Abstract
Background:
Preterm labor is a leading risk factor for neonatal death and long-term impairment and linked closely with inflammation. Non-obstetric surgery is occasionally needed during pregnancy and the anesthetic drugs or surgery itself can give rise to inflammation. Here, we examined the influence of propofol pretreatment on the expression of cyclooxygenase-2 (COX-2) and prostaglandin E2 (PGE2) after lipopolysaccharide (LPS) stimulation. In addition, we evaluated the expression of pro-inflammatory cytokines and nuclear factor kappa B (NF-κB).
Methods:
Human amnion-derived WISH cells were used to investigate the effect of propofol on the LPS-induced expression of inflammatory substances involved in preterm labor. For the experiment, WISH cells were pretreated with various concentrations propofol (0.01–10 μg/ml) for 1 h and then treated with LPS (1 μg/ml) for 24 h. Cytotoxicity was evaluated using MTT assay. PGE2 concentration was assessed by ELISA. Protein expressions of COX-2, PGE2 and NF-κB were analyzed by western blotting analysis. RT-PCR was used for analysis of mRNA expression of COX-2, PGE2, interlukin (IL)-1β and tumor necrosis factor (TNF)-α.
Results:
Propofol showed no cytotoxicity on the WISH cells. LPS-induced PGE2 production and COX-2 and PGE2 expression were decreased after propofol pretreatment. Propofol also attenuated the LPS-induced mRNA expression of IL-1β and TNF-α. Moreover, the activation of NF-κB was inhibited by propofol pretreatment on LPS-stimulated WISH cells.
Conclusion:
We demonstrated that propofol suppresses the expression of inflammatory substances enhanced by LPS stimulation. Furthermore, this inhibitory effect of propofol on the inflammatory substance expression is mediated by suppression of NF-κB activation.
Keywords: Amnion, NF-κB, Inflammation, Preterm labor, Propofol
Introduction
An estimated 15 million babies are born preterm (delivered before 37 complete weeks of gestation) every year and this number is rising [1]. Preterm birth increases the morbidity and mortality of neonates and is the second most common cause of death in children under 5 years of age, responsible for approximately 1 million deaths in 2015 [2, 3]. Infection and/or inflammation are known as common causes of preterm labor; in addition, the use of anesthetic agents is associated with preterm labor [4]. Anesthesia during pregnancy is occasionally needed for non-obstetric surgery, such as cervical cerclage, surgeries for appendicitis, ovarian disorders (torsion or neoplasm), and trauma [5]. The anesthetic agents used for pregnant patients during non-obstetric surgery may have an adverse effect on preterm labor or birth. Therefore, it is necessary to ensure the safety of anesthetic agents for use in pregnant patients.
Infection and/or inflammation are related to early preterm birth and, according to the literature, account for approximately 40% of spontaneous preterm birth [2, 6]. Microbial invasion to the amniotic membrane activates pro-inflammatory signaling cascades, which trigger the production of pro-inflammatory cytokines (e.g., TNF-α and IL-1β) and cyclooxygenase-2 (COX-2) expression, and sequentially induce prostaglandin (PG) production and myometrial contraction and cervical ripening leading to preterm labor [7, 8]. Among prostaglandins (PGs), prostaglandin E2 (PGE2) has been reported as a major labor-inducing factor, and the concentration of PGE2 increases in the amniotic tissue at the beginning of labor [9].
PGs are formed from arachidonic acid via the action of COX enzyme (COX-1 and COX-2). It is known that COX-2 has a major role in PG production in the amnion during labor. According to the literature, the mRNA concentration of COX-2, rather than COX-1, increases near to full term and with the onset of labor [10]. In the amnion, COX-2 expression is regulated by nuclear factor-kappa B (NF-κB) [11]. NF-κB is an inducible transcription factor, which regulates the expression of various genes involved in immune and inflammatory responses [12]. NF-κB is retained in the cytoplasm in an inactivated form by binding to inhibitory proteins. The activation of NF-κB is upregulated through the activation of various cytokines, TNF, or Toll-like receptors on the cell surface, which leads to the promotion of inflammatory gene expression, including pro-inflammatory cytokines and COX-2 [13, 14].
Propofol is a widely used as an intravenous anesthetic for general anesthesia and sedation. Despite concerns about the possibility of neonatal depression and teratogenicity, propofol has been used for the induction of general anesthesia during non-obstetrical surgery and cesarean section [15, 16]. In some studies, propofol suppressed PGE2 synthesis in human mononuclear cell lines and reduced PGE2 production and COX-2 expression in amniotic membrane cells [17, 18]. In addition, propofol is known to have antioxidant or anti-inflammatory effects [19, 20]. However, there are no studies on the effect of propofol on the expression of pro-inflammatory cytokines and the transcription factors involved in the stimulation of preterm labor.
