Abstract
Medicine is constantly looking for new and improved treatments for diseases, which need to have a high efficacy and be cost-effective, creating a large demand on scientific research to discover such new treatments. One important aspect of any treatment is the ability to be able to target only the illness and not cause harm to another healthy part of the body. For this reason, metallic nanoparticles have been and are currently being extensively researched for their possible medical uses, including medical imaging, antibacterial and antiviral applications. Superparamagnetic metal nanoparticles possess properties that allow them to be directed around the body with a magnetic field or directed to a magnetic implant, which opens up the potential to conjugate various bio-cargos to the nanoparticles that could then be directed for treatment in the body. Here we report on some of the current bio-medical applications of various metal nanoparticles, including single metal nanoparticles, functionalized metal nanoparticles, and core-shell metal nanoparticles using a core of Fe3O4 as well as synthesis methods of these core-shell nanoparticles.
Keywords: Nanoparticle, Drug delivery, Nanoparticle synthesis, Nanomedicine
Introduction
Metal nanomaterials represent a significant doorway for the future of medicine. Although there is still much unknown about the long-term safety of metal nanoparticles in medicine [1], these particles have already found their place within various biomedical applications such as site-specific imaging in vivo [2–4], cancer detection [5, 6], cancer therapy [7–10], neurodegenerative disease therapy [11–13], HIV/AIDS therapy [14–16], ocular disease therapy [17–19], and respiratory disease therapy [20, 21]. Figure 1 presents a range of current uses for nanoparticles in medicine. Despite the recent advances in nanomedicine, there are still many obstacles in the way of nano-therapy, such as it can be hard to achieve a synthesis route which produces easily repeatable results, with many nanoparticle synthesis methods producing a range in both size [22–24] and shape [25–28] of nanoparticles and/or do not produce the nanomaterials in a large enough quantity to make it economically viable [29]. Another key factor is that it is relatively unknown as to the long-term toxicity of some nanoparticles over a period of time due to how relatively new the field of research is [30–32]. Among the many possible uses of metal nanoparticles lies the area of drug delivery [33, 34]. Due to the large surface area that nanoparticles provide [35], they possess the ability to be able to deliver large quantities of drugs or other medical cargoes [36]. An alternative to single metal nanoparticles is to incorporate a core to the nanoparticle which has alternative properties to the shell material, and one example of this is to incorporate a magnetic core. One challenge that still presents itself is synthesizing core-shell nanoparticles; there are many ways to synthesize nanoparticles [37], but new challenges emerge when attempting to synthesize a core-shell nanoparticle [38].
Fig. 1.
Some of the current uses of nanoparticles in medicine
This review first focuses on some of the methods currently used to generate core-shell nanoparticles focusing on using cores of Fe3O4 and coatings of gold or silver. We then examine current bio-medical applications of single metal nanoparticles, their limitations, and how to overcome them with the application of magnetic cores.
Synthesis of Core-Shell Nanoparticles
Methods for the synthesis of metallic nanoparticles have been known for many years, for example, Stevenson et al. published a synthesis for gold nanoparticles via the reduction of HAuCl4 in 1951 [39]. Since then, there have been many different routes for nanoparticle synthesis such as gas deposition [40], sol-gel [41], and aerosol/vapor phase [42]. However, a new challenge presents itself when attempting to synthesize metal nanoparticles consisting of a core-shell structure, in which one metal forms the core and a second metal forms the shell, for example Fe particles degrade in water, whilst HAuCl4 is a strong oxidizing agent [38]. One such example that will be discussed further is using a Fe3O4 (iron oxide) core and gold as the coating shell. In the preparation of such core-shell metal nanoparticles, two of the biggest issues are attempting to control the rate of coating and controlling the uniformity of the coating to create a solution of nanoparticles which are all of very similar shape and size [43]. Coating of gold or silver onto an iron oxide core can be divided into two main categories: direct coating of gold/silver onto iron [44] or using an intermediary layer to act as a glue between the gold and the iron layer [45]. The former category will be discussed here. The following text describes some methods that have been devised to synthesize gold- and silver-coated Fe3O4 nanoparticles.
Reverse Micelle Synthesis
A popular route for synthesizing metal nanoparticles is to use the reverse micelle method, or sometimes called the microemulsion route [46]. This method was first introduced in the 1980s when colloidal solutions of rhodium, platinum, and palladium nanoparticles were first synthesized [47].
Micelles are formed when molecules with hydrophobic and hydrophilic constituent parts come into contact with either an aqueous or hydrophobic phase [48]. The micelles will organize themselves in such a way that allows the hydrophilic part to be in contact with the aqueous phase and the hydrophobic constituent facing the hydrophobic phase [49]. In essence, a spheroid is formed with an inner shielded phase, which can furthermore contain a cargo [43, 50–52].
There are different approaches to the microemulsion route and these include water-in-oil (w/o) [53] and water-in-supercritical CO2 (w/sc-CO2) [54]. A w/o emulsion occurs when water is dispersed in a hydrocarbon-based continuous phase [53], thermodynamically driven surfactant self-assembly, and then generates the reverse micelles, with spherical micelles being the most common shape [43]. Any added polar or ionic materials added to this mixture become compartmentalized within the micelles, and nanoparticles are then formed when the micelle membranes come into contract with each other through Brownian motion [55]. A w/sc-CO2 emulsion involves using a fluid (CO2) that is in a supercritical state, i.e., above both its critical pressure and temperature [56]. This method holds particular interest as it is a more “green” approach to nanoparticle synthesis as no toxic organic solvents are required. It is also easier to recoup the product by simply lowering the pressure and releasing the fluid as CO2 gas [57].
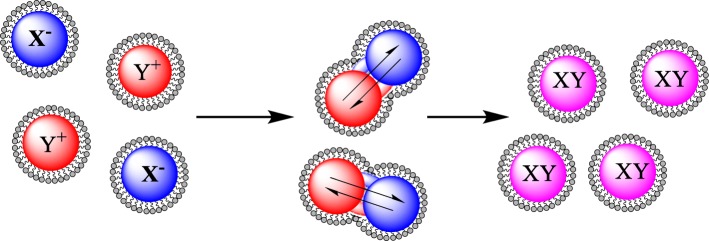
The reverse micelle route has been adapted from synthesizing metal nanoparticles to coating previously synthesized nanoparticles [58]. The first gold-coated iron oxide (Au-Fe3O4) nanoparticles synthesized in reverse micelles were done so almost 20 years ago [59]. This synthesis of Au-Fe3O4 nanoparticles was done using a H2O/CTAB (cetyltrimethyl ammoniumbromide) system to produce the micelles with sodium borohydride (NaBH4) as the reducing agent, reducing gold chloride (HAuCl4) onto the iron core. This synthesis produced a nanoparticle dispersion with an average size of 12 nm. Since this is the first production of Au-Fe3O4 NPs using microemulsions, there has been a range of Au-Fe3O4 NPs synthesis routes discovered [46, 60–63]. Figure 2 is a generic representation of how the nanoparticles are formed using the reverse micelle route.
Fig. 2.
A generic representation of the interaction of reverse micelles containing salts the react to form metal nanoparticles
Lin et al. published a slightly modified method to coat Fe3O4 with gold using a reverse micelle method [60]. The synthesis also employs a system using CTAB as the surfactant to form the reverse micelle, but with 1-butanol as a co-surfactant and octane as the oil phase, adding a water solution containing the metal ions using NaBH4 to reduce HAuCl4 onto the surface of the iron oxide nanoparticles. The reported optical results of the coated particles showed a shift in the absorbance peak of the UV/vis spectra from the gold colloid (526 nm) to the Au-Fe3O4 (555 nm). The TEM results of the coated particles indicated a size distribution of 5–15 nm, with an average size of 10 nm. This method was repeated by Pana et al. with a slightly larger size distribution of 5–35-nm-sized Au-Fe3O4 nanoparticles [63]. In addition, a very similar system has been employed by Seip et al. with the exception of using hydrazine to reduce the HAuCl4 [64].
The coating of Fe3O4 nanoparticles is not limited to just gold; Lopez Perez et al. reported on the synthesis of iron oxide nanoparticles using a system containing cyclohexane/Brij-97 (co-surfactant) and an aqueous phase with iron salts of FeSO4.7H2O and FeCl3.6H2O [65]. This system has been coated with both silver [58] and gold [46], producing 13-nm particles. An alternative method is reported by Tamer et al. for the synthesis of Au-Fe3O4 nanoparticles [62]. This method employs a co-precipitation of iron salts in NaOH, which were then washed in HClO4 to produce oxidized Fe3O4 nanoparticles. Coating of gold onto the Fe3O4 NPs occurred via the reduction of HAuCl4 by NaOH delivered to the system by CTAB micelles. Au-Fe3O4 NPs were produced with an average size of 23.5 nm. After characterization, particles were then modified with various functional groups to form a self-assembled monolayer (SAM) and further used for the capturing and detection of Escherichia coli.
A modified version of the reverse micelle synthesis has been done by Zhang et al. involving the use of a laser as the initiator for the coating of iron nanoparticles with gold [66]. The process involves making a reaction mixture of iron nanoparticles encapsulated in CTAB micelles, gold nanopowder in water, and octane, then irradiating with a pulsed laser while vigorously stirring the reaction. The laser irradiation facilitates the thermal decomposition of the gold nanoparticles. Gold atoms and clusters formed around the iron nanoparticles, forming gold-coated iron nanoparticles. The TEM results for the Au-Fe nanoparticles synthesized this way gave an average size of 18 nm with a size distribution of ±36 nm.
Thermal Synthesis
Among the various methods of gold shell-iron core nanoparticle synthesis lies a thermal route, wherein the reaction involves heating the reaction mixture to above its boiling point [67], and sometimes refluxing [68, 69]. There are two main categories for this type of synthesis: hydrothermal (water-based solvent) [70, 71] and solvothermal (organic-based solvent) [68, 72]. While there are many techniques for synthesizing metal nanoparticles via the thermal route [73–78], it is not possible to achieve the synthesis of the cores and coating of gold in a one pot reaction [68, 69, 72, 74, 77, 79–81], and in some cases, Fe3O4 cores are synthesized via a reverse micelle route [70] or a colloidal route [78] and then the particles are coated using a hydro- or solvothermal technique [70, 76, 78]. While there are a variety of solvent systems that are used in these synthetic methods, the majority of routes involve the addition of either iron oxide nanoparticles to boiling HAuCl4 or the inverse of HAuCl4 being added to boiling solutions of iron oxide nanoparticles [74, 79].
A method for the synthesis of Au-Fe3O4 nanoparticles has been done by Rudakovskaya et al. via a hydrothermal technique [76]. The principle of the method follows the addition of Fe3O4 nanoparticles to a boiling HAuCl4 solution. TEM analysis of these nanoparticles indicated an average size of 30 nm, with a general spherical shape and a size distribution between 20 and 35 nm; these images can be seen in Fig. 3.
Fig. 3.
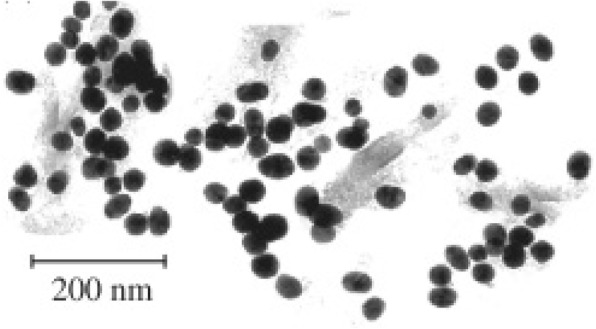
A TEM image of the nanoparticles synthesized by Rudakovskaya et al. As can be seen, the nanoparticles are roughly spherically shaped with an average size of 30 nm [76]
Colloidal Synthesis
Colloidal synthesis techniques offer a simple yet effective way of synthesizing metal nanoparticles [82]. Colloidal techniques often offer a level of simplicity over other techniques for nanoparticle synthesis, without the need for different solvents, or that it can be carried out at room temperature [83, 84]. The basic principles of the synthesis involve dispersing different metal ions in an aqueous phase, adding a reducing agent to the mixture, then mixing at a controlled temperature to form insoluble nanoparticles [39]. Colloidal synthesis routes offer the benefit of not having to involve potential toxic solvents in the synthesis (ideal if the nanoparticles are intended for biological use). However, there are some limitations to colloidal routes such as it can be hard to control the size distribution of the final synthesized nanoparticles [85] and the shape of the nanoparticles can be heavily influenced by reagent concentration [85]. On the positive side, it can however be easier to produce nanoparticles in a larger quantity [86]. This method for metal nanoparticle synthesis has been around for many years, being used for the synthesis of different types of nanoparticles such as silver [87] and gold [39, 88].
