Abstract
Pulses are the second most important source of food for humans after cereals. They hold an important position in human nutrition. They are rich source of proteins, complex carbohydrates, essential vitamins, minerals and phytochemicals and are low in lipids. Pulses are also considered the most suitable for preparing protein ingredients (concentrates and isolates) because of their high protein content, wide acceptability and low cost. In addition, pulse proteins exhibit functional properties (foaming and emulsification, water and fat absorption and gelation) as well as nutraceutical/health benefiting-properties which makes them healthier and low cost alternative to conventional protein sources like soy, wheat and animals. Proteins from different pulses (beans, peas, lentils, cowpeas, chickpeas, pigeon peas, etc.) differ in their composition and structure hence for finished product suitability. Therefore, this article aimed to review composition, structure–function relationship and current applications of different pulse proteins in the food industry.
Keywords: Application, Functional properties, Nutraceutical properties, Pulses, Secondary structure
Introduction
Pulses include Leguminosae crops harvested solely for dry seeds. Pulses are high in proteins, complex carbohydrates (resistant starch and dietary fibres), minerals, vitamins and other phytochemicals while low in fat, calories and sodium. These are sometimes also regarded as “poor man’s meat” due to their low cost and the presence of high amount of proteins. Besides high protein content, pulses also contain enzyme inhibitors, phytohemagglutinins/lectins, phytosterols, phytic and tannic acid and oligosaccharides which possess health-benefiting activities in humans (Tiwari and Singh 2012; Singh et al. 2017). As most of these constituents are concentrated in the hulls of the pulse seeds, it has been suggested that pulse seed coats may be considered as a source of phytochemicals, while cotyledons can be utilized as a source of vegetable proteins (Singh et al. 2017).
Lentils (Lens culinaris), kidney bean (Phaseolus vulgaris), mung bean (Phaseolus aureus), black gram/urad bean (Phaseolus mungo), chickpea (Cicer arietinum), field pea (Pisum sativum), and cowpea (Vigna unguiculata) are major pulses which are consumed throughout the Indian-Subcontinent. These pulses contain 20–30% proteins which are stored in the seed cotyledons as small spherical protein bodies. Protein content of different pulses varies with genotypes, germination, environmental conditions and application of fertilizers during growth and development (Singh 2017). The pulse proteins have essential amino acids composition complementary to cereals and are naturally gluten-free, thus, safe for the patients suffering from gluten intolerance/allergies. The proteins from pulses like beans and peas have been reported to be suitable for quality improvement of gluten-free muffins and preparation of edible films (Shevkani and Singh 2014, 2015; Shevkani et al. 2015a). Additionally, pulses are also considered appropriate for preparing protein-rich ingredients (protein concentrates and isolates) because of their low cost, high protein content and wide acceptability.
Despite these advantages, the effective utilization of pulse proteins in the food industry depends largely on their functional properties. The functional properties of proteins have been defined as the physical and chemical properties that affect the performance of proteins in food systems during processing, storage, and consumption (Kinsella and Melachouris 1976). These properties in turn depend on physicochemical and structural properties of proteins as well as processing conditions (Shevkani et al. 2014a, 2015a, b; Ghumman et al. 2016; Parmar et al. 2017). Proteins from different pulses vary in composition and structure and have different functional properties. Keeping the above in view, the present article attempts to review the composition, structure, functional properties and current applications of pulse proteins.
Composition
Pulse proteins chiefly comprise of globulins which are soluble in salt solutions and albumins (soluble in water) while prolamins (soluble in alcohol) and glutelins (soluble in dilute acid/base) are minor proteins and constitute a small portion, generally less than 5%. The ratio of albumins to globulins vary between 1:3 and 1:6.3 amongst different pulses (beans, lentils, black gram and chickpea) (Gupta and Dhillon 1993). Albumins primarily comprise metabolic proteins and include enzymatic and non-enzymatic proteins. They constitute only 10–20% of the total seed proteins. Pulse albumins are generally low in molecular weight (MW; 5–80 kDa) and higher in cysteine and methionine content than pulse globulins (Boulter and Croy 1997; Boye et al. 2010). Although, albumins are the most nutritive proteins in pulse seeds in terms of amino acid composition (Boulter and Croy 1997), they may also contain some anti-nutritional/bioactive constituents e.g. trypsin and/or chymotrypsin inhibitors, hemagglutinins/lectins, and amylase inhibitors (Boye et al. 2010). Globulins, in general, account for 70–80% of seed proteins and function primarily as storage proteins. Legumins and vicilins are major globulins/storage proteins in pulse seeds. Based on their different sedimentation coefficients, they are also referred as 7S globulins and 11–12S globulins, respectively. They were present in ratios of 10.5:1, 1:6–9, 1:9, 1–3:1 and 4–6:1, respectively in lentils, French beans, cowpeas, peas and chickpeas (Gupta and Dhillon 1993). Legumins, generally, have higher amount of sulfur-containing amino acids (methionine and cysteine) than vicilins (Boulter and Croy 1997).
Both vicilins and legumins are oligomeric proteins. Legumin is a hexamer of overall MW of 300–400 kDa. Each legumin subunit comprise of a larger acidic subunit of approximately 40 kDa and a smaller basic subunit of approximately 20 kDa that are disulfide bonded in the structure that disassociate under reducing conditions (Boulter and Croy 1997). The legumin acidic subunits are mostly situated on protein surface, while the basic subunits form the inner hydrophobic core (Gueguen and Cerleti 1994). Vicilin, on the other hand, is a trimer of monomers (identical or non-identical) of 50–70 kDa held together by non-covalent hydrophobic interactions (Boulter and Croy 1997). Vicilin shows an overall MW of 145–190 kDa. This globulin lacks cysteine residue in structure hence does not occur as disulfide-bonded protein. Vicilin is the major storage protein in kidney bean, cowpea, mung bean, red bean and urad bean in which it may account for up to 88% of total globulins (Meng and Ma 2001; Tang et al. 2009; Tang and Sun 2010; Shevkani et al. 2015a, b). On the other hand, legumins are the major globulin in chickpeas (Chavan et al. 1988) while the storage proteins in field pea and fava/faba bean comprise of both vicilins and legumins (Kimura et al. 2008; Shevkani et al. 2015a, b; Shevkani and Singh 2015).
Although vicilins are somewhat identical amongst pulses, their MW and composition vary amongst different pulses. Vicilins of about 133–140 kDa, 136–150 kDa, 162 kDa, 173 kDa, 155 kDa and 163 kDa were present in proteins from cowpea, kidney beans, mung bean, red bean, pea and fava/faba beans, respectively (Tang et al. 2009; Shevkani et al. 2015a, b; Vioque et al. 2012; Parmar et al. 2017). The vicilins also differed in terms of the attached carbohydrate groups. Vicilins (7S globulins) from lentils, cowpeas and French beans were glycosylated, whereas that from faba bean did not have carbohydrates in the structure (Gupta and Dhillon 1993; Kimura et al. 2008). Lentil vicilins contained about 2.8% carbohydrates (Gupta and Dhillon 1993). In addition to vicilins and legumins, peas also contained small amounts of a third globulin, convicilin, of about 70 kDa. Convicilin is a 7S globulin and in peas, it can occur in the form of a trimer of ~ 280–290 kDa composed of three convicilin molecules or as heteromeric trimers of convicilins and vicilins (Lam et al. 2018). Unlike vicilins, the convicilin contains sulphur-containing amino acids (McLean et al. 1974; Boye et al. 2010).
The composition of pulse protein depends on intrinsic as well as extrinsic factors. Mertens et al. (2012) observed a positive correlation of legumin to vicilin ratio with protein content for smooth pea varieties. The ratio of legumin to vicilin for pea and fava beans increased with seed development as legumins accumulated mostly during the last stages of growth (Chandler et al. 1984). The extrinsic factors like agronomic practice and environmental conditions during plant growth also affected pulse vicilins and legumins (Mertens et al. 2012). When grown under sulphur-deficient conditions, the proportion of vicilins was maintained throughout the seed development, while the synthesis of legumins was compromised or undetectable under severely deficient conditions (McLean et al. 1974).
