Abstract
Background:
Vitamin is a well-known co-factor for many metabolic processes and its roles in fertility and follicular growth have been studied. Vitamin supplementation is frequently achieved by daily ingestion in the form of a complex capsule. However, the role of single and complex vitamins in in vitro maturation of murine follicles is not fully elucidated.
Methods:
In this study, we evaluated the effects of two forms of vitamins. Pure L-ascorbic acid, and multi-vitamin (vitamin C + vitamin B complex) was treated at two different concentrations (50 and 100 µg/ml), to pre-puberty murine follicles during in vitro maturation. To determine the specific stage of growth that is affected by treatment with vitamins, the vitamins were treated from day 0, 4, 9, and 13. Growth of each follicle was assessed by measuring diameters of whole expanded area and of the granulosa cells. Expression of follicular and oocyte growth-related genes and the effect of vitamin on the viability of follicles was assessed using senescence associated β-galactosidase staining.
Results:
Treatment with vitamins promoted the in vitro growth of murine follicles and the upregulated the expression of granulosa cell- and oocyte-specific genes such as BMP15, Fsh receptor, and GDF9. The proliferation of the granulosa cells was enhanced by the treatment of vitamin. Fifty µg/ml concentration vitamin showed greater effects compared to higher concentration. The viability of in vitro grown follicles was also significantly improved in vitamin-treated follicles. The effects of single L-ascorbic acid and complex vitamin were not significantly different to those of day 4 and day 9 follicles. Vitamins promoted murine follicle development in vitro with different effects on specific growth stage.
Conclusion:
Supplementation of vitamins during in vitro maturation of murine follicles is an efficient strategy for in vitro expansion of follicular cells. These results could be customized to the sophisticated culture of follicles retrieved from aged or cancer-survived female that contain smaller number of follicles with reduced potential to develop into mature follicles.
Keywords: Vitamins, Murine follicle, In vitro maturation, L-ascorbic acid
Introduction
Ovarian follicles provide a specialized nest for the maturation of oocytes, and are developed through a complex mechanism involving the selection of dominant follicles to be grown and ovulated. Follicular development is a dynamic process that requires structural remodeling, energy consumption and synthesis of specific receptors for follicle-stimulating hormone (FSH) in granulosa (G) cells and of those for luteinizing hormone (LH) in theca (T) cells. Ovary contains many immature oocytes at birth. Until recruited and selected, immature oocyte remains within undeveloped follicles for more than years until puberty in humans and other mammals. During this folliculogenesis, the apoptosis of many follicles occurs at different developmental stage [1].
In vitro follicular maturation is strongly related to the maturation of oocytes during development of preantral follicles into mature follicles [2]. During this process, proliferation of G and T cells, and synthesis of estradiol due to stimulation of FSH, are accompanied [3]. The proliferation of G cells has important roles in follicular development, and is critically related to the maturation of oocytes. Several studies have previously evaluated the efficacy of different substances or conditions which could enhance the in vitro follicular growth with controversial data [4–6].
Vitamin C (L-ascorbic acid), is a well-known co-factor of metabolic pathways and acts as an anti-oxidant for scavenging reactive oxygen species (ROS). Its role in fertility and follicular growth has been studied in many animals. The ascorbic acid demonstrated a reduction in apoptosis of follicles in cow [7]. Also, ascorbic acid supplementation was related to the viability of caprine preantral follicles mediated by interaction with Fsh [8]. The other types of vitamins, vitamin B, also play important roles in normal reproduction [9] and development of the follicles in cattle [10, 11]. The ability of the follicles to uptake ascorbic acid confers an advantage in terms of granulosa cell survival in mice [12, 13]. Ascorbic acid prevented the adverse effects of endocrine disrupting chemical on rat ovary and exhibited the potential use as an antioxidant [14]. However, to date, few studies have investigated various concentrations of different vitamin preparations.
In vitro maturation of undeveloped follicles is important as an option of fertility preservation for aged women and cancer survivors. Therefore, the establishment of optimal in vitro follicular maturation condition that promotes nourishment of the granulosa cells is important [15–18]. Despite known important roles of vitamins in reproduction, those in in vitro follicle development and ovarian reserve are not fully elucidated [19–21]. Furthermore, single or combined type of vitamins may also be a determinant of follicular development.
Therefore, we tried to demonstrate the effects of single or multi vitamins on prolonged in vitro maturation of murine ovarian follicles in this study.
Materials and methods
Ethics
The protocols for animal experiments were previewed and approved by the IACUC of Seoul National University Hospital (IACUC No. 18-0029-S1A0) and all experimental procedures were performed under the regulation of Seoul National University Hospital, Department of Experimental Animal Research Unit.
Animal housing and ovary isolation
Mouse strain C57BL/6 was supplied from KoATech (Pyeongtaeg-si, Kyunggi-do, Korea) and maintained at animal facility of Seoul National University Hospital until experiment. The facility has a cycle of 12 h light and 12 h dark with air ventilation. Pre-sterilized feedstuff and water were daily provided. Health status was checked regularly by veterinarian.
