Abstract
In this study various coatings from apple press cake (AP) with immobilized antifungal bacterial cells were used for bread surface treatment to increase anti-moulding effect. The antifungal effect and technological properties of newly isolated Lactobacillus coryniformis LUHS71, L. curvatus LUHS51, L. farraginis LUHS206 and Leuconostoc mesenteroides LUHS225 strains. Then, the lactobacilli were tested for the effects of incorporation of sourdough on acrylamide formation in bread and antifungal effect against moulds commonly associated with bread spoilage. The addition of 15–20% of sourdoughs significantly (p = 0.0001) improved bread volume and crumb porosity depending on LAB strain, and reduced acrylamide formation on average by 23% (for LUHS51 and LUHS206) by 54% (for LUHS71 and LUHS225) compared to control bread. Additionally, the use of AP-LAB coatings prolonged shelf life from 3 to 6 days for control bread, and up to 9 days for sourdough breads. The combination of antifungal LAB sourdough and the AP-LAB coating leads to produce high quality bread with extended shelf life and would be a new and promising environmentally-friendly technological alternative.
Keywords: Sourdough, Acrylamide formation, Apple press cake, Coating, Moulding inhibition
Introduction
In the last years, consumer’s demands for preservative-free processed foods have increased as a result of growing awareness about the health hazards associated with chemicals. A major challenge of the food industry today is to produce products that are not only competitive and healthier for consumers but also more sustainable, and the use of natural antimicrobials presents a high potential to meet these demands (Luz et al. 2018).
Sourdough fermentation is an ecologically-friendly method for bread preservation (Zannini et al. 2012), and the sourdough bread technology can replace chemical preservatives, ensuring a safety of bread (Axel et al. 2017). Lactic acid bacteria have been extensively studied for their antibacterial and antifungal potential in order to be used as bio-preservatives (Esfahani et al. 2017; Cosentino et al. 2018). Different LAB species have been isolated from bread sourdoughs, the main from which are Lactobacillus, also species of Pediococcus, Leuconostoc, or Lactococcus spp. (Chavan and Chavan 2011; Bartkiene et al. 2013). However, the identification of LAB strains for specific application is still limited, and more strains need to be investigated.
Antifungal compounds of plant origin can be attractive candidates for natural preservation against fungal growth. The application of bio-preservatives derived from fruits/vegetables would be very perceptive to adapt plant processing residues for production of beneficial components. Our previous studies showed that cranberry-based compounds in combination with sourdough LAB could be promising as moulding preventing and acrylamide-reducing agents in bread making (Bartkiene et al. 2018). For instance, the fruit such as apple processing generates large amounts of food residues (Perussello et al. 2017), and apple processing by-products possess potent antioxidant properties because of the presence of beneficial phenolics (e.g., epicatechin, quercetin, chlorogenic acid, protocatechuic acid, ferulic acid, phloridzin, etc.) (Lohani and Muthukumarappan 2016; Rana et al. 2015). Apple pomace has versatile functional properties like glucose diffusion retardation index, emulsifying activity, water-/oil-holding capacity, and antimicrobial activity (Younis and Ahmad 2015), and could be utilised for the production of antimicrobial coatings for food protection.
Research studies attended on acrylamide described the potential health risk in carbohydrate-rich bakery products (Kumar et al. 2018). Fermentation processes performed with LAB and yeast could reduce the acrylamide content in bread, and this effect is mainly related to a decrease of pH rather than to the consumption of precursor nutrients (asparagine and reducing sugars) by microorganisms growing in sourdough (Nachi et al. 2018). For this reason, combined fermentation with LAB and yeast can be a good alternative for increasing wheat bread safety (Esfahani et al. 2017).
The objective of this study was to investigate the antifungal activity against moulds commonly associated with bread spoilage and the effect on sourdough and bread quality of four sourdough-isolated lactic acid bacteria strains (Lactobacillus coryniformis LUHS71, L. curvatus LUHS51, L. farraginis LUHS206 and Leuconostoc mesenteroides LUHS225). Then, the lactic acid bacteria (LAB) strains were tested for the effects of incorporation of sourdough on acrylamide formation in bread. To increase anti-moulding effect, various coatings from apple processing by-products (apple press cake, AP) with immobilized antifungal bacterial cells were used for bread surface treatment.
Materials and methods
Flours and lactic acid bacteria
Wheat flour type 550 (moisture 14.5%, protein 10.3%, falling number 320 s, ash 0.51%) obtained from the local market was used for the sourdough preparation and bread making. The L. coryniformis LUHS71, L. curvatus LUHS51, L. farraginis LUHS206 and Leuc. mesenteroides LUHS225 strains from the collection of the Lithuanian University of Health Sciences (Kaunas, Lithuania) were used for sourdough fermentations. The strains were stored and multiplicated according to previous study (Bartkiene et al. 2017). The LAB were characterized by the carbohydrate metabolism, the growth at different temperatures (10, 30, 37 and 45 °C), gas production and tolerance to high acidic conditions (at pH 2.5 for 2 h) (Bartkiene et al. 2017). Each samples was analysed in triplicate.
Sourdough fermentations
The wheat sourdough of 68% moisture content was prepared from wheat flour, tap water and LAB cell suspension (5% w/w), containing on average of 9.5 log10 colony-forming units (CFU)/mL of the individual LAB strain, and sourdough fermentation for 48 h at 30 °C. The number of bacterial cells, acidity parameters (pH and TTA), and enzyme (amylase and protease) activities were analysed after 24 and 48 h of fermentation.
Analyses
Methods of acidity parameters (pH, TTA) and microbial cell count determination are described in details in previous study (Bartkiene et al. 2018). The amylase levels in sourdough during fermentation were determined by the starch-iodine method as described by Nguyen et al. (2016). The mode of action of the protease was determined by a non-specific protease assay (Sigma’s Non-specific Protease Activity Assay - Casein as a Substrate. JoVE). Enzyme activities were calculated as a mean of three determinations and expressed as enzyme activity units (AU) per 100 g of sourdough.
Wheat bread making and quality evaluation
The wheat breads were prepared without (control bread) and with addition of different amounts of sourdough (10–20%) fermented with tested LAB. Methods of bread quality evaluation as well as bread making procedure are described in details in previous study (Bartkiene et al. 2018).
