Abstract
The antioxidant activities in vitro and hepatoprotective effects against carbon tetrachloride (CCl4) induced acute liver injury in vivo of myristic acid acylated derivative of phloridzin (PZM) were investigated. The PZM was obtained by enzymatic acylation of myristic acid and phloridzin (PZ). The antioxidant capability of PZM in vitro was evaluated by the ferric reducing antioxidant power assay (FRAP), 2,2′-Azinobis- 3-ethylbenzthiazoline-6-sulphonate (ABTS+·) and 2,2-diphenyl-1-picrylhydrazyl (DPPH·) radical scavenging assay. Mice were intragastrically treated with control or PZM (20, 40, and 80 mg/kg) for 5 days and intra-peritoneal injection with CCl4. The enzymatic acylated synthesis of myristic acid and phloridzin was region-selective taken place on 6″-OH of phloridzin glycoside moiety and achieved 93% yield. PZM had a significantly higher total antioxidant ability, same scavenging ABTS+· ability and weaker scavenging DPPH· ability when compared to the parent PZ. The of aminotransferase serum activity and malondialdehyde hepatic activity were elevated (P < 0.015) after treatment with CCl4, while the related liver enzymatic activities and glutathione concentration were lower. These changes were enhanced by PZM. Further studies showed that PZM reduced the interleukin-6 expression and stimulated liver regeneration caused by CCl4. PZM attained good antioxidant capacity in vitro and had excellent hepatoprotective effects in vivo and better bioactivity compared to the parent phloridzin. The significance of hepatoprotective effect of phloridzin derivative against CCl4-induced hepatotoxicity in mice is an important and new finding.
Keywords: Food safety, Natural product chemistry, Organic chemistry, Pharmaceutical chemistry
1. Introduction
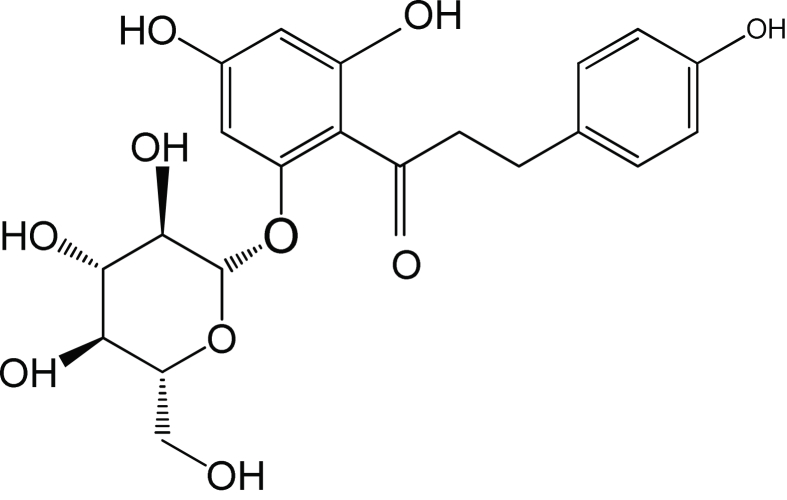
Phloridzin (C12H24O10·2H2O, recognized by different names like phlorizin, phlorrhizin, phlorhizin or phlorizoside, PZ) is a naturally occuring dihydrochalcone. It contains C6–C3–C6 skeleton with a β-D-glucopyranose moiety at position 2′ as shown in Fig. 1 (Ethrenkranz et al., 2005). It is a unique phenolic glucoside which has been mainly found in apple trees, apple buds and processed; apple products such as apple juice (Gosch et al., 2009, 2010; Parpinello et al., 2000). Since French scientist first found it from the bark of apple trees in 1835 (Petersen, 1835), PZ had attracted lots of scientific interests. Most studies on PZ and its derivatives are related to diabetes, antimicrobial activity, antioxidant activity, membrane permeability, gene expression antitumor activity (Sivakami and Rupasinghe, 2007; Gosch et al., 2010; Baldisserotto et al., 2012; Kobori et al., 2012; Fernando et al., 2016). These health benefits have contributed to the development of several industrial applications involving food, beverage, cosmetics and pharmaceuticals. However, the applications are somewhat hindered by the poor solubility of PZ in both hydrophobic and hydrophilic media (Mellou et al., 2006). The solubility of flavonoid glycosides can be enhanced by combining them with appropriate hydrophilic or hydrophobic moieties. In Som studies have used enzymatic modification of the flavonoids has also been used and shown to be feasible and region-selective (Chebil et al., 2006), and studies show that the enzyme-catalyzed flavonoid glycosides have increased lipophilicity and attenuated excellent biological activity (Salem et al., 2010; Ziaullah et al., 2013; Xu et al., 2014).
Fig. 1.
The structure of phloridzin (PZ).
Liver diseases are considered extremely serious health problems and give rise a high death rate (Li et al., 2013). Medical therapies for liver disease are limited applied, due to poor usability and efficiency, hence complementary and alternative medical (CAM) therapies were eagerly needed (Seeff et al., 2001). Previous studies have demonstrated that the liver injury may be controlled by blocking the process of oxidative stress and inflammation (Kim et al., 2010; Zhang et al., 2013). The fatty acid esters of phloridzin have also been shown to induce apoptosis in cancer cells (Nair and Rupasinghe, 2014).
The objective of this study was to investigate the antioxidant activity and CCl4-induced hepatotoxicity of phloridzin-6″-O-myristate (PZM) in mice using Silymarin for comparison. The authors believe this to be the first detailed study on the protective effects of the PZM on acute liver injury in mice.
2. Materials and methods
2.1. Materials
Phloridzin (PZ), myristic acid, 3 Å molecular sieves, 2,4,6-tris(2-pyridyl)-S-triazine (TPTZ) and 2,2-diphenyl-1-picrylhydrazyl (DPPH) were purchased from Aladdin (Shanghai, China) while the immobilized lipase B enzyme Novozyme 435® (from Candida antarctica and 10,000 propyl laurate unit activity) was obtained from Novo Industry A/S. Tween-80 and 2,2′-Azinobis-(3-ethylbenzthiazoline-6-sulphonate) (ABTS) were purchased from sigma, with phosphorus (V) oxide (P2O5), p-anisaldehyde, acetone, acetic acid, ethanol anhydrous, sodium acetate trihydrate, ferric chloride, vitamin C and carbon tetrachloride (CCl4) were obtained from Kemiou Chemical Reagent Co., Ltd (Tianjin, China) while olive oil was obtained from a local Pharmacy (Beijing, China). Silymarin was supplied by Madaus AG (Germany).
