Abstract
The microneedle (MN), a highly efficient and versatile device, has attracted extensive scientific and industrial interests in the past decades due to prominent properties including painless penetration, low cost, excellent therapeutic efficacy, and relative safety. The robust microneedle enabling transdermal delivery has a paramount potential to create advanced functional devices with superior nature for biomedical applications. In this review, a great effort has been made to summarize the advance of microneedles including their materials and latest fabrication method, such as three-dimensional printing (3DP). Importantly, a variety of representative biomedical applications of microneedles such as disease treatment, immunobiological administration, disease diagnosis and cosmetic field, are highlighted in detail. At last, conclusions and future perspectives for development of advanced microneedles in biomedical fields have been discussed systematically. Taken together, as an emerging tool, microneedles have showed profound promise for biomedical applications.
Key words: Microneedle patches, Biomedical applications, Microfabricated device, Drug delivery, Disease treatment, Disease diagnosis, Immunobiological administration, 3D printing
Graphical abstract
As a promising device, microneedles have made significant progress for revolutionizing the field of disease treatment, immunobiological administration, disease diagnosis, and cosmetic applications. We discuss a variety of materials and fabrication methods of microneedles. The conclusions and future perspectives for development of advanced microneedles in biomedical application are addressed.
1. Introduction
The microneedle was firstly presented in 1976 and an American patent was released synchronously concerning the microneedle for transdermal delivery1. After that, microneedle fabrication and application has made a huge progress with the rapid development of high precision microelectronics industry. The original applications of microneedles in biomedicine were concentrated on drug delivery. Drug delivery of pharmacologically active ingredients plays a crucial role in the fields of medicine and pharmacy. In general, active pharmaceutical compositions of drug can be delivered by the following routes, including oral administration2, parenteral route3, transdermal delivery4, and other penetration-enhancing methods5, 6, 7. Although oral delivery has lots of merits in respect of patient compliance, painless and low cost, numerous drugs often suffer from poor absorption caused by drug degradation resulted from the first-pass metabolism in the gastrointestinal route and microenvironment changes in pH, food, etc8, 9, 10. Parenteral route, injection with a hypodermic needle, has been widely used worldwide due to the large-dosed and effective method to deliver diverse types of drug molecules. Nevertheless, the clinical usage of this method is restricted by pain and needle-phobia accompanying with injections in some patients10, 11, 12. Other penetration-enhancing methods, such as iontophoresis, sonophoresis, and electrophoresis mainly caused a severe damage to stratum corneum structure13. As one of the robust routes for drug delivery, transdermal drug delivery systems (TDDS) have been extraordinarily studied over the past 50 years14, 15. In comparison to conventional delivery methods, the advent of microneedle-based transdermal delivery overcomes the obstacles like patient compliance, pain, the risk of infection and long-term treatment, etc16. Because microneedles only penetrate through the vigorous stratum corneum and the viable epidermis without reaching nerve endings and blood vessels, patient will not feel pain during the process17. Therefore, the emerging microneedle revolutionized the methods of drug delivery and shows the powerful potential in clinical applications.
Currently, the applications of microneedle expanded beyond their representative biomedical applications comprising disease long-term treatment, immunobiological administration, disease diagnosis, and cosmetic field. In addition to the small molecule drugs, microneedles can also be used to deliver a large amount of macromolecules in a controllable manner, such as insulin, growth hormones, immunobiological vaccine, receptor agonist, proteins and peptides, which could be transferred into the epidermis directly to improve theirs drug efficacy significantly for disease long-term treatment and immunobiological administration. It is well known that microneedles are divided into four types according to the different mechanisms in drug delivery10, 18, 19, 20, 21, 22. In terms of increasing penetration effect, dissolving microneedles in disease therapeutic efficacy are more prominent than the other three18. However, most of the above-mentioned microneedles can only be leaked out in one step leading to achieve long-term treatment difficultly23.
Recently, much attention has been focused on smart multifunction-responsive microneedles holding profound promise for revolutionizing the point-of-care (POC) disease diagnostics, disease prevention and personalized long-term treatment24, 25, 26, 27, 28, 29, 30. It has been reported that a swellable microneedles patch was exploited to transfer skin interstitial fluid (SIF) containing glucose and cholesterol for POC detection and personal healthcare monitoring31. In addition, a stretchable and wearable patch combined with graphene doped with gold mesh to enhance electrochemical signals not only timely measured the blood glucose concentration and the pH of human sweat, as well as temperature in sweat-control module, but also thermally induced to deliver metformin transcutaneously employing polymeric dissolving microneedles under hypoglycaemic conditions for diabetes real-time therapy. The results read out by a wearable electrochemical analyser were wirelessly transferred to application (APP) with Bluetooth technology, thereby realizing the integration of diagnosis and personalized long-term therapy26, 32. Moreover, significant progress in cosmetic field has been witnessed over the past decades. Microneedle can transport active cosmetic molecules into skin directly for reducing the risk of infection of injured skin and improving effectiveness of cosmeceutical products as well as safety. Hence, these unique selling points make cosmeceutical products promising to increase skin permeation of cosmeceuticals and heal the wound as rapidly as possible. In the end, we are dedicated to highlight the biomedical applications of microneedles patch and their future direction.
2. Microneedle classification and fabrication
In comparison with other transdermal delivery methods, microneedles have been typically fabricated as much as a depth of 200 μm without penetrating cross the dermis, so no pain is available33. A great progress has been made by the microelectronics industry since 1990, which is extremely beneficial for the microfabrication of microneedle. Microneedles are classified as solid microneedles for the pretreatment of skin, coated microneedles with water-soluble pharmaceutical formulations, dissolving microneedles without residual fragments, and hollow microneedles for liquid formulations. Alternatively, a series of microneedle arrays are listed in Table 125, 26, 32, 34, 35, 36, 37 according to their function.
Table 1.
The different types of functional microneedles fabricated out of various materials.
| Type | Material | Ref. |
|---|---|---|
| The disposable-manner microneedle | Carboxy-methyl-cellulose | 34 |
| Multi-round responsive microneedle | Alginate | 25 |
| Temperature responsive microneedle | Vinyl pyrrolidone | 26 |
| Glucose responsive microneedle | Hyaluronic acid | 32 |
| pH responsive microneedle | Hyaluronic acid | 35 |
| Swelling-shrinking microneedle | Hydrogel | 36 |
| Water-soluble microneedle | Dextrin | 37 |
2.1. Solid microneedles
Solid microneedles can be designed as skin pretreatment for producing large pores to deliver drugs. After the pores were formed, topical formulations (ointment, gel, and lotion) applying to treat skin are able to be transported into the dermis through the pores. Subsequently, they can be distributed in all parts of the body by systemic circulation38.
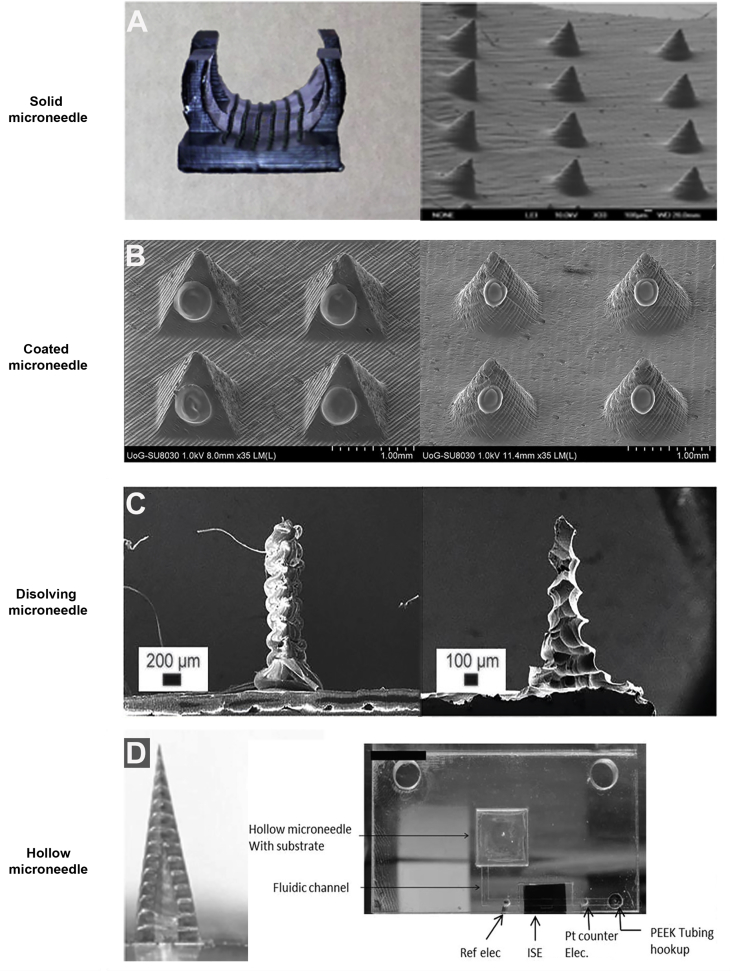
The shape and tips of microneedles are largely determined by the needle geometry devised upon the basis of simulation software. Silicon microneedles were fabricated in 1998 using ion etching method employing a conventional dry-etching process, which was a costly fabrication progress14. To get rid of this limit, the super-short microneedles were successfully manufactured using wet etching of silicon in 30% KOH at 80 °C39. SU-8, a distinguished ultraviolet (UV)-curable polymer, was associated with glass substrate inclined rotational at a certain angle, and thereby the designed solid microneedles with sharp tip were obtained40, 41. However, traditional MN fabrication techniques including dry and wet etching techniques are incapable of effectively fabricating MN array patches on curved surfaces. In other words, as conventional MN array is produced on the flat substrate surface, it is of difficulty to insert on uneven skin surface completely, which leads to low penetration efficiency. Lim et al.42 developed a novel microneedle splint using three-dimensional printing (3DP) on personalized curved surfaces to realize entire insertion into the undulating human skin (Fig. 1A)42. The personalized microneedle splint improved permeation effect significantly and delivered drugs transdermally through the skin for pain relief.
Figure 1.