Coating preparation
The apple press cake (AP) as a by-product after juice production (56% moisture) was obtained from MV Group Production wine factory (Anyksciai, Lithuania). Vacuum drying of the press cake was performed at 45 ± 2.0 °C and 6 × 10−3 mPa pressure in an XF020 vacuum dryer (France-Etuves, France). For the preparation of AP-LAB coating, apple press cake powder was mixed with sterile water (15:80, w/v), and the LAB suspension (containing an average of 9.5 log10 CFU/g) was added at a concentration of 5% (w/w). The samples were incubated for 6 h at optimal temperatures for the LAB (30/35 °C). For the bread surface treatment, the AP-LAB coating was spread on the bread surface right after baking.
Antifungal activity determination
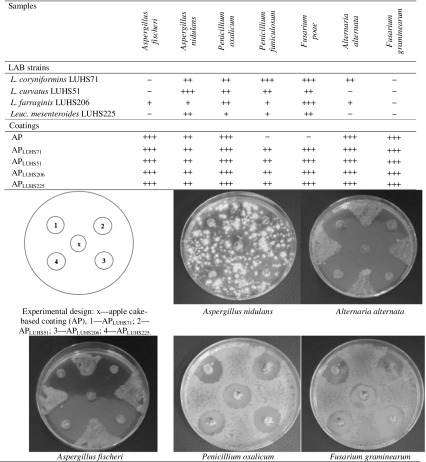
The antifungal activity of individual bacterial strains, the AP and their combinations against Penicillium funiculosum, P. oxalicum, Aspergillus nidulans, A. fischeri, Fusarium graminearum F. poae, and Alternaria alternata was analysed. The determination of antifungal activity of LAB was performed in duplicate by an agar well diffusion assay. For antifungal activity assay, the vacuum-dried apple press cake was milled to fine powder using a laboratory mill and was diluted with sterile water (20:80 w/v). The obtained suspension after careful mixing was used for analysis as described above.
Evaluation of bread moulding during storage
The mould formation on surface-coated bread prepared with different LAB sourdoughs, as well as coatings from AP in combination with the various LAB, was monitored during 10 days of storage For the mould spoilage analysis, bread samples were packed in plastic containers and stored at 24 ± 2 °C temperature. The surface of each sample was monitored daily for visible fungi colonies. Samples with (−) no visible colonies, (+) with one-two colonies (1–2 mm), (++) with three visible colonies (3–5 mm), (+++) pronounced mould growth (> 10 mm) were identified.
Statistical analysis
The sourdoughs with each LAB strain was prepared in duplicate. Bread with each sourdough was baked in duplicate, and each resulting sample was analysed at least in triplicate. The software of SPSS for Windows, ver. 25.0 (SPSS, Chicago, IL, USA) was used for statistical analysis. Analysis of variance (ANOVA) and Tukey’s significant difference (HSD) test were used to evaluate the differences between the tested samples. Pearson’s correlation was used to quantify the strength of the relationship between the variables. The results were considered statistically significant at p ≤ 0.05.
Results and discussion
Characterisation of LAB strains
Carbohydrate consumption capability of tested LAB strains are presented in Table 1. The highest carbohydrate fermentation diversity was shown by L. coryniformis LUHS71 and L. curvatus LUHS51 (23 and 22 carbohydrates from 49 analysed, respectively), the lowest was of L. farraginis LUHS206 (10 carbohydrates). In contrast to Edema (2010), in our study tested Leuc. mesenteroides LUHS225 was capable to ferment 20 from 49 analysed carbohydrates).
Table 1.
Carbohydrate metabolism, gas production, tolerance to temperature and low pH conditions (2 h at pH 2.5) of lactic acid bacteria (LAB) strains
| Lactobacillus coryniformins LUHS71 | Lactobacillus curvatus LUHS51 | Lactobacillus farraginis LUHS206 | Leuconostoc mesenteroides LUHS225 | |
|---|---|---|---|---|
| Glicerol | − | − | − | − |
| Erythritol | − | − | − | − |
| D-arabinose | − | − | − | − |
| L-arabinose | +++ | +++ | +++ | +++ |
| D-ribose | +++ | +++ | +++ | ++ |
| D-xylose | − | − | +++ | ++ |
| L-xylose | − | − | − | − |
| D-adonitol | − | − | − | − |
| Methyl-ßd-xYlopiranoside | − | − | − | − |
| D-galactose | +++ | +++ | + | ++ |
| d-glucose | +++ | +++ | − | +++ |
| d-fructose | +++ | +++ | +++ | +++ |
| d-mannose | +++ | +++ | − | +++ |
| L-sorbose | − | − | − | − |
| L-rhamnose | + | − | − | − |
| Dulcitol | − | − | − | − |
| Inositol | − | − | − | − |
| D-mannitol | +++ | +++ | − | − |
| D-sorbitol | +++ | +++ | − | − |
| Methyl-αD-mannopyranoside | ++ | + | − | − |
| Methyl-αD-glucopyranoside | − | − | − | +++ |
| N-acetylglucosamine | +++ | +++ | − | ++ |
| Amigdalin | +++ | +++ | − | ++ |
| Arbutin | +++ | +++ | − | − |
| Esculin | +++ | +++ | − | +++ |
| Salicin | +++ | +++ | − | +++ |
| D-cellobiose | +++ | +++ | − | +++ |
| d-maltose | +++ | +++ | +++ | +++ |
| d-lactose | +++ | +++ | − | − |
| D-melibiose | ++ | − | +++ | +++ |
| D-saccharose | +++ | +++ | − | +++ |
| D-trehalose | +++ | +++ | − | +++ |
| Inulin | − | − | − | − |
| D-melezitose | +++ | +++ | +++ | − |
| D-raffinose | − | − | − | +++ |
| Amidon | − | − | − | − |
| Glycogen | − | − | − | − |
| Xylitol | − | − | − | − |
| Gentiobiose | +++ | ++ | − | + |
| D-turanose | − | +++ | − | − |
| D-lyxose | − | − | − | − |
| D-tagatose | − | − | − | − |
| D-fucose | − | − | − | − |
| L-fucose | − | − | − | − |
| D-arabitol | − | − | − | − |
| L-arabitol | − | − | − | − |
| Potassium gluconate | − | + | ++ | + |
| Potassium 2-ketogluconate | − | − | − | − |
| Potassium 5-ketogluconate | − | − | ++ | − |
| Gas production (+/−) | − | − | − | + |
| Tolerance to temperature | ||||
| 10 °C | − | − | − | − |
| 30 °C | +++ | ++ | + | ++ |
| 37 °C | + | + | + | − |
| 45 °C | − | − | ++ | − |
| pH 2.5 | ||||
| 0 h log10 CFU/mL | 7.83 ± 0.1 | 8.31 ± 0.2 | 8.51 ± 0.2 | 8.14 ± 0.1 |
| 2 h log10 CFU/mL | 6.26 ± 0.1 | 3.50 ± 0.1 | 8.42 ± 0.1 | 2.69 ± 0.2 |
Interpretation of LAB growth in API 50 CH system: (+++), strong growth; (++), moderate growth; (+), weak growth; (−), no growth
All tested microbial strains were not able to grow at low temperature (10 °C) but showed strong (LUHS71) and moderate (LUHS51 and LUHS225) growing capability at 30 °C temperature conditions, also weak growth at 37 °C, except of L. farraginis LUHS206 (Table 1). It should be mentioned that Leuc. mesenteroides LUHS225 microorganisms were able to grow well only at 30 °C, while L. farraginis LUHS206 was only one able to grow at 45 °C.