Two week-old C57BL/6 female mice were sacrificed by cervical dislocation and abdomen was open using micro-scissor. The ovaries were isolated and transferred to Hank’s Balanced Salt Solution (HBSS; Invitrogen, Calsbad, CA, USA) for transport.
In vitro culture of murine ovarian follicles
Total number of animals used for the experiment was 60 (20 for each group) and of ovaries was 120 (40 for each group) and of follicles were 7500 (2500 for each group).
The method of ovarian follicle seeding and in vitro culture has been used in this study, which is previously reported [2, 4, 5, 13, 22]. Briefly, isolated ovaries were washed using pre-warmed HBSS and dissociated into single follicle by mechanical dissection using a 26 1/2 gauge syringe needle (BD Biosciences, San Jose, CA, USA). Among the individually isolated follicles, pre-antral follicles of diameter ranged from 100 to 140 µm were selected and put as a single follicle to 20 ul of a single media drop in a 60 mm tissue culture dish (Corning®, San Jose, CA, USA) containing 25 drops covered with mineral oil (Sigma-Aldrich, St. Louis, MO, USA). The medium consisted of MEM alpha (Cat. No. A10490, Invitrogen, Calsbad, CA, USA) supplemented with 5% fetal bovine serum (FBS; Invitrogen, Calsbad, CA, USA), 1× insulin-transferrin-selenium (ITS; Invitrogen, Calsbad, CA, USA) and 100 U/ml penicillin–streptomycin (P/S; Calsbad, CA, USA, Invitrogen). A final concentration of 200 mIU/ml of follicle stimulating hormone (FSH; Sigma-Aldrich, St. Louis, MO, USA) and 100 mIU/ml of luteinizing hormone (LH; Sigma-Aldrich, St. Louis, MO, USA) was added from the beginning of in the in vitro culture until induction of ovulation. The medium was refreshed every other day and total period of in vitro culture is 14 day and ovulation induction for 48 h. Growing follicles were observed daily and classified as well-grown or atresia based on the expansion of granulosa cell and distinct oocyte within follicle.
Treatment of vitamin reagents
Two groups indicated as Group I: complex vitamin (provided by Ildong Pharmaceutical Co. Ltd, Seoul, Korea) containing Thiamine-B1, Riboflavin-B2, Pyridoxine-B3 and ascorbic acid and Group S: vitamin C. Vitamin complex contained capsuled complex was homogenized using sterile mortar and pestle. Fifty or hundred µg/ml of each vitamin powder was dissolved in distilled water (DW) and mixed with culture media. Media with dissolved vitamins was administered from day 0, 4, 9 and 13 until induction of ovulation (Table 1).
Table 1.
Composition of media with vitamins
| I | S |
|---|---|
| MEM alpha w/o ascorbic acid | MEM alpha w/o ascorbic acid |
| Ascorbic acid | Ascorbic acid |
| Thiamine | |
| Riboflavin | |
| Pyridoxine |
I complex vitamin, S vitamin
Induction of ovulation
At day 14 of in vitro culture, grown follicles with morphology of Graffian follicle with antrum formation were selected for induction of ovulation and the ovulation was induced by treatment with 1.5 IU/ml of human chorionic gonadotropin (hCG; Sigma-Aldrich, St. Louis, MO, USA) and 5 ng/ml of epidermal growth factor (EGF; Invitrogen, Calsbad, CA, USA). Ovulation of cumulus-oocyte-complex (COC) was observed after 36 h using a phase-contrast microscope (Eclipse Ti-U; Nikon, Tokyo, Japan). Ovulated COC was incubated with 0.1 mg/ml of hyaluronidase (Sigma-Aldrich, St. Louis, MO, USA) for 5 min and washed with HBSS. Existence of polar body (PB) was observed and PB containing oocyte was determined as MII stage oocyte and ovulation rate is calculated as following
Measurement of in vitro follicular growth
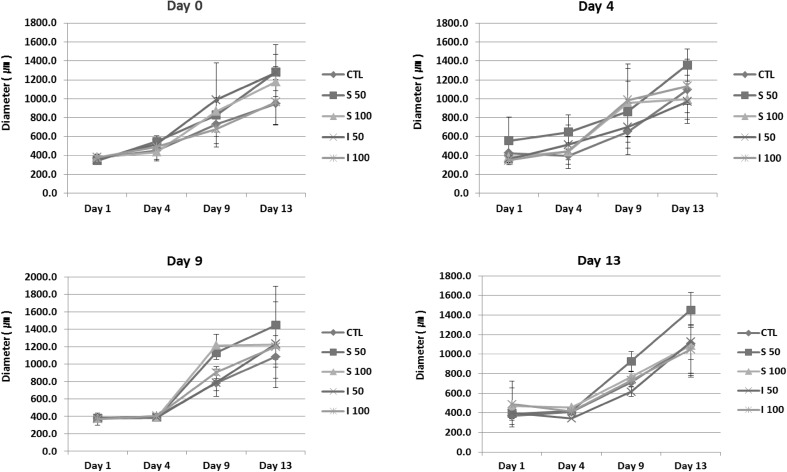
The diameter of each follicle was measured at day 1, 4, 9, 13 and 14 for each group of follicles. For the case of day 13 treatment, diameter was measured 24 h after treatments. The images of follicle was acquired using a phase-contrast microscope and the diameter was evaluated using i-Solutions program (IMT i-Solution Inc, Burnaby, BC, Canada) based on the area of expanded granulosa cells, and the whole area of follicle (Fig. 1). The average diameter was calculated and analyzed.