This basic method has been advanced and developed to produce different synthetic routes for the formation of gold-coated iron oxide nanoparticles [83, 84, 89–97]. Most of the methods for the synthesis of gold-coated iron oxide revolve around using various reducing agents to reduce HAuCl4 onto the surface of the iron oxide. Nadagouda et al. offer a proposed “green” synthetic route, using ascorbic acid to reduce HAuCl4 [84]. This method however seems to show little to no control over size or shape of the coated nanoparticles due to the lack of capping agent (an agent that binds to the outside of the nanoparticle that stops further “growth” of the nanoparticle) used in the synthesis [98]. A method which does show more control over the shape and size of synthesized coated particles is presented by Pal et al. [95] This method employs gold acetate as the gold salt, which is reduced onto the surface of 6-nm Fe3O4 nanoparticles to create 7-nm-sized Au–Fe3O4 particles, which are spherical in shape. A rapid method for coating Fe3O4 nanoparticles is presented by Rawal et al. which involves dispersing Fe3O4 nanoparticles in a solution of HAuCl4, then mixing with ethanol [83]. After 15 min at room temperature, the reaction was stopped and the Au-Fe3O4 nanoparticles were then separated with a magnet. TEM analysis of the purified solution showed that the particles produced ranged in size from 30 to 100 nm and had varied shapes across the sample; these images can be seen in Fig. 4. While this synthesis technique produced the coated nanoparticles quickly, it does not appear to be a very efficient synthesis for production of uniformly shaped and sized particles [83].
Fig. 4.
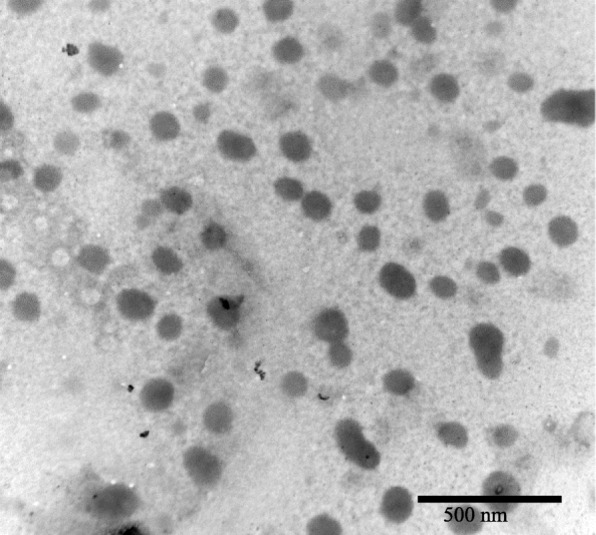
A TEM image of the nanoparticles synthesized by Rawal et al. These nanoparticles have a size distribution of 20–100 nm [83]
While some techniques offer just the reduction of gold salts, others prefer to put the reducing agent onto the surface of the iron, such as hydroxylamine [90, 93]. In many cases when Fe3O4 nanoparticles are coated with gold, the reduction of a gold salt yields standard gold nanoparticles as well [74], so the addition of the reducing agent onto the surface of the iron nanoparticles aims to improve the efficiency of the coating and is intended to lower the quantity of gold nanoparticles produced as a by-product [93].
Another technique involves seeding gold onto the surface of magnetic nanoparticles which provides a more direct route of getting gold to nucleate around the magnetic core of the nanoparticles [91, 92, 97]. This technique involves binding gold seeds, which are smaller than the iron oxide nanoparticles in solution, to the surface of the iron oxide. When the HAuCl4 is reduced in solution, the Au+ ions will seed onto the iron oxide and form a shell around the iron oxide nanoparticles. This gold seeding has been successfully employed by several groups; Goon et al. used polyethyleneimine to control the seeding of gold onto the surface of Fe3O4, producing fully coated nanoparticles. [91] However, the synthesized Au-Fe3O4 particles displayed high polydispersity, with particle size ranging from 40 to 110 nm. Levin et al. managed to produce gold shell-magnetic core nanoparticles with a size range of 50–70 nm, using a core functionalized with organosilane molecules to bind to the gold seeds [92]. Seeding of gold nanoparticles onto an iron core can be demonstrated with a variety of core shapes, for example, Wang et al. demonstrated gold seeding onto rice-shaped “Nano rice” Fe3O4 structures, which then led to a complete thick gold shell when gold was reduced onto the surface [97].
Bio-Medical Applications of Metal Nanoparticles
Antimicrobial Agents
Bacterial infections are very common, with antibiotics being a primary method of treatment since the discovery of penicillin in 1928 by Alexander Fleming [99]. Nanomedicine provides us with a new, broad range of possible treatment modalities, with metal nanoparticles being explored for future treatments [100]. Table 1 lists some of the nanoparticles that have been explored for antimicrobial applications. One material that has been examined for its potential use is silver, which has shown to have a variety of biomedical uses [101], for example, Sreekumar et al. utilized silver nanoparticles as part of a network of antimicrobial fibers. The nanoparticles varied in size from 20 to 120 nm, with an antibacterial efficacy against Escherichia coli as high as 94.3% compared to the fibers without silver nanoparticles [102]. While it has been shown that an antibiotic such as ampicillin is capable at achieving a kill rate of ≤ 99.9% in E. coli [103], the same study also reported the emergence of resistance to ampicillin in certain strains of E. coli. On this same note, it has been reported that E. coli can develop a resistance to silver nanoparticles; however, this resistance is not a genetic change, but it is a physical response that attempts to cause the colloidal nanoparticles to aggregate [104]. Also employing silver for its antibacterial properties, Holtz et al. designed a system of 60-nm silver vanadate nanowires ‘decorated’ with silver nanoparticles with a diameter of 1–20 nm [105]. This system showed to be promising against three Staphylococcus aureus strains and also interestingly had a much lower growth inhibiting concentration against methicillin-resistant Staphylococcus aureus (MRSA) than the antibiotic oxacillin.
Table 1.
List of antibacterial properties that have been exhibited by some metal nanoparticles and metal nanoparticle conjugates
| Type of nanoparticle | Size (nm) | Antimicrobial application | Mechanism of action | Ref |
|---|---|---|---|---|
| Silver as part of network of fibers | 20–120 | E. coli | Bacterial growth inhibition | [102] |
| Silver vanadate nanowires | 1–20 | S. aureus | Bacterial growth inhibition | [105] |
| Naked silver | 10–25 | C. albicans, P. fluorescens, E. coli | Bacterial growth inhibition | [106] |
| Thioguanine-capped gold | 3–4 | E. coli, A. fumigatus, P. aeruginosa, and anticancer effect against Hep2 | Bacterial growth inhibition, cellular toxicity | [107] |
| Naked gold | 25 | C. pseudotuberculosis | Vacuole formation in cell wall and agglomeration of NPs within cells | [108] |
| Naked gold | 6–40 | S. aureus, K. pneumonia, B. subtilis | Bacterial growth inhibition | [109] |
A silver nanoparticle synthesis was reported by Verma et al. where they employed their nanoparticles against the bacteria: Pseudomonas fluorescens, E. coli, and the fungus Candida albicans [106]. The silver nanoparticles had an average minimum inhibitory growth concentration of 5.83 μg/ml across the three strains, compared to some commonly used antibiotics such as ampicillin and neomycin which have minimum inhibitory growth concentrations of 4.0 μg/ml and 16.0 μg/ml, respectively, against strains of E. coli [110]. Of potential interest is the properties the nanoparticles displayed against P. fluorescens an C. albicans, both of which are associated with causing disease in immunocompromised patients [111]. Further investigations might find that the silver nanoparticles are a more efficient way to treat the pathogens than some of the most commonly used antibiotics, such as amphotericin B, which has extensive side effects [112].
The synthesis of thioguanine-capped gold nanoparticles has been reported by Selvaraj et al. where an enhanced antimicrobial effect against several bacterium, including: E. coli, Aspergillus fumigatus, and Pseudomonas aeruginosa [107]. It was found that the thioguanine-capped gold nanoparticles were more effective than unconjugated thioguanine as anticancer and antimicrobial agents, with their activities showing potential use as carriers for cancer drugs. In a similar manner, gold nanoparticles have been reported to have an antimicrobial effect on Corynebacterium pseudotuberculosis [108], nanoparticles with an average size of 25 nm, using a dose of 50 μg/ml showed a bacterial growth inhibition of 95% after 20 min of exposure. Similarly, naked gold nanoparticles were shown to have an antimicrobial effect on a variety of gram negative and gram positive bacteria including S. aureus, Klebsiella pneumonia, and Bacillus subtilis [109]. A dose of 1.35 μg/ml of AuNPs showed a growth inhibition of 46.4±0.4%, 38.3±0.2%, and 57.8±0.2% for S. aureus, K. pneumonia, and B. subtilis, respectively.
Antiviral
As with antibacterial applications, metal nanoparticles have shown to be promising in antiviral applications; Table 2 demonstrates a range of nanoparticles that have been shown to possess antiviral properties and could potentially be applied when treating viruses. Both naked and coated silver nanoparticles [113–116] have been shown to have a range of antiviral applications when in the nano-scale range.
Table 2.
Some of the metal nanoparticles and metal nanoparticle conjugates that have been demonstrated as having antiviral properties
| Type of nanoparticle | Size (nm) | Antiviral application | Mechanism of action | Ref |
|---|---|---|---|---|
| AgNPs | 10–50 | Hepatitis B virus (HBV) | Interaction with DNA and/or binding with virus particles | [113] |
| Ag-PS-NPs | 10–80 | Monkeypox virus (MPV) | Blocking of virus-host cell binding and penetration | [114] |
| PVP-AgNPs | 30–50 | Human immunodeficiency virus type 1 (HIV-1) | Prevention of HIV-1 transfection | [115, 116] |
| Au-MES | 4 | Herpes simplex virus type 1 (HSV-1) | Competition with host cell binding | [117] |
| Gold coated with an amphiphilic sulfate ligand | 2 | Human immunodeficiency virus type 1 (HIV-1) | Binding to gp120 | [118] |
| Copper iodide (CuI) nanoparticles | 100–400 | Feline calicivirus (FCV) | ROS generation and subsequent capsid protein oxidation | [119] |
| Copper iodide (CuI) nanoparticles | 160 | Influenza A of swine origin (H1N1) | Generation of hydroxyl radicals and degradation of viral proteins | [120] |
Hepatitis B (HBV) is a viral infection that currently affects 257 million people around the world and was responsible for 887,000 deaths in 2015 according to the World Health Organization [121]. Small (10–50 nm) naked silver nanoparticles have been tested as a possible treatment for HBV [113] and were shown to bind efficiently to HBV and further inhibit the production of HBV RNA. The mode of action is hypothesized to be due to the AgNPs binding to the HBV dsDNA (double-stranded DNA). Rogers et al. have demonstrated a use for silver nanoparticles, both naked and with a polysaccharide coating as an antiviral agent against monkeypox virus (MPV) [114]. The nanoparticles were tested in vitro against MPV at a range of concentrations between 12.5–100 μg/ml; the results of the study showed that all of the concentrations of polysaccharide-coated silver nanoparticles (Ag-PS-NPs) used were able to reduce MPV-induced plaque formations in vitro.
Silver nanoparticles may even have a role to play in the treatment of human immunodeficiency virus (HIV) [115, 116]. HIV is a major health concern, with WHO estimating that 36.7 million people are living with HIV as of 2016 [122]. It is important that treatments for HIV are discovered and implemented quickly and efficiently; Lara et al. have demonstrated the effect of silver nanoparticles (30–50 nm) on HIV-1 isolates showing inhibition of all strains of HIV-1 isolates [116]. The naked nanoparticles showed an overall IC50 of 0.44 mg/ml ± 0.3 against HIV-1, with the mechanism of viral inhibition shown to be the inhibition of virus-host cell binding, specifically the silver nanoparticles inhibit the interaction between the gp120 protein (an envelope glycoprotein) and the target cell membrane receptors. Also demonstrated by the same group was the ability for silver nanoparticles coated with polyvinylpyrrolidone (PVP) to prevent the transfection of HIV-1 into a human cervical tissue explant model [115]. Specifically, 0.15 mg/ml PVP-coated silver nanoparticles (PVP-AgNPs) inhibited infection by HIV-IIIB and HIV-AZT-RV isolates. This concentration of PVP-AgNPs also induced a proliferation of lymphocytes (immune cells) to the site of infection, in comparison to the control sample [115].
It is not only silver and coated silver nanoparticles that have been employed against viruses: 2-nm gold nanoparticles coated with an amphiphilic sulfate ligand were also shown to be effective against HIV-1 [118]. These particles were shown to target the fusion process of the virus and were shown in vitro to bind to gp120 protein and directly neutralize the HIV-1 infection. Mercaptoethanesulfonate-coated gold nanoparticles (Au-MES) showed an inhibition of herpes simplex virus type 1 (HSV-1) infection, possibly by inhibiting the virus binding to the host cell, cell to cell viral spreading, or alteration of cell susceptibility to viral infection induced by the presence of the nanoparticles [117].
Copper-iodide nanoparticles (CuI-NPs) have been shown to have antiviral properties on several different viruses: feline calicivirus (FCV) [119] and more interestingly influenza A virus of swine origin (H1N1) [120]. One hundred to 400 nm CuI-NPs showed an antiviral property when utilized against FCV, and it was hypothesized that monovalent Cu ions were responsible for the production of a reactive oxygen species (ROS) that caused subsequent capsid protein oxidation, leading to FCV inactivation. H1N1 virus was also shown to be inhibited by CuI-NPs, in a very similar manner, namely the production of hydroxyl radicals, leading to protein degradation. However, these radicals might also prove to be toxic to non-infected tissues, which would be important to determine before a treatment would be approved for use [123].
