Abstract
Refinement is usually used to remove undesired components to improve coconut oil quality. In the present study, crude coconut oil (CCO) was refined in four stages: conventional degumming, neutralization, bleaching, and deodorization. Physiochemical indices during the refinement were evaluated: peroxide value (PV), free fatty acid (FFA), fatty acid composition; fat composition; triacylglycerol profile, micronutrient (e.g., tocopherols and sterols) and contaminant (e.g., 3-monochloropropane-1,2-diol esters (3-MCPD esters), and glycidyl esters (GEs) levels. Compared to CCO, the quality of refined oil was improved by reducing PV and FFA levels. No significant changes in triacylglycerol compositions were found during refinement. However, unsaturated fatty acids like oleic and linoleic acids were decreased after refinement. Also, some micronutrients like tocopherol and sterol were decreased from 12.39 to 0.10 mg/kg and from 679.69 to 426.50 mg/kg, respectively. The undesired contaminants, i.e., 3-MCPD esters and GEs, increased markedly during refinement.
Electronic supplementary material
The online version of this article (10.1007/s13197-019-03810-w) contains supplementary material, which is available to authorized users.
Keywords: Coconut oil, Oil refinement, Fatty acid composition, Triacylglycerol, Micronutrient, Contaminant
Introduction
Coconut is known as the tree of life due to its versatility (Nitbani et al. 2016). It is currently cultivated in over 98 countries, particularly in the tropical regions of Southern Asia. Philippines, Indonesia, and India account for approximately 72% of the world’s planted coconut hectarage. According to a United States Department of Agriculture (USDA) report, the total production of dried coconut (known as ‘copra’) in 2016–2017 amounted to 5.41 million metric tons, producing 3.37 million metric tons of coconut oil (WMT 2017).
Coconut oil can be divided into virgin coconut oil (VCO) made from fresh coconuts and crude coconut oil (CCO) made from dried coconuts. VCO is extracted directly from coconut milk using a wet process under mild conditions (no harsh chemical treatment or solvent extraction), and gentle heating. CCO is obtained from copra by solvent extraction. According to a report by Krishna et al. (2010), VCO is mainly produced in Philippines, whereas CCO is produced in many countries including India and Sri Lanka. CCO dominates the industry for the following reasons: (1) removing the hard shell of the coconut is time-consuming; (2) the subsequent wet coconut kernel is susceptible to internal (including enzymatic) and external damage (Nathanael 1965); (3) the conservation of fresh kernels involves high transport costs; (4) the accumulation and retention of adequate levels of freshness are difficult; and (5) more extractable oil is obtained from copra than from fresh coconut (Krishna et al. 2010). Therefore, CCO has a dominant position in the circulation and distribution of coconut oil.
However, CCO contains many undesirable components including pigments, gums, waxes, trace metals, and odoriferous volatiles. Their presence has an adverse impact on the taste, smell, appearance, and storage stability of the oil. Therefore, it is necessary to refine CCO to remove unacceptable materials (Elias et al. 2005). However, oil loss and desirable compounds should be minimized to obtain a stable product with a bland or pleasant taste. Generally, the refinement process consists of four successive stages: degumming, neutralization, bleaching, and deodorization (Villarino et al. 2007).
Peroxide value (PV), the free fatty acid (FFA) content, and the composition of fatty acids, fats, and triglycerides are usually used to evaluate the nutritional value and oxidative stability for the oil. Micronutrients (tocopherols and sterols) have some biological activities and nutritional properties, such as anti-inflammatory and antitumor activities, and can lower the levels of plasma cholesterol and low-density lipoprotein (LDL) cholesterol (Piironen et al. 2000; Abu-Fayyad and Nazzal 2017). However, potentially hazardous contaminants—3-3-monochloropropane-1,2-diol esters (3-MCPD esters) and glycidyl esters (GEs)—may be generated during processing, especially in the high-temperature deodorization step (240 °C) (Pudel et al. 2015; Kuhlmann 2015).
The influence of the refinement process on the quality of refined, bleached, and deodorized coconut oil (RBDCO) has rarely been investigated. Moura and Jose revealed that unsaponifiable matter and total sterol content decreased during coconut oil refinement (Moura Fé 1971). Gordon and Rahman investigated the effects of various processing procedures on the indices (the contents of tocopherol, phosphorus, and minor metals) and oxidative stability of coconut oil (Gordon and Rahman 1991). Recently, characteristics and functional effects of VCO compared to RBDCO were studied by Yang et al. (2016) and Harris et al. (2017). To the best of knowledge, fat compositions, triglycerides, micronutrients (tocopherols and sterols), and contaminants (3-MCPD esters and GEs) have not been determined during the refinement process.
The aim of present study was to investigate the effect of each individual step of chemical refinement process on the major and minor components of coconut oil, which included an examination of the effect of chemical refinement on several parameters such as FFA content, fatty acid, fat and triglyceride compositions, and micronutrients (tocopherols and sterols) and contaminants (3-MCPD esters and GEs). Because most minor components are either beneficial or harmful to health, it is important to know which stages of chemical refinement affect the levels of minor components in the oil, to provide a theoretical basis for the moderate refinement of coconut oil.
Materials and methods
Materials
CCO was purchased from Fengyuan Chemical Co. Ltd. (Shenzhen, China). The CCO was processed using middle-scale refinement facilities supplied by Henan Huatai Food & Oil Machinery Engineering Co., Ltd. Samples obtained from each refinement step were allowed to cool to ambient temperature, then stored in darkness at 4 °C prior to analysis.