The highest viability after 2 h incubation at pH 2.5 showed the L. farraginis LUHS206, the negligible reduction in viable cells compared to initial count (8.51 log10 CFU/mL) was fixed (Table 1). The lower tolerance to acidic conditions showed L. coryniformis LUHS71 strain (reduction in viable cells 20%), the lowest viability showed L. curvatus LUHS51 and Leuc. mesenteroides LUHS225 strains (reduction in viable cells 57.9 and 67%, respectively).
As the bread properties are greatly affected by the sourdough stability, the stability of microbial strains is very important (Alfonzo et al. 2016). LAB stability in a sourdough ecosystem is influenced by various factors, including specific metabolic adaptations to the sourdough ecosystem (Vrancken et al. 2011) and metabolic interactions (De Vuyst and Neysens 2005). All of the isolated strains showed high fermentation activity on d-fructose, d-maltose and L-arabinose, the main soluble carbohydrates of sourdough (Passerini et al. 2013). Regarding to the results obtained, the L. coryniformis LUHS71 indicating a high tolerance to acidic conditions and broad carbohydrate metabolism can be recommended for sourdough bread production.
Antifungal properties of lactic acid (LAB) and apple processing by-products
The four tested lactobacilli species inhibited the growth of the A. nidulans, P. oxalicum, P. funiculosum, and F. poae in agar well diffusion tests, although the inhibition zones varied with the lactic acid bacteria as well as with the fungal strain tested (Table 2). No one of tested LAB was able to inhibit the growth of F. graminearum, and only the L. farraginis LUHS206 displayed weak antifungal activity against A. fischeri (+), and L. farraginis LUHS206. Also, the L. coryniformis LUHS71 showed low and moderate inhibition effect against A. alternata (+ and ++, respectively). However, the inhibition of the growth of all the tested fungal species was quite pronounced with the strain L. coryniformins LUHS71. The obtained results are highly promising because the use of sourdough fermented with LAB showing antifungal activity offers a natural alternative to chemical preservatives in bakery products (Axel et al. 2016).
Table 2.
Profile of inhibition of fungi by the lactic acid bacteria (LAB) strains and LAB-apple coatings
AP, apple press cake; APLUHS71, APLUHS51, APLUHS206, APLUHS225, apple by-products and L. coryniformins LUHS71, L. curvatus LUHS51, L. farraginis LUHS206, Leuc. mesenteroides LUHS225, respectively, combinations
Interpretation of inhibition: (−), no inhibition; (+), delay of spore formation; (++), delay of spore formation with a small clear zone of inhibition around the punchedwell; (+++), a very good inhibition of mycelium growth and sporulation with large clear zones around the punched well
The inhibition zone as well as inhibition effect was increased when the culture suspension was composed with AP coating, except for the case with P. funiculosum, when no one of tested LAB showed the inhibition of fungal growth. The AP coating inhibited the growth of four fungi except of P. funiculosum and F. poae. It was unexpected that all tested AP-LAB coatings did not inhibit the growth of P. funiculosum, although LAB’s alone were characterised by low to strong inhibitory activity (Table 2).
The antifungal activity of LAB is usually explained by the synergistic action of a mixture of their metabolites, as acetic, propionic, caproic, etc. acids, in which caproic acid plays a key role against moulds responsible for bread spoilage (Corsetti and Settanni 2007). LAB produce lactic and acetic acids during sourdough fermentation, which contribute to the antifungal activity when sourdough is included in bakery products. Also, lactobacilli able to produce the antifungal agents, of which phenyllactic acid is the most potential (Lavermicocca et al. 2000) against Aspergillus niger and Penicillium roqueforti and significantly prolong the shelf life of bread. According to Axel et al. (2016), the production of metabolites such as phenyl derivates (3-phenyllactic acid, 4-hydroxyphenyllactic acid and benzoic acid), hydroxy fatty acids or antifungal peptides during fermentation is specie- and substrate-specific. The antifungal mechanism is believed to originate from the complex synergistic interactions among these various compounds.
The growing interest in the application of antifungal plant ingredients in food has also led to extensive investigation of plants as sources of bio-preservatives. The major phenols of apple pomace encompass benzoic acids (gallic acid) and flavanols (rutin) (Grigoras et al. 2013). Apples are also a good source of aromatic compounds, like terpenes and derivatives as norisoprenoids and coumaran (Perussello et al. 2017). Most of these volatiles display strong antimicrobial activity, yet there are no previous studies about the antifungal properties of apple processing by-products and their possible application for bread moulding prevention. No prior in situ studies have examined the use of plant actives, essential oils, or both, regarding their application in bread production (Cruz Cabral et al. 2013). Our study demonstrates that the apple processing by-products, as well as their compositions with LAB could be potential as bio-preservatives and could be a new approach to sustainable bread production.
Characterization of LAB sourdoughs
Table 3 presents the characteristics of wheat sourdoughs produced with pure LAB starters. All the tested LAB showed good acidification rates in wheat flour medium, and the pH ranged from 3.55 to 4.47 after 24 h of fermentation, and the pH values of the sourdoughs were reduced on average by 40% during 48 h of fermentation. The lowest pH values were established for the L. coryniformis LUHS71 (pH 3.55 and 3.48) after 24 h and 48 h period, the highest—for the L. farraginis LUHS206 (pH 4.47) fermented samples after 24 h, and for the Leuc. mesenteroides LUHS225 (pH 3.73) fermented samples after 48 h fermentation.
Table 3.