Imaging
Magnetic resonance imaging (MRI) scanning is a very useful tool for medical diagnosis and provides clear anatomical images. Using MRI, one can visualize the blood flow, physiochemical traits, and the states of tissues and organs in the body [124]. Contrast agents are often employed in MRI for improved diagnostic sensitivity [125]. Conventionally used contrast agents are chelate-based, but the major drawbacks of current contrast agents are their biological stability and their toxicity levels when accumulated in cells [126]. For example, some contrast agents are iodine-based, and it has been reported that iodinated contrast media exposure is associated with subsequent development of incident hyperthyroidism and incident overt hypothyroidism [127]. Alternatives have been developed to provide an improved scanning efficacy by reducing the negative impact contrast agents can have on the body [128]. Alternatives include metal nanoparticles possibly conjugated with an agent which acts in a similar manner to a contrast agent for MRI scanning [129]. Figure 5 is an MRI contrast image of a rat cerebral cortex pre- and post-treatment of AuNPs [130].
Fig. 5.
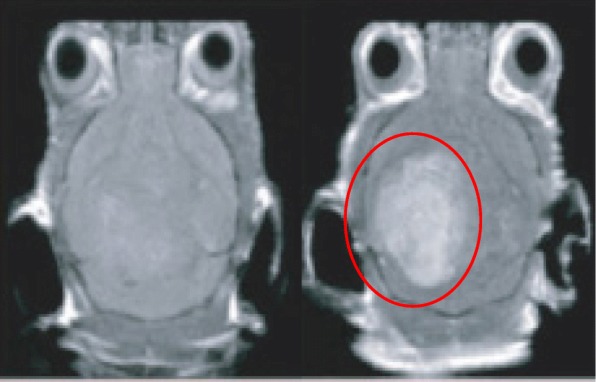
An MRI contrast image of a rat cerebral cortex pre- (left) and post-treatment (right). The area containing the AuNPs is ringed in red
Table 3 shows some of the nanoparticles that have been explored for use in medical imaging. Some computed tomography (CT) contrast agents have issues including short circulation half-lives [131] and potential tissue damage [130]. Due to this, metal nanoparticles have also been investigated for use in CT imaging [132]; Au nanoparticles show promising use in imaging due to their X-ray attenuation [133]. Kojima et al. showed that gold nanoparticles conjugated with a PEGylated dendrimer (PEG-AuNPs) made for a superior contrast agent in vitro as well as for X-ray computed tomography, compared to the commercially available iodine agent iopamidal [134]. The PEG-AuNPs showed a higher contrast efficiency than the commercially available iopamidal, with rapid excretion from the body [135]. The authors also noted that the PEG-AuNPs had photocytotoxic properties to enable photothermal therapy.
Table 3.
Some examples of metal nanoparticles and metal nanoparticle-conjugates that have been investigated for their use in medical imaging
Li et al. have demonstrated the use of coated AuNPs as an imaging tool for atherosclerosis; the AuNPs were applied in a type of medical imaging called “single-photon emission computed tomography” (SPECT) [136]. This type of imaging is very similar to using a gamma camera, but it is able to provide true 3D images that can be sliced, rotated, and manipulated to achieve a more accurate analytical technique [136]. The modified nanoparticles specifically targeted atherosclerosis plaques containing apoptotic macrophages, indicating a useful tool for invasively accurate detection of atherosclerosis plaques [136].
AuNPs have previously been demonstrated to be a possible agent for photoacoustic imaging (PA), showing high spatial resolution and sensitivity [137]. PA relies on the detection of ultrasonic waves which are emitted from tissues when exposed to non-ionizing pulsed laser irradiation [140]. The intensity/magnitude of the ultrasonic emission is responsible for the image contrast, therefore any agent that can both absorb the laser pulses and then give off heat as a result will increase the magnitude of the ultrasonic emission and AuNPs possess the ability to do both of these [141, 142]. AuNPs are potentially better than organic dyes due to the organic dyes’ susceptibility to photo-bleaching and rapid clearing from the blood [143]. AuNPs also have use in cell imaging for examining movement of nanoparticles within cells when conjugated with various cargoes. Figure 6 is a darkfield imaging of A431 lung cancer cells treated with AuNPs that target epidermal growth factor receptor, and the bright areas within the cells are the nanoparticles indicating their locations within the cells [144].
Fig. 6.
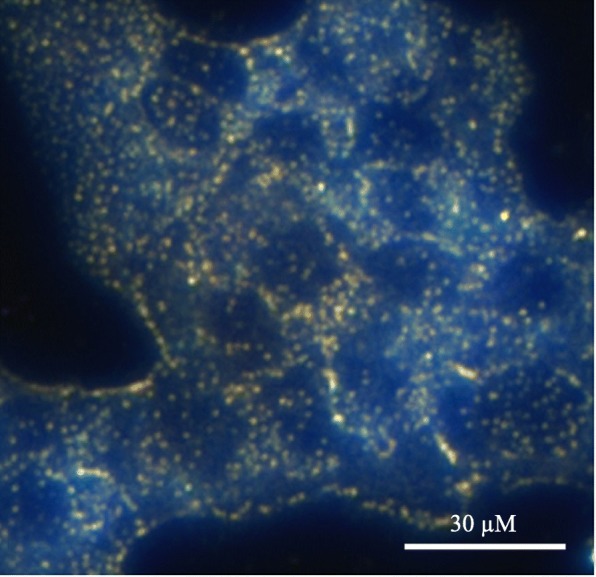
Darkfield imaging of A431 lung cancer cells treated with AuNPs; the bright yellow/orange dots are nanoparticles within the cells
Biomedical Cargo Delivery
Nanoparticles make for an ideal molecule for drug delivery due to the huge surface area to the volume ratio they provide when compared to their bulk material [145]. In addition, it is possible to engineer nanoparticles to either avoid or interact with the immune system in specific ways [146, 147]. For example, it has been demonstrated that an increased hydrophobicity of nanoparticles/sub-groups conjugated to the nanoparticles illicit and increased immune response by measuring cytokine mRNA levels in mice [146]. Focusing in the opposite direction, it has been suggested that nanoparticles can be conjugated with various ligands to directly activate the immune system to target the destruction of a tumor [148] or by accumulation in the liver or spleen for the generation of tolerance or immunity, respectively [147].
Gold nanoparticles have been extensively studied for their delivery of medical cargo, for example, Bhumkar et al. have explored the application of AuNPs for trans-mucosal delivery of insulin. Gold nanoparticles were synthesized in the presence of chitosan, which acts as a polymeric stabilizer [149]. These nanoparticles were then loaded with insulin and administered both nasally and orally to diabetic rats. The results showed an overall reduction in the rat’s blood glucose levels, an indication of successful movement of the nanoparticles through the mucosal membranes and into the bloodstream.
More recently “smart” AuNPs have been employed in PA [138]. These nanoparticles are roughly 10 nm in diameter and are functionalized with citraconic amide moieties which are susceptible to hydrolysis. The citraconic amides are converted into positively charged primary amino acids at a mildly acidic pH, while the surface molecules adopt negative charges at physiological pH [138]. Combined, these two properties cause the “smart” nanoparticles to adopt both positive and negative charges allowing them to aggregate rapidly due to electrostatic attraction. These nanoparticles are referred to as “smart” due to the nanoparticles presenting cancer-specific properties and accumulate rapidly and efficiently in cancer tissues and show a much lower accumulation in normal tissues [150].
Gold nanoparticles can also be used as a delivery system for nucleic acids [153], including oligonucleotides [151] and small interfering RNA (siRNA) [154]. Many different methods have been developed to functionalize AuNPs with nucleic acids, for example, Yonezawa et al. have synthesized gold nanoparticles modified with thiocholine, which then bound to DNA and formed a fusion of wire-like structures throughout the DNA [155]. Sandström et al. demonstrated the ability to bind nucleic acids onto gold nanoparticles [151], and a similar modification has been done by Rosi et al. where tetrathiol-modified antisense oligonucleotides were bound to 13-nm gold nanoparticles [152]. Being able to conjugate nucleic acids to nanoparticles opens up the possibility of targeted gene delivery, which could, for example, lead to genes coding for a specific protein to be delivered to a cell that was either deficient in that protein or could not produce the protein themselves [156]. It has also been exhibited that gold nanoparticles modified with DNA can transfect cancer cells [157] (Table 4).
Table 4.
A range of nanoparticle conjugates that have been examined for medical delivery of cargos
| Type of Nanoparticle | Size (nm) | Medical delivery application | Ref |
|---|---|---|---|
| Chitosan stabilized AuNPs | 10–50 | Delivery of insulin across transmucosal membranes | [149] |
| AuNPs conjugated to an oligonucleotide modified with thiol groups | 10–20 | Delivery of nucleic acids as a potential for gene therapy | [151] |
| AuNPs conjugated to antisense oligonucleotide modified with tetra-thiol groups | 13 | Delivery of nucleic acids as a potential for gene therapy | [152] |
Anticancer Drug Delivery
Cancer is one of the world’s leading killers with large areas of scientific research being dedicated to the fight against cancer, and nanoparticles offer a new doorway into methods to target and treat cancer. Table 5 presents a selection of nanoparticle/drug conjugates that have been tested for anticancer treatments. Paciotti et al. have investigated the application of PEGylated AuNPs as a carrier for tumor necrosis factor (TNF) which is a cell-signaling protein that possess the ability to induce apoptosis in healthy cells [158]. The Au-PEG-TNF nanoparticles were injected intravenously and agglomerated significantly more in MC-38 colon carcinoma cells compared to other healthy cells/tissues. The TNF not only gave therapeutic action on the MC-38 cells, but also seemed to possess a targeting property, indicated by the lack of agglomeration in healthy cells. Another interesting observation reported was the ability for the Au-PEG-TNF nanoparticles to diminish a tumor mass compared to “free” TNF.
Table 5.
A range of nanoparticle conjugates that have been examined for anticancer therapy
| Type of nanoparticle | Size (nm) | Medical delivery application | Ref |
|---|---|---|---|
| PEGylated AuNPs conjugated with TNF | 30–34 | Delivery of TNF to cancer cells targeted by the TNF itself, TNF induces cell apoptosis | [158] |
| AuNPs conjugated with folic acid using a PEG linker | 10 | Delivery of folic acid (vitamin B9), a precursor for nucleic acid production | [159] |
| AuNPs loaded with doxorubicin | 30–40 | Delivery of doxorubicin-loaded gold nanoparticles for tumor targeting/therapy | [160] |
| AuNPs coated with a tumor specific uptake peptide | 25–40 | Drug delivery to lymphoma cells with gold nanoparticles conjugated with cellular uptake peptides specific to lymphoma cells | [161] |
Doxorubicin is a widely used cancer therapeutic agent but has dose-limiting associations with cardiotoxicity. A gold nanoparticle-doxorubicin conjugate has been developed that demonstrates little no to cardiotoxicity to mice while being able to treat cancer [160]. Dixit et al. demonstrated the selective delivery of folic acid-coated AuNPs into folate receptor (FR) positive cancer cells, whereas when compared with a cell line that did not have folate receptors, uptake was shown to be minimal [159]. These results demonstrated the use of folate to target metal nanoparticles to FR-positive cancer cells for tumor imaging and ablation.
Limitations of Single Metal Nanoparticles and Overcoming Them
The principal obstacle with nanoparticle drug delivery is the ability to direct the nanoparticle to the target area [162, 163]. There are several methods in use for metal nanoparticle targeting such as antibodies [164–166] and homing peptides [167, 168]. There are however limitations to these methods, with the biggest being that before they even reach the desired target cells they have to pass through a variety of other barriers, such as blood vessels and the blood-brain barrier [169]. One way to overcome this targeting limitation is to use magnetic nanoparticles [170]. A magnetic nanoparticle-targeting system works by directing the nanoparticles to a target site using an external magnetic field, it has already been demonstrated that the magnetic anisotropy of the nanoparticle is a very important factor for medical treatments [171], with a change in anisotropy being able to the change the efficacy of hypothermia treatments for example [172]. Superparamagnetic metal nanoparticles have this property (they only present magnetic properties while in the presence of a magnetic field) [173]. However, the benefit of magnetic nanoparticles also presents a potential limitation, due to the toxicity of many magnetic materials [31, 174, 175]. Despite iron being approved for various imaging uses [5, 6, 31], it has been suggested in several studies that naked iron oxide nanoparticles may have some adverse effects when used in cell labeling [176–178]. One method that can be used to overcome any potential toxicity limitations is to coat the iron core [179]. A range of materials can be used as the coating material: silica [180–182], polymers [183, 184], gold [62, 93, 95, 185], or silver [58, 186]. Gold has low pharmaceutical activity [187] and silver has been used in biomedical applications for many years [188, 189],
The combination of a superparamagnetic core with an inert and safe metal coating produces metal nanoparticles with superior characteristics to non-magnetic metal particles [190]. As well as reducing toxicity, the coating also provides the potential for the conjugation of functionalized molecules onto the surface, such as drugs and biomolecules for application in the medical field [97, 140, 152]. It is of note that a core-shell nanoparticle still possesses the properties and uses of a nanoparticle made from the same material as just the shell, but the superparamagnetic core gives the ability to direct the nanoparticle in the body [191]. For example, a gold nanoparticle with an antibody is classified as a targeting nanoparticle, introducing the core would classify the nanoparticle as a directed targeting nanoparticle [173].