Thirty-seven standard fatty acid methyl esters, tocopherols (α, β, γ, and δ), β-sitosterol, campesterol, and stigmasterol standards were purchased from Sigma Chemical Co. (St Louis, MO, USA). Triacylglycerol standards consisting of LaLaLa and MMM (La, lauric; M, myristic) and partial triacylglycerols including 2-olein, 1,2-diolein, and 1,3-diolein acylglycerols were obtained from Larodan Fine Chemicals AB (Malmö, Sweden). D5-3-MCPD-1,2-bis-palmitoyl ester (98%), and 3-MCPD-1,2-bis-palmitoyl ester (98%) were purchased from Toronto Research Chemicals Inc. (North York, Canada). 3-MCPD (98%) was purchased from Maya Reagent (Jiaxing, China), and glycidyl stearate (98%) was obtained from Toronto Research Chemicals, Canada. The solvents used in the high-performance liquid chromatography (HPLC) analyses—including methanol, isohexane, ethyl acetate, diethyl ether, n-hexane, and isopropanol—were liquid chromatography grade. Other reagents and solvents were bought from Sinopharm Chemical Reagent (Shanghai, China).
Methods
Refinement process
The CCO refinement process comprised of degumming, neutralization, bleaching, and deodorization are shown as follows.
Degumming CCO was heated to 60 °C. Melted CCO was then thoroughly mixed with 85% phosphoric acid degumming agent (0.2% of oil) at an shaking rate of 50 rpm for 30 min, then allowed to rest for approximately 20 min. Most of gum was removed from the oil by phase separation. The oil was then washed with water by stirring the mixture slowly at 70 °C for 20 min before separation.
Neutralization Degummed oil was poured into a deacidifying tank. The oil was heated to 80 °C, after which a pre-determined amount of 15 Be’ NaOH solution (0.1%, w/w) was added. Initially, the mixture was stirred at 50 rpm for 30 min. Subsequently, the speed was adjusted to 20 rpm for 15 min to complete the saponification of the FFAs before centrifugation. The mixture was then allowed to stand for 30 min to let the aqueous soap stock settle. Oil–soap stock mixture was cooled to room temperature without further shaking. After separation, the oil was washed with distilled water at 60 °C to completely remove the soap, and dried under vacuum at 90 °C for 30 min.
Bleaching Neutralized coconut oil was poured into a bleaching tank. The oil was then heated to 105 °C and bleached with 1% activated clay (w/w). Bleaching was performed under vacuum and the mixture was shaken at 20 rpm. After 30 min, the slurry was allowed to cool (to 50 °C) and filtered through a filter bag using a vacuum pump.
Deodorization Bleached oil was pumped into a middle-scale (10-L) deodorization tower and heated to 230 °C using a thermal oil heater. A live steam was used to evaporate the volatile components and other odoriferous substances under vacuum. The oil spent approximately 90 min in the deodorizer. At the end of the process, we allowed the oil to cool to 40–50 °C before sample was collected.
Peroxide value (PV) and free fatty acid (FFA) content
The PV and FFA were measured according to American Oil Chemists’ Society (AOCS) official method (AOCS, 2009).
Fatty acid composition analysis
The analysis for fatty acid composition was carried out according to AOCS official method (AOCS 2009). Fatty acids were identified by comparing the retention times of the corresponding standards, and the contents of the analytes were presented in terms of relative proportions.
Fat composition
Triacylglycerol, diacylglycerol, and monoacylglycerol levels were determined according to the method described by Zeng et al. (2015). The mobile phase comprised a mixture of n-hexane, 2-propanol, and methanoic acid (15:1:0.003, v/v/v), and the flow rate was 0.8 mL/min. HPLC peaks were evaluated by comparing retention times with those of the reference standards. The results for each acylglycerol species were quoted as peak area percentages and were collected and processed using Waters 2695 integration software.
Triacylglycerol composition
The triacylglycerol composition of each oil sample was determined according to AOCS official method Ce 5c-93 using an Agilent 1200 HPLC system (Cunha and Oliveira 2006). The triacylglycerols were separated on two LiChroCART 18e columns (5 μm, 4.6 mm × 250 mm each; Merck KGaA, Germany) and detected using a refractive index detector. Each 10-μL sample was injected at a concentration of 30 mg/mL. The triacylglycerol groups were identified by comparing the retention times with those of the respective standards.
Micronutrient levels
Tocopherol content
The tocopherol content of each sample was determined using a Waters e2695 Separations Module HPLC system equipped with a Multi λ Fluorescence Detector (Waters 2475, Waters, USA) set at 295 nm. In brief, samples weighting 1 g (0.0001 g) were dissolved in a 10-mL brown volumetric flask. Each 10-μL sample was then injected into the system, and separation was carried out on a silica column (5 μm, 4.6 mm × 250 mm, Hanbon, China) using hexane/isopropanol (98.5/1.5, v/v) as the mobile phase at a flow rate of 1.0 mL/min. The column temperature was 30 °C. The α-, β-, γ-, and δ-tocopherols were identified by comparison with the standards, and the tocopherol contents were reported in mg/kg.
