Abstract
Purpose
To report two cases of neurotrophic keratitis (NK) after micropulse transscleral cyclophotocoagulation (MP-TCP).
Observations
Two patients with predisposing factors for decreased corneal sensation developed NK 1 month after MP-TCP. Both patients did not heal with initial treatment with topical antibiotic and preservative free artificial tears. One patient required use of a bandage contact lens and the other patient required tarsorrhaphy. Both eyes experienced recurrence of NK.
Conclusions and importance
NK can be triggered after MP-TCP in patients with underlying predisposing factors for decreased corneal sensation. This uncommon but vision-threatening complication should be discussed preoperatively with high-risk patients as a possible adverse event after MP-TCP and followed closely postoperatively.
Keywords: Glaucoma, Lasers solid-state, Micropulse transscleral cyclophotocoagulation, Neurotrophic keratitis
1. Introduction
Micropulse transscleral cyclophotocoagulation (MP-TCP) has been recently incorporated in the range of options for treating glaucoma after failure of medical treatment. With micropulse technology the diode laser is delivered to the pars plana in a series of repetitive short pulses of laser energy separated by rest periods. During the “ON period” the laser energy is absorbed preferentially by the targeted pigmented tissue, eventually reaching the coagulative threshold. While in the “OFF period” the adjacent non-pigmented tissues are able to “cool off,” thereby minimizing collateral tissue damage.1,2 The customized probe contacts the sclera and moves in a continuous painting fashion rather than individual burns.
MP-TCP has been reported to have similar success rates compared to the traditional transscleral cyclophotocoagulation but with lower incidence of complications.1 Although it has been suggested to have a safer profile in comparison with traditional transcleral cyclophotocoagulation (TCP), the following complications have been described with MP-TCP: hypotony, intraocular pressure (IOP) spike, prolonged anterior chamber inflammation, macular edema, corneal edema, phtisis bulbi, and transient hyphema in cases with neovascular glaucoma.1, 2, 3, 4, 5, 6 The aim of this case report is to present two cases of severe neurotrophic keratopathy (NK) after MP-TCP. To our knowledge, these post-MP-TCP complications have not been previously described in the literature.
2. Findings
2.1. Case 1
Patient is a 79-year old Asian female, with history of poorly controlled diabetes. Ocular history includes mixed mechanism glaucoma status post laser peripheral iridotomy (LPI) in both eyes, proliferative diabetic retinopathy with macular edema status post multiple intravitreal bevacizumab injections in both eyes, recurrent vitreous hemorrhage status post pars plana vitrectomy, endolaser in both eyes, lensectomy in right eye, and cataract surgery in left eye. Diabetic retinopathy has been quiescent since 2009. Patient was on topical travoprost, dorzolamide, timolol, and brimonidine in both eyes for her glaucoma. Best corrected visual acuity was hand motion in the right eye and 20/40 in the left eye preoperatively. IOP was 24 mmHg on right and 17 mmHg on left. Slit lamp examination was notable for clear cornea in both eyes, aphakia in the right eye, and pseudophakia in the left eye. Optic nerve examination showed enlarged cup disc ratio with inferior rim thinning and significant progression on the OCT. Visual field was unreliable in the right eye due to poor central vision secondary to macular edema. Given uncontrolled intraocular pressure, limited visual potential, and patient's desire to reduce eye drop burden, we elected to proceed with MP-TCP in the right eye.
2.2. Case 2
Patient is a 79-year old Hispanic female with well-controlled hypertension and diabetes mellitus without retinopathy. Ocular history includes cataract surgery in both eyes, herpetic keratitis status post penetrating keratoplasty in left eye, and advanced chronic angle closure glaucoma in both eyes status post Ahmed valve implantation in both eyes with subconjunctival adjunctive mitomycin-C over the plate at postoperative week one and month one.7 Preoperative best corrected visual acuity was 20/40 right eye and 20/150 left eye. Examination was notable for superficial punctate keratitis in the right eye and clear and compact corneal transplant in the left eye. Posterior examination was notable for severe glaucomatous cupping with 0.9 cup-to-disc ratio both eyes. Her IOP right eye was above goal in the high teens (16–22 mmHg) despite being on maximum tolerated medical therapy with timolol, dorzolamide, and brimonidine. The decision was made to proceed with MP-TCP in the right eye to better control the IOP with faster visual recovery in this functionally monocular patient with advanced glaucoma.