Secondary structure
Secondary structures of proteins can be estimated using Fourier transform infrared (FT-IR) spectroscopy. This technique is used for qualitative as well as quantitative estimation of protein secondary structures. This technique also allows evaluation of protein structure during and following thermal treatments (Law et al. 2008). FT-IR spectroscopy has been used by a number of workers for evaluating the structural characteristics of proteins from various pulses (Meng and Ma 2001; Carbonaro et al. 2012; Shevkani et al. 2015a; Shevkani and Singh 2015; Parmar et al. 2017). Amongst the different bands/regions observed when proteins are studied using FT-IR, the amide-I region is most sensitive to the protein secondary structure and hence is analysed to estimate the secondary structural components of pulse proteins. Table 1 represents the assignments of amide I band of FT-IR spectra to the secondary structure components of different plant proteins (Susi and Byler 1988; Kavanagh et al. 2000; Ellepola et al. 2005; Barth 2007; Carbonaro et al. 2012). The proportion of major secondary structural components of different plant proteins is shown in Table 2. In general, the secondary structure of pulse proteins consisted mainly of β-sheets, β-strands and β-turns, whereas α-helix were present in relatively lower proportion (Table 2). The pulse proteins with relatively higher content of β-structures (parallel and antiparallel β-sheets/β-turns) were thermally more stable and denatured at higher temperatures as compared to those with higher content of α-helix (Carbonaro et al. 2012; Shevkani et al. 2014a, 2015a, b; Parmar et al. 2017). The predominance of β-structures in the secondary structure also contribute to lower the digestibility of pulse proteins. A high content of β-sheets was reported to limit the access of proteolytic enzymes leading to lower protein digestibility (Yu 2005). Carbonaro et al. (2012) observed a strong negative correlation between the proportion of β-sheets digestibility of proteins from different sources (common bean, chickpea, lentils, soybean, barley, emmer, milk, cheese and chicken meat). It was also reported that β-sheets in legumes and intermolecular β-sheets aggregates formed as a result of their thermal treatment were primarily responsible for their lower digestibility (Carbonaro et al. 2012).
Table 1.
Assignment of FT-IR amide I (1600–1700 cm−1) band spectrum to secondary structure components of plant proteins
| Frequency (cm−1) | Assignment to secondary structure component |
|---|---|
| 1610–1615 | Protein aggregates as well as to absorption of amino acid side-chains |
| 1618–1620 | Antiparallel β-sheet, aggregated strand |
| 1623 | Anti-parallel β-sheet |
| 1629–1633 | β-Strands |
| 1630–1638 | β-Sheets |
| 1643–1645 | Unordered (random coil) |
| 1650–1660 | α-Helices |
| 1660–1670 | β-Turns/turns conformation |
| 1670–1680 | β-Turns/turns conformation |
| 1680–1688 | Antiparallel β-sheets |
| 1690–1695 | β-Type structures, protein aggregates as well as to absorption of amino acid side-chains |
Table 2.
Proportion of major secondary structural components of different plant proteins
| Protein | α-Helices (%) | β-Sheets (%) | β-Turns/turns (%) | Random coil (%) | β-Strands (%) | Antiparallel β-sheet (%) | Disordered structures (%) | Source |
|---|---|---|---|---|---|---|---|---|
| Rice globulins | About 30% | Over 50%b | About 20 | – | – | Ellepola et al. (2005) | ||
| Oat protein isolate | 19 | 74 | 7 | – | – | – | – | Liu et al. (2009) |
| Barleyd | 28.0 | 31.0 | 6.6 + 2.4 | – | – | 7.4 | – | Carbonaro et al. (2012) |
| Soybeand | 11.8 | 30.2 | 12.8 + 15.4 | – | – | 7.1 | – | Carbonaro et al. (2012) |
| Soy protein isolate | 29.6 | 36.8 | 16.0 | – | – | – | 18.5 | Withana-Gamage et al. (2011) |
| Soybean 7S globulns | 14.5 | 45.6 | 23.8 | – | – | – | 14.4 | Zhao et al. (2008) |
| Soybean 11S globulins | 17.0 | 47.3 | 19.3 | – | – | – | 16.5 | Zhao et al. (2008) |
| Chickpea protein isolates | 25.6–32.7 | 32.5–40.4 | 13.8–18.9 | – | – | – | 16.3–19.2 | Withana-Gamage et al. (2011) |
| Chickpead | 19.9 | 37.2 | 7.7 + 11.7 | – | – | 7.2 | – | Carbonaro et al. (2012) |
| Pea protein isolate | 28.0 | 30.0 | 21.7 | – | – | 6.9 | – | Shevkani et al. (2015b) |
| Kidney bean protein isolate | 22.8 | 38.6 | 20.1 | – | – | 8.4 | – | Shevkani et al. (2015b) |
| Dolichos lablab vicilins | 13.8 | 16.7 | 18.2 | 13.0 | 18.8 | – | – | Law et al. (2008) |
| Phaseolus calcaratus vicilins | 13.5 | 20.9 | 18.6 | 14.0 | 11.9 | – | – | Law et al. (2008) |
| Red bean (Phaseolus angularis) globulinsa | 23.2 | 17.9 | 30.0 | 28.9 | – | – | – | Meng and Ma (2002) |
| Lentilsd | 18.0 | 33.0 | 5.4 + 13.4 | – | – | 6.2 | – | Carbonaro et al. (2012) |
aCalculated from far-UV CD spectra
bβ-Sheets and turns
cMean values for 5 lines/genotypes
dReported as spectral weights of the secondary structure components
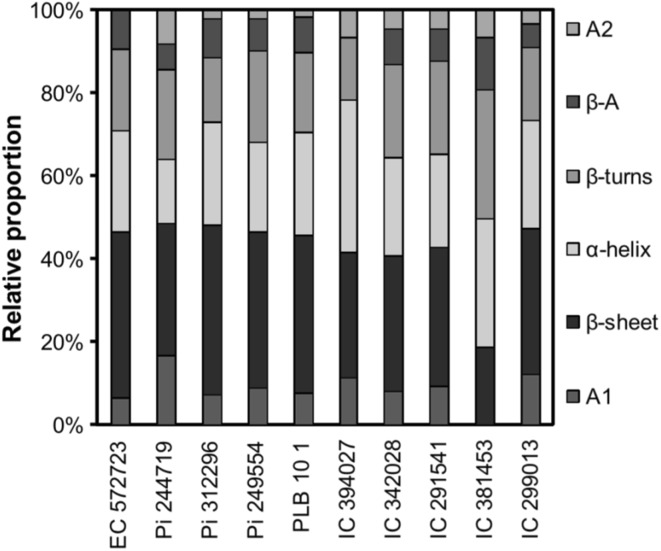
In comparison to barley proteins, the pulse proteins contained higher proportion of β-conformations (paralle and antiparallel β-sheets + β-strands + β-turns) and lower of α-helices while rice globulins and oat proteins had higher or somewhat similar content of β-sheets/turns (Table 2). The relative proportion of different secondary structure components of pulse proteins varied amongst different cultivars. The relative proportion of different secondary structure of five each of kidney bean and field pea genotypes/lines is shown in Fig. 1. In addition, the proteins from harder-to-cook kidney bean grains had higher β-sheets content than the proteins from the corresponding easy-to-cook/normal gains (Parmar et al. 2017).
Fig. 1.
Relative proportion of different secondary structural components of protein isolates from different kidney bean and field pea lines. EC 572723, Pi 244719, Pi 312296, Pi 249554, and PLB 10 1 are kidney bean lines while IC 394027, IC 342028, IC 291541, IC 381453 and IC 299013 are field pea lines
(Source: Shevkani et al. 2015b)
Thermal processing/heating, shift in pH, change in ionic strength and presence of salts and detergents alter the structure of pulse proteins. Heating can bring about un-ordering of secondary structure of proteins by affecting disulphide and hydrogen linkages. Heating of the plant globulins resulted in reorganization of the protein secondary structure (Tang and Ma 2009). Law et al. (2008) studied the effect of acidic (3 and 5) and basic pH (9 and 11), chaotropic salts (sodium chloride, sodium bromide, sodium iodide and sodium thiocyanate) and perturbants (ethylene glycol, sodium dodecyl sulfate, N-ethylmaleimide, urea and dithiothreitol) on the secondary structure of Dolichos lablab and Phaseolus calcaratus vicilins. They observed that acidic condition (pH 3 and 5) decreased α-helix and random coil content of Dolichos lablab vicilins, while extreme alkaline conditions brought about decrease in the content of α-helix and β-sheet; however, pH had less effect on α-helix content of Phaseolus calcaratus vicilins, yet the extreme acidic conditions (pH 3) decreased the content of the β-sheets and increased that of random coil of Phaseolus calcaratus vicilins. The authors also reported that the presence of chaotropic salts brought about transition in protein structure from ordered to disordered as indicated by decrease in β-sheet content and increase in the content of random coils for vicilins from both pulses while protein perturbants resulted in denaturation of vicilins (Law et al. 2008).