Fig. 1.
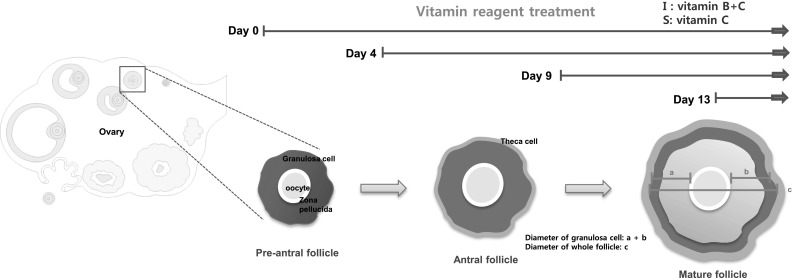
Experimental scheme of this study. Experimental scheme representing the measurement of diameter as indicated in drawing. a, b diameter of expanded granulosa cells, c diameter of whole expanded follicle
Senescence associated β-galactosidase (SA-β-gal) staining
All reactions were performed according to manufacturer’s protocol (Cell Biolabs, Inc., San Diego, CA, USA). The samples were washed with 1× PBS and fixed with 1× fixing solution for 5 min at room temperature. Further, samples were washed with 1× PBS and incubated with freshly prepared cell staining working solution at 37 °C in the dark conditions for 6 h. After 6 h of incubation, cell staining working solution was removed and the samples were washed three times with 1× PBS. The stained samples were then kept at 4 °C and images of positively stained region were captured using phase-contrast microscope (Eclipse Ti-U; Nikon, Tokyo, Japan).
Quantitative reverse transcription polymerase chain reaction (qRT-PCR)
Total RNAs of in vitro developed follicles (at day 1, day 4, day 9 and day 14) were isolated using TRIzol (Invitrogen, Calsbad, CA, USA) according to manufacturer’s instructions. Isolated RNAs were quantitated using Epoch plate reader (BioTek, Winooski, VT, USA) and 0.5 µg of RNA was reverse transcribed into cDNAs using Accute RT premix (Bioneer, Daejun, Korea) with oligo dT primers. cDNAs were mixed with QuantiTect SYBR® Green PCR Kits (Qiagen, Valencia, CA, USA) and gene-specific forward and reverse primers. The primer sequences used for PCR reaction are listed in Table 2. Templates were denatured for 5 min at 95 °C, and amplified as per the following protocol (30 cycles): denatured at 95 °C for 15 s, amplified at 58 °C for 60 s. All reactions were run in triplicate and the relative gene expression was normalized to GAPDH expression level as follows.
Table 2.
Primer sequences for qRT-PCR
| Gene | Sequences (5′ → 3′) | Sequences (3′ → 5′) |
|---|---|---|
| ESR1 | TACTGACCAACCTGGCAGACAG | TGGACCTGATCATGGAGGGT |
| ESR2 | AGTTGGCCGACAAGGAGTTG | CGCACTTGGTCGAACAGG |
| FSH Rc | GCTCACCAAGCTTCGAGTCA | GCCTTAAAATAGACTTGTTGC |
| BMP15 | CAGTAAGGCCTCCCAGACGT | AAGTTGATGGCGGTAAACCA |
| GDF9 | AACCCAGCAGAAGTCACCTC | AGGGGCTGAAGGAGGGAGG |
| ZP3 | CCGAGCTGTGCAATTCCCAGA | AACCCTCTGAGCCAAGGGTGA |
| GAPDH | GAAGGTCGGTGTGAACGAAT | TTTGATGTTAGCGGGGTCTC |
ESR1 estrogen receptor alpha, ESR2 estrogen receptor beta, FSH Rc follicle-stimulating hormone receptor, BMP15 bone morphogenetic protein 15, GDF9 growth differentiation factor 9, ZP3 zona pellucida glycoprotein 3
Statistical analysis
Statistical analysis was performed using SPSS version 21.0 (SPSS Inc., Chicago, IL, USA). Data are expressed as the mean ± standard errors and were analyzed by Kruskal–Wallis test. p value less than 0.05 considered to be statistically significant.
