Abstract
Purpose of Review
The concussion public health burden has increased alongside our knowledge of the pathophysiology of mild traumatic brain injury (mTBI). The purpose of this review is to summarize our current understanding of mTBI pathophysiology and biomechanics and how these underlying principles correlate with clinical manifestations of mTBI.
Recent Findings
Changes in post-mTBI glutamate and GABA concentrations seem to be region-specific and time-dependent. Genetic variability may predict recovery and symptom severity while gender differences appear to be associated with the neuroinflammatory response and neuroplasticity. Ongoing biomechanical research has shown a growing body of evidence in support of an “individual-specific threshold” for mTBI that varies based on individual intrinsic factors.
Summary
The literature demonstrates a well-characterized timeframe for mTBI pathophysiologic changes in animal models while work in this area continues to grow in humans. Current human research shows that these underlying post-mTBI effects are multifactorial and may correlate with symptomatology and recovery. While wearable sensor technology has advanced biomechanical impact research, a definitive concussion threshold remains elusive.
Keywords: Concussion pathophysiology, Mild traumatic brain injury, Neurometabolic cascade, Biomechanical impact, Concussion threshold, Linear-rotational acceleration
Introduction
Definitions
Despite the significant public health burden caused by mild traumatic brain injury (mTBI) in the United States (U.S.), we still have much to learn about the subject. The term “concussion” comes from the Latin concussio—meaning “to strike together.” A clear consensus regarding its clinical definition and pathophysiology remains a challenge, with some using the terms concussion and mTBI interchangeably, some using them distinctly and, more recently, even a call to eliminate the term completely [1, 2]. The most commonly referenced definition describes concussion as a brain injury induced by direct or indirect biomechanical force transmitted to the head, resulting in a reversible clinical syndrome manifested as signs and symptoms affecting the physical, cognitive, emotional, and sleep domains reflecting a predominantly functional, rather than structural injury [3, 4]. The most recent guidelines from the Centers for Disease Control (CDC), however, recommend clinical use of the single term, “mild traumatic brain injury” when referring to concussion [5]. Concurrently, a subconcussive impact is hypothesized to generate similar neuronal injury, without reaching an undefined “concussion threshold” resulting in an absence of acute symptoms following these head impacts.
Epidemiology
Incidence and prevalence data for mTBI in the U.S. are likely underrepresented, as most data comes from emergency department (ED) databases. A state-based report from 2013 showed approximately 640,000 TBI-related ED visits in the U.S., of which, approximately 70–90% were attributed to mTBI [6, 7]. A study using National Electronic Injury Surveillance System-All Injury Program (NEISS-AIP) estimated 430,000 sports and recreation-related mTBI visits to the ED—70% of which occurred in subjects between 0 and 19 years old [8]. The same dataset showed an increase in sports and recreation-related ED visits in both males and females from 2001 to 2012, with females from 15 to 19 years old experiencing the greatest increase (211.5%). When comparing mTBI rates by gender within the same sport (soccer, basketball, softball/baseball), females experience a higher rate of mTBI than their male counterparts at both high school and collegiate levels [9].
Increasingly recognized by the media and public, mTBI research has led to an enhanced understanding of its pathophysiology and the development of evidence-based approaches to diagnosis and management. Numerous guidelines and position statements for the prevention, diagnosis, and treatment of mTBI have been proposed [3, 10–12]. Extensive research using animal mTBI models and increasing corroboration with human studies have created the foundation for these evolving clinical recommendations. The intricacies of mTBI pathophysiology and the translation to clinical symptomatology and long-term sequelae remain under rigorous investigation. This review aims to summarize our current understanding of the complex pathophysiology and biomechanics of mTBI and how these underlying principles correlate with clinical manifestations of mTBI.
The Biology of Concussion
Acute Neurometabolic Cascade
Ionic Flux and Neurotransmitter Release
Mechanical force transmitted to the brain causes a disturbance in cellular homeostasis, initiating complex biochemical alterations and neurometabolic changes [13] (Table 1). Shearing and stretching forces cause a temporary perturbation in the plasmalemmal membrane, termed “mechanoporation,” causing an outflow of intracellular potassium and subsequent diffuse neuronal depolarization [15, 16]. Immediate post-injury clinical symptoms are attributed to a diffuse neuronal depression (similar to the hypothesized migraine theory) following sudden depolarization [4].
Table 1.
Acute neurometabolic changes following mTBI
| Post-injury change | Mechanism | Pathophysiologic effect | Recovery timeline | |
|---|---|---|---|---|
| Glutamate | Immediate release from injured neurons followed by region-specific decrease | “Mechanoporation” produces neuronal depolarization and neurotransmitter release |
Promotes feedback loop of depolarization and neuron hyperexcitability Promotes influx of sodium and calcium |
Initial increase normalizes within minutes of injury Region-specific decrease at 72 h recovers by 2 weeks post-injury |
| Gamma-amino-butyric-acid (GABA) | Decreased in a region-specific and time-dependant manner | Loss of GABA-ergic interneurons has been suggested [14] | Decreased neuronal inhibitory effect | Region-specific decrease up to 2 weeks |
| Potassium | Extraneuronal increase | Glutamate stimulates potassium efflux via ligand-gated potassium channels | Stimulation of feedback loop of depolarization and hyperexcitability | Within 10 min from injury |
| Calcium | Intraneuronal increase/accumulation |
Initial neuronal “mechanoporation” Promoted by glutamate release |
Cell damage and mitochondrial impairment | Approximately 3 to 4 days after injury |
| Glucose | Increase followed by decrease | Increased neuronal glycolysis followed by hypometabolism + blood flow-uncoupling |
Decreased ATP from deficient oxidative metabolism Ineffective anaerobic metabolism |
Hyperglycolitic phase: • 30 min to 6 h Hypometabolic phase: • 5 to 10 days |
| Blood flow | Global as well as regional and time-dependant decreases |
Autoregulatory and vasoreactive disturbances induced by CO2 Local and diffuse structural vessel damage |
Promotes anaerobic metabolism “Window of vulnerability” to repeated head impacts |
Approximately 10 days |
Depolarization promotes the release of excitatory neurotransmitters involved in cortical activation and hyperexcitability resulting in cell damage and death [17, 18]. Glutamate stimulates potassium efflux via ligand-gated potassium channel and binds to N-methyl-d-aspartate (NMDA) receptors resulting in a feedback loop of depolarization and hyperexcitability [17, 19]. Animal mTBI models have shown a transient increase in glutamate concentration in the brain immediately post-mTBI that typically normalizes within hours [18]. A study using proton magnetic resonance spectroscopy found no difference in glutamate concentrations between mTBI athletes and controls in the premotor cortex (M1) post-injury. However, the authors found lower glutamate and gamma-amino-butyric-acid (GABA) concentrations at 72 h and 2 weeks in the dorso-lateral pre-frontal cortex (DLPFC) in the mTBI group. They also found a higher glutamate-to-GABA ratio 2 weeks post-injury [20••]. These findings suggest that post-mTBI changes in glutamate and GABA concentrations are likely region-specific and time-dependent.
Excitatory neurotransmitter release promotes an influx and accumulation of intracellular sodium and calcium, precipitating cell damage, and mitochondrial impairment rather than the cell death seen in severe TBI [21, 22]. Mutations in the CACNA1A gene, which are associated with different clinical migraine phenotypes, also correlate with increased symptomatology following mild TBI [23]. Concussed athletes carrying the CACNA1E gene polymorphism rs704326 (encoding for voltage-dependent calcium channels) experienced a prolonged recovery [24•]. Calcium channel blockers used in mTBI rat models have shown potential for limiting post-traumatic calcium accumulation and improving mTBI outcomes [25]. Given the overlap of typical post-concussive and migraine symptoms and the association of prior migraine history with prolonged recovery [26], genetics likely play a role in post-mTBI biometabolic recovery and symptom severity/duration.
Energy Crisis
In order to rapidly restore ionic homeostasis following mTBI, mitochondria must meet the increased cellular metabolic demand. Animal models show that the neuronal glycolytic rate increases 30–46% in the 30 min following fluid percussion injury and may persist for 6 h. This hypermetabolic period is followed by relative glucose hypometabolism lasting 5 to 10 days post-injury [27]. Simultaneously, there is ineffective oxidative metabolism and decreased cerebral blood flow. These two co-factors promote anaerobic metabolism with decreased ATP production and excessive lactate accumulation resulting in an acidic microenvironment [28, 29].
