 | C9H8O3 | 163 | 119, 153 | p-Coumaric acida,b |
| 33.5 | C15H10O8 | 317 | 137 | Myricetina |
| 36.8 | C15H10O7 | 301 | 271, 151 | Quercetina |
| 43.5 | C15H10O6 | 285 | 175, 153 | Kaempferola |
| 48.1 | C21H20O11 | 447 | 285 | Kaempferol-3-O-glycosidea |
| 49.7 | C21H20O12 | 463 | 301 | Quercetin 3-O-glycosidea |
a Compounds identified in ethanolic extract
b Compounds identified in CO2-EtOH extract
Ethanol by conventional extraction method allowed to recover more polarity phenolic compounds compared to the SFE with CO2-EtOH, for example, some flavonoids and their glycosides were only observed in the ethanoic extract. As expected, the use of CO2-EtOH as solvent extracted medium polarity compounds such as simple phenolic acids. Previous studies showed that ferulic acid and caffeic acid are soluble in CO2-EtOH, in addition, their solubility improves when the co-solvent amount is increased (Bitencourt et al. 2016, 2018). The phenolic composition observed in the ethanolic extract can be related with its high TPC and DPPH· scavenging activity; polyhydroxy phenolics have been reported as compounds with high reduction–oxidation potentials and free radical scavenging properties (Oroian and Escriche 2015). On the other hand, CO2-EtOH selectivity on the phenolic acids may be related with the high AA in the VEO observed for extract number 11.
Conclusion
The SFE using neat CO2 and CO2-EtOH was explored for the recovery of phenolic antioxidants from papaya agroindustrial wastes and compared with a conventional extraction method with ethanol. The ethanolic extract showed the higher extraction yield and TPC values. In SFE with neat CO2 the extraction pressure showed the higher effect on yield and TPC, and as expected, the addition of ethanol as co-solvent improved the extraction yield and TPC, allowing recover up to 44.81% of phenolic compounds. Ethanolic and CO2-EtOH extracts exhibited good DPPH· scavenging activity, while CO2-EtOH extracts showed the higher AA in VEO. On the other hand, the phenolic composition observed in the extracts analyzed indicates that CO2-EtOH is a selective solvent to extract phenolic acids. In general, the present study showed that papaya seeds are a promising source of phenolic extracts with interesting antioxidant properties. The SFE using CO2-EtOH is an attractive green extraction technique for the recovery of phenolic antioxidants (particularly phenolic acids) from this residue. Papaya seeds and its extracts can be of interest to food, pharmaceutical, and cosmetic industries as source of high value ingredients. This is an alternative for the most efficient use and potential valorization of papaya agroindustrial waste.
Acknowledgements
The authors are grateful to the DIB at Universidad Nacional de Colombia (Project: 201010021085), and to the Brazilian founding agency CNPq (Process Number 473153/2012-2) for the financial support. Also, Alimentos SAS and Duquesa (Bogotá-Colombia) for the papaya seeds and the VEO samples.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Banerjee J, Singh R, Vijayaraghavan R, MacFarlane D, Patti AF, Arora A. Bioactives from fruit processing wastes: green approaches to valuable chemicals. Food Chem. 2017;225:10–22. doi: 10.1016/j.foodchem.2016.12.093. [DOI] [PubMed] [Google Scholar]
- Bitencourt RG, Cabral FA, Meirelles AJA. Ferulic acid solubility in supercritical carbon dioxide, ethanol and water mixtures. J Chem Thermodyn. 2016;103:285–291. doi: 10.1016/j.jct.2016.08.025. [DOI] [Google Scholar]
- Bitencourt RG, Palma AM, Coutinho JAP, Cabral FA, Meirelles AJA. Solubility of caffeic acid in CO2 + ethanol: experimental and predicted data using Cubic Plus Association Equation of State. J Supercrit Fluids. 2018;138:238–246. doi: 10.1016/j.supflu.2018.04.008. [DOI] [Google Scholar]
- Castro-Vargas HI, Rodríguez-Varela LI, Ferreira SRS, Parada-Alfonso F. Extraction of phenolic fraction from guava seeds (Psidium guajava L.) using supercritical carbon dioxide and co-solvents. J Supercrit Fluids. 2010;51:319–324. doi: 10.1016/j.supflu.2009.10.012. [DOI] [Google Scholar]
