Abstract
Wheat protein contains a large number of side chain groups, amino, hydroxyl groups and sulfydryl, which influence on the quality of Chinese noodles has not been reported. Amino and thiol groups of wheat gluten were modified by chemical reactions, and acetylated gluten (AG) and reduced gluten with anhydrous sodium sulfite (SG) were obtained. Two types of noodles were made by addition of AG and SG, and the effects of AG and SG on texture and cooking properties were investigated. With the increase of AG amount in the original flour, the sedimentation value of reconstituted flour and the tensile force of fresh and cooked noodles decreased, whereas the hardness and adhesiveness increased. The gluten index and springiness of the reconstituted flour did not vary significantly compared to those of the original flour. In addition, most of the texture and cooking quality properties of the two types of noodles decreased except the adhesiveness and tensile force of fresh noodles with a rising trend along with the increase of SG. Furthermore, the cooking yield was reduced, whereas the cooking and protein losses increased along with the elevation of modified gluten levels. Our results indicated that significant differences (p < 0.05) were present between the texture and cooking properties of Chinese noodles made by flour with AG and SG and those of unmodified samples, except for the springiness of AG noodles, and the reduction of disulfide bond was disadvantageous for the quality of noodles. Therefore, the results of the present study indicate that amino and sulfhydryl groups of wheat gluten have an important role in obtaining high-quality noodles.
Keywords: Noodle quality, Gluten, Amino acetylation, Disulfide bonds reduction
Introduction
Chinese noodles are becoming increasingly popular worldwide due to their convenience of preparation, nutritional quality, and palatability. Reportedly, at least 12% of the global wheat production is used for processing Asian noodle products (Lü et al. 2014). Gluten proteins are the main constituents of the wheat grain, and their quantity and quality have a significant contribution to the quality characteristics of Chinese noodles since the content of glutenin (39% of all gluten proteins) determines dough elasticity, hardness, and tensility; gliadin (43% of all gluten proteins) is responsible for dough viscosity and extensibility (Hu et al. 2007; Oh et al. 1985). Other 18% are non-gluten protein, mainly consisting of albumin and globulin (Osborne. 1907). The elasticity, fracture energy, and relaxation strength of noodles with a high protein content are also higher than those of noodles with a low protein level as evidenced in earlier studies on the physical and chemical properties of noodles (Shimizu et al. 1958). The surface hardness of cooked Japanese noodles was determined by the gluten strength and noodle microstructure (Dexter et al. 1979). In another study, the strength of flour gluten was found to increase after the addition of α-, β-, and γ-alcohol-soluble protein and their subunits (Khatkar et al. 2002).
The content of the amino acids glutamine and proline in gluten is approximately 35–40%, and that of glycine is 30–35% of the content of all amino acids of gluten proteins, which are the main contributors of amino groups. The content of the acylamino group is about 80–90% of the total quantity of wheat gluten amide groups contained mainly in the low-molecular-weight glutenin (LMW) and alcohol-soluble protein, in which the content of nonpolar and charged amino acids is lower (Liao et al. 2015; Grosch and Wieser 1999). The content of cysteine in gluten proteins is about 2%, most of which in the oxidized form. In the process of flour milling, free thiol groups form a network of protein intramolecular or intermolecular disulfide bonds, which is important for gluten structure formation and stabilization (Arfvidsson et al. 2004; Anjum et al. 2007). The abundant glutamic acid in high-molecular-weight glutenin subunits (HMW-GS) forms hydrogen bonds between intra- and intermolecular, thus the quantity of glutamic acid correlates with the viscoelastic property (Belton et al. 1994). It is noteworthy that the hydrogen bonds in glutenin subunits and polymers determine the level of gluten viscoelasticity (Gilbert et al. 2000).
The impact of the quantity, quality, and composition of gluten proteins on the nutritional, sensory, and storage characteristics of Chinese noodles have been studied previously (Hu et al. 2007; Huang and Morrison 1988; Lu and Zhang 2005; Liu et al. 2011). However, there is limited information on the effect of side-chain polar substituents of wheat gluten chain on the quality characteristics of Chinese noodles. Therefore, the objectives of this research were to investigate the impact of amino and thiol groups of wheat gluten on the quality properties of Chinese noodles. In addition, the optimal conditions for modification of the amino and thiol groups of wheat gluten were established.
Materials and methods
Materials and chemicals
Wheat flour (brand: high-tensile wheat flour, manufactured by Jinyuan Mianye Industry Co., Ltd., Zhengzhou, China) was obtained from the local market and stored at 4 °C during the study. Protein (N × 5.7), carbohydrates, total fat, moisture and ash contents were 12.9%, 68.36%, 1.73%, 13.8% and 0.61% for wheat flour, respectively, which were determined by the Approved Methods 46-13, 76-13.01, 30-10, 44-15A and 08-01 of American Association of Cereal Chemists (AACC 2000). Sedimentation value and gluten index were determined by Approved Methods 56-70 and 38-12, respectively (AACC 2000). All other chemicals used in the experiments were analytical grade (Beijing Chemical Works, Beijing, China).
Extraction, fractionation, and determination of gluten content
The wheat gluten proteins were separated by a standard AACC dough washing procedure (38-10) with some modifications. The wheat flour (50 g) was wrapped in a muslin cloth and washed under a stream of running water until the starch was fully washed out. The obtained wheat gluten mass was collected from the cloth, washed with running water for additional 5 min, followed by determination of the starch content using KI/I reagent. The samples without starch were frozen at − 20 °C and freeze-dried (Cool Safe TM, Scanvae, Denmark). The obtained wheat gluten was milled to an 80-mesh size for further studies. The values of wet and dry gluten, and the gluten index were determined using a Glutomatic 2200 system (Perten Instruments Co., Beijing, China) according to the guidelines provided in AACC method 38-12A (2000).
Preparation and determination of acetylated gluten
The preparation of acetylated gluten was determined according to the method described by Zhang et al. (2002) with a minor modification. Gluten was suspended by continuous stirring in 1 M sodium hydroxide (pH 8.0–8.5) to form a uniform solution containing 10% (w/v) gluten proteins. Acetic anhydride (15% gluten weight) was added during the process of magnetic stirring at a temperature of 35 °C in a water bath. Sodium hydroxide (2 M) was used to modify pH of the solution to pH 8.0–8.5. The samples were then centrifuged at 4000×g for 10 min after the reaction, and the precipitate was freeze-dried and powdered to an 80-mesh size for further study.