The new microfabrication technique (3D printing) to fabricate different types of microneedles. (A) A proprietary resin, 3DM-castable, was employed to manufacture 3D printing of a microneedle array on the curved surfaces. Reproduced with permission from Ref. 42. Copyright © 2017, IOP Publishing Group. (B) Two microneedle designs (pyramid and cone) were built by 3D printing technology using a biocompatible resin, and then followed by inkjet printing coating process of insulin formulations. Reproduced with permission from Ref. 49. Copyright © 2018, Elsevier. (C) Various customized MN length and shapes were designed and printed within 1 h using a US Food and Drug Administration (FDA)-approved biodegradable material (polylactic acid). Reproduced with permission from Ref. 55. Copyright © 2018, Royal Society of Chemistry. (D) The strategy allows for a 3D printing hollow microneedle to draw fluid associated with a microfluidic chip applying a three-electrode system, which provides a robust on-body sensing system for monitoring potassium (K+). Reproduced with permission from Ref. 60. Copyright © 2014, WILEY-VCH.
2.2. Coated microneedles
Coated microneedles have two main functions. One is to pierce skin and the other is to deliver desired drugs applying on the surface of microneedle. Unfortunately, the maximum drug dose is less than 1 mg. This is the reason for limiting the development of coated microneedles43. Using Layer-by-layer coating techniques to further increase the drug loading, coated microneedles were required to dip or spray by aqueous solution with high viscosity. With the aid of electrostatic attraction, negatively charged DNA or virus were absorbed on positively charged microneedle easily to attain microneedle coating44. Coated microneedles were confirmed as a versatile device, due to the extensive scope of coated drugs (small molecules, macromolecules, vaccines, DNA, micron-scale particles)45, 46, 47, 48. The different shapes of coated microneedles are fabricated to promote permeation and drug loading. As compared to previous fabrication techniques, such as micromolding and lithographie, galvanoformung und abformung (LIGA) technique, those methods often suffered from cumbersome master templates and tedious preparation processes, thereby lacking the versatility of fabrication steps and the capability of changing design quickly. Pere et al.49 employed 3DP technique to build pyramid and cone MN designs for the delivery of insulin (Fig. 1B)49. MN arrays integrated with 3DP are manufactured in a one-step manner to feature MN with different geometries rapidly. This effective method for the mass production of microneedle patches shows profound promise for commercial applications.
2.3. Dissolving microneedles
Dissolving microneedles manufactured from safe materials, such as biodegradable polymers and natural polymers, can control the release of drugs or vaccine embedded in the polymer. That is, dissolving microneedles controlling the release of encapsulated pharmaceutical agents are painless and safe in the application of disease diagnostics and treatment50. Dissolving microneedles were fabricated by micromoulding methodology at the room temperature51. Birchall et al.52 reported that biodegradable sugar glass microneedles were prepared under vacuum conditions at low temperature utilizing micromolding to deliver methylene blue solution. Furthermore, a novel room temperature method to produce dissolving microneedles using melted maltose was developed for protein delivery53. Subsequently, to encapsulate thermally sensitive compounds, ultrasonic welding was applied in preparing dissolving microneedles for delivery of growth factors54. However, bulk micromachining and prototyping bespoke needle architectures are still deemed to be expensive and time consuming. Luzuriaga et al.55 presented a new micromachining strategy employing fused deposition modeling (FDM) 3DP to design and print quickly with customized microneedle density, length, and shape (Fig. 1C)55. Furthermore, a post fabrication chemical etching method incorporated with 3DP has been developed to improve the feature size resolution, which has access to the microneedle tip sizes as small as 1 μm.
2.4. Hollow microneedles
Hollow microneedles cannot be confined with the low dose, as compared to others which can lead to the poor clinical effect. Moreover, extracting tissue fluids through hollow microneedles has gained lots of attention owing to the satisfactory capacity to transport diverse molecules. However, hollow microneedles often suffer from the risk of fracture as a result of insufficient mechanical strength56.
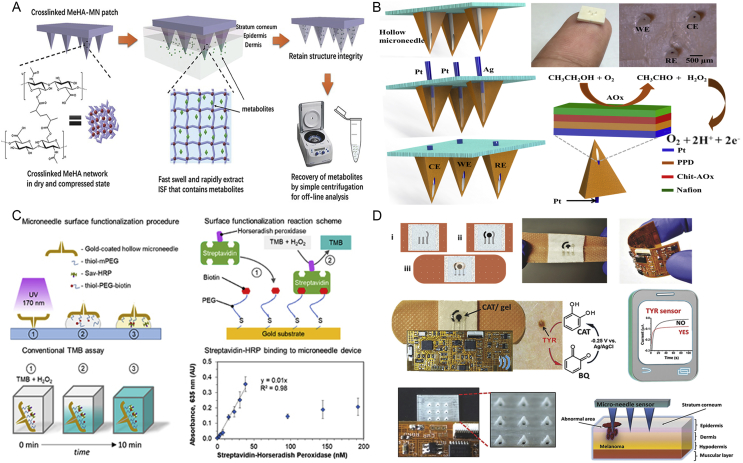
Several methods have been used to fabricate hollow microneedles. Microelectromechanical systems (MEMS) techniques were used to produce large batch hollow microneedles. Yu et al.57 fabricated cylindrical hollow microneedle on the basis of MEMS techniques undergoing three main procedures: photolithographic process, deep reactive ion etching Bosch process, and micromachining process. Glass hollow microneedles were prepared from conventional drawn glass micropipette techniques. Grinding the needle tip and briefly heating could make them smooth on the surface58. The LIGA technology associated with hot embossing process was utilized to fabricate hollow microneedle array, which involved in a vertical deep X-ray exposure. At the same time, the mask whose pattern was triangular arrays was exposed to the inclined deep X-ray. The shape was determined by the inclined angle of deep X-ray59. Recently, Miller et al.60 have exploited hollow microneedle-based transdermal sensor to extract fluid utilizing a laser direct write system for POC diagnostic applications (Fig. 1D)60. This new POC device by integrating hollow microneedle with a microfluidic chip has a potential for disease diagnostic. Together, the diversity of microneedle manufaction and the performance of different microneedle were shown in Table 214, 39, 42, 49, 51, 54, 55, 58, 59, 60, 61, 62, 63, 64.
Table 2.
Comparative efficacy and manufaction of diverse types of microneedles on the following performances presenting by low efficacy (+), moderate efficacy (++), high efficacy (+++).
| Type | Fabrication method | Drug loading | Sustained delivery | Molecular range | Ref. |
|---|---|---|---|---|---|
| Solid microneedles | Dry‒etching, wet etching, 3D‒printing, laser ablation, micromolding, magnetorheological drawing lithography, electroplating | + | + | ++ | 14, 39, 42, 49, 61, 62, 63 |
| Coated microneedles | As above | ++ | ++ | ++ | As above |
| Dissolving microneedles | Micromolding, ultrasonic welding, 3D‒printing, micromachining, ion etching | +++ | +++ | ++ | 51, 54, 55, 61, 64 |
| Hollow microneedles | Lithographic molding, X‒ray photolithography, 3D‒printing | +++ | +++ | +++ | 58, 59, 60 |
3. Applications of microneedles
Skin is not only a potent barrier, but also helpful for delivering bioactive agents. Thus it is well applied in molecular diagnosis and treatment. Microneedles were usually introduced for disease treatment by enhancing penetration and transporting drugs. At present, the application of microneedles is undergoing expansion to more fields including immunobiological administration, disease diagnosis and cosmetic uses.
3.1. Advantages of microneedles in transdermal drug delivery
It was in the beginning of 19th century that methods of enhancing the skin transport for transdermal delivery attracted an enormous amount of interests to perform extensive research1. As is known, transdermal drug diffusion across the skin is faced with great challenge due to the strong barrier, the intact stratum corneum65. Transdermal delivery possesses many overwhelming advantages as compared to intramuscular injection, subcutaneous injection and intradermal injection66. Therefore, microneedle is considered as an effective and painless device that is easy to use for patients, holding tremendous promise for delivering macromolecules in transdermal drug delivery field.
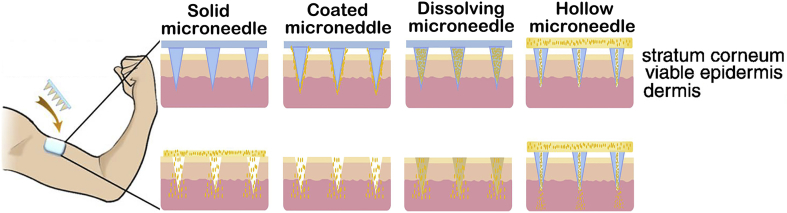
As described schematically in Fig. 210, the skin is made up of three regions: stratum corneum, viable epidermis and dermis. The outermost layer (10–20 μm), the stratum corneum, is filled with dead cells, which plays a potent role in deterring the entry of macromolecular. The epidermis layer (150–200 μm) contains viable cells without nerve networks and SIF connected to dermis by passive diffusion. This is why microneedles for transdermal delivery inserts to the skin without pain and SIF is seemed as ideal biomarkers for POC diagnosis and prognosis. The dermis (3–100 mm), comprising extensive nerve endings and vessels, is mainly to support the skin10, 17, 67, 68, 69.
Figure 2.
Methods of drug delivery to the skin using diverse microneedles patches on the human arm and the anatomy of the skin. Reproduced with permission from Ref. 10. Copyright © 2012, Elsevier.
3.2. Disease treatment
Most of the biotherapeutic drugs such as peptides, proteins, hormone and natural agent fail to be administered easily because of first pass metabolism. Therefore, hypodermic injection has to be considered in spite of pain with the insertion of needle.
The emerging devices, microneedle patches, are often regarded as potential candidates for hypodermic injection, which is painless, safe, and simple for patients to self-administer. Transdermal drug delivery for disease treatment is still the hot spot of application for microneedles.
3.2.1. Cancer
Traditional treatment regimens including surgery, chemotherapy and radiotherapy can lead to acute toxicity and side effects as well as tumor recurrence. A minimally invasive cancer treatment associated with microneedle patches always appeals broad interest as a result of advantageous controllability, easy applicability and predominant synergistic effect70.