In this study, we investigated the effect of propofol pretreatment on the expression of PGE2 and COX-2 on the lipopolysaccharide (LPS)-induced inflammatory response using amnion-derived WISH cells, and analyzed nitric oxide (NO) production, pro-inflammatory cytokines, and NF-κB expression.
Materials and methods
Cell culture
The establishment of a line (WISH) of human amnion cells was purchased from the American Type Culture Collection (ATCC® CCL25™, Manassas, VA, USA) and cultured in Dulbecco’s modified Eagle medium (DMEM; ATCC® 30-2003™, Manassas, VA, USA), supplemented with 10% fetal bovine serum (FBS; Gibco, Carlsbad, CA, USA) in a 5% CO2 atmosphere at 37 °C. After 3 days, adherent cells were removed, and the culture was continued, with the medium replaced twice per week.
Propofol treatment
This study used a commercially available propofol (Fresenius Kabi Austria GmbH, Hafnerstrabe, Austria), which was dissolved in dimethyl sulfoxide (DMSO; Biosesang, Seongnam, Korea) and further diluted in phosphate buffered saline (PBS). Propofol was added to cultured cells at concentrations between 0.01 and 10 μg/mL for 1 h and then LPS treatment (1 μg/mL) was applied for 24 h.
MTT assay
The cells (1 × 105/well) were seeded in 24-well plates and cultured for 24 h at 37 °C in a 5% CO2 incubator. Subsequently, the cells were exposed to propofol (0.01–10 µg/mL) for 1 h and then incubated with LPS (1 μg/mL) for 24 h. After treatment with propofol, the MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide; Affymetrix, Inc. Santa Clara, CA, USA] assay was performed by the addition of 100 μL MTT solution (5 mg/mL in PBS at pH 7.4) into each well and incubation of the plate at 37 °C. After 1 h, the medium was removed, and 100 μL DMSO was added into each well. The plate was gently rotated on an orbital shaker for 15 min to completely dissolve the precipitate. The absorbance at 540 nm was detected by using a microplate reader (Bio-Rad Model 680; Bio-Rad, Hercules, CA, USA). All experiments were repeated three times.
NO assay
WISH cells were seeded at 1 × 104 cells per well into 24-well plates. The concentration of NO in the culture supernatant was determined as nitrite (NO2−) using Griess reagent (Cell Signaling Technologies, Denver, MA, USA). Briefly, WISH cells were pretreated with propofol (0.01–10 μg/mL) for 1 h and then incubated with LPS (1 μg/mL) for 24 h. After incubation, the supernatant was collected and mixed with an equal amount of Griess reagent (1% sulfanilamide and 0.1% naphthyl ethylenediamine dihydrochloride in 2.5% orthophosphoric acid). The samples were incubated at room temperature for 10 min, and absorbance at 540 nm was detected by using a microplate reader (Bio-Rad Model 680). Each assay was performed in triplicate.
Measurement of PGE2 concentration
WISH cells were seeded in 6-well plates at a density of 1 × 105 cells/1.5 mL and cultured at 37 °C in a 5% CO2 incubator. Then, the cells were pretreated with propofol (0.01, 0.1, 1, and 10 µg/mL) for 1 h and incubated in the presence of LPS (1 µg/mL) for 24 h. Following the manufacturer’s instructions, a volume of 100 mL of cell lysate was collected for the determination of PGE2 concentration by using the Correlate-EIA high-sensitivity PGE2 enzyme immunoassay (ELISA) (Enzo Life Sciences, Plymouth Meeting, PA, USA).
Western blotting
All cells were extracted with chilled RIPA buffer (50 mM Tris pH 7.5, 150 mM NaCl, 5 mM EDTA, 0.5% NP40, 5 mM DTT, 0.2 mM sodium orthovanadate, 100 mM NaF, and 1 mM PMSF) containing protease inhibitor/phosphatase Inhibitor Cocktail (Cell Signaling Technology, Danvers, MA, USA). Samples (25 μg protein/well) were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to nitrocellulose membranes (Bio-Rad, Hercules, CA, USA).
