1. Introduction
Drug-induced liver injury (DILI), defined as liver injury caused by a drug and/or its metabolites, is a common clinical adverse drug reaction1, 2, 3, 4. This type of injury can cause acute liver failure and even death in severe cases5. DILI accounts for many drug warnings and is an important cause of failure in the clinical development of new drugs and new forms of traditional Chinese medicine (TCM). Thus, liver injury from drugs has attracted close attention from medical professionals, the pharmaceutical industry, and the general public6, 7, 8.
For thousands of years, TCM has contributed to the prevention and treatment of disease, to the development of the Chinese nation, and to the quality of life of Chinese people. For many individuals, TCM remains an essential component of modern life. However, continuous improvements in monitoring adverse drug reactions/events (ADRs/ADEs), along with increased TCM use worldwide has frequently identified TCM-associated safety issues such as DILI9, 10. This situation imposes serious obstacles to the research and development of new, safe TCM drugs and the viability of TCM industries.
Due to the lack of specific diagnostic indicators, an exclusion diagnosis of DILI has been commonly adopted, but this approach has shown a high rate of misdiagnosis. TCM DILI is often overlooked because of the pharmaceutical complexity of TCM, the limited basic information on the quantities of TCM components, as well as the increasingly common use of drug combinations. The causal relationship between liver injury and TCM is also difficult to clarify. In addition, potential TCM toxicity is often overlooked by the public since TCM preparations are commonly regarded as “natural, without toxic and side effects”. For these reasons and because developers and enterprises have paid insufficient attention to ADRs, the prevention and control of TCM safety risks is extremely difficult11. Therefore, the establishment of an objective and scientific technical system for assessing and identifying TCM DILI is needed.
To this end, with strengthening the risk management of the entire drug life cycle as the main goal, the China Food and Drug Administration (CFDA)of China organized experts in related fields to develop the Guidance for Clinical Evaluation of Traditional Chinese Medicine-Induced Liver Injury. This task was accomplished by integrating the consensus of Chinese and international experts with research progress in the fields of medicine and pharmacy as well as in clinical and scientific research. This document aims to provide advice and assistance to relevant institutions and personnel in identifying the risk signals of TCM DILI; scientifically assess the causal relationship between patients׳ liver injury and TCM; effectively reduce misdiagnosis; fully evaluate the safety, risks and benefits of relevant TCM; and purposefully develop prevention and control measures for TCM DILI. These activities are needed to reduce the failure rate of new TCM drug research and development (R&D) and the risk of clinical use as well as to promote a viable TCM industry in China.
This guide is designed for the evaluation of DILI and for risk control throughout the entire life cycle of TCM products, including the research and development stages of new drugs and post-marketing stage. It should also be used for TCM R&D, as well as by medical and regulatory agencies. Each drug marketing authority must carry the main responsibility for the product and not only strengthen the management of the entire lifecycle of the product but also take realistic and effective risk control measures to ensure the safety of public drug use.
The TCMs (including ethnomedicines) in this guide consist of the TCM preparations that are being studied and those already on the market. This guide can be used as a reference for clinically used TCM decoctions and tablets, formulated TCM granules, TCM extract, homemade herbal medicines, TCM-containing health products and TCM health foods as well as relevant excipients.
Many problems that urgently require in-depth study and solutions still exist in the field of TCM DILI. This guidance will be continuously revised and improved in accordance with research progress on TCM DILI and regulatory requirements.
2. Definition and epidemiological overview of TCM DILI
2.1. Definition of TCM DILI
TCM DILI refers to the liver injury caused by TCM itself and/or the metabolites of its components. TCM DILI belongs within the DILI category and is a common clinical adverse reaction to TCM1, 12.
TCM DILI can be divided into two types: an intrinsic type and an idiosyncratic type. In general, the intrinsic type of liver injury is closely related to drug dosage and associated factors, such as the duration of treatment, etc., does not depend significantly on individual differences, and follows a predictable course of illness. The idiosyncratic type of liver injury often does not show an obvious dependence with drug dosage or the duration of treatment, etc.; instead, this type of injury is closely associated with immunity, metabolism, heredity and other body factors. Individual differences are large for the idiosyncratic type of injury, and the incidence is often difficult to predict based on dosage and pharmacological action type.
2.2. The epidemiological overview of TCM DILI
According to epidemiological data published outside China, the incidence of DILI in the general population is estimated to be 1–20/100,00013, 14. At present, the exact incidence of TCM DILI in China and abroad is still unclear. Current estimates of TCM DILI mainly rely upon the composition ratio of TCM DILI versus the total DILI although the statistical data vary significantly among different countries and regions15, 16, 17, 18, 19. Research data from the US Drug-Induced Liver Injury Network (DILIN) estimate that liver injury caused by herbs and dietary supplements has increased rapidly, with the percentage of liver injuries involving these substances rising dramatically from 7% in 2005 to 19% in 201220. Data from the Asia-Pacific region suggest that Chinese herbal medicine was the main cause of DILI in Korea and Singapore21. Single- and multi-center retrospective clinical studies in China with large samples showed that the composition ratio of TCM DILI to the total DILI was approximately 20%22. At present, multi-center, large-scale, prospective epidemiological studies have not been performed.
To scientifically judge the overall status and trends of TCM DILI, a stratified comparison method is needed to analyze the composition ratio of Chinese and Western medicines associated with DILI. That is, a primary classification categorizes drugs that cause liver injury into TCMs, synthetic drugs and biological preparations. A secondary classification categorizes and compares TCMs, synthetic drugs and biological preparations according to their efficacy or indications. For example, TCMs can be divided into heat clearing, toxin relieving, blood circulation promoting and blood stasis removing, etc. Correspondingly, synthetic drugs can be divided into antibiotics and antitumor drugs, among others. A tertiary classification compares a specific TCM product, synthetic drug and biological preparations. For the classification of TCM prescriptions, please refer to the Pharmacopoeia of the People׳s Republic of China, clinical medication instructions23.
3. Main risk factors for TCM DILI
The risk factors for TCM DILI are relatively complex and should be analyzed from the perspectives of drugs, body and their interactions. In particular, for idiosyncratic liver injuries, the influence of immunity, metabolism, heredity and other body factors should be considered to more purposefully obtain information regarding liver injury risk factors. When evaluating TCM DILI, interference factors, such as poor drug quality and medication errors, should be excluded.
3.1. Drug factor
3.1.1. Sources and quality of Chinese herbal medicines, decoctions, tablets and excipients
Using the same name for different materials and mixing with impurities as well as improper formulation and processing are often the important interference factors that affect the assessment of TCM DILI. When evaluating the risk factors for TCM DILI, comprehensive inspections should be conducted on Chinese materia medica origin, place of origin, medicinal parts, harvesting time, processing and formulation method, and exogenous contaminants, such as impurities, agricultural and farm chemicals and heavy metal residues; in particular, microbial toxins should be controlled strictly. In addition, the influence of excipients involved in the TCM production process should be considered as well; such substances include concoction and preparation excipients as well as packaging materials and containers that have direct contact with the drug.
Biological assays are recommended on the basis of routine quality inspection when examining the quality and safety of TCMs. In particular, biological (toxic) potency, biomarkers, etc., should be used to perform quality evaluation and control. Molecular genetic markers can be used to identify the origin of confusing TCMs. For related methods, please refer to Chinese Herbal Medicine Quality Evaluation Methodology Guidance issued by the China Association of Chinese Medicine24.