Current Medicinal Uses of Gold-Coated Iron Oxide Nanoparticles
Core-shell superparamagnetic nanoparticles have already been assessed for their biomedical uses, with a wide range of uses already being applied [192] and with a majority of research investigating into the use of gold as a shell for the nanoparticles, in part due to its biocompatibility and ability to easily bind to a variety of materials. As such, this section will deal exclusively with gold shell nanoparticles. One of these uses is as a magnetic carrier for drug targeting [192–196]. Kayal and Ramanujan have tested an in vitro apparatus that simulates the human circulatory system as a test for the magnetic delivery of gold-coated iron oxide nanoparticles (Au-Fe3O4) loaded with doxorubicin [194]. Their system had various magnetic fields of increasing strength next to a capillary through which the doxorubicin-loaded particles were passed. A significant percentage of these nanoparticles were captured within the magnetic fields, strongly indicating the potential for the use of magnetic nanoparticles in drug delivery. Another use for a targeted system is the application of Au-Fe3O4 nanoparticles in photothermal therapy. Bhana et al. demonstrated the use of a core-shell system used in combination therapy deployed against two different cancer cell lines; head and neck (KB-3-1) and breast (SK-BR-3) with a reported decrease in cell viability of 64% when they exposed cell lines to a combined photothermal and photodynamic therapy, compared to each modality used on its own [197]. In photothermal therapy, gold nanoparticles are coated with a ligand, such as PEG [142], and these nanoparticles are irradiated with a laser, with a wavelength that matches the UV-vis λ-max of the gold nanoparticles [194]. The nanoparticles vibrate at the laser’s frequency which causes heat to be released causing the death of the surrounding tissue [198], introducing a core which is superparamagnetic can allow for a more accurate targeting for use in this therapy. Similarly, it has been reported by Kirui et al. that gold hybrid nanoparticles were deployed against SW1222 colorectal cancer in photothermal therapy, showing an increased case of cellular apoptosis after therapy, with their conclusion being that the cells showed an increased uptake, leading to a reduced laser power required to reach threshold therapeutic levels [199]. The use of core-shell nanoparticles for photothermal therapy of cancer has also been reported by other groups [200, 201].
Metal nanoparticles have already shown to have a place in contrast imaging, for example core-shell nanoparticles can also be used in T1- and T2-weighted imaging in MRI [202]. Research by Cho et al. demonstrated that gold-coated iron nanoparticles can be successfully used in MRI imaging, as well as opening the route for conjugating various ligands for use in biosensors [202]. A magnetic carrier capable of imaging and photothermal therapy has been reported by Cheng et al. They demonstrated the magnetic targeting of multi-functional nanoparticles to a tumor in a mouse model, which could be imaged inside the tumor and showed a reduction in the tumor size when combined with photothermal therapy [203]. It is also of note that in this work, both the nanoparticle dosage (1.6 mg/kg) and laser power (1 W/cm2) are among the lowest applied for in vivo photothermal therapy. Moreover, there was no obvious toxicity from the nanoparticles reported. Table 6 presents some of the currently reported uses of core-shell nanoparticles.
Table 6.
Examples of the medical uses already been demonstrated for gold-coated iron magnetic nanoparticles
| Type of nanoparticle | Medical application | Ref |
|---|---|---|
| Gold-coated iron oxide | Targeted delivery of doxorubicin | [194] |
| Gold-coated iron oxide | Photothermal and photodynamic combination anticancer treatment | [197] |
| Gold hybrid nanoparticles | Photothermal anticancer therapy | [199] |
| Gold-coated iron nanoparticles | T1- and T2-MRI imaging | [202] |
| Multifunctional gold nanoparticle | Magnetically directed tumor targeting in mice for phototherapy and imaging of the particles | [203] |
| Multifunctional gold-coated iron oxide | Cancer diagnosis and therapy | [204] |
| Gold-coated iron oxide | Cancer therapy | [205] |
| Gold-coated iron oxide | MRI/PA imaging | [206] |
Another medical area where such core-shell metal nanoparticles have been suggested to make an impact is in directed enzyme prodrug therapy (DEPT) [170, 191]. DEPT is a promising method of cancer treatment, with several therapies making it through to clinical trials [207, 208]. The main principal of DEPT is the targeted delivery of a prodrug-activating enzyme to a tumor site. Upon arrival at the tumor site, the enzyme enters the target cells where it can later activate an administered prodrug. However, the efficacy of the therapy depends on the ability to direct the enzyme to the tumor site, with current directional techniques relying on passive targeting methods such as viruses [207, 209] or antibodies [210, 211], rather than an active targeting system for enzyme delivery. A novel therapy proposed by Gwenin et al. potentially overcomes the targeting issue [170, 212]. This approach involves conjugating a genetically modified prodrug-activating enzyme onto the surface of a gold-coated iron oxide superparamagnetic nanoparticle (AuMNP), then directing the AuMNP-enzyme conjugate to the target site using a magnetic field to increase the efficacy of the targeted therapy. Figure 7 presents some of the uses of a core-shell nanoparticle.
Fig. 7.
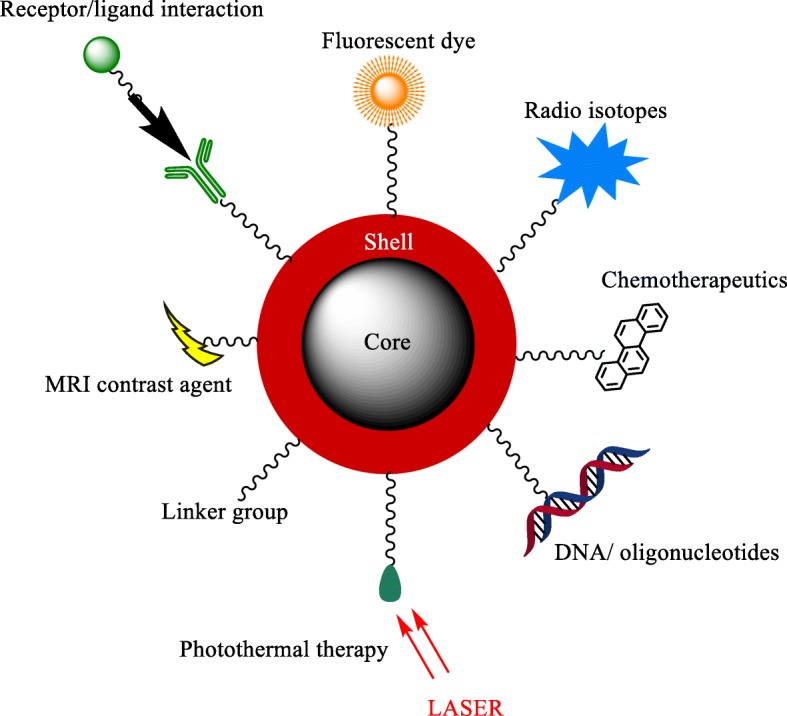
A pictorial representation of the applications of core/shell nanoparticles
Conclusions
In brief, single metal nanoparticles have been shown to currently possess a wide range of biomedical applications, with more application for these nanoparticles being discovered. One of the limiting factors that these nanoparticles face in medical treatments is to find a way for precise accurate targeting of areas within the body, be it for targeting of a drug delivery or for therapies involving the nanoparticles directly. A way to overcome this is to employ a magnetic core to create core-shell nanoparticles that can then be directed around a body using a magnetic field. There are a variety of methods that can be used to synthesize these core-shell nanoparticles, with each method having its own advantages and disadvantages. There remain many obstacles for core-shell nanoparticles before they can be routinely applied in the medical field and these include
Achieving a synthesis route which produces easily repeatable results;
Producing particles of a set size [22–24] and shape [25–28]; and
Producing large enough quantities to make it economically viable [29].
Another key factor is the relatively unknown toxicity of some nanoparticles over an extended period of time due to how relatively new the field of research is.
Acknowledgements
Not applicable
Abbreviations
- (o/w)
Oil-in-water
- (w/o)
Water-in-oil
- (w/sc-CO2)
Water-in-supercritical CO2
- AgNP
Silver nanoparticle
- Ag-PS-NPs
Polysaccharide-coated silver nanoparticles
- Au-Fe3O4
Gold-coated iron oxide nanoparticle
- Au-MES
Mercaptoethane sulfate-coated gold nanoparticle
- AuNP
Gold nanoparticle
- Au-PEG-TNF
Polyethylene glycol-coated tumor necrosis factor-loaded gold nanoparticles
- CT
Computed tomography
- CTAB
Cetyltrimethylammonium bromide
- CuI NPs
Copper-iodine nanoparticles
- DNA
Deoxyribonucleic acid
- FCV
Feline calicivirus
- FR
Folate receptor
- Gp120
Glycoprotein 120
- H1N1
Influenza A of swine origin
- HBV
Hepatitis B virus
- HIV
Human immune-deficiency virus-1
- HSV-1
Herpes simplex virus 1
- KB-3-1
Head and neck cancer
- MC-38
Colon carcinoma
- MPV
Monkeypox virus
- MRI
Magnetic resonance imaging
- PA
Photoacoustic imaging
- PEG
Polyethylene glycol
- PVP-AgNP
Polyvinylpyrrolidone-coated silver nanoparticle
- RNA
Ribonucleic acid
- ROS
Reactive oxygen species
- siRNA
Small interfering ribonucleic acid
- Sk-BR-3
Breast cancer
- SPECT
Single-photon emission computed tomography
- TNF
Tumor necrosis factor
Authors’ Contributions
SDA coordinated the content, compilation, and writing of all sections, VVG proofed the paper and guided the content, tables and text. CDG coordinated the editing of all sections and final editing of the paper. All authors read and approved the final manuscript.
Funding
The authors thank the School of Chemistry at Bangor University for their support throughout this project, as well as funding from Welsh Government, the Life Sciences Research Network Wales, Cancer Research Wales and the Knowledge Economy Skills Scholarship (KESS).