Acidity characteristics, amylase and protease activities (AU/100 g) and bacterial cell counts (log10 CFU/g) of sourdoughs produced with tested lactic acid bacteria (LAB) strains
| Sourdough samples | pH | TTA (°N) | Amylase activity | Protease activity | LAB cell count | ||||
|---|---|---|---|---|---|---|---|---|---|
| Fermentation time (h) | |||||||||
| 0 | 24 | 48 | 0 | 24 | 48 | ||||
| LUHS71 | 5.97 ± 0.02a | 3.55 ± 0.03a | 3.48 ± 0.02a | 0.4 ± 0.01a | 4.0 ± 0.01d | 5.8 ± 0.02c | 159.2 ± 9.3b | 215.3 ± 5.1c | 8.10 ± 0.3b |
| LUHS51 | 5.96 ± 0.01a | 3.63 ± 0.03b | 3.60 ± 0.01b | 0.4 ± 0.02a | 3.2 ± 0.02b | 4.5 ± 0.01b | 153.7 ± 4.8a,b | 165.9 ± 3.1a | 7.49 ± 0.2a |
| LUHS206 | 6.07 ± 0.02c | 4.47 ± 0.02d | 3.60 ± 0.01b | 0.5 ± 0.01b | 2.4 ± 0.02a | 6.8 ± 0.01d | 147.5 ± 8.0a | 171.5 ± 3.9a,b | 8.12 ± 0.1b |
| LUHS225 | 6.01 ± 0.01b | 3.75 ± 0.02c | 3.73 ± 0.02c | 0.8 ± 0.01c | 3.4 ± 0.02c | 4.3 ± 0.02a | 142.1 ± 7.5a | 169.3 ± 6.4a | 7.96 ± 0.1b |
| Statistical analysis | ||||
|---|---|---|---|---|
| Factor | Dependent variable | Mean square | F | p |
| LAB strains | pH after 24 h | 0.533 | 819.692 | 0.0001 |
| pH after 48 h | 0.031 | 125.100 | 0.0001 | |
| TTA after 24 h | 1.310 | 4030.769 | 0.0001 | |
| TTA after 48 h | 4.130 | 16,520.000 | 0.0001 | |
| Amylase | 830.594 | 0.470 | 0.712 | |
| protease | 1630.640 | 71.060 | 0.0001 | |
| LAB count | 0.259 | 6.903 | 0.013 | |
Data expressed as a mean values (n = 3) ± SD; SD, standard deviation
Mean values with different letters are significantly different (p ≤ 0.05)
AU, activity units
Strains: LUHS71, L. coryniformins LUHS71; LUHS51, L. curvatus LUHS51; LUHS206, L. farraginis LUHS206; LUHS225, Leuc. mesenteroides LUHS225
These results identify a 24 h fermentation as suitable for L. coryniformis LUHS71, L. curvatus LUHS51 and Leuc. mesenteroides LUHS225, whereas 48 h is recommended for sourdough preparation with L. farraginis LUHS206. Leuconostoc species have been previously characterised as showing slow growth rates and weak acidifying properties (Edema 2010), and information about the uses of L. coryniformis LUHS71, L. curvatus LUHS51 and L. farraginis LUHS206 strains for sourdough preparation is not available in the literature.
The highest TTA at 24 h corresponded to L. coryniformis LUHS71 sourdough (4.0 °N). In comparison, sourdoughs fermented with L. curvatus LUHS51, L. farraginis LUHS206 and Leuc. mesenteroides LUHS225 showed 20, 40 and 15% lower TTA, respectively. After 48 h of fermentation, the highest TTA was recorded for L. farraginis LUHS206 sourdough (6.8 °N), while L. coryniformis LUHS71, L. curvatus LUHS51 and Leuc. mesenteroides LUHS225 sourdoughs had TTA lower by 14.7, 33.8 and 36.8% respectively.
The growth and adaptation of the microorganisms in the medium is primarily linked to the growth associated-enzymes. While the microbial proteolytic enzymes are involved in the promotion of microbial cell growth by the provision of essential amino acids (Matthews et al. 2004), several amylase activities are required to hydrolyse starch to its glucose units. Also, proteolysis improves nutritional and organoleptic features of baked goods (Rizzello et al. 2014).
The highest activities of amylolytic (on average 156.4 AU/100 g) enzyme activity was determined for L. coryniformis LUHS71 and L. curvatus LUHS51strains. Slightly lower amylolytic activity (on average 144.8 AU/100 g) was determined for L. farraginis LUHS206 and Leuc. mesenteroides LUHS225 strains. Strain L. coryniformis LUHS71 can be characterised as to have the highest proteolytic activity (215.3 AU/100 g), while other strains had by 20% (LUHS206) and by 22% (LUHS51 and LUHS225) lower proteolytic activities (Table 3).
The statistical analysis showed that sourdough acidity parameters (pH and TTA) were significantly affected by fermentation time and LAB strain used for sourdough production (Table 3). With the reference to the data analysis, amylolytic and proteolytic activities of LAB seems to be specific in terms of species and strains. A moderate positive relation (r = 0.6965) was found between the LAB count and proteolytic enzyme activities in wheat sourdoughs. The lowest LAB count was found in L. curvatus LUHS51 sourdough (7.49 log10 CFU/g), whereas the cell number was by 6–8% higher in L. coryniformis LUHS71 and L. farraginis LUHS206, and Leuc. mesenteroides LUHS225 sourdoughs, respectively. Usually, the amylolytic activity of LAB is expected to increase the availability of energy sources for sourdough microorganisms, contributing to a rapid pH decrease. In this study, a moderate negative effect of amylolytic enzyme activity on the pH of sourdoughs was established (r = 0.4029).
Bread quality parameters
Table 4 lists the quality characteristics of wheat bread produced with tested LAB sourdoughs. Data analysis showed the significant effect of single LAB strain and quantity of sourdough on bread crumb TTA and bread specific volume, porosity and overall acceptability. Results showed that addition of sourdoughs up to 15% improved bread volume by 6.8–10.6% and crumb porosity by 11.7–12.4% compared to control bread. The highest specific volume was established for the breads prepared with 15% of L. coryniformis LUHS71 and Leuc. mesenteroides LUHS225 sourdoughs (3.38 and 3.35 cm3/g, respectively), and 20% of L. curvatus LUHS51 and L. farraginis LUHS206 of sourdoughs (3.29 and 3.17 cm3/g, respectively). Similar tendency was observed for the bread porosity, and the above-mentioned bread samples showed the highest values (on average 84.21%) compared to control bread (73.42 cm3/g). In case of LUHS225 and LUHS71 sourdoughs, addition of 20% sourdough significantly decreased specific volume and porosity as well as acceptability of bread.