Results
Effects of vitamins on in vitro growth of follicles and granulosa cells
The entire experimental scheme is demonstrated in Fig. 1. Pre-antral follicles of 2-week-old mice were expanded for 14 days (Fig. 2). Follicles were treated with single vitamin, L-ascorbic acid (indicated as S) or complex vitamin containing vitamin B and C (indicated as I). Treated murine follicles showed normal growing morphology at each observed stage and fully matured follicle ovulated at a higher rate than non-treated control (CTL).
Fig. 2.
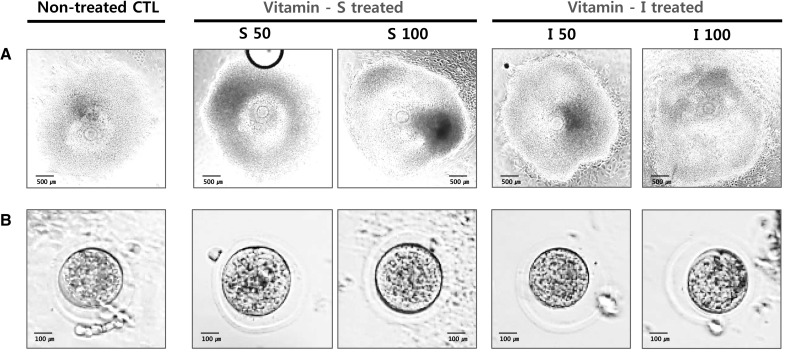
Observation of in vitro developing murine ovarian follicles. The follicles were grown with or without each vitamin and the ovulation was induced at day 14. AIn vitro matured morphology of follicles and oocytes cultured for 2 weeks from pre-puberty mice. B Morphology of ovulated oocytes from each group
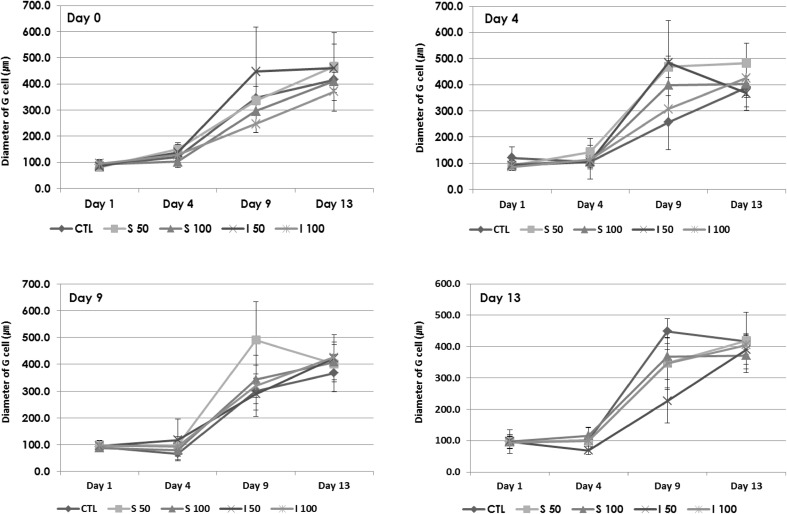
The expansion of follicles was calculated by measuring the diameter of whole follicle (Fig. 3) and the expansion of granulosa cells (Fig. 4). The diameters of the treated group on day 4 and day 9 were 1.5-fold larger compared to those of non-treated CTL. Diameters of treated group on day 0 (early) and day 13 (late stage) were not significantly affected by treatment of vitamins at both concentrations (50 µg/ml and 100 µg/ml). Treatment with I at 50 and 100 µg/ml showed more prominent effects in day 0 and 4 treated groups (*p < 0.05), while S at 50 and 100 µg/ml showed more highly proliferative effects in day 9 and day 13 treated groups (*p < 0.05). Vitamin C treatment enhanced the proliferation of granulosa cells. Evaluation of granulosa cell diameter, excluding the diameter of oocyte, showed that I at 50 µg/ml was more effective on the proliferation of granulosa cells (*p < 0.05).
Fig. 3.
Measured diameters of in vitro grown whole follicle. The diameter of each follicle was measured at day 1, 4, 9, 13 and 14. For the case of day 13 treatment, diameter was measured 24 h after treatments. The average diameter of grown follicles showed that the vitamins showed stimulatory effects on the growth of follicles (*p < 0.05). However, the effect of vitamins from different resource showed differential effects
Fig. 4.
Measured diameters of granulosa cells (G cells) in in vitro grown follicles. The diameter of granulosa cells was calculated by excluding the diameter of oocyte from that of whole follicle
Taken together, treatment at day 4 and day 9 enhanced the expansion effectively and the effects were more prominent at 50 µg/ml concentration in both vitamin groups.