3.1.2. Liver-injury-related risk substances
TCM DILI-related risk substances include both the prototype compounds of TCMs and the metabolites produced inside the body. At present, multiple prototype compounds and metabolites of TCMs that cause liver injury have been identified25, such as triptolide from Tripterygium wilfordii (Tripterygium wilfordii Hook f.) and other diterpenoids, as well as monocrotaline from Gynura japonica (Gynura japonica (Thunb.) Juel) and other pyrrolizidine alkaloids26. If the prescription contains potential liver-damaging TCMs or relevant compounds, an evaluation of risk-benefit balance is recommended.
3.1.3. Rationality of prescription
For liver injury caused by the compound prescription of TCMs, the rationality of the prescription should be analyzed systematically from the perspectives of theory, method, prescription and herbs by combining TCM theory with modern research data to explore the possible liver injury risk factors. From the viewpoint of safe drug use, attention must be paid with TCM prescription to incompatibility taboos, such as “mutual inhibition” and “antagonism”. Of these taboos, “eighteen incompatible medicaments” and “nineteen medicaments of mutual restraint” are important for understanding incompatibility taboos by TCM theory. In principle, it is suggested to not use TCMs that have “mutual inhibition” and “antagonism” in the development of TCM new drugs. If a prescription involves incompatibility taboos, the developer of TCM new drugs must provide the necessary research evidence to prove its rationality and safety. The adverse interactions between different TCM ingredients in a prescription can be analyzed from the aspects of chemistry and biology.
In addition, the dosage, usage, product specifications and dispensing of TCM are also important aspects of assessing the rationality of prescriptions.
3.1.4. Method of drug administration
Changes in the method of drug administration can affect the absorption, distribution and metabolism as well as the effectiveness and safety of TCMs in vivo and in vitro. Changes in the route of drug administration and dosage form may increase the safety risk, especially the change from external use to internal administration, from topical to systemic use and from oral administration to drug injection. New drug release technologies or delivery systems may also increase the risk of liver injury. If any of the above changes occurs, the developer of TCM new drugs must provide the necessary research evidence to prove the rationality and safety of the new formulation or route of administration.
3.2. Body factors
3.2.1. Individual differences
The evaluation of TCM DILI, especially idiosyncratic TCM DILI, should consider the effect of TCM on the susceptibility to liver injury of patient body factors, including immunity, heredity, metabolism, underlying diseases and body constitution type27, 28, 29. For example, immune disorders, such as abnormal immune activation or defects in immune tolerance30, may increase the susceptibility of the liver to drug toxicity, thereby inducing TCM DILI31. When taking drugs with a potential risk of liver injury, the effects of body factors, such as immunity, heredity and underlying diseases, on TCM DILI should be examined.
3.2.2. Special populations
Advanced age is not only an important DILI susceptibility factor but also an independent risk factor for chronic DILI. Children have a relatively low ability to metabolically detoxify certain drugs, which may increase their risk of liver injury32. Although no data on the risk of TCM DILI exists for pregnant women or fetuses, the safety risks of TCM use in pregnancy should be fully considered.
The following recommendations apply if the evaluated TCM could be used clinically in children or senior populations, as well as gestational and breastfeeding women, etc. Investigators should pay attention to the drug risk in special populations during the preclinical assessment and clinical trials and as part of post-marketing surveilance. Risks should be clearly stated in the product manual according to the research results to guide safe clinical medication use.
3.2.3. Patients with underlying liver disease
When liver injury occurs after TCM use in patients with underlying liver disease, caution should be taken to distinguish DILI from a recurrence of the underlying liver disease33. If the evaluated TCM may be used clinically in patients with underlying liver disease, investigators are advised to pay attention to the risk of medication use in patients with underlying liver disease during the preclinical assessment and clinical trials and after the drug goes to market. The risk should be stated in the product manual according to the research results to guide safe clinical medication use.
3.3. Clinical medication factors
For different diseases and individual patient characteristics, attention should be paid to the influence on TCM DILI of the indications, contraindications, dosage, course of treatment, and route of clinical use of TCM as well as to the drug use recommendations for special populations34, 35, 36.
3.3.1. Dosage and course of treatment
The dosage and course of treatment in TCM are closely related to drug safety. Any clinical overdose of TCM and/or drug used over an extended treatment course should be supported by safety research data. For the same patient or subject, in addition to monitoring the dose of a single-component TCM prescription, attention should be paid to the TCM dose prescribed as a combined or multiple-drug medication or for the treatment of another disease.
3.3.2. Correspondence between the prescription and the illness
A lack of correspondence between a prescription (medicine) and an illness is one of the common irrational uses of TCMs that may also be an important factor in inducing adverse TCM reactions. In clinical practice, the basic principle of determining the treatment based on the differentiation of illness and syndrome should be followed to avoid irrational drug use.
3.3.3. Combined medication
Combined or multiple-component medication includes the joint use of more than a single TCM (including proprietary TCMs and decoctions), TCM with synthetic drugs and TCM with biological preparations. In addition to combined medications based on doctors׳ prescriptions, special attention should be paid to the use of over-the-counter medicines or other health products used by patients themselves.
Improper combined medication may increase the risk of TCM DILI. The composition, efficacy and rationality of TCM prescriptions cannot be viewed in isolation when investigating and analyzing TCM DILI and other adverse reactions. The interactions between different treatment methods and different drugs in the treatment practice should also be considered. The risk of adverse reactions to TCM may increase when the composition, efficacy or indications of two or more drugs are similar.
At present, some compounded preparations of Chinese and Western medicines exist in China. Whether a medicine contains specific synthetic drug ingredients is often difficult to judge from the name of the medicine alone. In such cases, synthetic drug ingredients may have been added to TCM preparations. Such compounding, whether inadvertent or not may be illegal and dangerous. This possibility should be carefully considered and excluded when analyzing the TCM DILI risk factors.
3.4. Other factors
Improper dietary or environmental factors may cause liver injury or increase the risk of certain TCM DILIs. Such factors, including alcohol, hair dyes, renovation pollutants or other environmental toxicants, should be carefully identified and excluded when analyzing TCM DILI risk factors.
4. Risk signals associated with TCM DILI
Risk signals of DILI are indicators associated with liver injury or dysfunction and mainly include clinical symptoms, signs, biochemical findings, histopathological characteristics, imaging changes and biomarkers37. The sources of risk signal include literature reviews, preclinical safety assessment, premarketing clinical trials, postmarketing adverse reaction monitoring and safety-related experience. Careful assembly and review of the TCM DILI risk signals can help to effect the early detection and prevention of TCM DILI and control the TCM DILI risk.
4.1. Risk signals associated with TCM DILI
4.1.1. Clinical symptoms and signs
The clinical manifestations of DILI vary in severity, and some patients may have no obvious clinical discomfort. Common clinical manifestations include fatigue, a loss of appetite, nausea, an uncomfortable oily feeling, deep yellow or brown urine, upper abdominal pain and liver discomfort, etc. These manifestations are sometimes accompanied by fever and rash, and patients with severe cases may have conditions such as coagulopathy (tar-like stool) and even coma. Patients with mild cases may have no significant signs, whereas those with severe cases may have yellow skin and sclera, a dull complexion, palmar erythema, signs of ascites and abdominal wall varicose veins38, 39.
4.1.2. Main biochemical indicators
The main indicators of DILI include alanine aminotransferase (ALT) and aspartate aminotransferase (AST) that reflect hepatocyte injuries; alkaline phosphatase (ALP) and γ-glutamyl transpeptidase (GGT) that reflect bile duct injuries; and serum total bilirubin (TBil), albumin, cholinesterase, prothrombin time (PT), prothrombin activity (PTA) and the international normalized ratio (INR) that reflect liver dysfunction1, 2, 12.
