Abstract
The effects of indigenous fermentation on volatile compound profiles in a Theobroma cacao L, TSH565 clone, resistant to Moniliophtora perniciosa and Phytophthora spp. were evaluated in Southern Brazil. Sixty-three volatile flavor compounds in pulp and 36 in grains were identified by SPME-HS/GC–MS and classified as terpenes, alcohols, esters, ketones and aldehydes, among others. The relative amount of these compounds and their evolution until the end of the fermentation process were assessed in both fresh and fermented grains/pulp masses. β-myrcene and β-cis-ocimene, among terpenes, were detected in high amounts and are associated to a fine chocolate aroma. The sensory evaluation of chocolates manufactured from the fermented cocoa was performed by trained panelists, which defined 15 sensory descriptors. Chocolates from the TSH565 cultivar were characterized by a rich, fruity, intense cocoa flavor and bitterness, which are valuable sensorial and commercial attributes.
Electronic supplementary material
The online version of this article (10.1007/s13197-019-03736-3) contains supplementary material, which is available to authorized users.
Keywords: Theobroma cacao L, Resistant to ceratocystis wilt and witches’ broom, HS-SPME volatile extraction, GC-coupled to mass spectrometry, Descriptive sensory analysis
Introduction
Cocoa (Theobroma cacao L.) is a cash crop of huge economic significance worldwide and the key raw material for chocolate manufacturing (Ho et al. 2015). Cocoa pulp has been shown to be rich in fermentable sugars at about 9–13% w/w, including glucose, fructose and sucrose, presenting high acidity (pH 3.0–3.5) conferred by diverse organic acids, mainly citric acid, and comprising protein content ranging from 0.4 to 0.6% w/w (Lima et al. 2011).
Flavor is an important quality attribute of cocoa grains and their derived products, such as chocolate and, consequently, contributes to product acceptance (Afoakwa et al. 2008; Owusu et al. 2012). The characteristic flavors of cocoa grains are due to a rich volatile fraction composed of a mixture of hundreds of compounds (Magi et al. 2012). Currently, over 600 flavor compounds have been identified in cocoa grains and cocoa products, comprising nitrogen-and-oxygen heterocyclic compounds, aldehydes, ketones, esters, alcohols, hydrocarbons, nitriles, sulphides, pyrazines, ethers, furans, thiazoles, pyrones, acids, phenols, imines, amines, oxazoles and pyrroles (Kingor et al. 2016).
Flavor compounds in cocoa grains are formed during roasting from flavor precursors generated during fermentation and drying processes, and are, thus, influenced by several factors, such as type of cocoa (its genotype), bean composition, soil conditions, the age of the cocoa trees, postharvest treatments (such as pulp pre-conditioning, fermentation and drying), and finally, cocoa processing, including roasting and storage, and transportation to a manufacturing plant (Afoakwa et al. 2013; Crafack et al. 2014).
As mentioned previously, volatile cocoa powder constituents are important in imparting flavor notes to the end product (Li et al. 2012). For example, several studies have reported differences in cocoa grain constituents sampled from different parts of the world, including a recent study comparing the fatty acid composition of grains from Ecuador and Ghana (Torres-Moreno et al. 2015, Capriolia et al. 2016).
The quality and flavor of the chocolate typically depend on the origin of the cocoa grains, but ingredient proportions and chocolate producer practices must differ in order to attend consumer preferences (Lanza et al. 2011). Sensory evaluation is a powerful scientific tool applied to identify differences in sensory attributes and assess product acceptability.
In this study, the spontaneous fermentation of cocoa grain/pulp mass (Theobroma cacao L) from a special clonal variety belonging to the Trinidad Selected Hybrid (TSH) collection was assessed. A TSH 565 clonal variety from Southern Bahia, Brazil, was selected for its productivity and its resistance to Moniliophthora perniciosa and Phytophthora spp., that cause the infections known as witches broom (WB) and ceratocystis wilt (CR), but also because it maintains traditional fine flavor bean quality when fermented by indigenous microbiota (Maharaj et al. 2011). The natural cocobiota in the spontaneous fermentation of this cocoa hybrid in Southern Brazil was predominantly composed by yeasts, including Hanseniaspora spp., Saccharomyces spp. and Pichia spp. and by bacteria, like Lactobacillus spp., Leuconostoc spp. and Acetobacter spp. (Bastos et al. 2018).
Thus, the aim of the present study was to evaluate the formation kinetics of volatile compounds throughout the 144 h of the TSH565 cocoa grain/pulp mass fermentation. These volatile compounds were analyzed by SPME-HS/GC–MS and were related to previously reported desirable notes and off-flavor, in order to identify the flavor notes of the produced chocolate, evaluated by sensory tests.
Materials and methods
Cocoa bean fermentation
Ripen pods of the TSH565 cocoa clonal variety were harvested at 6 months from plantations located at the Cacao Plan Executive Committee (Comissão Executiva do Plano da Lavoura Cacaueira—Centro de Desenvolvimento e Capacitação Tecnológica do Centro de Pesquisas do Cacau/CEPLAC) at the manufacturing facilities headquartered in the municipality of Ilhéus (14°47′20″S, 39°2′56″W), Southern Brazil. At 48 h post-harvesting, the harvested pods were cut with unwashed machetes and the grains plus their surrounding pulp were scooped out by hand and transferred to a 125 kg load wooden box (50 × 50 × 50 cm) with holes at the bottom to drain the liquid generated during fermentation. The wet cocoa grains were covered with fresh banana leaves to ensure adequate insulation. Natural fermentation proceeded for 6 days at room temperature ranging from 23 °C at night to 29 °C during the day. Turnings were performed after 48 h from the beginning of the process and at each subsequent 24 h until the end of the fermentation (144 h).
At each 24 h, 200 g samples were collected at approximately 25 cm from the upper surface of the fermenting wort, transferred to sterile bags and stored at − 20 °C, until analytical assays were performed.
Volatile compound identification
The volatile compounds were extracted from cocoa pulp/grains mass using solid phase micro-extraction (SPME), and three-phase 50/30 µm divinylbenzene/carboxene/polydimethylsiloxane (DVB/CAR/PDMS) fibers (Supelco, PA, USA). Ground cacao grains (1 g) were weighed in 40 ml vials and sealed with septum caps. The extraction conditions and analysis were previously optimized by the exposure time of the fiber at different temperatures, as previously described, with minor modifications (Ho et al. 2015). One microliter of octadecane in water (387 mg/g) was used as internal standard (IS). For the HS-SPME extraction, the samples were heated at 60 °C for 15 min to reach equilibrium and for a further 30 min to expose the fiber to the volatile compounds in the HS, at 60 °C.
Volatile compounds were analyzed on an Agilent 6890 gas chromatograph coupled to an Agilent 5973 N mass selective detector (GC/MS) (Agilent, NY, USA) with a DB-5 column 30 m × 0.25 mm × 0.25 µm (J & W Scientific, CA, USA). Helium was used as the carrier gas at a flow rate of 1.0 mL/min. The oven temperature was programmed to 40 °C for 5 min, increased until 200 °C, at a rate of 5 °C/min, with subsequent 10 °C/min increases until 280 °C. The injector, transfer line, ion source and quadrupole temperatures were 260 °C, 250 °C, 230 °C and 150 °C, respectively. The mass detector was operated in the electronic ionization mode (70 eV) at 3.15 scan/s with a mass ranging from 40 to 550 u. Compounds were identified according to their mass spectra using the Wiley mass spectrometer library (Enhanced data analysis software (Agilent) and the retention index was calculated and compared to literature data using the linear retention index (LRI) (Adams 2007). Samples were analyzed in triplicate.
Aroma descriptors were obtained for each detected compound using the online databases Flavornet (Arn and Acree 1998), included in the TGSC information system from The Good Scents Company (2015), as suggested previously (Cevallos-Cevallos et al. 2018; Dunkel et al. 2009) and in the National Institute of Standards and Technology (NIST) (webbook.nist.gov/chemistry/name-ser.html).
Statistical analyses
Data were expressed as mean ± SD and significances were determined through an ANOVA and Tukey tests with p value = 5%, using the GraphPad Prism v. 5 software (GraphPad Software, CA, USA).
Chocolate manufacturing
Chocolates were processed at 23 °C and 68% relative humidity using the CEPLAC manufacturing facilities. Fermented and dry grains were roasted during for 5–35 min at 121–162 °C in a rotating cylindrical boiler with a moisture extractor (Jaf Inox, SP, BRA). Roasted grains were ground and peeled and seeds were removed, obtaining cocoa nibs that were homogenized by a slicer (Jaf Inox) and transformed into liquor. The formulation of bitter chocolate was prepared according to the French “Salon du Chocolat” contest protocol, consisting of cocoa mass (65.1%), sugar (31.55%), cocoa butter (3.0%) and lecithin (0.35%). Ingredient mixture, mass refining and conching were performed in a melanger (Jaf Inox), a large metal cylinder with two rotating granite wheels that grind and refine the chocolate into small particles. Conching was performed at 65 °C for 36 h following the tempering process, the controlled process of raising, lowering and raising the chocolate temperature again to form exactly the right type of crystals. The melted chocolate (15 g) was then poured into plastic bar-shaped molds. Once cooled, the bars were wrapped in aluminum foil (Cromos, BRA) and stored at 18 °C.