Expression of specific genes altered by vitamins treatment
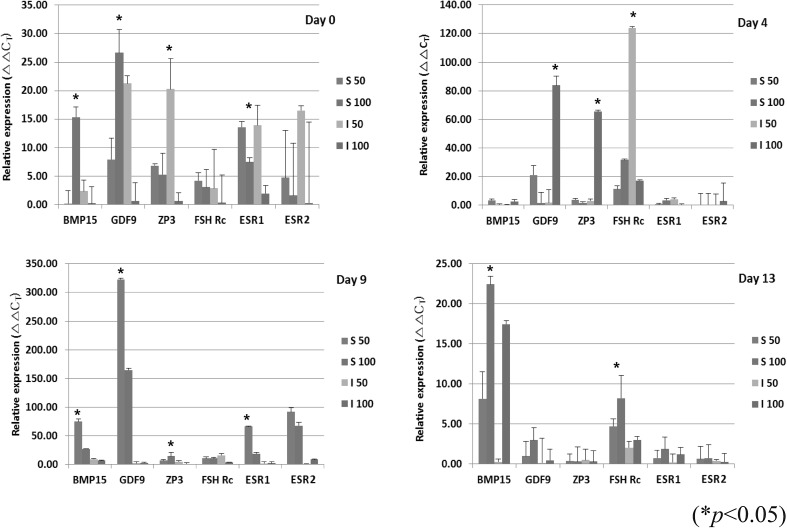
The expression of bone morphogenetic protein 15 (BMP15), growth differentiation factor 9 (GDF9), zona pellucida glycoprotein 3 (ZP3), follicle stimulating hormone receptor (Fsh Rc), estrogen receptor alpha (ESR1), and estrogen receptor beta (ESR2) was evaluated in CTL and vitamin groups at day 0, 4, 9, and 13 (Fig. 5). The expression of BMP15 and GDF9 were slightly upregulated in groups treated with vitamin at day 4, 9 and 13. The expressions of ESR1 and ESR2 were upregulated in both groups treated on day 4 and 9 (*p < 0.05). The expression of GDF9 and ZP3 was highest with treatment of I at 50 µg/ml on day 0.
Fig. 5.
Expression of oocyte-specific genes in in vitro cultured follicles. The expression of genes in groups treated with vitamin at day 0, 4, 9 and 13 was analyzed by qRT-PCR. The relative expression was calculated by normalizing with corresponding to GAPDH expression CT value (*p < 0.05)
Taken together, these results demonstrated that vitamins have up-regulating effects on the proliferation of granulosa cells and oocyte-specific genes involved in in vitro murine follicular maturation.
Effects on senescence of in vitro matured follicles
Staining degree of SA-β-gal, a common biochemical marker for the senescence, was observed in each group of follicles. Granulosa cells of central and peripheral regions were positively-stained and the theca cells of outer region are relatively weakly stained. Treatment with vitamins significantly reduced the intensity of positively-stained region in day 9-treated follicles (Fig. 6).
Fig. 6.
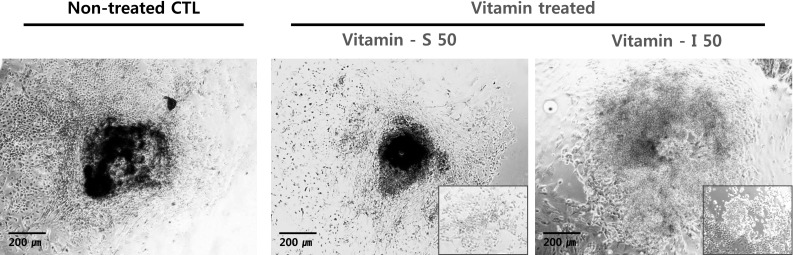
Biochemical staining of vitamin-treated, cultured follicles. The proliferated cells catalyze the hydrolysis of X-gal and produced a blue color. The degree of staining was diminished in vitamin-treated groups
These results indicated that treatment with vitamins retarded the senescence of early developed granulosa cells during in vitro maturation of follicles.
Discussion
The unique property of follicles is critically related to the infertility and reproductive life span of women. Therefore, maintenance and acquisition of healthy and stable status of follicles or oocytes is extremely important. Many molecules have been presented as protective or enhancing elements that may act as indirect or direct supplement during in vitro maturation of oocytes [2]. With growing age of pregnancy and in vitro fertilization (IVF) application, maintenance of healthy follicle, especially for assisted reproductive technology, is an emerging issue in reproductive medicine field [23].
Vitamin C (L-ascorbic acid), is one of the most well-known anti-oxidants and dietary supplement, and its direct correlation with generation of ROS may lead to the inference that vitamin C could be a viable supplement for the health of follicle [24]. The effects of vitamin C or vitamin family on fertility have been reported by many studies [25–27]. However, those studies were focused on whole ovary [28] or follicle level with L-ascorbic acid only. In this study, we demonstrated the effects of vitamins, single (S) or combined complex (I), on in vitro development of follicles retrieved from pre-pubertal mice.