Availability of Data and Materials
Not applicable
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hofmann-Amtenbrink M, Grainger DW, Hofmann H. Nanoparticles in medicine: current challenges facing inorganic nanoparticle toxicity assessments and standardizations. Nanomedicine. 2015;11:1689–1694. doi: 10.1016/j.nano.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 2.Gao X, Cui Y, Levenson RM, et al. In vivo cancer targeting and imaging with semiconductor quantum dots. Nat Biotechnol. 2004;22:969–976. doi: 10.1038/nbt994. [DOI] [PubMed] [Google Scholar]
- 3.Akerman ME, Chan WCW, Laakkonen P, et al. Nanocrystal targeting in vivo. Proc Natl Acad Sci. 2002;99:12617–12621. doi: 10.1073/pnas.152463399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim S, Lim YT, Soltesz EG, et al. Near-infrared fluorescent type II quantum dots for sentinel lymph node mapping. Nat Biotechnol. 2004;22:93–97. doi: 10.1038/nbt920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harisinghani MG, Barentsz J, Hahn PF, et al. Noninvasive detection of clinically occult lymph-node metastases in prostate cancer. N Engl J Med. 2003;348:2491–2499. doi: 10.1056/NEJMoa022749. [DOI] [PubMed] [Google Scholar]
- 6.Huh Y-M, Jun Y, Song H-T, et al. In vivo magnetic resonance detection of cancer by using multifunctional magnetic nanocrystals. J Am Chem Soc. 2005;127:12387–12391. doi: 10.1021/ja052337c. [DOI] [PubMed] [Google Scholar]
- 7.Duncan R. The dawning era of polymer therapeutics. Nat Rev Drug Discov. 2003;2:347–360. doi: 10.1038/nrd1088. [DOI] [PubMed] [Google Scholar]
- 8.Allen TM, Cullis PR. Drug delivery systems: entering the mainstream. Science. 2004;303:1818–1822. doi: 10.1126/science.1095833. [DOI] [PubMed] [Google Scholar]
- 9.Micha JP, Goldstein BH, Birk CL, et al. Abraxane in the treatment of ovarian cancer: the absence of hypersensitivity reactions. Gynecol Oncol. 2006;100:437–438. doi: 10.1016/j.ygyno.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 10.Sengupta S, Eavarone D, Capila I, et al. Temporal targeting of tumour cells and neovasculature with a nanoscale delivery system. Nature. 2005;436:568–572. doi: 10.1038/nature03794. [DOI] [PubMed] [Google Scholar]
- 11.Garcia-Garcia E, Andrieux K, Gil S, Couvreur P. Colloidal carriers and blood-brain barrier (BBB) translocation: a way to deliver drugs to the brain? Int J Pharm. 2005;298:274–292. doi: 10.1016/j.ijpharm.2005.03.031. [DOI] [PubMed] [Google Scholar]
- 12.Schlachetzki F, Zhang Y, Boado RJ, Pardridge WM. Gene therapy of the brain: the trans-vascular approach. Neurology. 2004;62:1275–1281. doi: 10.1212/01.WNL.0000120551.38463.D9. [DOI] [PubMed] [Google Scholar]
- 13.Popovic N, Brundin P. Therapeutic potential of controlled drug delivery systems in neurodegenerative diseases. Int J Pharm. 2006;314:120–126. doi: 10.1016/j.ijpharm.2005.09.040. [DOI] [PubMed] [Google Scholar]
- 14.Olbrich C, Bakowsky U, Lehr CM, et al. Cationic solid-lipid nanoparticles can efficiently bind and transfect plasmid DNA. J Control Release. 2001;77:345–355. doi: 10.1016/S0168-3659(01)00506-5. [DOI] [PubMed] [Google Scholar]
- 15.Tabatt K, Sameti M, Olbrich C, et al. Effect of cationic lipid and matrix lipid composition on solid lipid nanoparticle-mediated gene transfer. Eur J Pharm Biopharm. 2004;57:155–162. doi: 10.1016/j.ejpb.2003.10.015. [DOI] [PubMed] [Google Scholar]
- 16.De Jaeghere F, Allémann E, Kubel F, et al. Oral bioavailability of a poorly water soluble HIV-1 protease inhibitor incorporated into pH-sensitive particles: effect of the particle size and nutritional state. J Control Release. 2000;68:291–298. doi: 10.1016/S0168-3659(00)00272-8. [DOI] [PubMed] [Google Scholar]
- 17.Pignatello R, Bucolo C, Spedalieri G, et al. Flurbiprofen-loaded acrylate polymer nanosuspensions for ophthalmic application. Biomaterials. 2002;23:3247–3255. doi: 10.1016/S0142-9612(02)00080-7. [DOI] [PubMed] [Google Scholar]
- 18.Pignatello R, Bucolo C, Ferrara P, et al. Eudragit RS100® nanosuspensions for the ophthalmic controlled delivery of ibuprofen. Eur J Pharm Sci. 2002;16:53–61. doi: 10.1016/S0928-0987(02)00057-X. [DOI] [PubMed] [Google Scholar]
- 19.Ludwig A. The use of mucoadhesive polymers in ocular drug delivery. Adv Drug Deliv Rev. 2005;57:1595–1639. doi: 10.1016/j.addr.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 20.JOHN ALISON E., LUKACS NICHOLAS W., BERLIN AARON A., PALECANDA AIYAPPA, BARGATZE ROBERT F., STOOLMAN LLOYD M., NAGY JON O. Discovery of a potent nanoparticle P-selectin antagonist with anti-inflammatory effects in allergic airway disease. The FASEB Journal. 2003;17(15):2296–2298. doi: 10.1096/fj.03-0166fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kumar M, Xiaoyuan K, Behera AK et al (2003) Chitosan IFN-γ-pDNA nanoparticle (CIN) therapy for allergic asthma. Genet Vaccines Ther 1:1-10 [DOI] [PMC free article] [PubMed]
- 22.Teranishi T, Miyake M. Size control of palladium nanoparticles and their crystal structures. Chem Mater. 1998;10:594–600. doi: 10.1021/cm9705808. [DOI] [Google Scholar]
- 23.Sun S, Zeng H. Size-controlled synthesis of magnetite nanoparticles. J Am Chem Soc. 2002;124:8204–8205. doi: 10.1021/ja026501x. [DOI] [PubMed] [Google Scholar]
- 24.Pileni M-P. The role of soft colloidal templates in controlling the size and shape of inorganic nanocrystals. Nat Mater. 2003;2:145–150. doi: 10.1038/nmat817. [DOI] [PubMed] [Google Scholar]
- 25.Sun Y, Xia Y. Shape-controlled synthesis of gold and silver nanoparticles. Science. 2002;298:2176–2179. doi: 10.1126/science.1077229. [DOI] [PubMed] [Google Scholar]
- 26.Manna L, Scher EC, Alivisatos AP. Synthesis of soluble and processable rod-, arrow-, teardrop-, and tetrapod-shaped CdSe nanocrystals. J Am Chem Soc. 2000;122:12700–12706. doi: 10.1021/ja003055+. [DOI] [Google Scholar]
- 27.Gadogbe M, Ansar SM, Chu IW, et al. Comparative study of the self-assembly of gold and silver nanoparticles onto thiophene oil. Langmuir. 2014;30:11520–11527. doi: 10.1021/la502574p. [DOI] [PubMed] [Google Scholar]
- 28.Klajn R, Pinchuk AO, Schatz GC, Grzybowski BA. Synthesis of heterodimeric sphere-prism nanostructures via metastable gold supraspheres. Angew Chem Int Ed. 2007;46:8363–8367. doi: 10.1002/anie.200702570. [DOI] [PubMed] [Google Scholar]
- 29.Wang X, Hall JE, Warren S, et al. Synthesis, characterization, and application of novel polymeric nanoparticles. Macromolecules. 2007;40:499–508. doi: 10.1021/ma0613739. [DOI] [Google Scholar]
- 30.Mazzola L. Commercializing nanotechnology. Nat Biotechnol. 2003;21:1137–1143. doi: 10.1038/nbt1003-1137. [DOI] [PubMed] [Google Scholar]
- 31.Markides H, Rotherham M, El Haj AJ. Biocompatibility and toxicity of magnetic nanoparticles in regenerative medicine. J Nanomater. 2012;2012:1–11. doi: 10.1155/2012/614094. [DOI] [Google Scholar]
- 32.Buzea C, Pacheco I (2019) Toxicity of nanoparticles. In: Nanotechnology in eco-efficient construction. Elsevier, pp 705–754
- 33.Faraji AH, Wipf P. Nanoparticles in cellular drug delivery. Bioorganic Med Chem. 2009;17:2950–2962. doi: 10.1016/j.bmc.2009.02.043. [DOI] [PubMed] [Google Scholar]
- 34.Liao H, Nehl CL, Hafner JH. Biomedical applications of plasmon resonant metal nanoparticles. Nanomedicine (Lond) 2006;1:201–208. doi: 10.2217/17435889.1.2.201. [DOI] [PubMed] [Google Scholar]
- 35.Lanone S, Boczkowski J. Biomedical applications and potential health risks of nanomaterials: molecular mechanisms. Curr Mol Med. 2006;6:651–663. doi: 10.2174/156652406778195026. [DOI] [PubMed] [Google Scholar]
- 36.Singh R, LJ W. Nanoparticle-based targeted drug delivery. Exp Mol Pathol. 2009;86:215–223. doi: 10.1016/j.yexmp.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kruis FE, Fissan H, Peled A. Synthesis of nanoparticles in the gas phase for electronic, optical and magnetic applications—a review. J Aerosol Sci. 1998;29:511–535. doi: 10.1016/S0021-8502(97)10032-5. [DOI] [Google Scholar]
- 38.Chen M, Yamamuro S, Farrell D, Majetich SA. Gold-coated iron nanoparticles for biomedical applications. J Appl Phys. 2003;93:7551–7553. doi: 10.1063/1.1555312. [DOI] [Google Scholar]
- 39.Stevenson PC, Turkevich J, Hillier J. A study of the nucleation and growth processes in the synthesis of. Discuss Faraday Soc. 1951;11:55–75. doi: 10.1039/df9511100055. [DOI] [Google Scholar]
- 40.Cuenya BR. Synthesis and catalytic properties of metal nanoparticles: size, shape, support, composition, and oxidation state effects. Thin Solid Films. 2010;518:3127–3150. doi: 10.1016/j.tsf.2010.01.018. [DOI] [Google Scholar]
- 41.Laurent S, Forge D, Port M, et al. Magnetic iron oxide nanoparticles: synthesis, stabilization, vectorization, physicochemical characterizations, and biological applications. Chem Rev. 2008;108:2064–2110. doi: 10.1021/cr068445e. [DOI] [PubMed] [Google Scholar]
- 42.Ling D, Hyeon T. Chemical design of biocompatible iron oxide nanoparticles for medical applications. Small. 2013;9:1450–1466. doi: 10.1002/smll.201202111. [DOI] [PubMed] [Google Scholar]
- 43.Eastoe J, Hollamby MJ, Hudson L. Recent advances in nanoparticle synthesis with reversed micelles. Adv Colloid Interf Sci. 2006;128–130:5–15. doi: 10.1016/j.cis.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 44.Moraes Silva S, Tavallaie R, Sandiford L, et al. Gold coated magnetic nanoparticles: from preparation to surface modification for analytical and biomedical applications. Chem Commun. 2016;52:7528–7540. doi: 10.1039/C6CC03225G. [DOI] [PubMed] [Google Scholar]
- 45.Fang C-L, Qian K, Zhu J, et al. Monodisperse α-Fe2O3@ SiO 2@ Au core/shell nanocomposite spheres: synthesis, characterization and properties. Nanotechnology. 2008;19:125601. doi: 10.1088/0957-4484/19/12/125601. [DOI] [PubMed] [Google Scholar]
- 46.Iglesias-Silva E, Vilas-Vilela JL, López-Quintela MA, et al. Synthesis of gold-coated iron oxide nanoparticles. J Non-Cryst Solids. 2010;356:1233–1235. doi: 10.1016/j.jnoncrysol.2010.04.022. [DOI] [Google Scholar]
- 47.Boutonnet M, Kizling J, Stenius P, Maire G. The preparation of monodisperse colloidal metal particles from microemulsions. Colloids Surf. 1982;5:209–225. doi: 10.1016/0166-6622(82)80079-6. [DOI] [Google Scholar]
- 48.Tanford C. Theory of micelle formation in aqueous solutions. J Phys Chem. 1974;78:2469–2479. doi: 10.1021/j100617a012. [DOI] [Google Scholar]
- 49.Pileni MP. Nanosized particles made in colloidal assemblies. Langmuir. 1997;13:3266–3276. doi: 10.1021/la960319q. [DOI] [Google Scholar]
- 50.Holmes JD, P A B, B A K, Johnston KP. Synthesis of cadmium sulfide Q particles in water-in-CO 2 microemulsions. Langmuir. 1999;15:6613–6615. doi: 10.1021/la990510a. [DOI] [Google Scholar]
- 51.Ohde H, Ye X-R, Wai CM, Rodriguez JM. Synthesizing silver halide nanoparticles in supercritical carbon dioxide utilizing a water-in-CO2 microemulsion. Chem Commun. 2000;2354:2353–2354. doi: 10.1039/b005924m. [DOI] [Google Scholar]
- 52.Ohde H, Wai CM, Kim H, et al. Hydrogenation of olefins in supercritical CO 2 catalyzed by palladium nanoparticles in a water-in-CO 2 microemulsion. J Am Chem Soc. 2002;124:4540–4541. doi: 10.1021/ja012232j. [DOI] [PubMed] [Google Scholar]
- 53.Carpenter EE, Seip CT, O’Connor CJ. Magnetism of nanophase metal and metal alloy particles formed in ordered phases. J Appl Phys. 1999;85:5184–5186. doi: 10.1063/1.369118. [DOI] [Google Scholar]
- 54.Lim KT, Hwang HS, Ryoo W, Johnston KP. Synthesis of TiO2 nanoparticles utilizing hydrated reverse micelles in CO2. Langmuir. 2004;20:2466–2471. doi: 10.1021/la035646u. [DOI] [PubMed] [Google Scholar]