Similarly in humans, using fluorodeoxyglucose (FDG)-PET imaging, we see a pattern of post-mTBI hyperglycolysis followed by glucose hypometabolism. Peskind et al. showed prolonged post-mTBI regional hypometabolism in veterans exposed to repetitive blast injuries compared with age-matched controls [30]; however, longitudinal within-subject assessments are lacking [31]. Animal and human studies have shown a decrease in post-injury metabolic biomarkers including N-acetyl-aspartate (NAA); however, a definitive clinical correlate with NAA levels has not been found [32].
Animal studies have shown poorer cognitive outcomes in the setting of repeated head injury during the vulnerable window of glucose hypometabolism [33, 34]. This is the foundation for the “second impact syndrome” theory, one that remains controversial. While there is clear biological vulnerability when repeated mTBI occurs in close succession, this is distinct from the clinically rare but catastrophic “second impact syndrome” described with malignant cerebral edema and high mortality. The duration of the post-injury window of metabolic vulnerability is variable and requires additional research to better quantify its length and co-factors [4]. Current evidence supports the clinical practice of prophylactic activity modification to reduce the likelihood of a second injury within this vulnerable window.
Lactate serves as an alternative fuel source for the brain and is presumably utilized during the post-mTBI energy crisis, showing neuroprotective effects in mild to severe TBI rat models [35, 36], as well as beneficial cerebral metabolic effects in severe TBI in humans [37]. While research has elucidated a number of aspects of post-mTBI brain metabolism, the metabolic derangements are likely more complex and multifactorial than currently understood.
Blood Flow and Neurovascular Changes
The triphasic (hypo-hyper-hypo) response in cerebral blood flow (CBF) seen in severe TBI [38] has been postulated to occur in mTBI. Data is limited however, given the challenges in evaluating CBF immediately post-injury [39]. Researchers hypothesize that this triphasic response is stimulated by autoregulatory compromise, vasoreactive disturbances, and regional perfusion variability [39] partly due to carbon dioxide production from cerebral metabolic derangements [40]. Recent studies in a moderate TBI rat model using a novel vessel-painting technique provide structural evidence for these hemodynamic alterations; researchers found decrements in vessel junctions and vessel length [41•]. Rat models show a decrease in global and regional CBF [4, 41•, 42] paralleling the period of vulnerability following mTBI [39].
Churchill and colleagues recently showed MRI-based measures of CBF and function in adult athletes with mTBI during the first-week post-injury. Regional blood flow was decreased in the frontal and temporal lobes with functional blood flow effects seen in regions involved in autonomic regulation and emotion processing (cingulum, insula, and hippocampal gyri) [43•]. Thiebault et al. studied dynamic and pathophysiologic timeframe differences in CBF using transcranial doppler (TCD) in concussed athletes. While no significant decrease in CBF volume was found versus controls, they saw a trend towards reduced CBF velocity in immediate post-injury measurements warranting further investigation [44]. Functional MRI studies have shown that CBF changes correlate with initial symptom severity, returning to baseline slower than symptom reporting and neurocognitive testing [45, 46]. The present research suggests variability in CBF alterations post-mTBI that are region and time-dependent, affected by structural vessel changes, and may persist after clinical symptoms have resolved.
Axonal and Cytoskeletal injury
Rapid head deceleration induces neuronal shearing injury due to linear and rotational forces transferred to the neuron. The subsequent microstructural axonal damage is well described in the current literature [47]. Although diffuse axonal injury is more pronounced in severe TBI, it is seen in the full spectrum of TBI [48]. Research has shown that impact velocity rather than impact force better predicts axonal injury, stemming from the viscoelastic properties of tau protein [49, 50]. Mechanical deformation of neurofilaments and microtubules results in disruption of axonal transport and accumulation of beta-amyloid precursor protein (b-APP) [12, 51]. This occurs in the 6 h post-injury via phosphorylation or calpain-mediated protein breakdown [52]. High calcium levels can further destabilize microtubules 6–24 h post-injury [53, 54]. In vitro stretch injury models demonstrate post-stretch axonal undulations, beading, and axolemmal permeability—only some of which are reversible [55].
Subacute Pathophysiology
Ongoing Axonal and Cytoskeletal Problems
Using APP as an index for axonal pathology, peak damage occurs in the initial 24 h following injury with a subsequent graded return to baseline [56, 57]. With the advancement of neuroradiology, the quantification of selective white matter loss sits at the forefront of mTBI research. Animal research has shown that unmyelinated axons from an immature brain are more vulnerable to injury than myelinated fibers subjected to repeated mTBI [58, 59]. Recent work evaluated immature rats 7 days post-single mTBI vs. repeated mTBI. Neuroimaging (DTI and MR spectroscopy) and immunohistochemistry showed significant neurochemical and white matter changes between groups [60•]. Utilizing fractional anisotropy (FA), a measure of linear water diffusion related to white matter tract disturbance, investigators reported a decrease in FA in the ipsilateral corpus callosum, hippocampus, and external capsule in mTBI versus sham injury, with accumulation of b-APP only seen with mTBI [60•].
A pediatric population with mild and moderate TBI showed subacute decreases in FA in white matter subcortical regions but no changes in the corpus callosum [61]. Acutely, pediatric patients with mTBI show increased FA in the corpus callosum with a strong correlation between FA and post-concussive symptoms [62, 63]. These transient FA increases are hypothesized to arise from acute axonal swelling. Corpus callosum function following moderate to severe TBI has been studied using event-related potentials to calculate inter-hemispheric transfer time (IHTT). A slower IHTT was associated with decreased performance scores in a pediatric population [64]. More recently, multicomponent driven equilibrium single-pulse observation of T1 and T2 (mcDESPOT) was used to evaluate myelin water fraction (MWF), the ratio of myelin-associated water to total water after mTBI [65••]. Increased MWF was found in contact sports players compared with non-contact sports players in season and 3 months post-season, which was interpreted as an ongoing re-myelination process after cessation of contact sports. Current evidence provides a foundation for research investigating the underlying neuropathophysiology of post-mTBI white matter changes and the neuronal recovery process.
Impaired Synaptic Plasticity
Synaptic remodeling occurs predominantly during development through the rearrangement of dendritic spines [66, 67]. Animal studies suggest that those raised in enriched environments show increased cognition, cortical density, and complexity of dendritic arbors [68]. An in vivo rat model showed that mTBI causes changes in ligand-gated NMDA excitatory receptors and inhibitory GABA-ergic interneurons, subsequently affecting normal developmental plasticity, electrophysiology, and memory in a young rat model [69].
Research with immature rodent models compared development after rearing in an enriched environment between mTBI versus uninjured animals. Animals in the mTBI group did not show the anatomic or cognitive enhancement in adulthood seen with uninjured animals [70]. Impaired long-term potentiation (LTP) is seen 2 days post-injury and partially recovers by day seven [71]. However, LTP may be affected up to 28 days after injury in the juvenile female hippocampus compared to male counterparts [72•]. Furthermore, repeat mTBI rat models showed an increased neuronal loss in the hippocampus 28 days post-injury with attenuated NMDA receptor-mediated responses and impaired LTP [73]. More recent studies suggest that environmental enrichment strategies improved memory, decreased anxiety, and promoted exploratory behavior after repeated mTBI, possibly by mitigating post-injury NMDA subunit synaptic changes [74].
While human research is more limited, we see similar findings in LTP in college football players with a history of two or more concussions. DeBeaumont et al. concluded that GABA-mediated intracortical inhibition suppressed LTP and long-term depression-like plasticity and decreased implicit motor learning [75]. A study of high school athletes with mTBI who self-rated their activity showed that those with highest or lowest levels of post-injury activity reported more symptoms and showed worse cognitive performance than those with moderate levels of activity [76]. Limited human research suggests that there is a variable period of impaired neural activation and neuroplasticity following mTBI that may be mitigated with environmental interventions including exercise.
Neuroinflammation
Neuroinflammation involves the activation and upregulation of microglia and inflammatory cytokines [77•] and may contribute to ongoing cellular damage [78]. Microglia and macrophages (MG/M) are likely the main propagators of tissue inflammation beyond the core injury site in preclinical studies of more severe TBI [79•]. Recent studies show subacute gender differences in the MG/M response; male mice exhibit more rapid and pronounced microglial activation and astrogliosis compared to their female counterparts [79•]. Interleukin 1β (IL-1B) and interleukin 6 (IL-6) are primarily expressed following TBI, mediating the neuroinflammatory response [80]. Additionally, circulating neutrophils, monocytes, and lymphocytes leak through the damaged BBB within 1 day of injury, increasing neuroinflammation [81].