The membranes were blocked in TBS-0.1% Tween 20 (TBST) containing 3% skim milk for 1 h at room temperature. Subsequently, the membranes were then incubated with α-tubulin (1:1000; Santa Cruz, CA, USA), NF-κB p65 (1:1000; Santa Cruz, CA, USA), phospho-NF-kB p65 27.Ser 536 (1:500; Santa Cruz, CA, USA), PGE synthase 2 (A-2) antibody (1:1000; Cell Signaling Technology, Danvers, MA, USA), or COX-2(D5H5) rabbit mAb (1:1000; Santa Cruz, CA, USA) overnight at 4 °C in TBST with 3% skim milk. After three washes with TBST, the membranes were incubated with horseradish peroxidase (HRP)-conjugated anti-rabbit (1:1000; Enzo Life Sciences) and anti-mouse (1:1000; Santa Cruz, CA, USA) secondary antibodies for 1 h at room temperature. After three washes with TBST, the bands were visualized by use of the enhanced chemiluminescence detection reagents (Promega, Madison, WI, USA). α-Tubulin expression was used as the control; the target protein bands were normalized relative to the control band by using the NIH image program (ImageJ Launcher, Bethesda, MD, USA).
RNA extraction and reverse transcriptase-polymerase chain reaction (RT-PCR)
Total RNA was extracted from WISH cells by using the TRIzol reagent (Invitrogen, Carlsbad, CA, USA). Total mRNA (1 μg) was synthesized to cDNA using oligo (dT) PrimeScript™ first-strand cDNA Synthesis Kits (TaKaRa Clontech, BD Biosciences, Palo Alto, CA, USA) in accordance with the manufacturer’s instructions. RT-PCR was performed on a SimpliAmp Thermal Cycler (Applied Biosystems, Life Technologies, Carlsbad, CA, USA). Polymerase chain reaction (PCR) primers were as follows: COX-2, Forward: 5′-CCT TCC TCC TGT GCC TGA TG-3′, Reverse: 5′-CTG GCC TCG CTT ATG ATC T-3′; PGE2, Forward: 5′-TGA AGG CTG TGA ACG AGC AG-3′, Reverse: 5′-CAT TGG GGG AGA TCA GGT GC-3′; IL-1β, Forward: 5′- CTC GCC AGT GAA ATG ATG GCT-3′, Reverse: 5′-GTC GGA GAT TCG TAG CTG GAT-3′; TNF-α, Forward: 5′-CCA GGC AGT CAG ATC ATC TTC-3′, Reverse: 5′-GTT ATC TCT CAG CTC CAC GC-3′; β-actin, Forward: 5′-GAC CTG ACT GAC TAC CTC ATG-3′, Reverse: 5′-CGC TCA TTG CCA ATG GTG ATG-3′. The amplification of COX-2, PGE2, IL-1β, and β-actin was performed for 35 cycles of 95 °C for 30 s, 55 °C for 30 s, and 72 °C for 30 s, with a final extension period of 72 °C for 7 min. The amplification of TNF-α and β-actin was performed for 35 cycles of 94 °C for 30 s, 54 °C for 30 s, and 72 °C for 30 s, with a final extension period of 72 °C for 10 min. The PCR-amplified products were separated on 1.5% agarose gels and stained. The Gel Doc ImageQuant LAS 500 System (GE Healthcare Bio-Sciences AB, Uppsala, Sweden) was used to assay the PCR products; all target genes were normalized to β-actin. The data were analyzed by using the NIH image program (ImageJ Launcher, Bethesda, MD, USA).
Statistical analysis
Data values are presented as the mean ± standard deviation (SD). All experiments were repeated three times. Statistical significance was determined by using SigmaPlot v10 software. P value of < 0.05 was considered to indicate statistically significant.
Results
Propofol and LPS were not toxic to WISH cells
The effects of propofol and LPS on the viability of WISH cells were analyzed by MTT assay. WISH cells were pretreated with various concentration of propofol (0.01–10 μg/mL) and then exposed to LPS (1 μg/mL). As shown in Fig. 1, there were no significant differences in cell viability at the propofol concentrations tested.
Fig. 1.
Propofol and LPS did not affect the viability of WISH cells in MTT assay. WISH cells were cultured in media with various concentrations propofol (0.01–10 μg/mL) for 1 h and then exposed to LPS (1 μg/mL) for 24 h. Data are presented as mean ± SD. All experiments were repeated three times
Propofol did not affect NO production
NO is known to be involved in the relaxation of the smooth muscle myometrial cells, which leads to uterine quiescence throughout gestation [21, 22]. To investigate the effect of propofol on NO production in WISH cells, the concentrations of the stable nitrite end products in all the culture supernatants were measured by the Griess reaction microassay. As shown in Fig. 2, there was no difference in NO production at the pretreated propofol concentrations.
Fig. 2.