2.3. Surgical procedure
After general anesthesia was conducted, the G6 micropulse probe (IRIDEX Inc., Mountain View, CA) was placed at the limbus with the notch facing the limbus and the probe perpendicular to the surface of the globe. Laser settings were 2000 mW with a duty cycle of 31.33%. One hundred eighty degrees were treated by moving the probe in a continuous painting fashion, over 80 seconds. The other hemifield was then treated using the same procedure to achieve 360° of treatment. Care was taken to avoid the 3 and 9 o'clock position. During the postoperative period, prednisolone 1% was given 4 times a day with a weekly taper over the first month.
3. Postoperative period
3.1. Case 1
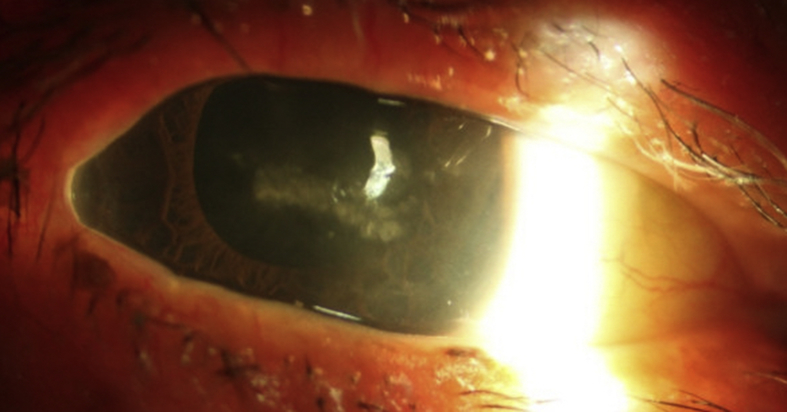
At postoperative week 1, vision was counting fingers with an IOP of 8 mmHg. Examination showed intact cornea epithelium without any epithelial defect. At the 1-month postoperative visit, visual acuity remained stable at counting fingers and IOP was 10 mmHg on the same preoperative glaucoma medications. Slit lamp examination of the right eye showed mild conjunctival injection and a large epithelial defect measuring 2 mm vertical x 6 mm horizontal (Fig. 1) in the interpalpebral region with heaped epithelial margins. Patient reported mild discomfort but was otherwise asymptomatic. Corneal sensation was reduced in all quadrants and no lagophthalmos was noted. The patient was treated with Trimethoprim/polymyxin B eyedrops and preservative free artificial tears plus lubricant ointment at night. Prednisolone was discontinued. Two weeks later the corneal epithelial defect persisted without improvement, thus a bandage contact lens was applied. The corneal epithelial defect healed after two weeks of therapeutic bandage contact lens wear (postoperative month 2).
Fig. 1.
Slit lamp photography of the right eye of Patient 1 depicting a large epithelial defect stained with fluorescein in the interpalpebral zone with rolled epithelial margins.
The patient subsequently developed a recurrent epithelial defect at postoperative month 3 and 5 that responded to topical antibiotic, lubrication, and bandage contact lens. She was given proper instruction on eye drop administration and monitored frequently. At postoperative month 7 her epithelium was intact and the IOP was 19 mmHg in the right eye with travatan at night and timolol 0.5% twice a day.
3.2. Case 2
At postoperative week 1, the IOP in the right eye was 6 mmHg and all glaucoma medications were discontinued. Atropine 1% was added for shallow peripheral choroidal effusions.
