Abstact
Background:
In recent years, researchers discovered that menstrual blood-derived stem cells (MenSCs) have the potential to differentiate into a wide range of tissues including the chondrogenic lineage. In this study, we aimed to investigate the effect of MenSCs encapsulated in fibrin glue (FG) on healing of osteochondral defect in rabbit model.
Methods:
We examined the effectiveness of MenSCs encapsulated in FG in comparison with FG alone in the repair of osteochondral defect (OCD) lesions of rabbit knees after 12 and 24 weeks.
Results:
Macroscopical evaluation revealed that the effectiveness of MenSCs incorporation with FG is much higher than FG alone in repair of OCD defects. Indeed, histopathological evaluation of FG + MenSCs group at 12 weeks post-transplantation demonstrated that defects were filled with hyaline cartilage-like tissue with proper integration, high content of glycosaminoglycan and the existence of collagen fibers especially collagen type II, as well as by passing time (24 weeks post-transplantation), the most regenerated tissue in FG + MenSCs group was similar to hyaline cartilage with relatively good infill and integration. As the same with the result of 12 weeks post-implantation, the total point of microscopical examination in FG + MenSCs group was higher than other experimental groups, however, no significant difference was detected between groups at 24 weeks (p > 0.05).
Conclusion:
In summary, MenSCs as unique stem cell population, is suitable for in vivo repair of OCD defects and promising for the future clinical application.
Keywords: Menstrual blood stem cells, Fibrin glue, Osteochondral defect, Rabbit knee
Introduction
Articular cartilage has limited capacity to self-repair caused by joint injury, ageing, and developmental disorders as a result of the tissue avascularity along with the low mitotic activity of the chondrocytes [1–4]. The poor regenerative properties of this tissue have remained one of the most challenging for orthopedic studies. To date, the field of cartilage tissue engineering has ushered in new methodologies for the treatment of cartilage defects for eliminating the drawbacks associated with conventional treatments like autografts and allografts, microfracture, debridement, and total and partial joint replacements [5]. Up to now, significant progress has been achieved in the subject of cartilage repair using chondrocyte-based therapy [6–8]. However, this technique still has some limitations. For example, the dedifferentiation of patients’ chondrocytes during in vitro culture, and decreased human chondrocyte number or cellularity with aging may impair and/or even result in the repair failure [2]. On the other hand, other chondrocyte sources such as allogeneic or xenogeneic chondrocytes can potentially induce immune responses and transmit diseases [2]. Therefore, there is much effort to explore better alternative cell sources such as stem cells that could be used effectively to overcome the limitations of the native chondrocytes.
In recent studies, menstrual blood has been identified as an easily accessible, replenishable, and renewable source of stem cells with properties similar to mesenchymal stem cells [9–14]. Other characteristics such as the high proliferation rate, immunomodulatory activity, no legal considerations associated with their collection, lack of ethical concerns and restrictions, the appropriated capacity of in vitro expansion and self-renewal in culture, absence of teratogenic potency, lack of karyotypic abnormalities, broad differentiation potential, and high tolerating cryopreservation have made menstrual blood stem cells (MenSCs) as an appropriate stem cell resource for tissue engineering and regenerative medicine [9–13, 15].
With the aim of tissue engineering and regenerative medicine using MenSCs, different lineages like osteoblast [9], adipocyte [16], hepatocyte [10, 17, 18], cardiomyocyte [19–21], neural lineage [22, 23] from these cells have been established. Moreover, differentiation potential of MenSCs into chondrogenic lineage is reported by a few studies. Patel et al. showed chondrogenic differentiation of MenSCs by demonstration of sulfated proteoglycans development using alcian blue staining [13]. Furthermore, we indicated that in vitro MenSCs differentiation in scaffold enhances chondrogenic commitment compared to two-dimensional (2D) culture system [11]. Despite differentiation potential of MenSCs into the chondrogenic lineage, there is no report about the effectiveness of MenSCs in the repair of osteochondral defects in animal models and so, for the first time, we studied the potency of MenSCs in restoring of osteochondral defects in a rabbit model. For better cell delivery, MenSCs were encapsulated in fibrin glue (FG) as a carrier. Fibrin glue as a tissue-derived natural material, could be utilised to create new functional structures for cartilage regeneration [24]. It is a suitable matrix for attachment, proliferation, and biosynthesis of cartilaginous matrix components [25]. Moreover, FG has a positive effect on differentiation ability of stem cells and production of collagen II and proteoglycan depends on stem cell type [26].
A few studies are reporting in vivo regeneration of osteochondral defects using FG. Johnson et al. [27] demonstrated that articular, auricular, and costal chondrocytes in FG could produce the new cartilaginous matrix similar to native cartilage in nude mice. Other studies revealed that FG is a suitable polymer for the formation of injectable tissue-engineered cartilage in the nude mouse and rabbit for the regeneration of hyaline articular cartilage [28, 29]. Based on this knowledge, we examined the effectiveness of MenSCs encapsulated in FG in comparison with FG alone in the repair of osteochondral defects of rabbit knees. The success of these studies may have significant clinical implication for patients in future.
Materials and methods
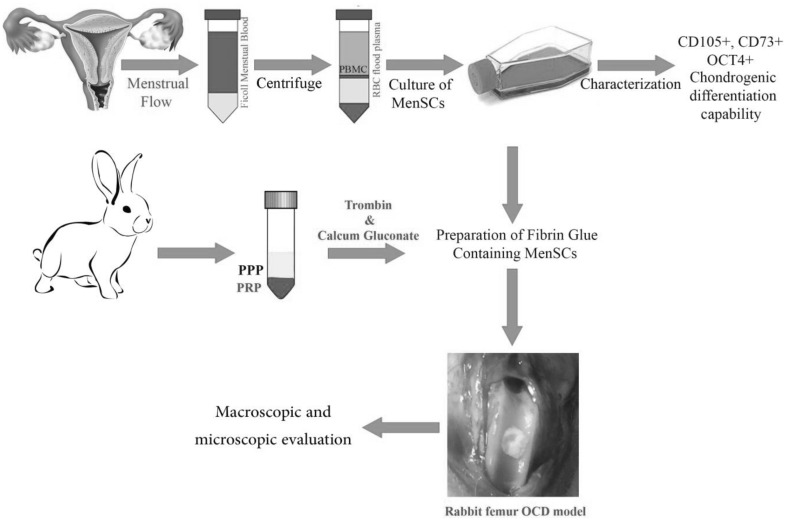
Figure 1 indicates a schematic diagram of methodology road map. The detail protocols of each part are as following.
Fig. 1.
The schematic diagram of MenSCs isolation, characterization, encapsulation in fibrin glue and transplantation to osteochondral defect (OCD) in rabbit model
Isolation and culture of MenSCs
Menstrual blood was collected from five healthy, non-smoking, female volunteers with mean age 28 years (25–45 years) using a Diva cup (Diva International Co., Lunette, Finland) during the first menstrual blood flow initiated. All participants in the study were evaluated underwent a standard medical history and physical examination. HIV, viral hepatitis, and papillomavirus positive donors were excluded from this study. Also, all volunteers in the study gave consent to use of the material for research purposes and signed an informed consent form approved by Medical Ethics Committee of Avicenna Research Institute (IACUC no. IR.ACECR.Avicenna.REC.1395.14). The contents of Diva cup were transferred into the collection tube containing 2.5 µg/mL fungizone (Gibco, Scotland, UK), 100 µg/mL streptomycin, 100 U/mL penicillin and 0.5 mM EDTA (all from Sigma-Aldrich, St. Louis, MO, USA) in phosphate buffered saline (PBS) without Ca2+ or Mg2+. The isolation and culture of stem cells from menstrual blood was carried out according to our previous procedure [9]. In brief, the mononuclear cells were separated from menstrual blood under standard conditions in ficoll gradient. The obtained cell pellet was cultivated in complete Dulbecco’s Modified Eagle’s Medium-F12 (DMEM-F12) (Sigma-Aldrich, St. Louis, MO, USA) at 37 °C in a humidified 5% CO2 incubator. Following a couple of days of incubation, the non-adherent cells were rinsed remaining the adherent cell population that was raising as fibroblastic cells in colonies. Culture medium was changed every 3–4 days to allow growing up adherent cells with mesenchymal morphology. The cultured cells at passages 2–4 were characterized and used for further experiments.