- 55.Bommarius AS, Holzwarth JF, Wang DIC, Hatton TA. Coalescence and solubilizate exchange in a cationic four-component reversed micellar system. J Phys Chem. 1990;94:7232–7239. doi: 10.1021/j100381a051. [DOI] [Google Scholar]
- 56.Liu J, Raveendran P, Shervani Z, et al. Synthesis of Ag and AgI quantum dots in AOT-stabilized water-in-CO 2 microemulsions. Chem A Eur J. 2005;11:1854–1860. doi: 10.1002/chem.200400508. [DOI] [PubMed] [Google Scholar]
- 57.Liu J, Ikushima Y, Shervani Z. Environmentally benign preparation of metal nano-particles by using water-in-CO2 microemulsions technology. Curr Opin Solid State Mater Sci. 2003;7:255–261. doi: 10.1016/j.cossms.2003.09.005. [DOI] [Google Scholar]
- 58.Iglesias-Silva E, Rivas J, León Isidro LM, López-Quintela MA. Synthesis of silver-coated magnetite nanoparticles. J Non-Cryst Solids. 2007;353:829–831. doi: 10.1016/j.jnoncrysol.2006.12.050. [DOI] [Google Scholar]
- 59.Carpenter EE, Sangregorio C, Connor CJ. Effects of shell thickness on blocking temperature of nanocomposites of metal particles with gold shells. IEEE Trans Magn. 1999;35:3496–3498. doi: 10.1109/20.800568. [DOI] [Google Scholar]
- 60.Lin J, Zhou W, Kumbhar A, et al. Gold-coated Iron (Fe@ Au) nanoparticles: synthesis, characterization, and magnetic field-induced self-assembly. J Solid State Chem. 2001;159:26–31. doi: 10.1006/jssc.2001.9117. [DOI] [Google Scholar]
- 61.Cho S-J, Idrobo J-C, Olamit J, et al. Growth mechanisms and oxidation resistance of gold-coated iron nanoparticles. Chem Mater. 2005;17:3181–3186. doi: 10.1021/cm0500713. [DOI] [Google Scholar]
- 62.Tamer U, Cetin D, Suludere Z, et al. Gold-coated iron composite nanospheres targeted the detection of Escherichia coli. Int J Mol Sci. 2013;14:6223–6240. doi: 10.3390/ijms14036223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pana O, Teodorescu CM, Chauvet O, et al. Structure, morphology and magnetic properties of Fe-Au core-shell nanoparticles. Surf Sci. 2007;601:4352–4357. doi: 10.1016/j.susc.2007.06.024. [DOI] [Google Scholar]
- 64.Seip CT, O’Connor CJ. Fabrication and organization of self-assembled metallic nanoparticles formed in reverse micelles. Nanostruct Mater. 1999;12:183–186. doi: 10.1016/S0965-9773(99)00094-X. [DOI] [Google Scholar]
- 65.Lopez Perez JA, Lopez Quintela MA, Mira J, et al. Advances in the preparation of magnetic nanoparticles by the microemulsion method. J Phys Chem B. 1997;101:8045–8047. doi: 10.1021/jp972046t. [DOI] [Google Scholar]
- 66.Zhang J, Post M, Veres T, et al. Laser-assisted synthesis of superparamagnetic Fe@ Au core-shell nanoparticles. J Phys Chem B. 2006;110:7122–7128. doi: 10.1021/jp0560967. [DOI] [PubMed] [Google Scholar]
- 67.Takami S, Sato T, Mousavand T, et al. Hydrothermal synthesis of surface-modified iron oxide nanoparticles. Mater Lett. 2007;61:4769–4772. doi: 10.1016/j.matlet.2007.03.024. [DOI] [Google Scholar]
- 68.Park H-Y, Schadt MJ, Wang L, et al. Fabrication of magnetic core@ shell Fe oxide@ Au nanoparticles for interfacial bioactivity and bio-separation. Langmuir. 2007;23:9050–9056. doi: 10.1021/la701305f. [DOI] [PubMed] [Google Scholar]
- 69.Liu HL, Hou P, Zhang WX, Wu JH. Synthesis of monosized core-shell Fe3O4/Au multifunctional nanoparticles by PVP-assisted nanoemulsion process. Colloids Surfaces A Physicochem Eng Asp. 2010;356:21–27. doi: 10.1016/j.colsurfa.2009.12.023. [DOI] [Google Scholar]
- 70.Banerjee S., Raja S. O., Sardar M., Gayathri N., Ghosh B., Dasgupta A. Iron oxide nanoparticles coated with gold: Enhanced magnetic moment due to interfacial effects. Journal of Applied Physics. 2011;109(12):123902. doi: 10.1063/1.3596760. [DOI] [Google Scholar]
- 71.Sun X, Zheng C, Zhang F, et al. Size-controlled synthesis of magnetite (Fe3O4) nanoparticles coated with glucose and gluconic acid from a single Fe(III) precursor by a sucrose bifunctional hydrothermal method. J Phys Chem C. 2009;113:16002–16008. doi: 10.1021/jp9038682. [DOI] [Google Scholar]
- 72.Aneesh PM, Vanaja KA, Jayaraj MK. Synthesis of ZnO nanoparticles by hydrothermal method. 2007. p. 66390J. [Google Scholar]
- 73.Brust Mathias, Walker Merryl, Bethell Donald, Schiffrin David J., Whyman Robin. Synthesis of thiol-derivatised gold nanoparticles in a two-phase Liquid–Liquid system. J. Chem. Soc., Chem. Commun. 1994;0(7):801–802. doi: 10.1039/C39940000801. [DOI] [Google Scholar]
- 74.Ban Z, Barnakov YA, Li F, et al. The synthesis of core–shell iron@ gold nanoparticles and their characterization. J Mater Chem. 2005;15:4660. doi: 10.1039/b504304b. [DOI] [Google Scholar]
- 75.Zhai Y, Zhai J, Wang Y, et al. Fabrication of iron oxide core/gold shell submicrometer spheres with nanoscale surface roughness for efficient surface-enhanced Raman scattering. J Phys Chem C. 2009;113:7009–7014. doi: 10.1021/jp810561q. [DOI] [Google Scholar]
- 76.Rudakovskaya PG, Beloglazkina EK, Majouga AG, Zyk NV. Synthesis and characterization of terpyridine-type ligand-protected gold-coated Fe3O4 nanoparticles. Mendeleev Commun. 2010;20:158–160. doi: 10.1016/j.mencom.2010.05.012. [DOI] [Google Scholar]
- 77.Mohammad F, Balaji G, Weber A, et al. Influence of gold nanoshell on hyperthermia of super paramagnetic iron oxide nanoparticles (SPIONs) J Phys Chem C Nanomater Interfaces. 2010;114:19194–19201. doi: 10.1021/jp105807r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhou H, Lee J, Park TJ, et al. Ultrasensitive DNA monitoring by au-Fe 3O 4 nanocomplex. Sensors Actuators B Chem. 2012;163:224–232. doi: 10.1016/j.snb.2012.01.040. [DOI] [Google Scholar]
- 79.Wang L, Luo J, Fan Q, et al. Monodispersed core-shell Fe3O4@Au nanoparticles. J Phys Chem B. 2005;109:21593–21601. doi: 10.1021/jp0543429. [DOI] [PubMed] [Google Scholar]
- 80.Robinson I, Tung LD, Maenosono S, et al. Synthesis of core-shell gold coated magnetic nanoparticles and their interaction with thiolated DNA. Nanoscale. 2010;2:2624. doi: 10.1039/c0nr00621a. [DOI] [PubMed] [Google Scholar]
- 81.Dahal N, Chikan V, Jasinski J, Leppert V. Synthesis of water-soluble iron− gold alloy nanoparticles. Chem Mater. 2008;17:6389–6395. doi: 10.1021/cm800389r. [DOI] [Google Scholar]
- 82.Wei Y, Han B, Hu X, et al. Synthesis of Fe3O4 nanoparticles and their magnetic properties. Procedia Eng. 2012;27:632–637. doi: 10.1016/j.proeng.2011.12.498. [DOI] [Google Scholar]
- 83.Rawal R, Chawla S, Pundir CS. An electrochemical sulfite biosensor based on gold coated magnetic nanoparticles modified gold electrode. Biosens Bioelectron. 2012;31:144–150. doi: 10.1016/j.bios.2011.10.007. [DOI] [PubMed] [Google Scholar]
- 84.Nadagouda MN, Varma RS. A greener synthesis of Core (Fe, cu)-shell (au, Pt, Pd, and Ag) nanocrystals using aqueous vitamin C. Cryst Growth Des. 2007;7:2582–2587. doi: 10.1021/cg070554e. [DOI] [Google Scholar]
- 85.Ahmad T, Wani IA, Ahmed J, Al-Hartomy OA. Effect of gold ion concentration on size and properties of gold nanoparticles in TritonX-100 based inverse microemulsions. Appl Nanosci. 2014;4:491–498. doi: 10.1007/s13204-013-0224-y. [DOI] [Google Scholar]
- 86.Sau TK, Murphy CJ. Room temperature, high-yield synthesis of multiple shapes of gold nanoparticles in aqueous solution. J Am Chem Soc. 2004;126:8648–8649. doi: 10.1021/ja047846d. [DOI] [PubMed] [Google Scholar]
- 87.Nowack B, Krug HF, Height M. 120 years of nanosilver history: implications for policy makers. Environ Sci Technol. 2011;45:1177–1183. doi: 10.1021/es103316q. [DOI] [PubMed] [Google Scholar]
- 88.Enustun BV, Turkevich J. Coagulation of colloidal gold. J Am Chem Soc. 1963;85:3317–3328. doi: 10.1021/ja00904a001. [DOI] [Google Scholar]
- 89.Xu Z, Hou Y, Sun S. Magnetic core/shell Fe3O4/Au and Fe3O4/Au/Ag nanoparticles with tunable plasmonic properties. J Am Chem Soc. 2007;129:8698–8699. doi: 10.1021/ja073057v. [DOI] [PubMed] [Google Scholar]
- 90.Brown KR, Walter DG, Natan MJ. Seeding of colloidal Au nanoparticle solutions. 2. Improved control of particle size and shape. Chem Mater. 2000;12:306–313. doi: 10.1021/cm980065p. [DOI] [Google Scholar]
- 91.Goon IY, Lai LMH, Lim M, et al. Fabrication and dispersion of gold-shell-protected magnetite nanoparticles: systematic control using polyethyleneimine. Chem Mater. 2009;21:673–681. doi: 10.1021/cm8025329. [DOI] [Google Scholar]
- 92.Levin CS, Hofmann C, Ali TA, et al. Magnetic−plasmonic core−shell nanoparticles. ACS Nano. 2009;3:1379–1388. doi: 10.1021/nn900118a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lyon JL, Fleming DA, Stone MB, et al. Synthesis of Fe oxide core/Au shell nanoparticles by iterative hydroxylamine seeding. Nano Lett. 2004;4:719–723. doi: 10.1021/nl035253f. [DOI] [Google Scholar]
- 94.Ma LL, Borwankar AU, Willsey BW, et al. Growth of textured thin Au coatings on iron oxide nanoparticles with near infrared absorbance. Nanotechnology. 2013;24:025606. doi: 10.1088/0957-4484/24/2/025606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Pal S, Morales M, Mukherjee P, Srikanth H. Synthesis and magnetic properties of gold coated iron oxide nanoparticles. J Appl Phys. 2009;105:1–4. doi: 10.1063/1.3055268. [DOI] [Google Scholar]
- 96.Wagstaff AJ, Brown SD, Holden MR, et al. Cisplatin drug delivery using gold-coated iron oxide nanoparticles for enhanced tumour targeting with external magnetic fields. Inorganica Chim Acta. 2012;393:328–333. doi: 10.1016/j.ica.2012.05.012. [DOI] [Google Scholar]
- 97.Wang H, Brandl DW, Le F, et al. Nanorice: a hybrid plasmonic nanostructure. Nano Lett. 2006;6:827–832. doi: 10.1021/nl060209w. [DOI] [PubMed] [Google Scholar]
- 98.Phan CM, Nguyen HM. Role of capping agent in wet synthesis of nanoparticles. J Phys Chem A. 2017;121:3213–3219. doi: 10.1021/acs.jpca.7b02186. [DOI] [PubMed] [Google Scholar]
- 99.Diggins FWE. The true history of the discovery of penicillin, with refutation of the misinformation in the literature. Br J Biomed Sci. 1999;56:83–93. [PubMed] [Google Scholar]
- 100.Bao G, Mitragotri S, Tong S. Multifunctional nanoparticles for drug delivery and molecular imaging. Annu Rev Biomed Eng. 2013;15:253–282. doi: 10.1146/annurev-bioeng-071812-152409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Rai M, Deshmukh SD, Ingle AP, et al. Metal nanoparticles: the protective nanoshield against virus infection. Crit Rev Microbiol. 2014;42:1–11. doi: 10.3109/1040841X.2013.879849. [DOI] [PubMed] [Google Scholar]
- 102.Sreekumar TV, Das A, Chandra L, et al. Inherently colored antimicrobial fibers employing silver nanoparticles. J Biomed Nanotechnol. 2009;5:115–120. doi: 10.1166/jbn.2009.040. [DOI] [PubMed] [Google Scholar]
- 103.White CA, Toothaker RD, Smith AL, Slattery JT. In vitro evaluation of the determinants of bactericidal activity of ampicillin dosing regimens against Escherichia coli. Antimicrob Agents Chemother. 1989;33:1046–1051. doi: 10.1128/AAC.33.7.1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Panáček A, Kvítek L, Smékalová M, et al. Bacterial resistance to silver nanoparticles and how to overcome it. Nat Nanotechnol. 2018;13:65–71. doi: 10.1038/s41565-017-0013-y. [DOI] [PubMed] [Google Scholar]
- 105.Holtz RD, Souza Filho a G, Brocchi M, et al. Development of nanostructured silver vanadates decorated with silver nanoparticles as a novel antibacterial agent. Nanotechnology. 2010;21:185102. doi: 10.1088/0957-4484/21/18/185102. [DOI] [PubMed] [Google Scholar]
- 106.Verma VC, Kharwar RN, Gange AC. Biosynthesis of antimicrobial silver nanoparticles by the endophytic fungus Aspergillus clavatus. Nanomedicine. 2010;5:33–40. doi: 10.2217/nnm.09.77. [DOI] [PubMed] [Google Scholar]