Sterol content
According to the procedures described in our previous studies (Jin et al. 2016), 200 mg of coconut oil was first mixed with 0.5 mL of 0.5 mg/mL 5-α-cholestane and 2 mL of 2 mol/L KOH-CH3CH2OH. The mixture was heated at 85 °C for 1 h, then cooled and mixed with 5 mL of hexane and 2 mL of distilled water three times to extract the unsaponifiable material. The product was dried under nitrogen, silylated with 200 μL of N,O-bis(trimethylsilyl)trifluoroacetamide (BSTFA) + trimethylchlorosilane (TMCS) at 70 °C for 0.5 h, and finally dissolved in 1 mL of hexane. The prepared samples (1 μL) were injected into the gas chromatography system and separated on a DB-5 capillary column (0.25 μm, 30 m × 0.25 mm, Agilent, USA). The column temperature was initially set at 200 °C for 0.5 min, then raised to 300 °C at a rate of 10 °C/min, and maintained for 18 min. The flame ionisation detector (FID) temperature and the injector temperature were both 280 °C. The carrier gas (helium) was supplied at 1 mL/min and the split ratio was 1:100. The ion source and transmission line temperatures were set at 250 and 280 °C, respectively. The ionization mode was electron ionization (EI) and the mass range (m/z) was 50–500. Sterol contents were reported as mg/kg.
Contaminants
AOCS official method Cd 29c-13 was used to determine the contents of 3-MCPD esters and GEs (Zwagerman and Overman 2016).
Statistical analysis
Experiments were conducted in triplicate, and the results are expressed as mean ± standard deviation. Statistical analysis was performed by one-way analysis of variance (ANOVA) using the SPSS Statistics 19.0 software package (SPSS Inc., Chicago, IL, USA). P-values less than 0.05 were considered statistically significant.
Results and discussion
PV, FFA, and fat composition
The changes in PV, FFA, and fat composition during the coconut oil refinement process were shown in Table 1 (Table S1). The CCO had the highest PV index (6.02 meq/kg), which was markedly reduced to 0.24 meq/kg in the deodorized coconut oil. Higher value in CCO resulted from the joint action of enzymes and atmospheric oxygen during the oil extraction process (Elias et al. 2005). The refinement process affected the decrease in the peroxide values compared to the published data (P < 0.05) (Franke et al. 2009). FFA content decreased significantly by 94.5% during the neutralization process. The bleached oil had a higher FFA content compared with the neutralized oil because the bleaching earth was acidic, and some of the residual soap was converted to FFA. Furthermore, the acidic bleaching earth may have catalysed the hydrolysis of the triacylglycerols. It should be noted that the PV increased slightly during bleaching compared with the neutralized oil, possible owing to oxidation catalysed by the acidic earth. Marina et al. (2009) reported low PV (0.21–0.57 meq/kg) and FFA (0.15–0.25%) in VCO. Triacylglycerol (TAG) levels increased during the refinement process, whereas 1,3-diacylglycerol levels decreased, and 1,2(2,3)-diacylglycerol levels remained constant. No monoglycerides were detected in any of the oil samples, and the total content of diacylglycerols was in the range of 3.27–4.56%, which was in agreement with the previously reported results (Dayrit et al. 2011).
Table 1.
Effects of processing on peroxide value and fat compositions of coconut oilsa
| PV | Fat composition (%) | |||||
|---|---|---|---|---|---|---|
| (meg/kg) | TAG | FFA | 1,3-DAG | 1,2(2,3)-DAG | Total DAG | |
| Crude | 6.02 ± 0.22a | 87.15 ± 0.01b | 5.70 ± 0.03a | 3.40 ± 0.25ab | 0.84 ± 0.04a | 4.23 ± 0.28ab |
| Degummed | 5.16 ± 0.14b | 86.27 ± 0.46b | 5.87 ± 0.14a | 3.65 ± 0.15a | 0.92 ± 0.06a | 4.56 ± 0.21a |
| Neutralized | 2.60 ± 0.06d | 94.37 ± 0.22a | 0.32 ± 0.01b | 3.06 ± 0.01b | 0.87 ± 0.11a | 3.92 ± 0.11ab |
| Bleached | 3.52 ± 0.10c | 94.32 ± 0.47a | 0.39 ± 0.02b | 2.87 ± 0.01bc | 0.89 ± 0.01a | 3.75 ± 0.00bc |
| Deodorized | 0.24 ± 0.00e | 95.45 ± 0.06a | 0.12 ± 0.01b | 2.47 ± 0.04c | 0.80 ± 0.06a | 3.27 ± 0.09c |
aMeans with different letters in the same column are significantly different at the 5% level (test of Tukey, P < 0.05)
TAG triacylglycerol, DAG diacylglycerol, FFA free fatty acid, PV peroxide value
Fatty acid composition
The changes to the fatty acid composition during the coconut oil refinement process are shown in Table 2. The fatty acid profiles of the crude, degummed, neutralized, bleached, and deodorized oils were not significantly different from those reported in the literature; lauric acid (C12:0), myristic acid (C14:0), oleic acid (C18:1), and palmitic acid (C16:0) were present in the highest concentrations (Gordon and Rahman 1991; Appaiah et al. 2014). The contents of oleic and linoleic acids decreased markedly over the whole refinement process (P < 0.05); isomerization lowered the relative levels of both acids, and increased the proportion of saturated fatty acids, such as caprylic acid (C8:0), capric acid (C10:0), and stearic acid (C18:0), especially at high temperatures (Hénon et al. 1999; Amaral et al. 2006).
Table 2.