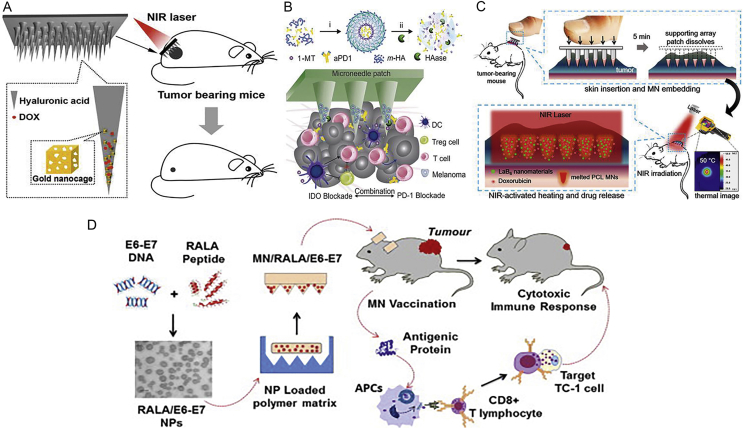
To meet these requirements, Dong et al.71 developed hyaluronic acid (HA) dissolving microneedle arrays containing chemotherapeutic drug doxorubicin (DOX) and gold nanocages to combine chemotherapy with photothermal therapy for treating superficial tumors synergistically (Fig. 3A)71. Additionally, cancer immunology is known as the immune checkpoint inhibitor in treating tumor72, 73. As seen in Fig. 3B30, Ye et al.30 indicated that an HA-based microneedle platform was encapsulated anti-PD1 antibody (aPD1) to prevent immune evasion and 1-methyl-d,l-tryptophan (1-MT) to block immunosuppressive enzyme indoleamine 2,3-dioxygenase (IDO) with synergistic transcutaneous delivery for melanoma immunotherapy. HA-based microneedle platform was dissociated by an overexpressed hyaluronidase (HAase) in the tumor microenvironment thus delivering checkpoint inhibitors (aPD1 and 1-MT). Furthermore, Chen et al.74 demonstrated that the dissolvable poly(vinyl alcohol)/polyvinylpyrrolidone (PVA/PVP) supporting array including photothermal nanoparticles and an antitumor drug can eradicate 4T1 tumors within 1 week without tumor recurrence (Fig. 3C)74. Thus, the combinational therapeutics have a potent alternative to long-term and multiple cancer therapy. To induce strong functional cytotoxic and potent T-cell responses, van der Maaden et al.75 developed hollow microneedle injection system combined with the synthetic long peptide as a therapeutic cancer vaccine. As compared to intradermal injections, the promising strategy enables much lower volumes, further leading to improve immunogenicity and effectiveness of tumor vaccine formulations. RNA interference has become a novel therapeutic approach to target specific genes for cancer treatment. Tang et al.76 evaluated the efficacy of the microneedle array for delivering siRNA into the tumor region and inhibiting tumor progression activity. With the exception of small drugs and RNA for tumor treatment, McCarthy group presented an important breakthrough technology, which utilized the RALA peptide to encapsulate the E6/E7 plasmid DNA in the dissolving microneedle patch. The microneedle platform can delay tumor initiation and slow tumor growth in a therapeutic model (Fig. 3D)77.
Figure 3.
(A) Dissolvable microneedle arrays made from HA containing chemotherapeutic drug DOX were integrated with gold nanocages followed by exposure to NIR light to combine chemotherapy with photothermal therapy for synergistically treating superficial tumors. Reproduced with permission from Ref. 71. Copyright © 2016, American Chemical Society. (B) A microneedle platform based on HA was encapsulated anti-PD1 antibody to combine with the PD1 and 1-MT to inhibit IDO for melanoma immunotherapy. Reproduced with permission from Ref. 30. Copyright © 2018, American Chemical Society. (C) A light-activatable microneedle patch was composed of dissolvable PVA/PVP as supporting material containing polycaprolactone formulation, which consisted of photothermal nanoparticles and an antitumor drug for treating skin tumors synergistically. While exposed to NIR light, the microneedle patch was melted at 50 °C to release DOX for locoregional cancer therapy. Reproduced with permission from Ref. 74. Copyright © 2015, American Chemical Society. (D) The polymeric polyvinylpyrrolidone microneedle patch brought together two main components including the peptide RALA and DNA vaccine for the treatment of cervical cancer. Reproduced with permission from Ref. 77. Copyright © 2017, Elsevier.
3.2.2. Diabetes
The intricate and multiple daily injection is prone to decrease the patient's compliance. Furthermore, the risk of hypoglycemia owing to frequent mealtime-related administration was significantly improved78.
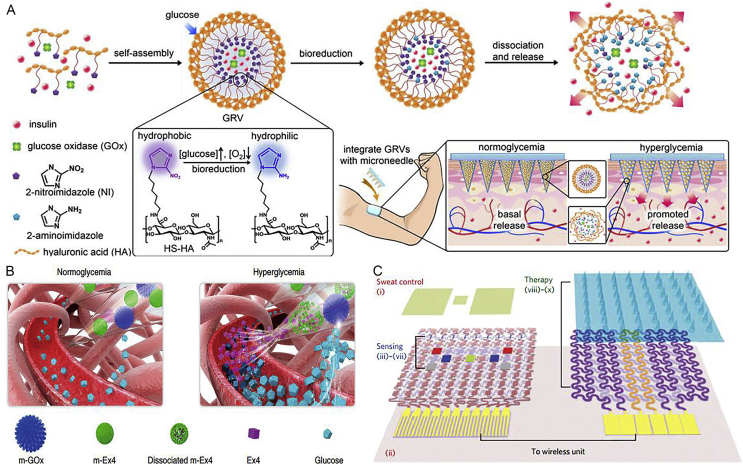
To overcome these challenges, Yu et al.32 demonstrated that a glucose-responsive microneedle array patches regulated the glucose effectively in blood by delivering insulin for type 1 diabetes treatment (Fig. 4A)32. Glucose oxidase served as a potent enzyme, which converted glucose to gluconic acid with the consumption of oxygen. The hyperglycemic state or hypoxic condition could reduce oleophilic 2-nitroimidazole to hydrophilic 2-aminoimidazoles accompanying with the dissociation of hyaluronic acid vesicles spontaneously followed by release of insulin. The smart insulin patch using self-assembled artificial vesicles offered an alternative opportunity for rapid glucose-responsive, pain-free and safe diabetes therapy. Remarkably, Chen et al.25 reported a smart and pH-responsive exendin-4 (Ex4) delivery microneedle patch based on alginate for type 2 diabetes therapy (Fig. 4B)25. This patch loaded with dual mineralized peptide/protein nanoparticles could separately control the release of Ex4 to avoid rapid loss without multiple daily injections. To construct long-term glucose responsiveness, copper phosphate mineralized particles containing glucose oxidase (m-GOx) played a potent role in converting glucose to H+ signals under hyperglycemic conditions, while calcium phosphate mineralized particles encapsulating Ex4 (m-Ex4) as a pH-sensitive biomaterial can be dissolved to release Ex4 under acidic condition. An alginate-based microneedle patch would be an effective candidate to realize facile administration for diabetes 2 patients.
Figure 4.
(A) The glucose-responsive microneedle array patches regulated the glucose effectively in blood by delivering insulin smartly for type 1 diabetes. Reproduced with permission from Ref. 32. Copyright © 2015, PNAS Group. (B) The smart and pH-responsive Ex4 delivery microneedle patch based on alginate for type 2 diabetes therapy was integrated with m-GOx and m-Ex4. Reproduced with permission from Ref. 25. Copyright © 2017, Nature Publishing Group. (C) A wearable/portable electrochemical device based on graphene integrated with a gold mesh, which not only monitored pH, temperature, humidity, and glucose level timely in sweat, but also was induced thermally to release metformin synchronously by dissolving microneedles for diabetes therapy. Reproduced with permission from Ref. 26. Copyright © 2016, Nature Publishing Group.
Lee et al.26 developed a wearable/portable electrochemical device, which can not only monitor pH, temperature, humidity and glucose level timely in sweat, but also be actuated thermally to release metformin transcutaneously for diabetes therapy (Fig. 4C)26. The advanced devices have a great potential to revolutionize POC detection and personalized treatment. The POC patch was made up of stretchable water-proof film (silicone), which consists of the sweat-control layer (i and ii), sensing layer (iii–vii) and therapy layer (viii–x). Simultaneously, the real-time data could be transmitted wirelessly to achieve POC diagnosis and treatment of diabetes.
3.2.3. Obesity
There are two different types of adipose: brown adipose tissue (BAT) and white adipose tissue (WAT). BAT plays a crucial role in producing heat thus increasing body energy expenditure79, 80. Whereas the WAT stores exceeded energy resulting in weight gain, it may further deteriorate the disease due to reactive oxygen species and free fatty acids.
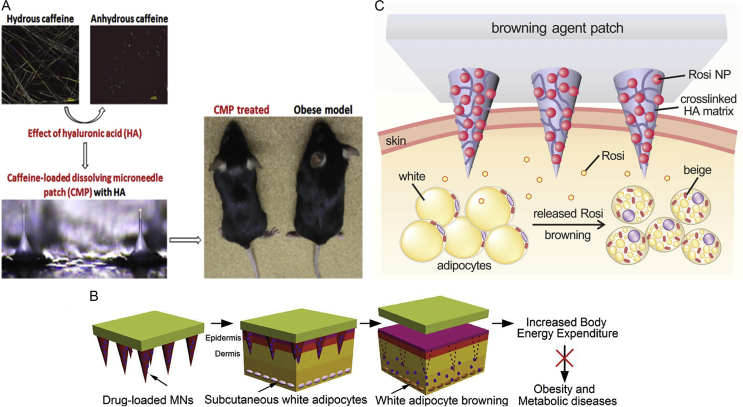
Caffeine extracted from tea or coffee was reported to possess anti-obesity activity without harmful effects for mankind81, 82. Dangol et al.83 found that HA-based dissolving microneedle loading caffeine can lead to the significant weight loss of high-fat diet-induced obese mice (Fig. 5A)83. Further, this study indicated that caffeine possesses outstanding therapeutic activity against obesity by improving the efficiency of transdermal delivery of caffeine. Moreover, a new strategy employing dissolving HA-based microneedles for localized treatment of obesity was realized (Fig. 5B)28. Browning agents including β3-adrenoceptor agonist and thyroid hormone T3 can convert WAT to BAT by transdermal dissolving microneedles to release slowly, and thereby this method can suppress the gaining of weight and enable long-term management. This transdermal delivery approach enables long-term POC treatment due to the long dissolving time. In addition, as illustrated in Fig. 5C35, Zhang et al.35 reported that a locally induced browning patch encapsulating rosiglitazone (Rosi) not only inhibited the gaining of body fat, but also improved insulin sensitivity. The transdermal HA-based device was embedded nanoparticle encapsulating Rosi in the dextran as a browning agent, and glucose oxidase to provide acid environment, and catalase (CAT) to consume undesired H2O2. Then, the ideal device could deliver the browning agent to increase white fat energy expenditure, thus reducing adipose tissue for obesity treatment.