Sensory evaluation
Chocolates produced from the TSH565 cacao variety fermentation were evaluated using a quantitative descriptive analysis procedure to describe and quantify their sensorial characteristics. The judges were trained at the Laboratório de Análise Sensorial do Setor de Tecnologia de Alimentos at SETEA/CEPEC/CEPLAC, and were able to recognize several flavors, including artificial fruits, herbs, spices, acetic acid, smoke, floral, empyreumatic, dairy, sweet, citrus fruits, burnt, and alcoholic aromas. Seven judges with high score for flavor identification were considered able to perform the ranking descriptive analysis (RDA) (Richter et al. 2010) for the subsequent sensory evaluation of the manufactured chocolate samples.
A “dark” commercial chocolate (Barry Callebaut, IL, USA) was used as the reference to compare the intensity of each attribute. Sample bars were tested by the seven judges, according to Brazilian ABNT standard NBR14140 recommendations (ABNT 1998), in a single session conducted in a spacious, enclosed area with adequate lighting at 23 °C. First, the reference sample was tested, and the judges were asked to rinse their mouth with water between samples. Two tasting sessions were conducted, allowing the judges to taste the chocolates in duplicate. The recognition of an attribute by one judge was marked in the corresponding box questionnaire. The samples were blind-labeled and the order of presentation was completely randomized, as recommended by Valentin et al. (2012). The questionnaire results were converted to binominal data, where ‘1’ indicates attribute recognition, whilst ‘0’ marks an attribute not ticked by the judges. Statistical analyses were performed to verify significant differences in the CATA profiles using the software SPSS Statistics version 19 (IBM, NY, USA).
Aroma profile analyses were performed by applying an unstructured seven point-scale, varying from absent (0) to very intense (7). The grades given by the judges were averaged and compared to the reference by the ANOVA and Tukey tests, in order to evaluate differences at a 5% significance level.
Results and discussion
Volatile compounds in fresh and fermented cocoa pulp and grains
Sixty-three volatile compounds were identified in pulp and thirty-six in cocoa grains, clustered into six main functional chemical groups (Table 1). Twenty-eight and twenty-two volatile compounds were identified in the TSH565 clone pulp at the beginning (0 h) and after 144 h of fermentation, respectively, while 19 and 28 compounds were detected simultaneously in the grains (Table 1).
Table 1.
Volatile compounds identified in fresh and fermented cocoa pulp and grain from the TSH 565 clonal variety by MEFS-HS-CG/EM
| Retention time (min) | Pulp | Grain | Compound | Odor descriptiona | Retention Indexb | Retention Indexc |
|---|---|---|---|---|---|---|
| Ketones | ||||||
| 2.75 | + | 2-Pentanone | Fruity, Thinner, Acetone | – | 686 | |
| 7.89 | + | + | 4-Heptanone | Fruity, cheesy, sweet, tutti-frutti, cognac and pineapple nuances | 871 | 869 |
| 8.52 | + | + | 2-Heptanone | Fruity, acetone, green banana, with a creamy nuance | 888 | 889 |
| 10.26 | + | 3-Hepten-2-one | Sweet, fruity, acetone, with a green woody nuance | 936 | 940 | |
| 12.63 | + | 5-Hepten-2-one, 6-methyl | Citrus green, musty, lemongrass apple | 1001 | 1003 | |
| 14.66 | + | + | Acetophenone | Sweet, cherry pit and coumarin | 1062 | 1065 |
| 15.61 | + | + | 2-Nonanone | Fruity, sweet, waxy, green herbaceous, coconut like | 1090 | 1091 |
| 21.64 | + | + | 2-Undecanone | Waxy, fruity, acetone with fatty pineapple nuances | 1293 | 1291 |
| Terpenes | ||||||
| 12.23 | + | + | β-Myrcene | Herbaceous, woody with a rosy celery and carrot nuance | 990 | 991 |
| 13.3 | + | o-Cymene | Fresh citrus, woody spice | 1021 | 1021 | |
| 13.32 | + | m-Cymene | Citrus, herb flower | 1022 | 1026 | |
| 13.46 | + | + | D-Limonene | Citrus orange fresh sweet | 1026 | 1030 |
| 13.86 | + | + | β-cis-Ocimene | Warm floral herb flower sweet | 1038 | 1037 |
| 14.18 | + | β-trans-Ocimene | Sweet herbal | 1047 | 1050 | |
| 14.49 | + | γ-Terpinene | Sweet, citrus, with tropical and lime nuances | 1056 | 1059 | |
| 14.95 | + | cis-Linalool oxide | Sweet, Woody, Floral, Creamy, Slight earthy | 1070 | 1074 | |
| 15.44 | + | (Z)-Linalool oxide | Floral | 1085 | 1088 | |
| 15.91 | + | + | Linalool | Citrus floral, woody, green blueberry | 1099 | 1098 |
| 16.81 | + | (Z)-Neo-allo-ocimene | (Off-flavor) | 1128 | 1129 | |
| 17.16 | + | Camphor | Camphor | 1140 | 1139 | |
| 17.81 | + | Linalool oxide | Floral honey | 1161 | 1163 | |
| 18.63 | + | α-Terpineol | Pine, citrus, woody, floral | 1188 | 1191 | |
| 23.53 | + | + | Cyclosativene | Earthy (off-flavor) | 1364 | 1368 |
| 23.82 | + | + | Copaene | Woody (off-flavor) | 1375 | 1376 |
| 24.94 | + | Caryophyllene | Woody and terpenic (off-flavor) | 1419 | 1418 | |
| 25.74 | + | (E)-Geranylacetone | Fresh green fruity, waxy rose, magnolia tropical | 1451 | 1453 | |
| 26.61 | + | Guaiene | Sweet woody dry guaiac wood spicy powdery | 1486 | 1491 | |
| 27.13 | + | α-Farnesene | Woody, green vegetative with a hint of a floral nuance | 1508 | 1508 | |
| 27.37 | + | Naphthalene | Oil tar (off-flavor) | 1518 | 1513 | |
| 27.5 | + | δ-Cadinene | Herbal woody | 1523 | 1526 | |
| 28.44 | + | Nerolidol | Floral green, citrus, woody | 1563 | 1559 | |
| Alcohols | ||||||
| 2.91 | + | + | 2-pentanol | Green, Fruity, Sweet, Pungent, Plastic | – | 730 |
| 3.53 | + | Isoamyl alcohol | Balsamic fruit | – | 747 | |
| 5.35 | + | 2,3-Butanediol | Fruity, creamy, buttery | 802 | 802 | |
| 7.8 | + | 1-Hexanol | Flower, green | 869 | 867 | |
| 9.06 | + | + | 2-Heptanol | Fresh lemon, grass herbal, sweet floral, earthy (off-flavor) | 903 | 905 |
| 11.56 | + | 1-Heptanol | Musty, pungent, leafy green (off-flavor) | 971 | 969 | |
| 12.6 | + | 2-Octanol | Fresh, spicy green, earthy (off-flavor) | 1000 | 997 | |
| 13.57 | + | 1-Hexanol, 2-ethyl | Citrus, fresh floral, oily sweet | 1029 | 1028 | |
| 13.72 | + | + | Benzyl Alcohol | Floral rose phenolic balsamic | 1033 | 1032 |
| 15.97 | + | + | 2-Nonanol | Citrus orange, with slight fruity undernotes | 1101 | 1098 |
| 16.22 | + | + | Phenylethyl Alcohol | Floral rose, dried rose flower, rose water | 1110 | 1111 |
| Esters | ||||||
| 7.22 | + | Ethyl 3-methyl butyrate | Sweet, fruity | 853 | 856 | |
| 8.09 | + | + | Isoamyl Acetate | Sweet fruity banana solvent | 877 | 876 |
| 8.17 | + | 1-Butanol, 2-methyl-, acetate | Sweet, banana, fruity, ripe, fruit-like note | 879 | 880 | |
| 12.58 | + | Ethyl hexanoate | Sweet, fruity, pineapple, green banana nuance | 999 | 996 | |
| 18.02 | + | + | Ethyl benzoate | Sweet, fruity, medicinal, cherry, grape | 1168 | 1170 |