At postoperative week 3, the patient presented with painless corneal epithelial defect measuring 4.5 mm vertical x 7 mm horizontal with rolled edges. Trimethoprim/polymyxin B eye drops were added along with preservative free artificial tears. One week later, the corneal epithelial defect persisted without improvement, thus a bandage contact lens was placed. At postoperative week 5, the corneal epithelial defect remained unchanged without improvement despite medical therapy. Corneal sensation again was reduced in all quadrants suggesting NK. Culture was obtained and was negative for infection. Given the lack of improvement, the epithelial edges were scraped to promote healing and a temporary lateral tarsorrhaphy was placed. The corneal epithelial defect healed within 2 weeks.
At postoperative month 3.5, the patient developed a recurrence of the epithelial defect, measuring 3 mm vertical x 5 mm horizontal. A temporary lateral tarsorrhaphy was again performed.
At postoperative month 6, best corrected visual acuity of the right eye was 20/70 and IOP was 9 mmHg without medication. Unfortunately, the corneal epithelial defect recurred despite a lateral tarsorrhaphy at the area where the cornea was exposed. A contact bandage lens was added and 1 week later the epithelial defect was healed with epithelial irregularity in the zone of the previous defect (Fig. 2). IOP in the right eye was 11 mmHg without glaucoma medications.
Fig. 2.
Right eye slit lamp photo of temporal tarsorraphy with central corneal epithelium irregularity in the zone of the recurrent epithelial defect due to neurotrophic keratitis.
4. Discussion
MP-TCP is an alternative form of cyclodestructive procedure in the treatment of uncontrolled glaucoma. The delivery of diode laser is fragmented into pulses, separated by rest periods, to minimize collateral tissue damage.1,2 Compared to traditional TCP, MP-TCP has a better safety profile with lower complication rates and can be used in eyes with better visual potential. Corneal complication is uncommon with two cases of corneal edema reported by Williams et al.3 Here we present two patients who developed NK after MP-TCP.
NK is characterized by reduced corneal sensitivity, spontaneous epithelial breakdown, and impairment of corneal healing despite frequent lubrication.8 NK is often difficult to treat with a high rate of recurrence of epithelial breakdown and may predispose patients to the development of infectious corneal ulcers. Both patients presented in our report had risk factors for decreased corneal sensation for many years but never developed NK prior to the MP-TCP treatment. Another potential confounder for the NK in patient 2 was the adjunctive mitomycin-C following the Ahmed valve implantation. However, we routinely perform adjunctive mitomycin-C following Ahmed valve at our institution and we have not seen a case of NK as reported previously.7 The temporal relationship of the events is highly suggestive that MP-TCP was the inciting factor for the development of NK.
NK has been described previously after traditional TCP, but not with MP-TCP.8,9 All cases described in the literature had predisposing conditions for decreased corneal innervation. The risk factors described among the published cases after traditional TCP were multiple prior ocular surgeries with corneal incisions, poorly controlled diabetes with neovascular glaucoma, chronic use of topical beta blockers, and corneal dystrophies.8,9
The pathophysiology behind the development of NK after traditional TCP was thought to be related to the emitted diode laser damaging the long ciliary nerves, thus it is important for the surgeon to avoid ablating the 3 and 9 o'clock position where the long ciliary nerves innervate the anterior segment. The ciliary nerves are responsible for sensation and play a critical role in the blink reflex, tear production and secretion, as well as the integrity and function of the corneal epithelium. The neuropeptides are especially important in epithelial wound healing.10 The thermal effect has been shown previously with traditional TCP, in which the continuous application of the diode laser causes coagulative necrosis of the pigmented cells and adjacent tissue.11 However, there are no histologic studies evaluating corneal nerves after TCP, although Raivio et al.12 showed no difference in the corneal sensation and corneal nerves density after traditional TCP in eyes without predisposing conditions that affects the corneal sensory nerves. Future study can be considered to evaluate the corneal nerves density before and after MP-TCP using confocoal microscopy.