Fatty acid composition of different refinement stages of coconut oilsa
| Crude | Degummed | Neutralized | Bleached | Deodorized | |
|---|---|---|---|---|---|
| C6:0 | 0.39 ± 0.10a | 0.39 ± 0.09a | 0.44 ± 0.01a | 0.46 ± 0.01a | 0.43 ± 0.01a |
| C8:0 | 5.04 ± 0.52a | 4.97 ± 0.46a | 5.47 ± 0.01a | 5.75 ± 0.04a | 5.55 ± 0.09a |
| C10:0 | 4.07 ± 0.14a | 4.04 ± 0.09a | 4.26 ± 0.01a | 4.49 ± 0.03a | 4.49 ± 0.02a |
| C12:0 | 38.11 ± 0.13a | 38.01 ± 0.27a | 38.51 ± 0.20a | 39.79 ± 0.13a | 40.31 ± 0.13a |
| C14:0 | 19.43 ± 0.46a | 19.42 ± 0.35a | 19.10 ± 0.11a | 19.09 ± 0.10a | 19.54 ± 0.28a |
| C16:0 | 13.17 ± 0.27a | 13.25 ± 0.23a | 12.85 ± 0.03a | 12.37 ± 0.05a | 12.50 ± 0.08a |
| C18:0 | 2.50 ± 0.09a | 2.52 ± 0.08a | 2.49 ± 0.01a | 2.54 ± 0.00a | 2.59 ± 0.01a |
| C18:1 | 12.52 ± 0.19b | 12.62 ± 0.25a | 12.29 ± 0.30c | 11.38 ± 0.21d | 10.80 ± 0.20e |
| C18:2 | 4.76 ± 0.01b | 4.78 ± 0.04a | 4.60 ± 0.02c | 4.12 ± 0.01d | 3.79 ± 0.18e |
| ∑SFA | 82.72 ± 0.19d | 82.60 ± 0.28e | 83.12 ± 0.32c | 84.50 ± 0.20b | 85.41 ± 0.36a |
| ∑MUFA | 12.52 ± 0.19a | 12.62 ± 0.25a | 12.29 ± 0.30a | 11.38 ± 0.21a | 10.80 ± 0.20a |
| ∑PUFA | 4.76 ± 0.01a | 4.78 ± 0.04a | 4.60 ± 0.02a | 4.12 ± 0.01a | 3.79 ± 0.18a |
aValues followed by different letters in the same row differed by at least 5% significance (test of Tukey, P < 0.05)
Triacylglycerol composition
Effects of chemical refinement on the triacylglycerol composition were further analysed. As illustrated in Table 3, the major TAGs in the coconut oils were LaLaM, LaLaP, LaLaLa, CpLaLa, and LaLaO. They accounted for approximately 53.33–59.61% of the total TAG content. Refinement process did not significantly change the content of CyLaLa, LaLaLa, LaLaM, and LaLaO (P > 0.05). There were minor significant differences among the percentages of di-unsaturated and tri-saturated triacylglycerols (Di-UTAG and Tri-TAG) in all the oil samples. CCO had a higher total Mono-UTAG content than the oils sampled at other stages of refinement. For triacylglycerol profiles, Di-UTAG and Tri-STAG remained constant throughout the whole refinement process.
Table 3.
Triacylglycerol compositions of different refining stages of coconut oilsab
| Triacylglycerols (%)b | Crude | Degummed | Neutralized | Bleached | Deodorized |
|---|---|---|---|---|---|
| CLaLa | 1.13 ± 0.12a | 1.02 ± 0.06a | 1.20 ± 0.14a | 1.27 ± 0.08a | 1.02 ± 0.24a |
| CyLaLa | 7.03 ± 0.05a | 6.75 ± 0.23a | 7.49 ± 0.20a | 8.14 ± 0.16a | 7.42 ± 0.22a |
| CCyO | 0.88 ± 0.07a | 0.90 ± 0.24a | 0.85 ± 0.03a | 0.77 ± 0.14a | 0.80 ± 0.22a |
| CpLaLa | 11.34 ± 0.11a | 10.89 ± 0.30a | 11.81 ± 0.00a | 12.38 ± 0.28a | 11.50 ± 0.30a |
| LaLaLa | 10.55 ± 0.14a | 11.63 ± 0.76a | 11.12 ± 0.29a | 12.11 ± 1.06a | 12.43 ± 0.63a |
| CyCpO | 4.90 ± 0.08a | 3.65 ± 0.94a | 4.66 ± 0.31a | 4.06 ± 0.87a | 3.60 ± 0.48a |
| LaLaM | 14.23 ± 1.09a | 15.05 ± 0.03a | 14.19 ± 1.08a | 14.14 ± 1.52a | 14.87 ± 1.45a |
| CyLaO | 1.64 ± 0.03a | 1.56 ± 0.01a | 1.66 ± 0.03a | 1.65 ± 0.02a | 1.40 ± 0.05a |
| LaLaP | 11.43 ± 0.37a | 11.84 ± 0.09a | 11.27 ± 0.09a | 11.12 ± 0.03a | 12.00 ± 0.02a |
| CpLaO | 1.36 ± 0.05a | 1.38 ± 0.07a | 1.37 ± 0.02a | 1.33 ± 0.09a | 1.24 ± 0.03a |
| LaLaO | 6.68 ± 0.08a | 7.15 ± 0.08a | 6.61 ± 0.01a | 6.43 ± 0.01a | 7.09 ± 0.03a |
| LaLaL | 2.80 ± 0.36a | 2.69 ± 0.18a | 2.86 ± 0.28a | 2.73 ± 0.40a | 2.65 ± 0.24a |
| LaPS | 2.86 ± 0.10a | 3.14 ± 0.07a | 2.70 ± 0.12a | 2.72 ± 0.05a | 3.27 ± 0.12a |