4.1.3. Pathological manifestation of liver tissue
Pathological manifestations of the liver include nonspecific pathological changes, such as hepatocyte degeneration and necrosis, inflammatory immune cell infiltration, fibrous hyperplasia, bile duct injury and vascular lesions. DILI caused by Gynura japonica, Senecio vulgaris L., etc., exhibits relatively specific hepatic histopathological features that can lead to hepatic sinusoidal obstruction syndrome (HSOS)/hepatic veno-occlusive disease (HVOD). Typical pathological manifestations include hepatic sinusoidal dilatation, hyperemia and thrombus, mainly in zone III of the hepatic lobule; hepatocyte swelling; necrosis and atrophy of liver cell plates; and subintimal fibrosis of the hepatic veins, with a thickening of the wall and a narrowing of the lumen.
4.1.4. Imaging changes
Imaging examinations, such as B-mode ultrasound, computed tomography (CT) or magnetic resonance imaging (MRI), can be helpful in assessing the risk of DILI. In patients with acute liver injury, B-mode ultrasound of liver mostly shows no significant change or only a mild enlargement. In patients with acute liver failure, the liver volume may decrease. Patients with chronic disease may have imaging findings such as cirrhosis, splenomegaly and portal hypertension. CT/MRI has great diagnostic value for HSOS/HVOD caused by Gynura japonica, etc. Hepatomegaly is apparent as map-like changes which are observable in the enhanced portal phase; visualization of hepatic veins is unclear, and ascites can be detected. Transient elastography can reflect changes in liver stiffness.
4.1.5. Biomarkers
At present, no recognized biomarker exists that can be used for the differential diagnosis of DILI, and development of such specific biomarkers are needed40, 41. Biomarkers that are extensively studied and of certain value include cytokeratin 18 (CK-18), high mobility group protein B1 (HMGB1), microRNA 122 (miR-122), glutamate dehydrogenase (GLDH), kidney injury molecule 1 (KIM-1) and colony-stimulating factor 1 (CSF-1)42, 43. Acetaminophen (APAP)–cysteine adduct (APAPA) is specific for APAP-induced liver injury and can be used for the identification of hepatotoxic components in a TCM compound preparation that is doped with APAP. However, this biomarker is inconvenient for clinical testing and is limited only to research applications at present.
4.2. Assessment of risk signals for TCM DILI
TCM DILI risk signals should be collated throughout the process of drug development, production and use. Relevant institutions and personnel, such as new drug developers and drug marketing license holders, should review the TCM DILI risk signals from the literature, preclinical safety evaluations, clinical trials and postmarketing evaluations and make full use of safety-related drug data to fully understand the potential safety risks. These data may originate from various levels of adverse drug reaction and rational drug use monitoring agencies as well as medical and scientific research institutions in China44, 45.
4.2.1. Review of TCM DILI risk information based on literature, experience and media
If a TCM is found to have the following characteristics, attention should be paid to its possible liver injury risk during preclinical safety evaluation, premarketing clinical trials and post-marketing adverse reaction monitoring.
-
(1)
TCMs and their closely related TCM varieties with the risk of liver injury documented in literature;
-
(2)
TCMs containing a structure identical or similar to those of substances with liver injury risk, as documented in the literature or a known database;
-
(3)
TCMs or herbs with liver injury risks suggested by medicinal or food experience in China and abroad; and
-
(4)
TCMs or herbs with liver injury risks suggested by media information in China and abroad.
4.2.2. Review of TCM DILI risk signals based on preclinical safety evaluation
Preclinical toxicology evaluation is an important part of assessing the risk of liver injury by TCM drugs. Preclinical safety evaluation should include studies conducted on basic toxicity, target organ toxicity, and the toxicity mechanism and pharmacokinetics (toxicokinetics). These studies should be performed according to the requirements of the International Council on Harmonization (ICH) regarding technical requirements for the registration of pharmaceuticals for human use, with particular attention to the close monitoring of liver-function-related biochemical indicators and pathological changes46, 47, 48. For the medicines that have been identified as risks for liver injury from preclinical safety evaluation, a careful review is needed of the research data regarding the substances causing the risk, the types of liver injury, the mechanism, and the relationships among the drug dosage, exposure time, toxicity and efficacy. Attention should also be paid to individual and species differences as well as to differences in the liver injury risk of different disease models in experimental animals49. For the idiosyncratic type of TCM DILI, the collection of research data on liver injury based on the disease model or the susceptibility model should be considered.
4.2.3. Assessment of TCM DILI risk signals based on clinical practice
Regardless of the premarketing clinical trial stage or the postmarketing clinical application stage, all possible information related to TCM DILI should be assembled and reviewed. In clinical practice, in addition to collecting information such as age, gender, ethnicity, physique and a history of allergy, drinking and underlying diseases, information collection in patients with liver injury should focus on relevant clinical manifestations and a detailed medication history. For details, please refer to the “Table of survey report of traditional Chinese medicine-induced liver injury” (see Supporting Information Table S1 of this guidance).
A patient medication history includes information on the type of drug used, prescription composition, usage, dosage and the course of treatment. In particular, attention should be paid to clarifying the temporal relationship between medication usage and disease onset. Detailed information on synthetic drugs, biological preparations, health products, foods, etc., that are being taken should also be collected to provide a basis for a differential diagnosis and the diagnosis of liver injury from a combined medication. The medication history is mainly based on the medications taken within 6 months but may be extended to 1 year or more if necessary. In addition, information should be collected regarding the existence of irrational drug use, such as a poor correspondence of drug to illness or TCM syndrome.
5. Clinical diagnosis of TCM DILI
TCM DILI lacks specific diagnostic indicators and is mainly an exclusion diagnosis. The injury can be identified by carefully understanding the medical history (especially the medication history) and the physical, etiological, immunological, genetic, biochemical and imaging examinations as well as the liver diseases triggered by other causes according to the following sources: Guidelines for the Diagnosis and Treatment of Drug-Induced Liver Injury issued by the China Society of Hepatology (CSH), Chinese Medical Association, and Guidelines for the Diagnosis and Management of Herb-Induced Liver Injury issued by the China Association of Chinese Medicine1, 2, 12. Liver histopathological examination is of important value in the diagnosis and differential diagnosis of DILI, especially for the differential diagnosis of autoimmune liver disease50, 51, 52. For suspicious cases potentially misdiagnosed as TCM DILI, consultations conducted by liver disease experts and clinical pharmacy experts are recommended.
5.1. Differential diagnosis
Liver diseases that need to be distinguished from DILI include the following: all types of viral hepatitis (especially sporadic hepatitis E), nonalcoholic fatty liver disease, alcoholic liver disease, autoimmune hepatitis, primary biliary cholangitis, primary sclerosing cholangitis, immunoglobulin G4 (IgG4)-related diseases and the genetic metabolic liver diseases, such as hepatolenticular degeneration (Wilson׳s disease), α-antitrypsin deficiency and hemochromatosis50. Attention needs to be paid to distinguishing cholestatic DILI from intrahepatic and extrahepatic bile duct obstruction diseases, such as gallstones, tumors, and clonorchiasis.
Other confusing diseases that need to be distinguished can include non-hepatotropic infections, such as Epstein–Barr (EB) virus, cytomegalovirus and herpes simplex virus; vascular diseases, such as Budd–Chiari syndrome and acute hypoxic necrosis of lobular central cells (hypoxic hepatitis); and hyperthyroidism. Systemic hypoxic injury in tissues and organs caused by bacterial infections and other pathogens, heart failure, hypotension or shock, vascular occlusion and pulmonary insufficiency should also be excluded. DILI should also be distinguished from poisoning caused by industrial, environmental or food toxins.