| 18.87 | + | + | Ethyl octanoate | Sweet, musty, pineapple and fruity | 1196 | 1194 |
| 20.17 | + | + | Ethyl phenylacetate | Floral honey, rosy with balsamic dark chocolate and anisic black licorice nuances | 1241 | 1243 |
| 24.14 | + | Ethyl 9-decenoate | Fruity fatty (off-flavor) | 1386 | 1388 | |
| 24.3 | + | + | Ethyl decanoate | Sweet, fruity, apple | 1394 | 1394 |
| 29.14 | + | Ethyl dodecanoate | Sweet, floral | 1594 | 1595 | |
| 33.54 | + | Ethyl myristate | Sweet | 1794 | 1793 | |
| Aldehydes | ||||||
| 2.50 | + | + | 3-Methylbutanal | Malty, chocolate | – | 655 |
| 5.21 | + | Hexanal | Green, fruity and with a woody nuance | 798 | 800 | |
| 10.97 | + | (E)-2-Heptenal | Intense green, oily, with fruity overtones | 955 | 957 | |
| 11.03 | + | + | Benzaldehyde | Sweet, almond, cherry | 957 | 961 |
| 13.93 | + | + | Benzeneacetaldehyde | Honey, floral powdery, fermented, chocolate | 1040 | 1043 |
| 14.45 | + | (E)-2-Octenal | Fresh cucumber, banana, green leaf | 1055 | 1056 | |
| 15.99 | + | Nonanal | Rose fresh, orange peel | 1102 | 1098 | |
| 19.09 | + | Decanal | Sweet aldehydic, orange peel, citrus floral | 1203 | 1204 | |
| 20.66 | + | (E)-2-Decenal | Earthy, mushroom, aldehydic with a chicken and pork fat nuance | 1259 | 1261 | |
| Others | ||||||
| 4.22 | + | Toluene | Caramel, synthetic (off-flavor) | – | 762 | |
| 7.37 | + | Ethylbenzene | (off-flavor) | 857 | 856 | |
| 8.36 | + | 2,6-Dimethyl-pyridine | Nutty, amine, | 884 | 885 | |
| 8.46 | + | Styrene | Sweet balsam, floral plastic (off-flavor) | 887 | 890 | |
| 13.76 | + | 2(3H)-Furanone, 5-ethenyldihydro-5-methyl- | Fruity, minty (off-flavor) | 1035 | 1039 | |
| 18.96 | + | n-Dodecane | Alkane (off-flavor) | 1199 | 1199 | |
| 21.79 | + | + | n-Tridecane | Alkane (off-flavor) | 1299 | 1299 |
| 24.44 | + | + | n-Tetradecane | Mild (off-flavor) | 1399 | 1399 |
| 26.92 | + | n-Pentadecane | Waxy (off-flavor) | 1499 | 1500 | |
| 29.27 | + | n-Hexadecane | (Off-flavor) | 1599 | 1600 | |
| 33.64 | + | n-Octadecane | Alkane (off-flavor) | 1799 | 1800 | |
aAccording to NIST Chemistry WebBook, SRD 69: www. http://webbook.nist.gov/chemistry/name-ser.html
bCalculated linear retention index
cLinear retention index obtained from literature data
Some of these compounds have been reported to confer desirable note flavors or off-flavors in cocoa grains during the fermentation, drying and/or roasting processes (Rodriguez-Campos et al. 2012).
A similar study was previously carried out using distinct cacao variety clones resistant to witches broom and provided by CEPLAC. When the hybrids PH6 and PH9 were used, 37 volatile compounds were detected at the beginning (0 h) and 34 volatile compounds, at the end (144 h) of fermentation (Magalhães et al. 2017). In another study carried out with four distinct hybrids, the authors detected 36 and 41 volatile compounds, respectively, at the beginning and at the end of the fermentation process of grains from the CEPEC2004 clone, and 35 and 42 compounds at 0 h and 144 h of fermentation, respectively for the PH15 hybrid. The highest number of volatile compounds, 38 and 46 were found at 0 h and 144 h, respectively, in the spontaneous fermentation of the PS1319 hybrid, while 31 volatile compounds were detected at 0 h and 42 at the end of fermentation of the grains from the SJ02 hybrid, (Magalhães et al. 2018).
The highest number of compounds in pulp is produced from sugars by yeast fermentation combined with the production of flavoring molecules such as esters, alcohols, acids, aldehydes and ketones, among others. Sixty-six volatiles were identified in cocoa pulp from Colombian plantations (Quijano et al. 2010).
The chemical signature of chocolate flavor is complex, since over 600 volatile and non-volatile compounds contribute to the production of desirable notes and are considered indicative of the quality of the cocoa grains. The influence of volatile compounds formed during fermentation on the final chocolate flavor is not yet defined, since some of them migrate to inside the grains and others can evaporate or be chemically transformed during roasting (Aprotosoaie et al. 2016).
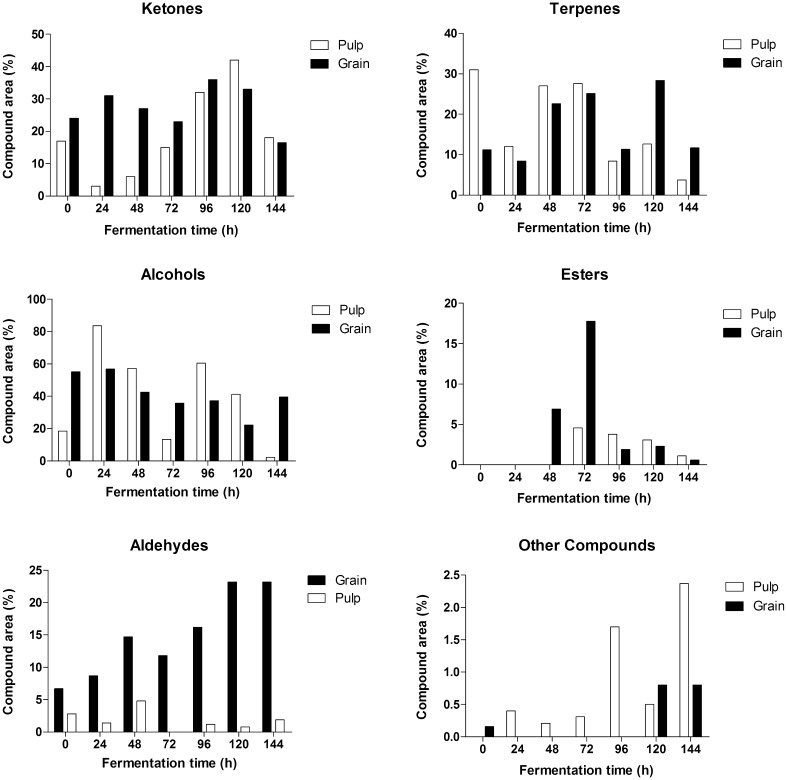
The different classes of volatile compounds produced during the cocoa pulp and grain fermentation processes and their variations are listed (Fig. 1). Through the cocoa fermentation process, the proteolytic reactions inside the grains release small peptides and free amino acids, which influence chocolate flavor in the later processing steps (Voigt and Lieberei 2014).
Fig. 1.
Relative concentrations (%) of volatile compounds formed in the cocoa pulp and in the grains during the fermentation. Volatiles from each sample were adsorbed into a solid phase micro extraction fiber and analyzed by gas chromatography-mass spectrometry a ketones, b terpenes, c alcohols, d esters, e aldehydes and f other compounds
The microbial community dynamics involved in spontaneous TSH565 fermentation were evaluated by enumerations in specific culture media followed by molecular identification of isolated microorganisms by random amplified polymorphic DNA (RAPD) and partial DNA sequencing. The results indicate that the cocoa pulp of this hybrid is first bioconverted by the yeast community (anaerobic phase), followed by lactic acid bacteria (aerobic phase), producing distinct volatile compounds and organic acids (acetic and lactic acid), which permeate the cocoa grain tissue (Bastos et al. 2018).
The relative amount of alcohols, aldehydes, esters, ketones, and terpenes detected in fresh pulp and grains was altered according to fermentation duration (Table 1).
Ketones
Ketones were detected at the beginning of the fermentation process (0 h) representing 16.2% and 25.1% of total volatile content in cocoa pulp and grains, respectively (Fig. 1). Concentrations were higher in the pulp after 120 h (41.4%), while ketones peaked at 96 h (36.4%). In grains, however, ketone production showed no alterations during the fermentative process. Ketone production has been ascribed to the yeasts in both grains and pulp of the TSH565 hybrid (Bastos et al. 2018).
Three identified ketone compounds—2-heptanone, acetophenone and 2-nonanone—were maintained only in the grains during the entire process (Tables 2, 3). Similar results have been previously reported, where 2-heptanone was the major compound found, but in both pulp and grains (Ho et al. 2015). The relative amount of acetophenone in grains was sustained during fermentation, conferring floral notes and a sweet flavor to the final product. The same ketone compounds have been reported in cocoa grains fermented and dried at 60 °C or 70 °C (Rodriguez-Campos et al. 2012). High ketone concentrations are propitious to the production of good quality cocoa, with fruity and floral notes.
Table 2.