Figure 5.
(A) HA-based dissolving microneedle loading caffeine (natural products reducing fat by lipolysis) could improve the efficiency of transdermal delivery of caffeine due to the inhibition of its crystal growth in dry microneedle and the failure to first pass metabolism. Reproduced with permission from Ref. 83. Copyright © 2017 Elsevier. (B) The dissolving poly(lactic-co-glycolic acid)-based microneedle patches loaded β3-adrenoceptor agonist and thyroid hormone T3 (prominent browning effects) were capable of promoting excess WAT browning and reducing the accumulation of body fat. Reproduced with permission from Ref. 28. Copyright © 2017, WILEY-VCH. (C) The pH-responsive microneedle patch based on HA device can effectively control the release of browning agent in a sustained manner. Reproduced with permission from Ref. 35. Copyright © 2017, American Chemical Society.
3.2.4. Other diseases
Microneedle patches for transdermal drug delivery were also applied in the treatment of other diseases. Alzheimer's disease (AD), whose clinical symptoms are memory loss and language problems, is regarded as a chronic neuro-degenerative disorder. Donepezil hydrochloride (DPH) was considered as a safe and long half-life drug, which was approved by FDA for the effective treatment of AD84. Transdermal delivery of 95% DPH in the tips was transported into porcine skin in 5 min. Obviously, tip-loaded dissolving microneedles encapsulating DPH was more effective as compared with oral administration (Fig. 6A)34. Neuropathic pain resulting from nerve injury causes frequent complaints for patients, which is difficult to treat85. Anti-calcitonin gene related peptide (A-CGRP) is able to selectively block CGRP receptors, thereby inhibiting CGRP signaling to relieve the pain (Fig. 6B)86. It was reported that the analgesic microneedle patches have an effective and simple alternative to relieve localized neuropathic pain by transdermally delivering the high specificity of A-CGRP as compared with clinical treatments. Moreover, neonatal infections including sepsis have become a leading cause of childhood mortality in resource-limited countries87. As is known, intramuscular gentamicin (GEN) and oral amoxicillin were applied to prevent serious bacterial infection in hospital. However, GEN was deposited in vials and required to select an appropriate dose according to patient's weight. Weighing GEN repeatedly leads to bacterial infection easily and injecting GEN has a low therapeutic effect88. Therefore, González-Vázquez et al.89 developed three doses of microneedle patches including low, medium and high doses of GEN to decrease the risk of infection of neonates and young infants avoiding frequent calculation of GEN (Fig. 6C)89. In addition, dihydroergotamine mesylate (DHE) was demonstrated to treat moderate to severe acute migraine attacks by subcutaneous injection and nasal spray which possesses many limitations such as pain and poor bioavailability. To overcome these limitations, a new delivery method integrated with PVP-based dissolving microneedle arrays loading with DHE enabled painless self-administration with high bioavailability (Fig. 6D)90. For long-term treatment of various eye diseases, Than et al.91 reported a strategy for localized, efficient and controlled ocular drug delivery, using a flexible polymeric eye patch equipped with a row of detachable microneedles (Fig. 6E)91. These biodegradable microneedles can penetrate the ocular barriers and then be self-implanted as an array of independent drug reservoirs for controlled drug release.
Figure 6.
(A) An effective method using dissolving microneedles based on hydroxy-propyl-methyl-cellulose was developed to deliver DPH for the treatment of Alzheimer's disease. Reproduced with permission from Ref. 34. Copyright © 2016, Elsevier. (B) Dissolvable microneedle patches mediating transdermal delivery of A-CGRP directly achieved local analgesia treatment successfully. Reproduced with permission from Ref. 86. Copyright © 2016, American Chemical Society. (C) Dissolving microneedles arrays delivered GEN transdermally for the treatment of neonatal sepsis. Reproduced with permission from Ref. 88. Copyright © 2017, Elsevier. (D) Dissolving microneedles patches based on PVP was able to treat acute migraine by the delivery of DHE. Reproduced with permission from Ref. 90. Copyright © 2017, Elsevier. (E) The flexible polymeric eye patch was assembled by an array of biodegradable and detachable microneedles. Reproduced with permission from Ref. 91. Copyright © 2018, Nature Publishing Group.
4. Immunobiological administration
Conventional routes to deliver vaccines or antibodies are administered by hypodermic injection, intramuscular injection or intradermal injection for preventive inoculation. However, conventional vaccination procedures have several shortcomings like needle phobia and the pain accompanying puncture of needle into the dermis. Needle-free vaccination devices, such as liquid jet injectors and microneedles, have been extensively studied92, 93.
To some extent, the stability of vaccines and the durability of antigen have a short life at high temperature. A newly alternative vaccine delivery, the recombination of three formulations, is available to prolong life by needle injection for hepatitis B94.
Microneedle arrays can form transient conduits to increase the transport of vaccine molecules. Ding et al.95 indicated that the clinical response to cholera toxin using microneedle is superior to conventional intramuscular injection.
Dissolving microneedles have to take into consideration of complete penetration and dissolving time. Li et al.96 delivered monoclonal antibody using maltose microneedles in 1 min as compared to solid microneedle for 24 h. Additionally, dissolving microneedles were able to deliver a small dose of hormones and organic compounds.
Sullivan et al.97 introduced biocompatible polymer microneedles integrated with inactivated influenza virus vaccine to improve vaccine immunogenicity, which was superior to intramuscular injection under the same condition. This work indicated that lung virus clearance using dissolving microneedles at the same dose was 1000 times more effective than above-mentioned methods.
Recently, Raphael et al.98 optimized the proportion of formulation made up of mannitol, sucrose, trehalose, and sorbitol to achieve their required vaccine stability. To improve vaccine effect, nanoparticle vaccines were employed for protein antigen to control immune response using hollow microneedle. The results suggested hollow microneedle delivery combined with nanoparticle is a potent method for intradermal injection of nanoparticle vaccines to increase cellular immune responses99. De Groot et al.100 testified that ceramic nanoporous microneedle arrays have a better capability of transporting diphtheria toxoid and tetanus toxoid. Obviously, microneedles transcend other methods depending on their overwhelming advantages including painless, portability, individual use and rapid delivery of vaccine (Table 3)99, 100, 101, 102, 103, 104, 105.
Table 3.
Lists of the latest key finding of immunobiological administration through microneedles.
| Microneedle type | Material | Vaccine (type) | Key finding | Ref. |
|---|---|---|---|---|
| Dissolving microneedle | Hydroxyethyl starch 70000 | Hepatitis B surface antigen (protein) | The antigenicity of the Hepatitis B adjuvant maintained for 6 months at 50 °C with only a 10% loss | 101 |
| Dissolving microneedle | Hyaluronic acid | Live attenuated BCG (bacteria) | Vaccination using microneedle array mixed with Bacille Calmette–Guerin (BCG) powder did not provoked severe inflammation and bruise as compared to intradermal injection | 102 |
| Dissolving microneedle | Sucrose | Rabies vaccination (nucleic acid) | A rabies DNA vaccine integrated with dissolving microneedle was ten-fold lower vaccine dose than full-dose intramuscular vaccination | 103 |
| Dissolving microneedle | Poly-vinyl alcohol | Influenza (virus) | Microneedle patch was capable of improving vaccine immunogenicity and increasing vaccination coverage | 104 |
| Hollow microneedle | Polyimide | IgG and IgG1 (protein) | Nanoparticulate vaccines were discovered for protein antigen to control immune response using hollow microneedle | 99 |
| Solid microneedle and hollow microneedle | Silicone | Ovalbumin (nucleic acid) | Hollow microneedle patches delivered ovalbumin efficiently for in vivo immunization in contrast to solid microneedle patches | 105 |
| Coated microneedle | Ceramic | Diphtheria and tetanus toxoid (peptide) | Ceramic nanoporous microneedle arrays have a better capability of transporting diphtheria toxoid and tetanus toxoid | 100 |
5. Disease diagnosis
Hollow microneedles can puncture the skin into the epidermis to withdraw SIF by capillary force or using vacuum. The extracted SIF metabolites were applied to diagnose several diseases like cancer, atherosclerosis, thrombosis, cardiovascular disorders and diabetes31.
The glucose in the extracted SIF was first monitored by a glucose test strip, using solid glass microneedles to penetrate skin. Then the SIF was extracted by borosilicate glass capillary tubing106. Obviously, the procedure was too complicated and the glass microneedle might have a severe risk of breaking off in skin after usage. Subsequently, microneedles with diverse sensors have been focused in the previous decades. Amaral et al.107 made an effort to develop an alternative neuroscience device integrated with magnetoresistive sensors for neurons in individual cells.
To overcome the drawback of long-time extracting, polymer and hydrogel microneedle patch made it possible to withdraw SIF rapidly. Mandal et al.108 created a microneedle-based technology by coating a cross-linked biocompatible polymer on the surface of solid microneedle. When inserting into skin, the alginate-coated microneedles would swell gradually forming a porous matrix for local leukocyte infiltration. Additionally, Chang et al.31 developed a swellable polymeric microneedle patch for disease diagnosis (Fig. 7A)31. However, the procedure was too cumbersome since the subsequent analysis required centrifugation.
Figure 7.
(A) A swellable polymeric microneedle patch was made of methacrylated hyaluronic acid extracting about 1.4 mg SIF in 1 min. Subsequently, the extracted SIF metabolites were collected by centrifugation to analyse the real-time level of glucose and cholesterol. Reproduced with permission from Ref. 31. Copyright © 2017, WILEY-VCH. (B) The microneedle-based enzyme electrode was functionalized with multi-layer reagent to monitor ethanol in artificial interstitial fluid. Reproduced with permission from Ref. 109. Copyright © 2017, Elsevier. (C) The biosensor utilizing a hollow metallic microneedle connected to microfluidics and photonic components was fabricated to detect target analytes (streptavidin-horseradish peroxidase) rapidly and reach the low limit of detection (60.2 nmol/L). Reproduced with permission from Ref. 110. Copyright © 2018, IOP Publishing Group. (D) A minimally invasive hollow microneedle device with stretchable or wearable POC sensor based on bandage was capable of screening skin melanoma in a portable and wireless mobile device. Reproduced with permission from Ref. 111. Copyright © 2014, WILEY-VCH.
