Abstract
The bioaccessibility, antioxidant activity, and bioactive and volatile compounds of freeze-dried Asian pear powder (FDAPP) and hot air-dried Asian pear powder (HDAPP) were investigated. Compared to FDAPP, HDAPP exhibited significantly (p < 0.05) higher total phenolic, arbutin, and bioaccessible phenolic contents and the ferric reducing ability of plasma for the free phenolic fraction. However, all antioxidant activities for the bound phenolic fraction were lower in HDAPP, which could contribute to the release of bound antioxidant components due to cell rupture in the HDAPP. Based on the volatile profile, hot air drying provided a sweet as well as attractive flavor in the Asian pear powder (APP). Considering economic viability, higher levels of bioactive compounds, and desirable aromatic properties, hot air drying is the better option compared to freeze-drying for APP production, which could be used as a functional ingredient in food products.
Keywords: Asian pear powder, Drying methods, Bioaccessibility, Antioxidant activity, Bioactive compounds, Volatile compounds
Introduction
Asian pear is a valued fruit with health-promoting properties including richness in dietary fiber, relatively high content of vitamin C, and bioactive compounds. As previously reported, the Asian pear has a high amount of arbutin (Jiang et al. 2018), and contains other individual phenolic compounds (Yim and Nam 2016). The phenolic compounds present in Asian pears are strongly correlated with its antioxidant activities. However, some Asian pear cultivars have observed softening and core browning during early storage, reducing the fruit quality, which limits its consumption and leads to great economic losses.
For this reason, one possible conservation method is developing food ingredients such as Asian pear powder (APP) that can extend the shelf life. Dehydrated fruits can be easily handled and transported as well as stored for long times because of the reduction in their volume (Karam et al. 2016). APP could also be developed into a new natural sweetener or functional ingredient that could be used as a substitute in various foods, such as ice cream or baking products. The addition of natural functional ingredients such as fruit powders in food formulations attracts consumer attention and could increase profitability. In fact, few studies have reported the addition of APP as a functional ingredient in food products (Park et al. 2011).
In regards to different drying methods, freeze-drying (FD) and hot air drying (HD) have been frequently used for the production of dehydrated vegetable and fruit products. Dehydrated food obtained via FD tend to exhibit high porosity, negligible collapse, and a high rehydration ratio with minimal color deterioration and the preservation of nutraceutical components (Karam et al. 2016). However, this technique requires a long-term drying process and is an expensive preservation method because of the low drying rate by refrigeration and vacuum systems, which increases the energy costs (Karam et al. 2016). In contrast, HD is one of the most common drying methods and is widely used in the food industry because of its low cost. However, HD results in reduced product quality. This drying process involves exposure to a high temperature and oxygen, influencing the product’s chemical composition and volatile compounds (Nunes et al. 2016). A few authors reported that HD could increase phenolic compounds of the powder likely by the formation of phenolic substances during the drying process (Que et al. 2008). To determine the quality of the APP derived using these drying methods, it is necessary to investigate the resulting phenolic compounds and antioxidant activities.
Until now, there have been few systematic studies on the comparison of different drying techniques for obtaining APP. Therefore, the objective of this study was to investigate the effects of FD and HD methods on the chemical profile, bioaccessibility, antioxidant activity, and volatile compounds of APP. In addition, changes in the microstructure of dried Asian pear slices produced using both drying methods were observed.
Materials and methods
Materials
The Asian pear (Pyrus pyrifolia Nakai cv. Niitaka) used in this experiment was purchased from a farmhouse located in Gwangju, South Korea and was stored in refrigerator at 4 °C for one and half month after purchasing and prior to processing.
Preparation of APP by FD and HD
Asian pears were peeled with a hand peeler and cut into slices (3-mm thickness) using a slicing machine (HFS 350G, Fujee, Suwon, Korea). The fresh Asian pears were dehydrated both in a freeze dryer (FDU-7003, Operon, Gimpo, Korea) at − 70 °C for 72 h and a hot air dryer (Dasol Scientific Co. Ltd., Seoul, Korea) operating with an airflow rate of 2.0 m/s at 65 °C for 26 h. After that, the dried slices were ground to make powder and sieved through a 60 mesh screen. The freeze-dried Asian pear powder (FDAPP) and hot air-dried Asian pear powder (HDAPP) were sealed under air in aluminum laminated film bags and stored at − 20 °C until analysis. The moisture contents of FDAPP and HDAPP were 11.6% and 10.2%, respectively (AOAC 1990).
Preparation of free and bound phenolic extracts
Extraction of free phenolic content was carried out according to the method of Aydin and Gocmen (2015). The liquid fraction of free phenolic content was stored at − 20 °C until further analysis.
The remaining residue was used for the determination of bound phenolic compounds according to an alkaline extraction method described by Uribe et al. (2015). The dried extract was dissolved in 10 mL of methanol for bound phenolic compounds and stored at − 20 °C. The free and bound fractions were used for measuring the content of phenolic compounds and antioxidant activities.
Free and bound phenolic contents
The free and bound phenolic contents of APP samples were determined by the Folin–Ciocalteu colorimetric method as described by Eghdami and Sadeghi (2010). Samples were analyzed by a UV spectrophotometer and absorbance was measured at 765 nm. The free and bound phenolic contents were expressed as mg gallic acid equivalent (GAE)/100 g dry weight (DW).