Table 4.
The influence of different LAB sourdoughs on bread quality parameters and acrylamide (AA) formation
| Bread samples | Sourdough content (%) | v (cm3 g−1) | Porosity (%) | TTA (°N) | Overall acceptability | AA (µg kg−1) |
|---|---|---|---|---|---|---|
| Control | 3.01 ± 0.02b | 73.42 ± 0.11b | 1.5 ± 0.2a | 123 ± 12a | 15.10 ± 0.09 l | |
| L. coryniformins LUHS71 | 10 | 3.27 ± 0.03f | 79.76 ± 0.08d | 2.9 ± 0.3e | 125 ± 9a | 9.31 ± 0.04f |
| 15 | 3.38 ± 0.04g | 84.50 ± 0.10e | 3.1 ± 0.2e | 148 ± 5c | 6.93 ± 0.12d | |
| 20 | 2.80 ± 0.03a | 71.15 ± 0.04a | 3.4 ± 0.1e | 141 ± 6c | 4.20 ± 0.05a | |
| L. curvatus LUHS51 | 10 | 3.00 ± 0.02b | 75.48 ± 0.09c | 2.1 ± 0.3c | 135 ± 4b | 14.93 ± 0.05k |
| 15 | 3.05 ± 0.03c | 79.39 ± 0.08d | 2.4 ± 0.4d | 134 ± 6b | 11.38 ± 0.12h | |
| 20 | 3.29 ± 0.04f | 84.14 ± 0.03e | 2.8 ± 0.2e | 139 ± 4b | 8.62 ± 0.06e | |
| L. farraginis LUHS206 | 10 | 2.96 ± 0.03b | 79.16 ± 0.09d | 2.0 ± 0.1b | 134 ± 10b | 14.41 ± 0.07j |
| 15 | 3.00 ± 0.02b | 79.29 ± 0.11d | 2.1 ± 0.1c | 134 ± 9b | 11.61 ± 0.06i | |
| 20 | 3.17 ± 0.04d | 83.41 ± 0.12f | 2.5 ± 0.5d | 144 ± 2d | 8.64 ± 0.08e | |
| Leuc. mesenteroides LUHS225 | 10 | 3.24 ± 0.05e | 80.14 ± 0.06d | 3.0 ± 0.6e | 140 ± 8b,c | 10.65 ± 0.06g |
| 15 | 3.35 ± 0.03g | 84.79 ± 0.08e | 4.2 ± 0.2f | 135 ± 8b,c | 5.19 ± 0.11c | |
| 20 | 3.21 ± 0.02e | 76.24 ± 0.09c | 5.1 ± 0.2g | 114 ± 11a | 4.70 ± 0.11b |
| The influence of different LAB on bread parameters | ||||
|---|---|---|---|---|
| Factor | Dependent variable | Mean square | F | p |
| LAB | Specific volume | 0.078 | 77.604 | 0.0001 |
| Porosity | 190,712.769 | 1434.677 | 0.0001 | |
| TTA | 2.474 | 2725.698 | 0.0001 | |
| Overall acceptability | 597,516.436 | 9857.505 | 0.0001 | |
| AA | 3571.488 | 517,030.527 | 0.0001 | |
| Quantity of sourdough | Specific volume | 0.024 | 23.709 | 0.0001 |
| Porosity | 732.089 | 5.507 | 0.010 | |
| TTA | 0.026 | 28.341 | 0.0001 | |
| Overall acceptability | 59.250 | 0.977 | 0.390 | |
| AA | 102.115 | 14,782.763 | 0.0001 | |
| LAB × quantity of sourdough | Specific volume | 0.129 | 128.972 | 0.0001 |
| Porosity | 578.486 | 4.352 | 0.004 | |
| TTA | 0.005 | 5.426 | 0.001 | |
| Overall acceptability | 367.250 | 6.059 | 0.0001 | |
| AA | 1.741 | 251.991 | 0.0001 | |
Data expressed as a mean values (n = 3) ± SD; SD, standard deviation
Mean values within a column with different letters are significantly different (p ≤ 0.05)
TTA, total titratable acidity; v, specific volume; Control, wheat bread without sourdough
In all instances, increasing the sourdough content the bread TTA increases from 1.5 °N (control bread) to 2.5–2.8 °N for LUHS206 and LUGS51, to 3.4 °N for LUHS71 and to 5.1 °N for LUHS225. A moderate positive correlation existed (r = 0.4840) between the TTA and the specific volume of bread, whereas the crumb porosity has not a significant relation to TTA (r = 0.1857). Comparing bread samples prepared with different quantities of sourdough within same bacterial strain, the most acceptable bread samples had the highest porosity, and these parameters were strongly correlated (r = 0.5897).
The amylase and protease activities of tested bacteria (Table 3) determined during sourdough fermentations showed a significant relation to bread texture parameters and overall acceptability (Table 4). The sourdough fermentations with strains indicating higher amylase activity (LUHS71 and LUHS51) influenced the production of bread with higher specific volume and porosity values compared to lower amylase active strains, moreover, the bread prepared with higher proteolytic activity (LUHS71 and LUHS206) sourdoughs were indicated as the higher acceptability sourdough breads (Table 4).
Sourdough bread making technology have received considerable attention in the last years, largely because of the many advantages of sourdough over yeast (Mamhoud et al. 2016). According to the literature (Kranenburg et al. 2002), starter lactic acid bacteria provide the enzymes that may be involved in flavour-forming reactions, and hence the potential for formation of specific flavour compounds. Proteolysis and lipolysis during sourdough fermentation increase free amino acid content in the dough, which are major precursors for volatile aroma compounds.
Acrylamide content in sourdough bread
The levels of acrylamide in different sourdough bread samples produced by the different LAB were investigated (Table 4). Acrylamide reduction depended on sourdough additive and LAB used for fermentation. In all cases, sourdough addition reduced the acrylamide content in bread, on average by 54.90% (LUHS71), 22.90% (LUHS51), 23.51% (LUHS206) and 54.64% (LUHS225) compared with control sample. With increasing of sourdough content from 10 to 20%, acrylamide content can be reduced on average from 34 to 70% for L. coryniformis LUHS71 and Leuc. mesenteroides LUHS225, and from 24 to 42% for L. curvatus LUHS51 and L. farraginis LUHS206 (Table 4). Thus, selection of the LAB starters according to the bread formula seems to be very important (Bartkiene et al. 2013).