Relative amount of volatile compounds found in pulp during fermentation
| Compound areas (%) | Odor descriptiona | Fermentation time (h) | ||||||
|---|---|---|---|---|---|---|---|---|
| Ketones | 0 | 24 | 48 | 72 | 96 | 120 | 144 | |
| 2-Pentanone | Fruity, Thinner, Acetone | 2.26 ± 0.03 | 1.89 ± 0.01 | 0 | 0.68 ± 0.02 | 0 | 0 | 2.02 ± 0.46 |
| 4-Heptanone | Fruity, cheesy, sweet, tutti-frutti, cognac and pineapple nuances | 0 | 0 | 0 | 0 | 0 | 0 | 0.21 ± 0.04 |
| 2-Heptanone | Fruity, acetone, green banana, with a creamy nuance | 9.52 ± 0.51 | 0 | 0 | 6.22 ± 0.55 | 22.6 ± 1.06 | 35.5 ± 1.1 | 0 |
| 5-Hepten-2-one, 6-methyl | Citrus green, musty, lemongrass apple | 0.6 ± 0.01 | 0 | 0 | 0.34 ± 0.01 | 1.27 ± 0.12 | 0 | 1.06 ± 0.13 |
| Acetophenone | Sweet, cherry pit and coumarinic | 2.31 ± 0.19 | 1.29 ± 0.02 | 4.0 ± 0.04 | 2.36 ± 0.08 | 6.9 ± 0.4 | 1.43 ± 0.03 | 5.29 ± 0.73 |
| 2-Nonanone | Fruity, sweet, waxy, green herbaceous, coconut like | 1.1 ± 0.01 | 0 | 2.4 ± 0.1 | 5.5 ± 0.4 | 0 | 4.5 ± 0.08 | 7.81 ± 0.6 |
| 2-Undecanone | Waxy, fruity, acetone with fatty pineapple nuances | 0.39 ± 0.01 | 0 | 0 | 0 | 0 | 0 | 0.1 ± 0.01 |
| Terpenes | ||||||||
| β-Myrcene | Herbaceous, woody with a rosy celery and carrot nuance | 8.0 ± 1.09 | 0 | 1.02 ± 0.1 | 0.72 ± 0.110 | 0 | 0 | 0 |
| o-Cymene | Fresh citrus, woody spice | 0 | 0.26 ± 0.02 | 0.2 ± 0.01 | 0 | 0.56 ± 0.05 | 0.098 ± 0.0 | 0 |
| m-Cymene | Citrus, herb flower | 0.18 ± 0.03 | 0 | 0 | 0.11 ± 0.01 | 0 | 0 | 0 |
| D-Limonene | Citrus orange fresh sweet | 1.68 ± 0.27 | 1.05 ± 0.1 | 0.51 ± 0.09 | 0.39 ± 0.04 | 2.72 ± 0.53 | 0.38 ± 0.03 | 1.43 ± 0.21 |
| β-cis-Ocimene | Warm floral herb flower sweet | 0.83 ± 0.05 | 0.61 ± 0.03 | 0.89 ± 0.05 | 0.4 ± 0.03 | 0 | 0 | 0 |
| γ-Terpinene | Sweet, citrus, with tropical and lime nuances | 0 | 0.39 ± 0.006 | 0 | 0 | 0.51 ± 0.05 | 0 | 0 |
| cis-Linalooloxide | Sweet, Woody, Floral, Creamy, Slight earthy | 10.6 ± 1.6 | 8.61 ± 0.84 | 5.29 ± 0.61 | 0 | 0 | 0 | 0 |
| Linalool | Citrus floral, woody, green blueberry | 6.23 ± 0.25 | 0 | 18.98 ± 0.86 | 25.28 ± 1.01 | 0 | 11.1 ± 0.25 | 0 |
| Camphor | Camphor | 0 | 0 | 0 | 0 | 0.89 ± 0.03 | 0.11 ± 0.01 | 0 |
| Linalool oxide | Floral honey | 2.47 ± 0.09 | 0 | 0 | 0 | 0 | 0.5 ± 0.04 | 0 |
| α-Terpineol | Pine, citrus, woody, floral | 0 | 0 | 0 | 0 | 0.8 ± 0.05 | 0.22 ± 0.02 | 0 |
| Cyclosativene | Earthy (off-flavor) | 0.14 ± 0.006 | 0 | 0.32 ± 0.04 | 0.26 ± 0.006 | 0.57 ± 0.08 | 0 | 0.51 ± 0.04 |
| Copaene | Woody (off-flavor) | 0.24 ± 0.006 | 0 | 0.29 ± 0.04 | 0.23 ± 0.01 | 0 | 0.2 ± 0.03 | 0.43 ± 0.04 |
| Caryophyllene | Woody and terpene (off-flavor) | 0 | 0 | 0 | 0.15 ± 0.01 | 0 | 0 | 0.3 ± 0.03 |
| (E)-Geranylacetone | Fresh green fruity, waxy rose, woody magnolia tropical | 0 | 0 | 0.67 ± 0.11 | 0.43 ± 0.03 | 0.76 ± 0.06 | 0 | 0.95 ± 0.02 |
| Guaiene | Sweet woody dry guaiac wood spicy powdery | 0 | 0 | 0.22 ± 0.006 | 0.14 ± 0.01 | 0 | 0 | 0 |
| α-Farnesene | Woody, green vegetative with a hint of a floral nuance | 0 | 0 | 0 | 0.21 ± 0.02 | 0 | 0 | 0.19 ± 0.04 |
| Selina-3,7(11)-diene | Not found (off-flavor) | 0 | 0 | 0 | 0 | 0.52 ± 0.09 | 0 | 0 |
| δ-Cadinene | Herbal woody | 0.32 ± 0.006 | 0 | 0 | 0.21 ± 0.02 | 0 | 0 | 0.33 ± 0.02 |
| Nerolidol | Floral green, citrus, woody | 0 | 0 | 0.26 ± 0.03 | 0 | 0 | 0 | 0 |
| Alcohols | ||||||||
| 2-pentanol | Green, Fruity, Sweet, Pungent, Plastic | 8.25 ± 0.18 | 12.23 ± 0.15 | 6.07 ± 0.12 | 3.68 ± 0.14 | 11.1 ± 0.39 | 8.3 ± 0.11 | 0.81 ± 0.21 |
| Isoamyl alcohol | Balsamic fruit | 0 | 2.05 ± 0.02 | 3.54 ± 0.12 | 2.2 ± 0.08 | 4.35 ± 0.04 | 1.23 ± 0.01 | 0 |
| 1-Hexanol | Flower, green | 1.13 ± 0.11 | 1.12 ± 0.08 | 0 | 0 | 0 | 0 | 0 |
| 2-Heptanol | Fresh lemon, grass herbal, sweet floral, earthy (off-flavor) | 41.04 ± 2.2 | 43.3 ± 1.98 | 33.2 ± 1.49 | 28.4 ± 1.3 | 29.5 ± 1.46 | 27.7 ± 0.64 | 0 |
| 1-Heptanol | Musty, pungent, leafy green (off-flavor) | 0 | 0 | 0.36 ± 0.07 | 0 | 0 | 0 | 0 |
| 1-Hexanol, 2-ethyl | Citrus, fresh floral, oily sweet | 0 | 0 | 0.46 ± 0.007 | 0.24 ± 0.03 | 0.83 ± 0.02 | 0.21 ± 0.02 | 0.65 ± 0.11 |
| Benzyl Alcohol | Floral rose phenolic balsamic | 0 | 0 | 0 | 0 | 0 | 0.28 ± 0.03 | 0 |
| Phenylethyl Alcohol | Floral rose, dried rose flower, rose water | 0 | 2.15 ± 0.03 | 4.56 ± 0.27 | 4.68 ± 0.28 | 13.0 ± 0.45 | 3.48 ± 0.17 | 0.62 ± 0.006 |
| 2-Nonanol | Citrus orange, with slight fruity undernotes | 0 | 23.5 ± 1.5 | 4.86 ± 0.12 | 0 | 0 | 0 | 0 |
| Esters | ||||||||
| Isoamyl acetate | Sweet fruity banana solvent | 0 | 0 | 0.57 ± 0.02 | 0.91 ± 0.09 | 0 | 0 | 0 |
| 1-Butanol, 2-methyl-, acetate | Sweet, banana, fruity, ripe, fruit-like note | 0 | 0 | 0 | 2.35 ± 0.006 | 0 | 0 | 0 |
| Ethyl benzoate | Sweet, fruity, medicinal, cherry, grape | 0 | 0 | 0 | 0.4 ± 0.02 | 0 | 0 | 0 |
| Ethyl octanoate | Sweet, musty, pineapple and fruity | 0 | 0 | 2.3 ± 0.3 | 1.21 ± 0.13 | 0.83 ± 0.11 | 0 | 0 |
| Ethyl phenylacetate | Floral honey, rosy with balsamic dark chocolate and licorice nuances | 0 | 0 | 1.57 ± 0.06 | 2.38 ± 0.1 | 0 | 0 | 0 |
| Ethyl 9-decanoate | Fruity fatty | 0 | 0 | 0 | 0.24 ± 0.01 | 0 | 0 | 0 |
| Ethyl decanoate | Sweet, fruity, apple | 0 | 0 | 2.29 ± 0.37 | 3.96 ± 0.51 | 0 | 0 | 0 |
| Ethyl dodecanoate | Sweet, floral | 0 | 0 | 0 | 5.32 ± 0.13 | 0 | 1.37 ± 0.03 | 0.85 ± 0.02 |
| Ethyl myristate | Sweet | 0 | 0 | 0 | 0.11 ± 0.01 | 0.36 ± 0.02 | 0 | 0 |
| Aldehydes | ||||||||
| 3-Methylbutanal | Malty, chocolate | 0 | 0 | 0 | 0 | 0 | 0 | 0.29 ± 0.02 |
| Hexanal | Green, fruity and with a woody nuance | 0 | 0 | 0.63 ± 0.09 | 0 | 0 | 0 | 0 |
| (E)-2-Heptenal | Intense green, oily, with fruity overtones | 0 | 0 | 0.09 ± 0.01 | 0 | 0 | 0 | 0 |
| Benzaldehyde | Sweet, almond, cherry | 0 | 0 | 0.27 ± 0.006 | 0 | 0 | 0 | 0.42 ± 0.04 |
| Benzeneacetaldehyde | Honey, floral powdery, fermented, chocolate | 0.32 ± 0.02 | 0.4 ± 0.02 | 0.34 ± 0.006 | 0 | 0 | 0.38 ± 0.01 | 0.55 ± 0.04 |