5.2. Clinical classification
The common clinical types of DILI include the hepatocyte injury type, the most common clinical type, and the cholestatic and mixed types. In clinical practice, the classification is mainly based on the clinical phenotype and serum ALT, ALP and R values, where R=(measured ALT value/ALT ULN)/(measured ALP value/ALP ULN) and ULN refers to the upper limit of normal value.
-
(1)
Hepatocellular injury type: R≥5;
-
(2)
Cholestatic injury type: R≤2; and
-
(3)
Mixed injury type: 2<R<5.
In recent years, there is a tendency toward use of a new R value (nR) which is a higher ratio calculated as the nR of "measured ALT value/ALT ULN" or "measured AST value/AST ULN".
The CSH Guidelines for the Diagnosis and Treatment of Drug-Induced Liver Injury released by the Chinese Medical Association in 2015 added the hepatic vascular injury type, a typical representative of which is the HSOS/HVOD caused by taking Gynura japonica2. The injured target cells can be the endothelial cells of hepatic sinusoids, hepatic veins and portal veins.
5.3. Clinical staging
Liver injury can be divided into acute and chronic stages according to differences in the treatment course. The onset of acute liver injury is fast, with rapid recovery of liver function; liver function can usually be restored within 6 months after onset to the pre-onset level. In chronic DILI, liver function does not return to the pre-onset level within 6 months of the injury, or the patient shows the symptoms and signs as well as the imaging and histological evidence of chronic liver injury or portal hypertension53.
5.4. Grading of severity
The CSH Guidelines for the Diagnosis and Treatment of Drug-Induced Liver Injury issued by the Chinese Medical Association and the Guidelines for the Diagnosis and Management of Herb-Induced Liver Injury issued by the China Association of Chinese Medicine are recommended references for the assessment of TCM DILI severity. A severity grading scale is shown in Table 1.
Table 1.
Grading of DILI severity.
| Grade | Severity | DefinitionS |
|---|---|---|
| 0 | No liver injury | Patients can tolerate drug exposure without a hepatotoxicity response |
| 1 | Mild liver injury | Serum ALT and/or ALP show a reversible increase, with TBil <2.5 × ULN and INR <1.5 |
| 2 | Moderate liver injury | Serum ALT and/or ALP are increased, and TBil≥2.5×ULN or INR≥1.5, even without a TBil increase |
| 3 | Severe liver injury | Serum ALT and/or ALP are increased, TBil≥5×ULN, with or without INR≥1.5 |
| 4 | Acute liver failure | Serum ALT and/or ALP are increased and TBil≥10×ULN or a daily increase of ≥17.1 μmol/L (1.0 mg/dL) and INR≥2.0 or PTA<40%. The following can occur simultaneously: ascites or hepatic encephalopathy and other organ failure associated with TCM DILI. |
| 5 | Lethal injury | Death due to TCM DILI or the need for liver transplantation to survive |
5.5. Evaluation of liver injury severity in drug clinical trials
Severe liver injury may lead to failure of new-drug clinical trials. Hy׳s Law is an important means for evaluating the prognosis of severe liver injury in drug clinical trials. Approximately 10% of the cases that meet Hy׳s law are characterized by fatal liver injury. The cases meeting Hy׳s Law should satisfy the following three conditions:
-
1.
The drug causes hepatocellular type injury, the patient׳s serum ALT (or AST) increase ≥3×ULN;
-
2.
At the same, the patient shows a serum TBil increase >2×ULN, but no evidence of biliary obstruction occurs (serum ALP increases); and
-
3.
Other causes of a simultaneous increase in serum ALT (or AST) and TBil can be excluded.
According to the experience of the US Food and Drug Administration (FDA), if a single Hy׳s law case occurs during drug clinical trials, then the risk for the occurrence of fatal liver injury necessitates high vigilance; if 2 Hy׳s law cases occur, then fatal liver injury is strongly suggested and extremely likely to occur when the drug is used in an expanded population. When Hy׳s law is used for clinical evaluation, the eDISH software, which is recommended by the US FDA, can be used to assist the judgement.
6. Systematic causality assessment for TCM DILI
Causality assessment is the key to the clinical evaluation of TCM DILI and the basis for the prevention and control of TCM DILI risk. In this guide, the assessment of causality for TCM DILI includes 3 levels: first, the relationship between liver injury and drugs; second, the relationship between liver injury and TCM; and third, the relationship between liver injury and a certain TCM product.
6.1. Methods and criteria for the causality assessment of TCM DILI
The essential need for research and development of new TCM drugs requires the implementation of standardized methodologies to identify TCM DILI. Therefore, the use of the strategies and methods listed below is recommended for evaluating causality in TCM DILI. Critical sources in development of these methodologies are the CFDA General Principles for Clinical Study on New Traditional Chinese Medicines and the Guidelines for the Diagnosis and Management of Herb-Induced Liver Injury published by the China Association of Chinese Medicine.
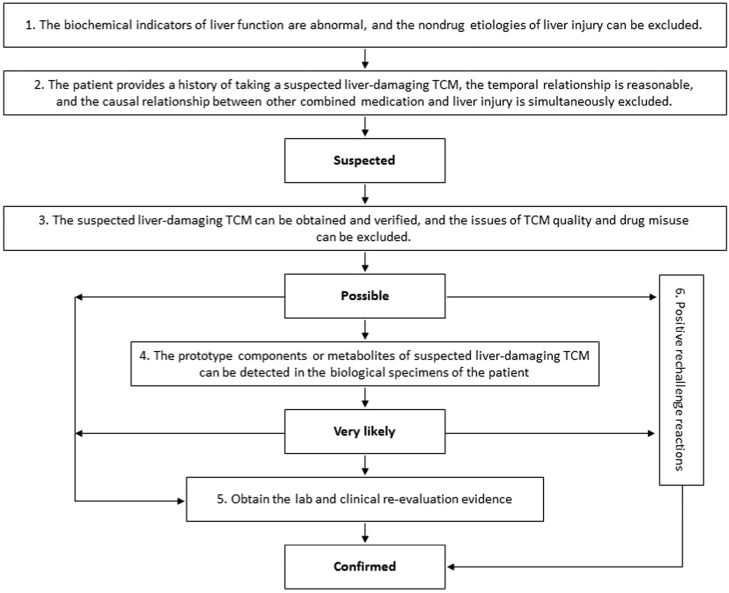
The specific process delineated below is also shown in Fig.11, 12, 54, 55, 56. The Roussel Uclaf Causality Assessment Method57, 58 (RUCAM) Scale (see the Supporting Information Table S2 of this guidance) can also be used to assess the degree of correlation between TCM and DILI.
-
1.
Liver function biochemical tests are abnormal, and the non-drug etiologies of liver injury (see the “Differential Diagnosis” section of this guidance) can be excluded. The determination of the abnormality of liver biochemical indicators is based on the biochemistry standards of DILI, that is, when any one of the following situations occurs: 1) ALT≥5×ULN; 2) ALP≥2×ULN, especially when this result is accompanied by an increase in 5′-nucleotidase or GGT, and an increase in ALP caused by bone disease is excluded; and 3) ALT≥3×ULN and TBil≥2×ULN.