Nitric oxide (NO) production was not changed after LPS and propofol treatment in WISH cells. Stable nitrite end products were measured by the Griess reaction microassay. The results are presented as mean ± SD. All experiments were repeated three times
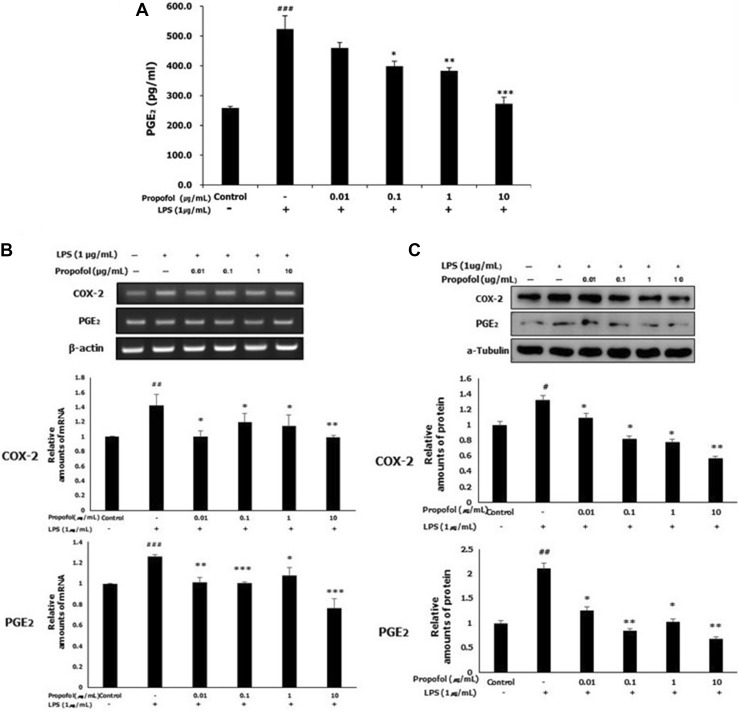
Propofol pretreatment decreased PGE2 production and COX-2 and PGE2 expression in LPS-stimulated WISH cells
We used an ELISA to investigate the effect of propofol on the LPS-induced production of PGE2. As shown in Fig. 3A, the level of PGE2 was significantly elevated by LPS stimulation compared with that in the control cells. This elevation was significantly inhibited by propofol pretreatment at 0.1, 1 and 10 μg/mL concentrations compared with that in the LPS group.
Fig. 3.
LPS-induced PGE2 production and COX-2 and PGE2 expression were inhibited by propofol. WISH cells were cultured in media after treatment with propofol (0.01–10 μg/mL) for 1 h and/or LPS (1 μg/mL) for 24 h. A PGE2 production was analyzed using ELISA. B The mRNA expression of COX-2 and PGE2 was analyzed using RT-PCR analysis. Relative density of the mRNA expression of COX-2 and PGE2 was normalized by β-actin. C The protein expression of COX-2 and PGE2 was evaluated by western blotting. Relative density analysis was performed using NIH Image program and normalized by α-tubulin. Values are mean ± SD of three independent experiments. #p < 0.05, ##p < 0.01, ###p < 0.001 versus control group; *p < 0.05, **p < 0.01, ***p < 0.001 versus LPS group
To examine the effect of propofol on the mRNA expression of COX-2 and PGE2, we used RT-PCR analysis. As shown in Fig. 3B, propofol significantly restricted the LPS-induced mRNA expression of COX-2 and PGE2 at all concentrations of propofol.
The protein expression of COX-2 and PGE2 was evaluated by western blotting. After treatment with LPS (1 μg/mL), the expression of COX-2 and PGE2 was significantly increased compared with that in the control group. Propofol pretreatment before LPS exposure led to a dose-dependent decrease in COX-2 expression between 0.01 and 10 μg/mL propofol, with significant differences compared with that in the LPS group. In addition, PGE2 expression showed a significant decrease at all concentrations of propofol (0.01–10 μg/mL) compared with those in the LPS group (Fig. 3C).
Propofol pretreatment reduced the mRNA expression of IL-1β and TNF-α in LPS-stimulated WISH cells
To evaluate the effect of propofol on the LPS-induced mRNA expression of pro-inflammatory cytokines (IL-1β and TNF-α), we used RT-PCR analysis. As shown in Fig. 4, LPS increased the mRNA expression of IL-1β and TNF-α significantly compared with the control group. Propofol pretreatment exerted an inhibitory effect on the mRNA expression of IL-1β at concentrations of 0.01 and 10 μg/mL, which was statistically significant. However, mRNA expression of TNF-α was significantly reduced by propofol pretreatment at all concentrations (Fig. 4).
Fig. 4.