The neuroinflammatory response following mTBI has been hypothesized to correlate with concussion symptomatology and symptom duration [82]. Interestingly, mild systemic inflammation seems to influence the mTBI recovery process. Subjects with initial post-injury elevations in high-sensitivity C-reactive protein (hsCRP), an inflammatory biomarker, were more likely to experience persistent post-concussive symptoms, cognitive impairment, and ongoing psychological issues 3 months after mTBI [83]. A recent review highlights the significant role inflammation plays in mTBI pathophysiology, proposing its central role in persistent concussive symptoms [77•]. While research is too limited to confirm the contribution of neuroinflammation in mTBI prognosis and recovery, the neuroinflammatory response clearly plays a vital role in mTBI pathophysiology. Importantly, it also serves as a potential target for future interventions.
Blood-Brain Barrier Dysfunction
The BBB is an intricate capillary system that maintains a stable extracellular environment by regulating the transit of blood products into the brain [84]. Despite the scarcity of molecular models to support post-mTBI changes in BBB permeability, accumulated data suggest that there is an increase in the number of endothelial caveolae and decreased expression of junctional adhesion proteins hours to days after TBI [85, 86]. Direct shear injury is followed by secondary metabolic perturbations including ischemia, hypoxia, and vasospasm, all of which may perpetuate BBB dysfunction [87].
Animal studies have identified variable post-injury BBB recovery times. Some suggest dysfunction resolving within a few hours of TBI [87, 88]. Others support a biphasic course with early-phase BBB disruption (3 to 6 h post-injury) followed by delayed BBB dysfunction (1 to 3 days post-injury) [89, 90]. “Late” BBB dysregulation is seen following immunoglobulin G deposition around callosal blood vessels 3 months post-injury [91]. Johnson et al. evaluated BBB disruption in a swine model of mTBI and found marked acute (6 to 72 h) permeability of the BBB with an acute astroglial response [92].
Neuroimaging evidence of BBB disruption is seen after mild and moderate TBI [93] with BBB integrity restored in days to weeks [94]. Using an impermeable radiotracer, a study using PET-CT found BBB permeability in 73% of subjects [95]. We also see BBB disruption in American football players as a result of exposure to “subconcussive” head impacts [96]. A range of inflammatory genetic markers have been reported in the subset of individuals after mTBI with evidence of meningeal enhancement on MRI [97]. There is sufficient evidence to support BBB dysfunction following TBI; however, the extent and time course of disruption following human mTBI remains poorly understood.
Cell Death
While cell death is commonly seen in moderate and severe TBI, animal models of mTBI have shown limited cell death [58, 98–100]. Neuronal apoptosis occurs in the cortex and anterior thalamus of immature rats after mTBI [101] with the appearance of cognitive deficits and tissue loss after a single cortical impact [102] as well as repeated impacts [34]. Human research is limited but has progressed in recent years with the advent of quantitative MRI to evaluate longitudinal changes in brain volume. One study showed greater diffuse volume loss after single mTBI compared to age-matched controls 1 year post-injury, with atrophy in the limbic system and precuneal cortex correlating with neuropsychiatric testing performance [103]. Lower hippocampal volume is seen after single remote TBI in middle-aged men compared to controls [104], after repeat mTBI in boxers [105] and in college football players with history of concussion. [106]. A study comparing patients with recent mTBI to controls found significantly smaller volumes in the caudate, putamen, and thalamus 2 months after injury. One year post-injury, however, these initial brain volume differences had resolved suggesting a subsequent normalization of brain tissue [107]. Despite the lack of structural changes seen on standard neuroimaging, advances in neuroimaging techniques have helped to identify short and long-term region-specific morphologic changes.
Biomechanics and Impact Monitoring
Biomechanical Principles
While objective measures (e.g., balance testing, reaction time, visual tracking) are routinely used for concussion diagnosis and management, the scientific community is still in search of definitive diagnostic tools. Current research is seeking to identify the kinematic signature of concussion. However, existing technology only allows for kinematic correlates of brain biomechanics via spatial tracking of the skull [47]. Traditional force measurements include linear acceleration (LA) and rotational acceleration (RA) [108]; the former measured as gravitational force units (g) and the latter in units of radians per second squared (rad/s2) [47].
Linear Acceleration
Earlier biomechanics research focused on the correlation between linear acceleration and mTBI, searching for theoretical injury “thresholds.” The research found a strong correlation between the LA and intracranial pressure [109]. Parallel TBI research has shown that transient increases in intracranial pressure cause neurologic dysfunction, with the level of dysfunction correlating with the peak intracranial pressure achieved during injury [110]. Animal models of pressure-induced brain injury revealed that LA-induced pressure gradients are less significant than those created by equivalent RA [111].
Rotational Acceleration
In the 1940s, Holbourn pioneered work on the tensile and shear strain caused by RA, exposing primates and rats to sudden rotation using inertial loading [47]. Given its physical properties, brain tissue deforms easily when exposed to shear forces [112]. A series of surrogate model studies reinforced the association between RA-induced shear deformation and mTBI [113, 114]. More recently, research using finite element models has supported the strong relationship between rotational acceleration and brain strain [115].
Linear-Rotational Correlation
Studying isolated RA is extremely difficult in vivo. The head rotation experienced during injury inherently couples both linear and angular forces. Furthermore, studies have shown that mTBI is almost exclusively produced in primates when both forces were applied, requiring twice the level RA to induce mTBI when LA was absent [114]. In addition, head-impact kinematic research in football has proposed a correlation between LA and RA with the development of a theoretical injury risk curve. Rowson et al. presented a cohort of cases where this correlation holds true [116]. Research demonstrates that linear and rotational forces play a significant role in mTBI [117], and that ongoing biomechanical and equipment research is vital to further injury understanding and prevention.
Monitoring Impacts
Measuring Exposure
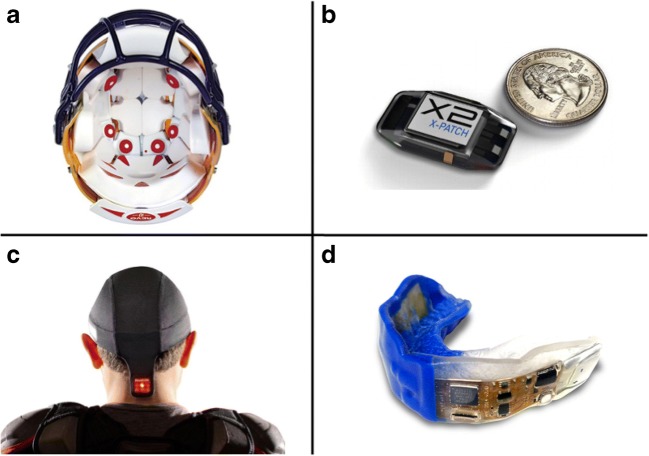
Despite the inherent challenges in evaluating mTBI in vivo, the use of sensors (Fig. 1) has helped understand the biomechanical aspects of mTBI and study head-impact forces in sport. Sport-related mTBI is quantified as the number concussions per “athlete exposure,” with athlete exposures (AE) including games, practices, or events. While not accounting for total time of exposure, AEs offer a reasonable exposure estimate to allow for comparison mTBI risk amongst sports. mTBI rates are also often reported for athletes and teams as “per-season” rates [118]. Head-impact exposure data from high school and collegiate populations have led to changes in practice rules that limit contact activities. These rule changes have attributed to a 50% reduction in head impacts in one study of youth football [119].
Fig. 1.
Examples of head-impact sensor technologies; a helmet sensor system, b mastoid process neck sensor, c skullcap-based sensor, d embedded mouth guard sensor
Helmet-Based Sensors
Helmeted devices for head-impact research have traditionally been used in football, with recent increasing use in boxing, snow sports, ice hockey, and soccer. The availability of helmet-based devices has grown in recent years. The Riddell Head Impact Telemetry System (HITS) sensor device and the six-degree-of-freedom (6DOF) system are the two most commonly used research systems [108]. Despite their increasing popularity, these systems remain costly and inaccurate due to poor coupling with the head. Helmet-detected acceleration can be up to ten times greater than true head acceleration [120]. A study using the 6DOF system and HITS devices both within Riddell helmets found that resultant RA was overestimated [116].
Non-helmeted Sensors and Other Approaches
Mouth guard, base of skull, and ear canal sensors produce better coupling with the skull [121]. The most commonly used research systems are the X-Patch, an adhesive patch worn behind the ear affixed to the mastoid process, and the X-Guard, a device embedded in a custom mouth guard. Both use a triaxial accelerometer and gyroscope to measure head acceleration and impacts in non-helmeted sports [104]. Limitations of these systems include signal noise from skin motion and variable mouth guard fit. Specifically, the X-patch has shown up to a 290% error in the RA resultant peak value [121] and high false-positive rates yielding a low positive predictive value (16.3%) [122].
Other non-helmeted sensor fixation systems include headbands, skullcaps, and armbands. While some devices capture acceleration magnitudes, most only record impact number and location. None of these devices are peer-reviewed and, thus, lack scientific validation [108]. An additional approach to mTBI surveillance includes quantitative and qualitative video analyses. This analysis monitors the number of head impacts, injury mechanism, and clinical signs of mTBI. The clinical and sideline utility of these devices and observational techniques require further investigation to support their validity.
Impacts and Biological Threshold for Concussion
Our current understanding of brain kinematics comes predominantly from helmeted collision sports. A body of high school and collegiate football research has identified an average peak linear acceleration of 100 g as a theoretical “threshold” for mTBI [123]. This threshold is supported by a recent systematic review showing a mean peak linear head acceleration of 98.68 g (95% CI 82.36–115.00) and mean peak rotational head acceleration of 5776.60 rads/s2 (95% CI 4583.53–6969.67) associated with mTBI [124]. Conversely, the highly variable range of reported head impact forces inducing mTBI seems to refute a finite concussion threshold. One large football study found mTBI-inducing forces ranging between 29 and 205 g of LA, and 183 and 10,484 rad/s2 of RA [125]. Scientists have proposed that high-level American football athletes may be a self-selected “impact tolerant” population. More concerning, however, is the theory that cultural differences in sports may result in variable reporting of mTBI rather than tolerance of higher impacts [47]. Nonetheless, methodological limitations, underreporting, and a previous history of concussions are all noteworthy confounders in many of these studies.
Current research has led to the concept of the “individual-specific threshold.” This personalized threshold accounts for the many intrinsic factors proposed to play a role in ones’ ability to tolerate head impact forces. This concept aligns with research showing the variable relationship between biomechanical forces and mTBI. Research has not found an association between head-impact magnitude or location and the presence of mTBI symptoms, SCAT3 scores [126••], clinical outcomes, balance testing, or neuropsychological performance [127]. While research has documented a wide range of LA (54–94 g) and RA (2640–4468 rad/s2), 90% of mTBI cases occurred following one of the top five highest magnitude accelerations subjects had ever experienced [126••]. This work further supports the “individual-specific threshold” concept.
The existing literature provides evidence of individualized mTBI vulnerability. Predisposing intrinsic factors including age, genetics, epigenetics, cerebrospinal fluid levels, susceptibility of brain tissue to injury, and extrinsic factors such as muscular strength, helmet type, sport position, and impact anticipation may explain the variable response to head impacts [125, 126••, 127, 128]. Additionally, a history of subconcussive impacts or prior mTBI may affect an individual’s threshold for future mTBI [125]. As technology and research advances, we can better address these ongoing questions and enhance the safety of our athletes and military personnel. While our current impact sensor systems have significant limitations, existing work shows promise in these systems. Future advances in wearable sensors could allow for enhanced mTBI detection and the identification of individual-specific mTBI thresholds.
Conclusion
Advances in animal model research, neuroimaging, and biomechanical impact kinematics have led to our increasing knowledge of mTBI pathophysiology. The post-impact neurobiochemical cascade that is well supported by basic science literature needs additional translational human research. We know that mechanically induced brain injury initiates ionic, metabolic, inflammatory, and neurovascular changes in the CNS, that may lead to acute and chronic neurologic sequelae. Despite the increasing association between acute pathophysiology with clinical signs and symptoms of mTBI, ongoing research continues to elucidate the relationship between time and region-specific neurologic changes. The individual-specific threshold for mTBI seems to better explain the variability in head-impact tolerance and subsequent clinical presentation. There is still limited but growing evidence to support a host of intrinsic and extrinsic co-factors contributing to one’s mTBI threshold. Newer technology and future impact sensor research may provide important insights into the underlying mTBI pathophysiology, personalized mTBI impact thresholds, and further individualized assessment and treatment of mTBI.
Compliance with Ethical Standards
Conflict of Interest
Rafael Romeu-Mejia and Joshua T. Goldman each declare no potential conflicts of interest.
Christopher C. Giza reports grants from NINDS, NCAA, US Department of Defense, UCLA Steve Tisch BrainSPORT Program, Easton Clinic for Brain Health, UCLA Brain Injury Research Center, and Avanir (2017–2018). Dr. Giza reports personal fees from Highmark Interactive (2018), Neural Analytics (2015–2016), and Medicolegal cases: 0–2 annually. Dr. Giza also reports book royalties from Blackwell Publishing.
Human and Animal Rights and Informed Consent
All reported studies/experiments with human or animal subjects performed by the authors have been previously published and complied with all applicable ethical standards (including the Helsinki declaration and its amendments, institutional/national research committee standards, and international/national/institutional guidelines).
Footnotes
This article is part of the Topical Collection on Concussion
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
- 1.Sharp DJ, Jenkins PO. Concussion is confusing us all. Pract Neurol. 2015;15:172–186. doi: 10.1136/practneurol-2015-001087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.DeMatteo CA, Hanna SE, Mahoney WJ, Hollenberg RD, Scott LA, Law MC, et al. My child doesn’t have a brain injury, he only has a concussion. Pediatrics. 2010;125:327–334. doi: 10.1542/peds.2008-2720. [DOI] [PubMed] [Google Scholar]
- 3.McCrory P, Meeuwisse W, Dvořák J, Aubry M, Bailes J, Broglio S, Cantu RC, Cassidy D, Echemendia RJ, Castellani RJ, Davis GA, Ellenbogen R, Emery C, Engebretsen L, Feddermann-Demont N, Giza CC, Guskiewicz KM, Herring S, Iverson GL, Johnston KM, Kissick J, Kutcher J, Leddy JJ, Maddocks D, Makdissi M, Manley GT, McCrea M, Meehan WP, Nagahiro S, Patricios J, Putukian M, Schneider KJ, Sills A, Tator CH, Turner M, Vos PE. Consensus statement on concussion in sport—the 5th international conference on concussion in sport held in Berlin, October 2016. Br J Sports Med. 2017;51:838–847. doi: 10.1136/bjsports-2017-097699. [DOI] [PubMed] [Google Scholar]
- 4.Giza CC, Hovda DA. The new neurometabolic cascade of concussion. Neurosurgery. 2014;75:S24–S33. doi: 10.1227/NEU.0000000000000505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lumba-Brown A, Yeates KO, Sarmiento K, Breiding MJ, Haegerich TM, Gioia GA, Turner M, Benzel EC, Suskauer SJ, Giza CC, Joseph M, Broomand C, Weissman B, Gordon W, Wright DW, Moser RS, McAvoy K, Ewing-Cobbs L, Duhaime AC, Putukian M, Holshouser B, Paulk D, Wade SL, Herring SA, Halstead M, Keenan HT, Choe M, Christian CW, Guskiewicz K, Raksin PB, Gregory A, Mucha A, Taylor HG, Callahan JM, DeWitt J, Collins MW, Kirkwood MW, Ragheb J, Ellenbogen RG, Spinks TJ, Ganiats TG, Sabelhaus LJ, Altenhofen K, Hoffman R, Getchius T, Gronseth G, Donnell Z, O’Connor RE, Timmons SD. Centers for disease control and prevention guideline on the diagnosis and management of mild traumatic brain injury among children. JAMA Pediatr. 2018;172:e182853. doi: 10.1001/jamapediatrics.2018.2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taylor CA, Bell JM, Breiding MJ, Xu L. Traumatic brain injury–related emergency department visits, hospitalizations, and deaths — United States, 2007 and 2013. MMWR Surveill Summ. 2017;66:1–16. doi: 10.15585/mmwr.ss6609a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Faul M, Xu L, Wald MM, Coronado VG. Traumatic brain injury in the United States: emergency department visits, hospitalizations, and deaths. Centers Dis Control Prev Natl Cent Inj Prev Control 2010;891–904.
- 8.Coronado VG, Haileyesus T, Cheng TA, Bell JM, Haarbauer-Krupa J, Lionbarger MR, Flores-Herrera J, McGuire LC, Gilchrist J. Trends in sports-and recreation-related traumatic brain injuries treated in US emergency departments: the National Electronic Injury Surveillance System-All Injury Program (NEISS-AIP) 2001-2012. J Head Trauma Rehabil. 2015;30:185–197. doi: 10.1097/HTR.0000000000000156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Covassin T, Moran R, Elbin RJ. Sex differences in reported concussion injury rates and time loss from participation: an update of the national collegiate athletic association injury surveillance program from 2004-2005 through 2008-2009. J Athl Train. 2016;51:189–194. doi: 10.4085/1062-6050-51.3.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giza CC, Kutcher JS, Ashwal S, Barth J, Getchius TSD, Gioia GA, Gronseth GS, Guskiewicz K, Mandel S, Manley G, McKeag DB, Thurman DJ, Zafonte R. Summary of evidence-based guideline update: evaluation and management of concussion in sports: report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology. 2013;80:2250–2257. doi: 10.1212/WNL.0b013e31828d57dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Herring SA, Cantu RC, Guskiewicz KM, Putukian M, Kibler WB. Concussion (mild traumatic brain injury) and the team physician. Med Sci Sports Exerc. 2011;43:2412–2422. doi: 10.1249/MSS.0b013e3182342e64. [DOI] [PubMed] [Google Scholar]
- 12.Institute NSS. Interassociation consensus: diagnosis and management of sport-related concussion best practices. 2017;1–19. Doi:10.1002/app.21943.