Aliquots of 1 mL of 0.1% ninhydrin and 1 mL of 1% gluten were mixed until homogeneity was achieved, and heated in boiling water for 5 min, then cooled to 25 °C. A volume of 100 mL distilled water was added to the mixture, and measurements were performed at a wavelength of 580 nm against a blank sample consisting of 1 mL of 0.1% ninhydrin without gluten solution. The degree of acetylation was calculated by the following formula:
Reduction and determination of disulfide bonds
Reduction and determination of disulfide bonds was determined according to the method described by Liu et al. (2015) with a minor modification. Different weight of ahydrous sodium sulfite was added to the uniform suspension containing 10% gluten. The final solutions contained a series of concentration of anhydrous sodium sulfite to gluten 3, 5, 8, 10, 15 and 20 μmol/g, respectively. The mixture was stired for 20 min, centrifuged at 6000×g for 10 min and freeze-dried, then powdered to an 80-mesh size.
The content of free thiol groups (sulfhydryl, -SH) in the noodles dough was determined according to the method described by Liu et al. (2015, 2011) with a minor modification. Freeze-dried noodles dough powder (75 mg) was suspended in 2.0 mL of 0.2 M Tris-Gly reaction buffer (pH 8.0) for 2 h, then added 4.7 g guanidine hydrochloride. After all the reagents were dissolved, the solution was diluted to a constant volume 10 mL with 0.2 M Tris-Gly. After shaking, 4 mL of the mixture of 8 M urea and 5 M guanidine hydrochloride and 50 μL of 10 mM DTNB (5,5′-Dithiobis-(2-nitrobenzoic acid))were added to 1 mL of each of the above mentioned samples, followed by shaking for 30 min at 180 rpm on the shaking table. The samples were then centrifuged at 6000 x g for 10 min, and the absorbance of the supernatant was measured at 412 nm. The content of free thiol groups was calculated with the average of the three times experiments by the following equation:
where, A412 is the absorbance at 412 nm; D is the dilution factor (sulfhydryl of factor is 5.02); C is the concentration of samples (mg/mL).
Fourier transform infrared spectroscopy (FTIR) measurement
The lyophilized gluten (original and two kinds of modified gluten) were grinded and filtered through a 80-mesh sieve. The samples about 2 mg were weighed and ground with 400 mg KBr for mixing completely. The mixture was pressed into pellets and the secondary structure of protein was measured using an FTIR spectrophotometer (IRPrestige-21, SHIMADZU, Japan). The resolution was 4 cm−1 and the scan range were set from 4000 to 400 cm−1. The data were processed using Peak Fit software (Version 4.12). Gaussian–Lorentzian second derivative was applied to resolve individual band, due to the overlap of bands. The percentages of each secondary structures were calculated by the ratio of the corresponding area to total area of amide I band.
Noodle preparation
Noodles were prepared using the method described by Wang et al. (2011), and the dough consisted of 100 g of reconstitution flour and 35% (w/w) distilled water (30 °C). The main ingredients in the reconstituted flour contain original flour 50 g, starch 42 g, modified and unmodified gluten (0 + 8, No. 0; 2 + 6, No. 1, 4 + 4, No. 2; 6 + 2, No. 3; 8 + 0, No. 4; g). Two kinds of modified gluten are acetylated gluten (AG) and reduced gluten with anhydrous sodium sulfite (SG). Therefore, these five groups of sample was defined as 0, 2%, 4%, 6% and 8% of two kinds of modified gluten, respectively. The dough was formed using a multifunction mixer (HM730, Hanshang Industrial Co., Ltd., Qingdao, China) and mixed with a slow and a fast speed for 5 min and 2 min, respectively. The prepared flocculated mixture was left undisturbed under two layers of wet gauze for 20 min. Using a noodle press (DMT-10A, Fuxing Machinery Co., Ltd., Longkou, China), the dough was sheeted twice successively between a set of rollers with 2-mm and 3.5-mm gaps. The sheet was folded and rolled through six times, each by gradually decreasing the size of roller gaps of 3.5 mm, 3 mm, 2.5 mm, 2 mm, 1.5 mm, and 1 mm. After a 20-min rest period under a four-layer gauze at room temperature, the final dough sheet was initially cut into 2-mm-wide noodles with a roller cutter and then into 44-cm-long strips, and stored at room temperature for further studies.
SDS-PAGE analysis
Reduction and determination of disulfide bonds was determined by the electrophoresis analysis. Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), according to the method described by Li et al. (2014) with a minor modification, was performed using 12% separating gel (pH 8.3) and 5% stacking gel (pH 6.8). The sample contained 8–12 μL of 6 M wheat flour water solutions, 4 M mixture of wheat gluten and modified gluten water solutions in 0.06 M Tris–HCl buffer with pH 6.8. Each sample was loaded on a 5% (v/v) polyacrylamide gel in an electrode buffer containing 0.025 M Tris, pH 8.3; 0.192 M glycine; 0.1% SDS (w/v) and mounted on a Mini 2-D (Bio-Rad, Richmond, CA, USA) vertical electrophoresis cell. Electrophoresis was performed at 40 mA for the separating gel and at 80 mA for the stacking gel. The gel staining solution consisted of 0.1% (w/v) Coomassie brilliant blue R-250, 45% (v/v) methanol, 10% (v/v) acetic acid. The destaining solution was composed of 10%(v/v) methanol, 10%(v/v) acetic acid, and 80%(v/v) deionized water.
Cooking characteristics
The optimum cooking time and the cooking loss of noodles were determined according to Approved Method 66-50 of AACCI (2000) with a minor modification. Cooking yield was determined as described by Wang et al. (2011) and Mirhosseini et al. (2015) with minor modifications. Cooking time was measured using 10 fresh noodles of 44 cm. Further, the noodles were cut off in the middle and cooked in 500 mL of boiling water. Optimum cooking time was determined by removing a piece of noodles every 15 s and pressing the cooked noodle between two of glass slides until the white hard-core line disappeared. The cooking time was the average of three individual measurements conducted for each sample.
Cooking yield and cooking loss were determined on 20 fresh noodles of 22 cm cooked in 500 mL of boiling water to the optimum cooking times determined as described above. Cooked noodles were drained for 5 min on the filter paper and were then weighed. One part of the cooking water was evaporated at 105 °C to a constant weight for calculating the cooking loss. A volume of 50 mL of cooking water was evaporated at 105 °C for 1 h, and then the content of crude protein was determined to calculate the protein loss. The determination of crude protein content is referring to Kjeldahl method. All samples were analyzed in triplicate to obtain the mean value. The cooking yield, cooking loss and protein loss were calculated by the following equations:
Texture analysis
According to the method mentioned in the reference (Guo et al. 2017), textural properties of fresh and cooked noodles were measured using a TA-XT2i Texture Analyzer (Stable Micro Systems, London, UK) under the optimal test conditions at room temperature. For TPA analysis, the instrument was calibrated using 5 kg load cell with a return trigger path of 15 mm. Every sample was composed of five strands of noodles on the test board in a parallel form, upright to the probe cutter. Pasta firmness/stickiness rig code HDP/PFS probe was used to measure and calculate tensile strength, initial distance, etc. based on texture profile analysis (TPA). The settings utilized were: pretest, test, and post-test speed of 2.0, 0.8, and 0.8 mm/s, respectively; strain, 70%; trigger type, auto-5 g; interval time, 1 s.