Propofol attenuated the LPS-induced mRNA expression of IL-1β and TNF-α. RT-PCR was used to detect the mRNA expression of IL-1β and TNF-α in WISH cells and assessed by band densitometry. Relative mRNA level was normalized by β-actin and presented as mean ± SD of three independent experiments. ##p < 0.01, ###p < 0.001 versus control group; *p < 0.05, **p < 0.01 versus LPS group
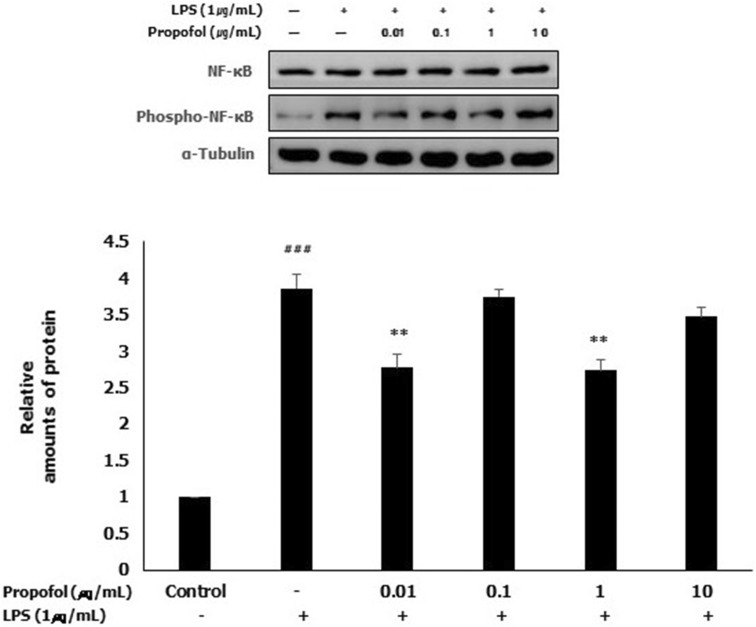
LPS-induced NF-κB activation was inhibited by propofol pretreatment
To investigate the effect of propofol on the activation of NF-κB, which plays a key role in regulation of the expression of various inflammatory genes, western blotting was performed. To analyze the activation of NF-κB, the expression of phospho-NF-κB (phospho-p65, the activated form of NF-κB) was examined by western blotting. In the LPS group, the expression of phospho-NF-κB was significantly increased compared with that in the control group. The expression of phospho-NF-κB was significantly decreased by propofol pretreatment at 0.01 and 1 μg/mL compared with that in the LPS group. Although there was no significant difference in the expression of phospho-NF-κB at 0.1 and 10 μg/mL propofol, it was decreased slightly compared with that in the LPS group (Fig. 5).
Fig. 5.
LPS-induced NF-κB activation was inhibited by propofol pretreatment in WISH cells. Protein expression of activated NF-κB was examined using western blotting analysis by immunoblotting for phospho-NF-κB (phospho-p65). Relative amounts of protein were analyzed by triplicate experiments and normalized by NF-κB. ### p < 0.001 versus control group; **p < 0.01 versus LPS group
Discussion
In this study, propofol decreased PGE2 production and the expression of COX-2 and PGE2 in LPS-stimulated WISH cells. This result is in agreement with other recent studies that have reported that propofol suppressed PGE2 production through the inhibition of COX expression in human immune cells or amniotic membranes [17, 18, 23]. Here, we stimulated amnion-derived WISH cells with LPS to induce inflammatory responses that lead to the upregulation of pro-inflammatory cytokines as well as the synthesis of PGs. Bacterial LPS has been used widely in experiments on inflammation because LPS stimulates the release of many inflammatory cytokines, such as TNF-α, IL-1β, and IL-6 [24]. Intrauterine infection or infection-led activation of inflammatory responses have been considered as a main risk factor related to spontaneous preterm birth [25, 26]. Therefore, we stimulated the inflammatory response in WISH cells with LPS after propofol pretreatment to verify whether propofol exerts a protective effect against the production of inflammatory substances in WISH cells.
We showed that NO production was not affected by propofol pretreatment in LPS-stimulated WISH cells. Luo et al. [27] reported that NO production was increased by propofol concentrations between 12.5 and 100 μM in human umbilical vein endothelial cells. In our study, we evaluated the NO concentration in WISH cells using propofol concentrations of 0.01–10 μg/mL (0.056–56 μM). The difference in results between the study of Luo et al. [27] and our study may arise from the cell types in each experiment. Each cell type has a different sensitivity to drug and different effect on the activation of nitric oxide synthase (NOS). This is the first study to investigate the effect of propofol on NO production in amnion-derived WISH cells, but the results are inconclusive. Therefore, further study is required to demonstrate the influence of propofol on NO production in the amnion.
In previous studies, the molecule NO, a free radical generated from the amino acid l-arginine by NOS, is known to induce uterine quiescence during pregnancy [28, 29]. However, some literature showed that NOS activity and localization was not changed in fetal membranes and that NO metabolites were increased in amniotic fluid during labor [30–32]. Therefore, the role of NO produced by the fetal membrane and placenta is still under debate.