Attention should be paid to 2 situations. First, not all the patients with DILI or TCM DILI have an ALT that is greater than 5×ULN. In clinical trials of new TCMs, the possibility of DILI should be considered if a patient shows an ALT≥3×ULN, especially when the increase is accompanied by a TBil increase, an INR increase and/or significant clinical symptoms; if the occurrence of abnormal liver biochemical indicators has a reasonable temporal relationship with drug administration or withdrawal; and if other causes of non-DILI can be excluded. Second, when 3×ULN≤ALT≤5×ULN and these elevations are unaccompanied by TBil and INR abnormalities and clinical manifestations such as fatigue, loss of appetite, etc.; the change in ALT level should be viewed dynamically. An “adaptive response" of the body to TCM rather than typical DILI is suggested if the ALT level returns to normal by itself.
-
2.
If a patient provides a history of taking suspected liver-damaging TCM and the temporal relationship is reasonable, the causal relationship between other combined or sequential medications and liver injury should be assessed simultaneously. Note that patients sometimes do not report all medications to doctors or researchers, especially nonprescription drugs, Chinese herbal medicines, empirical prescriptions, folk prescriptions and health products. Therefore, a patient should be carefully queried. The time span of the medication history to be investigated should range from the onset of liver injury to 6 months or more before the onset. For combined medication, not only the types of drugs and the usage and dosage require consideration but also the starting and ending date of the combined medication as well as the existence of a reasonable temporal relationship with liver injury. The use of the following table is recommended in assembling the medication history: “Table of survey report of traditional Chinese medicine-induced liver injury” (see Supporting Information Table S1 of this guidance).
-
3.
Suspected liver-damaging TCM can be identified at the same time that the TCM quality issue and drug misuse are excluded. The assessed TCM and its related data should be verified, including the remaining TCM and the names of its manufacturer and suppliers, approval document number, production batch number, product manual, etc. The quality inspection of TCMs includes origin identification and a quality compliance inspection as well as the elimination of counterfeit TCMs, contamination by exogenous harmful substances, illegal synthetic additives, drug misuse, and errors in drug prescription, formulation and administration.
-
4.
The prototype components and/or metabolites of suspected liver-damaging TCMs can be detected in patient׳s biological specimens, such as serum, urine, liver tissue or hair59. In clinical trials of new TCMs, if the suspected liver-damaging TCM is used in a clinical trial, and if confirmation that the subject has taken the evaluated TCM as instructed is possible, then the detection of the prototype components and/or metabolites in the biological specimens can be exempted.
-
5.
A variety of toxicology and histology methods, including TCM safety evaluation models and methods that are associated with clinical syndromes, are used to obtain laboratory re-evaluation evidence. Both prospective and retrospective clinical studies are used for this purpose, combined with clinical biological specimen analysis to obtain clinical re-evaluation evidence.
-
6.
A positive drug rechallenge reaction is a reliable basis for the clinical evaluation of DILI causality; however, a negative rechallenge reaction cannot be used as evidence to exclude DILI.
Figure 1.
Flowchart for evaluating causality between TCM use and liver injury60.
Based on the above six evaluation criteria, the TCM DILI causality assessment can be divided into five levels: excluded, suspected, possible, very likely and confirmed. The evaluation criteria are as follows:
-
•
Suspected: 1 + 2;
-
•
Possible: Suspected + 3;
-
•
Very likely: Possible + 4;
-
•
Confirmed: Very likely + 5 or 6; Possible + 5 or 6; and
-
•
Under the following conditions, the causality assessment level is ‘excluded’: the causal relationship of liver injury can be attributed to clear nondrug causes, the relationship between the occurrence of liver injury and the time of taking the evaluated TCM is not reasonable, and the causal relationship of liver injury with drugs can be attributed to the other drugs rather than the evaluated TCM.
6.2. Basic requirements for the evaluation report on TCM DILI causality
The evaluation report on the causal relationship of TCM DILI consists of two parts:
-
1.
Diagnostic conclusions regarding liver injury, such as diagnostic nomenclature, clinical type, disease course, severity degree, etc.
-
2.
Conclusions on the assessment of the causality between liver injury and TCM, such as the name of the liver-damaging TCM and the results of the causality assessment. The information such as the name and composition of the liver-damaging TCMs should be recorded accurately. For example, name and dosage should be recorded for Chinese herbs, TCM decoctions and tablets and formulated TCM granules. For TCM decoctions, the component herbs and their ratio (dosage) should be recorded. For Chinese patent medicines and related preparations, information regarding the product and the manufacturer should be recorded.
7. Risk prevention and control of TCM DILI61
For TCMs with a liver injury risk, clinical and laboratory re-evaluation should be conducted to confirm the liver injury risk signals and liver injury type. Re-assessment should clarify the susceptible population, risk substances, injury mechanisms and influencing factors, and weigh against TCM benefits. This re-evaluation of TCMs should be performed according to their clinical treatment value and the incidence of liver injury or the number of reported injury cases, injury degree, clinical classification and prognosis, combined with information on patient physique, purpose of treatment and alternative medicine use. In accordance with TCM premarketing and post-marketing characteristics and requirements, risk control measures are developed separately to achieve the monitoring, management and control of the TCM safety risk during the entire life cycle of the drug. These risk control measures include close observation, adjustment of treatment plans or discontinuation of medication, suspension of clinical trials, revision of instructions, drug circulation and use restrictions and drug withdrawal from market.
7.1. Main prevention and control measures for the risk of TCM DILI before marketing
The following control measures should be taken for the risk of liver injury that occurs during premarketing TCM clinical trials.
7.1.1. Close observation
Once a DILI-related risk signal appears, close observation should be performed. Initial examination should include ALT, AST, ALP, GGT, TBil, PTA and/or INR. According to the severity of DILI, the monitoring indicators and monitoring frequency (weekly, semimonthly, monthly, etc.) should be determined to continuously monitor the changes in liver biochemical indicators. If the monitored indicators do not change or the symptoms disappear after discontinuing the medication, the monitoring frequency can be reduced accordingly. Follow-up is recommended until 6 months after all abnormal indicators return to normal or reach the baseline level. The continued presence of abnormal liver biochemical indicators during long-term follow-up after drug withdrawal suggests that the injury may have developed into chronic DILI.
Studies have shown that compared with the liver injury caused by synthetic drugs, the latency of liver injury caused by TCM is longer, the injury is more hidden, and the proportion that develops into chronic liver injury is higher. For TCMs suggested to pose a risk of liver injury, whether to extend the duration of follow-up and observation during clinical trials and post-marketing evaluation is an option that should be considered.
7.1.2. Discontinuation of medication
When the health of patients or subjects is impaired, please refer to the criteria recommended by the US FDA concerning the immediate withdrawal of a drug due to liver injury in clinical trials. A drug should be stopped immediately if any of the following sets of conditions is met:
-
(1)
Serum ALT or AST>8×ULN;
-
(2)
ALT or AST>5×ULN for 2 weeks;
-
(3)
ALT or AST>3×ULN and TBil>2×ULN or INR> 1.5; and
-
(4)
ALT or AST>3×ULN, accompanied by progressively increased fatigue, nausea, vomiting, right upper abdominal pain or tenderness, fever, rash and/or eosinophilia >5%.
When the above conditions occur in clinical trials, emergency unblinding must be performed, and affected subjects should quit their clinical trial and receive treatment and follow-up. The researchers should immediately report the condition to the clinical trial applicant, the ethics committee and/or the national drug administration agency according to the drug clinical trial quality management specifications.