- Castro-Vargas HI, Baumann W, Parada-Alfonso F. Valorization of agroindustrial wastes: identification by LC-MS and NMR of benzylglucosinolate from papaya (Carica papaya L.) seeds, a protective agent against lipid oxidation in edible oils. Electrophoresis. 2016;37:1930–1937. doi: 10.1002/elps.201500499. [DOI] [PubMed] [Google Scholar]
- da Silva AC, Jorge N. Bioactive compounds of oils extracted from fruits seeds obtained from agroindustrial waste. Eur J Lipid Sci Technol. 2017;119:1–5. [Google Scholar]
- Dávila JA, Hernández V, Castro E, Cardona CA. Economic and environmental assessment of syrup production. Colombian case. Bioresour Technol. 2014;161:84–90. doi: 10.1016/j.biortech.2014.02.131. [DOI] [PubMed] [Google Scholar]
- Desai U, Wagh A. Papaya. In: Salunkhe DK, Kadam SS, editors. Handbook of fruit science and technology: production, composition, storage and processing. 1. New York: Marcel Dekker Inc.; 1995. pp. 297–314. [Google Scholar]
- Eskandani M, Hamishehkar H, Ezzati J. Cytotoxicity and DNA damage properties of tert-butylhydroquinone (TBHQ) food additive. Food Chem. 2014;153:315–320. doi: 10.1016/j.foodchem.2013.12.087. [DOI] [PubMed] [Google Scholar]
- Faisal RS, Raju CV, Lakshmisha IP, Singh RR. Antioxidant and antimicrobial properties of grape and papaya seed extracts and their application on the preservation of Indian mackerel (Rastrelliger kanagurta) during ice storage. J Food Sci Technol. 2016;53:104–117. doi: 10.1007/s13197-015-1983-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAO-Food and Agriculture Organization of the United Nations FAOSTAT (2016) http://www.fao.org/faostat/es/#data/QC/visualize. Accessed 22 Oct 2018
- Gogna N, Hamid N, Dorai K. Metabolomic profiling of the phytomedicinal constituents of Carica papaya L. leaves and seeds by 1H NMR spectroscopy and multivariate statistical analysis. J Pharm Biomed Sci. 2015;115:74–85. doi: 10.1016/j.jpba.2015.06.035. [DOI] [PubMed] [Google Scholar]
- Guillén-Sans R, Guzmán-Chozas M. The thiobarbituric acid (TBA) Reaction in foods: a review. Crit Rev Food Sci Nutr. 1998;38:315–330. doi: 10.1080/10408699891274228. [DOI] [PubMed] [Google Scholar]
- Herrero M, Sanchez-Camargo A, Cifuentes A, Ibañez E. Plants, seaweeds, microalgae and food by-products as natural sources of functional ingredients obtained using pressurized liquid extraction and supercritical fluid extraction. Trends Anal Chem. 2015;71:26–38. doi: 10.1016/j.trac.2015.01.018. [DOI] [Google Scholar]
- Kadiri O, Akanbi CT, Olawoye BT, Gbadamosi SO. Characterization and antioxidant evaluation of phenolic compounds extracted from the protein concentrate and protein isolate produced from pawpaw (Carica papaya Linn.) seeds. Int J Food Prop. 2017;20:2423–2436. doi: 10.1080/10942912.2016.1230874. [DOI] [Google Scholar]
- Kao TH, Chen BH. Fruits and vegatables. In: Chandrasekaran M, editor. Valorization of food processing by-products. 1. Boca Raton: CRC Press; 2013. pp. 517–557. [Google Scholar]
- Kazutoshi F, Takayuki S. Formation of genotoxic dicarbonyl compounds in dietary oils upon oxidation. Lipids. 2004;39:481–486. doi: 10.1007/s11745-004-1254-y. [DOI] [PubMed] [Google Scholar]
- Khor E, Wong N. Comparison study of therapeutic properties of proteins and secondary metabolites from Carica papaya. Int. J. Pharm. Pharm. 2016;8:153–158. doi: 10.22159/ijpps.2016v8i10.13298. [DOI] [Google Scholar]
- Kothari V, Seshadri S. Antioxidant activity of seed extracts of Annona squamosa and Carica papaya. Food Sci Nutr. 2010;40:403–408. doi: 10.1108/00346651011062050. [DOI] [Google Scholar]
- Li YM, Su N, Yang HQ, Bai XP, Zhu QX, Liu HX, Li JQ. The extraction and properties of Carica papaya seed oil. Adv J Food Sci Tech. 2015;7:773–779. doi: 10.19026/ajfst.7.1736. [DOI] [Google Scholar]
- Lin C, Pfaltzgraff LA, Herrero-Davila L, Mubofu EB, Abderrahim S, Clark JH, Koutinas AA, Kopsahelis N, Stamatelatou K, Dickson F, Thankappan S, Mohamed Z, Brocklesby R, Luque R. Food waste as a valuable resource for the production of chemicals, materials and fuels. Current situation and global perspective. Energy Environ Sci. 2013;6:426–464. doi: 10.1039/c2ee23440h. [DOI] [Google Scholar]