Bioaccessible phenolics and phenolic bioaccessibility
Bioaccessible phenolics of samples was determined by an in vitro gastrointestinal digestion (GID) mediated enzymatic extraction method described by Aydin and Gocmen (2015). After carrying out GID, the mixture solution was filtered through a Whatman No. 1 filter and then the filtrate was used for determination of bioaccessible phenolics. Bioaccessible phenolics were also determined using Folin–Ciocalteu colorimetric method and expressed as mg GAE/100 g (DW). Bioaccessibility was calculated as the percentage of total phenolic content.
Antioxidant activity
The 2,2-diphenyl-1-picrylhydrazyl (DPPH), 2,2-azinobis (3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) and the ferric reducing antioxidant power (FRAP) assays were carried out to measure respective antioxidant activities according to the methods described by Thaipong et al. (2006). Absorbance values of DPPH, ABTS and FRAP assays were measured by spectrophotometer at 515, 734 and 593 nm, respectively. The results were expressed as micromole Trolox equivalents (μmol TE/g, DW), and the standard curve was linear between 25 and 800 mM Trolox.
Arbutin, chlorogenic acid, and p-coumaric acid contents
Major phenolic compounds were measured as described by Yim and Nam (2016), using a Shimadzu LC-20AVP system (Shimadzu Co. Tokyo, Japan) equipped with a UV–Vis detector. An HPLC C18 column (Phenomenex BondClone 10 μm, 300 × 3.9 mm, Phenomenex, Torrance, California, USA) was used at a flow rate of 1.0 mL/min at 30 °C. A gradient elution was performed with the following two phases: phase A containing 2% (v/v) aqueous acetic acid and phase B containing 0.5% (v/v) acetic acid in 50% acetonitrile by the gradient as follows: 2% B from 0 to 5 min; 2 to 55% B, from 5 to 55 min; 100% B, from 55 to 65 min; followed by 10% B from 65 to 70 min; and post run with 2% A for 10 min. The samples were filtered through 0.45-μm filters, and 20 μL of samples and standard solutions were injected into the HPLC system. The chromatograms were recorded at 280 nm for arbutin, and 320 nm for chlorogenic acid and p-coumaric acid. Five concentrations (10–150 ppm) were used to prepare the calibration curve. The regression equations were found by plotting the area of the standard solutions against concentrations. The amounts of individual phenolic compound were obtained by comparing the retention times of the samples with those of the standard solutions, and the data are expressed as mg/100 g (DW).
Extraction of volatile compounds
Solid phase micro-extraction (SPME) fibers were used to extract odor components from the headspace of APP samples. SPME fibers (50/30 μm divinylbenzene/carboxen/polydimethylsiloxane; Supelco, Bellefonte, PA, USA) were conditioned at 250 °C for 5 min prior to and at intervals in between the analyses. Each APP sample (1.6 g) was placed in a headspace vial (20 mL) with a screw cap. The tightly capped vial was stirred for 20 min in a thermostatic bath (70 °C), and fibers were exposed to the headspace of the vial for 30 min.
GC–MS analysis
SPME fibers were injected into the port of a GC–MS system (Agilent GC 6890, Palo Alto, CA, USA) coupled to a mass-selective detector (Agilent GC 6890) and retained for thermal desorption at 250 °C for 2 min. A fused silica capillary column DB WAX (60 m × 0.25 mm × 0.25 μm) was used with an oven temperature programmed at 40 °C for 3 min, increased by 2 °C/min to 150 °C, increased by 4 °C/min to 200 °C, and held at 200 °C for 5 min. Helium was used as a carrier gas at a flow rate of 1 mL/min. The MS interface temperature was set at 250 °C, the ion source temperature was 230 °C, the MS quadrupole temperature was 150 °C, and the transfer line temperature was 280 °C. The split ratio was 10:1. Mass spectra (MS, Varian Saturn 2000, Agilent, Santa Clara, CA, USA) of volatile compounds were compared with those of the mass spectral database of the National Institute of Standards and Technology (NIST). The relative amounts present were calculated on the basis of peak-area ratios.
Scanning electron microscopy (SEM)
The microstructure of the dried Asian pear slices was observed using low vacuum scanning electron microscopy (SEM; JSM-7500F, JEOL Ltd., Tokyo, Japan) at 15 kV. The samples were cut regularly and fixed on steel supports with charcoal glue. Then, samples were coated with gold using an ion sputter and were observed at a magnification of 100× and 300×.
Statistical analysis
All experiments were performed in triplicate. Statistical analysis was performed by SPSS program (ver. 18.0) (SPSS Inc., Chicago, IL, USA). The difference between the means a and b of the results collected after assaying the APP was evaluated by the Tukey’s test, stating as null hypothesis that the difference a − b = 0. The null hypothesis was rejected and the difference between the means was judged significantly different from zero, when the probability level p of the test was found lower than the significance level set at p < 0.05.