During in vitro follicular maturation, granulosa cells drastically expanded. This in vitro environment for maturation of cells usually lasts at least ten days or more without sub-culture [2, 5, 13, 21, 29] and this kind of long-term culture system leads to a decrease in the proliferation of early-onset proliferating cells, therefore, differentiated granulosa cells of outer layer that lie with theca cells possibly had decreased proliferation. Treatment of vitamins drastically reduced the degree of SA-β-gal staining in follicles during development in G cells. Granulosa cells are those facing the oocytes and the status of this cell type is critically related to the maturation of oocyte through secretion of paracrine factors and nourishing of estradiol. In our study, vitamins significantly enhanced the expansion of G cells and this result is similar to that obtained in previous studies [12]. This phenomenon was observed for both concentrations of vitamins, however, the effects were more prominent in lower dose of single L-ascorbic acid at middle stage (day 9) (*p < 0.05) and of complex vitamin at early stage (day 4) (*p < 0.05). There results imply that treatment of vitamin has positive effects on developing follicles and complex vitamin is more efficient, especially on those of early day 0 and day 4 seeded follicles.
These results were in line with previous reports. Treatment of vitamin C significantly enhanced the survival and reduced the apoptosis in the follicles [12]. Rossetto et al. reported that vitamin C contributed to caprine follicle vitality and interaction with Fsh [8]. However, they only used L-ascorbic acid as the source of vitamin.
In this study, we compared the effects of L-ascorbic acid and vitamin complex (B and C) prepared as combined (I) and L-ascorbic acid (S). In vitro maturation of follicle is an emerging technology for fertility preservation in cancer survivors, with premature ovarian failure, or in peri-menopausal age women [30]. Ovaries of these patients have reduced number of hormone responsive follicles, instead their major follicle population is composed of primordial follicles with no response to hormones [31]. Therefore, the effects of vitamins as single or combined form on follicle health and in vitro maturation should be demonstrated in this setting.
Our study only focused the effects of vitamins in pre-puberty mice. As aforementioned, in vitro follicular maturation is a promising alternative technology for improving fertility in aged women, therefore, the effects should be evaluated in an aged model or other mammals in the future [32]. Vitamins have known to affect ovulation related symptoms such as polycystic ovary syndrome [33]. Since vitamin families are generally taken as a subtype or combination, in the form of capsule, the combined effects of two or more vitamin families also have to be studied in-depth regarding their effects on in vitro follicular maturation. Furthermore, these effects should also be clinically confirmed in those who are infertile women and who has genetic or epigenetic incompetence [34–37].
Vitamins promote murine follicle development in vitro with different effects on specific growth stage. Treatment with vitamin drastically enhanced expansion of follicles during in vitro maturation process. These results also demonstrated that vitamins could be used for in vitro follicle maturation either alone or in combination with hormones.
Acknowledgements
The authors would like to express sincere thanks to the technical assistance of Mi Ae Lee, Da Young Song and Se Hoon Park. This research was supported by the grants of Ildong Pharmaceutical Co. Ltd (0620154170), Ministry of Science and ICT (2016R1E1A1A01943455) and Seoul National University Hospital (0320170280), Republic of Korea.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical statement
The protocols for animal experiments were previewed and approved by the IACUC of Seoul National University Hospital (IACUC No. 18-0029-S1A0) and all experimental procedures were performed under the regulation of Seoul National University Hospital, Department of Experimental Animal Research Unit.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Byeong Cheol Kang, Phone: 82-2-2072-1971, Email: bckang@snu.ac.kr.
Seung-Yup Ku, Phone: 82-2-2072-1901, Email: jyhsyk@snu.ac.kr.