A tensile test was performed with another probe described by Lu et al. (2009) with a mini-modification. The tensile force and tension distance were determined by a A/SPR probe in the mode of “Measure Force in Tension”. The distance calibration was performed with a return trigger path at 50 mm. The settings were: pre-test and test speed, 2 mm/s; post-test speed, 10 mm/s; trigger distance, 120 mm; trigger type, auto-5 g. Texture measurements of the noodles were replicated six times for each group sample. Then, the average value was calculated after discarding the maximum and minimum values.
Scanning electron microscopy
According to the method described by Lu et al. (2013) with a minor modification, Chinese noodles samples were freeze-dried for 2 days. The dried noodles were fractured and the cross-section was mounted onto aluminium stubs with conductive carbon cement. The mounted samples were coated with gold under vacuum and viewed using an FEI Quanta-200 electron microscope (Eindhoven, Netherlands) at an accelerating voltage of 20 kV. The micrographs were taken using 400× magnification.
Statistical analysis
Data were expressed as mean ± SD (n ≥ 3). Analysis of variance (ANOVA) was conducted, and the significant differences among the means of three replications were evaluated by the Duncan’s multiple range test at a significance level of p < 0.05 using the SPSS system software 17.0. The figures were prepared using GraphPad Prism 5.0 software (GraphPad Software, San Diego, CA, USA).
Results and discussion
Electrophoretic profiles
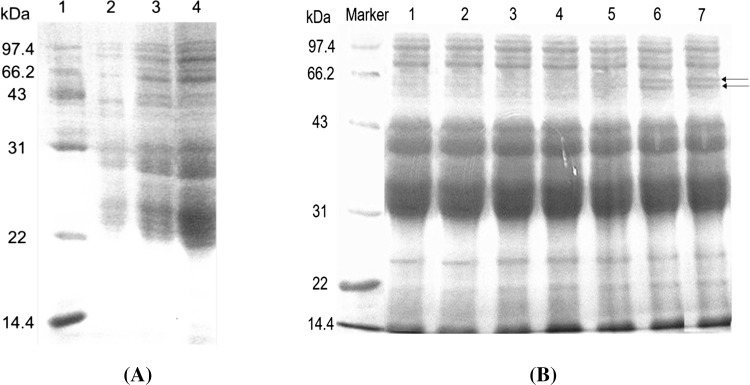
Wheat gluten proteins are mainly composed of glutenins and gliadins. Glutenins are comprised of aggregated proteins in which individual subunits are cross-linked via interchain disulfide bonds, leading to a wide molecular weight (Mw) distribution from 102 to 104 kDa. Gliadins constitute a heterogeneous mixture of monomeric proteins with Mw ranging from 30 to 80 kDa (Carceller and Aussenac 2001; Wahlund et al. 1996). Monomeric proteins (unextractable and extractable) in various flour properties amongst Indian wheat varieties ranged from 23.83% to 51.97% and 48.03% to 76.17%, respectively. Majority of varieties with HMW-GS combinations of 91 kDa + 80 kDa + 78 kDa + 74 kDa PPs showed very high grain hardness index (97–100) (Katyal et al. 2016). In our study, SDS-PAGE was performed to characterize the changes between the original and modified gluten. As seen from Fig. 1a, no evident changes were caused in the flour protein, original and AG below the Mw of 97.4 k Da, which suggests that the flour protein was not hydrolyzed obviously due to the acetylated modification, and the change of noodle quality was possibly generated by the reduction of amino groups of gluten proteins.
Fig. 1.
SDS-PAGE of a 1—marker of low molecular weight protein, 2—wheat flour, 3—original gluten, 4—AG; b 1—original gluten, 2–7—SG with the concentration of anhydrous sodium sulfite were 3, 5, 8, 10, 15 and 20 μmol/g Pro, respectively. The place arrow refers to is the difference in 6,7 and others
As seen from Fig. 1b, the comparison between SG and the original gluten revealed an exceedingly remarkable reduction in the appearance of bands intensity on the top of the separation gel for the samples of 15 and 20 μmol/g Pro between the Mw of 43 to 66.2 k Da, while no evident changes were observed for the samples of 3 and 10 μmol/g Pro. This result suggested that the interchain disulfide bonds played a dominant role in the formation of protein aggregates, and the gluten polymer is hydrolyzed along with the increase of the concentration of sodium sulfite. On the other hand, the spherical three-dimensional structure of gliadin may be destroyed, that increases molecular weight due to interaction with other proteins. Thus, the concentration of 10 μmol/g Pro SG was selected to study its effect on the change in the quality of noodles caused by the created bonds between the free thiol and disulfide group.
Degree of acetylation, sedimentation value, and gluten index
Acetylated gluten was obtained as a result of the reaction between the gluten in the flour and acetic anhydride in the presence of sodium hydroxide (Zukowska et al. 2008):
The absorbance of free amino groups and ninhydrin in the reaction sample was detected at a wavelength of 580 nm, and the degree of acetylation of the modified gluten proteins was found to be 53.48%. Sedimentation value is a comprehensive index which reflects the quantity and quality of gluten proteins, and the larger the sedimentation value, the greater the gluten strength is (p < 0.05). To a certain extent, increased protein quality contributes positively to noodle sensory scores and cooking quality. However, a too high protein quality (wet gluten content > 35%, sedimentation value > 60 mL, stability time > 16 min) decreases the appearance quality of noodles. The wheat protein quality criteria contributing to a high quality of the noodle produced were as follows: flour protein content 12–14%, wet gluten content 28–34%, sedimentation value 40–45 mL (Liu et al. 2011). In an earlier study, a positive correlation between sedimentation value and gluten index was found (Axtord et al. 1979). Unextractable polymeric proteins also showed a positive correlation with gluten index (Katyal et al. 2016). In our investigation, the sedimentation value of the original flour was the highest (43.75 ± 0.3 mL), whereas that of the reconstituted flour declined slightly (41.85–41.05 mL) along with the increase of AG, suggesting that for noodle-making purposes, the quality of the original flour gluten was better than that of AG. In addition, there was a small difference between the gluten index value of the original flour and that of the reconstituted flour with AG (95 ± 1.2–94 ± 1.0). In brief, the quality properties of noodles, including toughness, viscosity, and appearance quality, were not improved by acetylation-modified gluten.