Functional properties
Functional properties of proteins decide their ultimate application in various food systems. Protein solubility, emulsification, water and fat absorption, foaming and gelation/rheological behaviours are considered as the most important functional properties of protein isolates in foods. The proteins from different pulses show wide variations for these properties which has been attributed to differences in protein molecular features (such as amino acid composition, MW, secondary structure, charge distribution, hydrophobicity, etc.), processing conditions and environmental factors (Tang and Sun 2011; Shevkani et al. 2014a, 2015a, b).
Protein solubility
Protein solubility is an important property of food protein isolates because of its association with emulsifying, foaming and gelation properties. The protein solubility of protein isolates is dependent primarily on the balance of hydrophilic to hydrophobic residues in the structure (Damodaran 2008). In addition, surface charge, pH of the system as well as type and concentration of salts also affect the protein solubility of pulse protein isolates. Hydrophobic patches on the surface of proteins hinder protein solubility whereas charge on protein surface favour solubility. A negative relation between protein solubility and surface hydrophobicity (Karaca et al. 2011) while positive between solubility and surface charge (magnitude of zeta potential) has been reported for pea, faba bean and kidney bean protein isolates (Karaca et al. 2011; Shevkani and Singh 2015; Shevkani et al. 2015b).
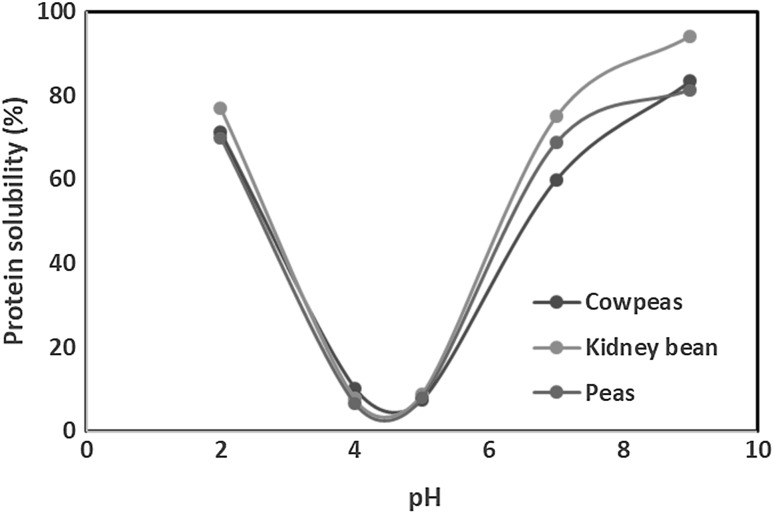
The pH of medium has significant effect on the solubility of pulse proteins. Proteins from different pulses and their cultivars/varieties generally showed higher solubility at alkaline and acidic pH values whereas they were sparingly soluble at pH around their isoelectric point which generally ranged between pH 4.0 and 5.0 for most pulses (Karaca et al. 2011; Shevkani et al. 2015a, b; Ghumman et al. 2015; Joshi et al. 2017). The proteins carried no net charge at their isoelectric point, consequently there was no repulsion between the charged molecules and the protein–protein interaction favoured aggregation. Representative protein solubility-pH profiles of kidney bean, pea and cowpea proteins are shown in Fig. 2.
Fig. 2.
Typical U shaped protein solubility-pH profiles of proteins isolated from three different pulses
Tang and Sun (2010) highlighted that electrostatic repulsions or interactions between proteins were important for protein solubility of mung bean globulins. As salts in the solution can screen electric diffuse and stern layers surrounding proteins, they may affect solubility of pulse proteins by influencing surface charge (zeta potential) and electrostatic repulsive forces and, depending on the salt concentration and type, they may experience salt-induced solubilization (salting-in) or salt-induced aggregation/insolubilization (salting-out) (Tiwari and Singh 2012; Lam et al. 2018). Thiocyanates, perchlorates, barium and calcium salts increased solubility by promoting interactions between proteins and water and ordering the surrounding hydration layers while sulfates, hydrogen phosphate, potassium and ammonium salts promoted ion-water interactions, disrupting the hydration layers surrounding the proteins and exposed hydrophobic moieties resulting in aggregation/precipitation of proteins depending on ionic strength of the solution and hydrophobicity of proteins (Damodaran 2008).
Emulsifying and foaming properties
An emulsion may be defined as a dispersion/suspension of two immiscible liquids (mostly oil/fat and water in foods) in which one liquid is dispersed in the form of globules within the continuous phase of another liquid. Such a system is thermodynamically unstable because of increased interfacial surface tension. Proteins can stabilize such system by decreasing interfacial tension and preventing coalescence because of their ability to adsorb at the interface and forming elastic/cohesive layers surrounding the oil droplets (Dickinson 2010). Proteins further contribute to the stability of emulsion by increasing the viscosity of the continuous phase that decreases the rate of movement of oil droplets in the system (Sikorski 2001).
The ability of proteins to stabilize emulsion is regarded as one of the most important functional properties that decide their applications in food products like comminuted meats, batters, doughs, coffee/tea whiteners, ice-creams, soups, cakes, mayonnaise, etc. Emulsifying properties of pulse proteins can be measured as emulsifying activity index (EAI) and emulsion stability index (ESI). Proteins from different pulses and their varieties differ widely for emulsifying properties. Protein isolates form lentils, horse gram, kidney beans, field peas and cowpeas showed EAI and ESI varying in the range from 4.74 to 26.60 m2/g and 7.18 to 95.40 min (Shevkani et al. 2015a, b; Ghumman et al. 2016). Karaca et al. (2011) reported higher EAI (47.90, 44.29, 44.51, 42.87 and 44.20 m2/g, respectively) and ESI (82.94, 69.39, 86.79, 12.40 and 85.97 min, respectively) for protein isolates from chickpeas, faba beans, lentils, peas and soybeans, respectively. The solubility and hydrophobicity are two major factors that determine adsorption and emulsifying properties of proteins. Kato and Nakai (1980) reported relation between surface hydrophobicity, interfacial tension and emulsifying properties of proteins. In addition, charge on protein surface (magnitude of zeta potential) together with hydrophobicity strongly affects the adsorption of proteins at interface (Magadassi and Kamishny 1996). Furthermore, composition and molecular flexibility of pulse proteins have also been attributed for the differences in emulsifying properties of proteins from different pulses. Vicilins from peas exhibited superior emulsifying properties than corresponding legumins (Kimura et al. 2008), which has been attributed to their greater solubility (Koyoro and Powers 1987) and higher surface hydrophobicity (Boye et al. 2010). However, in a relatively recent study on proteins from different kidney bean and field pea lines/cultivars (Shevkani et al. 2015a, b), it was observed that the kidney bean proteins, despite lower hydrophobicity and protein solubility, showed higher EAI than proteins from different field pea lines/cultivars. Similarly, the protein isolate from white cowpea with higher vicilins showed higher emulsifying properties than that from red cowpea with relatively lesser vicilin content (Shevkani et al. 2015a). This was attributed partly to lower MW and greater flexibility in the structure of vicilins which made them relatively easy to reorient at the interface after being adsorbed there (Shevkani et al. 2015a, b).
Foaming properties determine the applications of proteins in food products where aeration and overrun is essentially required (Shevkani et al. 2014b). Chiffon cakes, fudges, confectionery products, whipped toppings, soufflés and mousses, ice-cream mixes, etc. are some of the examples of such products. Foaming properties of pulse proteins are measured as foaming capacity (FC) and foam stability (FS). FC is an indicator of the increase in volume after whipping. It depends on the ability of proteins to diffuse to the interface, reorient, and form a viscous film without excessive aggregation. FS reflects the ability of proteins to maintain the foam. Cowpea protein isolate with more soluble proteins (higher protein solubility) showed greater foaming and vice versa (Shevkani et al. 2015a). A positive relation of foaming properties with surface charge (magnitude of zeta potential) and solubility for protein isolates from various kidney bean and field pea lines was also reported by Shevkani et al. (2015b). It was suggested that greater charge on the surface of proteins contributed to greater foaming by weakening hydrophobic interactions, increasing protein solubility and flexibility, which allowed their rapid spreading on the interface and rapidly encapsulate air particles. Ghumman et al. (2015) reported higher FC and FS for albumins from lentils (76.7% and 66.7%, respectively) and horse gram (79% and 55%, respectively) as compared to the globulins (FC = 16.7% and 8.9%, respectively; FS = 6.7% and 4.4%, respectively). The lower foaming for globulins was attributed to their reduced ability to unfold/reorient at the interface, which limits encapsulation of air bubbles (Sai-Ut et al. 2009), while albumins on the other hand, were highly soluble hence showed increased ability to foam due to enhanced protein unfolding (Mundi and Aluko 2012). The soluble pulse proteins can migrate to the interface quickly to accommodate change in conformation for formation of stable foam (Yin et al. 2010; Adebiyi and Aluko 2011; Shevkani et al. 2015a, b).