- 13.Steenerson K, Starling AJ. Pathophysiology of sports-related concussion. Neurol Clin Elsevier Inc. 2017;35:403–408. doi: 10.1016/j.ncl.2017.03.011. [DOI] [PubMed] [Google Scholar]
- 14.Cantu D, Walker K, Andresen L, Taylor-Weiner A, Hampton D, Tesco G, Dulla CG. Traumatic brain injury increases cortical glutamate network activity by compromising GABAergic control. Cereb Cortex. 2015;25:2306–2320. doi: 10.1093/cercor/bhu041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Farkas O. Mechanoporation induced by diffuse traumatic brain injury: an irreversible or reversible response to injury? J Neurosci. 2006;26:3130–3140. doi: 10.1523/JNEUROSCI.5119-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Katayama Y, Becker DP, Tamura T, Hovda DA. Massive increases in extracellular potassium and the indiscriminate release of glutamate following concussive brain injury. J Neurosurg. 1990;73:889–900. doi: 10.3171/jns.1990.73.6.0889. [DOI] [PubMed] [Google Scholar]
- 17.Mc Fie S, Abrahams S, Patricios J, Suter J, Posthumus M, September AV. Inflammatory and apoptotic signalling pathways and concussion severity: a genetic association study. J Sports Sci Routledge. 2018;36:2226–2234. doi: 10.1080/02640414.2018.1448570. [DOI] [PubMed] [Google Scholar]
- 18.Giza CC, Hovda DA. The neurometabolic cascade of concussion. J Athl Train. 2001;36:228–235. [PMC free article] [PubMed] [Google Scholar]
- 19.Yi JH, Hazell AS. Excitotoxic mechanisms and the role of astrocytic glutamate transporters in traumatic brain injury. Neurochem Int. 2006;48:394–403. doi: 10.1016/j.neuint.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 20.Yasen AL, Smith J, Christie AD. Glutamate and GABA concentrations following mild traumatic brain injury: a pilot study Alia L. Yasen. J Neurophysiol. 2018;120:1318–1322. doi: 10.1152/jn.00896.2017. [DOI] [PubMed] [Google Scholar]
- 21.Cheng G, Kong RH, Zhang LM, Zhang JN. Mitochondria in traumatic brain injury and mitochondrial-targeted multipotential therapeutic strategies. Br J Pharmacol. 2012;167:699–719. doi: 10.1111/j.1476-5381.2012.02025.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barkhoudarian G, Hovda DA, Giza CC. The molecular pathophysiology of concussive brain injury. Clin Sports Med Elsevier Inc. 2011;30:33–48. doi: 10.1016/j.csm.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 23.Monaghan KG, Jackson CE, KuKuruga DL, Feldman GL. Mutation analysis of the CACNA1A calcium channel subunit gene in 27 patients with sporadic hemiplegic migraine. Am J Med Genet. 2000;94:120–124. doi: 10.1002/1096-8628(20000911)94:2<120::aid-ajmg4>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 24.McDevitt J. CNS voltage-gated calcium channel gene variation and prolonged recovery following sport-related concussion. Orthop J Sport Med. 2016;4:2325967116S0007. [Google Scholar]
- 25.Gurkoff GG, Shahlaie K, Lyeth BG, Berman RF. Voltage-gated calcium channel blockers for the treatment of traumatic brain injury. New Ther Trauma Brain Inj. Elsevier; 2017.
- 26.Mihalik JP, Stump JE, Collins MW, Lovell MR, Field M, Maroon JC. Posttraumatic migraine characteristics in athletes following sports-related concussion. J Neurosurg. 2005;102:850–855. doi: 10.3171/jns.2005.102.5.0850. [DOI] [PubMed] [Google Scholar]
- 27.Yoshino A, Hovda DA, Kawamata T, Katayama Y, Becker DP. Dynamic changes in local cerebral glucose utilization following cerebral conclusion in rats: evidence of a hyper- and subsequent hypometabolic state. Brain Res. 1991;561:106–119. doi: 10.1016/0006-8993(91)90755-k. [DOI] [PubMed] [Google Scholar]
- 28.Kawamata T, Katayama Y, Hovda DA, Yoshino A, Becker DP. Lactate accumulation following concussive brain injury: the role of ionic fluxes induced by excitatory amino acids. Brain Res. 1995;674:196–204. doi: 10.1016/0006-8993(94)01444-m. [DOI] [PubMed] [Google Scholar]
- 29.Kalimo H, Rehncrona S, Söderfeldt B, Olsson Y, Siesjö BK. Brain lactic acidosis and ischemic cell damage: 2. Histopathol J Cereb Blood Flow Metab. 1981;1:313–327. doi: 10.1038/jcbfm.1981.35. [DOI] [PubMed] [Google Scholar]
- 30.Peskind ER, Petrie EC, Cross DJ, Pagulayan K, McCraw K, Hoff D, et al. Cerebrocerebellar hypometabolism associated with repetitive blast exposure mild traumatic brain injury in 12 Iraq war Veterans with persistent post-concussive symptoms. Neuroimage. Elsevier B.V.; 2011;54:S76–82. [DOI] [PMC free article] [PubMed]
- 31.Byrnes KR, Wilson CM, Brabazon F, Von Leden R, Jurgens JS, Oakes TR, et al. FDG-PET imaging in mild traumatic brain injury: a critical review. Front Neuroenerg. 2014;6:13. doi: 10.3389/fnene.2013.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vagnozzi R, Signoretti S, Cristofori L, Alessandrini F, Floris R, Isgró E, et al. Assessment of metabolic brain damage and recovery following mild traumatic brain injury: a multicentre, proton magnetic resonance spectroscopic study in concussed patients. Brain. 2010;133:3232–3242. doi: 10.1093/brain/awq200. [DOI] [PubMed] [Google Scholar]
- 33.Prins ML, Alexander D, Giza CC, Hovda DA. Repeat mild traumatic brain injury: mechanisms of cerebral vulnerability. J Neurotrauma. 2013;30:30–38. doi: 10.1089/neu.2012.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.DeFord SM, Wilson MS, Rice AC, Clausen T, Rice LK, Barabnova A, et al. Repeated mild brain injuries result in cognitive impairment in B6C3F1 mice. J Neurotrauma. 2002;19:427–438. doi: 10.1089/08977150252932389. [DOI] [PubMed] [Google Scholar]
- 35.Prins ML, Hovda DA. The effects of age and ketogenic diet on local cerebral metabolic rates of glucose after controlled cortical impact injury in rats. J Neurotrauma. 2009;26:1083–1093. doi: 10.1089/neu.2008.0769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Appelberg KS, Hovda DA, Prins ML. The effects of a ketogenic diet on behavioral outcome after controlled cortical impact injury in the juvenile and adult rat. J Neurotrauma. 2009;26:497–506. doi: 10.1089/neu.2008.0664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bouzat P, Sala N, Suys T, Zerlauth JB, Marques-Vidal P, Feihl F, Bloch J, Messerer M, Levivier M, Meuli R, Magistretti PJ, Oddo M. Cerebral metabolic effects of exogenous lactate supplementation on the injured human brain. Intensive Care Med. 2014;40:412–421. doi: 10.1007/s00134-013-3203-6. [DOI] [PubMed] [Google Scholar]
- 38.Martin NA, Patwardhan RV, Alexander MJ, Africk CZ, Lee JH, Shalmon E, Hovda DA, Becker DP. Characterization of cerebral hemodynamic phases following severe head trauma: hypoperfusion, hyperemia, and vasospasm. J Neurosurg. 1997;87:9–19. doi: 10.3171/jns.1997.87.1.0009. [DOI] [PubMed] [Google Scholar]
- 39.Choe MC, Babikian T, Difiori J, Hovda DA, Giza CC. A pediatric perspective on concussion pathophysiology. Curr Opin Pediatr. 2012;24:689–695. doi: 10.1097/MOP.0b013e32835a1a44. [DOI] [PubMed] [Google Scholar]
- 40.Clausen M, Pendergast DR, Willer B, Leddy J. Cerebral blood flow during treadmill exercise is a marker of physiological postconcussion syndrome in female athletes. J Head Trauma Rehabil. 2016;31:215–224. doi: 10.1097/HTR.0000000000000145. [DOI] [PubMed] [Google Scholar]
- 41.Obenaus A, Ng M, Orantes AM, Kinney-Lang E, Rashid F, Hamer M, et al. Traumatic brain injury results in acute rarefication of the vascular network. Sci Rep. 2017;7:1–14. doi: 10.1038/s41598-017-00161-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yamakami I, McIntosh TK. Alterations in regional cerebral blood flow following brain injury in the rat. J Cereb Blood Flow Metab. 1991;11:655–660. doi: 10.1038/jcbfm.1991.117. [DOI] [PubMed] [Google Scholar]