Aspartate aminotransferase (AST), alanine aminotransferase (ALT), superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GSH-Px), glutathione (GSH), malondialdehyde (MDA) and interleukin-6 (IL-6) detection kits were purchased from Nanjing Jiancheng Institute of Biotechnology (Nanjing, China). Enhanced Bicinchoninic Acid (BCA) Protein Assay Kit was obtained from Beijing Kangwei Institute of Biotechnology (Beijing, China).
2.2. General
The chemical structure of the PZM was determined by 1H and 13C NMR spectroscopic analysis in DMSO-d6 on Bruker AVANCE 500MHZ spectrometer (Bruker Corp., Billerica, MA, USA). The internal standard was tetra-methyl-silane (TMS, C4H12Si), chemical shifts expressed in δ ppm, coupling constants in Hertz, and spin multiplicities by the symbols s (singlet), d (doublet), dd (doublet of doublets), t (triplet), q (quartet), m (multiplet), and br (broad).
2.3. Synthesis
PZM was synthesizd from PZ through an enzymatic reaction process as detailed in Sekhon-Loodu et al. (2015). Briefly, under inert conditions, PZ (0.50 g; 1.15 mM), dry acetone (9 mL), myristic acid (1.70 g, 7.44 mM), and Novozyme lipase 435® (1.58 g) were taken in a dried round bottom flask having 3 Ǻ molecular sieves, stirred continuously at 46 °C and 120 rpm for 42–48 h. Thin layer chromatography (acetone/toluene; 2:3) was used to confirm the completion of the reaction. Subsequently, the mixture was filtered, evaporated, and passed through a column chromatography (first with acetone/toluene; from 1:6 to 35:75 and then from 35:75 to 1:1) to elute pure PZM.
The structure of the final product PZM as analyzed by 1H NMR and 13C NMR spectroscopy is shown below:
Phloridzin-6″-O-myristate: yellow solid; yield: 93.4%. 1H NMR (DMSO-d6,500MHz): δ 10.52 (s, 1H, ArOH), 9.08 (br s, 1H, ArOH), 7.04 (d, 2H, J = 7.5 Hz, H-2, H-6), 6.66 (d, 2H, J = 7.5 Hz, H-3, H-5), 6.11 (s, 1H, H-3′), 5.98 (s, 1H, H-5′), 5.41 (br s, 1H, OH), 5.37 (br s, 2H, 2OH), 4.99 (d, 1H, J = 6.8 Hz, H-1″), 4.33 (br d, 1H, J = 11.3 Hz, H-6a′′), 4.14 (dd, 1H, J = 11.4 Hz, 6.9 Hz, H-6b′′), 3.61 (br t, J = 6.9 Hz, H-4″), 3.36-3.21 (m, 6H, 2×Hα, H-2″, H-3″, H-5″, OH), 2.78 (br t, 2H, J = 6.9Hz, 2×Hβ), 2.28 (t, 2H, J = 6.9 Hz, 2×H-2‴), 1.47 (m, 2H, 2×H-3‴), 1.22-1.17 (m, 20H, 2(CH2)), 0.87 (br t, 3H, J = 6.8 Hz, CH3); 13C NMR (DMSO-d6, 125MHz): δ 205.09 (CO), 173.26 (OCO), 165.84 (C-4′), 164.89 (C-6′), 161.07 (C-2′), 155.75 (C-4), 131.93 (C-1), 129.55 (C-2, C-6), 115.41 (C-3, C-5), 105.66(C-1′), 101.09 (C-1″), 97.39 (C-3′), 94.99 (C-5′), 76.89 (C-3″), 74.37 (C-5″), 73.58 (C-2″), 70.28(C-4″), 63.52 (C-6″), 45.43 (Cα), 33.87 (C-2‴), 31.76 (Cβ), 29.52, 29.35, 29.17 (C-4‴, C-5‴, C-6‴, C-7‴, C-8‴, C-9‴, C-10‴, C-11‴, C-12‴), 24.84(C-3‴), 22.55 (C-13‴), 14.39 (C-14‴).
2.4. The ferric reducing antioxidant power (FRAP) assay
The total antioxidant capacity of the PZM and phloridzin was determined based on the FRAP assay reported in Benzie and Strain (1996). The FRAP reagent was prepared fresh from 300 mM acetate buffer (pH 3.6), 20 mM ferric chloride and 10 mM TPTZ in 40 mM HCl mixed together in the ratio of 10:1:1. Vitamin C was used as a standard. The appropriate quality of the vitamin was dissolved in 95% ethanol to obtain 70, 80, 90, 100, 110, 120 and 130 μM concentrations to obtain a calibration curve. PZM and PZ were also dissolved in 95% ethanol to prepare desired concentrations. To perform the assay, 100 μL of blank, standard or samples was made to react with 900 μL of FRAP solution at 37 °C. Absorbance was recorded at 593 nm after 10 min reaction time. Antioxidant capacity was calculated as μM ascorbic acid equivalent/L of 1 mM of solution.
2.5. ABTS·+ radical scavenging assay
The ABTS·+ radical scavenging assay of the PZM and PZ was carried out using the method of Re et al. (1999) with some modifications. ABTS·+, a blue/green free radical, has absorption maxima at wavelengths 645, 734 and 815 nm, but turns colorless by antioxidants. ABTS and potassium persulfate were prepared in water at 7 mM and 14 mM concentration, respectively. ABTS·+ was produced by reaction of 5 mL ABTS·+ solution with 88 μL potassium persulfate. The mixture was held in dark at room temperature for 12–16 h to complete the reaction. For the study of PZM and PZ, the ABTS·+ solution was diluted with 95% ethanol to an absorbance of 0.70 (±0.2) at 734 nm to form the test reagent. The reaction mixture containing 50 μL test sample and 1mL test reagent was incubated at room temperature for 5 min. The absorbance was recorded at 734 nm exactly after 1 min. Ascorbic acid was used as a standard material. The appropriate quality ascorbic acid was dissolved in 95% ethanol to obtain 300, 400, 500, 600 and 700 μM concentration and a calibration curve was prepared. Antioxidant capacity was calculated as μM ascorbic acid equivalent/L of 1 mM of solution.