Recently, the microneedle-based enzyme electrode was functionalized with multi-layer reagents to monitor ethanol in SIF (Fig. 7B)109. Pt and Ag wires were used as electrode through the aperture of hollow microneedle immobilizing alcohol oxidase, followed by the biocatalytic reaction. In addition, an alternative device integrated with real-time sensors for biomarker detection has attracted great attention (Fig. 7C)110. These microneedle devices were modified with thiol-PEG-biotin, then producing a reaction with streptavidin-horseradish peroxidase. At last, the enzyme-catalyzed 3,3′,5,5′-tetramethylbenzidine (TMB) oxidation absorbance was quantified at 635 nm to gain a direct binding curve about the amount of streptavidin, because TMB solution containing H2O2 was catalyzed with streptavidin-horseradish peroxidase. Ranamukhaarachchi et al.110 reported that a hollow metallic microneedle connected to microfluidics and photonic components was fabricated to selectively detect target biomarker according to ELISA reaction. Surprisingly, Ciui et al.111 discovered that a minimally invasive hollow microneedle device with stretchable or wearable POC sensor based on bandage was able to diagnose skin tumors timely (Fig. 7D)111. Melanoma biomarker, tyrosinase enzyme, was detected by amperometer integrated with the soft flexible electronics, generating the oxidation reaction with catechol substrate to obtain benzoquinone which was reduced to CAT at −0.25 V. The signal was wirelessly transmitted by Bluetooth to smart device in real time. These integrated and wearable devices hold tremendous promise for decentralized POC diagnosis.
6. Cosmetic field
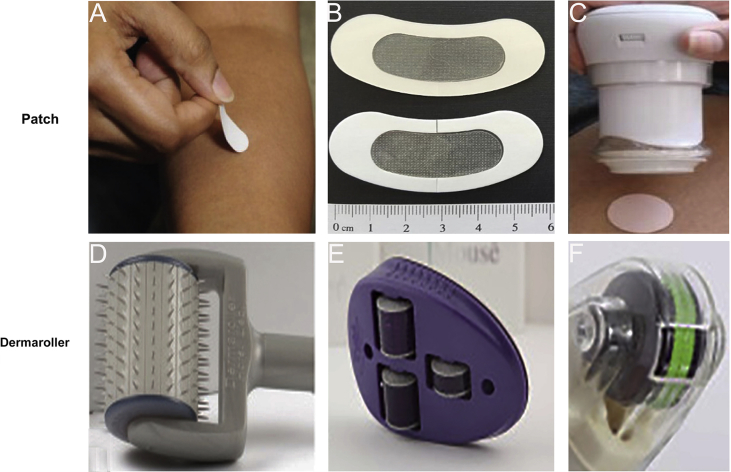
In recent years, cosmetic combined with microneedle treatment has made a great progress. The fact that a large number of cosmeceutical products were manufactured proves that cosmetic application is promising. In general, cosmetic applications are mainly divided into two parts. One is to promote the natural healing of the injured skin. The other is to enhance skin permeation of cosmeceuticals. Minimally invasive delivery of microneedle created transient holes to enhance the penetration, triggering wound repair mechanism spontaneously112. As expected, microneedle fails to cause severe erythema and postinflammatory hyperpigmentation compared with laser for atrophic acne scars treatment113. In addition, microneedle can transport active cosmetic molecules into skin directly for improving effectiveness and safety, creating micro-channels without reaching nerve. Therefore, a novel cosmetic patch loading retinyl retinoate and ascorbic acid were successfully produced for anti-wrinkle without side effects such as allergies114. With the expansion of cosmetic market, commercial scale manufacturing will witness the boom of microneedle product. This can be divided into two broad categories: patches and rollers. Penetrating microneedles patch into the skin at low velocity (e.g., slow insertion by hand) may cause low penetration effect, which is mainly due to the highly elastic skin (Fig. 8A and B)115, 116. To overcome this limitation, it is necessary to apply a high-velocity applicator to improve the skin's instantaneous stiffness and thereby achieving complete penetration efficiency, which is inevitable to increase the cost of microneedles patch (Fig. 8C)115. Microneedle rollers, applied extensively in cosmetic field, were prepared from soft polymer films by inclined rotational UV lithography117, 118. Beauty Mouse® (Fig. 8D)61, produced by the Dermaroller® (Germany), is more beneficial to enhance the skin penetration thanks to three rollers (total number: 480; needles width: 50 mm) as compared to a standard Dermaroller® with 192 needles (Fig. 8E)61. It is noteworthy that the newest device, the DermaFrac™ (Fig. 8F)61, combined with light emitting devices with functional wavelengths is designed for personalized customization only.
Figure 8.
(A) The microneedle patch is manufactured for manual application into the skin without an applicator. Reproduced with permission from Ref. 115, Copyright © 2017, Annual Reviews. (B) HA microneedle eye patch integrated with skin depigmentation ingredients for skin-brightening would be regarded as a promising functional cosmetic product. Reproduced with permission from Ref. 116. Copyright © 2017 WILEY-VCH. (C) A high-velocity applicator as a separate, reusable device is used to achieve complete skin insertion. Reproduced with permission from Ref. 115. Copyright © 2017, Annual Reviews. (D)‒(F) The evolution of typical Dermaroller® designs with microneedles for skin pretreatment. Reproduced with permission from Ref. 61. Copyright © 2017, Elsevier.
7. Conclusions and future perspectives
As an emerging device, microneedles possess characteristic advantages (painless and rapid delivery) as compared to other systemic administration and enable other representative biomedical applications. Even though microneedles have made significant progress for revolutionizing the field of immunobiological administration, disease diagnosis, disease long-term treatment, and cosmetic applications, it is still faced with many problems to be addressed. For example, there are lots of urgent and unmet requirements for the development of wearable and smart device to realize long-term disease treatment. At the same time, the wearable device based microneedle patches will be desirable to integrate disease diagnosis and treatment in the future. Moreover, a large amount of attention should be paid to improve desired drug effectiveness and therapeutic safety in the field of immunobiological administration. Furthermore, disease diagnosis based on microneedle should be associated with APP so as to transmit personalized health data wirelessly. Only in this way can we manage our own health to achieve personalized diagnostics through telemedicine and cloud medicine. At last, the simplicity and economic of cosmetic products based on microneedles may achieve their rapid growth and broader prospects over the next decades. In summary, microneedle holds great promise for biomedical applications.
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (Grants Nos. 21874066, 81601632 and 21563009, China) and the Natural Science Foundation of Jiangsu Province (BK20160616, China), the Fundamental Research Funds for Central Universities (China), the Shuangchuang Program of Jiangsu Province (China), and Thousand Talents Program for Young Researchers (China).
Footnotes
Peer review under responsibility of Institute of Materia Medica, Chinese Academy of Medical Sciences and Chinese Pharmaceutical Association.
Contributor Information
Yunzhi Fu, Email: yzhfu@hainu.edu.cn.
Yujun Song, Email: ysong@nju.edu.cn.