- Lorenzo JM, Pateiro M, Domínguez R, Barba FJ, Putnik P, Kovačević DB, Shpigelman A, Granato D, Franco D. Berries extracts as natural antioxidants in meat products: a review. Food Res Int. 2018;106:1095–1104. doi: 10.1016/j.foodres.2017.12.005. [DOI] [PubMed] [Google Scholar]
- Manna L, Bugnone CA, Banchero M. Valorization of hazelnut, coffee and grape wastes through supercritical fluid extraction of triglycerides and polyphenols. J Supercrit Fluids. 2015;104:204–211. doi: 10.1016/j.supflu.2015.06.012. [DOI] [Google Scholar]
- Martins N, Ferreira ICFR. Wastes and by-products: upcoming sources of carotenoids for biotechnological purposes and health-related applications. Trends Food Sci Technol. 2017;62:33–48. doi: 10.1016/j.tifs.2017.01.014. [DOI] [Google Scholar]
- Mazzutti S, Gonçalves Rodrigues LG, Mezzomo N, Venturi V, Ferreira SRS. Integrated green-based processes using supercritical CO2 and pressurized ethanol applied to recover antioxidant compounds from cocoa (Theobroma cacao) bean hulls. J Supercrit Fluids. 2018;135:52–59. doi: 10.1016/j.supflu.2017.12.039. [DOI] [Google Scholar]
- Mukhopadhyay M. Natural extracts using supercritical carbon dioxide. Boca Raton: CRC Press; 2000. [Google Scholar]
- Nieto Calvache J, Cueto M, Farroni A, Pla M, Gerschenson LN. Antioxidant characterization of new dietary fiber concentrates from papaya pulp and peel (Carica papaya L.) J Funct Foods. 2016;27:319–328. doi: 10.1016/j.jff.2016.09.012. [DOI] [Google Scholar]
- Oboh G, Olabiyi AA, Akinyemi AJ. Inhibitory effect of aqueous extract of different parts of unripe pawpaw (Carica papaya) fruit on Fe2+-induced oxidative stress in rat pancreas in vitro. Pharm Biol. 2013;51:1165–1174. doi: 10.3109/13880209.2013.782321. [DOI] [PubMed] [Google Scholar]
- Oliveira DA, Angonese M, Gomes C, Ferreira SRS. Valorization of passion fruit (Passiflora edulis sp.) by-products: sustainable recovery and biological activities. J Supercrit Fluids. 2016;111:55–62. doi: 10.1016/j.supflu.2016.01.010. [DOI] [Google Scholar]
- Oroian M, Escriche I. Antioxidants: characterization, natural sources, extraction and analysis. Food Res Int. 2015;74:10–36. doi: 10.1016/j.foodres.2015.04.018. [DOI] [PubMed] [Google Scholar]
- Pasquel JL, Machado APDF, Barbero GF, Rezende CA, Martínez J. Extraction of antioxidant compounds from blackberry (Rubus sp.) bagasse using supercritical CO2 assisted by ultrasound. J Supercrit Fluids. 2014;94:223–233. doi: 10.1016/j.supflu.2014.07.019. [DOI] [Google Scholar]
- Patthamakanokporn O, Puwastien P, Nitithamyong A, Sirichakwal PP. Changes of antioxidant activity and total phenolic compounds during storage of selected fruits. J Food Compost Anal. 2008;21:241–248. doi: 10.1016/j.jfca.2007.10.002. [DOI] [Google Scholar]
- Pérez C, Ruiz del Castillo ML, Gil C, Blanch GP, Flores G. Supercritical fluid extraction of grape seeds: extract chemical composition, antioxidant activity and inhibition of nitrite production in LPS-stimulated Raw 264.7 cells. Food Func. 2015;8:2607–2613. doi: 10.1039/C5FO00325C. [DOI] [PubMed] [Google Scholar]
- Salla S, Sunkara R, Ogutu S, Walker LT, Verghese M. Antioxidant activity of papaya seed extracts against H2O2 induced oxidative stress in HepG2 cells. LWT- Food Sci Tech. 2016;66:293–297. doi: 10.1016/j.lwt.2015.09.008. [DOI] [Google Scholar]
- Srinivasan M, Sudheer AR, Menon VP. Ferulic acid: therapeutic potential through its antioxidant property. J Clin Biochem Nutr. 2007;40:92–100. doi: 10.3164/jcbn.40.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valadez-Carmona L, Ortiz-Moreno A, Ceballos-Reyes G, Mendiola JA, Ibáñez E. Valorization of cacao pod husk through supercritical fluid extraction of phenolic compounds. J Supercrit Fluids. 2018;131:99–105. doi: 10.1016/j.supflu.2017.09.011. [DOI] [Google Scholar]
- Zetzel C, Brunner G, Meireles MAA (2003) Standardized low-cost batch SFE Units for University education and comparative research. In: 6th International symposium on supercritical fluids. Versailles France
- Zunjar V, Mammen D, Trivedi BM. Antioxidant activities and phenolics profiling of different parts of Carica papaya by LCMS-MS. Nat Prod Res. 2015;29:2097–2099. doi: 10.1080/14786419.2014.986658. [DOI] [PubMed] [Google Scholar]