| (E)-2-Octenal | Fresh cucumber, banana, green leaf | 0 | 0 | 1.21 ± 0.17 | 0 | 0 | 0 | 0 |
| Nonanal | Rose fresh, orange peel | 1.54 ± 0.02 | 0 | 0 | 0 | 0 | 0 | 0 |
| Decanal | Sweet aldehyde, orange peel, citrus floral | 0.62 ± 0.01 | 0.52 ± 0.04 | 0.41 ± 0.05 | 0 | 0.77 ± 0.12 | 0.09 ± 0.002 | 0 |
| (E)-2-Decenal | Earthy, mushroom, aldehyde with a chicken and pork fat nuance | 0 | 0 | 1.9 ± 0.17 | 0 | 0 | 0 | 0 |
| Others | ||||||||
| Ethylbenzene | (Off-flavor) | 0 | 0.22 ± 0.006 | 0 | 0 | 0 | 0 | 0 |
| 2,6-Dimethyl-pyridine | Nutty, amine | 0 | 0 | 0 | 0 | 0 | 0 | 0.45 ± 0.08 |
| 2(3H)-Furanone, 5-ethenyldihydro-5-methyl | Fruity, minty (off-flavor) | 0 | 0 | 0 | 0 | 0 | 0 | 1.82 ± 0.44 |
| n-Dodecane | Alkane (off-flavor) | 0 | 0 | 0 | 0 | 0 | 0.11 ± 0.01 | 0 |
| n-Tridecane | Alkane (off-flavor) | 0 | 0 | 0 | 0 | 0.42 ± 0.06 | 0.13 ± 0.01 | 0 |
| n-Tetradecane | Mild (off-flavor) | 0 | 0.14 ± 0.01 | 0 | 0 | 0 | 0.1 ± 0.02 | 0 |
| n-Pentadecane | Waxy (off-flavor) | 0 | 0 | 0 | 0 | 0.73 ± 0.14 | 0.19 ± 0.03 | 0 |
| n-Hexadecane | — (off-flavor) | 0 | 0 | 0 | 0 | 0.46 ± 0.03 | 0 | 0 |
| n-Octadecane | Alkane (off-flavor) | 0 | 0 | 0 | 0.22 ± 0.01 | 0 | 0 | 0 |
aAccording to NIST Chemistry WebBook, SRD 69: www. http://webbook.nist.gov/chemistry/name-ser.html
Table 3.
Changes in the relative concentrations of volatile compounds formed during cocoa grain fermentation
| Compound Areas (%) | Odor descriptiona | Fermentation time (h) | ||||||
|---|---|---|---|---|---|---|---|---|
| Ketones | 0 | 24 | 48 | 72 | 96 | 120 | 144 | |
| 4-Heptanone | Fruity, cheesy, sweet, tutti-frutti, cognac and pineapple nuances | 0 | 0 | 0 | 0 | 0 | 0 | 0.09 ± 0.02 |
| 2-Heptanone | Fruity, acetone, green banana, with a creamy nuance | 16.9 ± 0.1 | 19.6 ± 0.1 | 18.1 ± 0.2 | 11.4 ± 0.5 | 17.6 ± 0.5 | 20.8 ± 1.2 | 15.5 ± 0.7 |
| 3-Hepten-2-one | Sweet, fruity, acetone, with a green woody nuance | 0 | 0 | 0 | 0 | 0 | 0.2 ± 0.04 | 0 |
| Acetophenone | Sweet, cherry pit and coumarin | 1.6 ± 0.1 | 1.0 ± 0.05 | 1.8 ± 0.04 | 1.5 ± 0.1 | 2.3 ± 0.1 | 2.1 ± 0.1 | 1.5 ± 0.1 |
| 2-Nonanone | Fruity, sweet, waxy, green herbaceous, coconut like | 6.6 ± 0.1 | 9.7 ± 0.1 | 6.7 ± 0.4 | 9.2 ± 0.4 | 16.5 ± 0.1 | 9.3 ± 0.3 | 9.04 ± 0.08 |
| 2-Undecanone | Waxy, fruity, acetone with fatty pineapple nuances | 0 | 0 | 0 | 0 | 0.04 ± 0.006 | 0 | 0 |
| Terpenes | ||||||||
| β-Myrcene | Herbaceous, woody with a rosy celery and carrot nuance | 4.4 ± 0.07 | 3.7 ± 0.1 | 7.2 ± 0.1 | 5.08 ± 0.3 | 4.1 ± 0.3 | 8.5 ± 0.1 | 7.8 ± 0.2 |
| D-Limonene | Citrus orange fresh sweet | 0.4 ± 0.05 | 0.18 ± 0.04 | 0 | 0.41 ± 0.04 | 0.44 ± 0.05 | 0.33 ± 0.04 | 0.33 ± 0.04 |
| β-cis-Ocimene | Warm floral herb flower sweet | 3.02 ± 0.06 | 3.4 ± 0.2 | 5.9 ± 0.4 | 6.08 ± 0.3 | 4.3 ± 0.1 | 13.5 ± 0.5 | 0 |
| β-trans-Ocimene | Sweet herbal | 0.4 ± 0 | 0 | 1.1 ± 0.2 | 1.2 ± 0.1 | 0.7 ± 0.05 | 1.6 ± 0.1 | 0 |
| (Z)-Linalool oxide | Floral | 1.6 ± 0.1 | 1.01 ± 0.05 | 0 | 1.3 ± 0.1 | 1.2 ± 0.07 | 0.44 ± 0.01 | 1.5 ± 0.06 |
| Linalool | Citrus floral, woody, green blueberry | 0 | 0 | 6.9 ± 0.7 | 10.5 ± 0.5 | 0 | 0 | 0 |
| (Z)-Neo-allo-ocimene | Not found (off-flavor) | 0.4 ± 0.05 | 0.4 ± 0.08 | 1.1 ± 0.1 | 0.8 ± 0.1 | 0.64 ± 0.06 | 1.2 ± 0.1 | 0.8 ± 0.1 |
| Cyclosativene | Earthy (off-flavor) | 0.08 ± 0.01 | 0.04 ± 0.003 | 0 | 0 | 0 | 0 | 0 |
| Copaene | Woody (off-flavor) | 0.1 ± 0.01 | 0.02 ± 0.006 | 0 | 0 | 0 | 0 | 0 |
| Alcohols | ||||||||
| 2-pentanol | Green, Fruity, Sweet, Pungent, Plastic | 6.2 ± 0.5 | 9.9 ± 0.5 | 0.7 v 0.05 | 3.3 ± 0.06 | 0.8 ± 0.02 | 0.8 ± 0.01 | 0.7 ± 0.01 |
| Isoamyl alcohol | Balsamic fruit | 0 | 0.67 ± 0.04 | 0 | 1.8 ± 0.05 | 0 | 0 | 0.61 ± 0.03 |
| 2,3-Butanediol | Fruity, creamy, buttery | 0 | 0 | 0 | 0.44 ± 0.03 | 0.85 ± 0.07 | 0 | 0 |
| 2-Heptanol | Fresh lemon, grass herbal, sweet floral, earthy (off-flavor) | 43.6 ± 1.1 | 38.2 ± 0.9 | 37.7 ± 1.8 | 22.9 ± 0.4 | 26.6 ± 1.4 | 13.05 ± 0.5 | 28.4 ± 0.7 |
| 2-Octanol | Fresh, spicy green, earthy (off-flavor) | 0.6 ± 0.06 | 0.5 ± 0.07 | 0.5 ± 0.05 | 0 | 0.6 ± 0.06 | 0.47 ± 0.08 | 0.4 ± 0.06 |
| Benzyl Alcohol | Floral rose phenolic balsamic | 0 | 0 | 0 | 0 | 0 | 0 | 0.17 ± 0.03 |
| 2-Nonanol | Citrus orange, with slight fruity undernotes | 1.4 ± 0.08 | 4.19 ± 0.1 | 2.39 ± 0.2 | 2.2 ± 0.05 | 6.2 ± 0.2 | 4.13 ± 0.08 | 2.8 ± 0.1 |
| Phenylethyl Alcohol | Floral rose, dried rose flower, rose water | 0 | 0 | 0.53 ± 0.1 | 2.1 ± 0.1 | 1.2 ± 0.07 | 2.4 ± 0.2 | 5.6 ± 0.1 |
| Esters | ||||||||
| Ethyl 3-methyl butyrate | Sweet, fruity | 0 | 0 | 0 | 0 | 0 | 0 | 0.067 ± 0.0 |
| Isoamyl acetate | Sweet fruity banana solvent | 0 | 0 | 0.11 ± 0.006 | 2.2 ± 0.1 | 1.53 ± 0.03 | 0.65 ± 0.01 | 0.17 ± 0.0 |
| Ethyl hexanoate | Sweet, fruity, pineapple, green banana nuance | 0 | 0 | 0 | 0.84 ± 0.1 | 0.41 ± 0.02 | 0.39 ± 0.02 | 0.19 ± 0.03 |
| Ethyl benzoate | Sweet, fruity, medicinal, cherry, grape | 0 | 0 | 0 | 0 | 0 | 0.27 ± 0.01 | 0.19 ± 0.03 |
| Ethyl octanoate | Sweet, musty, pineapple and fruity | 0 | 0 | 0 | 0.77 ± 0.09 | 0.37 ± 0.05 | 0.56 ± 0.01 | 0.31 ± 0.02 |
| Ethyl phenylacetate | Floral honey, rosy with balsamic dark chocolate nuances | 0 | 0 | 0 | 0.46 ± 0.006 | 0.47 ± 0.01 | 0.22 ± 0.006 | 0.23 ± 0.006 |
| Ethyl decanoate | Sweet, fruity, apple | 0 | 0 | 0 | 0 | 0 | 0.18 ± 0.006 | 0 |
| Aldehydes | ||||||||
| 3-Methylbutanol | Malty, chocolate | 2.32 ± 0.07 | 1.04 ± 0.05 | 2.2 ± 0.1 | 3.2 ± 0.01 | 1.29 ± 0.04 | 4.99 ± 0.04 | 4.3 ± 0.1 |
| Benzaldehyde | Sweet, almond, cherry | 2.14 ± 0.03 | 0.37 ± 0.01 | 0.41 ± 0.04 | 0.72 ± 0.04 | 1.03 ± 0.01 | 1.49 ± 0.01 | 1.15 ± 0.0 |
| Benzeneacetaldehyde | Honey, floral powdery, chocolate | 7.64 ± 0.4 | 5.56 ± 0.21 | 6.01 ± 0.16 | 10.76 ± 0.09 | 8.86 ± 0.4 | 9.79 ± 0.16 | 16.73 ± 0.37 |
| Others | ||||||||
| Toluene | Caramel, synthetic (off-flavor) | 0 | 0 | 0 | 0 | 0 | 0.113 ± 0.0 | 0.09 ± 0.0 |