7.1.3. Adjustment of the research strategy, investigator manual and informed consent
The applicant, clinical researcher and ethics committee should comprehensively evaluate the drug risks and benefits according to the safety risk during the drug clinical trial period, combined with the development prospects of the new drug and the treatment status of the proposed indications. If the risk factor is controllable and the current medication risk is less than the potential benefits, then the research plan, investigator׳s manual and informed consent form can be adjusted to further reinforce the protection of subjects. For example, measures such as stricter restrictions on the subject population, dosage reduction and shortening the course of treatment can be taken to change the medication plan to minimize the known risks. Simultaneously, the investigator׳s manual and the informed consent form should be improved in a timely manner based on the exposed risk signals, and all clinical investigators and subjects who are about to participate in clinical trials should be alerted to possible risks during clinical trials. The applicant should submit the adjusted research plan, researcher׳s manual and informed consent form to the national drug evaluation agency in a timely manner for the record.
7.1.4. Suspension of clinical trials
When the degree of TCM DILI is severe and/or the frequency of its occurrence is high enough to cause severe damage to the health of the subjects, the applicant, clinical researchers, ethics committees and related institutions are advised to comprehensively assess the drug risks and benefits, to reconsider the development prospects of the new drug and the treatment status of the proposed indications. When the risk is greater than the potential benefits, the clinical trial should be suspended immediately. The national drug evaluation agency will also order the developer to immediately suspend the new-drug clinical trials based on the safety monitoring information during drug development.
7.2. Major postmarketing risk prevention and control measures for TCM DILI
After a new TCM is placed on the market, its use is more widespread and complex than that of clinical trials. Drug marketing authorization holders, drug manufacturers and drug marketing companies are advised to perform large-scale population observation and confirmation for the liver injury signals that appeared in the preclinical safety evaluation and/or in the clinical trials before marketing, according to the Measures for Reporting, Monitoring and Managing Drug Adverse Reactions (the 81st order of the Ministry of Health, China)62. For new drugs with a relatively short clinical trial cycle before marketing and an insufficient exposure of risk signals, long-term and large-scale population monitoring after marketing should be conducted to collect possible liver injury signals. These signals should be confirmed in a timely manner, and the risk should be handled promptly63. For post-marketing TCMs, the safety control of TCM quality and the guidance for reasonable clinical medication should also be strengthened. The major post-marketing TCM DILI risk prevention and control measures are as follows.
7.2.1. Avoid using outside product instruction
After new TCMs are put on the market, use of outside indications, overdose, use of outside indicated treatment course and use of outside indicated populations should be avoided. In particular, attention should be paid to the medication safety risk for special populations (pregnant women, children, elders, etc.) and the population whose age differs from that of the subjects participating in the clinical trials. In addition, care should be taken to prevent medication errors.
7.2.2. Safety-related post-marketing evaluation and research should be conducted
For TCMs without a risk of liver injury, the drug marketing authorization holder, drug producer and marketing companies should continuously monitor ADRs and report them within the regulated time frame. The drug safety supervision and management agency may adopt emphasized monitoring and spot check methods, when necessary, to fully understand the occurrence of TCM DILI and assess TCM risks and benefits. In conjunction with laboratory research, studies should be conducted on specific populations susceptible to TCM DILI, risk substances and injury mechanisms; measures to reduce the risk of TCM DILI should be developed; and the post-marketing risk management plan should be modified and improved. Relevant risk information and prevention and control measures should be shared as much as possible among the institutions that and individuals who conduct TCM research and development, production, application, marketing and supervision as well as among patients.
7.2.3. Modification of product instruction
When the benefits of the drug outweigh the risks, the most common risk management method is to revise the drug instruction by adding the information regarding the high-risk populations that are susceptible to liver injury caused by the drug, the clinical manifestations and the degree of severity. Additionally, the patients who take the drug are advised to monitor liver function (maybe regularly if needed). For drugs that can induce liver injury with confirmed evidence, a mandatory warning should be added to the drug instruction according to the incidence of liver injury or its frequency and degree of severity. Moreover, corresponding risk prevention measures should be developed, such as strengthening the safety education of health care personnel, pharmacists and patients regarding the product risks to enhance risk awareness.
7.2.4. Restriction of use
For TCMs that can induce liver injury with confirmed evidence, product risk control measures should be developed to restrict their usage (such as restricting their prescription by doctors and their usage by pharmacists) to control the risk that may arise from the use of this type of drug by medical institutions and populations. These measures should be developed according to the incidence or frequency of the injury, the degree of severity, prognosis, alternative drug availability, risk and benefits.
7.2.5. Suspension of production and sales or direct withdrawal from the market
If serious adverse drug reactions occur but the abovementioned measures cannot effectively mitigate the drug safety risks and the withdrawal of the product from the market will not significantly affect the treatment status of the relevant indication areas, the national drug administration agency may suspend its production and sales or ban the drug approval license directly.
Acknowledgments
The authors acknowledged to Zhuo Shi and Zixin Han for the primary language translation.
Footnotes
Peer review under responsibility of Institute of Materia Medica, Chinese Academy of Medical Sciences and Chinese Pharmaceutical Association.
Supplementary data associated with this article can be found in the online version at https://doi.org/10.1016/j.apsb.2018.12.003.
Contributor Information
Xiaohe Xiao, Email: pharmacy302@126.com.
Jianyuan Tang, Email: dr.jytang@sohu.com.
Appendix A. Supplementary material
Supplementary material
.
References
- 1.China Association of Chinese Medicine . China Press of Traditional Chinese Medicine; Beijing: 2016. Guideline for diagnosis and treatment of herb-induced liver injury (T/CACM 005-2016) [Google Scholar]
- 2.China Society of Hepatology . Shanghai Scientific & Technical Publishers; Shanghai: 2015. Chinese Medical Association. CSH clinical guideline: the diagnosis and management of drug-induced liver injury. [Google Scholar]
- 3.Björnsson E.S., Bergmann O.M., Björnsson H.K., Kvaran R.B., Olafsson S. Incidence, presentation, and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology. 2013;144:1419–1425. doi: 10.1053/j.gastro.2013.02.006. [DOI] [PubMed] [Google Scholar]
- 4.Miguel A., Azevedo L.F., Araújo M., Pereira A.C. Frequency of adverse drug reactions in hospitalized patients: a systematic review and meta- analysis. Pharmacoepidemiol Drug Saf. 2012;21:1139–1154. doi: 10.1002/pds.3309. [DOI] [PubMed] [Google Scholar]
- 5.Andrade R.J., Lucena M.I., Kaplowitz N., García M.B., Borraz Y., Pachkoria K. Outcome of acute idiosyncratic drug-induced liver injury: long-term follow-up in a hepatotoxicity registry. Hepatology. 2006;44:1581–1588. doi: 10.1002/hep.21424. [DOI] [PubMed] [Google Scholar]
- 6.U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER), Center for Biologics Evaluation and Research (CBER). Guidance for industry drug-induced liver injury: premarketing clinical evaluation. 2009. Available from: 〈http://www.fda.gov/downloads/Drugs/.../Guidances/UCM174090.pdf〉.
- 7.U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER), Center for Biologics Evaluation and Research (CBER). Guidance for industry good pharmacovigilance practices and pharmacoepidemiologic assessment. 2005. Available from: 〈https://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM071696.pdf〉.
- 8.U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER), Center for Biologics Evaluation and Research (CBER). Guidance for industry postmarketing studies and clinical trials-implementation of section 505(o)(3) of the Federal Food, Drug, and Cosmetic Act. 2011. Available from: 〈https://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM172001.pdf〉.