References
- 1.Prausnitz M.R., Mitragotri S., Langer R. Current status and future potential of transdermal drug delivery. Nat Rev Drug Discov. 2004;3:115–124. doi: 10.1038/nrd1304. [DOI] [PubMed] [Google Scholar]
- 2.Wang J.S., Hung Y.J., Lu Y.C., Tsai C.L., Yang W.S., Lee T.I. Difference between observed and predicted glycated hemoglobin at baseline and treatment response to vildagliptin-based dual oral therapy in patients with type 2 diabetes. Diabetes Res Clin Pract. 2018;138:119–127. doi: 10.1016/j.diabres.2018.02.002. [DOI] [PubMed] [Google Scholar]
- 3.Shakya P., Madhav N.V., Shakya A.K., Singh K. Palatal mucosa as a route for systemic drug delivery: a review. J Control Release. 2011;151:2–9. doi: 10.1016/j.jconrel.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 4.Del Río-Sancho S., Serna-Jiménez C.E., Sebastián-Morelló M., Calatayud-Pascual M.A., Balaguer-Fernández C., Femenía-Font A. Transdermal therapeutic systems for memantine delivery. Comparison of passive and iontophoretic transport. Int J Pharm. 2017;517:104–111. doi: 10.1016/j.ijpharm.2016.11.038. [DOI] [PubMed] [Google Scholar]
- 5.Mitragotri S., Kost J. Low-frequency sonophoresis: a review. Adv Drug Deliv Rev. 2004;56:589–601. doi: 10.1016/j.addr.2003.10.024. [DOI] [PubMed] [Google Scholar]
- 6.Denet A.R., Vanbever R., Préat V. Skin electroporation for transdermal and topical delivery. Adv Drug Deliv Rev. 2004;56:659–674. doi: 10.1016/j.addr.2003.10.027. [DOI] [PubMed] [Google Scholar]
- 7.Cevc G. Lipid vesicles and other colloids as drug carriers on the skin. Adv Drug Deliv Rev. 2004;56:675–711. doi: 10.1016/j.addr.2003.10.028. [DOI] [PubMed] [Google Scholar]
- 8.Gill H.S., Prausnitz M.R. Coated microneedles for transdermal delivery. J Control Release. 2007;117:227–237. doi: 10.1016/j.jconrel.2006.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.He H., Lu Y., Qi J., Zhao W., Dong X., Wu W. Biomimetic thiamine- and niacin-decorated liposomes for enhanced oral delivery of insulin. Acta Pharm Sin B. 2018;8:97–105. doi: 10.1016/j.apsb.2017.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim Y.C., Park J.H., Prausnitz M.R. Microneedles for drug and vaccine delivery. Adv Drug Deliv Rev. 2012;64:1547–1568. doi: 10.1016/j.addr.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nir Y., Paz A., Sabo E., Potasman I. Fear of injections in young adults: prevalence and associations. Am J Trop Med Hyg. 2003;68:341–344. [PubMed] [Google Scholar]
- 12.Hamilton J.G. Needle phobia: a neglected diagnosis. J Fam Pract. 1995;41:169–175. [PubMed] [Google Scholar]
- 13.Prausnitz M.R. Microneedles for transdermal drug delivery. Adv Drug Deliv Rev. 2004;56:581–587. doi: 10.1016/j.addr.2003.10.023. [DOI] [PubMed] [Google Scholar]
- 14.Zhang Q., Xu C., Lin S., Zhou H., Yao G., Liu H. Synergistic immunoreaction of acupuncture-like dissolving microneedles containing thymopentin at acupoints in immune-suppressed rats. Acta Pharm Sin B. 2018;8:449–457. doi: 10.1016/j.apsb.2017.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ma X., Song Q., Gao X. Reconstituted high-density lipoproteins: novel biomimetic nanocarriers for drug delivery. Acta Pharm Sin B. 2018;8:51–63. doi: 10.1016/j.apsb.2017.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thomas B.J., Finnin B.C. The transdermal revolution. Drug Discov Today. 2004;9:697–703. doi: 10.1016/S1359-6446(04)03180-0. [DOI] [PubMed] [Google Scholar]
- 17.Hegde N.R., Kaveri S.V., Bayry J. Recent advances in the administration of vaccines for infectious diseases: microneedles as painless delivery devices for mass vaccination. Drug Discov Today. 2011;16:1061–1068. doi: 10.1016/j.drudis.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 18.Lee J.W., Park J.H., Prausnitz M.R. Dissolving microneedles for transdermal drug delivery. Biomaterials. 2008;29:2113–2124. doi: 10.1016/j.biomaterials.2007.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jain A.K., Lee C.H., Gill H.S. 5-Aminolevulinic acid coated microneedles for photodynamic therapy of skin tumors. J Control Release. 2016;239:72–81. doi: 10.1016/j.jconrel.2016.08.015. [DOI] [PubMed] [Google Scholar]
- 20.Chen G., Hao B., Ju D., Liu M., Zhao H., Du Z. Pharmacokinetic and pharmacodynamic study of triptolide-loaded liposome hydrogel patch under microneedles on rats with collagen-induced arthritis. Acta Pharm Sin B. 2015;5:569–576. doi: 10.1016/j.apsb.2015.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gardeniers H.J., Luttge R., Berenschot E.J., de Boer M.J., Yeshurun S.Y., Hefetz M. Silicon micromachined hollow microneedles for transdermal liquid transport. J Microelectromech Syst. 2003;12:855–862. [Google Scholar]
- 22.Zhang S., Qiu Y., Gao Y. Enhanced delivery of hydrophilic peptides in vitro by transdermal microneedle pretreatment. Acta Pharm Sin B. 2014;4:100–104. doi: 10.1016/j.apsb.2013.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tai W., Mo R., Di J., Subramanian V., Gu X., Buse J.B. Bio-inspired synthetic nanovesicles for glucose-responsive release of insulin. Biomacromolecules. 2014;15:3495–3502. doi: 10.1021/bm500364a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Song Y., Zhang Y., Bernard P.E., Reuben J.M., Ueno N.T., Arlinghaus R.B. Multiplexed volumetric bar-chart chip for point-of-care diagnostics. Nat Commun. 2012;3:1283. doi: 10.1038/ncomms2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen W., Tian R., Xu C., Yung B.C., Wang G., Liu Y. Microneedle-array patches loaded with dual mineralized protein/peptide particles for type 2 diabetes therapy. Nat Commun. 2017;8:1777. doi: 10.1038/s41467-017-01764-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee H., Choi T.K., Lee Y.B., Cho H.R., Ghaffari R., Wang L. A graphene-based electrochemical device with thermoresponsive microneedles for diabetes monitoring and therapy. Nat Nanotechnol. 2016;11:566–572. doi: 10.1038/nnano.2016.38. [DOI] [PubMed] [Google Scholar]
- 27.Song Y., Huang Y.Y., Liu X., Zhang X., Ferrari M., Qin L. Point-of-care technologies for molecular diagnostics using a drop of blood. Trends Biotechnol. 2014;32:132–139. doi: 10.1016/j.tibtech.2014.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Than A., Liang K., Xu S., Sun L., Duan H., Xi F. Transdermal delivery of anti-obesity compounds to subcutaneous adipose tissue with polymeric microneedle patches. Small Methods. 2017;1:1700269. [Google Scholar]
- 29.Song Y., Wang Y., Qi W., Li Y., Xuan J., Wang P. Integrative volumetric bar-chart chip for rapid and quantitative point-of-care detection of myocardial infarction biomarkers. Lab Chip. 2016;16:2955–2962. doi: 10.1039/c6lc00561f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ye Y., Wang J., Hu Q., Hochu G.M., Xin H., Wang C. Synergistic transcutaneous immunotherapy enhances antitumor immune responses through delivery of checkpoint inhibitors. ACS Nano. 2016;10:8956–8963. doi: 10.1021/acsnano.6b04989. [DOI] [PubMed] [Google Scholar]
- 31.Chang H., Zheng M., Yu X., Than A., Seeni R.Z., Kang R. A swellable microneedle patch to rapidly extract skin interstitial fluid for timely metabolic analysis. Adv Mater. 2017;29:1702243. doi: 10.1002/adma.201702243. [DOI] [PubMed] [Google Scholar]
- 32.Yu J., Zhang Y., Ye Y., DiSanto R., Sun W., Ranson D. Microneedle-array patches loaded with hypoxia-sensitive vesicles provide fast glucose-responsive insulin delivery. Proc Natl Acad Sci U S A. 2015;112:8260–8265. doi: 10.1073/pnas.1505405112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xie Y., Xu B., Gao Y. Controlled transdermal delivery of model drug compounds by MEMS microneedle array. Nanomedicine. 2005;1:184–190. doi: 10.1016/j.nano.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 34.Kim J.Y., Han M.R., Kim Y.H., Shin S.W., Nam S.Y., Park J.H. Tip-loaded dissolving microneedles for transdermal delivery of donepezil hydrochloride for treatment of Alzheimer's disease. Eur J Pharm Biopharm. 2016;105:148–155. doi: 10.1016/j.ejpb.2016.06.006. [DOI] [PubMed] [Google Scholar]
- 35.Zhang Y., Liu Q., Yu J., Yu S., Wang J., Qiang L. Locally induced adipose tissue browning by microneedle patch for obesity treatment. ACS Nano. 2017;11:9223–9230. doi: 10.1021/acsnano.7b04348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Donnelly R.F., Larrañeta E. Microarray patches: potentially useful delivery systems for long-acting nanosuspensions. Drug Discov Today. 2018;23:1026–1033. doi: 10.1016/j.drudis.2017.10.013. [DOI] [PubMed] [Google Scholar]
- 37.Ito Y., Yoshimitsu J.I., Shiroyama K., Sugioka N., Takada K. Self-dissolving microneedles for the percutaneous absorption of EPO in mice. J Drug Target. 2006;14:255–261. doi: 10.1080/10611860600785080. [DOI] [PubMed] [Google Scholar]
- 38.Blagus T., Markelc B., Cemazar M. In vivo real-time monitoring system of electroporation mediated control of transdermal and topical drug delivery. J Control Release. 2013;172:862–871. doi: 10.1016/j.jconrel.2013.09.030. [DOI] [PubMed] [Google Scholar]
- 39.Li W.Z., Huo M.R., Zhou J.P., Zhou Y.Q., Hao B.H., Liu T. Super-short solid silicon microneedles for transdermal drug delivery applications. Int J Pharm. 2010;389:122–129. doi: 10.1016/j.ijpharm.2010.01.024. [DOI] [PubMed] [Google Scholar]
- 40.Kim K., Park D.S., Lu H.M., Che W., Kim K., Lee J.B. A tapered hollow metallic microneedle array using backside exposure of SU-8. J Micromech Microeng. 2004;14:597–603. [Google Scholar]
- 41.Yoon Y.K., Park J.H., Allen M.G. Multidirectional uv lithography for complex 3-D mems structures. J Microelectromech Syst. 2006;15:1121–1130. [Google Scholar]
- 42.Lim S.H., Ng J.Y., Kang L. Three-dimensional printing of a microneedle array on personalized curved surfaces for dual-pronged treatment of trigger finger. Biofabrication. 2017;9:015010. doi: 10.1088/1758-5090/9/1/015010. [DOI] [PubMed] [Google Scholar]