| LaMO | 2.76 ± 0.00a | 2.63 ± 0.13a | 2.65 ± 0.02a | 2.49 ± 0.10a | 2.51 ± 0.06a |
| LaML | 1.06 ± 0.02a | 1.07 ± 0.02a | 0.95 ± 0.02a | 0.96 ± 0.06a | 0.98 ± 0.03a |
| LaPO | 2.71 ± 0.01a | 1.03 ± 0.03a | 0.98 ± 0.03a | 0.88 ± 0.06a | 0.70 ± 0.09a |
| MOP | 1.58 ± 0.01a | 1.55 ± 0.07a | 1.51 ± 0.06a | 1.37 ± 0.09a | 1.50 ± 0.14a |
| MLP | 1.55 ± 0.00a | 1.56 ± 0.03a | 1.50 ± 0.01a | 1.32 ± 0.08a | 1.51 ± 0.39a |
| PPS | 1.07 ± 0.04a | 1.06 ± 0.09a | 1.11 ± 0.05a | 1.11 ± 0.15a | 0.85 ± 0.04a |
| POP | 1.18 ± 0.15a | 1.18 ± 0.04a | 1.19 ± 0.03a | 1.17 ± 0.14a | 0.73 ± 0.17a |
| POS | 1.05 ± 0.24a | 0.97 ± 0.00a | 0.91 ± 0.09a | 0.97 ± 0.35a | 1.01 ± 0.13a |
| POO | 0.86 ± 0.10a | 0.96 ± 0.00a | 0.96 ± 0.08a | 0.83 ± 0.11a | 0.77 ± 0.07a |
| Mono-UTAG | 29.12 ± 2.00a | 27.30 ± 0.57b | 27.27 ± 0.54b | 27.69 ± 0.05b | 27.3 ± 0.61b |
| Di-UTAG | 0.88 ± 0.12a | 0.96 ± 0.00a | 0.99 ± 0.03a | 0.96 ± 0.08a | 0.90 ± 0.00a |
| Tri-STAG | 60.79 ± 0.44a | 61.38 ± 0.39a | 60.93 ± 1.02a | 60.87 ± 0.93a | 61.26 ± 0.38a |
aValues followed by an identical letter in the same row did not differ beyond a 5% significance (test of Tukey, P < 0.05)
bTri-STAG trisaturated triacylglycerols, Di-UTAG diunsaturated triacylglycerols, Mono-UTAG monounsaturated triacylglycerol, C caproic, Cy caprylic, Cp capric, La lauric, M myristic, P palmitic, S stearic, O oleic, L linoleic
Micronutrient levels
Tocopherols
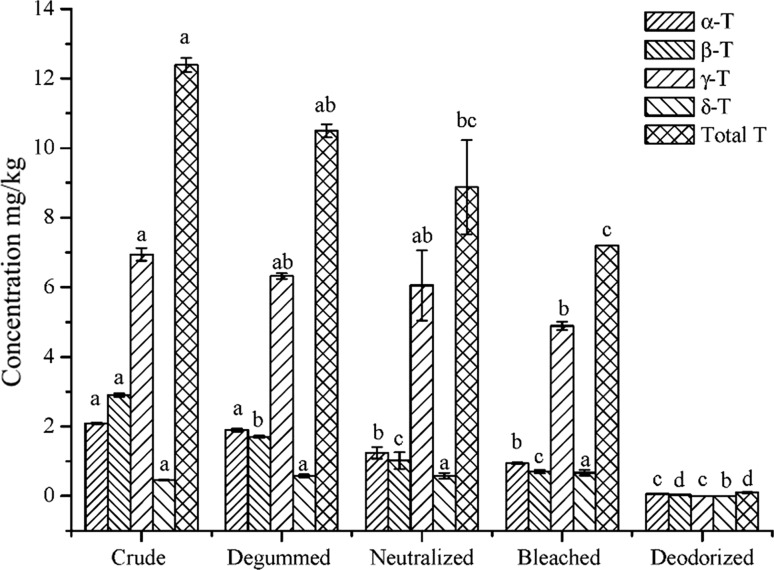
The changes in the contents of total tocopherol and tocopherol species during coconut oil processing are presented in Fig. 1. CCO contained 12.39 mg/kg total tocopherols, and γ-tocopherol (6.94 mg/kg) accounted for the majority (56%). γ-Tocopherol has also been reported to be dominant (Appaiah et al. 2014), contrary to the result reported by Kumar and Krishna (2015), in which α-tocopherol was dominant. Different varieties of coconut might be responsible for these discrepancies. There was a continuously falling trend in the contents of total tocopherols in the degummed (10.5 mg/kg), neutralized (8.8 mg/kg), bleached (7.2 mg/kg), and deodorized (0.1 mg/kg) oils. The results revealed that the contents of total tocopherol and tocopherol species declined significantly (P < 0.05) during the refinement process. In the early stages of refinement, tocopherols were only slightly affected. However, the contents of total tocopherols and isomers was markedly affected by deodorization as a result of distillation. We observed a marked reduction in the levels of α-, β-, γ-, and δ-tocopherol (≦ 0.1 mg/kg) at the deodorization stage. Throughout the refinement process, the relative contents of α- and β-tocopherols decreased (from 16.8 to 13.1%, and from 23.4 to 9.7%, respectively). The tocopherol composition influenced by the refinement process. Van Hoed et al. (2006) reported that throughout the refinement process, the relative contents of γ- and δ-tocotrienols decreased, whereas the α-tocopherol and α-tocotrienol contents increased, and the γ-tocopherol content remained constant. The discrepancies are probably due to differences in the refinement conditions and the various kinds of oil. Mansor et al. (2012) and Appaiah et al. (2014) reported that VCO contained 0.09–69.4 mg/kg of total tocopherols. In present study, the total tocopherol content of RBDCO was close to the lower reported limit for VCO.