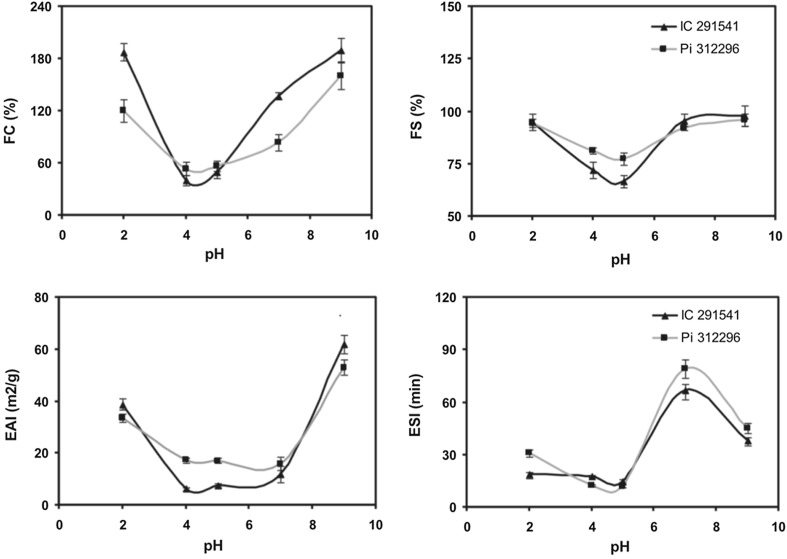
Emulsifying and foaming properties of pulse proteins are controlled by pH of the system. EAI/ESI-pH and FC/FS-pH curves of pulse proteins generally resemble the protein solubility-pH curve (Fig. 3). Proteins from different pulses (beans, peas and chickpeas) showed higher emulsification and foaming in the acidic and alkaline pH, while lower values at pH around isoelectric point (Zhang et al. 2009; Adebiyi and Aluko 2011; Shevkani et al. 2015a, b). Charge on proteins should be high enough to succeed various attractive forces that lead to stabilize electrostatic repulsive forces between oil droplets (McClements 2005), while at isoelectric point proteins have zero net charge. Furthermore, it has also been suggested that for entrapping oil droplets or air bubbles the proteins need to be soluble in the liquid phase so that they can rapidly migrate to the interface and rearrange there to form elastic film (Yin et al. 2010; Adebiyi and Aluko 2011; Toews and Wang 2013).
Fig. 3.
Foaming (FC foaming capacity and FS foam stability) and emulsifying properties (EAI emulsifying activity index and ESI emulsion stability index) of protein isolates from kidney bean (Pi 312296) and field pea (IC 291541) at different pH
(Source: Shevkani et al. 2015b)
Water and fat absorption
Water and fat/oil absorption of proteins are related with texture, mouthfeel and flavour retention of foods (Shevkani et al. 2015a). Water absorption capacity (WAC) is particularly a critical property of proteins in viscous foods, such as, soup, dough, custard and baked foods, which are supposed to imbibe water without dissolution of the proteins (Sreerama et al. 2012) whereas high fat/oil absorption capacity (FAC/OAC) of protein is required in ground meals, meat replacers and extenders and baked foods in which fat/oil contribute to the texture of finished foods. In general, pulse protein isolates show higher water and fat absorption capacities as compared to the corresponding flours (Tiwari and Singh 2012).
Water absorption capacity may be defined as the ability of proteins to physically hold water against gravity (Kinsella and Melachouris 1976) and it is most commonly expressed as mass of water that can be absorbed by known weight of protein isolate. WAC of pulse protein products is mainly due to the hydrophilic parts like polar and charged side chains of proteins as well as of carbohydrates which have an affinity for water molecules (Kinsella and Melachouris 1976). WAC of pulse proteins vary widely amongst different pulses and their variety. Proteins from different kidney bean, field pea and cowpea varieties/lines showed WAC varying between 1.6 and 4.8 g/g (Shevkani et al. 2015a, b). Vioque et al. (2012) reported a value of 2.6 g/g for Vicia faba protein isolate while Kaur and Singh (2007) observed WAC between 2.3 and 3.5 g/g for chickpea protein isolates and Kaur et al. (2007) reported WAC of 2.8–2.9 g/g for lentil protein isolates.
Fat/oil absorption capacity of proteins mainly determines mouthfeel and flavour retention of foods (Kinsella and Melachouris 1976). FAC/OAC may be defined as the amount of fat/oil that can be absorbed by protein. In general, proteins interact with lipids through the binding of nonpolar side chains of amino acids with aliphatic chains of oils/fats. Proteins from different pulses differed for FAC. Vioque et al. (2012) reported a value of 2.31 g/g for Vicia faba protein isolate. Protein isolates from different kidney bean, field pea and cowpea varieties/lines showed FAC varying between 4.7–6.9 g/g, 5.5–7.2 g/g and 1.4–2.0 g/g, respectively (Shevkani et al. 2015a, b).
Gelation and rheological properties
Gelation is an important functional property of proteins in viscous foods such as puddings, soups, gels, curds, heated-minced meats, etc. The protein gels can be formed by application heat, pressure and ions, though heat-induced gelation is most commonly involved in the processing of various heat-set protein products. However, excessive heating of proteins at high temperatures (> 100 °C) may cause scission of peptide bonds which may prevent gelation (Joshi et al. 2017).
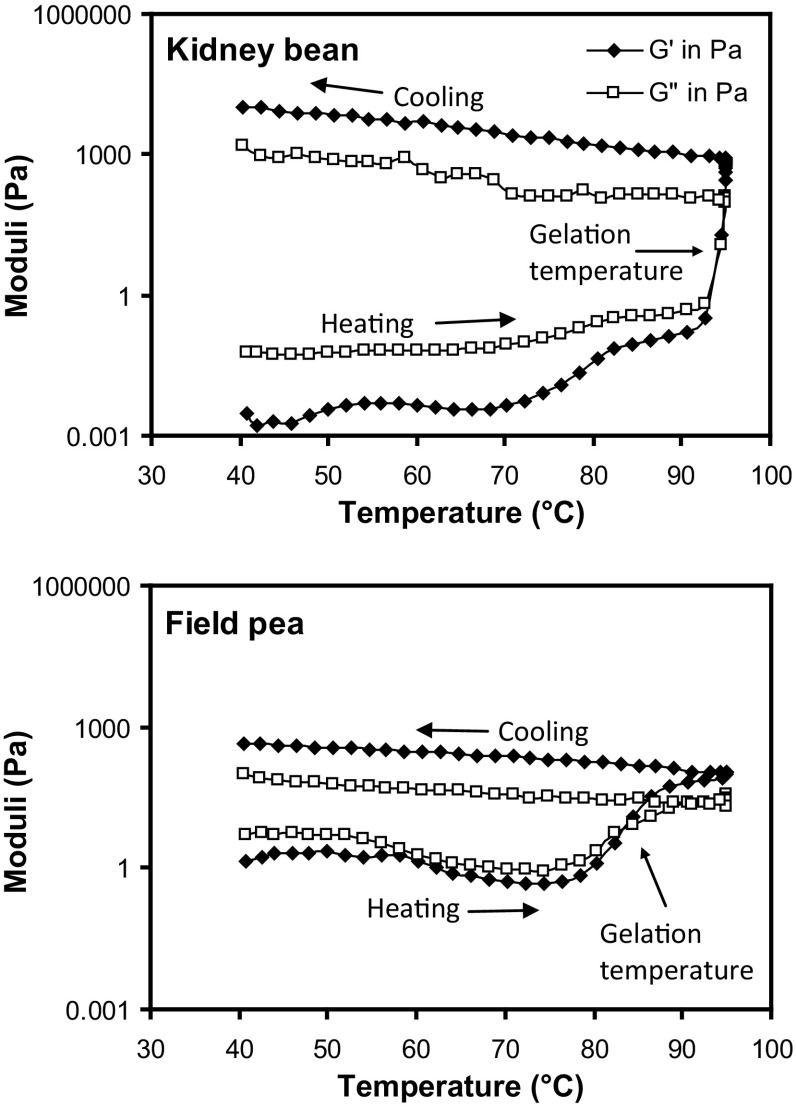
Dynamic rheometers are widely used to study heat-induced gelation of pulse proteins. Parameters obtained from dynamic rheological measurements of protein suspensions include G′ and G″ which are indicators of the gel strength. G′ determines elastic component of gel network and indicates the strength of the structure contributing to three-dimensional network, whereas G″ represents the interactions not contributing to the three-dimensional network (Sun and Arntfield 2010). Gelation/gel temperature i.e. minimum temperature for gel formation is generally detected as the crossover temperature between G′ and G″ during the temperature sweep rheological measurements (Fig. 4). Heat-induced gelation of pulse proteins depends on a number of factors including protein structure, composition and pH of medium. Gelation temperature of pulse proteins is generally dependent on thermal stability of proteins and is higher than the thermal denaturation temperature, as denaturation is a prerequisite for heat-induced gelation (Nagano et al. 1992). Gelation temperatures in the range from 87.4 to 94.5 °C and 84.0 to 93.1 °C, respectively were reported for protein isolates from different kidney bean and field pea lines having denaturation temperatures of 85.7–93.3 °C and 82.7–85.5 °C, respectively (Shevkani et al. 2015a, b). Higher gelation temperature for kidney bean protein isolates than field pea isolates was attributed to higher relative proportion of β-sheets that contributed to higher thermal stability of proteins through large surface area and greater intermolecular interaction. Mession et al. (2015) studied the effect of fractionation of globular proteins (salt extracted and ultra-filtered) from smooth yellow peas on heat-induced aggregation and acid cold-set gelation. They separated vicilins and legumins from pea globulins obtained by salt extraction and ultrafiltration and showed that the denaturation temperature of pea proteins increased with increasing legumin content. The effects of varying pH between 3 and 10 on gelation properties of pea protein isolate (salt extracted) at different salt concentrations (0–2.0 M) was investigated by Sun and Arntfield (2011). It was shown that the stiffest gel was formed using 0.3 M concentration of NaCl at pH 4.0.