Results and discussion
Total phenolic content and its bioaccessibility
The total phenolic contents of FDAPP and HDAPP and their bioaccessibilities are shown in Table 1. The total phenolic (sum of free and bound phenolic) contents of FDAPP and HDAPP were 254.91 mg GAE/100 g (DW) and 261.96 mg GAE/100 g (DW), respectively. A similar result for total phenolic content of 10 pear cultivars was reported by Yim and Nam (2016). In the comparison of total phenolic contents of FDAPP and HDAPP, a significantly (p < 0.05) higher total phenolic content was observed for HDAPP, and a similar result was found by Aydin and Gocmen (2015) for total phenolic content in HD pumpkin flour as compared to pumpkin flour produced by FD. For detailed monitoring of phenolic content variation, phenolic content of APP in the free and bound fractions was measured. Thus, the free phenolic content of HDAPP was significantly (p < 0.05) higher than that of FDAPP, whereas bound phenolic content was lower for HDAPP. This may be due to exposure to the HD temperature, which produced changes in the plant cell wall by disruption, and thus bound phenolics were released more easily as compared to in the FD process. This is in agreement with Jeong et al. (2004), who observed an increase in phenolic content in heated citrus peels due to the breakdown of the matrix.
Table 1.
Total phenolics, bioaccessible phenolics, and their bioaccessibility in freeze-dried Asian pear powder (FDAPP) and hot air-dried Asian pear powder (HDAPP)
| FDAPP | HDAPP | |
|---|---|---|
| Free phenolic (mg GAE/100 g, DW) | 229.01 ± 1.57b,* | 240.08 ± 1.81a |
| Bound phenolic (mg GAE/100 g, DW) | 26.08 ± 0.16a | 22.01 ± 1.98b |
| Total phenolic (mg GAE/100 g, DW) | 255.21 ± 1.74b | 262.00 ± 3.79a |
| Bioaccessible phenolic (mg GAE/100 g, DW) | 196.32 ± 10.23b | 220.01 ± 4.76a |
| Phenolic bioaccessibility (%) | 77.00 ± 3.81b | 84.02 ± 0.70a |
*Values are expressed as mean ± standard deviation (SD) of triplicate analysis
a,bMeans followed by different letters on the row are significantly different (p < 0.05)
Another possible explanation for the increase in total phenolic content might be due to the increase in the free hydroxyl groups as a consequence of hydrolysis of glycosidic groups or other substituents (Giovanelli and Paradiso 2002). Moreover, according to Que et al. (2008), an increase in total phenolic content might be due to availability of precursors of phenolic molecules by non-enzymatic interconversion between phenolic molecules of pumpkin flour during drying at 70 °C. Piga et al. (2003) and Nunes et al. (2016) reported that plums and guava powders dried at 85 and 55 °C exhibit increased total phenolic and free phenolic contents. Thus, drying temperature is a very important factor in the drying of fruits and vegetables, contributing to high levels of total phenolic content, and similar results were found in this study.
Phenolic bioaccessibility was investigated in order to assess APP as an accessible phenolic source. Until now, few studies on the phenolic bioaccessibility in fresh pear and its dried products have been reported. The bioaccessible phenolics content in measured FDAPP and HDAPP and their bioavailability are shown in Table 1. The bioaccessible phenolics content of HDAPP was 219.58 mg GAE/100 g (DW), indicating that its value was significantly higher than that of FDAPP. The higher tendency of HDAPP bioaccessible phenolic content might be due to slight increase in total phenolic content as compared to FDAPP. The phenolic bioaccessibility of HDAPP was found to be higher than that of FDAPP, and its value was 83.82%. Similar results were reported by Aydin and Gocmen (2015), who found that a higher bioaccessible phenolics content and bioaccessibility were observed in HD pumpkin flour when compared to pumpkin flour obtained by FD. It is possible that drying affected the microstructure of Asian pears and thus changed the phenolic bioaccessibility. The phenolic bioaccessibility in FDAPP and HDAPP of 76.64% and 83.82%, respectively, was higher than those of HD pumpkin flour (30.76%) and FD pumpkin flour (29.19%) (Aydin and Gocmen 2015), but similar to that of grapes (88.80%) (Tagliazucchi et al. 2010). The differences in phenolic bioaccessibility might be attributed to different plant cell wall matrices, phenolic profiles, and sample preparations. TPC for wide range of fruits and vegetables have been reported by Singh et al. (2016). The fruit included pomegranate, kinnow, mango, banana, jambolan, grapes and sapodilla, whereas vegetables comprised of beetroot, brinjal, orange carrot, bitter gourd, mentha and spinach. Overall, the TPC for different fruits and vegetables were found in ranges of 354.90–1639.70 mg GAE/100 g and 179.30–1028.60 mg GAE/100 g, respectively. Higher TPC was exhibited by the fruit peels as compared to pulp. Among all fruits, the highest TPC was shown by pomegranate peel and pulp whereas lowest TPC was found in case of kinnow.