Fig. 1.
Concentration of tocopherols in different processing steps (mg/kg). Different letters (a–d) indicated significant differences between concentrations of tocopherol content in the studied rice bran oils (test of Tukey, P < 0.05)
Sterols
The changes to the total sterol and individual sterol contents during coconut oil processing are shown in Fig. 2. The total sterol content in CCO was 679.69 mg/kg, and β-sitosterol was the most abundant sterol (440.61 mg/kg), followed by stigmasterol (161.16 mg/kg), and campesterol (77.92 mg/kg). The relative proportions of the individual sterols did not change during the refinement process, in agreement with the results reported by Ferrari et al. (1996). This result was in contrasted with that reported by Moura Fé (1971), who found that there was a proportional decrease in campesterol during refinement, whereas the levels of stigmasterol and β-sitosterol increased. The total sterol content decreased by 37% in the fully refined coconut oil, compared to the CCO. The contents of β-sitosterol, stigmasterol, and campesterol in the fully refined coconut oil decreased by 35, 42, and 39%, respectively, compared to the CCO. The contents of total and individual sterols declined markedly (P < 0.05) as refinement progressed, consistent with the results reported by Van Hoed et al. (2019). The sterol content was markedly reduced by the neutralization process, which probably formed micelles with soaps that were then transferred into the soap stock (Kochhar 1983), consistent with the results reported by Xie (2012) and Verleyen et al. (2002). Both Marina et al. (2009) and Appaiah et al. (2014) have reported average total sterols contents in the range of 306.6–960 mg/kg in VCO samples. The RBDCO in present study contained similar levels of sterols.
Fig. 2.
Concentration of sterols during different processing steps (mg/kg). Different letters (a–c) indicated significant differences between concentrations of sterol content in the studied rice bran oils (test of Tukey, P < 0.05)
Contaminants
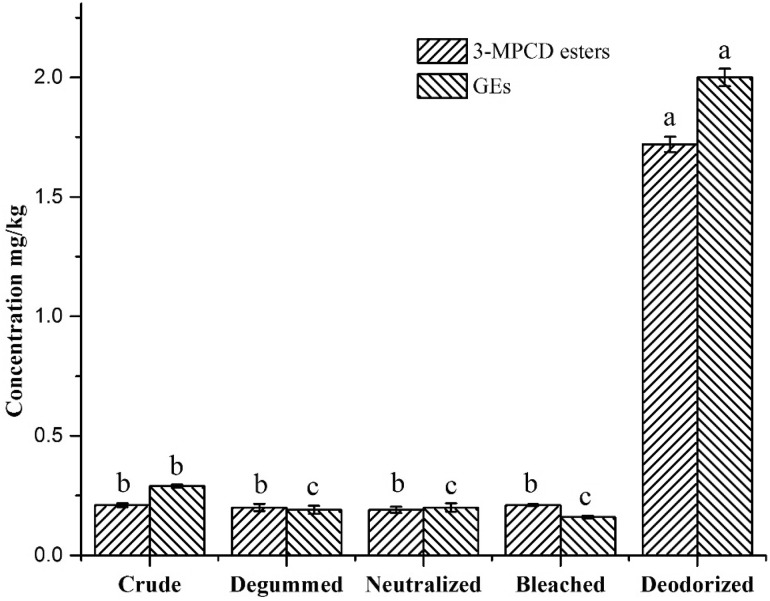
Figure 3 illustrated the changes in the levels of 3-MCPD esters and GEs in the sample oils during the refinement process. The levels of 3-MCPD esters and GEs in the CCO were 0.21 mg/kg and 0.29 mg/kg, respectively. Approximately same levels of 3-MCPD esters (0.19–0.21 mg/kg) in the degummed, neutralized, and bleached oils were detected. The reduction in GEs content in the bleaching step may have resulted from the use of activated clay, which can effectively absorb harmful substances in edible oil (Ouyang et al. 2014). There were marked increases in the contents of 3-MCPD esters and GEs (719.1% and 596.6%, respectively) compared to the CCO. 3-MCPD esters and GEs are the main contaminants formed during the deodorization step when vegetable oils are refined, as previously observed by Pudel et al. (2015), Becalski et al. (2015) and Cheng et al. (2017). To the best of our knowledge, the present paper is the first report of changes in the levels of both contaminants during the refinement of coconut oil.
Fig. 3.
Concentration of contaminants during different processing steps (mg/kg). Different letters (a–c) indicated significant differences between concentrations of 3-MCPD esters and GE content in the studied rice bran oils (test of Tukey, P < 0.05)
Conclusion
PV and contents of FFA, diacylglycerol, and unsaponifiable material (including tocopherols and sterols) declined markedly during the refinement process, whereas the levels of triacylglycerol and contaminants (3-MCPD esters and GEs) increased significantly. Triacylglycerol composition changed little throughout the whole refinement process. The main loss of tocopherols and the increase in the levels of 3-MCPD esters and GEs occurred in the deodorization step. The levels of all the sterol components decreased markedly during neutralization. Therefore, measures should be taken to maximize the retention of beneficial micronutrients and minimize the generation of contaminants by focusing on the neutralization and deodorization process parameters (e.g., NaOH solution concentration, duration of treatment, and temperature). This would facilitate the future production of high-quality RBDCO for human consumption.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant No. 31872895), the China Postdoctoral Science Foundation (No. 2017M621635), the Fund of “Shanghai Technological Innovation Action Plan: Technical standard project (2018 No. 18DZ2200300) and the national first-class discipline program of Food Science and Technology (JUFSTR20180202).
Compliance with ethical standards
Conflict of interest
No conflict of interest exits in the submission of this manuscript, and manuscript is approved by all authors for publication.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Ming Chang, Phone: +86 510-85876799, Email: chang@jiangnan.edu.cn.
Xingguo Wang, Email: wangxg1002@gmail.com.
References
- Abu-Fayyad A, Nazzal S. Gemcitabine-vitamin E conjugates: synthesis, characterization, entrapment into nanoemulsions, and in vitro deamination and antitumor activity. Int J Pharm. 2017;528:463–470. doi: 10.1016/j.ijpharm.2017.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaral JS, Casal S, Seabra RM, Oliveira BP. Effects of roasting on hazelnut lipids. J Agric Food Chem. 2006;54:1315–1321. doi: 10.1021/jf052287v. [DOI] [PubMed] [Google Scholar]
- AOCS . Official methods and recommended practices of the AOCS. Champaign: AOCS Press; 2009. [Google Scholar]
- Appaiah P, Sunil L, Kumar PP, Krishna AG. Composition of coconut testa, coconut kernel and its oil. J Am Oil Chem Soc. 2014;91:917–924. doi: 10.1007/s11746-014-2447-9. [DOI] [Google Scholar]
- Becalski A, Feng S, Lau PY, Zhao T. A pilot survey of 2- and 3-monochloropropanediol and glycidol fatty acid esters in foods on the Canadian market 2011–2013. J Food Compos Anal. 2015;37:58–66. doi: 10.1016/j.jfca.2014.09.002. [DOI] [Google Scholar]
- Cheng WW, Liu GQ, Wang LQ, Liu ZS. Glycidyl fatty acid esters in refined edible oils: a review on formation, occurrence, analysis, and elimination methods. Compr Rev Food Sci Food Saf. 2017;16:263–281. doi: 10.1111/1541-4337.12251. [DOI] [PubMed] [Google Scholar]
- Cunha SC, Oliveira MBPP. Discrimination of vegetable oils by triacylglycerols evaluation of profile using HPLC/ELSD. Food Chem. 2006;95:518–524. doi: 10.1016/j.foodchem.2005.03.029. [DOI] [Google Scholar]
- Dayrit FM, Dimzon IKD, Valde MF, Santos JER, Garrovillas MJM, Villarino BJ. Quality characteristics of virgin coconut oil: comparisons with refined coconut oil. Pure Appl Chem. 2011;83:1789–1799. doi: 10.1351/PAC-CON-11-04-01. [DOI] [Google Scholar]
- Elias CC, Yvonne TVA, Evangekube AM, Economico P, Jr., María Luz JB, et al. Coconut oil. In: Shahidi F, et al., editors. Bailey’s industrial oils and fats products. 6. Hoboken: Wiley; 2005. [Google Scholar]
- Ferrari RA, Schulte E, Esteves W, Brühl L, Mukherjee K. Minor constituents of vegetable oils during industrial processing. J Am Oil Chem Soc. 1996;73:587–592. doi: 10.1007/BF02518112. [DOI] [Google Scholar]
- Franke K, Strijowski U, Fleck G, Pudel F. Influence of chemical refining process and oil type on bound 3-chloro-1, 2-propanediol contents in palm oil and rapeseed oil. LWT Food Sci Technol. 2009;42:1751–1754. doi: 10.1016/j.lwt.2009.05.021. [DOI] [Google Scholar]
- Gordon MH, Rahman IA. Effect of processing on the composition and oxidative stability of coconut oil. J Am Oil Chem Soc. 1991;68:574–576. doi: 10.1007/BF02660153. [DOI] [Google Scholar]
- Harris M, Hutchins A, Fryda L. The impact of virgin coconut oil and high-oleic safflower oil on body composition, lipids, and inflammatory markers in postmenopausal women. J Med Food. 2017;20:345–351. doi: 10.1089/jmf.2016.0114. [DOI] [PubMed] [Google Scholar]