Fig. 4.
Changes in the dynamic rheological moduli (G′ and G″) of protein isolates from kidney bean and field pea during heating and cooling. Arrow showing gelation temperature of protein as crossover temperature between moduli
(Source: Shevkani et al. 2015b)
Nutraceutical/bioactive properties
Traditionally, pulses have been of interest particularly because of high protein content. However, recently, pulse proteins have also been of interest owing to their bioactive properties such as those considered to be related with the management of type-2 diabetes and obesity and lowering of serum cholesterol and low-density lipoprotein-cholesterol levels (Boye et al. 2010; Li et al. 2017). Pulse protein ingredients may contain bioactive constituents like lectins/hemagglutinins and enzyme inhibitors, possessing health benefits like they may help in lowering serum glucose levels and alleviating obesity (Campos-Vega et al. 2010; Tiwari and Singh 2012; Shevkani et al. 2015b). In addition, the substitution of animal proteins with pulse proteins has also been found to decrease the levels of low-density lipoprotein cholesterol, non-high-density lipoprotein cholesterol, and apolipoprotein responsible for cardiovascular diseases (Li et al. 2017). Peptides from chickpea legumins contained histidine, tryptophan and phenylalanine having antioxidant activity (Torres-Fuentes et al. 2015). Li et al. (2008) observed that chickpea protein hydrolysate fraction (fra. IV) with the highest total hydrophobic amino acids content (38.94%) and hydrophobicity (125.62 kcal/mol amino acid residue) exhibited the strongest antioxidant activity. Common bean and mung bean protein hydrolysates and peptides exhibited angiotensin I converting enzyme inhibition, antioxidant capacity, antimicrobial and antitumor activities (Luna-Vital et al. 2015; Xie et al. 2019). Owing to theses nutraceutical properties the inclusion of pulse proteins in diets may be recommended for management of obesity and reducing the risk of chronic diseases like hypertension, cardiovascular diseases and cancers.
Applications
Pulse proteins as concentrates and isolates find applications in a number of food systems. The pulse protein isolates are often prepared from dehulled/defatted pulse flours by alkaline extraction and isoelectric precipitation method involving stirring in aqueous alkali (NaOH) solution at pH between 8 and 11, followed by stirring for periods varying from 30 to 180 min to maximize solubilisation of the proteins, filtration/centrifugation to remove insoluble material/residue and precipitation of proteins at isoelectric pH (Boye et al. 2010; Tiwari and Singh 2012). The inclusion of pulses and pulse proteins in diet is generally recommended because of nutritional as well as nutraceutical properties. Recently, pulse proteins have been explored for nutritional and functional improvement of a number of food products including cakes, muffins, crackers, pasta, meat products, beverages, etc.
Cereal-based (bakery and pasta) foods
Pulse proteins, as concentrates or isolates, are primarily used in novel and traditional foods as nutritional additives and ingredients to improve nutritional value and to impart necessary sensory characteristics (structure, texture, flavour, and colour). The essential amino acid content complimentary to cereals makes them particularly suitable for improving the protein quality of cereal-based foods. Pulse proteins find applications mainly due to their low cost, comparative functionality, acceptability and high nutritional and nutraceutical properties. In the cereal-based foods, pulse protein ingredients not only improved protein quality but also contributed to improved texture of finished products by increasing water absorption of dough/batters. The addition of thermally modified cowpea proteins improved water absorption and acceptability of wheat bread and sponge cake (Campbell et al. 2016). It was also reported that glycated and denatured cowpea proteins can be used to replace whole egg by 20% in sponge cake (Campbell et al. 2016). Chickpea proteins partially substituted soy (one-third of soy) in breads without negatively affecting the texture of finished product (Serventi et al. 2018).
Pulse proteins also improved technological quality attributes of gluten-free foods by increasing viscoelasticity of batters or by forming protein network in the dough systems. Protein isolates from kidney beans, peas and cowpeas were explored for possible application in gluten-free muffins (Shevkani and Singh 2014; Shevkani et al. 2015a). Kidney bean and pea protein isolates at incorporation level of 10% enhanced viscoelasticity (dynamic rheological moduli) of corn starch-based batters and resulted in muffins with quality attributes (crust colour, specific volume, springiness, cohesiveness, appearance and porosity) comparable to the muffins prepared with wheat gluten (Shevkani and Singh 2014). Cowpea proteins also improved technological quality attributes of rice muffins depending on level of incorporation and protein characteristics (Shevkani et al. 2015a). It was demonstrated that emulsification and foaming were desirable characteristics of protein isolates for quality improvement of rice muffins. Shaabani et al. (2018) recently studied the effect of addition of varying levels of chickpea protein isolate, transglutaminase and xanthan gum on the rheological properties and quality attributes of millet muffins and showed that chickpea proteins with transglutaminase contribute to the formation of protein network in the muffins.
Imitation milk and bean curds
Pulse proteins have also found applications in the preparation of non-dairy/imitation milks, beverages and bean curds. Protein isolates from different pulses were explored for their application in the preparation of imitation milk (Sosulski et al. 1978). The milks were preferred in the following order of functional and organoleptic properties: lima bean = mung bean = pea bean > northern bean = lupin > lentil = soybean > chickpea > field pea > faba bean. Cai et al. (2001) investigated the application of protein fractions from soybean, chickpeas, lentils, smooth peas, mung beans, and faba beans in the preparation of bean curds. They prepared bean curds through thermal denaturation of bean milks and coagulation with calcium sulphate and magnesium sulphate and reported that bean curds from chickpea and faba bean had textural properties comparable to soy proteins. It was also shown that protein concentration of 2.3–3% using 1.5% calcium sulphate as coagulant formed the best curd.
Meat products
Pulse proteins in comminuted cooked meat products improved textural properties and yield. Abdel-Aal et al. (1987) used chickpea and faba bean protein and flours (levels of 20–40%) as extender in sausages. They reported that proteins were more acceptable than flours for all substitution levels. Ghribi et al. (2018) recently investigated the effect of incorporation of lower levels of (1.5, 2.5 and 5%) chickpea protein concentrates on the properties of cooked sausage and reported that chickpea proteins not only reduced cooking yield and lipid oxidation but also increased colour stability, total antioxidant capacities, protein content and acceptability by consumers.