- 107.Selvaraj V, Nirmala Grace A, Alagar M, Hamerton I. Antimicrobial and anticancer efficacy of antineoplastic agent capped gold nanoparticles. J Biomed Nanotechnol. 2010;6:129–137. doi: 10.1166/jbn.2010.1115. [DOI] [PubMed] [Google Scholar]
- 108.Mohamed MM, Fouad SA, Elshoky HA, et al. Antibacterial effect of gold nanoparticles against Corynebacterium pseudotuberculosis. Int J Vet Sci Med. 2017;5:23–29. doi: 10.1016/j.ijvsm.2017.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Shamaila S, Zafar N, Riaz S, et al. Gold nanoparticles: an efficient antimicrobial agent against enteric bacterial human pathogen. Nanomaterials. 2016;6:71. doi: 10.3390/nano6040071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Salmon SA, Watts JL. Minimum inhibitory concentration determinations for various antimicrobial agents against 1570 bacterial isolates from turkey poults. Avian Dis. 2000;44:85. doi: 10.2307/1592511. [DOI] [PubMed] [Google Scholar]
- 111.Erdogan A, Rao SSC. Small intestinal fungal overgrowth. Curr Gastroenterol Rep. 2015;17:1–7. doi: 10.1007/s11894-015-0436-2. [DOI] [PubMed] [Google Scholar]
- 112.Zietse R, Zoutendijk R, Hoorn EJ. Fluid, electrolyte and acid-base disorders associated with antibiotic therapy. Nat Rev Nephrol. 2009;5:193–202. doi: 10.1038/nrneph.2009.17. [DOI] [PubMed] [Google Scholar]
- 113.Lu L, Sun RW-Y, Chen R, et al. Silver nanoparticles inhibit hepatitis B virus replication. Antivir Ther. 2008;13:253–262. [PubMed] [Google Scholar]
- 114.Rogers JV, Parkinson CV, Choi YW, et al. A preliminary assessment of silver nanoparticle inhibition of monkeypox virus plaque formation. Nanoscale Res Lett. 2008;3:129–133. doi: 10.1007/s11671-008-9128-2. [DOI] [Google Scholar]
- 115.Lara HH, Ixtepan-Turrent L, Garza-Treviño EN, Rodriguez-Padilla C. PVP-coated silver nanoparticles block the transmission of cell-free and cell-associated HIV-1 in human cervical culture. J Nanobiotechnology. 2010;8:15. doi: 10.1186/1477-3155-8-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lara HH, Ayala-Nuñez NV, Ixtepan-Turrent L, Rodriguez-Padilla C. Mode of antiviral action of silver nanoparticles against HIV-1. J Nanobiotechnology. 2010;8:1. doi: 10.1186/1477-3155-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Baram-Pinto D, Shukla S, Gedanken A, Sarid R. Inhibition of HSV-1 attachment, entry, and cell-to-cell spread by functionalized multivalent gold nanoparticles. Small. 2010;6:1044–1050. doi: 10.1002/smll.200902384. [DOI] [PubMed] [Google Scholar]
- 118.Di Gianvincenzo P, Marradi M, Martínez-Ávila OM, et al. Gold nanoparticles capped with sulfate-ended ligands as anti-HIV agents. Bioorg Med Chem Lett. 2010;20:2718–2721. doi: 10.1016/j.bmcl.2010.03.079. [DOI] [PubMed] [Google Scholar]
- 119.Shionoiri N, Sato T, Fujimori Y, et al. Investigation of the antiviral properties of copper iodide nanoparticles against feline calicivirus. J Biosci Bioeng. 2012;113:580–586. doi: 10.1016/j.jbiosc.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 120.Fujimori Y, Sato T, Hayata T, et al. Novel antiviral characteristics of nanosized copper(I) iodide particles showing inactivation activity against 2009 pandemic H1N1 influenza virus. Appl Environ Microbiol. 2012;78:951–955. doi: 10.1128/AEM.06284-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.World Health Organization . Global hepatitis report, 2017. 2017. [Google Scholar]
- 122.World Health Organization (WHO) Progress report 2016, prevent HIV, test and treat all. 2016. [Google Scholar]
- 123.Halliwell B, Gutteridge JMC. Oxygen toxicity, oxygen radicals, transition metals and disease. Biochem J. 1984;219:1–14. doi: 10.1042/bj2190001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Radiologists RC of. Padhani A, Allen C, Carey B. Recommendations for cross-sectional imaging in cancer management: computed tomography – CT magnetic resonance imaging – MRI positron emission tomography – PET-CT. 2014. [Google Scholar]
- 125.McRobbie DW, Moore EA, Graves MJ, Prince MR. MRI from picture to proton. Cambridge: Cambridge University Press; 2006. [Google Scholar]
- 126.Murphy KJ, Brunberg JA, Cohan RH. Adverse reactions to gadolinium contrast media: a review of 36 cases. Am J Roentgenol. 1996;167:847–849. doi: 10.2214/ajr.167.4.8819369. [DOI] [PubMed] [Google Scholar]
- 127.Rhee CM, Bahn I, Alexander EK, Brunelli SM. Association between iodinated contrast media exposure and incident hyperthyroidism and hypothyroidism. Arch Intern Med. 2012;172:153. doi: 10.1001/archinternmed.2011.677. [DOI] [PubMed] [Google Scholar]
- 128.Lee M, Lee G, Park S-S, et al. Synthesis of TiO 2 / SiO 2 nanoparticles in a water-in-carbon-dioxide microemulsion and their photocatalytic activity. 2005. pp. 379–389. [Google Scholar]
- 129.Blasiak B, Van Veggel FCJM, Tomanek B (2013) Applications of nanoparticles for MRI cancer diagnosis and therapy. J Nanomater 2013:13-15
- 130.Li W, Chen X. Gold nanoparticles for photoacoustic imaging. Nanomedicine. 2015;10:299–320. doi: 10.2217/nnm.14.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Stacul F, van der Molen AJ, Reimer P, et al. Contrast induced nephropathy: updated ESUR contrast media safety committee guidelines. Eur Radiol. 2011;21:2527–2541. doi: 10.1007/s00330-011-2225-0. [DOI] [PubMed] [Google Scholar]
- 132.van Schooneveld MM, Cormode DP, Koole R, et al. A fluorescent, paramagnetic and PEGylated gold/silica nanoparticle for MRI, CT and fluorescence imaging. Contrast Media Mol Imaging. 2010;5:231–236. doi: 10.1002/cmmi.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Cheheltani R, Ezzibdeh RM, Chhour P, et al. Tunable, biodegradable gold nanoparticles as contrast agents for computed tomography and photoacoustic imaging. Biomaterials. 2016;102:87–97. doi: 10.1016/j.biomaterials.2016.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kojima C, Umeda Y, Ogawa M, et al. X-ray computed tomography contrast agents prepared by seeded growth of gold nanoparticles in PEGylated dendrimer. Nanotechnology. 2010;21:245104. doi: 10.1088/0957-4484/21/24/245104. [DOI] [PubMed] [Google Scholar]
- 135.Umeda Y, Kojima C, Harada A, et al. PEG-attached PAMAM dendrimers encapsulating gold nanoparticles: growing gold nanoparticles in the dendrimers for improvement of their photothermal properties. Bioconjug Chem. 2010;21:1559–1564. doi: 10.1021/bc1001399. [DOI] [PubMed] [Google Scholar]
- 136.Li X, Wang C, Tan H, et al. Gold nanoparticles-based SPECT/CT imaging probe targeting for vulnerable atherosclerosis plaques. Biomaterials. 2016;108:71–80. doi: 10.1016/j.biomaterials.2016.08.048. [DOI] [PubMed] [Google Scholar]
- 137.Wang Y, Xie X, Wang X, et al. Photoacoustic tomography of a nanoshell contrast agent in the in vivo rat brain. Nano Lett. 2004;4:1689–1692. doi: 10.1021/nl049126a. [DOI] [Google Scholar]
- 138.Song J, Kim J, Hwang S, et al. “Smart” gold nanoparticles for photoacoustic imaging: an imaging contrast agent responsive to the cancer microenvironment and signal amplification via pH-induced aggregation. Chem Commun. 2016;52:8287–8290. doi: 10.1039/C6CC03100E. [DOI] [PubMed] [Google Scholar]
- 139.Ashton JR, Castle KD, Qi Y, et al. Dual-energy CT imaging of tumor liposome delivery after gold nanoparticle-augmented radiation therapy. Theranostics. 2018;8:1782–1797. doi: 10.7150/thno.22621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Xu Minghua, Wang Lihong V. Photoacoustic imaging in biomedicine. Review of Scientific Instruments. 2006;77(4):041101. doi: 10.1063/1.2195024. [DOI] [Google Scholar]
- 141.Huang X, El-Sayed IH, Qian W, El-Sayed MA. Cancer cell imaging and photothermal therapy in the near-infrared region by using gold nanorods. J Am Chem Soc. 2006;128:2115–2120. doi: 10.1021/ja057254a. [DOI] [PubMed] [Google Scholar]
- 142.Hirsch LR, Stafford RJ, Bankson JA, et al. Nanoshell-mediated near-infrared thermal therapy of tumors under magnetic resonance guidance. Proc Natl Acad Sci. 2003;100:13549–13554. doi: 10.1073/pnas.2232479100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Woodford O, Harriman A, McFarlane W, Wills C. Dramatic effect of solvent on the rate of photobleaching of organic pyrrole-BF 2 (BOPHY) dyes. ChemPhotoChem. 2017;1:317–325. doi: 10.1002/cptc.201600061. [DOI] [Google Scholar]
- 144.Ashton JR, Gottlin EB, Patz EF, et al. A comparative analysis of EGFR-targeting antibodies for gold nanoparticle CT imaging of lung cancer. PLoS One. 2018;13:1–20. doi: 10.1371/journal.pone.0206950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Buzea C, Pacheco II, Robbie K. Nanomaterials and nanoparticles: sources and toxicity. Biointerphases. 2007;2:MR17–MR71. doi: 10.1116/1.2815690. [DOI] [PubMed] [Google Scholar]
- 146.Moyano DF, Goldsmith M, Solfiell DJ, et al. Nanoparticle hydrophobicity dictates immune response. J Am Chem Soc. 2012;134:3965–3967. doi: 10.1021/ja2108905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Boraschi D, Duschl A. Nanoparticles and the immune system: safety and effects. Nanoparticles Immune Syst Saf Eff. 2013;11:2621–2624. [Google Scholar]
- 148.Lammers T, Kiessling F, Hennink WE, Storm G. Drug targeting to tumors: principles, pitfalls and (pre-) clinical progress. J Control Release. 2012;161:175–187. doi: 10.1016/j.jconrel.2011.09.063. [DOI] [PubMed] [Google Scholar]
- 149.Bhumkar DR, Joshi HM, Sastry M, Pokharkar VB. Chitosan reduced gold nanoparticles as novel carriers for transmucosal delivery of insulin. Pharm Res. 2007;24:1415–1426. doi: 10.1007/s11095-007-9257-9. [DOI] [PubMed] [Google Scholar]
- 150.Nam J, La WG, Hwang S, et al. PH-responsive assembly of gold nanoparticles and “spatiotemporally concerted” drug release for synergistic cancer therapy. ACS Nano. 2013;7:3388–3402. doi: 10.1021/nn400223a. [DOI] [PubMed] [Google Scholar]
- 151.Sandström P, Boncheva M, Åkerman B. Nonspecific and thiol-specific binding of DNA to gold nanoparticles. Langmuir. 2003;19:7537–7543. doi: 10.1021/la034348u. [DOI] [Google Scholar]
- 152.Rosi NL, Giljohann DA, Thaxton CS, et al. Oligonucleotide-modified gold nanoparticles for intracellular gene regulation. Science. 2006;312:1027–1030. doi: 10.1126/science.1125559. [DOI] [PubMed] [Google Scholar]
- 153.Ding Y, Jiang Z, Saha K, et al. Gold nanoparticles for nucleic acid delivery. Mol Ther. 2014;22:1075–1083. doi: 10.1038/mt.2014.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Giljohann DA, Seferos DS, Prigodich AE, et al. Gene regulation with polyvalent siRNA−nanoparticle conjugates. J Am Chem Soc. 2009;131:2072–2073. doi: 10.1021/ja808719p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Yonezawa T, Onoue S, Kimizuka N. Metal coating of DNA molecules by cationic, metastable gold nanoparticles. Chem Lett. 2002;31:1172–1173. doi: 10.1246/cl.2002.1172. [DOI] [Google Scholar]
- 156.Barron N, Piskareva O, Muniyappa M. Targeted genetic modification of cell lines for recombinant protein production. Cytotechnology. 2007;53:65–73. doi: 10.1007/s10616-007-9050-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Niidome T, Nakashima K, Takahashi H, Niidome Y (2004) Preparation of primary amine-modified gold nanoparticles and their transfection ability into cultivated cells. Electronic Supplementary Information (ESI) available: A TEM image of the complex at a w/w ratio of 11 Chem Commun 1978. See http://xlink.rsc.org/?DOI=b406189f. [DOI] [PubMed]
- 158.Paciotti GF, Myer L, Weinreich D, et al. Colloidal gold: a novel nanoparticle vector for tumor directed drug delivery. Drug Deliv J Deliv Target Ther Agents. 2004;11:169–183. doi: 10.1080/10717540490433895. [DOI] [PubMed] [Google Scholar]