- Hénon G, Kemény Z, Recseg K, Zwobada F, Kovari K. Deodorization of vegetable oils. Part I: modelling the geometrical isomerization of polyunsaturated fatty acids. J Am Oil Chem Soc. 1999;76:73–81. doi: 10.1007/s11746-999-0050-2. [DOI] [Google Scholar]
- Jin J, Warda P, Mu H, Zhang Y, Jie L, Mao J, Xie D, Huang J, Jin Q, Wang X. Characteristics of mango kernel fats extracted from 11 China-specific varieties and their typically fractionated fractions. J Am Oil Chem Soc. 2016;93:1115–1125. doi: 10.1007/s11746-016-2853-2. [DOI] [Google Scholar]
- Kochhar S. Influence of processing on sterols of edible vegetable oils. Prog Lipid Res. 1983;22:161–188. doi: 10.1016/0163-7827(83)90008-5. [DOI] [PubMed] [Google Scholar]
- Krishna AG, Gaurav R, Singh BA, Kumar PP, Preeti C. Coconut oil: chemistry, production and its applications-a review. Indian Coconut J. 2010;53:15–27. [Google Scholar]
- Kuhlmann J. Determination of bound 2,3-epoxy-1-propanol (glycidol) and bound monochloropropanediol (MCPD) in refined oils. Eur J Lipid Sci Technol. 2015;113:335–344. doi: 10.1002/ejlt.201000313. [DOI] [Google Scholar]
- Kumar PP, Krishna AG. Physicochemical characteristics of commercial coconut oils produced in India. Grasas Aceites. 2015;66:e062. doi: 10.3989/gya.0228141. [DOI] [Google Scholar]
- Mansor TST, Man YBC, Shuhaimi M, Abdul Afiq MJ, Ku Nurul FKM. Physicochemical properties of virgin coconut oil extracted from different processing methods. Food Res Int. 2012;19:837–845. [Google Scholar]
- Marina A, Man YC, Nazimah S, Amin I. Chemical properties of virgin coconut oil. J Am Oil Chem Soc. 2009;86:301–307. doi: 10.1007/s11746-009-1351-1. [DOI] [Google Scholar]
- Moura Fé J (1971) Changes in some components of the unsaponifiable fraction of coconut oil during refining. University of Arizona. Accessed May 2013. http://arizona.openrepository.com/arizona/bitstream/10150/287783/4/azu_td_7201709_sip1_w.pdf
- Nathanael W. Some aspects of copra deterioration. Ceylon Coconut Q. 1965;16:111–120. [Google Scholar]
- Nitbani FO, Jumina SD, Solikhah EN. Isolation and antibacterial activity test of lauric acid from crude coconut oil (Cocos nucifera L.) Procedia Chem. 2016;18:132–140. doi: 10.1016/j.proche.2016.01.021. [DOI] [Google Scholar]
- Oilseeds, WMT [world markets and trade] (2017). United States Department of Agriculture. https://apps.fas.usda.gov/psdonline/circulars/oilseeds.pdf
- Ouyang J, Hu Z, Wang W, Sun M, Zhou E, Feng W, Qi Y, Zhang W. Effects of different refining processes and conditions on 3-MCPD content. Cereals Oils Process. 2014;10(35–38):42. [Google Scholar]
- Piironen V, Lindsay DG, Miettinen TA, Toivo J, Lampi AM. Plant sterols: biosynthesis, biological function and their importance to human nutrition. J Sci Food Agric. 2000;80:939–966. doi: 10.1002/(SICI)1097-0010(20000515)80:7<939::AID-JSFA644>3.0.CO;2-C. [DOI] [Google Scholar]
- Pudel F, Benecke P, Fehling P, Freudenstein A, Matthäus B, Schwaf A. On the necessity of edible oil refining and possible sources of 3-MCPD and glycidyl esters. Eur J Lipid Sci Technol. 2015;113:368–373. doi: 10.1002/ejlt.201000460. [DOI] [Google Scholar]
- Van Hoed V, Depaemelaere G, Ayala JV, Santiwattana P, Verhé R, De Greyt W. Influence of chemical refining on the major and minor components of rice brain oil. J Am Oil Chem Soc. 2006;83:315–321. doi: 10.1007/s11746-006-1206-y. [DOI] [Google Scholar]
- Verleyen T, Sosinska U, Ioannidou S, Verhé R, Dewettinck K, Huyghebaert A, De Greyt W. Influence of the vegetable oil refining process on free and esterified sterols. J Am Oil Chem Soc. 2002;79:947–953. doi: 10.1007/s11746-002-0585-4. [DOI] [Google Scholar]
- Villarino BJ, Dy LM, Lizada MCC. Descriptive sensory evaluation of virgin coconut oil and refined, bleached and deodorized coconut oil. LWT Food Sci Technol. 2007;40:193–199. doi: 10.1016/j.lwt.2005.11.007. [DOI] [Google Scholar]
- Xie D. Effect of refining and storage on quality of rapeseed oil (master’s thesis) Wuxi: Jiangnan University; 2012. [Google Scholar]
- Yang S, Ningrat R, Eun J, Min B. Effects of supplemental virgin coconut oil and condensed tannin extract from pine bark in lactation dairy diets on ruminal fermentation in a dual-flow continuous culture system. Adv Dairy Res. 2016;4:1–6. doi: 10.4172/2329-888X.C1.002. [DOI] [Google Scholar]
- Zeng CX, Qi SJ, Xin RP, Yang B, Wang YH. Enzymatic selective synthesis of 1,3-DAG based on deep eutectic solvent acting as substrate and solvent. Bioprocess Biosyst Eng. 2015;38:2053–2061. doi: 10.1007/s00449-015-1445-0. [DOI] [PubMed] [Google Scholar]
- Zwagerman R, Overman P. A novel method for the automatic sample preparation and analysis of 3-MCPD-, 2-MCPD-, and glycidyl-esters in edible oils and fats. Eur J Lipid Sci Technol. 2016;118:997–1006. doi: 10.1002/ejlt.201500358. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.