Despite avoiding the 3 and 9 o'clock position during the MP-TCP treatment, two patients still developed NK after MP-TCP. We hypothesized that this may be due to thermal damage to the perilimbal nerve plexus surrounding the corneal limbus.13 The custom probe for the MP-TCP differs from the traditional TCP in that it has a curved contact point rather than a wedged contact tip, and may lead to inadvertent ablation of the perilimbal nerve plexus during the treatment with the probe being placed too anteriorly towards the cornea. Given this concern, we advise to avoid any overlapping of the laser probe over the corneal limbus to avoid the possibility of direct nerve damage. Unlike traditional TCP where energy duration and amount are titrated based on pigmentation, the laser energy for MP-TCP is standardized at 2000mW except for the treatment duration which may vary among providers. Several treatment durations have been described for MP-TCP, ranging from 100 to 360 seconds.2,3,5 Both of our patients received 160 seconds of MP-TCP, which is on the shorter duration. Thus, the NK from our report is unlikely to be a complication of overtreatment.
Regarding the time of onset of the NK after traditional TCP, Johnson SM.9 has described two cases in which the corneal epithelial defect was detected at postoperative month one. Fernández-Vega González Á et al.9 reported five cases of NK after traditional TCP in which the mean time for diagnosing the epithelial defect was at 22 days (range: 10–35 days). Classically, the neurotrophic ulcer does not present with pain, so the onset may be difficult to determine. Our patients had a similar presentation and onset time of these prior NK case reports described in the literature after traditional TCP. Both patients presented during their regular follow up at 3 or 4 weeks after the MP-TCP with asymptomatic large corneal epithelial defect. Given the improved safety profile compared to traditional TPC,1 MP-TCP has been utilized in eyes with better visual potential.14 Thus, it is important to identify at risk patients, particularly those with conditions that alter corneal sensory nerves,8 and to counsel them of the potential risk of developing postoperative NK and to follow closely in the initial postoperative period.
Treatment for NK has a step-ladder approach depending on the stage and severity of the disease.8,10 As presented in our patients, initial treatment includes preservative free artificial tears, topical antibiotics to prevent infection, and bandage contact lens to improve epithelialization. Nevertheless, many patients needed additional treatment such as debridement of the rolled edges of the epithelial defect, autologous serum eye drops, amniotic membrane, tarsorrhaphy, and others.8,10 Average healing time for epithelial defects is around 36 days with high recurrence rate.9
5. Conclusions
NK is a rare but serious complication that can develop after MP-TCP in patients with predisposing factors for decreased corneal sensation. The treatment of NK can be difficult and carries a high rate of recurrence. The risk of developing NK should be discussed preoperatively with high-risk patients and patients should be monitored closely postoperatively. When performing the laser, care should be taken to avoid treating the 3 and 9 o'clock position, and consideration should avoid placing the probe too anteriorly to the limbus. Future studies should evaluate prospectively the utility of measuring corneal sensation, quantifying corneal nerves using confocal microscopy before and after MP-TCP, and determining optimum probe placement.
Patient consent
Personal identifying information has been removed from this report because consent to publish such information was not obtained.
Acknowledgements and disclosures
Funding
None.
Conflict of interest
The authors have no financial disclosures related to the topic.
Authorship
All authors attest that they meet the current ICMJE criteria for Authorship.
Acknowledgements
Dr. Claudio Perez acknowledges the Pan-American Ophthalmological Foundation and the Retina Research Foundation for funding through the Gillingham Pan-American Fellowship.
This work was made possible in part, by NIH-NEI EY002162 - Core Grant for Vision Research and by the Research to Prevent Blindness Unrestricted Grant.
Footnotes
Supplementary data to this chapter can be found online at https://doi.org/10.1016/j.ajoc.2019.100469.