Free thiol group content of the noodle dough
In the present examination, dough properties, based largely on the cohesive elastic character of hydrated gluten, were probably affected by the reversible increment in disulfide bonds, which produced viscous-structured supramolecular cross-linking (Morel et al. 2002). Free -SH groups were usually quantified to confirm the cross-linking of the gluten proteins during the processes of gluten formation and dough and noodle production (Gujral and Rosell 2004; Li et al. 2014). The use of a reducing agent can lead to the reduction of disulfide bonds to free -SH. We found that the free -SH content of SG, reduced by anhydrous sodium sulfite with the final levels of 3, 5, 8, 10, 15, and 20 μmol/g Pro, considerably increased from 3 to 20 μmol/g Pro (p < 0.05), and the content was from 12.15 to 68.46 µmol/g.
Five different key time points in the course of noodle production were determined to study the changes in the content of free -SH groups, which in turn contributed to the formation of loose dough (T1), proofing for 20 min (T2); after rolling (T3); after rolling for 10 min (T4); after rolling for 20 min (T5). To measure the free -SH content, the samples were vacuum-dried after freezing at − 20 °C for 30 min. The free -SH content in the original flour declined considerably from T1 to T5 (p < 0.05) in the course of making noodles from T1 to T5 (5.51, 5.36, 3.94, 3.74, and 3.3), whereas the respective values for the reconstituted flour with SG during T1–T5 were substantially higher (50.2, 45.77, 44.49, 43.1, and 41.0). It is noteworthy that the free -SH content in the reconstituted flour with SG, was higher and its change more pronounced than those of the original flour. Oxidation of the free -SH group to disulfide bonds occurred because the gluten proteins were explored in the air, and the intermolecular distance was decreased due to the outside force and hydration, which led to the gradual increase of the quantity of the organized gluten network structure, and the starch granules were surrounded by a network of gluten- granules. Even during sodium sulfite reduction in the process of making noodles, it is difficult to avoid the oxidation of a part of the free -SH groups. Nevertheless, the effect on the reaction of oxidation of -SH groups can be ignored in the analysis of the results because the reaction conditions was uniform in the whole study.
FTIR spectroscopy analysis
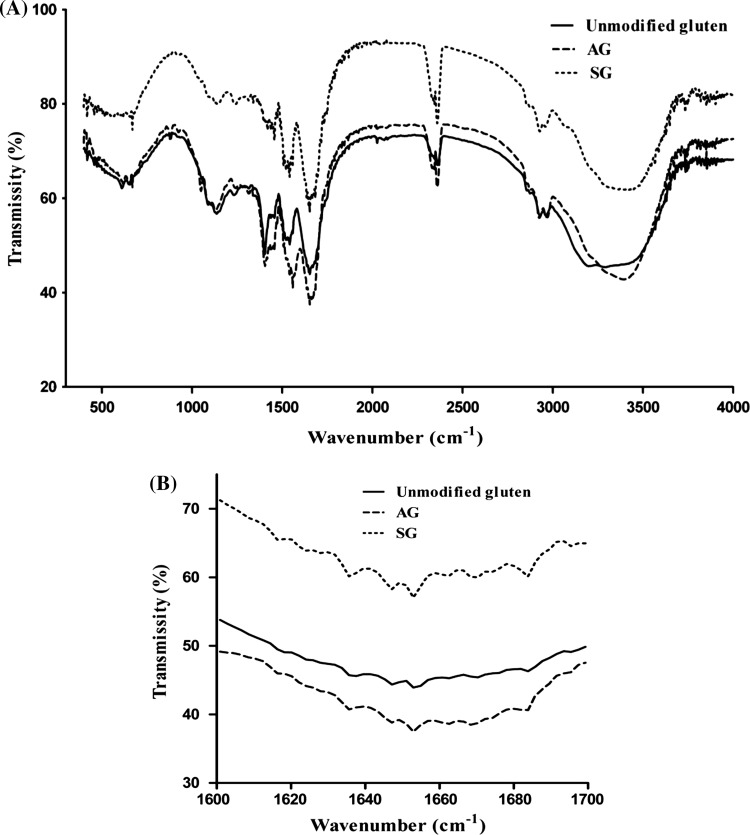
The amide A band (about 3500 cm−1) and amide B (about 3100 cm−1) originate from a Fermi resonance between the first overtone of amide II and the N–H stretching vibration. Amide I and amide II bands are two major bands of the protein infrared spectrum (Mazzaracchio et al. 2011). The contribution of the thiol group stretching vibrations in the region between 2550 and 2590 cm−1. The strength of peak was different from 3000 cm−1 to 3500 cm−1 between original gluten and AG, indicating there was less amino group in the acetylated gluten. Moreover, the peak strength of the thiol group stretching vibrations in SG was stronger than that of unmodified gluten because of more thiol groups in SG reduced with anhydrous sodium sulfite.
In addition, the gluten secondary structure was determined from the absorbance peak positions of Raman spectrum and areas of the amide I bands located between 1600 and 1700 cm−1 (Fig. 2b), which corresponded to four kinds of conformation of secondary structure of proteins: α-helix (20–30%, 1650–1660 cm−1), β-sheet (20–30%, 1605–1640 cm−1), β-turn (15–20%, 1660–1700 cm−1) and random coil (0–10%, 1640–165 cm−1) (Achouri et al. 2012). The results showed that β-sheet was the major component of the secondary structure of original gluten, SG and AG, while no significant change in random coils among three kinds of gluten, with the content 11.15% (p > 0.05). Compared with unmodified gluten, the components of secondary structure of SG and AG showed significant difference (p < 0.05) with α-helix, β-sheet and β-turn ranging from 10.89% to 11.06%, 46.73% to 44.38% and 31.25% to 33.30%, respectively, but the scope of change was not large so far. This suggested that modification of amino and thiol groups had some effect on the secondary structure of original gluten, which might be due to the change of tight connection between gluten protein chain. Better dough texture and enhanced gluten hydration might correspond to increased polymerization degree and molecular weight of protein, and more β-turn or α-helix structures (Liu et al. 2017). Therefore, the characteristics of Chinese noodles could be influenced by the quantity of amino and thiol groups.
Fig. 2.
FTIR spectra of gluten. a wavenumber from 400 to 4000 cm−1; b the amide I region
Noodle texture analysis
Texture is of a paramount concern to consumers of Chinese noodle products (Ajila et al. 2010). The textural attributes of fresh and cooked noodles used in our study are summarized in Tables 1 and 2. As seen from Table 1, both the fresh and cooked noodles made of AG flour had much higher hardness, springiness, and adhesiveness for all the reconstitution degrees except sample No. 4 than that made of original flour. This result is consistent with the findings of Zhang et al. (2002), who found that the number of free amino groups of gluten was reduced after acetylation, and the gluten network structure became loose and irregular due to interactions between amino and carboxyl groups and the reduction of ε- amino groups in the dough formation process. In addition, the charge in the balance between the protein molecules was disrupted by the weak electrostatic attraction and molecular aggregation caused by acetylation (Zhang et al. 2002). Overall, fresh and cooked noodles presented the highest hardness, springiness, adhesiveness, and chewiness for No. 3 reconstituted flour. Significant (p < 0.05) improvement in hardness, adhesiveness, and tensile force was found for AG fresh and cooked noodles. However, the influence of AG content on the springiness of fresh noodles and tension distance of cooked noodles was not significant (p > 0.05, Table 1). That might be due to the high content of acetylated reaction, which could have decreased the strength of the noodles, leading to the formation of a loose structure of the noodle dough and increased extensibility and flexibility of gluten proteins. This finding is consistent with the results obtained in the cooking loss test.
Table 1.
Effect of amino change on the textural parameters of the fresh and cooked noodles
| Hardness/g | Springiness/mm | Adhesiveness/-g·s | Chewiness/g | Tensile force/g·s | Tension distance/mm | |
|---|---|---|---|---|---|---|
| Fresh noodle | ||||||
| Control | 11634 ± 144b | 0.67 ± 0.04a | 8582 ± 85b | 16.96 ± 0.82b | 44.23 ± 3.58bc | |
| 2% AG | 12511 ± 170c | 0.67 ± 0.02a | 9685 ± 117c | 22.13 ± 0.17d | 48.48 ± 7.10c | |
| 4% AG | 12535 ± 427c | 0.71 ± 0.04a | 9517 ± 405c | 19.20 ± 0.55c | 35.68 ± 4.76a | |
| 6% AG | 13455 ± 272d | 0.76 ± 0.03a | 10395 ± 387d | 18.68 ± 0.87c | 38.29 ± 4.01ab | |
| 8% AG | 9697 ± 81a | 0.71 ± 0.12a | 6985 ± 73a | 13.80 ± 0.09a | 51.48 ± 6.07c | |
| Cooked noodle | ||||||
| Control | 5165 ± 227a | 0.87 ± 0.03a | 3475 ± 235a | 3019 ± 285a | 12.46 ± 1.21a | 64.25 ± 1.56a |
| 2% AG | 5043 ± 243a | 0.91 ± 0.02a | 3761 ± 82b | 3418 ± 148b | 16.85 ± 0.66c | 75.80 ± 4.71a |
| 4% AG | 5705 ± 440b | 0.90 ± 0.03a | 4134 ± 150c | 3727 ± 139b | 15.85 ± 0.21c | 73.74 ± 13.14a |
| 6% AG | 5910 ± 189b | 0.91 ± 0.06a | 4208 ± 251c | 3746 ± 417b | 15.81 ± 0.38c | 64.53 ± 6.27a |
| 8% AG | 5051 ± 51a | 0.91 ± 0.05a | 3785 ± 53b | 3431 ± 205b | 14.49 ± 0.36b | 75.96 ± 21.92a |
Values with the same letter are not significantly different (p < 0.05)
Table 2.
Effect of disulfide bonds change on the textural parameters of the fresh and cooked noodles
| Springiness/mm | Adhesiveness/-g·s | Chewiness/g | Tensile force/g·s | Tension distance/mm | |
|---|---|---|---|---|---|
| Fresh noodle | |||||
| Control | 0.67 ± 0.03a | 8265 ± 105c | 17.13 ± 062a | 44.28 ± 5.64a | |
| 2% SG | 0.66 ± 0.04a | 10100 ± 159b | 13.13 ± 0.12c | 20.43 ± 4.16b | |
| 4% SG | 0.66 ± 0.03b | 9916 ± 563b | 14.56 ± 0.51bc | 10.32 ± 2.16c | |
| 6% SG | 0.64 ± 0.03bc | 9985 ± 234a | 15.98 ± 0.89bc | 10.35 ± 2.35c | |
| 8% SG | 0.60 ± 0.04c | 10869 ± 164a | 16.53 ± 0.07b | 10.31 ± 1.20c | |
| Cooked noodle | |||||
| Control | 0.86 ± 0.02a | 4500 ± 334a | 4000 ± 275a | 12.23 ± 1.25a | 68.23 ± 6.55a |
| 2% SG | 0.84 ± 0.03b | 4498 ± 436b | 3758 ± 341b | 10.56 ± 0.53b | 67.80 ± 2.77b |
| 4% SG | 0.78 ± 0.01b | 4234 ± 260b | 3312 ± 289c | 10.24 ± 0.35bc | 60.03 ± 2.86c |
| 6% SG | 0.74 ± 0.02c | 4000 ± 258bc | 3310 ± 117c | 10.13 ± 0.67c | 37.56 ± 3.27d |
| 8% SG | 0.73 ± 0.03c | 3586 ± 253c | 2693 ± 265d | 10.12 ± 0.98c | 31.37 ± 1.24d |
As seen from Table 2, a significant (p < 0.05,) improvement was found in each of the texture parameters of the fresh and cooked SG noodles. With increasing rate of SG, the springiness and tension distance of fresh noodles, as well as the values of the five parameters of cooked noodles determined, decreased, while the tensile force of fresh noodles gradually increased. It is, therefore, reasonable to assume that the activity of sodium sulfite lies in the ability of its -SH groups to reduce the disulfide bridges and disrupt the cross-linking of the gluten network, resulting in poor noodle quality after cooking. Our results demonstrate that disulfide bonds can be of utmost importance for the properties of noodles because their quality worsens due to the reduction of disulfide bonds to -SH groups.
Cooking properties
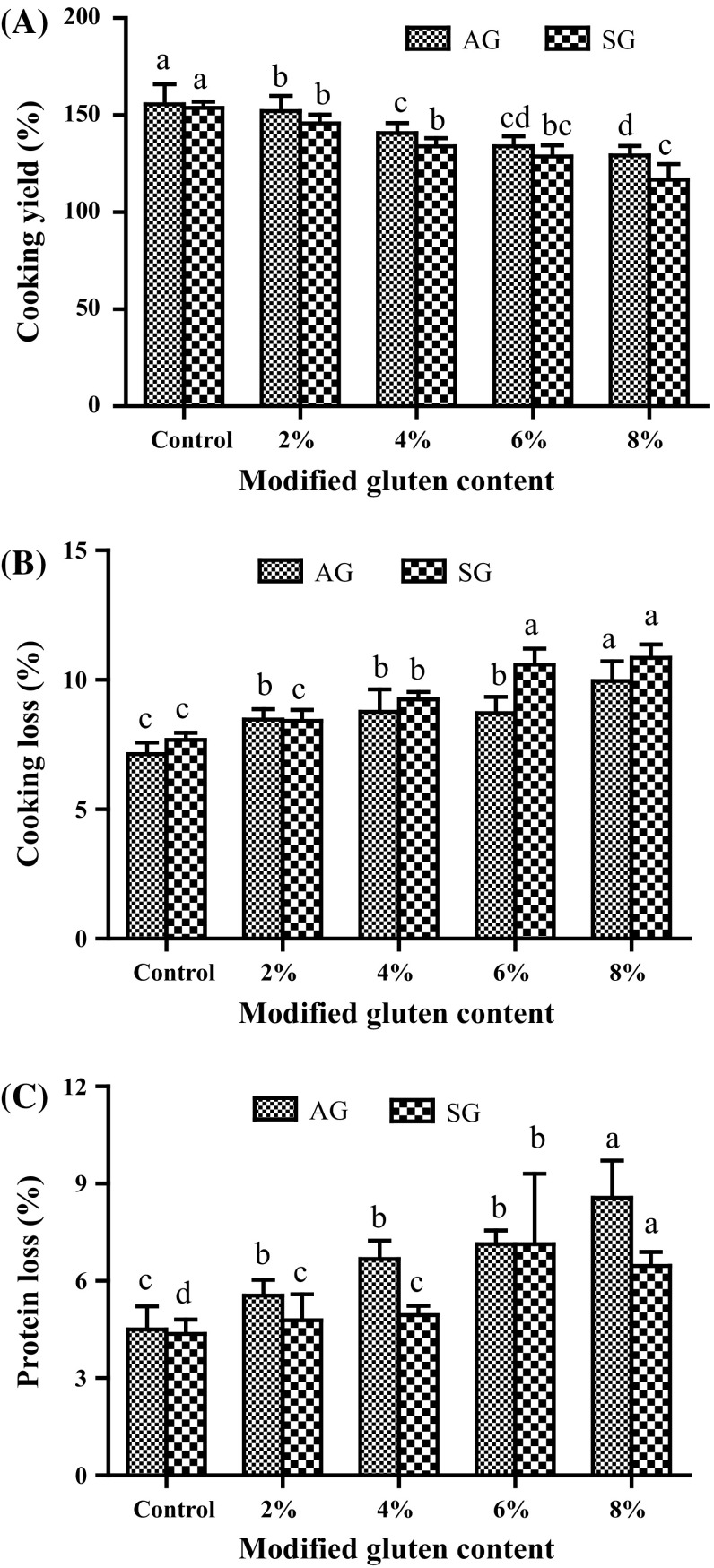
The cooking properties of the noodles, especially the cooking yield and protein loss, are closely related to their quality which can reflect the degree of muddy soup and affect the noodle processing parameters. The cooking properties of the noodles show a regular change with the increase of AG and SG: the cooking yield gradually decreased (Fig. 3a), whereas the cooking loss and protein loss steadily increased (Fig. 3b, c). Moreover, significant differences (p < 0.05) were observed in the values of the three above mentioned parameters, suggesting that the addition of AG and SG was disadvantageous to the cooking quality of noodles. Generally, disulfide bonds was formed by oxidation (Fischer 1985), which might be due to the sufficient interaction between wheat flour granules and water molecules, flour granules and flour granules, as well as among components of wheat flour, such as gluten and starch. As the protein molecules become more compact, some thiol amino acids might be brought closer to each other, leading to the formation of disulfide bonds and a better developed gluten network. Nonetheless, a loose gluten network was formed by the acetylation of amino groups and the reduction of the two disulfide bonds, and some components were dissolved in the soup. In addition, Noodle-cooking time related positively to the proportion of monomeric proteins but negatively to that of polymeric proteins and amylase content (Kaur et al. 2016). Because of less change in protein size distribution, the cooking time of noodles prepared from different reconstitution flours ranged between 330 and 382 s, and the influence of AG and SG content on the cooking time was not significant (p > 0.05).
Fig. 3.
Effects of modified gluten (AG and SG) on the cooking properties parameters of cooked noodles. a Cooking yield; b cooking loss; c protein loss. Different superscripts denote significant difference (p < 0.05)
Scanning electron microscopy
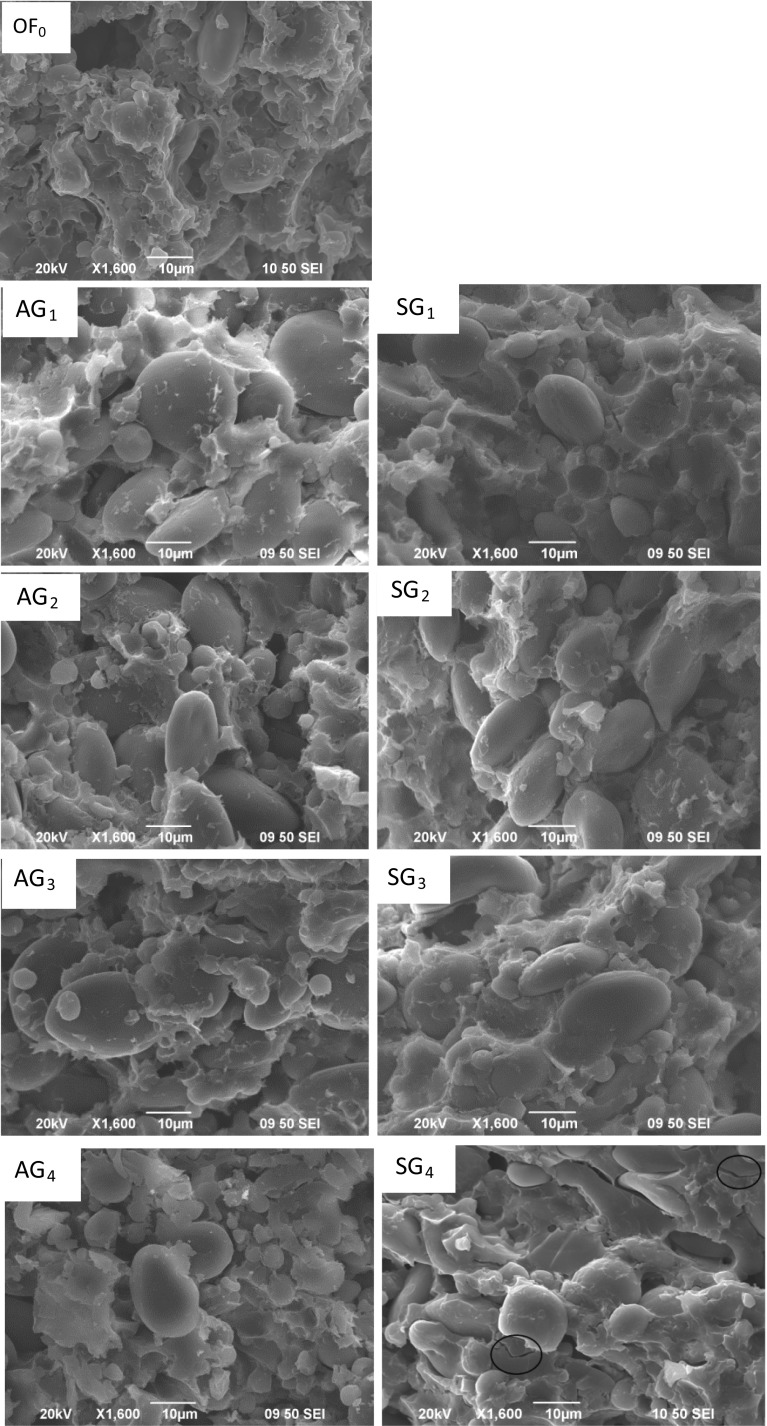
The scanning electron micrographs of cross-section are shown in Fig. 4 (AG1–4) which shows the microstructure of Chinese noodles strand made with AG reconstitution flour. Complete continuous gluten network structure can be seen from the noodles made with original flour, and the starch granules were distributed evenly among them. With the increase of the amount of AG, the gluten network structure of raw dried noodles is not complete, especially in the Fig. 4 (AG4), and some fragments can be seen clearly. The results show that the gluten network has been destroyed, which lead to detachment of part of protein from the network and starch granules exposed to the outside. As shown in Fig. 4 (SG1–4), a few fracture appears along with the increasing of SG. These microstructure characteristics give the cooked noodles the sensation of a smaller tensile force, lower chewiness and more cooking loss.