| Styrene | Sweet balsam, floral plastic (off-flavor) | 0 | 0 | 0 | 0 | 0 | 0.71 ± 0.08 | 0.64 ± 0.1 |
| n-Tridecane | Alkane (off-flavor) | 0.04 ± 0.006 | 0 | 0 | 0 | 0 | 0.073 ± 0.0 | 0.067 ± 0.0 |
| n-Tetradecane | Mild (off-flavor) | 0 | 0 | 0 | 0 | 0 | 0 | 0.093 ± 0.01 |
aAccording to NIST Chemistry WebBook, SRD 69: www. http://webbook.nist.gov/chemistry/name-ser.html
Terpenes
Twenty terpenes were observed in the pulp during the fermentation, specifically after 72 h (Table 2).
Nine terpenes were detected in grains during fermentation (Table 3). Linalool was the most relevant compound in this group, detected during the middle of the fermentation process (48–72 h), generally considered a biosynthesis product and found in its glycosidic bound form in the fruit pulp and in cocoa grain cotyledons. Linalool and its derivatives are often described as having a floral aroma and are considered key aroma components found in high concentrations in “noble-flavor” cocoas, such as Criollo and Arriba (Owusu et al. 2012).
β-myrcene and D-limonene were constantly detected in grains and pulps, respectively, during fermentation. Considering these compounds, β-myrcene was the only major compound detected at the end of grain fermentation (7.8%), while β-cis-ocimene presented its highest concentration (13.5%) after 120 h of fermentation, being described as responsible for a warm floral and herb flower sweet odor. β-myrcene and β-cis-ocimene were detected in the grains of the parental clone, SCA 6, and were associated to a fine chocolate aroma (Kadow et al. 2015).
Alcohols
Nine and eight distinct alcohols were identified in fresh and fermented grains and pulps, respectively (Table 1). Some of these compounds, like 2-heptanol, phenylethyl alcohol and benzyl alcohol, are responsible for odor production with desirable notes (Galvez et al. 2007).
After 24 h of pulp fermentation, an abrupt increase in alcohol content was observed (84.4%), followed by a gradual and constant decrease until reaching 2.1% at the end of the fermentation process (Fig. 1). These alcohols are formed by yeast metabolism, the predominant microorganisms in the first hours of fermentation, and the decrease in alcohol concentration may be due to their oxidation by acetic acid bacteria in the fermentative microbiota, as well as by evaporation, provoked by the increased temperature of the cocoa mass (Galvez et al. 2007). In grains, constant alcohol concentrations were observed during fermentation, reaching a relative percentage of 38.7% at the end of the process (Fig. 1).
The main volatile compound belonging to this class is 2-heptanol, which decreased during fermentation, with no detection in pulps but present at 28.4% relative amount in grains at the end of the fermentation process, overlapping pulp migration to the inside of the grains (Table 3). The high alcohol concentrations confer floral and sweet notes to the end-product (Aculey et al. 2010).
Benzyl alcohol, phenylethyl alcohol and 2-nonanol were produced during grain fermentation (Table 3). These alcohols were also detected during cocoa fermentation by Klesbsiella apiculata and Saccharomyces cerevisiae, and are desirable compounds found in high quality cocoa end products. It should be also considered that the migration of alcohol and ester aromas from the pulp into the grain tissue can be influenced by acetic acid, due to its ability to produce a permeable grain coat (Chetschik et al. 2018).
Esters
Nine main esters were formed during pulp fermentation and seven were synthesized in the fermented grains (Table 1). These compounds were detected after 48 h of fermentation in pulp and 72 h in grains but showed the highest concentrations after 72 h in both compartments, at 16.9 and 4.3%, respectively (Fig. 1).
The main esters were ethyl phenylacetate, isoamyl acetate, ethyl phenyl acetate and ethyl dodecanoate (Tables 2, 3). These compounds are well represented in fruit aromas and are the second most important group of volatile compounds, after pyrazines. In roasted cocoa grains, they confer a fruity flavor and are the typical aroma components (mainly acetates) that arise from amino acids in unroasted cocoa grains (Aprotosoaie et al. 2016).
The formation of amyl acetates during fermentation must be avoid because they are considered as indicators of flavour defects (Rodriguez-Campos et al. 2012).
The formation of amyl acetates during fermentation must be avoided, since they are considered flavor defect indicators (Rodriguez-Campos et al. 2012). Isoamyl acetate was detected in minor concentrations in the present study (0.11%).
The main source of ester production comes from yeast metabolism. Several yeast species have been detected in the spontaneous fermentation of the cocoa hybrid assessed herein, persisting during the entire fermentative process (Bastos et al. 2018), where some acids, such as citric acid, are consumed by yeast during the fermentation process. Ester production occurs in the stationary phase during ethanol utilization, when alcohols are enzymatically converted into their correspondent esters. Additionally, they can be produced during post-alcohol fermentation, through the chemical reaction between acids and alcohols, albeit in a minor scale when compared to the enzymatic reactions. The concentration of these compounds depends on yeast strains, fermentation temperature, aeration and sugar concentrations in the fermentative mass (Perestrelo et al. 2006).
Aldehydes
Nine aldehydes were detected in pulp but only benzaldehyde and benzeneacetaldehyde showed relative constant concentrations during fermentation. In the fresh grains, 3-methylbutanal, benzaldehyde and benzeneacetaldehyde were detected in high concentrations and persisted throughout the whole fermentation period (Table 1).