- 43.Churchill NW, Hutchison MG, Richards D, Leung G, Graham SJ, Schweizer TA. The first week after concussion: blood flow, brain function and white matter microstructure. NeuroImage Clin. 2017;14:480–489. doi: 10.1016/j.nicl.2017.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thibeault CM, Thorpe S, O’Brien MJ, Canac N, Ranjbaran M, Patanam I, Sarraf A, LeVangie J, Scalzo F, Wilk SJ, Diaz-Arrastia R, Hamilton RB. A cross-sectional study on cerebral hemodynamics after mild traumatic brain injury in a pediatric population. Front Neurol. 2018;9:200. doi: 10.3389/fneur.2018.00200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang Y, Nelson LD, LaRoche AA, Pfaller AY, Nencka AS, Koch KM, et al. Cerebral blood flow alterations in acute sport-related concussion. J Neurotrauma. 2016;33:1227–1236. doi: 10.1089/neu.2015.4072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Meier TB, Bellgowan PSF, Singh R, Kuplicki R, Polanski DW, Mayer AR. Recovery of cerebral blood flow following sports-related concussion. JAMA Neurol. 2015;72:530. doi: 10.1001/jamaneurol.2014.4778. [DOI] [PubMed] [Google Scholar]
- 47.Rowson S, Bland ML, Campolettano ET, Press JN, Rowson B, Smith JA, Sproule DW, Tyson AM, Duma SM. Biomechanical perspectives on concussion in sport. Sports Med Arthrosc. 2016;24:100–107. doi: 10.1097/JSA.0000000000000121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Choe MC, Valino H, Fischer J, Zeiger M, Breault J, McArthur DL, et al. Targeting the epidemic: interventions and follow-up are necessary in the pediatric traumatic brain injury clinic. J Child Neurol. 2016;31:109–115. doi: 10.1177/0883073815572685. [DOI] [PubMed] [Google Scholar]
- 49.MacFarlane MP, Glenn TC. Neurochemical cascade of concussion. Brain Inj. 2015;29:139–153. doi: 10.3109/02699052.2014.965208. [DOI] [PubMed] [Google Scholar]
- 50.Ahmadzadeh H, Smith DH, Shenoy VB. Viscoelasticity of tau proteins leads to strain rate-dependent breaking of microtubules during axonal stretch injury: predictions from a mathematical model. Biophys J Biophysical Society. 2014;106:1123–1133. doi: 10.1016/j.bpj.2014.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Johnson VE, Stewart W, Smith DH. Axonal pathology in traumatic brain injury. Exp Neurol. 2013;246:35–43. doi: 10.1016/j.expneurol.2012.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nixon RA. The regulation of neurofilament protein dynamics by phosphorylation: clues to neurofibrillary pathobiology. Brain Pathol. 1993;3:29–38. doi: 10.1111/j.1750-3639.1993.tb00723.x. [DOI] [PubMed] [Google Scholar]
- 53.William L, Povlishock JT, Graham DL, Al MET. A mechanistic analysis of nondisruptive axonal injury: a review. J Neurotrauma. 1997;14:419–440. doi: 10.1089/neu.1997.14.419. [DOI] [PubMed] [Google Scholar]
- 54.Pettus EH, Christman CW, Giebel ML, Povlishock JT. Traumatically induced altered membrane permeability: its relationship to traumatically induced reactive axonal change. J Neurotrauma. 1994;11:507–522. doi: 10.1089/neu.1994.11.507. [DOI] [PubMed] [Google Scholar]
- 55.Tang-Schomer MD, Johnson VE, Baas PW, Stewart W, Smith DH. Partial interruption of axonal transport due to microtubule breakage accounts for the formation of periodic varicosities after traumatic axonal injury. Exp Neurol. 2012;233:364–372. doi: 10.1016/j.expneurol.2011.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gultekin SH, Smith TW. Diffuse axonal injury in craniocerebral trauma. A comparative histologic and immunohistochemical study. Arch Pathol Lab Med. 1994;118:168–171. [PubMed] [Google Scholar]
- 57.Chen XH, Johnson VE, Uryu K, Trojanowski JQ, Smith DH. A lack of amyloid β plaques despite persistent accumulation of amyloid β in axons of long-term survivors of traumatic brain injury. Brain Pathol. 2009;19:214–223. doi: 10.1111/j.1750-3639.2008.00176.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Prins ML, Hales A, Reger M, Giza CC, Hovda DA. Repeat traumatic brain injury in the juvenile rat is associated with increased axonal injury and cognitive impairments. Dev Neurosci. 2011;32:510–518. doi: 10.1159/000316800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Reeves TM, Phillips LL, Povlishock JT. Myelinated and unmyelinated axons of the corpus callosum differ in vulnerability and functional recovery following traumatic brain injury. Exp Neurol. 2005;196:126–137. doi: 10.1016/j.expneurol.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 60.Fidan E, Foley LM, New LA, Alexander H, Kochanek PM, Hitchens TK, et al. Metabolic and structural imaging at 7 tesla after repetitive mild traumatic brain injury in immature rats. ASN Neuro. 2018;10:175909141877054. doi: 10.1177/1759091418770543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wozniak JR, Krach L, Ward E, Mueller BA, Muetzel R, Schnoebelen S, et al. Neurocognitive and neuroimaging correlates of pediatric traumatic brain injury: a diffusion tensor imaging (DTI) study. Arch Clin Neuropsychol. 2007;22:555–568. doi: 10.1016/j.acn.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mayer AR, Hanlon FM, Ling JM. Gray matter abnormalities in pediatric mild traumatic brain injury. J Neurotrauma. 2015;32:723–730. doi: 10.1089/neu.2014.3534. [DOI] [PubMed] [Google Scholar]
- 63.Wilde EA, McCauley SR, Hunter JV, Bigler ED, Chu Z, Wang ZJ, et al. Diffusion tensor imaging of acute mild traumatic brain injury in adolescents. Neurology. 2008;70:948–955. doi: 10.1212/01.wnl.0000305961.68029.54. [DOI] [PubMed] [Google Scholar]
- 64.Dennis EL, Ellis MU, Marion SD, Jin Y, Moran L, Olsen A, Kernan C, Babikian T, Mink R, Babbitt C, Johnson J, Giza CC, Thompson PM, Asarnow RF. Callosal function in pediatric traumatic brain injury linked to disrupted white matter integrity. J Neurosci. 2015;35:10202–10211. doi: 10.1523/JNEUROSCI.1595-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.•• Spader HS, Dean DC, LaFrance WC, Raukar NP, Cosgrove GR, Eyerly-Webb SA, et al. Prospective study of myelin water fraction changes after mild traumatic brain injury in collegiate contact sports. J Neurosurg. 2018;1–9. MWF at baseline was significantly higher in the bilateral basal ganglia, anterior and posterior corpora callosa, left corticospinal tract, and left anterior and superior temporal lobe in the brains of previously concussed athletes compared with the brains of controls. At 3 months after a concussive injury, MWF increase was also observed. These suggest acute/chronic MWF alterations in concussed athletes from previous injuries by theorizing possible re-myelination leading to hypermyelination. [DOI] [PMC free article] [PubMed]
- 66.Fu M, Zuo Y. Experience-dependent structural plasticity in the cortex. Trends Neurosci. 2011;34:177–187. doi: 10.1016/j.tins.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rosenzweig MR, Bennett EL. Psychobiology of plasticity: effects of training and experience on brain and behavior. Behav Brain Res. 1996;78:57–65. doi: 10.1016/0166-4328(95)00216-2. [DOI] [PubMed] [Google Scholar]
- 68.Giza CC, Griesbach GS, Hovda DA. Experience-dependent behavioral plasticity is disturbed following traumatic injury to the immature brain. Behav Brain Res. 2005;157:11–22. doi: 10.1016/j.bbr.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 69.Aungst SL, Kabadi SV, Thompson SM, Stoica BA, Faden AI. Repeated mild traumatic brain injury causes chronic neuroinflammation, changes in hippocampal synaptic plasticity, and associated cognitive deficits. J Cereb Blood Flow Metab Nat Publ Group. 2014;34:1223–1232. doi: 10.1038/jcbfm.2014.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fineman I, Giza CC, Nahed BV, Lee SM, Hovda DA. Inhibition of neocortical plasticity during development by a moderate concussive brain injury. J Neurotrauma. 2000;17:739–749. doi: 10.1089/neu.2000.17.739. [DOI] [PubMed] [Google Scholar]