In this study, we used propofol concentrations between 0.01 and 10 μg/mL with reference to previous study with a similar study design [18]. Our data demonstrate that propofol treatment at this range was not toxic to WISH cells. It was reported that the blood concentrations of propofol in clinical use were 0.8–1.0 μg/mL to induce awakening from anesthesia, 1–2 μg/mL for long-term intensive care unit sedation, and 3–11 μg/mL to maintain general anesthesia [33]. Therefore, the propofol concentrations used in this study are appropriate for clinical practice.
It has been demonstrated that NF-κB is important in the expression of COX-2 in amnion-derived WISH cells [34, 35]. Therefore, we hypothesized that the decrease in PGE2 and COX-2 induced by propofol may be mediated through blocking of NF-κB activation. NF-κB is composed of five subunits: NF-κB1 (encoding p50 and its precursor p105), NF-κB2 (encoding p52 and its precursor p100), RelA (p65), RelB, and c-Rel, mainly with p65 and p50 subunits [36]. NF-κB is sequestered in the cytoplasm in an activated form by IκB proteins (inhibitory protein) or by the p105 and p100 subunits, where they act as IκB proteins [37]. The activation of NF-κB occurs through two signaling pathways, the canonical and non-canonical pathways. Canonical NF-κB activation involves the degradation of IκB by IκB kinase (IKK) complex and p50/Rel A and p50/c-Rel dimers were predominant; in contrast, noncanonical NF-κB activation is mediated by the phosphorylation of p100, the NF-κB2 precursor protein. Subsequently, mature NF-κB2 p52 was generated and led to the nuclear translocation of p52/RelB [38, 39]. However, the mechanism of NF-κB activation in the amnion prior to labor is not established. In this study, we examined NF-κB activation through the detection of phosphorylated p65 expression by using western blotting and found that it was related to the canonical NF-κB pathway. However, additional experiments with regard to the noncanonical pathway are needed to confirm the mechanism of NF-κB activation in WISH cells.
In conclusion, we have shown that propofol attenuated LPS-induced PGE2 production, as well as the expression of COX-2, PGE2, IL-1β, and TNF-α in amnion-derived WISH cells. These results suggested that propofol exerts a protective effect on preterm labor induced by the inflammatory response. Moreover, this study showed that LPS-induced NF-κB activation was inhibited by propofol pretreatment at 0.01 and 1 μg/mL. On the basis of this result, we provided the evidence that a decrease in the expression of inflammatory substances induced by propofol may occurred through the inhibition of NF-κB activation. Although additional studies are necessary to identify the effect of propofol on preterm labor, we propose that propofol can confer protection against preterm labor induced by PGE2 and pro-inflammatory cytokines when used during pregnancy.
Acknowledgement
This study was supported by Dental Research Institute (PNUDH-DRI 2017-04), Pusan National University Dental Hospital.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Statement
There are no animal or human experiments carried out for this article.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Liu L, Oza S, Hogan D, Chu Y, Perin J, Zhu J, et al. Global, regional, and national causes of under-5 mortality in 2000–15: an updated systematic analysis with implications for the Sustainable Development Goals. Lancet. 2016;388:3027–3035. doi: 10.1016/S0140-6736(16)31593-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet. 2008;371:75–84. doi: 10.1016/S0140-6736(08)60074-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blencowe H, Cousens S, Oestergaard MZ, Chou D, Moller AB, Narwal R, et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet. 2012;379:2162–2172. doi: 10.1016/S0140-6736(12)60820-4. [DOI] [PubMed] [Google Scholar]