2.6. DPPH· radical scavenging assay
The DPPH· radical scavenging assay of PZM and PZ was carried out using a modified method of Brand-Williams et al. (1995). DPPH· is a stable free radical with an intense violet color with a maximum absorbance at 517 nm, and colorless as the unpaired electrons are scavenged by antioxidants. The reaction mixture containing 400 μL of 0.2 mM DPPH· solution (prepared in 95% ethanol) and 400μL control or vitamin C standard solution or test sample (prepared in 95% ethanol) were incubated in a water bath at 37 °C for 30 min, and the absorbance was measured at 517 nm. Vitamin C was used as the standard material and a calibration curve was prepared with 30, 40, 50, 60, 70 and 80 μM concentrations (in 95% ethanol). Antioxidant capacity was calculated as μM ascorbic acid equivalent/L of 1 mM of solution.
2.7. Animals and treatment
Male BALB/C mice weighing 18–22 g were obtained from the Experimental Animal Center of The Fourth Military Medical University (Xi'an, China) - production license number SCXK (army corps) 2012-0007. The animals were held in a controlled environment chamber at 23 ± 2 °C, 60 ± 5% relative humidity with 12 h light/dark cycles with a standard chow diet (The Fourth Military Medical University, Xi'an, China) and tap water ad libitum. The procedures involving animal care were complied with China National Institutes of Healthy Guidelines for the Care and Use of Laboratory Animals.
After acclimatizing the animals for 1 week, they were divided randomly into eight individual groups. Group I which represented normal control was given vehicles only at 10 ml/kg body weight. Group II was used as PZM control and fed PZM dissolved in 5% Tween-80 saline at 80 mg/kg. Group III which was treated as a model was given vehicles. Group IV was the positive control and given Silymarin dissolved in 5% Tween-80 saline at 40 mg/kg). Group V was used as the PZ control and given PZ at 40 mg/kg. Groups VI, VII and VIII (low, medium and high dosage) were administrated other levels of PZM (20, 40 and 80 mg/kg, respectively). After the specific diet for 5 days, and one hour after the final administration, the mice in Groups I and II were fed olive oil, while mice in Groups III–VIII were given 0.11% (v/v) CCl4 (10 ml/kg, dissolved in olive oil) injection intraperitoneally. Twenty-four hours after the CCl4 administration and followed by fasting (but given water ad libitum), the animals were anesthetized for obtaining the blood and then sacrificed to collect the livers. Blood samples were centrifuged at 3000 × g for 15 min at 4 °C to obtain serum. The excised livers were washed with cold phosphate buffered saline (pH 7.4), and the left lobe of the liver was fixed in 10% formalin to prepare paraffin sections and the remaining parts were stored at -80 °C for the other assays.
All of the experimental procedures with animals were carried out following the Guide for the Care and Use of Laboratory Animals: Eighth Edition, ISBN-10: 0-309-15396-4 and the animal protocol was approved by the animal ethics committee of Northwest A&F University. All experiments were carried out in ways to minimize suffering.
2.8. Determination of serum aminotransferase activities
The serum activities of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were measured to evaluate hepatotoxicity. An automatic biochemical analyzer (7180 series, Hitachi High-Tech Science Systems Co., Ltd, Japan) was used in the experiments. The enzyme activities are expressed in international units (U/L).
2.9. Preparation of liver protein homogenate
Liver tissues were homogenized with saline (1 part of liver tissue with 9 parts of saline) and centrifuged at 4,000 rpm for 10 min at 4 °C (Centrifuge 5804R, Eppendorf, Germany) to remove cell debris and nuclei. The supernatants were collected and stored at −80 °C for following various biochemical assays.
2.10. Measurement of protein
Protein concentrations from liver supernatants were determined by bicinchoninic acid (BCA) protein assay according to the manufacture's analysis. BCA was capable of monitoring and complexing cuprous ion (Cu+) produced in the reaction of protein and alkaline Cu2+ (Smith et al., 1985). The results are expressed as μg/μL in liver homogenates.
2.11. Determination of antioxidant enzymes activity
The activity of catalase (CAT), superoxide dismutase (SOD), and glutathione peroxidase activities (GSH-Px) were evaluated with the corresponding commercial kits according to the manufacturer's instructions. Briefly, CAT activity was determined based on the hydrogen peroxide decomposition method of Chance and Maehly (1955). The CAT activity was defined based on the amount (mL, units) of μmol H2O2 decomposing in one second. SOD activity was determined using xanthine substrate oxidized by xanthine oxidase (Beauchamp and Fridovic, 1971). Nitroblue tetrazolium (NBT) have the ability to inhibit superoxide anion radicals (O2-), which are generated as a result of xanthine oxidase activity on xanthine. SOD activity was based on the amount of SOD (unit, mL) resulting in a 50% inhibition of the NBT reduction in 1.0 mL of the reaction system. GSH-Px activity was determined by reduced glutathione GSH-H2O2 reaction system (Mohanda et al., 1984) and defined as amount of enzyme that reduced the level of GSH by 1.0 μmol/L per mg protein per min.
2.12. Measurement of the content of MDA and GSH
The content of MDA and GSH were evaluated by manufacturer's instructions. The amount of MDA was determined by the concentration of TBARS (Berton et al., 1998). MDA and the thiobarbituric acid (TBA) reacted to form a red complex with absorption maximum at 532 nm. The results are expressed as nmol/mg proteins in liver homogenates. The level of reduced GSH was evaluated by colorimetric analysis. The reduced GSH and dithio-bis-nitrobenzoic acid (DTNB) reacted to form a yellow complex with absorption maximum at 405 nm (Staal et al., 1969). The results are expressed as μmol/mg proteins in liver homogenates.
2.13. Assessment of pro-inflammatory cytokine
The liver Interleukin-6 (IL-6) level was quantified using a commercial TL-6 ELISA assay kit according to the manufacturer's instructions. The concentrations were expressed as ng/L.
2.14. Statistical analysis
All data obtained are expressed as a mean ± SD. Significance was considered at P˂0.05 and P˂0.01 using one-way analysis of variance (ANOVA) followed by Turkey's multiple comparison tests.
3. Results
3.1. Chemistry
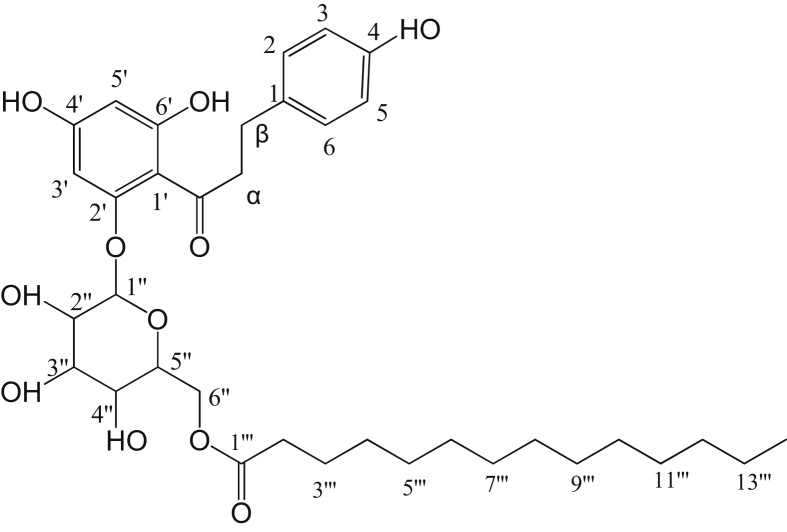
Fig. 1 shows the structure of phloridzin (PZ). NMR analysis of the purified product showed that acylation by lipase B did actually takes place on the 6″-OH of phloridzin glycoside moiety (Fig. 2), which agrees with the previous studies (Ardhaoui et al., 2004a, b).
Fig. 2.
Numbering of Phloridzin-6″-O-myristate.
3.2. Antioxidant capacity of phloridzin-6″-O-myristate (PZM)
The antioxidant capacity of PZM was expressed as ascorbic acid equivalent antioxidant capacity (AE). FRAP assay resulted in significantly higher (P˂0.05) AE for the PZM than for PZ (Table 1). The FRAR capacity of PZM was 1.28 folds higher than that of PZ. In ABTS·+ radical assay, the results demosntrated that both PZM and PZ were effective scavengers of the ABTS·+ radical, and there was no statistical difference between them (P˃0.05). Both PZ and PZM were stronger than the ascorbic acid standard. However, the DPPH· radical assay showed that PZM has a weaker DPPH· radical scavenging ability than PZ (P˂0.01). The reason for this lower DPPH· activity with PZM, which was unlike the other two antioxidant behavior, is not clear.
Table 1.
Antioxidant capacity measured by FRAP, ABTS·+ and DPPH· radical assays for phloridzin (PZ) and myristic acid ester of phloridzin (PZM).
| Compound | FRAP (μmol AE L−1) | ABTS (μmol AE L−1) | DPPH (μmol AE L−1) |
|---|---|---|---|
| Phloridzin | 77.9 ± 5.17 | 1794 ± 20.7 | 3.99 ± 0.3 |
| PZM | 95.9 ± 3.77* | 1792 ± 85.1 | 1.76 ± 0.003** |
Results were expressed as Means ± SD, *P˂0.05, **P˂0.01.
Antioxidant activity unit: μmol ascorbic acid equivalents/L of 1 mM of solution; PZM: Phloridzin myristate.
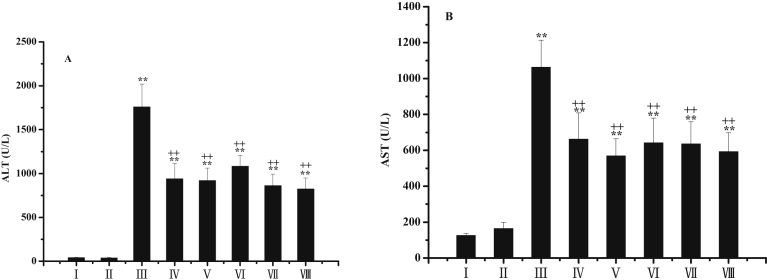
3.3. Effects of PZM on serum ALT and AST activities
Serum AST, ALT and histopathology were examined to evaluate the effects of PZM on CCl4-induced hepatotoxicity in mice. ALT and AST activities in the normal control group were 40.0 ± 4.20 and 125.5 ± 11.28 U·L−1, respectively. Serum ALT and AST activities showed a marked increase to 1459 ± 260.8 and 1063 ± 150.8 U·L−1 24 h after CCl4 injection (Fig. 3), indicating CCl4 administration induced remarkable liver damage. PZM control group showed no significance with normal control group, indicating that PZM was harmless to the mice. PZM treatment, in a dose independent manner, significantly reversed the CCl4-induced alteration of serum transaminase activity. Silymarin and PZ treatments also remarkably prevented the increase in AST and ALT. Even though the enzymatic activities in PZM treatment groups were still higher than those in the control group, obvious amelioration could be found (Gan et al., 2012; Gosch et al., 2011).
Fig. 3.
Effects of PZM on the activities of ALT (A) and AST (B) in serum against CCl4-induced hepatoxicity in mice. Group I: normal control; group II: PZM control; group III: model; group IV: Silymarin-treated; group V: PZ-treated; group VI: PZM-treated (20 mg/kg); group VII: PZM-treated (40 mg/kg), group VIII: PZM-treated (80 mg/kg). Values are expressed as mean ± SD.∗P < 0.05, ∗∗P < 0.01 compared to normal group. +P < 0.05, ++P < 0.01 compared to model group.
3.4. Effects of PZM on CCl4-induced changes in liver enzymatic activities
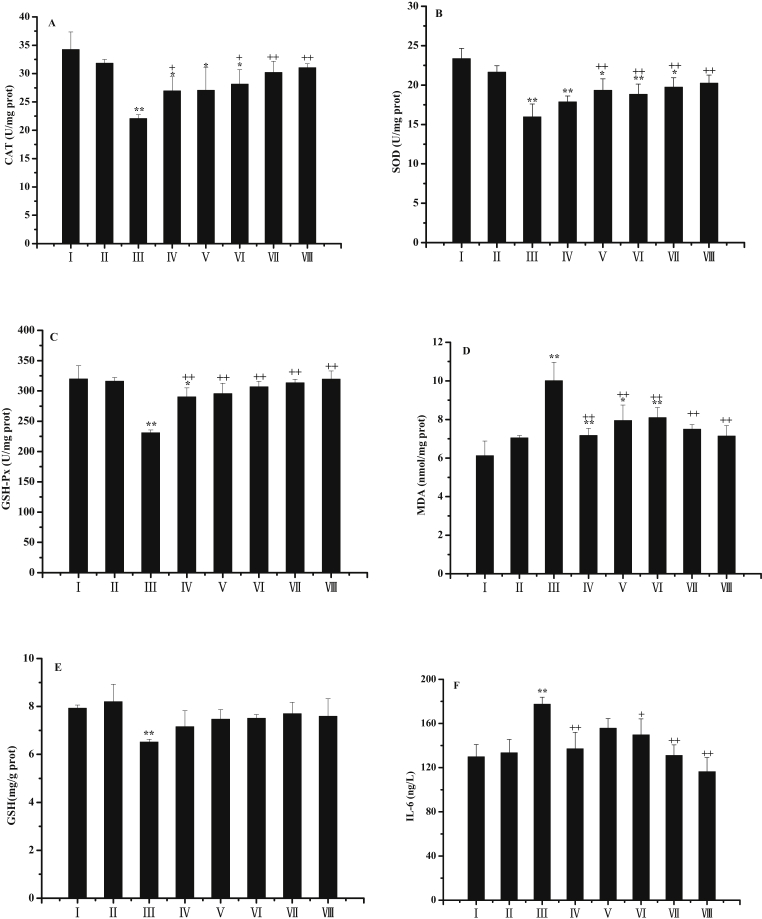
Liver enzymatic activities of CAT, SOD and GSH-Px were examined to evaluate the antioxidant ability of PZM on CCl4-induced acute liver injury. As shown in Fig. 4 (A–C), the levels of CAT, SOD and GSH-Px were significantly impaired by the treatment of CCl4 when compared with the control group. Administration of PZM significantly exhibited a notable increase in CAT, SOD and GSH-Px in a dose dependent fashion. The treatment of Silymarin and PZ also showed a remarkable effect compared to the model. The PZM at a dose of 80 mg/kg was more effective than phloridzin treatment in CAT, SOD and GSH-Px when compared to the model group.
Fig. 4.
Effects of PZM on the levels of CAT (A), SOD (B), GSH-Px (C), MDA (D) and GSH (E) against CCl4-induced hepatoxicity in mice. Group I: normal control; group II: PZM control; group III: model; group IV: Silymarin-treated; group V: PZ-treated; group VI: PZM-treated (20 mg/kg); group VII: PZM-treated (40 mg/kg), group VIII: PZM-treated (80 mg/kg). values are expressed as mean ± SD.∗P < 0.05, ∗∗P < 0.01 compared to normal group. +P < 0.05, ++P < 0.01 compared to model group.
3.5. Effects of PZM on the content of MDA and GSH
The content of MDA was determined to evaluate the effect of PZM treatment on CCl4-induced lipid peroxidation. CCl4 induced significantly elevation as compared to the control group (Fig. 4 D), which confirmed that CCl4 treatment had exactly induced oxidative damage in mice. PZM treatment, in a manner of dosage independent, significantly attenuated the alteration compared with the model group, so the treatment of Silymarin and PZ did. PZM at dose of 40 and 80 mg/kg showed a better effect than phloridzin treatment.
As shown in Fig. 4 E, compared with the control group, CCl4 had a notable decrease in the content of GSH, indicating the liver injury had been initiated by CCl4 treatment. Silymarin, phloridzin and PZM treatment elevated the GSH level, but no significant differences were observed when compared to the model group.
3.6. Effects of PZM on IL-6 expression
For a comprehensive evaluation of the effect of PZM on CCl4-induced hepatoxicity in mice, the level of IL-6 was investigated. IL-6 is a critical proregenerative factor and acute-phase inducer in the liver that also confers resistance to liver injury by hepatic toxins (2003 IL-6). As shown in Fig. 4F, CCl4-treated group exhibited a significant increase compared to the control group. PZM treatment, dose independently, inhibited remarkably the increase when compared with the model group, which reached approximately 34.4% by at dose of PZM at 80 mg/kg. Administration of Silymarin and PZ also reduced the level of IL-6 as compared to the model group. PZM, at dose of 40 and 80 mg/kg, shown a significant effect than PZ treatment (P < 0.05) and (P < 0.01).
4. Discussion
Enzymatic acylation of flavonoid compounds using lipase B (Novozym 435®) have been proved to be region-selective and easy reaction (Stevenson et al., 2006; Salem et al., 2010). Relatively high conversion yields of PZM (93%) could be obtained with ideal reaction condition. In the present investigation, high efficiency was achieved according to keep the reaction system under vacuum and the water content of the medium low.
Region-selective reactions of PZM ensured that some hydroxyl groups remain on the PZ chemical structure, preserving its antioxidant properties. The assessment of antioxidant capacity detailed earlier has shown that PZ and PZM have strong total antioxidant ability and good radical scavenging ability. Some differences were observed however between the two radical scavenging assays (DPPH· and ABTS·+), the reason for this is not very clear. Firstly, the ABTS·+ assay based on electron transfer behavior, and an end-point assay whereby different antioxidant compounds donate one or two electrons to scavenge the radical cation thereby providing the antioxidant activity. According to Huang et al. (2005), regardless of the donating potential of individual antioxidants they all have the time to react fully giving an accurate measurement of TAC at the end-point of the assay. The DPPH· assay on the other hand is based on the normal HAT reaction that occurs between antioxidants and the peroxyl radical. Instead of peroxyl radicals, more stable and less transient nitrogen radicals are created, with which some antioxidants can react more slowly than they would with the peroxyl radical in a biological system (Huang et al., 2005). Hence there could be some differences in the antioxidant behavior expressed by the two systems.
CCl4-induced liver acute injury is widely recognized and considered to be similar to liver injury caused by hepatotoxins in humans (Choi et al., 2011). CCl4 is a lipid-soluble effective hepatotoxic compound. Administration of CCl4 can alter permeability of the membrane and release the enzymes from the cells into circulation, resulting in abnormally elevation of ALT and AST (Drotman and Lawhorn, 1978). In this study, serum ALT and AST were increased significantly by administration of CCl4, indicating that cells in liver lost the functional integrity. However, the serum levels were remarkably attenuated by PZM, suggesting that PZM effectively protected the mice against CCl4-induced hepatoxicity.
CCl4 metabolism occurred in liver endoplasmic reticulum (ER) by cytochrome P450 and evoked a chain of reactive free radical formation, resulting in hepatocellular necrosis and lipid peroxidation in hepatic tissue (Wang et al., 2014). It is widely believed that CCl4 is transferred to trichloromethyl radical (CCl3·), and CCl3 forms the more toxic CCl3O2· when interact with oxygen. The liver tissue can develop some antioxidant defense to prevent the oxidative damage, including enzymatic activities (e.g., CAT, SOD and GSH-Px) and nonezymatic antioxidants (such as GSH) (Szymonik et al., 2003). Docosahexaenoic acid-acylated phloridzin (PZ-DHA) has been tested against tumorigenesis in the models of mammary carcinoma. The tumor volume and tumor weight and tumor sections have been investigated to evaluate the tumorigenesis of PZDHA; however, the relevant serum markers and biochemical indexes have not been studied. In present study, when compared to the control group, CCl4 treatment decreased significantly the enzymatic activities of CAT, SOD and GSH-Px, which suggested that CCl4 intoxication lead to inactivation of antioxidant enzymes. PZM treatment, in a dose-dependent manner, attenuated these decrease remarkably compared as the model group. The treatment of Silymarin and PZ also can alter the enzymatic activities of CAT, SOD and GSH-Px compared to the CCl4 group. For the CAT and GSH-Px, the low dose of PZM almost reached the same effect with PZ, while the medium and high dose of PZM showed a better protective tendency. For enzymatic activity of SOD, the medium and high dose of PZM showed similar effect as PZ. GSH is the most abundant redox system and plays a vital role in terminate oxidative damage caused by most hepatotoxins (Kim et al., 2010). The content of GSH was decreased significantly by injection of CCl4 compared to the control group. No statistic significant changes in the GSH level were observed for the treatment of Silymarin, PZ and PZM. It indicated that the enzymatic antioxidant system maybe played a more vital role in attenuating hepatotoxicity. The results of enzymatic activities suggested that PZM had a hepatoprotective effect, even though the PZM had no statistic significant difference with the parent compound PZ, the PZM still resisted against oxidative stress and maintained potent protective effect for liver tissue.
MDA, as an indicator of lipid peroxidation, reflects the level of oxidative stress directly (Gan et al., 2012). In the present study, a significant increase in the level of MDA was observed after CCl4 injection and treatment with PZM attenuated this increase. PZM significantly reduced the level of lipid peroxidation, that suggesting PZM might have a hepatoprotective effect by decreasing oxidative stress in the CCl4-induced acute liver injury.
In acute liver failure, CCl4 as hepatotoxins damaged cell membranes and released inflammatory mediators from activated hepatic macrophages, which enhanced the CCl4-induced hepatotoxicity (Shim et al., 2010). Cytokine, such as IL-6, promoted hepatic survival by regulating inherent and adaptive immunity, stimulating hemocytogenesis and liver regeneration (Taub, 2003). In our present study, administration of CCl4 significantly elevated the level of IL-6 compared to the control group, indicating that CCl4 exactly resulted hepatic apoptosis and inflammation. The alteration of IL-6 was remarkably attenuated by the treatment of PZM compared as the model group, suggesting PZM protected cytokine IL-6 and stimulating liver regeneration. The treatment of Silymarin and PZ also can reduce the level of IL-6 compared to the CCl4 group. For the level of IL-6, the low dose of PZM had reached a better effect than the parent PZ, while the medium and high dose of PZM almost reduced 15% and 25% IL-6 content compared to PZ treatment. The results of IL-6 showed that the derivative of phloridzin, PZM, maybe had a better bioactivity.
The synthesis, antioxidant capacity and hepatoprotective of myristic acid acylated derivative of phloridzin (PZM) were investigated. The synthesis of PZM achieved 93% yield, using lipase B (Novozym 435®) as catalyzing enzyme, acetone as solvent incubating in 45 °C. PZM had strong total antioxidant ability and defined ability to scavenge free radical of DPPH and ABTS. Compared to the parent PZ, PZM retained better antioxidant abilities. In the study of PZM against CCl4-induced acute liver injury in mice, when compared to the CCl4-induced hepatotoxicity group, treatment with PZM significantly reduced the serum level of ALT and AST, the l content of MDA and the cytokine IL-6, and remarkably elevated the enzymatic activities of CAT, SOD and GSH-Px, and the content of GSH. PZM reduced oxidative stress and suppressed liver inflammation. PZM had good hepatoprotective effects, and better biological activities than the parent PZ.
5. Conclusions
PZM demonstrated a significantly higher total antioxidant ability, similar scavenging ABTS+· ability but weaker scavenging DPPH· ability when compared to the parent PZ. The serum activities of aminotransferases and the hepatic level of malondialdehyde were confirmed to be elevated after CCl4 treatment, while the liver enzymatic activities (catalase, superoxide dismutase, and glutathione peroxidase) and the concentration of reduced glutathione were lower. However, these changes were attenuated by PZM treatment. Further, PZM treatment reduced the interleukin-6 expression and stimulated liver regeneration caused by CCl4. PZM demonstrated good antioxidant capacity in vitro, had excellent hepatoprotective effects in vivo and better bioactivity compared to the parent PZ. This is the first time report on the hepatoprotective effect of PZM against CCl4-induced hepatotoxicity in mice.
Declarations
Author contribution statement
Yamei Ren: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
Chunli Liu: Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Chunlong Yuan: Performed the experiments; Analyzed and interpreted the data.
Xiaolin Ren: Contributed reagents, materials, analysis tools or data.
Hosahalli Ramaswamy: Analyzed and interpreted the data; Wrote the paper.
Funding statement
This work was supported by Apple Modern Industrial System of Chinese Agriculture Ministry (Z225020701).
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Contributor Information
Hosahalli S. Ramaswamy, Email: Hosahalli.Ramaswamy@Mcgill.Ca.
Yamei Ren, Email: 715189648@qq.com.
References
- Ardhaoui M., Falcimaigne A., Engasser J.M., Moussou P., Pauly G., Ghoul M. Acylation of natural flavonoids using lipase of candida Antarctica as biocatalyst. J. Mol. Catal. B Enzym. 2004;29(1–6):63–67. [Google Scholar]
- Ardhaoui M., Falcimaigne A., Ognier S., Engasser J.M., Moussou P., Pauly G., Ghoul M. Effect of acyl donor chain length and substitutions pattern on the enzymatic acylation of flavonoids. J. Biotechnol. 2004;110(3):265–271. doi: 10.1016/j.jbiotec.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Baldisserotto A., Malisardi G., Scalambra E., Andreotti E., Romagnoli C., Vicentini C., Manfredini S., Vertuani S. Synthesis, antioxidant and antimicrobial activity of a new phloridzin derivative for dermo-cosmetic applications. Molecules. 2012;17(12):13275–13289. doi: 10.3390/molecules171113275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchamp C., Fridovic I. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 1971;44(1):276–287. doi: 10.1016/0003-2697(71)90370-8. [DOI] [PubMed] [Google Scholar]
- Berton T.R., Conti C.J., Mitchell D.L., Aldaz C.M., Lubet R.A., Fischer S.M. The effect of vitamin E acetate on ultraviolet-induced mouse skin carcinogenesis. Mol. Carcinog. 1998;23(3):175–184. doi: 10.1002/(sici)1098-2744(199811)23:3<175::aid-mc6>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Benzie I.F.F., Strain J.J. The ferric reducing ability of plasma (FRAP) as a masure of “antioxidant power”: the FRAP assay. Anal. Biochem. 1996;239(1):70∼76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- Brand-Williams W., Cuvelier M.E., Berset C. Use of a free radical method to evaluate antioxidant activity. Food Sci. Technol. 1995;28(1):25∼30. [Google Scholar]
- Chance B., Maehly A.C. Assay of catalase and peroxidases. Methods Enzymol. 1955;2:764–775. [Google Scholar]
- Chebil L., Humeau C., Falcimaigne A., Engasser J.M., Ghoul M. Enzymatic acylation of flavonoids. Proc. Biochem. 2006;41(11):2237–2251. [Google Scholar]
- Choi J.H., Kim D.W., Yun N., Choi J.S., Islam M.N., Kim Y.S., Lee S.M. Protective effects of hyperoside against carbon tetrachloride-induced liver damage in mice. J. Nat. Prod. 2011;74(5):1055–1060. doi: 10.1021/np200001x. [DOI] [PubMed] [Google Scholar]
- Drotman R.B., Lawhorn G.T. Serum enzymes as indicators of chemically induced liver damage. Drug Chem. Toxicol. 1978;1(2):163–171. doi: 10.3109/01480547809034433. [DOI] [PubMed] [Google Scholar]
- Ethrenkranz J.R.L., Lewis N.G., Ronald K.C., Roth J. Phlorizin: a review. Diabetes Metabol. Res. Rev. 2005;21(1):31–38. doi: 10.1002/dmrr.532. [DOI] [PubMed] [Google Scholar]
- Fernando W., Coombs M.R.P., Hoskin D.W., Rupasinghe H.P.V. Docosahexaenoic acid-acylatedphloridzin, a novel polyphenol fatty acid ester derivative, is cytotoxic to breast cancer cells. Carcinogenesis. 2016;37(10):1004–1013. doi: 10.1093/carcin/bgw087. [DOI] [PubMed] [Google Scholar]
- Gan D., Ma L., Jiang C., Wang M., Zeng X. Medium optimization and potential hepatoprotective effect of mycelial polysaccharides from Pholiota dinghuensis Bi against carbon tetrachloride-induced acute liver injury in mice. Food Chem. Toxicol. 2012;50(8):2681–2688. doi: 10.1016/j.fct.2012.05.003. [DOI] [PubMed] [Google Scholar]
- Gosch C., Flachowsky H., Halbwirth H., Thill J., Mjka-Wittmann R., Treutter D., Richterk K., Hanke M.V., Stich K. Substrate specificity and contribution of the glycosyltransferase UGT71A15 to phloridzin biosynthesis. Trees. 2011;26(1):259–271. [Google Scholar]
- Gosch C., Halbwirth H., Kuhn J., Miosic S., Stich K. Biosynthesis of phloridzin in apple (Malus domestica Borkh.) Plant Sci. 2009;176(2):223–231. [Google Scholar]
- Gosch C., Halbwirth H., Stich K. Phloridzin: biosynthesis, distribution and physiological relevance in plants. Phytochemistry. 2010;71(8-9):838–843. doi: 10.1016/j.phytochem.2010.03.003. [DOI] [PubMed] [Google Scholar]
- Huang D., Ou B., Prior R.L. The chemistry behind antioxidant capacity assays. J. Agric. Food Chem. 2005;53(6) doi: 10.1021/jf030723c. 1841∼185. [DOI] [PubMed] [Google Scholar]
- Kim H.Y., Kim J.K., Choi J.H., Jung J.Y., Oh W.Y., Kim D.C. Hepatoprotective effect of pinoresinol on carbon tetrachloride–induced hepatic damage in mice. J. Pharmacol. Sci. 2010;112(1):105–112. doi: 10.1254/jphs.09234fp. [DOI] [PubMed] [Google Scholar]
- Kobori M., Masumoto S., Akimoto Y., Oike H. Phloridzin reduces blood glucose levels and alters hepatic gene expression in normal BALB/c mice. Food Chem. Toxicol. 2012;50(7):2547–2553. doi: 10.1016/j.fct.2012.04.017. [DOI] [PubMed] [Google Scholar]
- Li L., Li W., Kim Y.H., Lee Y.W. Chlorella vulgaris extract ameliorates carbon tetrachloride-induced acute hepatic injury in mice. Exp. Toxicol. Pathol. 2013;5(1-2):73–80. doi: 10.1016/j.etp.2011.06.003. [DOI] [PubMed] [Google Scholar]