Antioxidant activity
Antioxidant activity of APP produced by FD and HD was measured using DPPH, ABTS, and FRAP assays. According to all assays, no significant differences in DPPH and ABTS of the free fraction for FDAPP and HDAPP were observed, while a higher FRAP (12.87 µmol TE/100 g DW) of the free fraction was observed for HDAPP. The DPPH and ABTS activities for HDAPP and FRAPP were as follows: DPPH (FDAPP: 7.43 µmol TE/100 g DW, HDAPP: 7.41 µmol TE/100 g DW) and ABTS (FDAPP: 5.16 µmol TE/100 g DW, HDAPP: 5.13 µmol TE/100 g DW). The results showed that DPPH and ABTS in the free fraction did not correspond to free phenolic content. However, the FRAP value in the free fraction contributed to free phenolic content, and an increase in the free phenolic content induced an increase in the FRAP value observed for HDAPP. This might be due to the fact that both assays rely on electron transfer mechanisms, which have strong correlations between the total phenolic (Folin–Ciocalteu assay) and FRAP assay (Boneza and Niemeyer 2018). Similar results with the high positive correlation between total phenolic content and FRAP value were found in other studies (Li et al. 2015). For the antioxidant activity of the bound fraction, HDAPP had a slightly lower value for DPPH, ABTS, and FRAP than FDAPP, which also corresponded to the lower content of bound phenolics for HDAPP. This variation could be explained due the fact that antioxidant compounds, such as phenolic components were supposed to be released from the inner cellular matrix of dried powder during long-term thermal process.
In this study, a higher drying temperature resulted in a higher antioxidant activity measured by the FRAP assay for the free fraction (12.87 µmol TE/100 g DW) as compared to FRAP value (12.37 µmol TE/100 g DW) from free fraction of FDAPP, and these results were consistent with those of pumpkin flour (Que et al. 2008). In case of bound fraction, FD led to increases in DPPH (1.44 µmol TE/100 g DW) ABTS (2.55 µmol TE/100 g DW) and FRAP (2.98 µmol TE/100 g DW) activities as compared to HDAPP (DPPH: 0.76 µmol TE/100 g DW, ABTS: 2.34 µmol TE/100 g DW and FRAP: 2.59 µmol TE/100 g DW). The authors explained that higher antioxidant activity might be due to the formation of phenolics by HD and may also be caused by the non-enzymatic browning, such as the Millard reaction. Along with Millard reaction products, increased scavenging activity might be attributed to the combined effects of reducing power, donation of hydrogen atoms, and scavenging of reactive oxygen species and hydroxyl radicals (Woo et al. 2009). DPPH and ABTS activities for various fruits and vegetables of Indian origin have been reported by Singh et al. (2016). The DPPH ranges for fruits and vegetables were 2.60–5.50 mM TE/g and 2.10–4.70 mM TE/g, respectively. Pomegranate peel and pulp exhibited the highest DPPH activity while sapodilla showed the lowest. In case of vegetables, black and orange carrot showed the highest and lowest DPPH activities, respectively. The ABTS ranges for fruits and vegetables were 3.0–6.30 mM TE/g and 2.0–5.0 mM TE/g, respectively. Pomegranate peel and pulp exhibited the highest ABTS activity while kinnow and banana showed the lowest ABTS values. Authors have concluded that the high values of scavenging activities were because of presence of betalains and anthocyanins in vegetables matrices.
Major phenolic compound contents
The results showed that the most abundant phenolic compounds were arbutin, followed by p-coumaric acid and chlorogenic acid in FDAPP and HDAPP in the free phenolic fraction, whereas bound phenolic fraction did not show these phenolic compounds. The arbutin contents of FDAPP and HDAPP were 93.95 and 121.63 mg/100 g (DW), respectively (Table 2). Similar results were reported by Yim and Nam (2016) about arbutin content which were in the range of 79.71–124.45 mg/100 g (DW) in Asian pears, such as ‘Wonwhang’, ‘Niitaka’, ‘Hanareum’, and ‘Chuwhang’ cultivars. Our findings indicated that the arbutin content was higher than that in various oriental pear cultivars (Cui et al. 2005). In contrast, HDAPP contained a nearly 1.29 times higher arbutin content than that of FDAPP. The results demonstrated that higher arbutin content corresponded to higher total phenolic content and FRAP antioxidant activity for HDAPP.
Table 2.
Major phenolic compounds contents of freeze-dried Asian pear powder (FDAPP) and hot air-dried Asian pear powder (HDAPP)
| Phenolic compounds | FDAPP | HDAPP |
|---|---|---|
| Arbutin (mg/100 g, DW) | 94.00 ± 2.35b,* | 122.0 ± 1.95a |
| Chlorogenic acid (mg/100 g, DW) | 10.02 ± 0.65a | 9.01 ± 0.51a |
| p-Coumaric acid (mg/100 g, DW) | 20.01 ± 0.28a | 18.68 ± 0.12b |
*Values are expressed as mean ± standard deviation (SD) of triplicate analysis
a,bMeans followed by different letters on the same row are significantly different (p < 0.05)
The chlorogenic acid contents of FDAPP and HDAPP were 9.56 and 8.84 mg/100 g (DW), respectively (Table 2). These values agreed with the report that various Asian pear cultivars like ‘Wonwhang’, ‘Niitaka’, ‘Hanareum’, and ‘Chuwhang’ pears have chlorogenic acid contents in the range of 5.51–11.21 mg/100 g (DW) (Yim and Nam 2016). In addition, no significant differences were observed in chlorogenic acid content between FDAPP and HDAPP. According to Aydin and Gocmen (2015), hot air-dried pumpkin flour contains more chlorogenic acid content than pumpkin flour produced by FD. Compared to those reports, our results did not show the same tendency for higher chlorogenic acid content of pea powder obtained by HD. These differences might be partially due to the drying process, resulting in a high or low content of chlorogenic acid depending on the type of plant material and the locality of the compound in the cell (An et al. 2016).