- 71.Sick TJ, Pérez-Pinzón MA, Feng ZZ. Impaired expression of long-term potentiation in hippocampal slices 4 and 48 h following mild fluid-percussion brain injury in vivo. Brain Res. 1998;785:287–292. doi: 10.1016/s0006-8993(97)01418-2. [DOI] [PubMed] [Google Scholar]
- 72.White ER, Pinar C, Bostrom CA, Meconi A, Christie BR. Mild traumatic brain injury produces long-lasting deficits in synaptic plasticity in the female juvenile hippocampus. J Neurotrauma. 2017;34:1111–1123. doi: 10.1089/neu.2016.4638. [DOI] [PubMed] [Google Scholar]
- 73.Reeves TM, Lyeth BG, Povlishock JT. Long-term potentiation deficits and excitability changes following traumatic brain injury. Exp Brain Res. 1995;106:248–256. doi: 10.1007/BF00241120. [DOI] [PubMed] [Google Scholar]
- 74.Liu X, Qiu J, Alcon S, Hashim J, Meehan WP, Mannix R. Environmental enrichment mitigates deficits after repetitive mild traumatic brain injury. J Neurotrauma. 2017;34:2445–2455. doi: 10.1089/neu.2016.4823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.De Beaumont L, Tremblay S, Poirier J, Lassonde M, Théoret H. Altered bidirectional plasticity and reduced implicit motor learning in concussed athletes. Cereb Cortex. 2012;22:112–121. doi: 10.1093/cercor/bhr096. [DOI] [PubMed] [Google Scholar]
- 76.Majerske CW, Mihalik JP, Ren D, Collins MW, Reddy CC, Lovell MR, et al. Concussion in sports: postconcussive activity levels, symptoms, and neurocognitive performance. J Athl Train. 2008;43:265. doi: 10.4085/1062-6050-43.3.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rathbone ATL, Tharmaradinam S, Jiang S, Rathbone MP, Kumbhare DA. A review of the neuro- and systemic inflammatory responses in post concussion symptoms: introduction of the “post-inflammatory brain syndrome” PIBS. Brain Behav Immun. 2015;46:1–16. doi: 10.1016/j.bbi.2015.02.009. [DOI] [PubMed] [Google Scholar]
- 78.Loane DJ, Byrnes KR. Role of microglia in neurotrauma. Neurotherapeutics. 2010;7:366–377. doi: 10.1016/j.nurt.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Villapol S, Loane DJ, Burns MP. Sexual dimorphism in the inflammatory response to traumatic brain injury. Glia. 2017;65:1423–1438. doi: 10.1002/glia.23171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Redell JB, Moore AN, Grill RJ, Johnson D, Zhao J, Liu Y, Dash PK. Analysis of functional pathways altered after mild traumatic brain injury. J Neurotrauma. 2013;30:752–764. doi: 10.1089/neu.2012.2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bélanger M, Allaman I, Magistretti PJ. Differential effects of pro- and anti-inflammatory cytokines alone or in combinations on the metabolic profile of astrocytes. J Neurochem. 2011;116:564–576. doi: 10.1111/j.1471-4159.2010.07135.x. [DOI] [PubMed] [Google Scholar]
- 82.Blaylock RL, Maroon JC. Immunoexcitotoxicity as a central mechanism in chronic traumatic encephalopathy—a unifying hypothesis. Surg Neurol Int. 2011;2:107. doi: 10.4103/2152-7806.83391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Su SH, Xu W, Li M, Zhang L, Wu YF, Yu F, et al. Elevated C-reactive protein levels may be a predictor of persistent unfavourable symptoms in patients with mild traumatic brain injury: a preliminary study. Brain Behav Immun. 2014;38:111–117. doi: 10.1016/j.bbi.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 84.Abbott NJ, Rönnbäck L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci. 2006;7:41–53. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- 85.Yeung D, Manias JL, Stewart DJ, Nag S. Decreased junctional adhesion molecule-a expression during blood-brain barrier breakdown. Acta Neuropathol. 2008;115:635–642. doi: 10.1007/s00401-008-0364-4. [DOI] [PubMed] [Google Scholar]
- 86.Nag S, Manias JL, Stewart DJ. Expression of endothelial phosphorylated caveolin-1 is increased in brain injury. Neuropathol Appl Neurobiol. 2009;35:417–426. doi: 10.1111/j.1365-2990.2008.01009.x. [DOI] [PubMed] [Google Scholar]
- 87.Barzó P, Marmarou A, Fatouros P, Corwin F, Dunbar J. Magnetic resonance imaging-monitored acute blood-brain barrier changes in experimental traumatic brain injury. J Neurosurg. 1996;85:1113–1121. doi: 10.3171/jns.1996.85.6.1113. [DOI] [PubMed] [Google Scholar]
- 88.Habgood MD, Bye N, Dziegielewska KM, Ek CJ, Lane MA, Potter A, Morganti-Kossmann C, Saunders NR. Changes in blood-brain barrier permeability to large and small molecules following traumatic brain injury in mice. Eur J Neurosci. 2007;25:231–238. doi: 10.1111/j.1460-9568.2006.05275.x. [DOI] [PubMed] [Google Scholar]
- 89.Baldwin SA, Fugaccia I, Brown DR, Brown LV, Scheff SW. Blood-brain barrier breach following cortical contusion in the rat. J Neurosurg. 1996;85:476–481. doi: 10.3171/jns.1996.85.3.0476. [DOI] [PubMed] [Google Scholar]
- 90.Başkaya MK, Rao AM, Doğan A, Donaldson D, Dempsey RJ. The biphasic opening of the blood-brain barrier in the cortex and hippocampus after traumatic brain injury in rats. Neurosci Lett. 1997;226:33–36. doi: 10.1016/s0304-3940(97)00239-5. [DOI] [PubMed] [Google Scholar]
- 91.Hay JR, Johnson VE, Young AMH, Smith DH, Stewart W. Blood-brain barrier disruption is an early event that may persist for many years after traumatic brain injury in humans. J Neuropathol Exp Neurol. 2015;74:1147–1157. doi: 10.1097/NEN.0000000000000261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Johnson VE, Weber MT, Xiao R, Cullen DK, Meaney DF, Stewart W, Smith DH. Mechanical disruption of the blood–brain barrier following experimental concussion. Acta Neuropathol. 2018;135:711–726. doi: 10.1007/s00401-018-1824-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Raghupathi R, Conti AC, Graham DI, Krajewski S, Reed JC, Grady MS, Trojanowski JQ, McIntosh TK. Mild traumatic brain injury induces apoptotic cell death in the cortex that is preceded by decreases in cellular Bcl-2 immunoreactivity. Neuroscience. 2002;110:605–616. doi: 10.1016/s0306-4522(01)00461-4. [DOI] [PubMed] [Google Scholar]
- 94.Shlosberg D, Benifla M, Kaufer D, Friedman A. Blood-brain barrier breakdown as a therapeutic target in traumatic brain injury. Nat Rev Neurol Nature Publishing Group. 2010;6:393–403. doi: 10.1038/nrneurol.2010.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Korn A, Golan H, Melamed I, Pascual-marqui R, Friedman A. Focal cortical dysfunction and blood – brain barrier disruption in patients with postconcussion syndrome. 2005;22:1–9. [DOI] [PubMed]
- 96.Weissberg I, Veksler R, Kamintsky L, Saar-Ashkenazy R, Milikovsky DZ, Shelef I, Friedman A. Imaging blood-brain barrier dysfunction in football players. JAMA Neurol. 2014;71:1453–1455. doi: 10.1001/jamaneurol.2014.2682. [DOI] [PubMed] [Google Scholar]
- 97.Livingston WS, Gill JM, Cota MR, Olivera A, O’Keefe JL, Martin C, et al. Differential gene expression associated with meningeal injury in acute mild traumatic brain injury. J Neurotrauma. 2017;34:853–860. doi: 10.1089/neu.2016.4479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gurkoff GG, Giza CC, Hovda DA. Lateral fluid percussion injury in the developing rat causes an acute, mild behavioral dysfunction in the absence of significant cell death. Brain Res. 2006;1077:24–36. doi: 10.1016/j.brainres.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 99.Lyeth BG, Jenkins LW, Hamm RJ, Dixon CE, Phillips LL, Clifton GL, Young HF, Hayes RL. Prolonged memory impairment in the absence of hippocampal cell death following traumatic brain injury in the rat. Brain Res. 1990;526:249–258. doi: 10.1016/0006-8993(90)91229-a. [DOI] [PubMed] [Google Scholar]