- 4.Romero R, Espinoza J, Gonçalves LF, Kusanovic JP, Friel LA, Nien JK. Inflammation in preterm and term labour and delivery. Semin Fetal Neonatal Med. 2006;11:317–326. doi: 10.1016/j.siny.2006.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Upadya M, Saneesh PJ. Anaesthesia for non-obstetric surgery during pregnancy. Indian J Anaesth. 2016;60:234–241. doi: 10.4103/0019-5049.179445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DiGiulio DB, Romero R, Kusanovic JP, Gómez R, Kim CJ, Seok KS, et al. Prevalence and diversity of microbes in the amniotic fluid, the fetal inflammatory response, and pregnancy outcome in women with preterm pre-labor rupture of membranes. Am J Reprod Immunol. 2010;64:38–57. doi: 10.1111/j.1600-0897.2010.00830.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rinaldi SF, Hutchinson JL, Rossi AG, Norman JE. Anti-inflammatory mediators as physiological and pharmacological regulators of parturition. Expert Rev Clin Immunol. 2011;7:675–696. doi: 10.1586/eci.11.58. [DOI] [PubMed] [Google Scholar]
- 8.Keelan JA, Blumenstein M, Helliwell RJ, Sato TA, Marvin KW, Mitchell MD. Cytokines, prostaglandins and parturition—a review. Placenta. 2003;24:S33–S46. doi: 10.1053/plac.2002.0948. [DOI] [PubMed] [Google Scholar]
- 9.Teixeira FJ, Zakar T, Hirst JJ, Guo F, Sadowsky DW, Machin G, et al. Prostaglandin endoperoxide-H synthase (PGHS) activity and immunoreactive PGHS-1 and PGHS-2 levels in human amnion throughout gestation, at term, and during labor. J Clin Endocrinol Metab. 1994;78:1396–1402. doi: 10.1210/jcem.78.6.8200943. [DOI] [PubMed] [Google Scholar]
- 10.Slater D, Dennes W, Sawdy R, Allport V, Bennett P. Expression of cyclo-oxygenase types-1 and -2 in human fetal membranes throughout pregnancy. J Mol Endocrinol. 1999;22:125–130. doi: 10.1677/jme.0.0220125. [DOI] [PubMed] [Google Scholar]
- 11.Allport VC, Pieber D, Slater DM, Newton R, White JO, Bennett PR. Human labour is associated with nuclear factor-kappaB activity which mediates cyclo-oxygenase-2 expression and is involved with the ‘functional progesterone withdrawal’. Mol Hum Reprod. 2001;7:581–586. doi: 10.1093/molehr/7.6.581. [DOI] [PubMed] [Google Scholar]
- 12.Oeckinghaus A, Ghosh S. The NF-kappaB family of transcription factors and its regulation. Cold Spring Harb Perspect Biol. 2009;1:a000034. doi: 10.1101/cshperspect.a000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lindström TM, Bennett PR. The role of nuclear factor kappa B in human labour. Reproduction. 2005;130:569–581. doi: 10.1530/rep.1.00197. [DOI] [PubMed] [Google Scholar]
- 14.Hayden MS, Ghosh S. Signaling to NF-κB. Genes Dev. 2004;18:2195–2224. doi: 10.1101/gad.1228704. [DOI] [PubMed] [Google Scholar]
- 15.Abboud TK, Zhu J, Richardson M, Peres Da Silva E, Donovan M. Intravenous propofol vs thiamylal-isoflurane for caesarean section, comparative maternal and neonatal effects. Acta Anaesthesiol Scand. 1995;39:205–209. doi: 10.1111/j.1399-6576.1995.tb04044.x. [DOI] [PubMed] [Google Scholar]
- 16.Higuchi H, Adachi Y, Arimura S, Kanno M, Satoh T. Early pregnancy does not reduce the C(50) of propofol for loss of consciousness. Anesth Analg. 2001;93:1565–1569. doi: 10.1097/00000539-200112000-00050. [DOI] [PubMed] [Google Scholar]
- 17.Inada T, Kubo K, Kambara T, Shingu K. Propofol inhibits cyclo-oxygenase activity in human monocytic THP-1 cells. Can J Anaesth. 2009;56:222–229. doi: 10.1007/s12630-008-9035-0. [DOI] [PubMed] [Google Scholar]
- 18.Kim JD, Ahn BM, Joo BS, Kwon JY, Chung HJ, Yu SB. Effect of propofol on prostaglandin E2 production and prostaglandin synthase-2 and cyclooxygenase-2 expressions in amniotic membrane cells. J Anesth. 2014;28:911–918. doi: 10.1007/s00540-014-1830-x. [DOI] [PubMed] [Google Scholar]
- 19.Aarts L, van der Hee R, Dekker I, de Jong J, Langemeijer H, Bast A. The widely used anesthetic agent propofol can replace alpha-tocopherol as an antioxidant. FEBS Lett. 1995;357:83–85. doi: 10.1016/0014-5793(94)01337-Z. [DOI] [PubMed] [Google Scholar]
- 20.Vasileiou I, Xanthos T, Koudouna E, Perrea D, Klonaris C, Katsargyris A, et al. Propofol: a review of its non-anaesthetic effects. Eur J Pharmacol. 2009;605:1–8. doi: 10.1016/j.ejphar.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 21.Yallampalli C, Garfield RE, Byam-Smith M. Nitric oxide inhibits uterine contractility during pregnancy but not during delivery. Endocrinology. 1993;133:1899–1902. doi: 10.1210/endo.133.4.8404632. [DOI] [PubMed] [Google Scholar]