Fig. 4.
Scanning electron micrographs of cross-section of noodles made by reconstitution flour with AG and SG. OF0: noodles made by original flour; AG1–4: noodles by reconstitution flour with different content of AG; SG1–4: noodles by reconstitution flour with different content of SG
Conclusion
In this paper, the change of amino and thiol groups of wheat gluten was found to impart the texture and cooking properties of Chinese noodles due to the certain macroscopic, structural, molecular changes in the protein components. Free sulfhydryl content increased along with the increase of reducing agent. Moreover, remarkable protein with a lower molecular weight were observed in SDS-PAGE. All these changes indicated the formation of worse gluten networks caused by acetylation and the reduction of disulfide bonds. In a word, amino and sulfhydryl groups of wheat gluten exerted obvious effects on the quality characteristics of Chinese noodles. Nevertheless, how to modify these groups in a reasonable range for an improvement of the quality properties of noodles, including springiness, chewiness, adhesiveness, etc., still needs a further study.
Acknowledgements
This work was supported by Fundamental Research Funds for Special Projects of Henan University of Technology (2018RCJH04), National Natural Science Foundation of China (21276065, 31401548), Science and technology research project of Henan province (182102110343) and Province Key Laboratory of Cereal Resource Transformation and Utilization, Henan University of Technology (001256, 001251).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Hua Li, Email: lixian78101@163.com.
Jingjing Wang, Email: 405702920@qq.com.
Li Pan, Email: Panli215@163.com.
Qiyu Lu, Phone: +8615038329349, Email: lixian78101@haut.edu.an.
References
- AACC . Method. 10. St. Paul, MN, USA: AACC; 2000. Approved methods of the AACC. [Google Scholar]
- Achouri A, Nail V, Boye JI. Sesame protein isolate, fractionation, secondary structure 507 and functional properties. Food Res Int. 2012;46(1):360–369. doi: 10.1016/j.foodres.2012.01.001. [DOI] [Google Scholar]
- Ajila CM, Aalami M, Leelavathi K, Prasada Rao UJS. Mango peel powder: a potential source of antioxidant and dietary fiber in macaroni preparations. Innov Food Sci Emerg Technol. 2010;11(1):219–224. doi: 10.1016/j.ifset.2009.10.004. [DOI] [Google Scholar]
- Anjum FM, Khan MR, Din A, Saeed M, Pasha I, Arshad MU. Wheat gluten: high molecular weight glutenin subunits-structure, genetics, and relation to dough elasticity. J Food Sci. 2007;72(3):56–63. doi: 10.1111/j.1750-3841.2007.00292.x. [DOI] [PubMed] [Google Scholar]
- Arfvidsson C, Wahlund K, Eliasson A. Direct molecular weight determination in the evaluation of dissolution methods for unreduced glutenin. J Cereal Sci. 2004;39(1):1–8. doi: 10.1016/S0733-5210(03)00038-9. [DOI] [Google Scholar]
- Axtord DW, McDermott EE, Redman DG. Note on the sodium dodecyl sulfate test of bread making quality: comparision with pelschenke and Zeleny test. Cereal Chem. 1979;56(6):582–584. [Google Scholar]
- Belton PS, Colquhoun IJ, Field JM, Grant A, Shewry PR, Tatham AS. 1H and 2H NMR relaxation studies of a high Mr subunit of wheat glutenin and comparison with elastin. J Cereal Sci. 1994;19(2):115–121. doi: 10.1006/jcrs.1994.1016. [DOI] [Google Scholar]
- Carceller JL, Aussenac T. Size characterisation of glutenin polymers by HPSEC-MALLS. J Cereal Sci. 2001;33(2):131–142. doi: 10.1006/jcrs.2000.0356. [DOI] [Google Scholar]
- Dexter JE, Matsuo RR, Dronzek BL. A scanning electron microscopy study of Japanese noodles. Cereal Chem. 1979;56(3):202–208. [Google Scholar]
- Fischer F. Oxidation and reduction electron-transfer is key to dough improvements. Bakers’ Digest. 1985;59(1):22. [Google Scholar]
- Gilbert SM, Wellner N, Belton PS, Greenfield JA, Siligardi G, Shewry PR. Expression and characterisation of a highly repetitive peptide derived from a wheat seed storage protein. BBA-Protein Struct Mol Enzymol. 2000;1479(1):135–146. doi: 10.1016/S0167-4838(00)00059-5. [DOI] [PubMed] [Google Scholar]
- Grosch W, Wieser H. Redox reactions in wheat dough as affected by ascorbic acid. J Cereal Sci. 1999;29(1):1–16. doi: 10.1006/jcrs.1998.0218. [DOI] [Google Scholar]
- Gujral HS, Rosell CM. Functionality of rice flour modified with a microbial transglutaminase. J Cereal Sci. 2004;39(2):225–230. doi: 10.1016/j.jcs.2003.10.004. [DOI] [Google Scholar]
- Guo XN, Wei XM, Zhu KX. The impact of protein cross-linking induced by alkali on the quality of buckwheat noodles. Food Chem. 2017;221:1178–1185. doi: 10.1016/j.foodchem.2016.11.041. [DOI] [PubMed] [Google Scholar]
- Hu XZ, Wei YM, Wang C, Kovacs MIP. Quantitative assessment of protein fractions of Chinese wheat flours and their contribution to white salted noodle quality. Food Res Int. 2007;40(1):1–6. doi: 10.1016/j.foodres.2006.05.003. [DOI] [Google Scholar]