- 43.Gill H.S., Prausnitz M.R. Coating formulations for microneedles. Pharm Res. 2007;24:1369–1380. doi: 10.1007/s11095-007-9286-4. [DOI] [PubMed] [Google Scholar]
- 44.DeMuth P.C., Su X., Samuel R.E., Hammond P.T., Irvine D.J. Nano-layered microneedles for transcutaneous delivery of polymer nanoparticles and plasmid DNA. Adv Mater. 2010;22:4851–4856. doi: 10.1002/adma.201001525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang Y., Brown K., Siebenaler K., Determan A., Dohmeier D., Hansen K. Development of lidocaine-coated microneedle product for rapid, safe, and prolonged local analgesic action. Pharm Res. 2012;29:170–177. doi: 10.1007/s11095-011-0524-4. [DOI] [PubMed] [Google Scholar]
- 46.Prow T.W., Chen X., Prow N.A., Fernando G.J., Tan C.S., Raphael A.P. Nanopatch-targeted skin vaccination against West Nile virus and Chikungunya virus in mice. Small. 2010;6:1776–1784. doi: 10.1002/smll.201000331. [DOI] [PubMed] [Google Scholar]
- 47.Chen X., Prow T.W., Crichton M.L., Jenkins D.W., Roberts M.S., Frazer I.H. Dry-coated microprojection array patches for targeted delivery of immunotherapeutics to the skin. J Control Release. 2009;139:212–220. doi: 10.1016/j.jconrel.2009.06.029. [DOI] [PubMed] [Google Scholar]
- 48.Chen X., Kask A.S., Crichton M.L., McNeilly C., Yukiko S., Dong L. Improved DNA vaccination by skin-targeted delivery using dry-coated densely-packed microprojection arrays. J Control Release. 2010;148:327–333. doi: 10.1016/j.jconrel.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 49.Pere C.P., Economidou S.N., Lall G., Ziraud C., Boateng J.S., Alexander B.D. 3D printed microneedles for insulin skin delivery. Int J Pharm. 2018;544:425–432. doi: 10.1016/j.ijpharm.2018.03.031. [DOI] [PubMed] [Google Scholar]
- 50.Gratieri T., Alberti I., Lapteva M., Kalia Y.N. Next generation intra- and transdermal therapeutic systems: using non- and minimally-invasive technologies to increase drug delivery into and across the skin. Eur J Pharm Sci. 2013;50:609–622. doi: 10.1016/j.ejps.2013.03.019. [DOI] [PubMed] [Google Scholar]
- 51.Donnelly R.F., Majithiya R., Singh T.R., Morrow D.I., Garland M.J., Demir Y.K. Design, optimization and characterisation of polymeric microneedle arrays prepared by a novel laser-based micromoulding technique. Pharm Res. 2011;28:41–57. doi: 10.1007/s11095-010-0169-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Martin C.J., Allender C.J., Brain K.R., Morrissey A., Birchall J.C. Low temperature fabrication of biodegradable sugar glass microneedles for transdermal drug delivery applications. J Control Release. 2012;158:93–101. doi: 10.1016/j.jconrel.2011.10.024. [DOI] [PubMed] [Google Scholar]
- 53.Sullivan S.P., Murthy N., Prausnitz M.R. Minimally invasive protein delivery with rapidly dissolving polymer microneedles. Adv Mater. 2008;20:933–938. doi: 10.1002/adma.200701205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Min J., Park J.H., Yoon H.H., Choy Y.B. Ultrasonic welding method to fabricate polymer microstructure encapsulating protein with minimum damage. Macromol Res. 2008;16:570–573. [Google Scholar]
- 55.Luzuriaga M.A., Berry D.R., Reagan J.C., Smaldone R.A., Gassensmith J.J. Biodegradable 3D printed polymer microneedles for transdermal drug delivery. Lab Chip. 2018;18:1223–1230. doi: 10.1039/c8lc00098k. [DOI] [PubMed] [Google Scholar]
- 56.Ito Y., Hagiwara E., Saeki A., Sugioka N., Takada K. Feasibility of microneedles for percutaneous absorption of insulin. Eur J Pharm Sci. 2006;29:82–88. doi: 10.1016/j.ejps.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 57.Yu L.M., Tay F.E., Guo D.G., Xu L., Yap K.L. A microfabricated electrode with hollow microneedles for ECG measurement. Sensor Actuat A Phys. 2009;151:17–22. [Google Scholar]
- 58.Wang P.M., Cornwell M., Hill J., Prausnitz M.R. Precise microinjection into skin using hollow microneedles. J Invest Dermatol. 2006;126:1080–1087. doi: 10.1038/sj.jid.5700150. [DOI] [PubMed] [Google Scholar]
- 59.Moon S.J., Lee S.S., Lee H.S., Kwon T.H. Fabrication of microneedle array using LIGA and hot embossing process. Microsyst Technol. 2005;11:311–318. [Google Scholar]
- 60.Miller P.R., Xiao X., Brener I., Burckel D.B., Narayan R., Polsky R. Microneedle-based transdermal sensor for on-chip potentiometric determination of K+ Adv Healthc Mater. 2014;3:876–881. doi: 10.1002/adhm.201300541. [DOI] [PubMed] [Google Scholar]
- 61.Bhatnagar S., Dave K., Venuganti V.V. Microneedles in the clinic. J Control Release. 2017;260:164–182. doi: 10.1016/j.jconrel.2017.05.029. [DOI] [PubMed] [Google Scholar]
- 62.Chen Z., Lin Y., Lee W., Ren L., Liu B., Liang L. Additive manufacturing of honeybee-inspired microneedle for easy skin insertion and difficult removal. ACS Appl Mater Interfaces. 2018;10:29338–29346. doi: 10.1021/acsami.8b09563. [DOI] [PubMed] [Google Scholar]
- 63.Sanjay S.T., Zhou W., Dou M., Tavakoli H., Ma L., Xu F. Recent advances of controlled drug delivery using microfluidic platforms. Adv Drug Deliv Rev. 2018;128:3–28. doi: 10.1016/j.addr.2017.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lim D.J., Vines J.B., Park H., Lee S.H. Microneedles: a versatile strategy for transdermal delivery of biological molecules. Int J Biol Macromol. 2018;110:30–38. doi: 10.1016/j.ijbiomac.2017.12.027. [DOI] [PubMed] [Google Scholar]
- 65.McAllister D.V., Wang P.M., Davis S.P., Park J.H., Canatella P.J., Allen M.G. Microfabricated needles for transdermal delivery of macromolecules and nanoparticles: fabrication methods and transport studies. Proc Natl Acad Sci U S A. 2003;100:13755–13760. doi: 10.1073/pnas.2331316100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Indermun S., Luttge R., Choonara Y.E., Kumar P., du Toit L.C., Modi G. Current advances in the fabrication of microneedles for transdermal delivery. J Control Release. 2014;185:130–138. doi: 10.1016/j.jconrel.2014.04.052. [DOI] [PubMed] [Google Scholar]
- 67.Lambert P.H., Laurent P.E. Intradermal vaccine delivery: will new delivery systems transform vaccine administration? Vaccine. 2008;26:3197–3208. doi: 10.1016/j.vaccine.2008.03.095. [DOI] [PubMed] [Google Scholar]
- 68.Teo A.L., Shearwood C., Ng K.C., Lu J., Moochhala S. Transdermal microneedles for drug delivery applications. Mater Sci Eng B. 2006;132:151–154. [Google Scholar]
- 69.Escobar-Chávez J.J., Bonilla-Martínez D., Angélica M., Villegas-González M., Molina-Trinidad E., Casas-Alancaster N. Microneedles: a valuable physical enhancer to increase transdermal drug delivery. J Clin Pharmacol. 2011;51:964–977. doi: 10.1177/0091270010378859. [DOI] [PubMed] [Google Scholar]
- 70.Hu Q., Sun W., Lu Y., Bomba H.N., Ye Y., Jiang T. Tumor microenvironment-mediated construction and deconstruction of extracellular drug-delivery depots. Nano Lett. 2016;16:1118–1126. doi: 10.1021/acs.nanolett.5b04343. [DOI] [PubMed] [Google Scholar]
- 71.Dong L., Li Y., Li Z., Xu N., Liu P., Du H. Au nanocage-strengthened dissolving microneedles for chemo-photothermal combined therapy of superficial skin tumors. ACS Appl Mater Interfaces. 2018;10:9247–9256. doi: 10.1021/acsami.7b18293. [DOI] [PubMed] [Google Scholar]
- 72.Topalian S.L., Drake C.G., Pardoll D.M. Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell. 2015;27:450–461. doi: 10.1016/j.ccell.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jeanbart L., Swartz M.A. Engineering opportunities in cancer immunotherapy. Proc Natl Acad Sci U S A. 2015;112:14467–14472. doi: 10.1073/pnas.1508516112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chen M.C., Lin Z.W., Ling M.H. Near-infrared light-activatable microneedle system for treating superficial tumors by combination of chemotherapy and photothermal therapy. ACS Nano. 2016;10:93–101. doi: 10.1021/acsnano.5b05043. [DOI] [PubMed] [Google Scholar]
- 75.van der Maaden K., Heuts J., Camps M., Pontier M., van Scheltinga A.T., Jiskoot W. Hollow microneedle-mediated micro-injections of a liposomal HPV E743-63 synthetic long peptide vaccine for efficient induction of cytotoxic and T-helper responses. J Control Release. 2018;269:347–354. doi: 10.1016/j.jconrel.2017.11.035. [DOI] [PubMed] [Google Scholar]
- 76.Tang T., Deng Y., Chen J., Zhao Y., Yue R., Choy K.W. Local administration of siRNA through microneedle: optimization, bio-distribution, tumor suppression and toxicity. Sci Rep. 2016;6:30430. doi: 10.1038/srep30430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ali A.A., McCrudden C.M., McCaffrey J., McBride J.W., Cole G., Dunne N.J. DNA vaccination for cervical cancer; a novel technology platform of RALA mediated gene delivery via polymeric microneedles. Nanomedicine. 2017;13:921–932. doi: 10.1016/j.nano.2016.11.019. [DOI] [PubMed] [Google Scholar]
- 78.Hou G.Y., Men L.H., Wang L., Zheng Z., Liu Z.Q., Song F.R. Quantitative analysis of urinary endogenous markers for the treatment effect of Radix Scutellariae on type 2 diabetes rats. Chin Chem Lett. 2017;28:1214–1219. [Google Scholar]
- 79.Wu J., Cohen P., Spiegelman B.M. Adaptive thermogenesis in adipocytes: is beige the new brown? Genes Dev. 2013;27:234–250. doi: 10.1101/gad.211649.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yoneshiro T., Matsushita M., Nakae S., Kameya T., Sugie H., Tanaka S. Brown adipose tissue is involved in the seasonal variation of cold-induced thermogenesis in humans. Am J Physiol Regul Integr Comp Physiol. 2016;310:R999–R1009. doi: 10.1152/ajpregu.00057.2015. [DOI] [PubMed] [Google Scholar]
- 81.Barquissau V., Léger B., Beuzelin D., Martins F., Amri E.Z., Pisani D.F. Caloric restriction and diet-induced weight loss do not induce browning of human subcutaneous white adipose tissue in women and men with obesity. Cell Rep. 2018;22:1079–1089. doi: 10.1016/j.celrep.2017.12.102. [DOI] [PubMed] [Google Scholar]