References
- 1.Markstrom E, Svensson ECh, Shao R, Svanberg B, Billig H. Survival factors regulating ovarian apoptosis—dependence on follicle differentiation. Reproduction. 2002;123:23–30. doi: 10.1530/rep.0.1230023. [DOI] [PubMed] [Google Scholar]
- 2.Kim YJ, Ku SY, Kim YY, Liu HC, Chi SW, Kim SH, et al. MicroRNAs transfected into granulosa cells may regulate oocyte meiotic competence during in vitro maturation of mouse follicles. Hum Reprod. 2013;28:3050–3061. doi: 10.1093/humrep/det338. [DOI] [PubMed] [Google Scholar]
- 3.Espey LL. The distribution of collagenous connective tissue in rat ovarian follicles. Biol Reprod. 1976;14:502–506. doi: 10.1095/biolreprod14.4.502. [DOI] [PubMed] [Google Scholar]
- 4.Park KE, Ku SY, Jung KC, Liu HC, Kim YY, Kim YJ, et al. Effects of urinary and recombinant gonadotropins on in vitro maturation outcomes of mouse preantral follicles. Reprod Sci. 2013;20:909–916. doi: 10.1177/1933719112468948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim YJ, Kim YY, Kang BC, Kim MS, Ko IK, Liu HC, et al. Induction of multiple ovulation via modulation of angiotensin II receptors in in vitro ovarian follicle culture models. J Tissue Eng Regen Med. 2017;11:3100–3110. doi: 10.1002/term.2214. [DOI] [PubMed] [Google Scholar]
- 6.Kim YY, Kim WO, Liu HC, Rosenwaks Z, Kim JW, Ku SY. Effects of paclitaxel and cisplatin on in vitro ovarian follicle development. Arch Med Sci. 2019 doi: 10.5114/aoms.2019.81730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thomas FH, Leask R, Srsen V, Riley SC, Spears N, Telfer EE. Effect of ascorbic acid on health and morphology of bovine preantral follicles during long-term culture. Reproduction. 2001;122:487–495. doi: 10.1530/rep.0.1220487. [DOI] [PubMed] [Google Scholar]
- 8.Rossetto R, Lima-Verde IB, Matos MH, Saraiva MV, Martins FS, Faustino LR, et al. Interaction between ascorbic acid and follicle-stimulating hormone maintains follicular viability after long-term in vitro culture of caprine preantral follicles. Domest Anim Endocrinol. 2009;37:112–123. doi: 10.1016/j.domaniend.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 9.Gaskins AJ, Chiu YH, Williams PL, Ford JB, Toth TL, Hauser R, et al. Association between serum folate and vitamin B-12 and outcomes of assisted reproductive technologies. Am J Clin Nutr. 2015;102:943–950. doi: 10.3945/ajcn.115.112185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ranjan R, Ranjan A, Dhaliwal GS, Patra RC. l-Ascorbic acid (vitamin C) supplementation to optimize health and reproduction in cattle. Vet Q. 2012;32:145–150. doi: 10.1080/01652176.2012.734640. [DOI] [PubMed] [Google Scholar]
- 11.Allison RD, Laven RA. Effect of vitamin E supplementation on the health and fertility of dairy cows: a review. Vet Rec. 2000;147:703–708. [PubMed] [Google Scholar]
- 12.Murray AA, Molinek MD, Baker SJ, Kojima FN, Smith MF, Hillier SG, et al. Role of ascorbic acid in promoting follicle integrity and survival in intact mouse ovarian follicles in vitro. Reproduction. 2001;121:89–96. doi: 10.1530/rep.0.1210089. [DOI] [PubMed] [Google Scholar]
- 13.Kim YJ, Ku SY, Rosenwaks Z, Liu HC, Chi SW, Kang JS, et al. MicroRNA expression profiles are altered by gonadotropins and vitamin C status during in vitro follicular growth. Reprod Sci. 2010;17:1081–1089. doi: 10.1177/1933719110377663. [DOI] [PubMed] [Google Scholar]
- 14.Soleimani Mehranjani M, Mansoori T. Stereological study on the effect of vitamin C in preventing the adverse effects of bisphenol a on rat ovary. Int J Reprod Biomed (Yazd) 2016;14:403–410. doi: 10.29252/ijrm.14.6.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dumesic DA, Meldrum DR, Katz-Jaffe MG, Krisher RL, Schoolcraft WB. Oocyte environment: follicular fluid and cumulus cells are critical for oocyte health. Fertil Steril. 2015;103:303–316. doi: 10.1016/j.fertnstert.2014.11.015. [DOI] [PubMed] [Google Scholar]
- 16.Matsuda F, Inoue N, Manabe N, Ohkura S. Follicular growth and atresia in mammalian ovaries: regulation by survival and death of granulosa cells. J Reprod Dev. 2012;58:44–50. doi: 10.1262/jrd.2011-012. [DOI] [PubMed] [Google Scholar]
- 17.Gao F, Zhang J, Wang X, Yang J, Chen D, Huff V, et al. Wt1 functions in ovarian follicle development by regulating granulosa cell differentiation. Hum Mol Genet. 2014;23:333–341. doi: 10.1093/hmg/ddt423. [DOI] [PubMed] [Google Scholar]
- 18.Lohr M, Kaltner H, Lensch M, André S, Sinowatz F, Gabius HJ. Cell-type-specific expression of murine multifunctional galectin-3 and its association with follicular atresia/luteolysis in contrast to pro-apoptotic galectins-1 and -7. Histochem Cell Biol. 2008;130:567–581. doi: 10.1007/s00418-008-0465-0. [DOI] [PubMed] [Google Scholar]