FDAPP showed a slightly higher tendency of p-coumaric acid content as compared that was found in HDAPP (Table 2). The probable cause of decrease in p-coumaric acid content of HDAPP could be thermal degradation of phenolic compounds (Buchner et al. 2006). These findings are in accordance with those of Kahoun et al. (2017), who also reported decreasing tendency of p-coumaric acid content in Mead products with corresponding rises in heating temperatures from 50 to 100 °C. Thermal processing is associated with the corresponding decreasing tendency of p-coumaric acid content and a future study is needed to elucidate the exact mechanism of this declining effect after exposure to thermal treatment.
Volatile compounds
Food flavor is one of the most important sensory quality attributes for fruit and fruit products. The volatile compounds in APP produced by different drying methods are shown in Table 3, and there were 22 and 28 kinds of compounds obtained by SPME from FDAPP and HDAPP, respectively. The different relative contents of volatile compounds in APP depended on which method was used in the drying process. The most important volatile compounds such as ethanol (18.30%), hexanal (17.66%), nonanal (13.64%), 2-hexenal (9.61%), and 1-hexanol (8.97%) were quantified in the FDAPP. Similar main volatile compounds were identified in the fresh ‘Niitaka’ pear, in which the flavor is mainly attributed to alcohols and aldehydes (Kim et al. 2008). Qin et al. (2012) reported that hexanal, 2-hexenal, 1-hexanol, and (E)-2-hexen-1-ol constitute the important volatiles from the fresh fruit of 33 Chinese Pyrus ussuriensis cultivars. These results proved that FDAPP had the characteristic alcohols and aldehydes of fresh Asian pear.
Table 3.
Relative contents of volatile compounds of freeze-dried Asian pear powder (FDAPP) and hot air-dried Asian pear powder (HDAPP)
| No. | Retention time (min) | Volatile compounds | Relative contents (peak area %) | |
|---|---|---|---|---|
| FDAPP | HDAPP | |||
| Alcohols | ||||
| 1 | 6.26 | Ethanol | 18.30 ± 0.56a,* | 8.37 ± 0.37b |
| 2 | 25.113 | 4-Hexen-1-ol | 3.47 ± 0.15 | ND |
| 3 | 27.649 | 1-Hexanol | 9.01 ± 0.27a | 2.78 ± 0.40b |
| 4 | 29.418 | 3-Hexen-1-ol | 2.09 ± 0.07 | ND |
| 5 | 30.794 | 2-Hexen-1-ol | 1.00 ± 0.06a | 0.25 ± 0.03b |
| 6 | 40.283 | 1-Octanol | 0.35 ± 0.02a | 0.10 ± 0.01b |
| Aldehydes | ||||
| 7 | 11.446 | Hexanal | 18.01 ± 1.45a | 5.19 ± 0.31b |
| 8 | 18.674 | 2-Hexenal | 9.61 ± 0.03a | 3.34 ± 0.35b |
| 9 | 23.285 | Octanal | 3.90 ± 0.08a | 4.01 ± 0.13a |
| 10 | 25.249 | 2-Heptenal | 0.42 ± 0.08a | 0.21 ± 0.02b |
| 11 | 29.89 | Nonanal | 14.01 ± 1.08a | 10.03 ± 1.82b |
| 12 | 31.894 | 2-Octenal | 1.00 ± 0.17a | 1.03 ± 0.14b |
| 13 | 33.062 | Methional | 0.42 ± 0.01b | 0.81 ± 0.13a |
| 14 | 33.563 | Furfural | 1.68 ± 0.10b | 30.02 ± 1.42a |
| 15 | 36.419 | Decanal | 1.20 ± 0.09b | 4.19 ± 0.26a |
| 16 | 37.029 | Benzaldehyde | 1.02 ± 0.09b | 1.75 ± 0.12a |
| Carboxylic acids and ketones | ||||
| 17 | 57.175 | Propanoic acid | 0.27 ± 0.06a | 0.09 ± 0.04b |
| 18 | 72.181 | Nonanoic acid | 0.59 ± 0.03b | 2.39 ± 0.49a |
| 19 | 66.133 | 2,5-Dimethyl-4-hydroxy-3(2H)-furanone | ND | 0.28 ± 0.01 |
| Esters | ||||
| 20 | 16.936 | Hexanal, 3-methyl- | 0.35 ± 0.03a | 0.19 ± 0.01b |
| 21 | 19.785 | Furan, 2-pentyl- | 1.22 ± 0.11a | 0.23 ± 0.02b |
| 22 | 36.113 | Ethanone, 1-(2-furanyl)- | ND | 0.56 ± 0.05 |
| 23 | 22.44 | Acetic acid, hexyl ester | ND | 0.24 ± 0.03 |
| 24 | 26.263 | 5-Hepten-2-one, 6-methyl- | 1.72 ± 0.01a | 0.15 ± 0.01b |
| 25 | 31.582 | 2(3H)-Furanone, 5-methyl- | ND | 0.41 ± 0.07 |
| 26 | 32.723 | Octanoic acid, ethyl ester | ND | 0.36 ± 0.04 |
| 27 | 40.157 | 2-Furancarboxaldehyde, 5-methyl- | ND | 1.88 ± 0.18 |
| 28 | 45.742 | Benzoic acid, ethyl ester | ND | 0.16 ± 0.01 |
| 29 | 74.864 | 4H-Pyran-4-one, 2,3-dihydro-3,5-dihydroxy-6-methyl- | 0.52 ± 0.05b | 17.88 ± 1.53a |
| 30 | 75.209 | Hexadecanoic acid, ethyl ester | ND | 0.76 ± 0.13 |
*Values are expressed as mean ± standard deviation (SD) of triplicate analysis
a,bMeans followed by different letters on the same row are significantly different (p < 0.05); ND not detected
Among all compounds, alcohols and aldehydes, especially ethanol, 4-hexen-1-ol, 1-hexanol, 3-hexen-1-ol, hexanal, and 2-hexenal, were most sensitive to the HD process, since they were greatly decreased compared to those obtained from the FD process. These changes could cause a sensation of reduced freshness and herbaceous aspect in the APP.