- Huang S, Morrison WR. Aspects of proteins in Chinese and British common (hexaploid) wheats related to quality of white and yellow Chinese noodles. J Cereal Sci. 1988;8:177–187. doi: 10.1016/S0733-5210(88)80028-6. [DOI] [Google Scholar]
- Katyal M, Virdi AS, Kaur A, Singh N, Kaur S, Ahlawat AK, Singh AM. Diversity in quality traits amongst Indian wheat varieties I: flour and protein characteristics. Food Chem. 2016;194:337–344. doi: 10.1016/j.foodchem.2015.07.125. [DOI] [PubMed] [Google Scholar]
- Kaur A, Shevkani K, Katyal M, Singh N, Ahlawat AK, Singh AM. Physicochemical and rheological properties of starch and flour from different durum wheat varieties and their relationships with noodle quality. J Food Sci Technol. 2016;53(4):2127–2138. doi: 10.1007/s13197-016-2202-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khatkar BS, Fido RJ, Tatham AS, Schofield JD. Functional properties of wheat gliadins. I. Effects on mixing characteristics and bread making quality. J Cereal Sci. 2002;35(3):299–306. doi: 10.1006/jcrs.2001.0429. [DOI] [Google Scholar]
- Li M, Zhu KX, Peng J, Guo XN, Amza T, Peng W, Zhou HM. Delineating the protein changes in Asian noodles induced by vacuum mixing. Food Chem. 2014;143:9–16. doi: 10.1016/j.foodchem.2013.07.086. [DOI] [PubMed] [Google Scholar]
- Liao L, Han XY, Li ZF, Zhao MM. Study of the effect of deamidation on the properties of degraded wheat gluten. Mod Food Sci Technol. 2015;31(1):21–25. [Google Scholar]
- Liu R, Wei YM, Zhang B. Review on relationship between wheat protein and noodle quality. J Triticeae Crops. 2011;31(6):1183–1187. [Google Scholar]
- Liu R, Xing YN, Zhang YQ, Zhang B, Jiang XJ, Wei YM. Effect of mixing time on the structural characteristics of noodle dough under vacuum. Food Chem. 2015;188:328–336. doi: 10.1016/j.foodchem.2015.04.045. [DOI] [PubMed] [Google Scholar]
- Liu R, Zhang YQ, Wu L, Xing YN, Kong Y, Sun JM, Wei YM. Impact of vacuum mixing on protein composition and secondary structure of noodle dough. LWT Food Sci Technol. 2017;85(184–190):197–203. doi: 10.1016/j.lwt.2017.07.009. [DOI] [Google Scholar]
- Lu QY, Zhang SB. Study on the effect of protein content and glutenin - to - gliadin ratio to noodle quality. J Chin Cereal Oil Ass. 2005;20:13–17. [Google Scholar]
- Lu QY, Guo SY, Zhang SB. Effects of flour free lipids on textural and cooking qualities of Chinese noodles. Food Res Int. 2009;42:226–230. doi: 10.1016/j.foodres.2008.11.007. [DOI] [Google Scholar]
- Lu QY, Zhang SB, Meng DD, Guo XY, Yuan YL. Effects of free lipid enrichment on the quality factors of wheat flour. J Food Agric Environ. 2013;11(1):198–202. [Google Scholar]
- Lü YG, Chen J, Li XQ, Ren L, He YQ, Qu LB. Study on processing and quality improvement of frozen noodles. LWT Food Sci Technol. 2014;59(1):403–410. doi: 10.1016/j.lwt.2014.05.046. [DOI] [Google Scholar]
- Mazzaracchio P, Tozzi S, Boga C, Luciano Forlani, Pifferi PG, Barbiroli G. Interaction between gliadins and anthocyan derivatives. Food Chem. 2011;129:1100–1107. doi: 10.1016/j.foodchem.2011.05.084. [DOI] [PubMed] [Google Scholar]
- Mirhosseini H, Rashid NFA, Amid BT, Cheong KW, Kazemi M, Zulkurnain M. Effect of partial replacement of corn flour with durian seed flour and pumpkin flour on cooking yield, texture properties, and sensory attributes of gluten free pasta. LWT - Food Sci Technol. 2015;63(1):184–190. doi: 10.1016/j.lwt.2015.03.078. [DOI] [Google Scholar]
- Morel MH, Redl A, Guilbert S. Mechanism of heat and shear mediated aggregation of wheat gluten protein upon mixing. Biomacromol. 2002;3(3):488–497. doi: 10.1021/bm015639p. [DOI] [PubMed] [Google Scholar]
- Oh NH, Seib PA, Ward AB. Noodles IV. Influence of flour protein, extraction rate, particle size, and starch damage on the quality characteristics of dry noodles. Cereal Chem. 1985;62(6):441–446. [Google Scholar]
- Osborne TB. The proteins of the wheat kernel. Washington: Carnegie Institution; 1907. [Google Scholar]
- Shimizu T, Fukawa H, Ichiba A. Physical properties of noodles. Cereal Chem. 1958;35(1):34–46. [Google Scholar]
- Wahlund KG, Gustavsson M, MacRitchie F, Nylander T, Wannerberger L. Size characterisation of wheat proteins, particularly glutenin, by asymmetrical flow field-flow fractionation. J Cereal Sci. 1996;23(2):113–119. doi: 10.1006/jcrs.1996.0011. [DOI] [Google Scholar]
- Wang F, Huang WN, Kim YS, Liu RS, Tilley M. Effects of transglutaminase on the rheological and noodle-making characteristics of oat dough containing vital wheat gluten or egg albumin. J Cereal Sci. 2011;54(1):53–59. doi: 10.1016/j.jcs.2011.02.010. [DOI] [Google Scholar]
- Zhang HY, Wang L, Xi YF, Zheng XD. Modification of wheat gluten by acylation. J Wuxi Univer Light Ind. 2002;21(3):239–243. [Google Scholar]
- Zukowska EA, Rudnik E, Kijenski J. Foaming properties of gluten and acetylated gluten-studies of the mathematical models to describe liquid drainage. J Cereal Sci. 2008;47(2):233–238. doi: 10.1016/j.jcs.2007.04.005. [DOI] [Google Scholar]