Preparation of fibrin glue containing MenSCs
Five mL of autologous blood was drawn from each rabbit and was combined with 0.5 mL of anticoagulant acid citrate solution (4%) (Merck KGaA, Darmstadt, Hesse, Germany) to prevent coagulation. The blood was first centrifuged at 200 g for 20 min at ambient temperature to separate the plasma containing the platelets from the red cells and buffy coat. In the next step, plasma containing the platelets were transferred to new tubes and was centrifuged again at 3000 g for 15 min at ambient temperature to produce a precipitate containing fibrinogen and a supernatant containing the thrombin. The precipitate is resuspended in a small volume of the supernatant and used as the fibrinogen component. The fibrinogen component was aliquoted to two tubes (500 µL in each tube). The first tube that was considered as main sample (fibrin glue containing MenSCs) was blended by 700 × 103 undifferentiated MenSCs suspended in100 µL serum free-culture medium. To another tube, 100 µL culture medium without stem cells was added. After that, 50 µL of calcium gluconate (100 mg/mL) and 20 µL of thrombin solution (500 IU/mL) was added to each tube to convert residual fibrinogen to dense fibrin network. Three minutes later, a flexible gel was formed in both tubes. The fibrin glue was prepared according to the described protocol previously with a minor modification [30].
Animals
24 New Zealand rabbits (female, 6–8 months old, weight ranging from 2 to 3.2 kg) were provided from Pasteur Research Institute (Tehran, Iran) and housed under standard conditions (temperature: 21 ± 1 °C; humidity: 55–60%) with food and water continuously available.
Surgical procedure and in vivo creation of osteochondral defects and scaffold implantation
The 24 rabbits were randomly assigned to two groups; 12 rabbits were considered as the treatment group, and 12 rabbits were assigned in control group. All animals underwent right stifle arthrotomies under general anaesthesia induced by of intramuscular injection of ketamine (35 mg/kg) and xylazine (5 mg/kg). Under sterile condition, a cylindrical osteochondral defect (3 × 4 mm) was made in the central flattened region of the trochlear groove of both stifles, 1 cm distal to the fused distal femoral growth plate, using a pneumatic drill under saline irrigation. In the treatment group, MenSCs encapsulated in fibrin glue, and fibrin glue alone was implanted in osteochondral lesions of right and left stifle respectively. In the control group, untreated defect as a negative control was created in left stifle. Enrofloxacin 5% (10 mg/kg) and Tramadol (2 mg/kg) were injected into each animal for 5 days after surgery. In the postoperative period, the animals were maintained in cages with standard postoperative care. During this period, the motion of the knee was not restricted, and full weight-bearing on the surgical joints was allowed. All physical exams of the animals including weighting gain or loss, nutritional status, physical activity, and the possibility of infection or inflammation post operationally were weekly registered in a designed table.
Gross morphological and histological evaluations of treated osteochondral defects samples
At 3 and 6 months after implantation, animals from each group were clinically examined, and then the animals were sacrificed, and knee joints were examined by gross observation and scored with Goebel method [31]. Then the tissue samples were collected and fixed with 10% neutral buffered formalin for 18 h at room temperature (RT) and then decalcified with 10% ethylenediaminetetraacetic acid (EDTA) (Merck KGaA, Darmstadt, Hesse, Germany) for 8 weeks and subsequently, were dehydrated, embedded in paraffin and cut into consecutive 4-mm-thick sections in the sagittal plane. These sections were routinely stained with hematoxylin and eosin, alcian blue, and Masson’s trichrome.
Additional sections were processed for type II collagen immunohistochemical evaluation. For immunohistochemical staining, the paraffin sections firstly were deparaffinized, rehydrated and were washed in PBS before immunostaining and then for antigen retrieval with enzymatic treatment, sections were incubated by 0.25% trypsin for 30 min at RT and 2.5% hyaluronidase (Sigma Aldrich, MO, USA) for 30 min at 37 °C. The slides were subsequently blocked with 20% sheep serum in 1% PBS-BSA for 30 min at RT. Endogenous peroxidase activity was blocked using 0.3% hydrogen peroxide. Samples were incubated with the primary antibody against type II collagen (Mouse monoclonal anti-collagen II; 1:100; Millipore: Billerica, MA, USA), and osteocalcin (Mouse monoclonal anti-octeocalcin (OC4-30) (ab13418); 1:100; Abcam, Cambridge, MA, USA) for 60 min at RT and overnight respectively. Then, the slides were incubated with peroxidase-conjugated secondary antibodies (REAL™ EnVision™/HRP, Rabbit/Mouse Kit, Dako; Hamburg, Germany) for 30 min at RT. Nuclei were counterstained with hematoxylin. Finally, the slides were visualised using an Olympus BX51 microscope and examined by a veterinary anatomical pathologist and then graded using a histologic scale of Mainil-Varlet et al. [32].
Statistical analysis
Differences between multiple groups at the one-time point and between two-time points in each group were statistically analysed using Mann–Whitney Nonparametric Test. P value < 0.05 was considered statistically significant. Statistics were performed using SPSS Software (IBM Corp, version 22, Belmont, CA, USA).