Predominant furfural content was observed in HDAPP, whereas little furfural content was observed in FDAPP. Furfural, which is a component found in coconut flavor (Norton and Macleod 1990), also contributes to the volatile compositions of sesame oil (Francisco et al. 2001). This compound is not only responsible for browning of fruit products but is also one of the main degradation products of ascorbic acid (Yuan and Chen 1998). Thus, a higher content of furfural in the HDAPP might be due to an increase in ascorbic acid degradation during the HD process. The analysis of furfural could be used to monitor the quality of products, considering it is a marker compound during the dehydration of Asian pears.
(4H)-Pyran-4-one, 2,3-dihydro-3,5-dihydroxy-6-methyl-(PDDM) is a reducing Maillard compound, exhibiting richer content in HDAPP as compared to FDAPP. This compound was found in high temperature-dried plum fruits, and was not detected in fresh plums, which also had strong antioxidant activity (Cechovská et al. 2011).
With regard to APP scent, FDAPP retained the characteristic aroma of fresh pear fruit, whereas HDAPP exhibited the typical aroma of thermally processed pear products including juice and jams. In fact, the FDAPP showed a higher relative content of volatile compounds, like those in green grass, related to the flavor of fresh pear. Thus, FDAPP could be applied to products to improve the fresh pear flavor. On the contrary, although there seemed to be a loss of volatile compounds related to fresh pear flavor during the HD process, there was an increase in the formation of other volatile compounds such as decanal, benzaldehyde, nonanoic acid, ethanone, 1-(2-furanyl)-, and 2(3H)-furanone, 5-methyl-, which could contribute to the typical sweet and attractive flavor of HDAPP. In addition, in regards to the furfural, these volatile compounds may offer an overall flavor associated with processed pears. Thus, HDAPP could be used in various products to enhance the aroma of the sweet and caramel-like flavor.
Microstructure
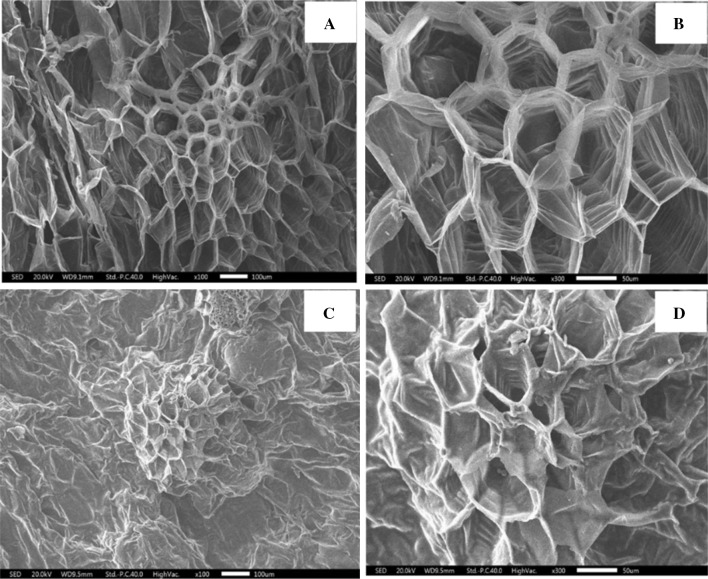
Figure 1 shows the microstructure of dried Asian pear slices produced by FD and HD. As shown in Fig. 1a, parenchyma cells were observed around the stone cell regions in the dried Asian pear slices obtained by FD, which were examined to determine the pore size and free volume in parenchyma cells. The microstructure of dried Asian pear slices produced by HD was characterized by small cavities and a high density of parenchyma cells (Fig. 1c). These phenomena can be explained by parenchyma cells, which had more shrinkage due to the thermal drying process.
Fig. 1.