- 82.Zheng G., Sayama K., Okubo T., Juneja L.R., Oguni I. Anti-obesity effects of three major components of green tea, catechins, caffeine and theanine, in mice. In Vivo. 2004;18:55–62. [PubMed] [Google Scholar]
- 83.Dangol M., Kim S., Li C.G., Lahiji S.F., Jang M., Ma Y. Anti-obesity effect of a novel caffeine-loaded dissolving microneedle patch in high-fat diet-induced obese C57BL/6J mice. J Control Release. 2017;265:41–47. doi: 10.1016/j.jconrel.2017.03.400. [DOI] [PubMed] [Google Scholar]
- 84.Tariot P., Salloway S., Yardley J., Mackell J., Moline M. Long-term safety and tolerability of donepezil 23 mg in patients with moderate to severe Alzheimer's disease. BMC Res Notes. 2012;5:283. doi: 10.1186/1756-0500-5-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Baron R. Mechanisms of disease: neuropathic pain-a clinical perspective. Nat Clin Pract Neuroll. 2006;2:95–108. doi: 10.1038/ncpneuro0113. [DOI] [PubMed] [Google Scholar]
- 86.Xie X., Pascual C., Lieu C., Oh S., Wang J., Zou B. Analgesic microneedle patch for neuropathic pain therapy. ACS Nano. 2017;11:395–406. doi: 10.1021/acsnano.6b06104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lawn J.E., Cousens S., Zupan J. 4 million neonatal deaths: when? where? why? Lancet. 2005;365:891–900. doi: 10.1016/S0140-6736(05)71048-5. [DOI] [PubMed] [Google Scholar]
- 88.Darmstadt G.L., Batra M., Zaidi A.K. Parenteral antibiotics for the treatment of serious neonatal bacterial infections in developing country settings. Pediatr Infect Dis J. 2009;28(1 Suppl):S37–S42. doi: 10.1097/INF.0b013e31819588c3. [DOI] [PubMed] [Google Scholar]
- 89.González-Vázquez P., Larrañeta E., McCrudden M.T., Jarrahian C., Rein-Weston A., Quintanar-Solares M. Transdermal delivery of gentamicin using dissolving microneedle arrays for potential treatment of neonatal sepsis. J Control Release. 2017;265:30–40. doi: 10.1016/j.jconrel.2017.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tas C., Joyce J.C., Nguyen H.X., Eangoor P., Knaack J.S., Banga A.K. Dihydroergotamine mesylate-loaded dissolving microneedle patch made of polyvinylpyrrolidone for management of acute migraine therapy. J Control Release. 2017;268:159–165. doi: 10.1016/j.jconrel.2017.10.021. [DOI] [PubMed] [Google Scholar]
- 91.Than A., Liu C., Chang H., Duong P.K., Cheung C.M., Xu C. Self-implantable double-layered micro-drug-reservoirs for efficient and controlled ocular drug delivery. Nat Commun. 2018;9:4433. doi: 10.1038/s41467-018-06981-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhou Z., Lin H., Li C., Wu Z. Recent progress of fully synthetic carbohydrate-based vaccine using TLR agonist as build-in adjuvant. Chin Chem Lett. 2018;29:19–26. [Google Scholar]
- 93.Moffatt K., Wang Y., Singh T.R., Donnelly R.F. Microneedles for enhanced transdermal and intraocular drug delivery. Curr Opin Pharmacol. 2017;36:14–21. doi: 10.1016/j.coph.2017.07.007. [DOI] [PubMed] [Google Scholar]
- 94.Hirschberg H.J., van de Wijdeven G.G., Kraan H., Amorij J.P., Kersten G.F. Bioneedles as alternative delivery system for hepatitis B vaccine. J Control Release. 2010;147:211–217. doi: 10.1016/j.jconrel.2010.06.028. [DOI] [PubMed] [Google Scholar]
- 95.Ding Z., Verbaan F.J., Bivas-Benita M., Bungener L., Huckriede A., van den Berg D.J. Microneedle arrays for the transcutaneous immunization of diphtheria and influenza in BALB/c mice. J Control Release. 2009;136:71–78. doi: 10.1016/j.jconrel.2009.01.025. [DOI] [PubMed] [Google Scholar]
- 96.Li G., Badkar A., Nema S., Kolli C.S., Banga A.K. In vitro transdermal delivery of therapeutic antibodies using maltose microneedles. Int J Pharm. 2009;368:109–115. doi: 10.1016/j.ijpharm.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 97.Sullivan S.P., Koutsonanos D.G., del Pilar Martin M. Dissolving polymer microneedle patches for influenza vaccination. Nat Med. 2010;16:915–920. doi: 10.1038/nm.2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Raphael A.P., Crichton M.L., Falconer R.J., Meliga S., Chen X., Fernando G.J. Formulations for microprojection/microneedle vaccine delivery: structure, strength and release profiles. J Control Release. 2016;225:40–52. doi: 10.1016/j.jconrel.2016.01.027. [DOI] [PubMed] [Google Scholar]
- 99.Du G., Hathout R.M., Nasr M., Nejadnik M.R., Tu J., Koning R.I. Intradermal vaccination with hollow microneedles: a comparative study of various protein antigen and adjuvant encapsulated nanoparticles. J Control Release. 2017;266:109–118. doi: 10.1016/j.jconrel.2017.09.021. [DOI] [PubMed] [Google Scholar]
- 100.De Groot A.M., Platteel A.C., Kuijt N., van Kooten P.J., Vos P.J., Sijts A.J. Nanoporous microneedle arrays effectively induce antibody responses against diphtheria and tetanus toxoid. Front Immunol. 2017;8:1789. doi: 10.3389/fimmu.2017.01789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Poirier D., Renaud F., Dewar V., Strodiot L., Wauters F., Janimak J. Hepatitis B surface antigen incorporated in dissolvable microneedle array patch is antigenic and thermostable. Biomaterials. 2017;145:256–265. doi: 10.1016/j.biomaterials.2017.08.038. [DOI] [PubMed] [Google Scholar]
- 102.Chen F., Yan Q., Yu Y., Wu M.X. BCG vaccine powder-laden and dissolvable microneedle arrays for lesion-free vaccination. J Control Release. 2017;255:36–44. doi: 10.1016/j.jconrel.2017.03.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Arya J.M., Dewitt K., Scott-Garrard M., Chiang Y.W., Prausnitz M.R. Rabies vaccination in dogs using a dissolving microneedle patch. J Control Release. 2016;239:19–26. doi: 10.1016/j.jconrel.2016.08.012. [DOI] [PubMed] [Google Scholar]
- 104.Littauer E.Q., Mills L.K., Brock N., Esser E.S., Romanyuk A., Pulit-Penaloza J.A. Stable incorporation of GM-CSF into dissolvable microneedle patch improves skin vaccination against influenza. J Control Release. 2018;276:1–16. doi: 10.1016/j.jconrel.2018.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Pamornpathomkul B., Niyomtham N., Yingyongnarongkul B.E., Prasitpuriprecha C., Rojanarata T., Ngawhirunpat T. Cationic niosomes for enhanced skin immunization of plasmid DNA-encoding ovalbumin via hollow microneedles. AAPS PharmSciTech. 2018;19:481–488. doi: 10.1208/s12249-017-0855-5. [DOI] [PubMed] [Google Scholar]
- 106.Wang P.M., Cornwell M., Prausnitz M.R. Minimally invasive extraction of dermal interstitial fluid for glucose monitoring using microneedles. Diabetes Technol Ther. 2005;7:131–141. doi: 10.1089/dia.2005.7.131. [DOI] [PubMed] [Google Scholar]
- 107.Amaral J., Pinto V., Costa T., Gaspar J., Ferreira R., Paz E. Integration of TMR sensors in silicon microneedles for magnetic measurements of neurons. IEEE Trans Magn. 2013;49:3512–3515. [Google Scholar]
- 108.Mandal A., Boopathy A.V., Lam L.K., Moynihan K.D., Welch M.E., Bennett N.R. Cell and fluid sampling microneedle patches for monitoring skin-resident immunity. Sci Transl Med. 2018;10:eaar2227. doi: 10.1126/scitranslmed.aar2227. [DOI] [PubMed] [Google Scholar]
- 109.Mohan A.M., Windmiller J.R., Mishra R.K., Wang J. Continuous minimally-invasive alcohol monitoring using microneedle sensor arrays. Biosens Bioelectron. 2017;91:574–579. doi: 10.1016/j.bios.2017.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ranamukhaarachchi S.A., Padeste C., Häfeli U.O., Stoeber B., Cadarso V.J. Design considerations of a hollow microneedle-optofluidic biosensing platform incorporating enzyme-linked assays. J Micromech Microeng. 2017;28:024002. [Google Scholar]
- 111.Ciui B., Martin A., Mishra R.K., Brunetti B., Nakagawa T., Dawkins T.J. Wearable wireless tyrosinase bandage and microneedle sensors: toward melanoma screening. Adv Healthc Mater. 2018;7:1701264. doi: 10.1002/adhm.201701264. [DOI] [PubMed] [Google Scholar]
- 112.Fabbrocini G., De Vita V., Monfrecola A., De Padova M.P., Brazzini B., Teixeira F. Percutaneous collagen induction: an effective and safe treatment for post-acne scarring in different skin phototypes. J Dermatol Treat. 2014;25:147–152. doi: 10.3109/09546634.2012.742949. [DOI] [PubMed] [Google Scholar]
- 113.Cachafeiro T., Escobar G., Maldonado G., Cestari T., Corleta O. Comparison of nonablative fractional erbium laser 1,340 nm and microneedling for the treatment of atrophic acne scars: a randomized clinical trial. Dermatol Surg. 2016;42:232–241. doi: 10.1097/DSS.0000000000000597. [DOI] [PubMed] [Google Scholar]
- 114.Kim M., Yang H., Kim H., Jung H., Jung H. Novel cosmetic patches for wrinkle improvement: retinyl retinoate- and ascorbic acid-loaded dissolving microneedles. Int J Cosmet Sci. 2014;36:207–212. doi: 10.1111/ics.12115. [DOI] [PubMed] [Google Scholar]
- 115.Prausnitz M.R. Engineering microneedle patches for vaccination and drug delivery to skin. Annu Rev Chem Biomol Eng. 2017;8:177–200. doi: 10.1146/annurev-chembioeng-060816-101514. [DOI] [PubMed] [Google Scholar]
- 116.Park K.Y., Kwon H.J., Lee C., Kim D., Yoon J.J., Kim M.N. Efficacy and safety of a new microneedle patch for skin brightening: a randomized, split-face, single-blind study. J Cosmet Dermatol. 2017;16:382–387. doi: 10.1111/jocd.12354. [DOI] [PubMed] [Google Scholar]
- 117.Park J.H., Choi S.O., Seo S., Choy Y.B., Prausnitz M.R. A microneedle roller for transdermal drug delivery. Eur J Pharm Biopharm. 2010;76:282–289. doi: 10.1016/j.ejpb.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 118.Coulman S.A., Birchall J.C., Alex A., Pearton M., Hofer B., O'Mahony C. In vivo, in situ imaging of microneedle insertion into the skin of human volunteers using optical coherence tomography. Pharm Res. 2011;28:66–81. doi: 10.1007/s11095-010-0167-x. [DOI] [PubMed] [Google Scholar]