Strategies for acrylamide reduction include pH controlling, decreasing the processing temperature and period, selection raw materials with low precursors (reducing sugars), adding of exogenous additives (e.g., amino acids, hydrogen carbonates, proteins or antioxidants) (Constantinou and Koutsidis 2016). According to Cheng et al. (2015), the acrylamide formation in bread is significantly correlated with the antioxidant activity of the additives.
In the current experiments, the LAB used showed versatile carbohydrate metabolism, and reduced the acrylamide level in bread. Although pure L. farraginis LUHS206 strain showed the least variability regarding carbohydrate fermentative ability, among the carbohydrates tested (Table 1), the LUHS206 sourdough showed good acidification rate and acrylamide-reducing ability. Finally, the acrylamide-reducing potential of lactobacilli is strain-specific. The best acrylamide reducing effect and highest antifungal activity showed L. coryniformins LUHS71 strain.
Influence of sourdough and coating combinations on bread moulding during storage
For the experiment, the bread samples of the highest quality and acceptability were selected for each LAB: breads prepared with 15% of LUHS71 or LUHS225 sourdough and 20% of LUHS51 or LUHS206 sourdough. The results of analysis of formation of mould on surface-coated bread prepared with different LAB sourdoughs during 10 days of storage are presented in Table 5. Importantly, we should indicate that the AP-LAB coatings did not have any influence on the bread acceptability (results not shown) because of palatable flavour of apples. In case of the influence of LAB fermentation on AP sensory attributes, though AP samples were not evaluated by using sensory analysis test, as unacceptable smell, as well as taste was not felt. Samples had the acidic flavour and odour.
Table 5.
Influence of different sourdoughs and surface treatment with apple by-products-LAB coatings on the bread mold colony growth during storage
| Bread samples | Intensity of the visible molds colonies on the bread surface | ||||||
|---|---|---|---|---|---|---|---|
| 4 days | 5 days | 6 days | 7 days | 8 days | 9 days | 10 days | |
| Control | + | + | ++ | +++ | +++ | +++ | +++ |
| Control + APLUHS71 | − | − | − | + | +++ | +++ | +++ |
| Control + APLUHS51 | − | − | − | + | +++ | +++ | +++ |
| Control + APLUHS206 | − | − | − | + | +++ | +++ | +++ |
| Control + APLUHS225 | − | − | − | + | +++ | +++ | +++ |
| Sourdough breads | |||||||
| 15% LUHS71 sourdough | − | − | − | − | + | +++ | +++ |
| 15% + APLUHS71 | − | − | − | − | − | − | + |
| 15% + APLUHS51 | − | − | − | − | − | − | + |
| 15% + APLUHS206 | − | − | − | − | − | − | + |
| 15% + APLUHS225 | − | − | − | − | − | − | + |
| 20% LUHS51 sourdough | − | − | − | + | ++ | ++ | +++ |
| 20% + APLUHS51 | − | − | − | − | − | − | + |
| 20% + APLUHS71 | − | − | − | − | − | − | + |
| 20% + APLUHS206 | − | − | − | − | − | − | + |
| 20% + APLUHS225 | − | − | − | − | − | − | + |
| 20% LUHS206 sourdough | − | − | − | − | + | +++ | +++ |
| 20% + APLUHS71 | − | − | − | − | − | − | + |
| 20% + APLUHS51 | − | − | − | − | − | − | + |
| 20% + APLUHS206 | − | − | − | − | − | − | + |
| 20% + APLUHS225 | − | − | − | − | − | − | + |
| 15% LUHS225 sourdough | − | − | − | + | ++ | +++ | +++ |
| 15% + APLUHS71 | − | − | − | − | − | − | + |
| 15% + APLUHS51 | − | − | − | − | − | − | + |
| 15% + APLUHS206 | − | − | − | − | − | − | + |
| 15% + APLUHS225 | − | − | − | − | − | − | + |
Control, wheat bread without sourdough; 15%, 20%, sourdough content; APLUHS, apple press cake and relevant LAB combination (coating)
Interpretation of growth: (−) no visible colonies, (+) with one-two colonies (1–2 mm), (++) with three visible colonies (3–5 mm), (+++) pronounced mould growth (> 10 mm)
The study showed that the use of AP-LAB coatings prolonged shelf life of control bread from 3 to 6 days (visible mould colonies were noticed on 4th day on the control bread surface) (Table 5). Mould colony on tested breads appeared after 7–8 days’ incubation and grew up to 10–12 mm in diameter in 10 days. Addition of sourdough fermented with certain LAB prolonged the shelf life of the wheat bread (control) to seven (LUHS51 and LUHS225 sourdough) or eight (LUHS71 and LUHS206 sourdough) days if compared to control bread. As can be seen from the results obtained, the higher quality and acceptability of bread and higher antimicrobial effect can be achieved using the selected amount of sourdough and antimicrobial AP-LAB coating which can inhibit the mould growth on bread surface up to 9 days. It was not found a significant difference in anti-moulding effect between different AP-LAB coatings. The possible extending the shelf life of bread by using 15% of sourdough was reported also by Denkova et al. (2014). However, high sourdough content (due to high acidification, the activity of amylolytic and proteolytic enzymes, among others) can lead to a negative influence on the porosity and specific volume, as well as the overall acceptability of bread. In our study, increasing the L. coryniformins LUHS71 and Leu. mesenteroides LUHS225 sourdough contents above 15% decreased the bread quality, whereas 20% L. curvatus LUHS51 and L. farraginis LUHS206 sourdoughs produced the highest quality breads.
Moreover, should be indicated that the mould inhibition effect strongly depends on the antimicrobial properties of LAB, amount of sourdough added and AP by-products used for coating preparation. According to the literature, LAB can function as a bio-preservative and improve the stability of the final product (coating), herewith, fruit juice supplemented with fruit pomace was able to induce a higher growth of LAB during fermentation (Furtado Dias et al. 2018). As our previous study showed, the apple pomace increased the viability of LAB during immobilization (for 24 h at 30 °C) (Bartkiene et al. 2017). Stimulatory role of grape pomace polyphenols on Lactobacillus acidophilus growth also it was reported by Hervert-Hernández et al. (2009).
In case the interactions between AP-LAB which can promote the preservation potential moreover, the combined action of LAB antifungal and antimicrobial activities and inhibitory effect of apple polyphenols against prominent bacterial food spoilers (Beermann et al. 2018).