- 9.Zhang P., Ye Y.A., Yang X.Z., Jiao Y. Systematic review on Chinese herbal medicine induced liver injury. Evid Based Complement Altern Med. 2016;2016:3560812. doi: 10.1155/2016/3560812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chalasani N.P., Hayashi P.H., Bonkovsky H.L., Navarro V.J., Lee W.M., Fontana R.J. ACG clinical guideline: the diagnosis and management of idiosyncratic drug-induced liver injury. Am J Gastroenterol. 2014;109:950–967. doi: 10.1038/ajg.2014.131. [DOI] [PubMed] [Google Scholar]
- 11.Farah M.H., Edwards R., Lindquist M., Leon C., Shaw D. International monitoring of adverse health effects associated with herbal medicines. Pharmacoepidemiol Drug Saf. 2000;9:105–112. doi: 10.1002/(SICI)1099-1557(200003/04)9:2<105::AID-PDS486>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 12.Wang J.B., Zhu Y., Bai Z.F., Wang F.S., Li X.H., Xiao X.H. Guidelines for the diagnosis and management of herb-induced liver injury. Chin J Integr Med. 2018;24:696–706. doi: 10.1007/s11655-018-3000-8. [DOI] [PubMed] [Google Scholar]
- 13.Larrey D. Epidemiology and individual susceptibility to adverse drug reactions affecting the liver. Semin Liver Dis. 2002;22:145–155. doi: 10.1055/s-2002-30105. [DOI] [PubMed] [Google Scholar]
- 14.Bjornsson E.S. Epidemiology and risk factors for idiosyncratic drug-induced liver injury. Semin Liver Dis. 2014;34:115–122. doi: 10.1055/s-0034-1375953. [DOI] [PubMed] [Google Scholar]
- 15.López-Gil S., Nuño-Lámbarri N., Chávez-Tapia N., Uribe M., Barbero-Becerra V.J. Liver toxicity mechanisms of herbs commonly used in Latin America. Drug Metab Rev. 2017;49:338–356. doi: 10.1080/03602532.2017.1335750. [DOI] [PubMed] [Google Scholar]
- 16.Valdivia-Correa B., Gómez-Gutiérrez C., Uribe M., Méndez-Sánchez N. Herbal medicine in Mexico: a cause of hepatotoxicity. Int J Mol Sci. 2016;17:235. doi: 10.3390/ijms17020235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Medina-Caliz I., Garcia-Cortes M., Gonzalez-Jimenez A., Cabello M.R., Robles-Diaz M., Sanabria-Cabrera J. Herbal and dietary supplement-induced liver injuries in the Spanish DILI registry. Clin Gastroenterol Hepatol. 2018;16:1495–1502. doi: 10.1016/j.cgh.2017.12.051. [DOI] [PubMed] [Google Scholar]
- 18.Vega M., Verma M., Beswick D., Bey S., Hossack J., Merriman N. The incidence of drug- and herbal and dietary supplement-induced liver injury: preliminary findings from gastroenterologist-based surveillance in the population of the state of Delaware. Drug Saf. 2017;40:783–787. doi: 10.1007/s40264-017-0547-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jing J., Teschke R. Traditional Chinese medicine and herb-induced liver injury: comparison with drug-induced liver injury. J Clin Transl Hepatol. 2018;6:57–68. doi: 10.14218/JCTH.2017.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Navarro V.J., Khan I., Björnsson E., Seeff L.B., Serrano J., Hoofnagle J.H. Liver injury from herbal and dietary supplements. Hepatology. 2017;65:363–373. doi: 10.1002/hep.28813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cho J.H., Oh D.S., Hong S.H., Ko H., Lee N.H., Park S.E. A nationwide study of the incidence rate of herb-induced liver injury in Korea. Arch Toxicol. 2017;91:4009–4015. doi: 10.1007/s00204-017-2007-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu J.M. A multicenter survey on hospital inpatients with drug-induced acute liver injury in China. Chin J Dig. 2007;27:439–442. [Google Scholar]
- 23.Chinese Pharmacopoeia Commission . China Medical Science Press; Beijing: 2010. Guidelines for clinical use of pharmacopoeia of the People׳s Republic. [Google Scholar]
- 24.China Association of Chinese Medicine. Chinese herbal medicine quality evaluation methodology guidance. (T/CACM 001-2017). 2017.
- 25.Liu C.H., Zhu C.W. Epidemic features, major causes, and diagnostic evaluation of herb-induced liver injury. Chin J Clin Hepatol. 2017;33:829–832. [Google Scholar]
- 26.Li C.Y., Niu M., Bai Z.F., Zhang C.N., Zhao Y.L., Li R.Y. Screening for main components associated with the idiosyncratic hepatotoxicity of a tonic herb Polygonum multiflorum. Front Med. 2017;11:253–265. doi: 10.1007/s11684-017-0508-9. [DOI] [PubMed] [Google Scholar]
- 27.Bai Z.F., Gao Y., Zuo X.B., Wang J.B., Xiao X.H. Progress in research on the pathogenesis of immune regulation and idiosyncratic drug-induced liver injury. Acta Pharm Sin. 2017;52:1019–1026. [Google Scholar]
- 28.Pais R., Rusu E., Ratziu V. The impact of obesity and metabolic syndrome on chronic hepatitis B and drug-induced liver disease. Clin Liver Dis. 2014;18:165–178. doi: 10.1016/j.cld.2013.09.015. [DOI] [PubMed] [Google Scholar]
- 29.Lucena M.I., Andrade R.J., Kaplowitz N., García-Cortes M., Fernández M.C., Romero-Gomez M. Phenotypic characterization of idiosyncratic drug-induced liver injury: the influence of age and sex. Hepatology. 2009;49:2001–2009. doi: 10.1002/hep.22895. [DOI] [PubMed] [Google Scholar]
- 30.Suzman D.L., Pelosof L., Rosenberg A. Hepatotoxicity of immune checkpoint inhibitors: an evolving picture of risk associated with a vital class of immunotherapy agents. Liver Int. 2018;38:976–987. doi: 10.1111/liv.13746. [DOI] [PubMed] [Google Scholar]
- 31.He L.Z., Yin P., Meng Y.K., Tang J.F., He T.T., Niu M. Immunological synergistic mechanisms of trans-/cis-stilbene glycosides in Heshouwu related idiosyncratic liver injury. Sci Bull. 2017;62:748–751. doi: 10.1016/j.scib.2017.04.020. [DOI] [PubMed] [Google Scholar]
- 32.Amin M.D., Harpavat S., Leung D.H. Drug-induced liver injury in children. Curr Opin Pediatr. 2015;27:625–633. doi: 10.1097/MOP.0000000000000264. [DOI] [PubMed] [Google Scholar]
- 33.Chalasani N., Regev A. Drug-induced liver injury in patients with preexisting chronic liver disease in drug development: how to identify and manage? Gastroenterology. 2016;151:1046–1051. doi: 10.1053/j.gastro.2016.10.010. [DOI] [PubMed] [Google Scholar]
- 34.Qian Y., Wang X.J. People׳s Medical Publishing House; Beijing: 2008. Rational use of traditional Chinese medicine in the treatment of liver disease and common Chinese medicine related liver injury. [Google Scholar]
- 35.Wang J.B., Zhao H.P., Zhao Y.L., Jin C., Liu D.J., Kong W.J. Hepatotoxicity or hepatoprotection pattern recognition for the paradoxical effect of the Chinese herb Rheum palmatum L. in treating rat liver injury. PLoS One. 2011;6:e24498. doi: 10.1371/journal.pone.0024498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xing X.Y., Zhao Y.L., Kong W.J., WangJB, Jia L., Zhang P. Investigation of the "dose-time-response" relationships of rhubarb on carbon tetrachloride-induced liver injury in rats. J Ethnopharmacol. 2011;135:575–581. doi: 10.1016/j.jep.2011.03.053. [DOI] [PubMed] [Google Scholar]