Edible/biodegradable films
Among various biodegradable polymers, pulse proteins have been regarded as a viable alternative for petroleum-based polymeric products and attracted increasing attention in both academic research and industries because of their excellent accessibility and low cost (Tang et al. 2009; Shevkani and Singh 2015). A flexible composite film was fabricated using kidney bean protein isolate and chitosan as antimicrobial food-packaging by Fan et al. (2014). They showed that the films produced at pH 3.0 and loaded with nisin had antimicrobial activity against Bacillus subtilis and Staphylococcus aureus (Fan et al. 2014). Shevkani and Singh (2015) investigated film-forming properties of kidney bean and field pea protein isolates. The films were casted from aqueous protein dispersions of varying pH (7, 8 and 9) and evaluated for optical properties, tensile strength, water resistance (water-solubility and water vapour permeability) and structure (FT-IR spectroscopy). The properties of the films related with protein characteristics (protein MW, surface charge, secondary and tertiary structure). In general, the field pea proteins were observed to be the most appropriate for film formation as the films were the lightest in colour, highest in tensile strength, and the lowest in opacity. It was also observed that basic pH increased transparency while heating increased tensile strength and water resistance (decreased water vapour permeability and water solubility) of films, these changes were observed to vary with the protein source depending on the extent of proteins ordered structure. Recently, it was reported that the incorporation of lentil proteins improved mechanical properties (Young modulus, stress at break) and water tolerance of starch films because of the enhanced crosslinking between protein and polymeric backbone (Yepes et al. 2019).
Encapsulation material
Pulse proteins have also been used as encapsulating material in the food industry to protect bioactive, volatiles and other components from deterioration as well as to mask the undesirable flavors in foods. Bioactive substances such as omega-3 fatty acids, phytosterols and carotenoids, can be encapsulated using the emulsions of vegetable proteins (Nesterenkoet al. 2013). Pea protein isolate stabilized emulsions effectively reduced oxidation of conjugated linoleic acid during storage (Lin et al. 2017). Chickpea protein encapsulation improved the stability of folate in processed foods (Ariyarathna and Karunaratne 2015).
Conclusion
Pulses are rich source of proteins and other nutrients. Proteins in different pulses comprise mainly globulins (vicilins and legumins). The composition of pulse proteins differs with genetics, stage of seed development, cultural/agronomic practices and environmental conditions during growth. Bean (kidney bean, mung bean, red bean and urad bean) and cowpea proteins contain vicilins as major globulins while chickpea proteins are high in legumins, whereas pea and fava/faba bean proteins comprise of both vicilins and legumins. In general, β-sheets, β-strands and β-turns are major secondary structure components in pulse proteins. They have been attributed partly to the lower digestibility of pulse proteins. Pulse proteins possess functional properties that make them suitable for utilization in a number of products. However, proteins from different pulses differ for their functional properties depending on composition, structure and processing conditions (ratios of albumins to globulins and legumis to vicilins, secondary structure, amino acid composition, molecular weight, hydrophobicity and surface charge as well as state of proteins i.e. native or denatured, medium pH and type and concentration of salt). In addition, pulse proteins also exhibit nutraceutical and disease preventing properties (such as lowering of blood/serum glucose and cholesterol levels, angiotensin I converting enzyme inhibition, antioxidant capacity, antimicrobial and antitumor activities) because of the presence of bioactive compounds like lectins/hemagglutinins, enzyme inhibitors, peptides and amino acids with antioxidant activity. Therefore, their inclusion in diet is recommended for reducing the risk of chronic diseases like hypertension, cardiovascular diseases and cancers and management of obesity and diabetes.
Acknowledgements
KS acknowledges UGC for BSR startup Grant. NS acknowledges DST for JC Bose National Fellowship.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abdel-Aal EM, Youssef MM, Shehata AA, El-Mahdy AR. Some legume proteins as bread fortifier and meat extender. Alex J Agric Res. 1987;32:179–189. [Google Scholar]
- Adebiyi AP, Aluko RE. Functional properties of protein fractions obtained from commercial yellow field pea (Pisum sativum L.) seed protein isolate. Food Chem. 2011;128:902–908. doi: 10.1016/j.foodchem.2011.03.116. [DOI] [Google Scholar]
- Ariyarathna IR, Karunaratne DN. Use of chickpea protein for encapsulation of folate to enhance nutritional potency and stability. Food Bioprod Process. 2015;95:76–82. doi: 10.1016/j.fbp.2015.04.004. [DOI] [Google Scholar]
- Barth A. Infrared spectroscopy of proteins. Biochim Biophys Acta. 2007;1767:1073–1101. doi: 10.1016/j.bbabio.2007.06.004. [DOI] [PubMed] [Google Scholar]
- Boulter D, Croy RRD. Legume seed storage proteins. Adv Bot Res. 1997;27:2–70. [Google Scholar]
- Boye J, Zare F, Pletch A. Pulse proteins: processing, characterization, functional properties and applications in food and feed. Food Res Int. 2010;43:414–431. doi: 10.1016/j.foodres.2009.09.003. [DOI] [Google Scholar]
- Cai R, Klamczynska B, Baik BK. Preparation of bean curds from protein fractions of six legumes. J Agric Food Chem. 2001;49:3068–3073. doi: 10.1021/jf0013398. [DOI] [PubMed] [Google Scholar]
- Campbell L, Euston SR, Ahmed MA. Effect of addition of thermally modified cowpea protein on sensory acceptability and textural properties of wheat bread and sponge cake. Food Chem. 2016;194:1230–1237. doi: 10.1016/j.foodchem.2015.09.002. [DOI] [PubMed] [Google Scholar]
- Campos-Vega R, Loarca-Piña G, Oomah BD. Minor components of pulses and their potential impact on human health. Food Res Int. 2010;43:461–482. doi: 10.1016/j.foodres.2009.09.004. [DOI] [Google Scholar]
- Carbonaro M, Maselli P, Nucara A. Relationship between digestibility and secondary structure of raw and thermally treated legume proteins: a Fourier transform infrared (FT-IR) spectroscopic study. Amino Acid. 2012;43:911–921. doi: 10.1007/s00726-011-1151-4. [DOI] [PubMed] [Google Scholar]
- Chandler PM, Spencer D, Randall PJ, Higgins TJ. Influence of sulfur nutrition on developmental patterns of some major pea seed proteins and their mRNAs. Plant Physiol. 1984;75:651–657. doi: 10.1104/pp.75.3.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavan JK, Kadan SS, Salunkhe DK. Biochemistry and technology of chickpea (Cicer arietinum L.) seeds. Crit Rev Food Sci Nutr. 1988;25:107–158. doi: 10.1080/10408398709527449. [DOI] [PubMed] [Google Scholar]
- Damodaran S. Amino acids, peptides, and proteins. In: Damodaran S, Parkin KL, Fennema OR, editors. Fennema’s food chemistry. Boca Raton: CRC Press; 2008. [Google Scholar]
- Dickinson E. Flocculation of protein-stabilized oil-in-water emulsions. Colloids Surf B Biointerfaces. 2010;81:130–140. doi: 10.1016/j.colsurfb.2010.06.033. [DOI] [PubMed] [Google Scholar]
- Ellepola SW, Choi SM, Ma Y. Conformational study of globulin from rice (Oryza sativa) seeds by Fourier-transform infrared spectroscopy. Int J Biol Macromol. 2005;37:12–20. doi: 10.1016/j.ijbiomac.2005.07.008. [DOI] [PubMed] [Google Scholar]