Results
Macroscopical evaluation
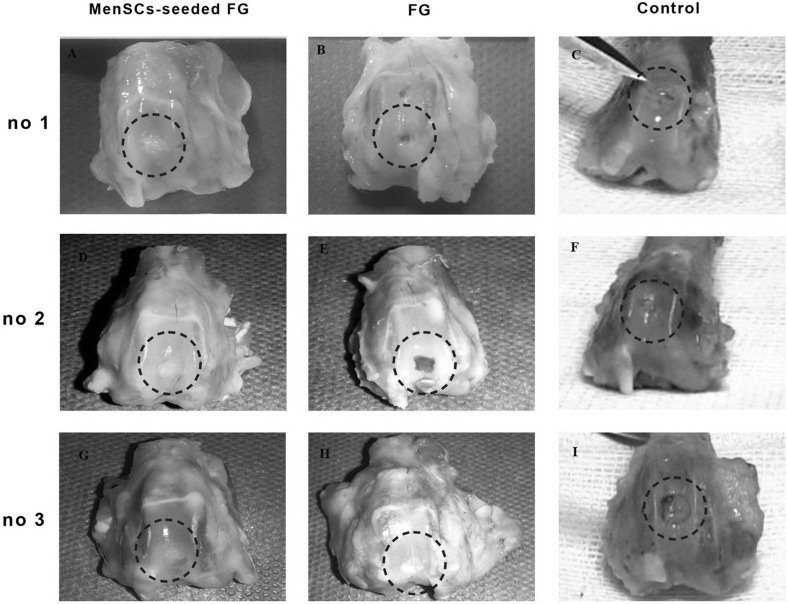
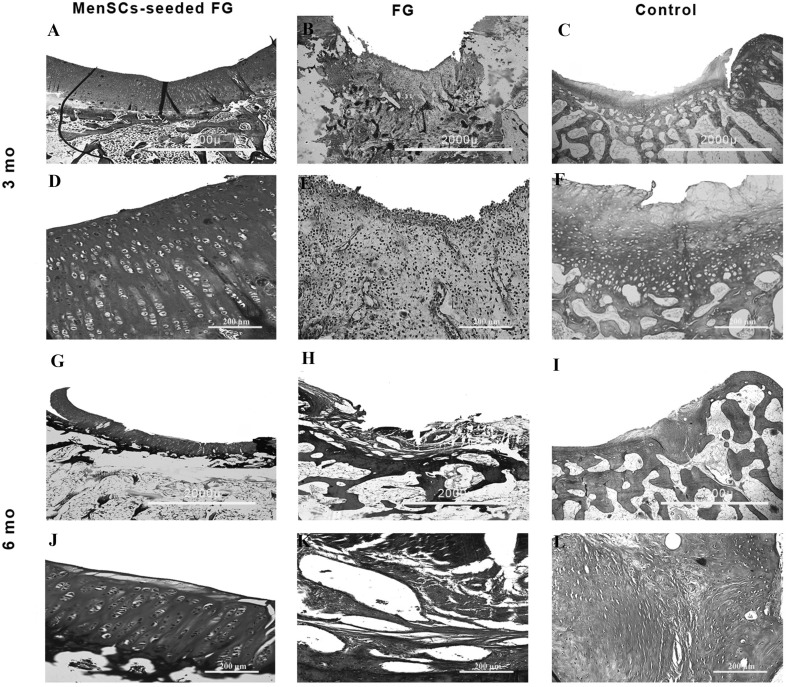
Macroscopic evaluation of the grafted defects with FG at 3 months after transplantation showed that the most defects were filled with a predominantly translucent, heterogeneous repaired tissue with relatively distinct borders. Some surfaces were still concave. In one case, the cancellous bone was exposed. Diffuse degenerative changes were detected in all specimen in this group (Fig. 2). In FG + MenSCs group, the defects in the most of the specimen were completely filled with firm, smooth, opaque hyaline cartilage-like tissue, which seemed relatively similar in colour and texture to the surrounding native articular cartilage that was statically significant compared to the control group (p < 0.05). The edges were properly integrated into the surrounding intact cartilage. The presence of blood vessels in repair tissue in both treated groups was lower than the control group. However, a significant correlation was only detected between FG + MenSCs and control groups (p < 0.05) (Fig. 2). The defects in the control group were mostly enclosed with fiber-like tissue and were depressed compared to the intact native cartilage level. The edges had no suitable integration, and relatively distinct borders could be distinguished from the surrounding intact articular cartilage. In addition, signs of osteoarthritis and vascular changes were seen in adjacent articular cartilage in control group (Fig. 2).
Fig. 2.
A–I Macroscopic appearance of the cartilage defect (dotted circle) healing at 3 months in three selected samples from each group; A, D, G (FG + MenSCs group), the defects were filled with hyaline cartilage-like tissue. B, E, H (FG group), the defects were not filled completely (B and E), and in (H), defect was filled with a heterogeneous white repair tissue. C, F, I (control group), note to the incomplete regenerative tissues that were depressed compared to the surrounding native cartilage level
As indicated in Table 1, the total point of the FG + MenSCs group was lower than other groups showed the best healing and statistical analysis showed that there was a significant relationship between FG + MenSCs group and the non-treated group at the 3 months (p < 0.05) (Table 1) (Fig. 2).
Table 1.
Scores according to macroscopic scoring system
| Parameters | Color of the repair tissue | Presence of blood vessels in the repair tissue | Surface of the repair tissue | Filling of the defect | Degeneration of adjacent articular cartilage | Total points | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Time groups | 3 mo | 6 mo | 3 mo | 6mo | 3 mo | 6 mo | 3 mo | 6 mo | 3 mo | 6 mo | 3 mo | 6 mo |
| MenSCs-seeded FG | 1 (0–2)† | 1 (0–2) | 0 (0–2)† | 0 (0–1) | 1 (0–3) | 1 (0–2) | 1 (0–1) | 0 (0–1)†* | 1 (0–2) | 2 (0–2) | 4.5 (1–8)† | 4 (1–8)† |
| FG | 1.5 (1–4) | 2 (2–4) | 0 (0–2) | 1.5 (1–4) | 1 (1–3) | 2 (1–3) | 1 (0–3) | 1 (0–3) | 2 (2–2) | 2 (2–3) | 6.5 (4–14) | 9 (6–16) |
| Control | 2 (2–3) | 2 (2–2) | 2 (1–3) | 1 (1–1) | 3 (1–3) | 1 (1–1) | 1 (1–2) | 2.5 (2–3) | 2 (0–2) | 1.5 (1–2) | 10 (7–11) | 8 (8–8) |
†MenSCs-seeded FG vs. control, p value < 0.05
*6 mo vs. 3 mo, p value < 0.05
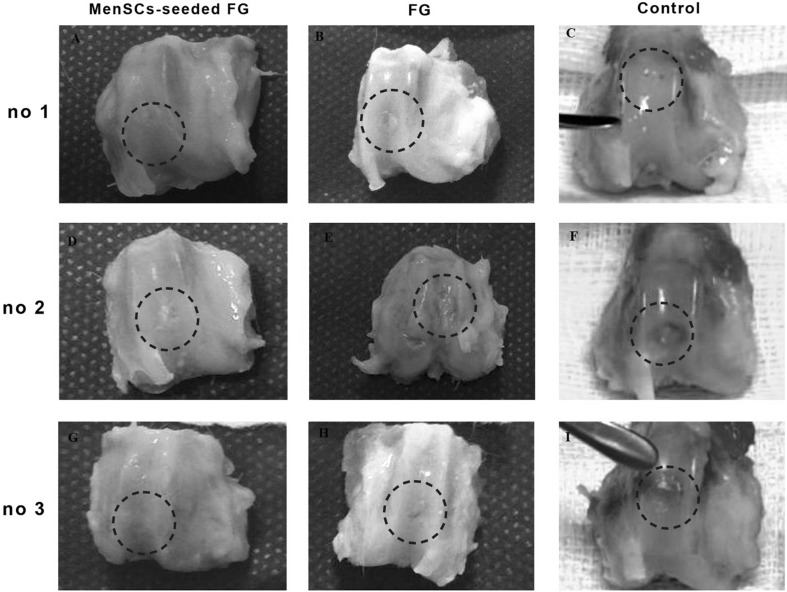
At 6 months after implantation, the surface of the most defects in FG group was covered with newly generated tissues with semi-translucent appearance. The surface was not as smooth as that of the normal articular cartilage. Though the regenerated tissue appeared relatively integrated with normal tissue, there was concavity in the centre of some defect (Fig. 3). In FG + MenSCs group, the surface of the most repaired defects exhibited glossy white and tan, closely well-integrated regenerated tissue. The amount of filling of the defect in FG + MenSCs group was significantly better than those of the control group (p < 0.05). The surface of repaired defects was remarkably smooth with margins appropriately connected to the surrounding native articular cartilage (Fig. 3). In the control group, the defects were filled with smooth heterogeneous tissue with some fissures on the surface and incompletely integrated with the intact native cartilage (Fig. 3).
Fig. 3.
A–I Macroscopic appearance of the cartilage defect (dotted circle) healing at 3 months in three selected samples from each group; A, D, G (FG + MenSCs group), the defects exhibited well-integrated, glossy, white to tan new regenerated tissue. B, E, H (FG group), note to incomplete regenerative tissues that could be distinguished from the adjacent normal articular cartilage. C, F, I (control group), the defects were filled with heterogeneous tissue and were depressed below the host native cartilage
As the same with the result of 3 months, the total point in FG + MenSCs group was lower than other experimental groups, and significant correlation was detected between FG + MenSCs and non-treated groups (p < 0.05) at the 6 months (Table 1).
As compared to two different time point, the total point of FG + MenSCs and defect groups after 6 months after implantation was lower than those of the 3 months after implantation, indicated better healing. Statistical analysis showed that there was a significant relationship infilling of the defect in FG + MenSCs group between 3 and 6 months (p < 0.05) (Table 1).
Microscopical evaluation
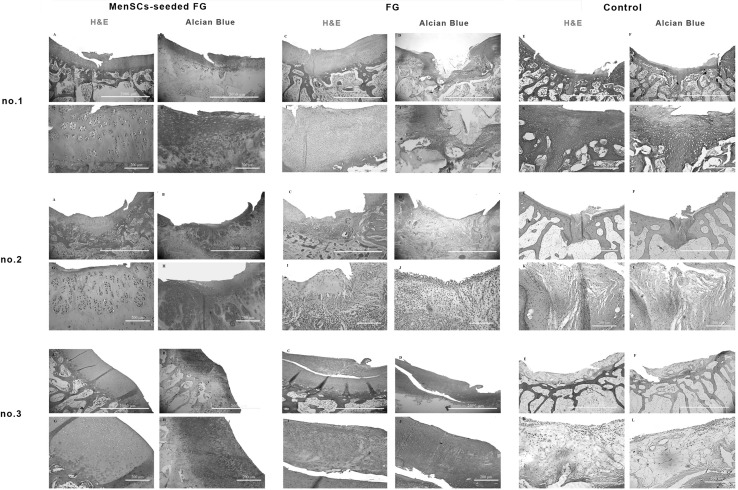
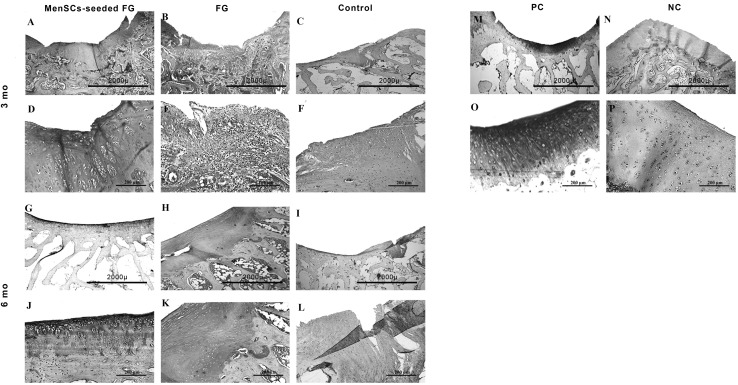
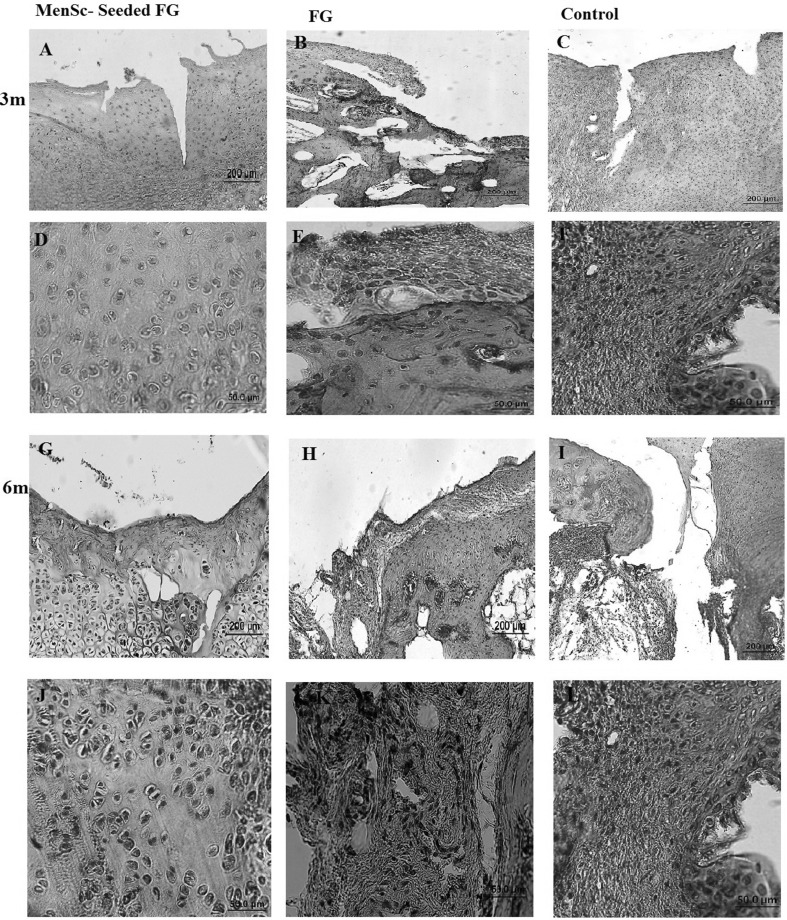
At 3 months post-implantation in FG group, the defects were filled with fibrocartilage, fibrous and immature repair tissues. In two case, subchondral destruction was detected. The surface of the most cases was the uneven and lateral integration of repair tissue to intact cartilaginous tissues was partially on both sides. Basal integration was relatively appropriate or not. The alcian blue staining exhibited no noticeable intensity. A complete tidemark was not detected in all cases in this group and its formation was relatively (Fig. 4). Masson’s trichrome staining showed that collagen fibers were produced in the extracellular matrices (Fig. 5). In addition, the amount of the collagen type II was less than native hyaline cartilage (Fig. 6).
Fig. 4.
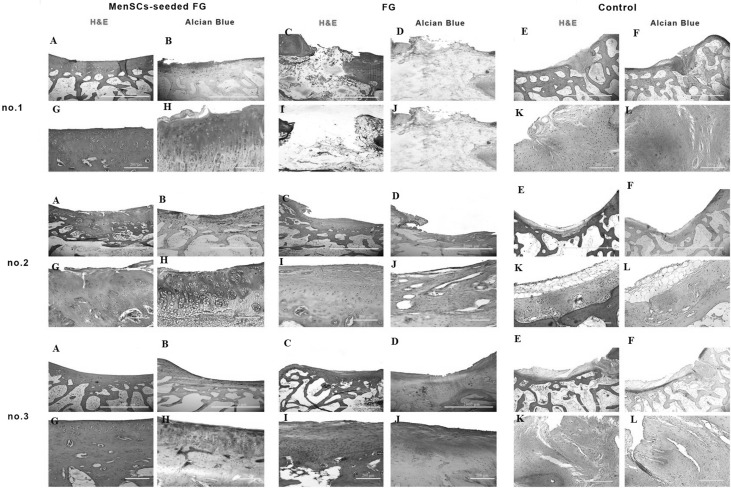
Microscopic appearance of the repaired cartilage defects at 3 months in three selected samples from each group (H and E and Alcian blue staining); (no.1 A, no.2 A, no.3 A): (FG + MenSCs group), the defects were filled with hyaline cartilage-like tissue. (no.1 G, no.2 G, no.3 G): higher magnification of previous slides, note to well-differentiated chondrocyte-like cells within lacunae with isogenic and/or columnar pattern. (no.1 B, no.2 B, no.3 B): intense staining of alcian blue staining in FG + MenSCs group indicted noticeable amount of proteoglycans. (no.1 H, no.2 H, no.3 H), higher magnification of previous slides. (no.1 C, no.2 C, no.3 C): (FG group), the defects were mostly filled with fibrocartilage and fibrous repair tissues. (no.1 I, no.2 I, no.3 I): higher magnification of previous slides, note to fibrocartilaginous tissue with disorganized chondrocyte. (no.1 D, no.2 D, no.3 D): The alcian blue staining exhibited variable intensity in FG group. (no.1 J, no.2 J, no.3 J): higher magnification of previous slides. (no.1 E, no.2 E, no.3 E): (control group), the defect was mainly filled with fibrocartilaginous repair tissue with infiltration to subchondral bone portion (no.1 E). In (no.2 E, no.3 E), the defects showed spontaneous healing and were mainly covered by an immature fibrous tissue. (no.1 K, no.2 K, no.3 K): higher magnification of previous slides. (no.1 F, no.2 F, no.3 F): the extra cellular matrixes of cases belonged to the control group showed slight to moderate intensity of alcian blue staining. (no.1 L, no.2 L, no.3 L): higher magnification of previous slides
Fig. 5.
Masson’s trichrome staining of the repaired cartilage defects. Samples have been stained by Masson’s trichrome at 3 and 6 months post-implantation in each group and shown in low and high magnification. Mo: month. A, D, G, J (FG + MenSCs group), note to the noticeable deposition of collagen fibers in defect area indicated by Masson’s trichrome staining. B, E, H, K (FG group), note to slight to moderate intensity of Masson’s trichrome staining. C, F, I, L (control group), note to lower intensity of Masson’s trichrome staining compared to normal hyaline cartilage
Fig. 6.
Immunostaining for collagen type II. Samples were stained at three and 6 months post-implantation in each group and shown in low and high magnification. Mo: month, A, D, G, J (FG + MenSCs group), note to noticeable expression of collagen type II indicated by brown color. B, E, H, K (FG group), the intensity of collagen type II expression was slight to moderate. C, F, I, L (control group), note to that the collagen II accumulation was less than compared to the native hyaline cartilage tissue. M, O PC, positive control (native hyaline cartilage). N, P NC, negative control
In the most cases of the FG + MenSCs group, defects were filled with hyaline cartilage-like tissue. The cellular morphology also displayed well-differentiated chondrocyte-like cells within lacunae and in some cases; the appropriate columnar pattern was seen like intact cartilage. The lateral integration was comparatively better than that of in the FG group. Alcian blue staining revealed a high content of glycosaminoglycan of the ECM in the most cases, which was slightly similar to normal cartilage tissue. Basal integration was perfect or relatively. The architecture of the surface and the amount of glycosaminoglycan was significantly better in the FG + MenSCs group compared to the FG group (p < 0.05) (Fig. 4). The Masson’s trichrome staining also proved the existence of collagen fibers especially collagen type II in repair tissues in this group like normal hyaline cartilage (Figs. 5, 6).
The defects in the untreated group showed spontaneous healing and were mainly covered by fibrous tissue. Fibrous tissues were hypertrophic, and collagen fibers were wrinkled. The lateral integration of repair tissue to intact native cartilaginous tissues was somewhat at both sides (Fig. 4).
Overall, as indicated in Table 2, the total point of microscopic examination in FG + MenSCs group was higher than other experimental groups. However, no significant relationship was detected between groups at the 3 months (Table 2).
Table 2.
Scores according to the modified histological grading scale
| Category | Tissue morphology | Matrix staining | Structural integrity | Chondrocyte clustering | Formation of tidemark | Architecture of the surface | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Time groups | 3 mo | 6 mo | 3 mo | 6 mo | 3 mo | 6 mo | 3 mo | 6 mo | 3 mo | 6 mo | 3 mo | 6 mo |
| MenSCs-seeded FG | 3 (1–3) | 3 (1–3) | 3 (1–3)† | 2 (1–3)‡ | 3 (1–4) | 3 (1–4)‡ | 1 (0–2) | 2 (1–2) | 2 (0–4) | 3 (0–4) | 2 (1–3)† | 2 (1–3) |
| FG | 2 (1–2) | 1 (0–2) | 1 (0–2) | 1 (0–2) | 2 (2–3) | 2 (1–2) | 2 (0–2) | 2 (1–2) | 3 (0–3) | 1 (0–2) | 1 (0–2) | 2 (1–2) |
| Control | 2 (2–2) | 1.5 (1–2) | 2 (2–2) | 1 (1–1)* | 2.5 (2–3) | 1.5 (1–2)* | 1 (1–1) | 2 (2–2)* | 1 (1–1) | 1.5 (1–2) | 2 (1–3) | 1 (1–1) |
| Category | Filling of the defect | Lateral integration | Basal integration | Inflammation | Total points | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Time groups | 3 mo | 6 mo | 3 mo | 6 mo | 3 mo | 6 mo | 3 mo | 6 mo | 3 mo | 6 mo |
| MenSCs-seeded FG | 4 (1–4) | 4 (2–4)‡ | 1 (1–2) | 1 (1–2) | 2.5 (0–3) | 2 (1–3) | 4 (4–4) | 4 (4–4) | 24 (14–32) | 26.5 |
| FG | 4 (1–4) | 2.5 (2–3) | 1 (0–2) | 1 (1–2) | 2 (0–3) | 2 (1–3) | 4 (0–4) | 4 (4–4) | 17.5 (14–23) | 20 (11–23) |
| Control | 3 (3–3) | 2 (2–2)* | 1 (1–1) | 1 (1–1) | 1.5 (1–2) | 1.5 (1–2) | 4 (4–4) | 4 (4–4) | 17 (15–19) | 20 (18–22) |
†MenSCs-seeded FG vs. FG, p value < 0.05
‡MenSCs-seeded FG vs. control, p value < 0.05
*6 mo vs. 3 mo, p value < 0.05
At 6 months post-implantation, in FG group, the type of repair tissues were fibrocartilage and/or fibrous tissue that was similar to the condition at 3 months post-implantation. The intensity of alcian blue staining was faint. The organization of chondrocyte-like cells involved some clusters of chondrocytes with irregular cellular orientation. The surfaces were not smooth completely. Peripheral and basal integration of repair tissue was moderately (Fig. 7). The Masson’s trichrome staining showed the presence of collagen fibers in repair zone (Fig. 5). However, the amount of the collagen type II was less than intact hyaline cartilage (Fig. 6).
Fig. 7.
Microscopic appearance of the repaired cartilage defects at 6 months in three selected samples from each group (H and E and Alcian blue staining); (no.1 A, no.2 A, no.3 A): (FG + MenSCs group), the defects were relatively filled with a homogeneous hyaline-like cartilage tissue. (no.1 G, no.2 G, no.3 G): higher magnification of previous slides. (no.1 B, no.2 B, no.3 B): note to high content of glycosaminoglycan of the ECM in FG + MenSCs group demonstrated by alcian blue staining. (no.1 H, no.2 H, no.3 H), higher magnification of previous slides. (no.1 C, no.2 C, no.3 C): (FG group), the defects were mainly filled with fibrous and/or fibrocartilaginous repair tissue. (no.1 I, no.2 I, no.3 I): higher magnification of previous slides. (no.1 D, no.2 D, no.3 D): The amount proteoglycans were less than native hyaline cartilage in FG group. (no.1 J, no.2 J, no.3 J): higher magnification of previous slides. (no.1 E, no.2, E, no.3 E): (control group), defects showed spontaneous healing and mainly filled by a disorganized and irregular fibrous tissue. (no.1 K, no.2 K, no.3 K): higher magnification of previous slides. (no.1 F, no.2 F, no.3 F): The repair tissues belonged to control group showed slight metachromatic staining. (no.1 L, no.2 L, no.3 L): higher magnification of previous slides
The most regenerated tissue in FG + MenSCs group was similar to hyaline cartilage with relatively good infill and integration. In the superficial portion, elongated to oval cells were organised parallel to the surface and in the deep zone; spherical cells were prepared in a columnar form comparable to native articular cartilage. The amount of cellularity decreased compared to 12 weeks repair, however, was still higher than that of intact adjacent cartilage. Mild fibrillation of the surface was apparent yet. In the cases with hyaline-like repair tissue, the intensity of the alcian blue staining was near to normal or slightly reduced compared with that of the adjacent native hyaline cartilage. The thickness of the repair cartilage was almost nearly that of the normal cartilage. Tidemark formation was perfect or relatively. No inflammation was noted in cases of this group. The amount of glycosaminoglycan, structural integrity, and filling of the defect in FG + MenSCs group was better than those in the untreated group (p < 0.05) (Fig. 7). The repair tissue filled the defect displayed a homogenously positive Masson’s trichrome staining (Fig. 5) and collagen fibers especially collagen type II was strongly positive in these samples like normal hyaline cartilage (Fig. 6).
In control group, the defects showed spontaneous healing and mainly filled by irregular fibrous tissue. In addition, there were some fibrocartilaginous structures in the periphery. In some cases, the integration of subchondral bone was lost, and infiltrations of fibrous and granular scar tissue with vascular invasion in these areas were detected. The repair surfaces were not smooth perfectly, but peripheral repair tissue to native cartilaginous tissues was suitable (Fig. 7).
As the same with the result 3 months after implantation, the total point of microscopical examination in FG + MenSCs group was higher than other experimental groups indicated better healing, however, no significant correlation was detected between groups at the 6 months (p > 0.05) (Table 2).
As compared to two different time points, the total point of all groups after 6 months after implantation was higher than those of the 3 months after implantation, indicated better healing. Statistical analysis showed that there was a significant relationship in the amount of glycosaminoglycan, structural integrity, chondrocyte clustering, and filling of the defect in the control group. However, no significant correlation was detected in other groups between 3 and 6 months (p > 0.05) (Table 2).
The evaluation of osteocalcin expression as a marker of chondrocyte hypertrophy [33], demonstrated no expression of this marker by the chondrocytes in neither of the experimented groups (Fig. 8).
Fig. 8.
Immunostaining for osteocalcin. Samples were stained at 3 and 6 months post-implantation in each group and shown in low and high magnification. Mo: month, A, D, G, J (FG + MenSCs group), B, E, H, K (FG group), C, F, I, L (control group), no expression of osteocalcin were seen in cartilaginous tissue of any group
Discussion
Nowadays, the scientists have been fascinated to MenSCs due to their exceptional advantages over other stem cells. High accessibility and availability, proliferative and self-renewal capacities, low immunogenicity and inflammatory reaction, and transdifferentiation potential, make MenSCs as a promising candidate for tissue engineering goals [9–13, 15, 16].
Recently, we demonstrated that MenSCs express mesenchymal markers like CD73, CD105 and also OCT-4 as embryonic marker. Moreover, these cells have the potential to differentiate into the chondrogenic lineage (as shown in Fig. 1). Chondrogenic differentiation ability of MenSCs was comparable to bone marrow stem cells (BMSCs) such differentiated MenSCs showed accumulation of proteoglycans and strong expression of collagen type II in a similar pattern with BMSCs [9, 11]. These data guided us to evaluate the efficiency of MenSCs in the repair of cartilage lesions and osteochondral defects in animal models such there is no in vivo study till now.
Based on the findings of the present study, transplantation of MenSCs could repair the OCD defects created in the rabbit knee with no sign of immuno rejection. Moreover, MenSCs encapsulated in FG was more effective in defect repair compared to FG alone. The repair potential of FG in osteochondral defects and cartilage regeneration was shown in several animal studies and even human trials [34–36], while there are some controversies in findings. Some studies reported that the using of FG alone or in combination with some growth factors could not promote OCD repair [36–38]. Homminga et al. [39]) showed that FG is a suitable matrix for attachment, proliferation, and biosynthesis of cartilaginous matrix components. Another study reported that composite of collagen and FG could support chondrocyte survival and synthetic activity in static cultures [26]. Eyrich et al. [28] indicated that bovine chondrocytes suspended in the fibrin gels proliferated well and produced a coherent cartilaginous extracellular matrix throughout the whole construct. Furthermore, the clinical use of platelet-rich fibrin glue with human autologous BMSCs in the treatment of articular cartilage defects in 5 patients indicated complete defect filling with complete congruity of the repaired articular surface in 3 patients at 12 months postoperatively whereas that of 2 patients showed incomplete congruity [34].
In the present study, application of FG alone resulted in better repair in gross evaluation compared to the no treated group at 12 week postoperatively, although the differences between groups were not significant. Six months post-implantation, the total score according to macroscopically healing criteria in FG group were higher than control group indicated weaker repair, however, no significant difference was detected. Also, as compared to two different time points, the total point of gross examination of FG group after 6 months post-implantation was higher than those of 3 months and revealed time-dependent degenerative changes, although, there were no significant relationships. In microscopical examination, there was also no significant relationship between FG and control groups according to the total score.
While there are some reports showing that subchondral bone exposion stimulates intristic MSC migration to defect area [40], in the present study, no significant spontaneous healing in control group was achieved in both time points.
Here, for the first time, we demonstrated that encapsulated MenSCs in FG could promote restoration of OCD compared to FG alone in a rabbit model by clinical and histopathological examinations. The overall clinical examination at 3 months after implantation showed that, in FG + MenSCs group, the defects in the most of the specimen were completely filled with hyaline cartilage-like that was statically significant compared to the FG group. The amount of glycosaminoglycan was significantly better in the FG + MenSCs group compared to the FG group. The Masson’s trichrome and IHC staining also proved the existence of collagen fibers especially collagen type II in repair tissues in this group like normal hyaline cartilage.
At 6 months after implantation, in FG + MenSCs group, there was a well-integrated regenerated tissue. The amount of filling, glycosaminoglycan and structural integrity of a defect in FG + MenSCs group was similar to hyaline cartilage with relatively good infill and integration and significantly better than those of the control group. The repair tissue filled the defect displayed a homogenously positive Masson’s trichrome staining and collagen fibers especially collagen type II was strongly positive in these samples like normal hyaline cartilage that was similar to the condition at 3 months post-implantation.
Macroscopical and microscopical examination at three and 6 months post-implantation showed the best healing in the FG + MenSCs group. In addition by microscopical evaluation, the total point of all groups after 6 months post-implantation indicated better healing than those of the 3 months after implantation. Statistical analysis showed that there was a significant relationship infilling of the defect in FG + MenSCs group between three and 6 months. Our results were consistent with other resreches which suggested that the combination of FG with chondrocytes or stem cells could promote remodelling and repair of OCD [34, 35, 38].
The more efficiency of MenSCs encapsulated in FG compared to FG alone suggests that FG alone has no enough innate ability to call endogenous chondrocytes and stem cells and so exogenous stem cell administration is required. Moreover, although proliferation and chondrogenic differentiation of MenSCs appears to be controlled by numerous cues in their microenvironment, it is suggested that a cocktail of growth factors and cytokines released by FG [30, 41, 42], or secreted via the paracrine effect of MenSCs [43, 44] plays a role in MenSCs differentiation into chondrocytes, calling home cells, mediate their adhesion and proliferation and the secretion of extracellular matrix (ECM). As a result, interaction among cytokines and growth factors by FG and MenSCs sounds to be main step forward for directing the chondrogenic differentiation of these cells. Furthermore, FG as a carrier for MenSCs, provide an appropriate topography and mechanical properties for cell attachment, proliferation, and integrate with host tissue and preserve cell survival after implantation [45, 46]. It is suggested that the high content of fibronectin in FG assists peripheral integration of repaired tissue to the adjacent native cartilaginous tissue [47].
To conclude, in the present study, we demonstrated that encapsulated MenSCs with FG have suitable in vivo regenerative capacity of osteochondral defects in a rabbit model and could be suggested for future application in clinical trial studies. The success of these studies may have significant clinical implications for patients in future.
Acknowledgements
The authors would like to thank the authorities of Avicenna Research Institute due to its financial support (Grant Number: 950106-030) and providing facilities in performing this research. The authors would like to thank Miss. Sayeh Khanjani for their assistance in lab works and Mr Farhad Hosseini for helping in animal surgeries.
Compliance with ethical standards
Conflict of interest
The authors declare no potential conflicts of interest.
Ethical statement
The study was approved by bio-ethic committee of Avicenna Research Institute (IACUC no. IR.ACECR.Avicenna.REC.1395.14). All animals received human care in compliance with the Guide for Care and Use of Laboratory Animals published by the National Institutes of Health (NIH publication No. 85-23, revised 1985).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Manijeh Khanmohammadi and Hannaneh Golshahi are co-first authors.
References
- 1.Chang KY, Cheng LW, Ho GH, Huang YP, Lee YD. Fabrication and characterization of poly (gamma-glutamic acid)-graft-chondroitin sulfate/polycaprolactone porous scaffolds for cartilage tissue engineering. Acta Biomater. 2009;5:1937–1947. doi: 10.1016/j.actbio.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 2.Zhang L, Hu J, Athanasiou KA. The role of tissue engineering in articular cartilage repair and regeneration. Crit Rev Biomed Eng. 2009;37:1–57. doi: 10.1615/CritRevBiomedEng.v37.i1-2.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Karuppal R. Current concepts in the articular cartilage repair and regeneration. J Orthop. 2017;14:A1–A3. doi: 10.1016/j.jor.2017.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Salzmann GM, Niemeyer P, Hochrein A, Stoddart MJ, Angele P. Articular cartilage repair of the knee in children and adolescents. Orthop J Sports Med. 2018;6:2325967118760190. doi: 10.1177/2325967118760190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martin I, Miot S, Barbero A, Jakob M, Wendt D. Osteochondral tissue engineering. J Biomech. 2007;40:750–765. doi: 10.1016/j.jbiomech.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 6.Brittberg M, Peterson L, Sjögren-Jansson E, Tallheden T, Lindahl A. Articular cartilage engineering with autologous chondrocyte transplantation: a review of recent developments. J Bone Joint Surg Am. 2003;85:109–115. doi: 10.2106/00004623-200300003-00017. [DOI] [PubMed] [Google Scholar]
- 7.Tuli R, Li WJ, Tuan RS. Current state of cartilage tissue engineering. Arthritis Res Ther. 2003;5:235–238. doi: 10.1186/ar991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kazemnejad S, Khanmohammadi M, Baheiraei N, Arasteh S. Current state of cartilage tissue engineering using nanofibrous scaffolds and stem cells. Avicenna J Med Biotechnol. 2017;9:50–65. [PMC free article] [PubMed] [Google Scholar]
- 9.Darzi S, Zarnani AH, Jeddi-Tehrani M, Entezami K, Mirzadegan E, Akhondi MM, et al. Osteogenic differentiation of stem cells derived from menstrual blood versus bone marrow in the presence of human platelet releasate. Tissue Eng Part A. 2012;18:1720–1728. doi: 10.1089/ten.tea.2011.0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khanjani S, Khanmohammadi M, Zarnani AH, Talebi S, Edalatkhah H, Eghtesad S, et al. Efficient generation of functional hepatocyte-like cells from menstrual blood-derived stem cells. J Tissue Eng Regen Med. 2015;9:E124–E134. doi: 10.1002/term.1715. [DOI] [PubMed] [Google Scholar]
- 11.Khanmohammadi M, Khanjani S, Bakhtyari MS, Zarnani AH, Edalatkhah H, Akhondi MM, et al. Proliferation and chondrogenic differentiation potential of menstrual blood-and bone marrow-derived stem cells in two-dimensional culture. Int J Hematol. 2012;95:484–493. doi: 10.1007/s12185-012-1067-0. [DOI] [PubMed] [Google Scholar]
- 12.Gargett CE, Masuda H. Adult stem cells in the endometrium. Mol Hum Reprod. 2010;16:818–834. doi: 10.1093/molehr/gaq061. [DOI] [PubMed] [Google Scholar]
- 13.Patel AN, Park E, Kuzman M, Benetti F, Silva FJ, Allickson JG. Multipotent menstrual blood stromal stem cells: isolation, characterization, and differentiation. Cell Transplant. 2008;17:303–311. doi: 10.3727/096368908784153922. [DOI] [PubMed] [Google Scholar]
- 14.Faramarzi H, Mehrabani D, Fard M, Akhavan M, Zare S, Bakhshalizadeh S, et al. The potential of menstrual blood-derived stem cells in differentiation to epidermal lineage: a preliminary report. World J Plast Surg. 2016;5:26–31. [PMC free article] [PubMed] [Google Scholar]
- 15.Meng X, Ichim TE, Zhong J, Rogers A, Yin Z, Jackson J, et al. Endometrial regenerative cells: a novel stem cell population. J Transl Med. 2007;5:57. doi: 10.1186/1479-5876-5-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khanmohammadi M, Khanjani S, Edalatkhah H, Zarnani AH, Heidari-Vala H, Soleimani M, et al. Modified protocol for improvement of differentiation potential of menstrual blood-derived stem cells into adipogenic lineage. Cell Prolif. 2014;47:615–623. doi: 10.1111/cpr.12133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khanjani S, Khanmohammadi M, Zarnani AH, Akhondi MM, Ahani A, Ghaempanah Z, et al. Comparative evaluation of differentiation potential of menstrual blood-versus bone marrow-derived stem cells into hepatocyte-like cells. PLoS One. 2014;9:e86075. doi: 10.1371/journal.pone.0086075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mou XZ, Lin J, Chen JY, Li YF, Wu XX, Xiang BY, et al. Menstrual blood-derived mesenchymal stem cells differentiate into functional hepatocyte-like cells. J Zhejiang Univ Sci B. 2013;14:961–972. doi: 10.1631/jzus.B1300081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rahimi M, Zarnani AH, Mohseni-Kouchesfehani H, Soltanghoraei H, Akhondi MM, Kazemnejad S. Comparative evaluation of cardiac markers in differentiated cells from menstrual blood and bone marrow-derived stem cells in vitro. Mol Biotechnol. 2014;56:1151–1162. doi: 10.1007/s12033-014-9795-4. [DOI] [PubMed] [Google Scholar]
- 20.Rahimi M, Mohseni-Kouchesfehani H, Zarnani AH, Mobini S, Nikoo S, Kazemnejad S, et al. Evaluation of menstrual blood stem cells seeded in biocompatible Bombyx mori silk fibroin scaffold for cardiac tissue engineering. J Biomater Appl. 2014;29:199–208. doi: 10.1177/0885328213519835. [DOI] [PubMed] [Google Scholar]
- 21.Hida N, Nishiyama N, Miyoshi S, Kira S, Segawa K, Uyama T, et al. Novel cardiac precursor-like cells from human menstrual blood-derived mesenchymal cells. Stem Cells. 2008;26:1695–1704. doi: 10.1634/stemcells.2007-0826. [DOI] [PubMed] [Google Scholar]
- 22.Azedi F, Kazemnejad S, Zarnani AH, Soleimani M, Shojaei A, Arasteh S. Comparative capability of menstrual blood versus bone marrow derived stem cells in neural differentiation. Mol Biol Rep. 2017;44:169–182. doi: 10.1007/s11033-016-4095-7. [DOI] [PubMed] [Google Scholar]
- 23.Azedi F, Kazemnejad S, Zarnani AH, Behzadi G, Vasei M, Khanmohammadi M, et al. Differentiation potential of menstrual blood-versus bone marrow-stem cells into glial-like cells. Cell Biol Int. 2014;38:615–624. doi: 10.1002/cbin.10245. [DOI] [PubMed] [Google Scholar]