Appendix A. Supplementary data
The following is the supplementary data to this article:
References
- 1.Aquino M.C., Barton K., Tan A.M. Micropulse versus continuous wave transscleral diode cyclophotocoagulation in refractory glaucoma: a randomized exploratory study. Clin Exp Ophthalmol. 2015;43(1):40–46. doi: 10.1111/ceo.12360. [DOI] [PubMed] [Google Scholar]
- 2.Tan A.M., Chockalingam M., Aquino M.C. Micropulse transscleral diode laser cyclophotocoagulation in the treatment of refractory glaucoma. Clin Exp Ophthalmol. 2010;38(3):266–272. doi: 10.1111/j.1442-9071.2010.02238.x. [DOI] [PubMed] [Google Scholar]
- 3.Williams A.L., Moster M.R., Rahmatnejad K. Clinical efficacy and safety profile of micropulse transscleral cyclophotocoagulation in refractory glaucoma. J Glaucoma. 2018;27(5):445–449. doi: 10.1097/IJG.0000000000000934. [DOI] [PubMed] [Google Scholar]
- 4.Emanuel M.E., Grover D.S., Fellman R.L. Micropulse cyclophotocoagulation: initial results in refractory glaucoma. J Glaucoma. 2017;26(8):726–729. doi: 10.1097/IJG.0000000000000715. [DOI] [PubMed] [Google Scholar]
- 5.Lee J.H., Shi Y., Amoozgar B. Outcome of micropulse laser transscleral cyclophotocoagulation on pediatric versus adult glaucoma patients. J Glaucoma. 2017;26(10):936–939. doi: 10.1097/IJG.0000000000000757. [DOI] [PubMed] [Google Scholar]
- 6.Kuchar S., Moster M.R., Reamer C.B. Treatment outcomes of micropulse transcleral cyclophotocoagulation in advanced glaucoma. Lasers Med Sci. 2016;31(2):393–396. doi: 10.1007/s10103-015-1856-9. [DOI] [PubMed] [Google Scholar]
- 7.Alvarado J.A., Hollander D.A., Juster R.P., Lee L.C. Ahmed valve implantation with adjunctive mitomycin C and 5-fluorouracil: long-term outcomes. Am J Ophthalmol. 2008 Aug;146(2):276–284. doi: 10.1016/j.ajo.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 8.Johnson S.M. Neurotrophic corneal defects after diode laser cycloablation. Am J Ophthalmol. 1998;126(5):725–727. doi: 10.1016/s0002-9394(98)00175-5. [DOI] [PubMed] [Google Scholar]
- 9.Fernández-Vega González Á., Barraquer Compte R.I., Cárcamo Martínez A.L. Neurotrophic keratitis after transscleral diode laser cyclophotocoagulation. Arch Soc Esp Oftalmol. 2016;91(7):320–326. doi: 10.1016/j.oftal.2015.12.001. [DOI] [PubMed] [Google Scholar]
- 10.Dua H.S., Messmer E.M., Rolando M. Neuropathic keratopathy. Prog Retin Eye Res. 2018 Sep;66:107–131. doi: 10.1016/j.preteyeres.2018.04.003. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 11.Lin S.C., Chen M.J., Lin M.S. Vascular effects on ciliary tissue from endoscopic versus scleral cyclophotocoagulation. Br J Ophthalmol. 2006;90(4):496–500. doi: 10.1136/bjo.2005.072777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Raivio V.E., Vesaluoma M.H., Tervo T.M. Corneal innervation, corneal mechanical sensitivity, and tear fluid secretion after transscleral contact 670-nm diode laser cyclophotocoagulation. J Glaucoma. 2002;11(5):446–453. doi: 10.1097/00061198-200210000-00014. [DOI] [PubMed] [Google Scholar]
- 13.Shaheen B.S., Bakir M., Jain S. Corneal nerves in health and disease. Surv Ophthalmol. 2014 May-Jun;59(3):263–285. doi: 10.1016/j.survophthal.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Masis Solano M., Huang G., Lin S.C. When should we give up filtration surgery: indications, techniques and results of cyclodestruction. Dev Ophthalmol. 2017;59:179–190. doi: 10.1159/000458496. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.