Aldehyde concentrations were higher in grains compared to pulps (Tables 2, 3). These compounds are frequently associated to lipid degradation and can confer pleasurable or disgusting notes (Bryant and McClung 2011), depending on their concentrations in the end product. The pleasant notes conferred by aldehydes are sweet and bitter flavors, such as cherry and almond aromas.
Other compounds
Another nine distinct compounds were detected in pulp and four compounds in grains but in relatively lower concentrations (Table 1). Among the compounds found in the pulp, 2,6-dimethyl-pyridine and 2(3H)-furanone, 5-ethenyldihydro-5-methyl were identified at the end of the fermentation, at relative concentrations of 0.4 and 1.8%, respectively. In grains, styrene was found at 0.6% concentration in the same phase of fermentation (Table 3).
Sensorial evaluation of the chocolate produced
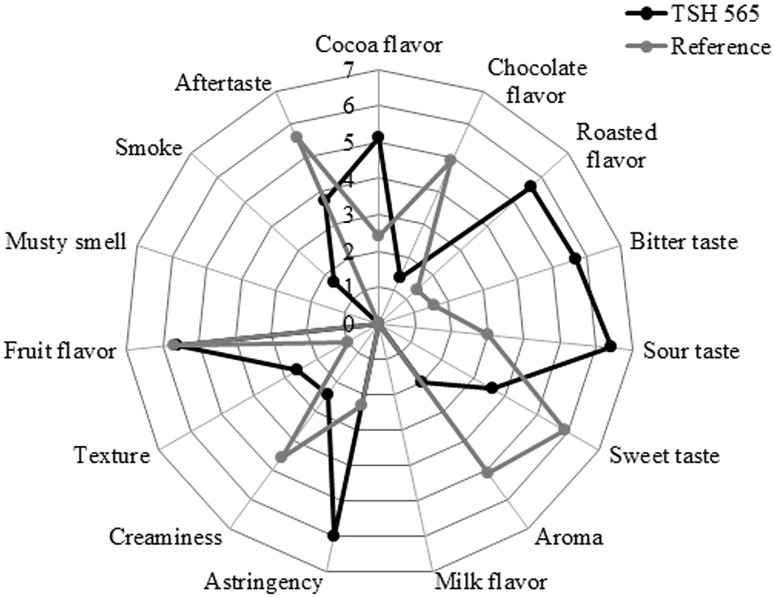
The sensory analysis of the chocolates produced after the spontaneous fermentation of the TSH565 cocoa clone showed taste differences when compared to a commercial chocolate. The judges generated a list of 15 descriptive attributes—cocoa flavor, chocolate flavor, roasted flavor, bitter taste, sour taste, sweet taste, aroma, milk flavor, astringency, creaminess, texture, fruit flavor, musky smell, smoke and aftertaste—with minimum and maximum values for the sensory characterization of chocolate samples. The means for each attribute was then calculated (Fig. 2).
Fig. 2.
Sensory evaluation of the chocolate produced from the spontaneous fermentation of TSH565 cocoa hybrid. The zero point of the descriptor scale is in the center of the figure and intensity increases toward the extremities. The mean value attributed to each trained panelist was marked in the corresponding axis and the sensory profile was draw by connecting the points. A dark Barry Callebaut commercial chocolate was used as the reference to compare the intensity of each sensory attribute
Regarding the aforementioned attributes, the chocolate produced from the TSH565 clone was perceived as an intense cocoa, roasted, bitter, sour and astringent and the scores conferred to these attributes allow for the end-product to be called “strong”. Alternatively, sweet and flavor attributes were perceived in low intensities, receiving low scores. In addition, the judges also identified a smoky smell in the chocolate produced from TSH565 cocoa.
The low intensity of a sweet chocolate flavor from the TSH565 clone could be masked by the high intensity of the bitter taste perceived by the judges. The bitter taste can be associated to the incomplete volatilization of acetic acid formed during fermentation, affecting the taste of the produced chocolate. A sour taste cannot be particularly attributed to this variety of cocoa grains, but can be acquired during fermentation, when the grains absorb acids and other substances produced by microorganisms involved in the process. The popularity of cocoa products is related to their unique sensory and pleasant melt-in-the-mouth properties (Torres-Moreno et al. 2015).
The fruit attributes presented close scores—5.6 and 5.7—when comparing the chocolates produced from the TSH565 clone and the commercial sample, respectively. A musty smell was observed in the commercial sample but not in the TSH565 chocolate. Unlike other studies, which indicated flower and fruit aromas as descriptors, the panelists did not perceive flower aromas, but the TSH565 chocolate presented acidity and roasting odors, characteristics previously identified in chocolates from Soconusco, Mexico (Vázquez-Ovando et al. 2015).
Regarding a weaker “aftertaste” attribute, the panelists mentioned a strong taste similar to “brown sugar” in the TSH565 chocolate, with a sandy texture and less creaminess than the reference sample. Concerning texture attribute, TSH565 chocolate was perceived as sandy, with a grainier texture, whereas the reference chocolate was perceived as creamier (Fig. 2).
The sensory attributes of the end-products depend on the genetic background of the cocoa fruits and the composition of endogenous microbiota, which confer a unique flavor to the chocolate, since processing methods, such as roasting, conching and the ingredient ratio of the chocolate manufacturing can be easily controlled.
During fermentation, pulp components are used by yeast and bacteria to develop precursors, such as ethanol and organic acids, that kill the grains, by slowly penetrating them, leading to swelling and stimulating the enzyme reactions that yield precursors, which are then transformed into the characteristic flavors and aroma notes during the roasting of grains (Engeseth and Pangan 2018). The diffusion rate of the organic acids into the beans, the timing of initial entry, length of permanence at optimum pH and the pH reached at the end of fermentation are crucial for optimal flavor formation, and cocoa fermentation adjustments can reduce acid notes and maximize chocolate flavors (Afoakwa et al. 2008). Free amino acids and peptides are generated from the enzymatic degradation of cocoa proteins, which, in turn, can form the typical cocoa aroma during the subsequent roasting process (Afoakwa et al. 2013). In addition to the formation of flavor precursors, a significant increase in volatile compounds after fermentation is also observed, and phenolic compounds are oxidized and polymerized to insoluble high molecular-weight compounds (tannins), leading to a significant decrease in their concentrations and, consequently, reducing the bitterness and astringency of the final product to acceptable levels (Magalhães et al. 2018).
The development of chocolate flavor is complex and over 500 non-volatile and volatile compounds contribute to characteristic chocolate flavors (Ho et al. 2015). Each cocoa hybrid presents a distinct fermentation dynamic in forming different volatile compounds, in different amounts, and contributes to the unique flavor sensations of chocolate products.
Conclusion
This study aids in organizing the key flavor compounds in the cocoa hybrid TSH565 pulp and grains. The identification of the main compounds produced during cocoa grains fermentation can aid in the search for off-flavor indicators and fermentation indices, such as cyclosativene, copaene, 2-heptanol, 2-octanol and ethyl-dodecanoate. Identification of the main compounds produced during traditional cocoa fermentation may aid in deciding when to stop the fermentative process, to avoid the production of off-flavor compounds, and the determination of the odor profile of the pulp/grains may be considered an indicator of the flavor quality of cocoa grains. The assumption is that the volatile profile of the grains reflects the odor and flavor properties of the fermented cocoa grains, which, in turn, will influence the sensory quality of chocolate.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors acknowledge financial support from CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico), CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior), FAPERJ (Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro), IFS (Instituto Federal de Sergipe, Campus Nossa Senhora da Gloria) and CEPLAC (Comissão Executiva do Plano da Lavoura Cacaueira).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Aculey PC, Snitkjaer P, Owusu M, Bassompiere M, Takrama J, Norgaard L, Petersen MA, Nielsen DS. Ghanaian cocoa bean fermentation characterized by spectroscopic and chromatographic methods and chemometrics. J Food Sci. 2010;75:S300–S307. doi: 10.1111/j.1750-3841.2010.01710.x. [DOI] [PubMed] [Google Scholar]
- Adams RP. Identification of essential oil components by gas chromatography/mass spectrometry. Carol Stream: Allured Publishing Corporation; 2007. [Google Scholar]
- Afoakwa EO, Paterson A, Fowler M, Ryan A. Flavor formation and character in cocoa and chocolate: a critical review. Crit Rev Food Sci Nutr. 2008;48:840–857. doi: 10.1080/10408390701719272. [DOI] [PubMed] [Google Scholar]
- Afoakwa EO, Kongor JE, Takrama J, Budu AS. Changes in nib acidification and biochemical composition during fermentation of pulp pre-conditioned cocoa (Theobroma cacao) beans. Int Food Res J. 2013;20:1843–1853. [Google Scholar]
- Aprotosoaie AC, Luca SV, Miron A. Flavor chemistry of cocoa and cocoa products—An overview. Compr Rev Food Sci Food Saf. 2016;15:73–91. doi: 10.1111/1541-4337.12180. [DOI] [PubMed] [Google Scholar]
- Arn H, Acree TE. Flavornet: a database of aroma compounds based on odor potency in natural products. Dev Food Sci. 1998;40:27. doi: 10.1016/S0167-4501(98)80029-0. [DOI] [Google Scholar]
- Associação Brasileira de Normas Técnicas – ABNT . Escalas utilizadas em análise sensorial de alimentos e bebidas—NBR 14141. Rio de Janeiro: ABNT; 1998. [Google Scholar]
- Bastos VS, Santos MFS, Gomes LP, Leite AMO, Paschoalin VMF, Del Aguila EM. Analysis of the cocobiota and metabolites of Moniliophthora perniciosa-resistant (Theobroma cacao L.) beans during spontaneous fermentation in Southern Brazil. J Sci Food Agric. 2018;98:4963–4970. doi: 10.1002/jsfa.9029. [DOI] [PubMed] [Google Scholar]
- Bryant RJ, McClung AM. Volatile profiles of aromatic and non-aromatic rice cultivars using SPME/GC–MS. Food Chem. 2011;124:501–513. doi: 10.1016/j.foodchem.2010.06.061. [DOI] [Google Scholar]
- Capriolia G, Fiorinia D, Maggia F, Nicolettib M, Ricciutellia M, Toniolob C, Prosperc B, Vittoria S, Sagratinia G. Nutritional composition, bioactive compounds and volatile profile of cocoa beans from different regions of Cameroon. Int J Food Sci Nutr. 2016;67:422–430. doi: 10.3109/09637486.2016.1170769. [DOI] [PubMed] [Google Scholar]
- Cevallos-Cevallos JM, Gysel L, Maridueña-Zavala MG, Molina-Miranda MJ. Time-related changes in volatile compounds during fermentation of bulk and fine-flavour cocoa (Theobroma cacao) beans. J Food Qual. 2018;2018:Article ID 1758381. doi: 10.1155/2018/1758381. [DOI] [Google Scholar]
- Chetschik I, Kneubul M, Chatelain K, Schluter A, Bernath K, Huhn T. Investigations on the aroma of cocoa pulp (Theobroma cacao L.) and its influence on the odor of fermented cocoa beans. J Agric Food Chem. 2018;66:2467–2472. doi: 10.1021/acs.jafc.6b05008. [DOI] [PubMed] [Google Scholar]
- Crafack M, Keul H, Eskildsen CE, Petersen MA, Saerens S, Blennow A, Skovmand-Larsen M, Swiegers JH, Petersen GB, Heimdal H, Nielsen DS. Impact of starter cultures and fermentation techniques on the volatile aroma and sensory profile of chocolate. Food Res Int. 2014;63:306–316. doi: 10.1016/j.foodres.2014.04.032. [DOI] [Google Scholar]
- Dunkel M, Schmidt U, Struck S, Berger L, Gruening N, Hossbach J, Jaeger IS, Effmert U, Piechulla B, Eriksson R, Knudsen J, Preissner R. SuperScent—a database of flavors and scents. Nucleic Acids Res. 2009;37:D291–D294. doi: 10.1093/nar/gkn695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engeseth NJ, Pangan MF. Current context on chocolate flavor development— a review. Curr Opin Food Sci. 2018;21:84–91. doi: 10.1016/j.cofs.2018.07.002. [DOI] [Google Scholar]
- Ho VTT, Zhao J, Fleet G. The effect of lactic acid bacteria on cocoa bean fermentation. Int J Food Microbiol. 2015;205:54–67. doi: 10.1016/j.ijfoodmicro.2015.03.031. [DOI] [PubMed] [Google Scholar]
- Kadow D, Niemenak N, Rohn S, Lieberei R. Fermentation like incubation of cocoa seeds (Theobroma cacao L) Food Sci Technol. 2015;62:357–361. [Google Scholar]
- Kingor JE, Hinneh M, Walle DV, Afoakwa EO, Boeckx P, Dewettinck K. Factors influencing quality variation in cocoa (Theobroma cacao) bean flavour profile—a review. Food Res Int. 2016;82:44–52. doi: 10.1016/j.foodres.2016.01.012. [DOI] [Google Scholar]
- Lanza CM, Mazzaglia A, Pagliarini E. Sensory profile of a specialty Sicilian chocolate. Int J Food Sci. 2011;23:36–44. [Google Scholar]
- Li Y, Feng Y, Zhu S, Luo C, Ma J, Zhong F. The effect of alkalization on the bioactive and flavor-related components in commercial cocoa powder. J Food Compos Anal. 2012;25:17–23. doi: 10.1016/j.jfca.2011.04.010. [DOI] [Google Scholar]
- Lima LJR, Almeida MH, Rob Nout MJ, Zwietering MH. Theobroma cacao L., “The Food of the Gods”: quality determinants of commercial cocoa beans, with particular reference to the impact of fermentation. Crit Rev Food Sci Nutr. 2011;51:731–761. doi: 10.1080/10408391003799913. [DOI] [PubMed] [Google Scholar]
- Magi E, Bono L, Di Carro M. Characterization of cocoa liquors by GC–MS and LC–MS/MS: focus on alkylpyrazines and flavanols. J Mass Spectrom. 2012;47:1191–1197. doi: 10.1002/jms.3034. [DOI] [PubMed] [Google Scholar]
- Magalhães VMI, de Figueiredo LV, da Cruz Pedroso MGM, Santos C, Lima N, Schwan RF. Impact of a microbial cocktail used as a starter culture on cocoa fermentation and chocolate flavor. Molecules. 2017;22:766–791. doi: 10.3390/molecules22050766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magalhães VMI, de Figueiredo LV, Santos C, Lima N, Schwan RF. Volatile compounds and protein profiles analyses of fermented cocoa beans and chocolates from different hybrids cultivated in Brazil. Food Res Int. 2018;109:196–203. doi: 10.1016/j.foodres.2018.04.012. [DOI] [PubMed] [Google Scholar]
- Maharaj K, Maharaj P, Bekele FL, Ramnath D, Bidaisee GG, Bekele I, Persad C, Jennings K, Sankar R. Trinidad selected hybrids: an investigation of the phenotypic and agro-economic traits of 20 selected cacao cultivars. Trop Agric (Trinidad) 2011;88:175–185. [Google Scholar]
- Owusu M, Petersen MA, Heimdal H. Effect of fermentation method, roasting and conching conditions on the aroma volatiles of dark chocolate. J Food Proc Preserv. 2012;36:446–456. doi: 10.1111/j.1745-4549.2011.00602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perestrelo R, Fernandes A, Albuquerque FF, Marques JC, Câmara JS. Analytical characterization of the aroma of Tinta Negra Mole red wine: identification of the main odorants compounds. Anal Chimic Acta. 2006;563:154–164. doi: 10.1016/j.aca.2005.10.023. [DOI] [Google Scholar]
- Quijano CE, Pino AJ, Ceballos L. Headspace volatiles of Theobroma cacao L. pulp from Colombia. J Essent Oil Res. 2010;22:113–115. doi: 10.1080/10412905.2010.9700383. [DOI] [Google Scholar]
- Richter V, Almeida T, Prudencio S, Benassi M. Proposing a ranking descriptive sensory method. Food Qual Prefer. 2010;21:611–620. doi: 10.1016/j.foodqual.2010.03.011. [DOI] [Google Scholar]
- Rodriguez-Campos J, Escalona-Buendía H, Contreras-Ramos S, Orozco-Avila I, Jaramillo-Flores E, Lugo-Cervantes E. Effect of fermentation time and drying temperature on volatile compounds in cocoa. Food Chem. 2012;132:277–288. doi: 10.1016/j.foodchem.2011.10.078. [DOI] [PubMed] [Google Scholar]
- The Good Scents Company (2015) The Good Scents Company Information System. 1. http://www.thegoodscentscompany.com/misc/about.html. Accessed 10 Jan 2019
- Torres-Moreno M, Torrescasana E, Salas-Salvado J, Blanch C. Nutritional composition and fatty acids profile in cocoa beans and chocolates with different geographical origin and processing conditions. Food Chem. 2015;166:125–132. doi: 10.1016/j.foodchem.2014.05.141. [DOI] [PubMed] [Google Scholar]
- Valentin D, Chollet S, Lelièvre M, Abdi H. Quick and dirty but still pretty good: a review of new descriptive methods in food science. Int J Food Sci Technol. 2012;47:1563–1578. doi: 10.1111/j.1365-2621.2012.03022.x. [DOI] [Google Scholar]
- Vázquez-Ovando A, Chacón-Martínez L, Betancur-Ancona D, Escalona-Buendía H, Salvador-Figueroa M. Sensory descriptors of cocoa beans from cultivated trees of Soconusco, Chiapas, Mexico. Food Sci Technol. 2015;35:285–290. doi: 10.1590/1678-457X.6552. [DOI] [Google Scholar]
- Voigt J, Lieberei R. Biochemistry of cocoa fermentation. In: Schwan RF, Fleet GH, editors. Cocoa and coffee fermentation. Boca Raton: CRC Press; 2014. pp. 193–227. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.