As was shown in our previous study (Bartkiene et al. 2018), the strongest anti-molding effect (no moulding up to 8 days) was achieved using 20% of Leu. mesenteroides LUHS242, L. paracasei LUHS244 and L. plantarum LUHS135 sourdough for wheat bread preparation, while the combination with bread surface treatment using cranberry-based coating allowed to inhibit mould formation on bread surface up to 10 days. In this case, all of tested LAB can be suitable for the antifungal sourdough AP-based coating production.
Conclusion
The tested LAB strains showed versatile carbohydrate fermentation and fast growth and acidification rates in wheat sourdough medium. However, to obtain the qualitative wheat bread the sourdough amount should be optimised. In this regard, the 15% of L. coryniformis LUHS71 and Leuc. mesenteroides LUHS225 sourdough or 20% of L. curvatus LUHS51 and L. farraginis LUHS206 sourdough can be recommended for acceptable bread making. In addition, acrylamide formation in wheat flour bread could be controlled by the sourdough content; the acrylamide level can be reduced significantly by the addition of 15–20% of sourdough. Moreover, it should be indicated that the mould inhibition effect strongly depends on the antimicrobial properties of LAB, amount of sourdough added and AP by-products used for coating preparation. These technological mean leads to wheat bread with enhanced quality (high acceptable), safety (lower acrylamide content) and extended shelf life (up to 10 days) without the use of chemical improvers and preservatives. As indicated above, the application of apple processing by-products containing antimicrobial components for antifungal coating preparations becomes highly relevant and would be a new and promising environmentally-friendly alternative.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Elena Bartkiene, Email: elena.bartkiene@lsmuni.lt.
Vadims Bartkevics, Email: vadims.bartkevics@bior.gov.lv.
Vita Lele, Email: vita.lele@lsmuni.lt.
Iveta Pugajeva, Email: iveta.pugajeva@bior.gov.lv.
Paulina Zavistanaviciute, Email: paulina.zavistanaviciute@lsmuni.lt.
Daiva Zadeike, Email: daiva.zadeike@ktu.lt.
Grazina Juodeikiene, Email: grazina.juodeikiene@ktu.lt.
References
- Alfonzo A, Urso V, Corona O, Francesca N, Amato G, Settanni L, Di Miceli G. Development of a method for the direct fermentation of semolina by selected sourdough lactic acid bacteria. Int J Food Microbiol. 2016;239:65–78. doi: 10.1016/j.ijfoodmicro.2016.06.027. [DOI] [PubMed] [Google Scholar]
- Axel C, Brosnan B, Zannini E, Furey A, Coffey A, Arendt EK. Antifungal sourdough lactic acid bacteria as biopreservation tool in quinoa and rice bread. Int J Food Microbiol. 2016;239:86–94. doi: 10.1016/j.ijfoodmicro.2016.05.006. [DOI] [PubMed] [Google Scholar]
- Axel C, Zannini E, Arendt EK. Mould spoilage of bread and its biopreservation: a review of current strategies for bread shelf life extension. Crit Rev Food Sci Nutr. 2017;57:3528–3542. doi: 10.1080/10408398.2016.1147417. [DOI] [PubMed] [Google Scholar]
- Bartkiene E, Jakobsone I, Juodeikiene G, Vidmantiene D, Pugajeva I, Bartkevics V. Effect of lactic acid fermentation of lupine wholemeal on acrylamide content and quality characteristics of wheat-lupine bread. Int J Food Sci Nutr. 2013;64:890–896. doi: 10.3109/09637486.2013.805185. [DOI] [PubMed] [Google Scholar]
- Bartkiene E, Vizbickiene D, Bartkevics V, Pugajeva I, Krungleviciute V, Zadeike D, Zavistanaviciute P, Juodeikiene G. Application of Pediococcus acidilactici LUHS29 immobilized in apple pomace matrix for high value wheat-barley sourdough bread. LWT Food Sci Technol. 2017;83:157–164. doi: 10.1016/j.lwt.2017.05.010. [DOI] [Google Scholar]
- Bartkiene E, Bartkevics V, Lele V, Pugajeva I, Zavistanaviciute P, Mickiene R, Zadeike D, Juodeikiene G. A concept of mould spoilage prevention and acrylamide reduction in wheat bread: application of lactobacilli in combination with a cranberry coating. Food Control. 2018;91:284–293. doi: 10.1016/j.foodcont.2018.04.019. [DOI] [Google Scholar]
- Beermann C, Gruschwitz N, Walkowski K, Göpel A. Growth modulating properties of polyphenolic apple pomace extract on food associated microorganisms. J Microbiol Biotechnol Food Sci. 2018;3(2):176. [Google Scholar]
- Chavan RS, Chavan SR. Sourdough technology-A traditional way for wholesome foods: a review. Compr Rev Food Sci Food Saf. 2011;10:69–182. [Google Scholar]
- Cheng J, Chen X, Zhao S, Zhang Y. Antioxidant-capacity-based models for the prediction of acrylamide reduction by flavonoids. Food Chem. 2015;168:90–99. doi: 10.1016/j.foodchem.2014.07.008. [DOI] [PubMed] [Google Scholar]
- Corsetti A, Settanni L. Lactobacilli in sourdough fermentation. Food Res Int. 2007;40:539–558. doi: 10.1016/j.foodres.2006.11.001. [DOI] [Google Scholar]
- Constantinou C, Koutsidis G. Investigations on the effect of antioxidant type and concentration and model system matrix on acrylamide formation in model Maillard reaction systems. Food Chem. 2016;197:769–775. doi: 10.1016/j.foodchem.2015.11.037. [DOI] [PubMed] [Google Scholar]
- Cosentino S, Viale S, Deplano M, Fadda ME, Pisano MB. Application of autochthonous Lactobacillus strains as bopreservatives to control fungal spoilage in Caciotta Cheese. Biomed Res Int. 2018;2018:915615. doi: 10.1155/2018/3915615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz Cabral L, Fernandez Pinto V, Patriarca A. Application of plant derived compounds to control fungal spoilage and mycotoxin production in foods. Int J Food Microbiol. 2013;166:1–14. doi: 10.1016/j.ijfoodmicro.2013.05.026. [DOI] [PubMed] [Google Scholar]
- De Vuyst L, Neysens P. The sourdough microflora: biodiversity and metabolic interactions. Trend Food Sci Technol. 2005;16:43–56. doi: 10.1016/j.tifs.2004.02.012. [DOI] [Google Scholar]