- 100.Dikranian K, Cohen R, Mac Donald C, Pan Y, Brakefield D, Bayly P, Parsadanian A. Mild traumatic brain injury to the infant mouse causes robust white matter axonal degeneration which precedes apoptotic death of cortical and thalamic neurons. Exp Neurol. 2008;211:551–560. doi: 10.1016/j.expneurol.2008.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Smith DH, Chen X-H, Pierce JES, Wolf JA, Trojanowski JQ, Graham DI, et al. Progressive atrophy and neuron death for one year following brain trauma in the rat. J Neurotrauma. 1997;14:715–727. doi: 10.1089/neu.1997.14.715. [DOI] [PubMed] [Google Scholar]
- 102.Pullela R, Raber J, Pfankuch T, Ferriero DM, Claus CP, Koh SE, Yamauchi T, Rola R, Fike JR, Noble-Haeusslein LJ. Traumatic injury to the immature brain results in progressive neuronal loss, hyperactivity and delayed cognitive impairments. Dev Neurosci. 2006;28:396–409. doi: 10.1159/000094166. [DOI] [PubMed] [Google Scholar]
- 103.Zhou Y, Kierans A, Kenul D, Ge Y, Rath J, Reaume J, Grossman RI, Lui YW. Mild traumatic brain injury: longitudinal regional brain volume changes. Radiology. 2013;267:880–890. doi: 10.1148/radiol.13122542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Monti JM, Voss MW, Pence A, McAuley E, Kramer AF, Cohen NJ. History of mild traumatic brain injury is associated with deficits in relational memory, reduced hippocampal volume, and less neural activity later in life. Front Aging Neurosci. 2013;5:1–9. doi: 10.3389/fnagi.2013.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.McKee AC, Stein TD, Nowinski CJ, Stern RA, Daneshvar DH, Alvarez VE, et al. The spectrum of disease in chronic traumatic encephalopathy. Brain. 2013;136:43–64. doi: 10.1093/brain/aws307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Singh R, Meier TB, Kuplicki R, Savitz J, Mukai I, Cavanagh LM, Allen T, Teague TK, Nerio C, Polanski D, Bellgowan PSF. Relationship of collegiate football experience and concussion with hippocampal volume and cognitive outcomes. J Am Med Assoc. 2014;311:1883–1888. doi: 10.1001/jama.2014.3313. [DOI] [PubMed] [Google Scholar]
- 107.Zagorchev L, Meyer C, Stehle T, Wenzel F, Young S, Peters J, Weese J, Paulsen K, Garlinghouse M, Ford J, Roth R, Flashman L, McAllister T. Differences in regional brain volumes two months and one year after mild traumatic brain injury. J Neurotrauma. 2016;33:29–34. doi: 10.1089/neu.2014.3831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.O’Connor KL, Rowson S, Duma SM, Broglio SP. Head-impact–measurement devices: a systematic review. J Athl Train. 2017;52:206–227. doi: 10.4085/1062-6050.52.2.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Meaney DF, Smith DH. Biomechanics of concussion. Clin Sports Med. 2011;30:19–31. doi: 10.1016/j.csm.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Nahum AM, Smith R, Ward CC. Intracranial pressure dynamics during head impact. Proceedings, 21st Stapp Car Crash Conf., SAE Paper No. 770922. 1977.
- 111.Thibault LE, Meaney DF, Anderson BJ, Marmarou A. Biomechanical aspects of a fluid percussion model of brain injury. J Neurotrauma. 1992;9:311–322. doi: 10.1089/neu.1992.9.311. [DOI] [PubMed] [Google Scholar]
- 112.Prange MT, Meaney DF, Margulies SS. Defining brain mechanical properties: effects of region, direction, and species. Stapp Car Crash J. 2000;44:205–213. doi: 10.4271/2000-01-SC15. [DOI] [PubMed] [Google Scholar]
- 113.Adams JH, Graham DI, Murray LS, Scott G. Diffuse axonal injury due to nonmissile head injury in humans: an analysis of 45 cases. Ann Neurol. 1982;12:557–563. doi: 10.1002/ana.410120610. [DOI] [PubMed] [Google Scholar]
- 114.Ommaya AK, Hirsch AE, Martinez JL. The role of whiplash in cerebral concussion. Proceedings of the 10th Stapp Car Crash Conference. 1996;314–3243.
- 115.Kimpara H, Iwamoto M. Mild traumatic brain injury predictors based on angular accelerations during impacts. Ann Biomed Eng. 2012;40:114–126. doi: 10.1007/s10439-011-0414-2. [DOI] [PubMed] [Google Scholar]
- 116.Rowson S, Duma SM, Beckwith JG, Chu JJ, Greenwald RM, Crisco JJ, Brolinson PG, Duhaime AC, McAllister TW, Maerlender AC. Rotational head kinematics in football impacts: an injury risk function for concussion. Ann Biomed Eng. 2012;40:1–13. doi: 10.1007/s10439-011-0392-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Post A, Blaine HT. Rotational acceleration, brain tissue strain, and the relationship to concussion. J Biomech Eng. 2015;137:030801. doi: 10.1115/1.4028983. [DOI] [PubMed] [Google Scholar]
- 118.Kerr ZY, Roos KG, Djoko A, Dalton SL, Broglio SP, Marshall SW, Dompier TP. Epidemiologic measures for quantifying the incidence of concussion in national collegiate athletic association sports. J Athl Train. 2017;52:167–174. doi: 10.4085/1062-6050-51.6.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Cobb BR, Urban JE, Davenport EM, Rowson S, Duma SM, Maldjian JA, Whitlow CT, Powers AK, Stitzel JD. Head impact exposure in youth football: elementary school ages 9–12 years and the effect of practice structure. Ann Biomed Eng. 2013;41:2463–2473. doi: 10.1007/s10439-013-0867-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Manoogian S, McNeely D, Duma S, Brolinson G, Greenwald R. Head acceleration is less than 10 percent of helmet acceleration in football impacts. Biomed Sci Instrum. 2006;42:383–388. [PubMed] [Google Scholar]
- 121.Wu LC, Nangia V, Bui K, Hammoor B, Kurt M, Hernandez F, Kuo C, Camarillo DB. In vivo evaluation of wearable head impact sensors. Ann Biomed Eng. 2016;44:1234–1245. doi: 10.1007/s10439-015-1423-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Press JN, Rowson S. Quantifying head impact exposure in collegiate women’s soccer. Clin J Sport Med. 2017;27:104–110. doi: 10.1097/JSM.0000000000000313. [DOI] [PubMed] [Google Scholar]
- 123.McCrory P, Feddermann-Demont N, Dvořák J, Cassidy JD, McIntosh A, Vos PE, Echemendia RJ, Meeuwisse W, Tarnutzer AA. What is the definition of sports-related concussion: a systematic review. Br J Sports Med. 2017;51:877–887. doi: 10.1136/bjsports-2016-097393. [DOI] [PubMed] [Google Scholar]
- 124.Brennan JH, Mitra B, Synnot A, McKenzie J, Willmott C, McIntosh AS, et al. Accelerometers for the assessment of concussion in male athletes: a systematic review and meta-analysis. Sport Med Springer International Publishing. 2017;47:469–478. doi: 10.1007/s40279-016-0582-1. [DOI] [PubMed] [Google Scholar]
- 125.Beckwith JG, Greenwald RM, Chu JJ, Crisco JJ, Rowson S, Duma SM, et al. Timing of concussion diagnosis is related to head impact exposure prior to injury. Med Sci Sports Exerc. 2013;45:747–754. doi: 10.1249/MSS.0b013e3182793067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Rowson S, Duma SM, Stemper BD, Shah A, Mihalik JP, Harezlak J, et al. Correlation of concussion symptom profile with head impact biomechanics: a case for individual-specific injury tolerance. J Neurotrauma. 2018;35:681–690. doi: 10.1089/neu.2017.5169. [DOI] [PubMed] [Google Scholar]
- 127.Guskiewicz KM, Mihalik JP. Biomechanics of sport concussion. Exerc Sport Sci Rev. 2011;39:4–11. doi: 10.1097/JES.0b013e318201f53e. [DOI] [PubMed] [Google Scholar]
- 128.Caccese JB, Buckley TA, Tierney RT, Arbogast KB, Rose WC, Glutting JJ, et al. Head and neck size and neck strength predict linear and rotational acceleration during purposeful soccer heading. Sport Biomech Routledge. 2017;3141:1–15. doi: 10.1080/14763141.2017.1360385. [DOI] [PubMed] [Google Scholar]