- Fan JM, Ma W, Liu GQ, Yin SW, Tang CH, Yang XQ. Preparation and characterization of kidney bean protein isolate (KPI)–chitosan (CH) composite films prepared by ultrasonic pretreatment. Food Hydrocoll. 2014;36:60–69. doi: 10.1016/j.foodhyd.2013.08.024. [DOI] [Google Scholar]
- Ghribi AM, Amira AB, Gafsi IM, Lahiani M, Bejar M, Triki M, Zouaria A, Attia H, Besbes S. Toward the enhancement of sensory profile of sausage “Merguez” with chickpea protein concentrate. Meat Sci. 2018;143:74–80. doi: 10.1016/j.meatsci.2018.04.025. [DOI] [PubMed] [Google Scholar]
- Ghumman A, Kaur A, Singh N. Functionality and digestibility of albumins and globulins from lentil and horse gram and their effect on starch rheology. Food Hydrocoll. 2016;61:843–850. doi: 10.1016/j.foodhyd.2016.07.013. [DOI] [Google Scholar]
- Gueguen J, Cerleti P. Proteins of some legume seeds: soybean, pea, fababean and lupin. In: Hudson BJF, editor. New and developing sources of food proteins. Durango: Chapman and Hill; 1994. pp. 145–193. [Google Scholar]
- Gupta R, Dhillon S. Characterization of seed storage proteins of lentil (Lens culinaris M.) Ann Biol. 1993;9:71–78. [Google Scholar]
- Joshi M, Timilsena Y, Adhikari B. Global production, processing and utilization of lentil: a review. J Integr Agric. 2017;16:2898–2913. doi: 10.1016/S2095-3119(17)61793-3. [DOI] [Google Scholar]
- Karaca A, Low NH, Nickerson MT. Emulsifying properties of chickpea, faba bean, lentil and pea proteins produced by isoelectric precipitation and salt extraction. Food Res Int. 2011;44:2742–2750. doi: 10.1016/j.foodres.2011.06.012. [DOI] [Google Scholar]
- Kato A, Nakai S. Hydrophobicity determined by a fluorescence probe method and its correlation with surface properties of proteins. Biochim Biophys Acta. 1980;624:13–20. doi: 10.1016/0005-2795(80)90220-2. [DOI] [PubMed] [Google Scholar]
- Kaur M, Singh N. A comparison between the properties of seed, starch, flour and protein separated from chemically hardened and normal kidney beans. J Sci Food Agric. 2007;87:729–737. doi: 10.1002/jsfa.2798. [DOI] [Google Scholar]
- Kavanagh GM, Clark AH, Ross-Murphy SB. Heat-induced gelation of globular proteins. Part 3. Molecular studies on low pH b-lactoglobulin gels. Int J Biol Macromol. 2000;28:41–50. doi: 10.1016/S0141-8130(00)00144-6. [DOI] [PubMed] [Google Scholar]
- Kimura A, Fukuda T, Zhang M, Motoyama S, Maruyama N, Utsumi S. Comparison of physicochemical properties of 7S and 11S globulins from pea, fava bean, cowpea, and French bean with those of soybean-french bean 7S globulin exhibits excellent properties. J Agric Food Chem. 2008;56:10273–10279. doi: 10.1021/jf801721b. [DOI] [PubMed] [Google Scholar]
- Kinsella JE, Melachouris N. Functional properties of proteins in foods: a survey. Crit Rev Food Sci Nutr. 1976;7:219–280. doi: 10.1080/10408397609527208. [DOI] [Google Scholar]
- Kong J, Yu S. Fourier transform infrared spectroscopic analysis of protein secondary structures. Acta Biochim Biophys Sin. 2007;39:549–559. doi: 10.1111/j.1745-7270.2007.00320.x. [DOI] [PubMed] [Google Scholar]
- Koyoro H, Powers JR. Functional properties of pea globulin fractions. Cereal Chem. 1987;64:97–101. [Google Scholar]
- Lam ACY, Karaca A, Tyler RT, Nickerson MT. Pea protein isolates: structure, extraction, and functionality. Food Rev Int. 2018;34:126–147. doi: 10.1080/87559129.2016.1242135. [DOI] [Google Scholar]
- Law HY, Choi SM, Ma CY. Study of conformation of vicilin from Dolichos lablab and Phaseolus calcaratus by Fourier-transform infrared spectroscopy and differential scanning calorimetry. Food Res Int. 2008;41:720–729. doi: 10.1016/j.foodres.2008.05.004. [DOI] [Google Scholar]
- Li Y, Jiang B, Zhang T, Mu W, Liu J. Antioxidant and free radical-scavenging activities of chickpea protein hydrolysate (CPH) Food Chem. 2008;106:444–450. doi: 10.1016/j.foodchem.2007.04.067. [DOI] [Google Scholar]
- Li SS, Blanco Mejia S, Lytvyn L, Stewart SE, Viguiliouk E, Ha V, de Souza RJ, Leiter L, Kendall CWC, Jenkins DJA, Sievenpiper JL. Effect of plant protein on blood lipids: a systematic review and meta-analysis of randomized controlled trials. J Am Heart Assoc. 2017;6:e006659. doi: 10.1161/JAHA.117.006659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin D, Lu W, Kelly AL, Zhang L, Zheng B, Miao S. Interactions of vegetable proteins with other polymers: structure-function relationships and applications in the food industry. Trend Food Sci Technol. 2017;68:130–144. doi: 10.1016/j.tifs.2017.08.006. [DOI] [Google Scholar]
- Liu G, Li J, Shi K, Wang S, Chen J, Liu Y, Huang Q. Composition, secondary structure, and self-assembly of oat protein isolate. J Agric Food Chem. 2009;57:4552–4558. doi: 10.1021/jf900135e. [DOI] [PubMed] [Google Scholar]
- Luna-Vital DA, Mojica L, de Mejia EG, Mendoza S, Loarca-Piña G. Biological potential of protein hydrolysates and peptides from common bean (Phaseolus vulgaris L.): a review. Food Res Int. 2015;76:39–50. doi: 10.1016/j.foodres.2014.11.024. [DOI] [Google Scholar]
- McClements DJ. Emulsion stability. In: Clydesdale FM, editor. Food emulsions: principles, practices, and techniques. Boca Raton: CRC Press; 2005. [Google Scholar]
- McLean LA, Sosulski FW, Youngs CG. Effects of nitrogen and moisture on yield and protein in field peas. Can J Plant Sci. 1974;54:301–305. doi: 10.4141/cjps74-047. [DOI] [Google Scholar]
- Meng GT, Ma CY. Fourier-transform infrared spectroscopic study of globulin from Phaseolus angularis (red bean) Int J Biol Macromol. 2001;29:287–294. doi: 10.1016/S0141-8130(01)00178-7. [DOI] [PubMed] [Google Scholar]
- Meng G, Ma CY. Characterization of globulin from Phaseolus angularis (red bean) Int J Food Sci Technol. 2002;37:687–695. doi: 10.1046/j.1365-2621.2002.00601.x. [DOI] [Google Scholar]
- Mertens C, Dehon L, Bourgeois A, Verhaeghe-Cartrysse C, Blecker C. Agronomical factors influencing the legumin/vicilin ratio in pea (Pisum sativum L.) seeds. J Sci Food Agric. 2012;92:1591–1596. doi: 10.1002/jsfa.4738. [DOI] [PubMed] [Google Scholar]
- Mession JL, Chihi ML, Sok N, Saurel R. Effect of globular pea proteins fractionation on their heat-induced aggregation and acid cold-set gelation. Food Hydrocoll. 2015;46:233–243. doi: 10.1016/j.foodhyd.2014.11.025. [DOI] [Google Scholar]
- Mundi S, Aluko RE. Physicochemical and functional properties of kidney bean albumin and globulin protein fractions. Food Res Int. 2012;48:299–306. doi: 10.1016/j.foodres.2012.04.006. [DOI] [Google Scholar]
- Nagano T, Hirotsuka M, Mori H, Kohyama K, Nishinari K. Dynamic viscoelastic study on the gelation of 7S globulin from soybeans. J Agric Food Chem. 1992;40:941–944. doi: 10.1021/jf00018a004. [DOI] [Google Scholar]
- Nesterenko A, Alrica I, Silvestrea F, Durrieu V. Vegetable proteins in microencapsulation: a review of recent interventions and their effectiveness. Ind Crop Prod. 2013;42:469–479. doi: 10.1016/j.indcrop.2012.06.035. [DOI] [Google Scholar]
- Papalamprou EM, Doxastakis GI, Biliaderis CG, Kiosseoglou V. Influence of preparation methods on physicochemical and gelation properties of chickpea protein isolates. Food Hydrocoll. 2009;23:337–343. doi: 10.1016/j.foodhyd.2008.03.006. [DOI] [Google Scholar]
- Parmar N, Singh N, Kaur A, Virdi AS, Shevkani K. Protein and microstructure evaluation of harder-to-cook and easy-to-cook grains from different kidney bean accessions. LWT-Food Sci Technol. 2017;79:487–495. doi: 10.1016/j.lwt.2017.01.027. [DOI] [Google Scholar]
- Pastor-Cavada E, Juan R, Pastor JE, Alaiz M, Vioque J. Protein isolates from two Mediterranean legumes: Lathyrus clymenum and Lathyrus annuus. Chemical composition, functional properties and protein characterisation. Food Chem. 2010;122:533–538. doi: 10.1016/j.foodchem.2010.03.002. [DOI] [Google Scholar]