- 19.Kim YJ, Park KE, Kim YY, Kim H, Ku SY, Suh CS, et al. Effects of estradiol on the paracrine regulator expression of in vitro maturated murine ovarian follicles. Tissue Eng Regen Med. 2017;14:31–38. doi: 10.1007/s13770-016-0006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim YY, Kim YJ, Cho KM, Kim SH, Park KE, Kang BC, et al. The expression profile of angiotensin system on thawed murine ovaries. Tissue Eng Regen Med. 2016;13:724–731. doi: 10.1007/s13770-016-0009-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim YY, Tamadon A, Ku SY. Potential use of antiapoptotic proteins and noncoding RNAs for efficient in vitro follicular maturation and ovarian bioengineering. Tissue Eng Part B Rev. 2017;23:142–158. doi: 10.1089/ten.teb.2016.0156. [DOI] [PubMed] [Google Scholar]
- 22.Park KE, Kim YY, Ku SY, Baek SM, Huh Y, Kim YJ, et al. Effects of alginate hydrogels on in vitro maturation outcome of mouse preantral follicles. Tissue Eng Regen Med. 2012;9:170–174. doi: 10.1007/s13770-012-0170-x. [DOI] [Google Scholar]
- 23.Kim YJ, Ku SY, Jee BC, Suh CS, Kim SH, Choi YM, et al. A comparative study on the outcomes of in vitro fertilization between women with polycystic ovary syndrome and those with sonographic polycystic ovary-only in GnRH antagonist cycles. Arch Gynecol Obstet. 2010;282:199–205. doi: 10.1007/s00404-010-1401-9. [DOI] [PubMed] [Google Scholar]
- 24.Paszkowski T, Clarke RN. The Graafian follicle is a site of L-ascorbate accumulation. J Assist Reprod Genet. 1999;16:41–45. doi: 10.1023/A:1022597629622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuo SM, Lin CP. 17beta-estradiol inhibition of ascorbic acid accumulation in human intestinal Caco-2 cells. Eur J Pharmacol. 1998;361:253–259. doi: 10.1016/S0014-2999(98)00709-2. [DOI] [PubMed] [Google Scholar]
- 26.Luck MR, Jeyaseelan I, Scholes RA. Ascorbic acid and fertility. Biol Reprod. 1995;52:262–266. doi: 10.1095/biolreprod52.2.262. [DOI] [PubMed] [Google Scholar]
- 27.Huang M, Li J, Teoh H, Man RY. Low concentrations of 17beta-estradiol reduce oxidative modification of low-density lipoproteins in the presence of vitamin C and vitamin E. Free Radic Biol Med. 1999;27:438–441. doi: 10.1016/S0891-5849(99)00086-6. [DOI] [PubMed] [Google Scholar]
- 28.Meur SK, Sanwal PC, Yadav MC. Ascorbic acid in buffalo ovary in relation to oestrous cycle. Indian J Biochem Biophys. 1999;36:134–135. [PubMed] [Google Scholar]
- 29.Kim YY, Yun JW, Kim JM, Park CG, Rosenwaks Z, Liu HC, et al. Gonadotropin ratio affects the in vitro growth of rhesus ovarian preantral follicles. J Investig Med. 2016;64:888–893. doi: 10.1136/jim-2015-000001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Donnez J, Dolmans MM. Fertility preservation in women. Nat Rev Endocrinol. 2013;9:735–749. doi: 10.1038/nrendo.2013.205. [DOI] [PubMed] [Google Scholar]
- 31.Speroff L. The effect of aging on fertility. Curr Opin Obstet Gynecol. 1994;6:115–120. doi: 10.1097/00001703-199404000-00002. [DOI] [PubMed] [Google Scholar]
- 32.Kim YY, Ku SY, Jang J, Oh SK, Kim HS, Kim SH, et al. Use of long-term cultured embryoid bodies may enhance cardiomyocyte differentiation by BMP2. Yonsei Med J. 2008;49:819–827. doi: 10.3349/ymj.2008.49.5.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim JJ, Choi YM, Chae SJ, Hwang KR, Yoon SH, Kim MJ, et al. Vitamin D deficiency in women with polycystic ovary syndrome. Clin Exp Reprod Med. 2014;41:80–85. doi: 10.5653/cerm.2014.41.2.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim YY, Min H, Kim H, Choi YM, Liu HC, Ku SY. Differential MicroRNA expression profile of human embryonic stem cell-derived cardiac lineage cells. Tissue Eng Regen Med. 2017;14:163–169. doi: 10.1007/s13770-017-0051-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee SH, Lee S, Jun HS, Jeong HJ, Cha WT, Cho YS, et al. Expression of the mitochondrial ATPase6 gene and Tfam in down syndrome. Mol Cells. 2003;15:181–185. [PubMed] [Google Scholar]
- 36.Ku SY, Suh CS, Kim SH, Choi YM, Kim JG, Moon SY. A pilot study of the use of low dose human menopausal gonadotropin in ovulation induction. Eur J Obstet Gynecol Reprod Biol. 2003;109:55–59. doi: 10.1016/S0301-2115(02)00476-1. [DOI] [PubMed] [Google Scholar]
- 37.Kim YJ, Ku SY, Jee BC, Suh CS, Kim SH, Choi YM, et al. A comparative study on the outcomes of in vitro fertilization between women with polycystic ovary syndrome and those with sonographic polycystic ovary-only in GnRH antagonist cycles. Arch Gynecol Obstet. 2010;282:199–205. doi: 10.1007/s00404-010-1401-9. [DOI] [PubMed] [Google Scholar]