- Mellou F., Loutrari H., Stamatis H., Roussos C., Kolisis F.N. Enzymatic esterification of flavonoids with unsaturated fatty acids: effect of the novel esters on vascular endothelial growth factor release from K562 cells. Proc. Biochem. 2006;41(9):2029–2034. [Google Scholar]
- Mohanda J., Marshall J.J., Duggin G.G., Horvath J.S., Tiller D.J. Differential distribution of glutathione and glutathione-related enzymes in rabbit kidney-possible implications in analgesic nephropathy. Biochem. Pharmacol. 1984;33(11):1801∼1807. doi: 10.1016/0006-2952(84)90353-8. [DOI] [PubMed] [Google Scholar]
- Nair S.V.G., Rupasinghe H.P.V. Fatty acid esters of phloridzin induce apoptosis of human liver cancer cells through altered gene expression. PLoS One. 2014;9(9):1–16. doi: 10.1371/journal.pone.0107149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parpinello G.P., Versari A., Galassi S. Phloretin glycosides bioactive compounds in apple fruit, purées, and juices juices. J. Med. Food. 2000;3(3):149–151. doi: 10.1089/jmf.2000.3.149. [DOI] [PubMed] [Google Scholar]
- Petersen C. Analyse des Phloridzins. Annal. Pharm. 1835:15. [Google Scholar]
- Re R., Pellegrini N., Proteggente A., Pannala A., Yang M., Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999;26(9-10):1231–1237. doi: 10.1016/s0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- Salem J.H., Humeau C., Chevalot I., Harscoat-Schiavo C., Vanderesse R., Blanchard F., Fick M. Effect of acyl donor chain length on isoquercitrin acylation and biological activities of corresponding esters. Process Biochem. 2010;45(3):382–389. [Google Scholar]
- Seeff L.B., Linadsay K.L., Bacon B.R., Kresina T.F., Hoofnagle J.H. Complementary and alternative medicine in chronic liver disease. Hepatology. 2001;34(3):595–603. doi: 10.1053/jhep.2001.27445. [DOI] [PubMed] [Google Scholar]
- Sekhon-Loodu S., Ziaullah, Rupasinghe H.P.V. Docosahexaenoic acid ester of phloridzin inhibit lipopolysaccharide-induced inflammation in THP-1 differentiated macrophages. Int. Immunopharmacol. 2015;25(1):199–206. doi: 10.1016/j.intimp.2015.01.019. [DOI] [PubMed] [Google Scholar]
- Shim J.Y., Kim M.H., Kim H.D., Ahn J.Y., Yun Y.S., Song J.Y. Protective action of the immunomodulator ginsan against carbon tetrachloride-induced liver injury via control of oxidative stress and the inflammatory response. Toxicol. Appl. Pharmacol. 2010;242(3):318–325. doi: 10.1016/j.taap.2009.11.005. [DOI] [PubMed] [Google Scholar]
- Sivakami M., Rupasinghe H.P.V. Fruit phenolics as natural antimicrobial agents: selective antimicrobial activity of catechin, chlorogenic acid and phloridzin. J. Food Agric. Environ. 2007;5(3&4):81–85. [Google Scholar]
- Smith P.K., Krohn R.I., Hermanson G.T., Mallia A.K., Gartner F.H., Provenzano M.D., Fujimoto E.K., Goeke N.M., Olson B.J., Klenk D.C. Measurement of protein using bicinchoninic acid. Anal. Biochem. 1985;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Staal G.E.J., Visser J., Veeger C. Purification and properties of glutathione reductase of human erythrocytes. Biochim. Biophys. Acta Enzymol. 1969;185(1) doi: 10.1016/0005-2744(69)90280-0. [DOI] [PubMed] [Google Scholar]
- Stevenson D.E., Wibisono R., Jensen D.J., Stanley R.A., Cooney J.M. Direct acylation of flavonoid glycosides with phenolic acids catalysed by Candida Antarctica lipase B (Novozym 435®) Enzym. Microb. Technol. 2006;39:1236–1247. [Google Scholar]
- Szymonik L.S., Czechowska G., Stryjecka Z.M., Slomka M., Maldro A., Celinski K. Catalase, superoxide dismutase, and glutathione peroxidase activities in various rat tissues after carbon tetrachloride intoxication. J. Hepato Biliary Pancreatic Surg. 2003;10(4):309–315. doi: 10.1007/s00534-002-0824-5. [DOI] [PubMed] [Google Scholar]
- Taub R. Hepatoprotection via the IL-6/Stat3 pathway. J. Clin. Investig. 2003;112(7):978–980. doi: 10.1172/JCI19974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Sit W., Tipoe G.L., Wan J.M. Differential protective effects of extra virgin olive oil and corn oil in liver inJury: a proteomic study. Food Chem. Toxicol. 2014;74:131–138. doi: 10.1016/j.fct.2014.09.002. [DOI] [PubMed] [Google Scholar]
- Xu J., Qian J., Li S. Enzymatic acylation of isoorientin isolated from antioxidant of bamboo leaves with palmitic acid and antiradical activity of the acylated derivatives. Eur. Food Res. Technol. 2014;239(4):661–667. [Google Scholar]
- Zhang S., Lu B.N., Han X., Xu L.N., Qi Y., Yin L.H., Xu Y.W., Zhao Y.Y., Liu K.X., Peng J.Y. Protection of the flavonoid fraction from Rosa laevigata Michx fruit against carbon tetrachloride-induced acute liver injury in mice. Food Chem. Toxicol. 2013;55:60–69. doi: 10.1016/j.fct.2012.12.041. [DOI] [PubMed] [Google Scholar]
- Ziaullah B.K.S., Warnakulasuriya S.N., Rupasinghe H.P.V. Biocatalytic synthesis, structural elucidation, antioxidant capacity and tyrosinase inhibition activity of long chain fatty acid acylated derivatives of phloridzin and isoquercitrin. Bioorg. Med. Chem. 2013;21(3):684–692. doi: 10.1016/j.bmc.2012.11.034. [DOI] [PubMed] [Google Scholar]