- Roy F, Boye JI, Simpson BK. Bioactive proteins and peptides in pulse crops: pea, chickpea and lentil. Food Res Int. 2010;43:432–442. doi: 10.1016/j.foodres.2009.09.002. [DOI] [Google Scholar]
- Sai-Ut S, Ketnawa S, Chaiwut P, Rawdkuen S. Biochemical and functional properties of proteins from red kidney, navy and adzuki beans. Asian J Food Agro-Ind. 2009;2:493–504. [Google Scholar]
- Serventi L, Vittadini E, Vodovotz Y. Effect of chickpea protein concentrate on the loaf quality of composite soy-wheat bread. LWT-Food Sci Technol. 2018;89:400–402. doi: 10.1016/j.lwt.2017.11.012. [DOI] [Google Scholar]
- Shaabani S, Yarmand MS, Kiani H, Emam-Djomeh Z. The effect of chickpea protein isolate in combination with transglutaminase and xanthan on the physical and rheological characteristics of gluten free muffins and batter based on millet flour. LWT-Food Sci Technol. 2018;90:362–372. doi: 10.1016/j.lwt.2017.12.023. [DOI] [Google Scholar]
- Shevkani K, Singh N. Influence of kidney bean, field pea and amaranth protein isolates on the characteristics of starch-based gluten-free muffins. Int J Food Sci Technol. 2014;49:2237–2244. doi: 10.1111/ijfs.12537. [DOI] [Google Scholar]
- Shevkani K, Singh N. Relationship between protein characteristics and film-forming properties of kidney bean, field pea and amaranth protein isolates. Int J Food Sci Technol. 2015;50:1033–1043. doi: 10.1111/ijfs.12733. [DOI] [Google Scholar]
- Shevkani K, Singh N, Rana JC, Kaur A. Relationship between physicochemical and functional properties of amaranth (Amaranthus hypochondriacus) protein isolates. Int J Food Sci Technol. 2014;49:541–550. doi: 10.1111/ijfs.12335. [DOI] [Google Scholar]
- Shevkani K, Singh N, Kaur A, Rana JC. Physicochemical, pasting, and functional properties of amaranth seed flours: effects of lipids removal. J Food Sci. 2014;79:C1271–C1277. doi: 10.1111/1750-3841.12493. [DOI] [PubMed] [Google Scholar]
- Shevkani K, Kaur A, Kumar S, Singh N. Cowpea protein isolates: functional properties and application in gluten-free rice muffins. LWT-Food Sci Technol. 2015;63:927–933. doi: 10.1016/j.lwt.2015.04.058. [DOI] [Google Scholar]
- Shevkani K, Singh N, Kaur A, Rana JC. Structural and functional characterization of kidney bean and field pea protein isolates: a comparative study. Food Hydrocoll. 2015;43:679–689. doi: 10.1016/j.foodhyd.2014.07.024. [DOI] [Google Scholar]
- Sikorski ZE. Functional properties of proteins in food systems. In: Sikorski ZE, editor. Chemical and functional properties of food proteins. Boca Raton: CRC Press; 2001. [Google Scholar]
- Singh N. Pulses: an overview. J Food Sci Technol. 2017;54:853–857. doi: 10.1007/s13197-017-2537-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh B, Singh JP, Shevkani K, Singh N, Kaur A. Bioactive constituents in pulses and their health benefits. J Food Sci Technol. 2017;54:858–870. doi: 10.1007/s13197-016-2391-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sosulski FW, Chakraborty P, Humbert ES. Legume-based imitation and blended milk products. Can Inst Food Sci Technol J. 1978;11:117–123. doi: 10.1016/S0315-5463(78)73224-4. [DOI] [Google Scholar]
- Sreerama YN, Sashikala VB, Pratape V, Singh V. Nutrients and antinutrients in cowpea and horse gram flours in comparison to chickpea flour: evaluation of their flour functionality. Food Chem. 2012;131:462–468. doi: 10.1016/j.foodchem.2011.09.008. [DOI] [Google Scholar]
- Sun XD, Arntfield SD. Gelation properties of salt extracted pea protein induced by heat treatment. Food Res Int. 2010;43:509–515. doi: 10.1016/j.foodres.2009.09.039. [DOI] [Google Scholar]
- Sun XD, Arntfield SD. Gelation properties of salt-extracted pea protein isolate induced by heat treatment: effect of heating and cooling rate. Food Chem. 2011;124:1011–1016. doi: 10.1016/j.foodchem.2010.07.063. [DOI] [Google Scholar]
- Susi H, Byler DM. Fourier-transform infrared spectroscopy in protein conformation studies. In: Cherry JP, Barford RA, editors. Methods for protein analysis. Champaign: American Oil Chemists’ Society; 1988. pp. 235–250. [Google Scholar]
- Tang CH, Ma CY. Heat-induced modifications in the functional and structural properties of vicilin-rich protein isolate from kidney (Phaseolus vulgaris L.) bean. Food Chem. 2009;115:859–866. doi: 10.1016/j.foodchem.2008.12.104. [DOI] [Google Scholar]
- Tang CH, Sun X. Physicochemical and structural properties of 8S and/or 11S globulins from mungbean [Vigna radiate (L.) Wilczek] with various polypeptide constituents. J Agric Food Chem. 2010;58:6395–6402. doi: 10.1021/jf904254f. [DOI] [PubMed] [Google Scholar]
- Tang CH, Xiao ML, Chen Z, Yang XQ, Yin SW. Properties of cast films of vicilin-rich protein isolates from Phaseolus legumes: influence of heat curing. LWT-Food Sci Technol. 2009;42:1659–1666. doi: 10.1016/j.lwt.2009.05.020. [DOI] [Google Scholar]
- Tiwari BK, Singh N. Pulse chemistry and technology. Cambridge: Royal Society of Chemistry; 2012. [Google Scholar]
- Toews R, Wang N. Physicochemical and functional properties of protein concentrates from pulses. Food Res Int. 2013;52:445–451. doi: 10.1016/j.foodres.2012.12.009. [DOI] [Google Scholar]
- Torres-Fuentes C, del Mar Contreras M, Recio I, Alaiz M, Vioque J. Identification and characterization of antioxidant peptides from chickpea protein hydrolysates. Food Chem. 2015;180:194–202. doi: 10.1016/j.foodchem.2015.02.046. [DOI] [PubMed] [Google Scholar]
- Vioque J, Alaiz M, Giron-Calle J. Nutritional and functional properties of Vicia faba protein isolates and related fractions. Food Chem. 2012;132:67–72. doi: 10.1016/j.foodchem.2011.10.033. [DOI] [PubMed] [Google Scholar]
- Withana-Gamage TS, Wanasundara JP, Pietrasik Z, Shand PJ. Physicochemical, thermal and functional characterisation of protein isolates from Kabuli and Desi chickpea (Cicer arietinum L.): a comparative study with soy (Glycine max) and pea (Pisum sativum L.) J Sci Food Agric. 2011;91:1022–1031. doi: 10.1002/jsfa.4277. [DOI] [PubMed] [Google Scholar]
- Xie J, Du M, Shen M, Wu T, Lin L. Physico-chemical properties, antioxidant activities and angiotensin-I converting enzyme inhibitory of protein hydrolysates from Mung bean (Vigna radiate) Food Chem. 2019;270:243–250. doi: 10.1016/j.foodchem.2018.07.103. [DOI] [PubMed] [Google Scholar]
- Yepes OO, Di Giogio L, Goyanes S, Mauri A, Famá L. Influence of process (extrusion/thermo-compression, casting) and lentil protein content on physicochemical properties of starch films. Carbohydr Polym. 2019;208:221–231. doi: 10.1016/j.carbpol.2018.12.030. [DOI] [PubMed] [Google Scholar]
- Yin SW, Tang CH, Wen QB, Yang XQ, Yuan DB. The relationships between physicochemical properties and conformational features of succinylated and acetylated kidney bean (Phaseolus vulgaris L.) protein isolates. Food Res Int. 2010;43:730–738. doi: 10.1016/j.foodres.2009.11.007. [DOI] [Google Scholar]
- Yu P. Protein secondary structures (a-helix and b-sheet) at a cellular level and protein fractions in relation to rumen degradation behaviours of protein: a new approach. Br J Nutr. 2005;94:655–665. doi: 10.1079/BJN20051532. [DOI] [PubMed] [Google Scholar]
- Zhang T, Jiang B, Mu W, Wang Z. Emulsifying properties of chickpea protein isolates: influence of pH and NaCl. Food Hydrocoll. 2009;23:146–152. doi: 10.1016/j.foodhyd.2007.12.005. [DOI] [Google Scholar]
- Zhao X, Chen F, Xue W, Lee L. FTIR spectra studies on the secondary structures of 7S and 11S globulins from soybean proteins using AOT reverse micellar extraction. Food Hydrocoll. 2008;22:568–575. doi: 10.1016/j.foodhyd.2007.01.019. [DOI] [Google Scholar]