- 24.He Y, Lu F. Development of synthetic and natural materials for tissue engineering applications using adipose stem cells. Stem Cells Int. 2016;2016:5786257. doi: 10.1155/2016/5786257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang ZH, Zhang J, Zhang Q, Gao Y, Yan J, Zhao XY, et al. Evaluation of bone matrix gelatin/fibrin glue and chitosan/gelatin composite scaffolds for cartilage tissue engineering. Genet Mol Res. 2016;15:15038431. doi: 10.4238/gmr.15038431. [DOI] [PubMed] [Google Scholar]
- 26.Deponti D, Di Giancamillo A, Gervaso F, Domenicucci M, Domeneghini C, Sannino A, et al. Collagen scaffold for cartilage tissue engineering: the benefit of fibrin glue and the proper culture time in an infant cartilage model. Tissue Eng Part A. 2014;20:1113–1126. doi: 10.1089/ten.tea.2013.0171. [DOI] [PubMed] [Google Scholar]
- 27.Johnson TS, Xu JW, Zaporojan VV, Mesa JM, Weinand C, Randolph MA, et al. Integrative repair of cartilage with articular and nonarticular chondrocytes. Tissue Eng. 2004;10:1308–1315. doi: 10.1089/ten.2004.10.1308. [DOI] [PubMed] [Google Scholar]
- 28.Eyrich D, Brandl F, Appel B, Wiese H, Maier G, Wenzel M, et al. Long-term stable fibrin gels for cartilage engineering. Biomaterials. 2007;28:55–65. doi: 10.1016/j.biomaterials.2006.08.027. [DOI] [PubMed] [Google Scholar]
- 29.Park JS, Yang HN, Woo DG, Jeon SY, Park KH. Chondrogenesis of human mesenchymal stem cells in fibrin constructs evaluated in vitro and in nude mouse and rabbit defects models. Biomaterials. 2011;32:1495–1507. doi: 10.1016/j.biomaterials.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 30.Xie X, Wang Y, Zhao C, Guo S, Liu S, Jia W, et al. Comparative evaluation of MSCs from bone marrow and adipose tissue seeded in PRP-derived scaffold for cartilage regeneration. Biomaterials. 2012;33:7008–7018. doi: 10.1016/j.biomaterials.2012.06.058. [DOI] [PubMed] [Google Scholar]
- 31.Goebel L, Orth P, Müller A, Zurakowski D, Bücker A, Cucchiarini M, et al. Experimental scoring systems for macroscopic articular cartilage repair correlate with the MOCART score assessed by a high-field MRI at 9.4 T–comparative evaluation of five macroscopic scoring systems in a large animal cartilage defect model. Osteoarthritis Cartilage. 2012;20:1046–1055. doi: 10.1016/j.joca.2012.05.010. [DOI] [PubMed] [Google Scholar]
- 32.Mainil-Varlet P, Rieser F, Grogan S, Mueller W, Saager C, Jakob RP. Articular cartilage repair using a tissue-engineered cartilage-like implant: an animal study. Osteoarthritis Cartilage. 2001;9:S6–S15. doi: 10.1053/joca.2001.0438. [DOI] [PubMed] [Google Scholar]
- 33.van der Kraan PM, van den Berg WB. Chondrocyte hypertrophy and osteoarthritis: role in initiation and progression of cartilage degeneration? Osteoarthritis Cartilage. 2012;20:223–232. doi: 10.1016/j.joca.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 34.Haleem AM, Singergy AA, Sabry D, Atta HM, Rashed LA, Chu CR, et al. The clinical use of human culture–expanded autologous bone marrow mesenchymal stem cells transplanted on platelet-rich fibrin glue in the treatment of articular cartilage defects: a pilot study and preliminary results. Cartilage. 2010;1:253–261. doi: 10.1177/1947603510366027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Könst YE, Benink RJ, Veldstra R, van der Krieke TJ, Helder MN, van Royen BJ. Treatment of severe osteochondral defects of the knee by combined autologous bone grafting and autologous chondrocyte implantation using fibrin gel. Knee Surg Sports Traumatol Arthrosc. 2012;20:2263–2269. doi: 10.1007/s00167-012-1891-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vogt S, Wexel G, Tischer T, Schillinger U, Ueblacker P, Wagner B, et al. The influence of the stable expression of BMP2 in fibrin clots on the remodelling and repair of osteochondral defects. Biomaterials. 2009;30:2385–2392. doi: 10.1016/j.biomaterials.2009.01.016. [DOI] [PubMed] [Google Scholar]
- 37.Lo Monaco M, Merckx G, Ratajczak J, Gervois P, Hilkens P, Clegg P, et al. Stem cells for cartilage repair: preclinical studies and insights in translational animal models and outcome measures. Stem Cells Int. 2018;2018:9079538. doi: 10.1155/2018/9079538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Berninger MT, Wexel G, Rummeny EJ, Imhoff AB, Anton M, Henning TD, et al. Treatment of osteochondral defects in the rabbit’s knee joint by implantation of allogeneic mesenchymal stem cells in fibrin clots. J Vis Exp. 2013;75:e4423. doi: 10.3791/4423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Homminga GN, Buma P, Koot HW, van der Kraan PM, van den Berg WB. Chondrocyte behavior in fibrin glue in vitro. Acta Orthop Scand. 1993;64:441–445. doi: 10.3109/17453679308993663. [DOI] [PubMed] [Google Scholar]
- 40.Zhang W, Chen J, Tao J, Jiang Y, Hu C, Huang L, et al. The use of type 1 collagen scaffold containing stromal cell-derived factor-1 to create a matrix environment conducive to partial-thickness cartilage defects repair. Biomaterials. 2013;34:713–723. doi: 10.1016/j.biomaterials.2012.10.027. [DOI] [PubMed] [Google Scholar]
- 41.Lin PB, Ning LJ, Lian QZ, Xia Z, Xin Y, Sen BH, et al. A study on repair of porcine articular cartilage defects with tissue-engineered cartilage constructed in vivo by composite scaffold materials. Ann Plast Surg. 2010;65:430–436. doi: 10.1097/SAP.0b013e3181d6e38b. [DOI] [PubMed] [Google Scholar]
- 42.Hopfner U, Aitzetmueller MM, Neßbach P, Hu MS, Machens HG, Maan ZN, et al. Fibrin glue enhances adipose-derived stromal cell cytokine secretion and survival conferring accelerated diabetic wound healing. Stem Cells Int. 2018;2018:1353085. doi: 10.1155/2018/1353085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gargett GE, Schwab KE, Deane JA. Endometrial stem/progenitor cells: the first 10 years. Hum Reprod Update. 2016;22:137–163. doi: 10.1093/humupd/dmw011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gargett CE, Chan RW, Schwab KE. Endometrial stem cells. Curr Opin Obstet Gynecol. 2007;19:377–383. doi: 10.1097/GCO.0b013e328235a5c6. [DOI] [PubMed] [Google Scholar]
- 45.Bal BS, Rahaman MN, Jayabalan P, Kuroki K, Cockrell MK, Yao JQ, et al. In vivo outcomes of tissue-engineered osteochondral grafts. J Biomed Mater Res B Appl Biomater. 2010;93:164–174. doi: 10.1002/jbm.b.31571. [DOI] [PubMed] [Google Scholar]
- 46.Li Y, Meng H, Liu Y, Lee BP. Fibrin gel as an injectable biodegradable scaffold and cell carrier for tissue engineering. ScientificWorldJournal. 2015;2015:685690. doi: 10.1155/2015/685690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Park SH, Park SR, Chung SI, Pai KS, Min BH. Tissue-engineered cartilage using fibrin/hyaluronan composite gel and its in vivo implantation. Artif Organs. 2005;29:838–845. doi: 10.1111/j.1525-1594.2005.00137.x. [DOI] [PubMed] [Google Scholar]