Microstructure of freeze-dried Asian pear slices (FDAPS) and hot air-dried Asian pear slices (HDAPS). a FDAPS (× 100), b FDAPS (× 300), c HDAPS (× 100), d HDAPS (× 300)
In Fig. 1b, the microstructure of dried Asian pear slices produced by FD is shown in stone cells with cells that were swollen, bonded to each other, and had a high degree of cell compartmentalization. Moreover, the presence of numerous lamella located in the lumen of the cells was indicated. Moreno et al. (2011) reported a similar phenomenon in ‘Packham’s Triumph’ pears. However, dried Asian pear slices produced by HD showed cellular rupture and changed their shape in the stone cell (Fig. 1d). During the HD process, more lamella was lost and formation of a mucus-like substance in the cell lumen inside the stone cell was observed. These phenomena might be due to the release of polymeric compounds, concentrated sugars, or bioactive compounds from the cell membrane during the thermal drying process. These microstructural modification may be related to changes in phenolic content and antioxidant activity of APP. However, more research is needed to validate these results with more profound details.
Conclusion
In this study, the influences of different drying methods (FD and HD) were evaluated on the bioaccessibility, antioxidant, bioactive compounds and volatile flavoring compounds of APP. It was evident from the results that both FD and HD exhibited significant impacts on bioaccessibility, antioxidant activity, and bioactive and volatile compounds of APP. HD caused significant (p < 0.05) rises of total phenolic, arbutin, and bioaccessible phenolic contents and FRAP values in free phenolic fraction of APP. Moreover, volatile compounds in HD treated APP represented the typical sweet and attractive flavor of APP. HD cost is quite lower as compared to FD, and thus can be recommended for economical APP production. Moreover, APP produced by HD can be used as a substitute functional ingredient in functional foods, such as baking products, ice cream and infant formulas.
Acknowledgements
This study received funding from BK 21 Plus Program, Graduate School of Chonnam National University, Gwanju, South Korea.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- An KJ, Zhao DD, Wang ZF, Wu JJ, Xu YJ, Xiao GS. Comparison of different drying methods on Chinese ginger (Zingiber officinale Roscoe): changes in volatiles, chemical profile, antioxidant properties, and microstructure. Food Chem. 2016;197:1292–1300. doi: 10.1016/j.foodchem.2015.11.033. [DOI] [PubMed] [Google Scholar]
- AOAC . Official method of analysis of AOAC international. Method 950.46. 15. Arlington: Association of Official Analytical Chemists; 1990. [Google Scholar]
- Aydin E, Gocmen D. The influences of drying method and metabisulfite pre-treatment on the color, functional properties and phenolic acids contents and bioaccessibility of pumpkin flour. LWT Food Sci Technol. 2015;60:385–392. doi: 10.1016/j.lwt.2014.08.025. [DOI] [Google Scholar]
- Boneza MM, Niemeyer ED. Cultivar affects the phenolic composition and antioxidant properties of commercially available lemon balm (Melissa officinalis L.) varieties. Ind Crops Prod. 2018;112:783–789. doi: 10.1016/j.indcrop.2018.01.003. [DOI] [Google Scholar]
- Buchner N, Krumbein A, Rhon S, Kroh LW. Effect of thermal processing on the flavonols rutin and quercetin. Rapid Commun Mass Spectrom. 2006;20:3229–3235. doi: 10.1002/rcm.2720. [DOI] [PubMed] [Google Scholar]
- Čechovská L, Cejpek K, Konečný M, Velíšek J. On the role of 2,3-dihydro-3,5-dihydroxy 6-methyl-(4H)-pyran-4-one in antioxidant capacity of prunes. Eur Food Res Technol. 2011;233:367–376. doi: 10.1007/s00217-011-1527-4. [DOI] [Google Scholar]
- Cui T, Nakamura K, Ma L, Li JZ, Kayahara H. Analyses of arbutin and chlorogenic acid, the major phenolic constituents in oriental pear. J Agric Food Chem. 2005;53:3882–3887. doi: 10.1021/jf047878k. [DOI] [PubMed] [Google Scholar]
- Eghdami A, Sadeghi F. Determination of total phenolic and flavonoid contents in methanolic and aqueous extract of Achillea Millefolium. J Org Chem. 2010;2:81–84. [Google Scholar]
- Francisco JDC, Jarvenpaa EP, Huopalahti R, Sivik B. Comparison of Eucalyptus camaldulensis Dehn. oils from Mozambique as obtained by hydrodistillation and supercritical carbon dioxide extraction. J Agric Food Chem. 2001;49:2339–2342. doi: 10.1021/jf0013611. [DOI] [PubMed] [Google Scholar]
- Giovanelli G, Paradiso A. Stability of dried and intermediate moisture tomato pulp during storage. J Agric Food Chem. 2002;50:7277–7281. doi: 10.1021/jf025595r. [DOI] [PubMed] [Google Scholar]