- Denkova R, Ilieva S, Denkova Z, Georgieva L, Krastanov A. Examination of the technological properties of newly isolated strains of the genus Lactobacillus and possibilities for their application in the composition of starters. Biotechnol Biotechnol Equip. 2014;28:487–494. doi: 10.1080/13102818.2014.918701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edema MO. Effect of Leuconostoc mesenteroides on the visco-elastic properties of sour maize meal. Int Food Res J. 2010;17:55–61. [Google Scholar]
- Esfahani BN, Kadivar M, Shahedi M, Soleimanian-Zad S. Reduction of acrylamide in whole-wheat bread by combining lactobacilli and yeast fermentation. Food Addit Contam Part A. 2017;34:1904–1914. doi: 10.1080/19440049.2017.1378444. [DOI] [PubMed] [Google Scholar]
- Furtado Dias J, Duarte Simbras B, Beres C, dos Santos K, Correa Cabral LM, Lemos Miguel MA (2018) Acid lactic bacteria as a bio-preservant for grape pomace beverage. Front Sust Food Syst 2, Article 58
- Grigoras CG, Destandau E, Fougère L, Elfakir C. Evaluation of apple pomace extracts as a source of bioactive compounds. Ind Crops Prod. 2013;49:794–804. doi: 10.1016/j.indcrop.2013.06.026. [DOI] [Google Scholar]
- Hervert-Hernández D, Pintado C, Rotger R, Goñi I. Stimulatory role of grape pomace polyphenols on Lactobacillus acidophilus growth. Int J Food Microbiol. 2009;136:19–122. doi: 10.1016/j.ijfoodmicro.2009.09.016. [DOI] [PubMed] [Google Scholar]
- Kranenburg R, Kleerbezem M, van Hylckama Vlieg J, Ursing BM, Boekhorst J, Smit BA, Ayad EHE, Siezen RJ. Flavour formation from amino acids by lactic acid bacteria: predictions from genome sequence analysis. Int Dairy J. 2002;12:111–121. doi: 10.1016/S0958-6946(01)00132-7. [DOI] [Google Scholar]
- Kumar J, Das S, Teoh SL. Dietary acrylamide and the risks of developing cancer: facts to ponder. Front Nutr. 2018;5:14. doi: 10.3389/fnut.2018.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavermicocca P, Valerio F, Evidente A, Lazzaroni S, Corsetti A, Gobbetti M. Purification and characterization of novel antifungal compounds from the sourdough Lactobacillus plantarum strain 21B. Appl Environ Microbiol. 2000;66:4084–4090. doi: 10.1128/AEM.66.9.4084-4090.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohani UC, Muthukumarappan K. Application of the pulsed electric field to release bound phenolics in sorghum flour and apple pomace. Innov Food Sci Emerg Technol. 2016;35:29–35. doi: 10.1016/j.ifset.2016.03.012. [DOI] [Google Scholar]
- Luz C, Calpe J, Saladino F, Luciano FB, Fernandez-Franzón M, Mañes J, Meca G. Antimicrobial packaging based on ɛ-polylysine bioactive film for the control of mycotoxigenic fungi in vitro and in bread. J Food Process Preserv. 2018;42:e13370. doi: 10.1111/jfpp.13370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamhoud A, Nionelli L, Bouzaine T, Hamdi M, Gobbetti M, Rizzello CG. Selection of lactic acid bacteria isolated from Tunisian cereals and exploitation of the use as starters for sourdough fermentation. Int J Food Microbiol. 2016;225:9–19. doi: 10.1016/j.ijfoodmicro.2016.03.004. [DOI] [PubMed] [Google Scholar]
- Matthews A, Grimaldi A, Walker M, Bartowsky E, Grbin P, Jiranek V. Lactic acid bacteria as a potential source of enzymes for use in vinification. Appl Environ Microbiol. 2004;70:5715–5731. doi: 10.1128/AEM.70.10.5715-5731.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachi I, Fhoula I, Smida I, Taher IB, Chouaibi M, Jaunbergs J, Bartkevics V, Hassouna M. Assessment of lactic acid bacteria application for the reduction of acrylamide formation in bread. LWT Food Sci Technol. 2018;92:435–441. doi: 10.1016/j.lwt.2018.02.061. [DOI] [Google Scholar]
- Nguyen HT, Van der Fels-Klerx HJ, Peters RJB, Van Boekel MAJS. Acrylamide and 5-hydroxymethylfurfural formation during baking of biscuits: part I: effects of sugar type. Food Chem. 2016;192:575–585. doi: 10.1016/j.foodchem.2015.07.016. [DOI] [PubMed] [Google Scholar]
- Passerini D, Coddeville M, Le Bourgeois P, Loubière P, Ritzenthaler P, Fontagné-Faucher C, Cocaign-Bousquet M. The carbohydrate metabolism signature of Lactococcus lactis strain A12 reveals its sourdough ecosystem origin. Appl Environ Microbiol. 2013;79:5844–5852. doi: 10.1128/AEM.01560-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perussello CA, Zhang Z, Marzocchella A, Tiwari BK. Valorization of apple pomace by extraction of valuable compounds. Compr Rev Food Sci Food Saf. 2017;16:776–796. doi: 10.1111/1541-4337.12290. [DOI] [PubMed] [Google Scholar]
- Rana S, Gupta S, Rana A, Bhushan S. Functional properties, phenolic constituents and antioxidant potential of industrial apple pomace for utilization as active food ingredient. Food Sci Hum Well. 2015;4:180–187. doi: 10.1016/j.fshw.2015.10.001. [DOI] [Google Scholar]
- Rizzello CG, Curiel JA, Nionelli L, Vincentini O, Di Cagno R, Silano M, Coda R. Use of fungal proteases and selected sourdough lactic acid bacteria for making wheat bread with an intermediate content of gluten. Food Microbiol. 2014;37:59–68. doi: 10.1016/j.fm.2013.06.017. [DOI] [PubMed] [Google Scholar]
- Vrancken G, Rimaux T, Weckx S, Leroy F, De Vuyst L. Influence of temperature and backslopping time on the microbiota of a type I propagated laboratory wheat sourdough fermentation. Appl Environ Microbiol. 2011;77:2716–2726. doi: 10.1128/AEM.02470-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Younis K, Ahmad S. Waste utilization of apple pomace as a source of functional ingredient in buffalo meat sausage. Cogent Food Agric. 2015;1:1119397. [Google Scholar]
- Zannini E, Pontonio E, Waters DM, Arendt EK. Applications of microbial fermentations for production of gluten-free products and perspectives. Appl Microbiol Biotechnol. 2012;93:473–485. doi: 10.1007/s00253-011-3707-3. [DOI] [PubMed] [Google Scholar]