- 37.Chen C.W., Ma H.N., Fu Q.C., Mao Y.M., Xie Q., Yu Y.C. Shanghai Science and Technology Press; Shanghai: 2013. Drug-induced and toxic liver disease. [Google Scholar]
- 38.Zhu Y., Li Y.G., Wang Y., Wang L.P., Wang J.B., Wang R.L. Analysis of clinical characteristics in 595 patients with herb-induced liver injury. Chin J Integr Med. 2016;36:44–48. [PubMed] [Google Scholar]
- 39.Zhu Y., Niu M., Chen J., Zou Z.S., Ma Z.J., Liu S.H. Comparison between Chinese herbal medicine and Western medicine-induced liver injury of 1985 patients. J Gastroenterol Hepatol. 2016;31:1476–1482. doi: 10.1111/jgh.13323. [DOI] [PubMed] [Google Scholar]
- 40.Lou W.Q., Chen C.W. Recent advances in the diagnosis and risk assessment of drug-induced liver injury. Liver. 2017;22:774–778. [Google Scholar]
- 41.Clarke J.I., Dear J.W., Antoine D.J. Recent advances in biomarkers and therapeutic interventions for hepatic drug safety—false dawn or new horizon? Exp Opin Drug Saf. 2016;15:625–634. doi: 10.1517/14740338.2016.1160057. [DOI] [PubMed] [Google Scholar]
- 42.Nicoletti P., Aithal G.P., Bjornsson E.S., Andrade R.J., Sawle A., Arrese M. Association of liver injury from specific drugs, or groups of drugs, with polymorphisms in hla and other genes in a genome-wide association study. Gastroenterology. 2017;152:1078–1089. doi: 10.1053/j.gastro.2016.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Church RJ, Kullak-Ublick GA, Aubrecht J, Bonkovsky HL, Chalasani N, Fontana RJ, et al., Candidate biomarkers for the diagnosis and prognosis of drug-induced liver injury: Hepatology 2018. Available from: 10.1002/hep.29802. [DOI] [PMC free article] [PubMed]
- 44.Zhang L., Wong L.Y., He Y., Wong I.C. Pharmacovigilance in China: current situation, successes and challenges. Drug Saf. 2014;37:765–770. doi: 10.1007/s40264-014-0222-3. [DOI] [PubMed] [Google Scholar]
- 45.Tan EH, Low EXS, Dan YY, Tai BC. Systematic review and meta-analysis of algorithms used to identify drug-induced liver injury (DILI) in health record databases. Liver Int 2017. Available from: 10.1111/liv.13646. [DOI] [PubMed]
- 46.China Food and Drug Administration. Technical guidelines for acute toxicity study of traditional Chinese medicine and natural medicine. 2007. Available from: 〈http://www.cde.org.cn/zdyz.do?Method=largePage&id=2086〉.
- 47.China Food and Drug Administration. Technical guidelines for long-term toxicity study of traditional Chinese medicine and natural medicine. 2007. Available from: 〈http://www.cde.org.cn/zdyz.do?Method=largePage&id=1498〉.
- 48.Jiang Z.Z., Wang X.Z., Sun L.X., Wang T., Zhang P.H., Huang X. Technical methods and application of traditional Chinese medicine toxicity evaluation. Prog Pharm Sci. 2013;37:545–547. [Google Scholar]
- 49.Wang J.B., Cui H.R., Bai Z.F., Xiao X.H. Precision medicine-oriented safety assessment strategy for traditional Chinese medicines: disease-syndrome-based toxicology. Acta Pharm Sin. 2016;51:1681–1688. [PubMed] [Google Scholar]
- 50.Suzuki A., Brunt E.M., Kleiner D.E., Miquel R., Smyrk T.C., Andrade R.J. The use of liver biopsy evaluation in discrimination of idiopathic autoimmune hepatitis versus drug-induced liver injury. Hepatology. 2011;54:931–939. doi: 10.1002/hep.24481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kullak-Ublick G.A., Andrade R.J., Merz M., End P., Benesic A., Gerbes A.L. Drug-induced liver injury: recent advances in diagnosis and risk assessment. Gut. 2017;66:1154–1164. doi: 10.1136/gutjnl-2016-313369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shahbaz O., Mahajan S., Lewis J.H. Highlights of drug- and herb-induced liver injury in the literature from 2016: how best to translate new information into clinical practice? Exp Opin Drug Metab Toxicol. 2017;13:935–951. doi: 10.1080/17425255.2017.1362391. [DOI] [PubMed] [Google Scholar]
- 53.Dakhoul L., Ghabril M., Chalasani N. Drug-induced chronic liver injury. J Hepatol. 2018;69:248–250. doi: 10.1016/j.jhep.2018.01.001. [DOI] [PubMed] [Google Scholar]
- 54.China Food and Drug Administration. General principles of clinical research on traditional Chinese medicine. 2015. Available from: 〈http://samr.cfda.gov.cn/WS01/CL0087/134581.html〉.
- 55.Wang J.B., Li C.Y., Zhu Y., Song H.B., Bai Z.F., Xiao X.H. Integrated evidence chain-based identification of Chinese herbal medicine-induced hepatotoxicity and rational usage: exemplification by Polygonum Multiflorum (He shou wu) Chin Sci Bull. 2016;61:971–980. [Google Scholar]
- 56.He T.T., Gomg M., Bai Y.F., Zhu Y., Wang J.B., Niu M. Clinical analysis of two diagnosis methods for herb-induced liver injury. Chin J Chin Mat Med. 2016;41:3096–3099. doi: 10.4268/cjcmm20161626. [DOI] [PubMed] [Google Scholar]
- 57.Danan G., Benichou C. Causality assessment of adverse reactions to drugs—I. A novel method based on the conclusions of international consensus meetings: application to drug induced liver injuries. J Clin Epidemiol. 1993;46:1323–1330. doi: 10.1016/0895-4356(93)90101-6. [DOI] [PubMed] [Google Scholar]
- 58.Danan G., Teschke R. RUCAM in drug and herb induced liver injury: the update. Int J Mol Sci. 2016;17:14–46. doi: 10.3390/ijms17010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang J., Ma Z., Niu M., Zhu Y., Liang Q., Zhao Y. Evidence chain-based causality identification in herb-induced liver injury: exemplification of a well-known liver-restorative herb Polygonum multiflorum. Front Med. 2015;9:457–467. doi: 10.1007/s11684-015-0417-8. [DOI] [PubMed] [Google Scholar]
- 60.Wang J.B., Zhang L., Guo Y.M., Bai Z.F., Xiao X.H. Causality assessment strategies and methods for Chinese medicines-induced liver injury. Acta Pharm Sin. 2018;53:920–928. [Google Scholar]
- 61.Xiao X.H. Pharmaceutical diseases and their risk prevention and control. Prog Pharm Sci. 2018;42:161–163. [Google Scholar]
- 62.Ministry of Health of the People’s Republic of China. Administrative measures for the reporting and monitoring of adverse drug reactions. 2011. Available from: 〈http://www.nhc.gov.cn/zwgkzt/wsbysj/201105/51770.shtml〉.
- 63.Raschi E., De Ponti F. Drug- and herb-induced liver injury: progress, current challenges and emerging signals of post-marketing risk. World J Hepatol. 2015;7:1761–1771. doi: 10.4254/wjh.v7.i13.1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material