- 22.Sladek SM, Magness RR, Conrad KP. Nitric oxide and pregnancy. Am J Physiol. 1997;272:R441–R463. doi: 10.1152/ajpregu.1997.272.2.R441. [DOI] [PubMed] [Google Scholar]
- 23.Kambara T, Inada T, Kubo K, Shingu K. Propofol suppresses prostaglandin E(2) production in human peripheral monocytes. Immunopharmacol Immunotoxicol. 2009;31:117–126. doi: 10.1080/08923970802452046. [DOI] [PubMed] [Google Scholar]
- 24.Poltorak A, He X, Smirnova I, Liu MY, Van Huffel C, Du X, et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282:2085–2088. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- 25.Simmons LE, Rubens CE, Darmstadt GL, Gravett MG. Preventing preterm birth and neonatal mortality: exploring the epidemiology, causes, and interventions. Semin Perinatol. 2010;34:408–415. doi: 10.1053/j.semperi.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 26.Thaxton JE, Nevers TA, Sharma S. TLR-mediated preterm birth in response to pathogenic agents. Infect Dis Obstet Gynecol. 2010;2010:378472. doi: 10.1155/2010/378472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Luo T, Xia Z, Ansley DM, Ouyang J, Granville DJ, Li Y, et al. Propofol dose-dependently reduces tumor necrosis factor-alpha-Induced human umbilical vein endothelial cell apoptosis: effects on Bcl-2 and Bax expression and nitric oxide generation. Anesth Analg. 2005;100:1653–1659. doi: 10.1213/01.ANE.0000150945.95254.D8. [DOI] [PubMed] [Google Scholar]
- 28.Ledingham MA, Thomson AJ, Greer IA, Norman JE. Nitric oxide in parturition. BJOG. 2000;107:581–593. doi: 10.1111/j.1471-0528.2000.tb13297.x. [DOI] [PubMed] [Google Scholar]
- 29.Moncada S, Palmer RM, Higgs EA. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991;43:109–142. [PubMed] [Google Scholar]
- 30.Di Iulio JL, Gude NM, King RG, Brennecke SP. Human placental and fetal membrane nitric oxide synthase activity before, during and after labour at term. Reprod Fertil Dev. 1995;7:1505–1508. doi: 10.1071/RD9951505. [DOI] [PubMed] [Google Scholar]
- 31.Thomson AJ, Telfer JF, Kohnen G, Young A, Cameron IT, Greer IA. Nitric oxide synthase activity and localization do not change in uterus and placenta during human parturition. Hum Reprod. 1997;12:2546–2552. doi: 10.1093/humrep/12.11.2546. [DOI] [PubMed] [Google Scholar]
- 32.Marinoni E, Di Iorio R, Villaccio B, Alberini A, Rota F, Cosmi EV. Amniotic fluid nitric oxide metabolite levels and nitric oxide synthase localization in feto-placental tissues are modified in association with human labor. Eur J Obstet Gynecol Reprod Biol. 2000;89:47–54. doi: 10.1016/S0301-2115(99)00186-4. [DOI] [PubMed] [Google Scholar]
- 33.Short TG, Aun CS, Tan P, Wong J, Tam YH, Oh TE. A prospective evaluation of pharmacokinetic model controlled infusion of propofol in paediatric patients. Br J Anaesth. 1994;72:302–306. doi: 10.1093/bja/72.3.302. [DOI] [PubMed] [Google Scholar]
- 34.Allport VC, Slater DM, Newton R, Bennett PR. NF-kappaB and AP-1 are required for cyclo-oxygenase 2 gene expression in amnion epithelial cell line (WISH) Mol Hum Reprod. 2000;6:561–565. doi: 10.1093/molehr/6.6.561. [DOI] [PubMed] [Google Scholar]
- 35.Elliott CL, Allport VC, Loudon JA, Wu GD, Bennett PR. Nuclear factor-kappa B is essential for up-regulation of interleukin-8 expression in human amnion and cervical epithelial cells. Mol Hum Reprod. 2001;7:787–790. doi: 10.1093/molehr/7.8.787. [DOI] [PubMed] [Google Scholar]
- 36.Baeuerle PA, Henkel T. Function and activation of NF-kappa B in the immune system. Annu Rev Immunol. 1994;12:141–179. doi: 10.1146/annurev.iy.12.040194.001041. [DOI] [PubMed] [Google Scholar]
- 37.Sun SC. Non-canonical NF-kappaB signaling pathway. Cell Res. 2011;21:71–85. doi: 10.1038/cr.2010.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Karin M, Delhase M. The I kappa B kinase (IKK) and NF-kappa B: key elements of proinflammatory signalling. Semin Immunol. 2000;12:85–98. doi: 10.1006/smim.2000.0210. [DOI] [PubMed] [Google Scholar]
- 39.Sun SC. The noncanonical NF-kappaB pathway. Immunol Rev. 2012;246:125–140. doi: 10.1111/j.1600-065X.2011.01088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]