- Jeong SM, Kim SY, Kim DR, Jo SC, Nam KC, Ahn DU, Lee SC. Effect of heat treatment on the antioxidant activity of extracts from citrus peels. J Agric Food Chem. 2004;52:3389–3393. doi: 10.1021/jf049899k. [DOI] [PubMed] [Google Scholar]
- Jiang GH, Nam SH, Eun JB. Effects of peeling, drying temperature, and sodium metabisulfite treatment on physicochemical characteristics and antioxidant activities of Asian pear powder. J Food Process Pres. 2018;42(2):e13526. doi: 10.1111/jfpp.13526. [DOI] [Google Scholar]
- Kahoun D, Řezková S, Královský J. Effect of heat treatment and storage conditions on mead composition. Food Chem. 2017;219:357–363. doi: 10.1016/j.foodchem.2016.09.161. [DOI] [PubMed] [Google Scholar]
- Karam MC, Petit J, Zimmer D, Djantou EB, Scher J. Effects of drying and grinding in production of fruit and vegetable powders: a review. J Food Eng. 2016;188:32–49. doi: 10.1016/j.jfoodeng.2016.05.001. [DOI] [Google Scholar]
- Kim W, Shim SL, Ryu KY, Jun SN, Jung CH, Seo HY, Song HP, Kim KS. Effect of electron-beam irradiation on flavor components in pear (Pyrus pyrifolia cv. Niitaka) J Korean Soc Food Sci Nutr. 2008;37(2):195–202. doi: 10.3746/jkfn.2008.37.2.195. [DOI] [Google Scholar]
- Li YG, Ma DY, Sun DX, Wang CY, Zhang J, Xie YX, Guo TC. Total phenolic, flavonoid content, and antioxidant activity of flour, noodles, and steamed bread made from different colored wheat grains by three milling methods. Crop J. 2015;3:328–334. doi: 10.1016/j.cj.2015.04.004. [DOI] [Google Scholar]
- Moreno J, Simpson R, Sayas M, Segura I, Aldana O, Almonacid S. Influence of ohmic heating and vacuum impregnation on the osmotic dehydration kinetics and microstructure of pears (cv. Packham’s Triumph) J Food Eng. 2011;104:621–627. doi: 10.1016/j.jfoodeng.2011.01.029. [DOI] [Google Scholar]
- Norton ID, Macleod AJ. Food flavors part C. The flavor of fruits. New York: Elsevier; 1990. [Google Scholar]
- Nunes JC, Lago MG, Castelo-Branco VN, Oliveira FR, Torres AG, Perrone D, Monteiro M. Effect of drying method on volatile compounds, phenolic profile and antioxidant capacity of guava powders. Food Chem. 2016;197:881–890. doi: 10.1016/j.foodchem.2015.11.050. [DOI] [PubMed] [Google Scholar]
- Park YO, Choi JH, Choi JJ. Physicochemical characteristics of Yanggaeng with pear juice and dried pear powder added. Korean J Food Preserv. 2011;18:692–699. doi: 10.11002/kjfp.2011.18.5.692. [DOI] [Google Scholar]
- Piga A, Alessandra DC, Giampaola C. From plums to prunes: influence of drying parameters on polyphenols and antioxidant activity. J Agric Food Chem. 2003;51:3675–3681. doi: 10.1021/jf021207+. [DOI] [PubMed] [Google Scholar]
- Qin GH, Tao ST, Cao YF, Wu JY, Zhang HP, Huang WJ, Zhang SL. Evaluation of the volatile profile of 33 Pyrus ussuriensis cultivars by HS-SPME with GC-MS. Food Chem. 2012;134:2367–2382. doi: 10.1016/j.foodchem.2012.04.053. [DOI] [PubMed] [Google Scholar]
- Que F, Mao LC, Fang XH, Wu T. Comparison of hot air-drying and freeze-drying on the physicochemical properties and antioxidant activities of pumpkin (Cucurbita moschata Duch.) flours. Int J Food Sci Technol. 2008;43:1195–1201. doi: 10.1111/j.1365-2621.2007.01590.x. [DOI] [Google Scholar]
- Singh JP, Kaur A, Shevkani K, Singh N. Composition, bioactive compounds and antioxidant activity of common Indian fruits and vegetables. J Food Sci Technol. 2016;53:4056–4066. doi: 10.1007/s13197-016-2412-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagliazucchi D, Verzelloni E, Bertolini D, Conte A. In vitro bioaccessibility and antioxidant activity of grape polyphenols. Food Chem. 2010;120:599–606. doi: 10.1016/j.foodchem.2009.10.030. [DOI] [Google Scholar]
- Thaipong K, Boonprakob U, Crosby K, Cisneros-Zevallos L, Byrne DH. Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. J Food Compos Anal. 2006;19:669–675. doi: 10.1016/j.jfca.2006.01.003. [DOI] [Google Scholar]
- Uribe E, Delgadillo A, Giovagnoli-Vicuña C, Quispe-Fuentes I, Zura-Bravo L. Extraction techniques for bioactive compounds and antioxidant capacity determination of Chilean papaya (Vasconcellea pubescens) fruits. J Chem. 2015;8:347532. [Google Scholar]
- Woo KS, Hwang IG, Lee YR, Lee JS, Jeong HS. Characteristics of sucrose thermal degradation with high temperature and high pressure treatment. Food Sci Biotechnol. 2009;18:717–723. [Google Scholar]
- Yim SH, Nam SH. Physiochemical, nutritional and functional characterization of 10 different pear cultivars (Pyrus spp.) J Appl Bot Food Qual. 2016;89:73–81. [Google Scholar]
- Yuan J, Chen F. Degradation of ascorbic acid in aqueous solution. J Agric Food Chem. 1998;46:5078–5082. doi: 10.1021/jf9805404. [DOI] [Google Scholar]