- 159.Dixit V, Van Den Bossche J, Sherman DM, et al. Synthesis and grafting of thioctic acid-PEG-folate conjugates onto Au nanoparticles for selective targeting of folate receptor-positive tumor cells. Bioconjug Chem. 2006;17:603–609. doi: 10.1021/bc050335b. [DOI] [PubMed] [Google Scholar]
- 160.Du Y, Xia L, Jo A, et al. Synthesis and evaluation of doxorubicin-loaded gold nanoparticles for tumor-targeted drug delivery. Bioconjug Chem. 2018;29:420–430. doi: 10.1021/acs.bioconjchem.7b00756. [DOI] [PubMed] [Google Scholar]
- 161.Kalimuthu K, Lubin B-C, Bazylevich A, et al. Gold nanoparticles stabilize peptide-drug-conjugates for sustained targeted drug delivery to cancer cells. J Nanobiotechnology. 2018;16:34. doi: 10.1186/s12951-018-0362-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Gao H. Progress and perspectives on targeting nanoparticles for brain drug delivery. Acta Pharm Sin B. 2016;6:268–286. doi: 10.1016/j.apsb.2016.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Rosenblum D, Joshi N, Tao W et al (2018) Progress and challenges towards targeted delivery of cancer therapeutics. Nat Commun 9:1-12 [DOI] [PMC free article] [PubMed]
- 164.Friedman AD, Claypool SE, Liu R. The smart targeting of nanoparticles. Curr Pharm Des. 2013;19:6315–6329. doi: 10.2174/13816128113199990375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Fay F, Scott CJ. Antibody-targeted nanoparticles for cancer therapy. Immunotherapy. 2011;3:381–394. doi: 10.2217/imt.11.5. [DOI] [PubMed] [Google Scholar]
- 166.Carter T, Mulholland P, Chester K. Antibody-targeted nanoparticles for cancer treatment. Immunotherapy. 2016;8:941–958. doi: 10.2217/imt.16.11. [DOI] [PubMed] [Google Scholar]
- 167.Okur AC, Erkoc P, Kizilel S. Targeting cancer cells via tumor-homing peptide CREKA functional PEG nanoparticles. Colloids Surf B Biointerfaces. 2016;147:191–200. doi: 10.1016/j.colsurfb.2016.08.005. [DOI] [PubMed] [Google Scholar]
- 168.King A, Ndifon C, Lui S, et al. Tumor-homing peptides as tools for targeted delivery of payloads to the placenta. Sci Adv. 2016;2:1–16. doi: 10.1126/sciadv.1600349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Pardridge WM. Blood–brain barrier delivery. Drug Discov Today. 2007;12:54–61. doi: 10.1016/j.drudis.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 170.Gwenin VV, Gwenin CD, Kalaji M. Colloidal gold modified with a genetically engineered nitroreductase: toward a novel enzyme delivery system for cancer prodrug therapy. Langmuir. 2011;27:14300–14307. doi: 10.1021/la202951p. [DOI] [PubMed] [Google Scholar]
- 171.Obaidat I, Issa B, Haik Y. Magnetic properties of magnetic nanoparticles for efficient hyperthermia. Nanomaterials. 2015;5:63–89. doi: 10.3390/nano5010063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Bødker F, Mørup S, Linderoth S. Surface effects in metallic iron nanoparticles. Phys Rev Lett. 1994;72:282–285. doi: 10.1103/PhysRevLett.72.282. [DOI] [PubMed] [Google Scholar]
- 173.Mody VV, Cox A, Shah S, et al. Magnetic nanoparticle drug delivery systems for targeting tumor. Appl Nanosci. 2014;4:385–392. doi: 10.1007/s13204-013-0216-y. [DOI] [Google Scholar]
- 174.Donaldson JD, Beyersmann D. Ullmann’s encyclopedia of industrial chemistry. Weinheim: Wiley-VCH Verlag GmbH & Co. KGaA; 2005. Cobalt and cobalt compounds; pp. 467–497. [Google Scholar]
- 175.Crossgrove J, Zheng W. Manganese toxicity upon overexposure. NMR Biomed. 2004;17:544–553. doi: 10.1002/nbm.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Karlsson HL, Gustafsson J, Cronholm P, Möller L. Size-dependent toxicity of metal oxide particles-a comparison between nano- and micrometer size. Toxicol Lett. 2009;188:112–118. doi: 10.1016/j.toxlet.2009.03.014. [DOI] [PubMed] [Google Scholar]
- 177.Soto K, Garza KM, Murr LE. Cytotoxic effects of aggregated nanomaterials. Acta Biomater. 2007;3:351–358. doi: 10.1016/j.actbio.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 178.Brunner TJ, Wick P, Manser P, et al. In vitro cytotoxicity of oxide nanoparticles: comparison to asbestos, silica, and the effect of particle solubility. Environ Sci Technol. 2006;40:4374–4381. doi: 10.1021/es052069i. [DOI] [PubMed] [Google Scholar]
- 179.Chia SL, Leong DT. Reducing ZnO nanoparticles toxicity through silica coating. Heliyon. 2016;2:1–18. doi: 10.1016/j.heliyon.2016.e00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Mine E, Yamada A, Kobayashi Y, et al. Direct coating of gold nanoparticles with silica by a seeded polymerization technique. J Colloid Interface Sci. 2003;264:385–390. doi: 10.1016/S0021-9797(03)00422-3. [DOI] [PubMed] [Google Scholar]
- 181.Cerruti MG, Sauthier M, Leonard D, et al. Gold and silica-coated gold nanoparticles as thermographic labels for DNA detection. Anal Chem. 2006;78:3282–3288. doi: 10.1021/ac0600555. [DOI] [PubMed] [Google Scholar]
- 182.Kobayashi Y, Nagasu R, Shibuya K et al (2014) Synthesis of a colloid solution of silica-coated gold nanoparticles for X-ray imaging applications. J Nanopart Res 16:1-13
- 183.Tural B, Özkan N, Volkan M. Preparation and characterization of polymer coated superparamagnetic magnetite nanoparticle agglomerates. J Phys Chem Solids. 2009;70:860–866. doi: 10.1016/j.jpcs.2009.04.007. [DOI] [Google Scholar]
- 184.Wang Y, Dave RN, Pfeffer R. Polymer coating/encapsulation of nanoparticles using a supercritical anti-solvent process. J Supercrit Fluids. 2004;28:85–99. doi: 10.1016/S0896-8446(03)00011-1. [DOI] [Google Scholar]
- 185.Chen D, Li J, Shi C, et al. Properties of core-shell Ni-Au nanoparticles synthesized through a redox-transmetalation method in reverse microemulsion. Chem Mater. 2007;19:3399–3405. doi: 10.1021/cm070182x. [DOI] [Google Scholar]
- 186.Jana NR. Silver coated gold nanoparticles as new surface enhanced Raman substrate at low analyte concentration. Analyst. 2003;128:954. doi: 10.1039/b302409a. [DOI] [Google Scholar]
- 187.Dierks S. Gold “MSDS” electronic space products international. 2005. pp. 1–6. [Google Scholar]
- 188.Alexander JW. History of the medical use of silver. Surg Infect. 2009;10:289–292. doi: 10.1089/sur.2008.9941. [DOI] [PubMed] [Google Scholar]
- 189.Marx DE, Barillo DJ. Silver in medicine: the basic science. Burns. 2014;40:S9–S18. doi: 10.1016/j.burns.2014.09.010. [DOI] [PubMed] [Google Scholar]
- 190.Shubayev VI, Pisanic TR, Jin S. Magnetic nanoparticles for theragnostics. Adv Drug Deliv Rev. 2009;61:467–477. doi: 10.1016/j.addr.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Gwenin VV, Gwenin CD, Kalaji M (2009) Gold coated magnetic particles enabling nitroreductase delivery to cancer cells. European Union Patent PCT/EP2010/062871
- 192.McBain SC, Yiu HHP, Dobson J. Magnetic nanoparticles for gene and drug delivery. Int J Nanomedicine. 2008;3:169–180. doi: 10.2147/ijn.s1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Veiseh O, Gunn JW, Zhang M. Design and fabrication of magnetic nanoparticles for targeted drug delivery and imaging. Adv Drug Deliv Rev. 2010;62:284–304. doi: 10.1016/j.addr.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Kayal S, Ramanujan RV. Anti-Cancer drug loaded iron–gold core–shell nanoparticles (Fe@Au) for magnetic drug targeting. J Nanosci Nanotechnol. 2010;10:5527–5539. doi: 10.1166/jnn.2010.2461. [DOI] [PubMed] [Google Scholar]
- 195.Dobson J. Magnetic nanoparticles for drug delivery. Drug Dev Res. 2006;67:55–60. doi: 10.1002/ddr.20067. [DOI] [Google Scholar]
- 196.Arruebo M, Fernández-Pacheco R, Ibarra MR, Santamaría J. Magnetic nanoparticles for drug delivery. Nanotoday. 2007;2:22–32. doi: 10.1016/S1748-0132(07)70084-1. [DOI] [Google Scholar]
- 197.Bhana S, Lin G, Wang L, et al. Near-infrared-absorbing gold nanopopcorns with iron oxide cluster core for magnetically amplified photothermal and photodynamic cancer therapy. ACS Appl Mater Interfaces. 2015;7:11637–11647. doi: 10.1021/acsami.5b02741. [DOI] [PubMed] [Google Scholar]
- 198.Abadeer NS, Murphy CJ. Recent progress in cancer thermal therapy using gold nanoparticles. J Phys Chem C. 2016;120:4691–4716. doi: 10.1021/acs.jpcc.5b11232. [DOI] [Google Scholar]
- 199.Kirui Dickson K, Rey Diego A, Batt Carl A. Gold hybrid nanoparticles for targeted phototherapy and cancer imaging. Nanotechnology. 2010;21(10):105105. doi: 10.1088/0957-4484/21/10/105105. [DOI] [PubMed] [Google Scholar]
- 200.Lal S, Clare SE, Halas NJ. Photothermal therapy : impending clinical impact. Acc Chem Res. 2008;41:1842–1851. doi: 10.1021/ar800150g. [DOI] [PubMed] [Google Scholar]
- 201.Sotiriou GA, Starsich F, Dasargyri A, et al. Photothermal killing of cancer cells by the controlled plasmonic coupling of silica-coated Au/Fe2O3 nanoaggregates. Adv Funct Mater. 2014;24:2818–2827. doi: 10.1002/adfm.201303416. [DOI] [Google Scholar]
- 202.Cho S-J, Jarrett BR, Louie AY, Kauzlarich SM. Gold-coated iron nanoparticles: a novel magnetic resonance agent for T1 and T2 weighted imaging. Nanotechnology. 2006;17:640–644. doi: 10.1088/0957-4484/17/3/004. [DOI] [Google Scholar]
- 203.Cheng L, Yang K, Li Y, et al. Multifunctional nanoparticles for upconversion luminescence/MR multimodal imaging and magnetically targeted photothermal therapy. Biomaterials. 2012;33:2215–2222. doi: 10.1016/j.biomaterials.2011.11.069. [DOI] [PubMed] [Google Scholar]
- 204.Rajkumar S, Prabaharan M. Multi-functional core-shell Fe3O4@ Au nanoparticles for cancer diagnosis and therapy. Colloids Surf B Biointerfaces. 2019;174:252–259. doi: 10.1016/j.colsurfb.2018.11.004. [DOI] [PubMed] [Google Scholar]
- 205.Izadiyan Z, Shameli K, Miyake M, et al. Green fabrication of biologically active magnetic core-shell Fe3O4/Au nanoparticles and their potential anticancer effect. Mater Sci Eng C. 2019;96:51–57. doi: 10.1016/j.msec.2018.11.008. [DOI] [PubMed] [Google Scholar]
- 206.Kang N, Xu D, Han Y, et al. Magnetic targeting core/shell Fe3O4/Au nanoparticles for magnetic resonance/photoacoustic dual-modal imaging. Mater Sci Eng C. 2019;98:545–549. doi: 10.1016/j.msec.2019.01.013. [DOI] [PubMed] [Google Scholar]
- 207.Searle PF, Chen MJ, Hu L, et al. Nitroreductase: a prodrug-activating enzyme for cancer gene therapy. Clin Exp Pharmacol Physiol. 2004;31:811–816. doi: 10.1111/j.1440-1681.2004.04085.x. [DOI] [PubMed] [Google Scholar]
- 208.Patel P, Young JG, Mautner V, et al. A phase I/II clinical trial in localized prostate cancer of an adenovirus expressing nitroreductase with CB1984. Mol Ther. 2009;17:1292–1299. doi: 10.1038/mt.2009.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Palmer DH, Mautner V, Mirza D, et al. Virus-directed enzyme prodrug therapy: intratumoral administration of a replication-deficient adenovirus encoding nitroreductase to patients with resectable liver cancer. J Clin Oncol. 2004;22:1546–1552. doi: 10.1200/JCO.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 210.Bagshawe KD, et al. Antibody-directed enzyme prodrug therapy professor. In: Valentino S, Borchardt R, Hageman M, et al., editors. Prodrugs: challenges and rewards. New York: Springer; 2005. pp. 526–536. [Google Scholar]
- 211.Sharma SK, Chester KA, Bagshawe KD. Antibody-directed enzyme prodrug therapy (ADEPT) Handb Ther Antibodies Second Ed. 2014;1(4):475–486. [Google Scholar]
- 212.Gwenin CD, Kalaji M, Williams PA, Jones RM. The orientationally controlled assembly of genetically modified enzymes in an amperometric biosensor. Biosens Bioelectron. 2007;22:2869–2875. doi: 10.1016/j.bios.2006.12.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable