Highlights
-
•
Recombinant human dentin matrix protein 1 (DMP1) can be produced in Escherichia coli.
-
•
E. coli produced DMP1 could induce the expression of osteogenic-related genes and calcium deposition in human PDL cells.
-
•
This protein has potential to use for improving tooth repair and regeneration in the future.
Keywords: Dentin matrix protein 1 (DMP1), Prokaryotic expression, E. coli, Purification
Abstract
The study aimed to produce recombinant human dentin matrix protein 1 (DMP1) and to test, whether the recombinant DMP1 produced in Escherichia coli possesses functional activity. A gene construction comprising a gene encoding for DMP1 protein with polyhistidine sequence at its C-terminus was created using the pET22b plasmid and expressed in E. coli. The optimization of cultivation conditions has enabled the induction of the gene expression with 0.5 mM isopropyl β-D-1-thiogalactopyranoside (IPTG) and DMP1 recombinant protein production at 37 °C for 6 h. The recombinant protein was purified using Ni affinity chromatography. DMP1 influence on the viability, osteogenic differentiation and calcification of human periodontal ligament (PDL) cells was examined. The purified DMP1 could induce the expression of osteogenesis related genes and calcium deposition in PDL cells. These findings indicate that DMP1 produced in E. coli can induce the osteogenic differentiation of human PDL cells, leading to improved tooth repair and regeneration.
1. Introduction
Dentin matrix proteins (DMPs) are a group of noncollageneous proteins expressed in the extracellular matrix of dentin and bone. There are four different proteins: dentin matrix protein 1 (DMP1), dentin phosphoprotein (DPP) or dentin matrix protein 2 (DMP2), dentin sialoprotein (DSP) and DMP4. The first multifunctional protein identified from the dentin matrix was DMP1.
Dentin matrix protein 1 (DMP1) is a highly phosphorylated protein that belongs to the family of small integrin-binding ligand N-linked glycoproteins (SIBLINGs) [1]. DMP1 plays an important role in osteoblast differentiation and matrix mineralization [2,3]. DMP1 contains an RGD domain, which serves as a ligand for the integrin receptor on the cell membrane. Thus, DMP1 can regulate cell adhesion and promote selective cell attachment [4,5]. Moreover, DMP1 also plays important roles in the induction of bone formation, stem cell differentiation and biomineralization [6,7]. Therefore, DMP1 is an important signalling molecule in bone tissue engineering.
Among various recombinant protein expression systems, bacteria remain attractive. The bacterial expression system is a cost-effective platform that allows rapid biomass accumulation. The scale-up process is also simple. Therefore, protein expression in Escherichia coli is very promising on an industrial scale.
In this study, we optimized the conditions for recombinant human DMP1 expression in E. coli. DMP1 was purified using Ni affinity chromatography. In addition, the cytotoxicity, osteogenic differentiation and calcification of human periodontal ligament (PDL) cells were investigated.
2. Materials and methods
2.1. Expression of human DMP1 in E. coli
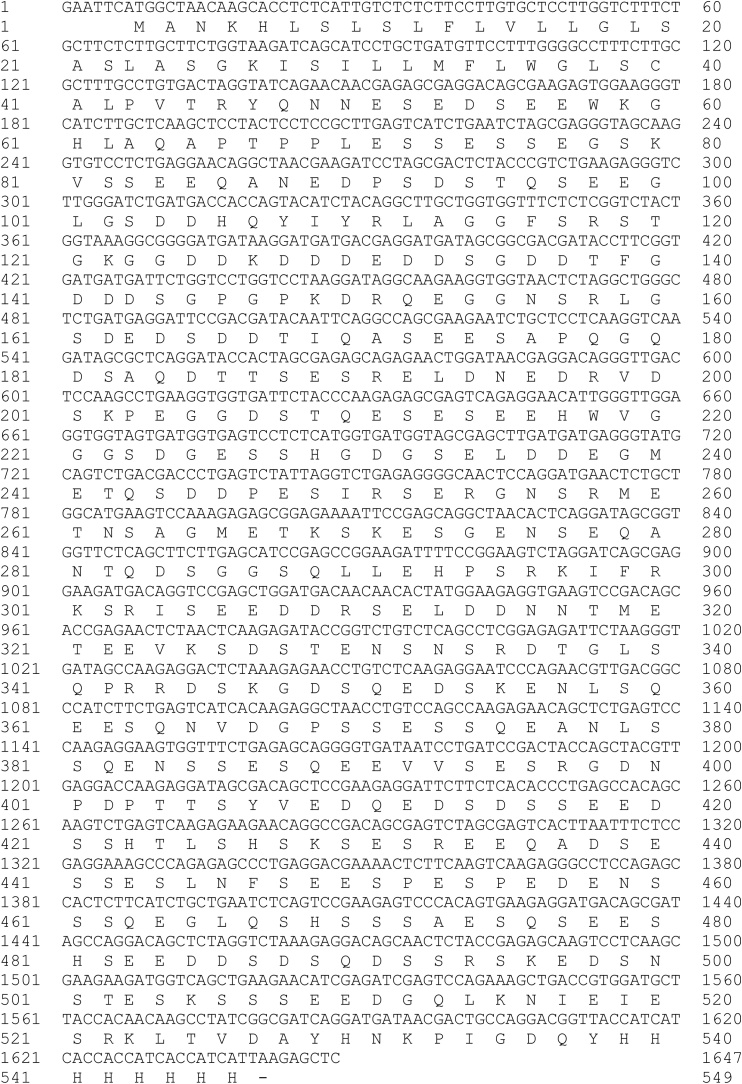
The human DMP1 gene with a 6xHis tag at the C-terminus was synthesized and reported (Fig. 1). The DMP1 gene was inserted into the pET22b expression vector (Merck, USA) by using EcoRI and SacI (New England Biolabs, UK) restriction enzymes. The pET22b-DMP1 plasmid was transformed into E. coli strain Rosetta (DE3) (Novagen, Germany). The bacterial cells were cultured in Luria Bertani (LB) media (HiMedia Laboratories, India) with 100 μg/ml ampicillin (ITW Reagents, Germany) at 37 °C with shaking at 200 rpm. After the OD600 of the culture reached 0.6, protein expression was induced by adding isopropyl-β-D-1-thiogalactopyranoside (IPTG) (Bio Basic, Canada) to final concentrations of 0.2, 0.5, and 1 mM. After IPTG induction, the incubation continued at either 28 °C or 37 °C on a rotary shaker at 200 rpm. The cells were harvested by centrifugation every 2, 4 and 6 h and suspended in 100 μl of 1X SDS loading dye buffer (125 mM Tris HCl, pH 6.8, 12% SDS, 10% glycerol, 22% β-mercaptoethanol, and 0.001% bromophenol blue).
Fig. 1.
Nucleotide and amino acid sequences of the human DMP1 gene.
2.2. SDS-PAGE and Western blot analysis
The proteins were separated using 10% SDS-PAGE under reducing conditions and stained with Coomassie brilliant blue. For Western blot analysis, proteins were transferred to a nitrocellulose membrane (Thermo Fisher Scientific, USA) and probed with HRP-conjugated goat anti-His (Abcam, UK) or rabbit anti-DMP1 (Abcam, UK) antibodies diluted at 1 : 5000 in 3% skim milk in 1 × TBS plus 0.5% Tween 20 (TBST) and HRP-conjugated goat anti-rabbit IgG antiserum (Jackson Immunology Research, USA) diluted at 1 : 5000 in 3% skim milk in 1 × TBST. The membrane was developed with chemiluminescence by using enhanced chemiluminescence (ECL) (GE Healthcare, UK) plus detection reagent.
2.3. Purification of DMP1
The cells were collected by centrifugation at 4000 g for 10 min, and the pellet was resuspended in lysis buffer (100 mM Tris, pH 7.4, 200 mM NaCl, and 5 mM imidazole) and sonicated using ultrasonic lysis (Sonics & Materials, Inc., USA). The solution was centrifuged at 6000 g for 30 min. The supernatant was filtered with a 0.45 μm syringe filter (Sigma-Aldrich, Germany) and loaded onto a Ni-NTA affinity column (Qiagen Gmbh, Germany). Then, the column was washed with washing buffer (100 mM Tris, pH 7.4, 200 mM NaCl, and 25 mM imidazole) and eluted with elution buffer (100 mM Tris, pH 7.4, 200 mM NaCl, and 250 mM imidazole). The fractions were determined by SDS-PAGE and Western blot analysis.
2.4. Cell culture
The protocol to obtain cells was approved by the Human Ethics Committee, Faculty of Dentistry, Chulalongkorn University. Cell explants were cultured in Dulbecco’s modified Eagle’s medium containing 10% foetal bovine serum (Gibco, USA), 2 mM L-glutamine (Gibco, USA), 100 U/ml penicillin (Gibco, USA), 100 μg/ml streptomycin (Gibco, USA) and 5 μg/ml amphotericin B (Gibco, USA) in 100% humidity at 37 °C with 5% carbon dioxide. Cultured medium was changed every 48–72 h. After reaching confluence, the cells were subcultured at a 1:3 ratio, and cells at passages 3–5 were used in the subsequent experiment. The three human periodontal ligament (hPDL) cells used in all experiments were isolated from three different donors.
2.5. Cell viability assay
Human periodontal ligament (hPDL) cells were seeded at a density of 50,000 cells per well in a 24-well plate. The hPDL cells were cultured for 24 and 72 h on DMP1 produced from E. coli (1 μg/ml and 2 μg/ml), and proteins from E. coli not containing the DMP1 protein eluted from the Ni affinity column were used as a negative control. The cells were assessed for viability by a thiazolyl blue tetrazolium bromide (USB Corporation, USA) assay as previously reported [8]. Then, 500 μl of 3-[4,5-dimethylthiazole-2-yl]-2,5-diphenyltetrazolium bromide (MTT) (0.5 mg/ml) solution was added to each well, followed by sample incubation at 37 °C for 30 min. Then, 500 μl of glycine buffer (100 mM NaCl, 100 mM glycine, pH 10) was added. DMSO (1:9) was added to the well. After the formazan crystals had dissolved, the absorbance was determined spectrophotometrically at 570 nm using a reference wavelength of 570 nm on an ELX800UV universal microplate reader (Bio-Tek Instruments, Inc., Vermont, USA).
2.6. Real-time PCR analysis for osteoblast differentiation markers
The hPDL cells were treated with and without DMP1 protein for 72 h. Total RNA from hPDL cells was extracted using Isol-RNA Lysis Reagent (Gene All Biotechnology, Korea), and 1 μg/ml of RNA per sample was converted to cDNA using a reverse transcriptase kit (Promega, Madison, WI, USA). Quantitative RT-PCR was performed using a Lightcycler Nano real-time polymerase chain reaction machine (Roche Applied Science, Indianapolis, IN, USA) using Fast Start Essential DNA Green Master (Roche Applied Science). Primers specific to ALP, BMP2, CBFA1, COL1, OPN, OSX and WNT3a were used for this protocol, and the RT-PCR conditions were set as follows: denaturation at 94 °C for 10 min, annealing at 60 °C for 10 s, and extension at 72 °C for 10 s for 45 cycles. GAPDH was used as a reference gene for the internal control. The primer sequences are shown in Table 1. The data were statistically analysed by two-way ANOVA.
Table 1.
Primer sequence for gene expression analysis using RT-PCR.
| Gene | Forward (F) or Reverse (R) | Sequences | Reference |
|---|---|---|---|
| GAPDH | F | 5’ CACTGCCAACGTGTCAGTGGTG 3’ | NM001289745.2 |
| R | 5’ GTAGCCCAGGATGCCCTTGAG 3’ | ||
| ALP | F | 5’ CGAGATACAAGCACTCCCACTTC 3’ | NM000478.3 |
| R | 5’ CTGTTCAGCTCGTACTGCATGTC 3’ | ||
| CBFA1 | F | 5’ ATGATGACACTGCCACCTCTGA 3’ | NM001024630.3 |
| R | 5’ GGCTGGATAGTGCATTCGTG 3’ | ||
| COL1 | F | 5’ GTGCTAAAGGTGCCAATGGT 3’ | NM000088.3 |
| R | 5’ ACCAGGTTCACCGCTGTTAC 3’ | ||
| OSX | F | 5’ GCCAGAAGCTGTGAAACCTC 3’ | NM001300837.1 |
| R | 5’ GCTGCAAGCTCTCCATAACC 3’ | ||
| OPN | F | 5’ AGGAGGAGGCAGAGCACA 3’ | NM001040060.1 |
| R | 5’ CTGGTATGGCACAGGTGATG 3’ | ||
| WNT3a | F | 5’ CTGTTGGGCCACAGTATTCC 3’ | NM033131.3 |
| R | 5’ GGGCATGATCTCCACGTAGT 3’ |
2.7. Osteogenic differentiation
Calcium deposition was evaluated on day 14 after cells were cultured in either general medium (Dulbecco’s modified Eagle’s medium containing 10% foetal bovine serum (Gibco, USA), 1% Glutamax I supplement (Gibco, USA) and 1% antibiotic-antimycotic (Gibco, USA)) or osteogenic medium (general medium supplemented with 50 mg/ml ascorbic acid, 10 mM β-glycerophosphate and 100 nM dexamethasone). Cells cultured in normal growth medium were employed as a control and analysed by using the Alizarin red S staining assay.
2.8. Alizarin red S staining
Cells were fixed with cold methanol for 10 min. After washing with phosphate-buffered saline (PBS), the cells were stained with 1% Alizarin red S staining solution (Sigma-Aldrich, St. Louis, MO) at room temperature. Each of the specimens was washed with deionized water and air-dried. The quantification was determined with 10% cetylpyridium chloride in aqueous 10 mM sodium phosphate solution at pH 7. The absorbance of the product was measured on a microplate reader at 570 nm. The 10% cetylpyridium chloride in aqueous 10 mM sodium phosphate solution was used as a blank. The data were statistically analysed by one-way ANOVA.
3. Results
3.1. Optimization of DMP1 expression in E. coli
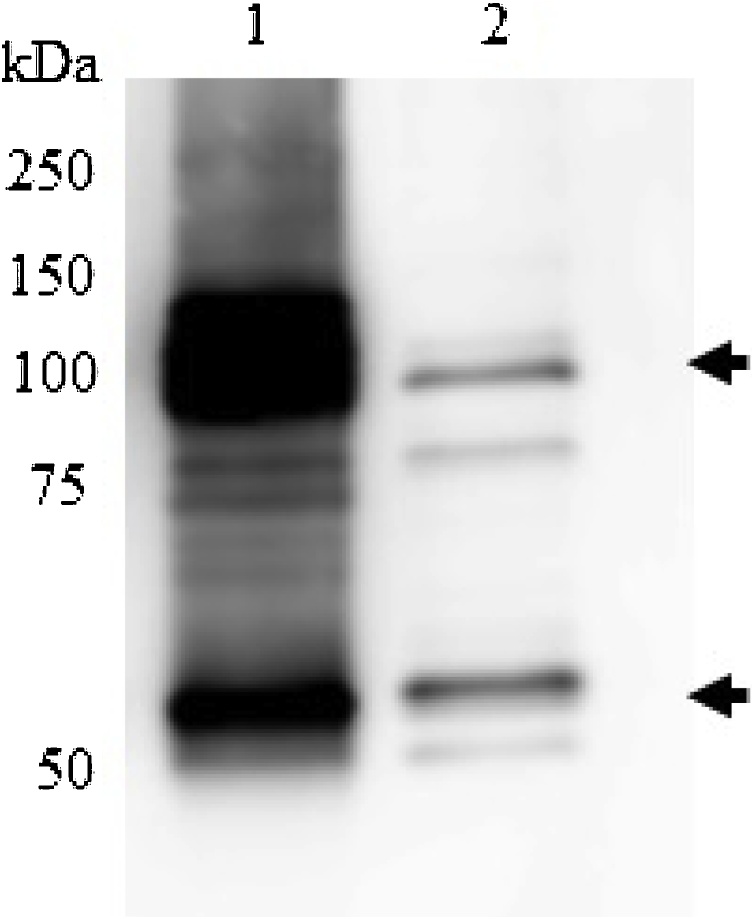
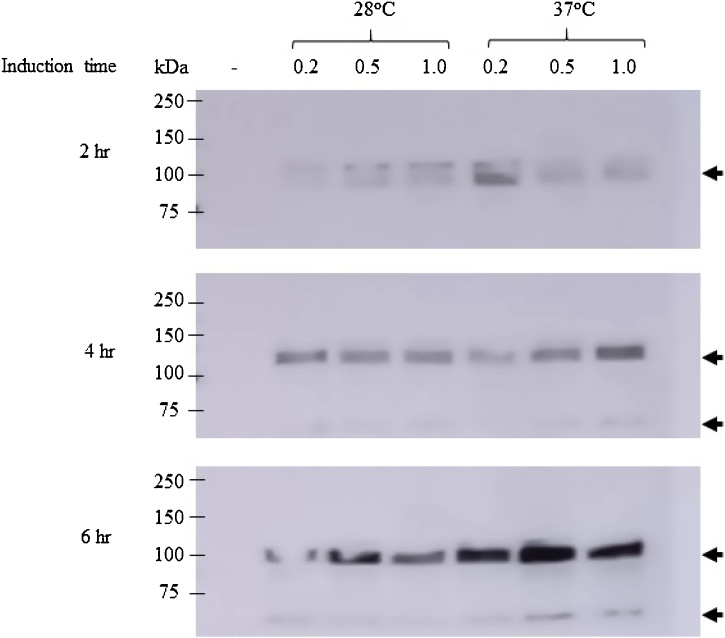
The gene encoding the DMP1 sequence (Fig. 1) was cloned into pET22b and transformed into E. coli strain Rosetta (DE3). The pET-DMP1 vector is predicted to encode a recombinant protein with a molecular size of 60 kDa. Western blot analysis using anti-human DMP1 protein confirmed DMP1 expression, with 2 major bands at approximately 60 and 120 kDa (Fig. 2). The results from liquid chromatography-electrospray ionization-tandem mass spectrometry (LC-ESI-MS) analysis of both the 60 and 120 kDa proteins showed that both identified proteins matched the product of the human DMP1 gene (Accession number: NP_004398.1), with scores of 581 and 301 for the 60 and 120 kDa proteins, respectively. This result confirmed that the purified protein obtained in this study was human DMP1 protein. To determine the optimal conditions for DMP1 expression in E. coli, the IPTG concentrations, temperature, and induction time were varied. The results showed that the highest level of DMP1 expression in E. coli was induced with 0.5 mM IPTG for 6 h at 37 °C (Fig. 3).
Fig. 2.
Expression of DMP1 in E. coli compared with the positive control (commercial DMP1 from R&D Systems, USA). Western blot analysis probed with rabbit anti-human DMP1 antiserum and HRP-conjugated goat anti-rabbit IgG antiserum. Lane 1: DMP1 produced from E. coli, Lane 2: commercial DMP1 (positive control).
Fig. 3.
Determination of the optimal conditions for recombinant DMP1 expression in E. coli using Western blot analysis probed with anti-histidine conjugated with HRP. The parameters included culturing temperature (28 °C and 37 °C), incubation time (2, 4 and 6 h), and concentration of IPTG (0.2 mM, 0.5 mM and 1 mM) with a negative control (E. coli without pET22b-DMP1).
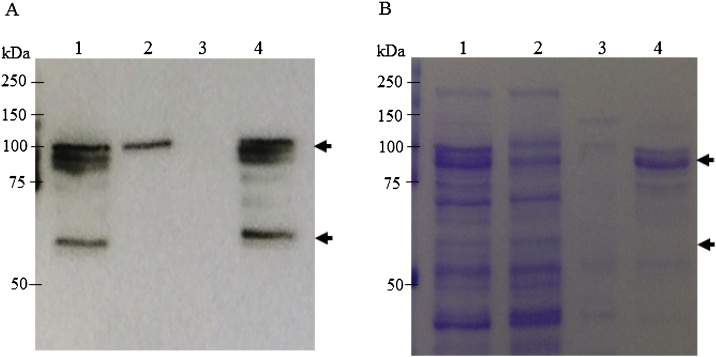
3.2. Purification of DMP1 protein using Ni affinity chromatography
The soluble proteins extracted from E. coli were purified under nondenaturing conditions with Ni affinity chromatography. The purification was confirmed by Western blot analysis (Fig. 4A) and SDS-PAGE (Fig. 4B). Our results demonstrated that DMP1 was lost in the flow through (Fig. 4A). However, the DMP1 protein in the elution fraction was purified up to 80%.
Fig. 4.
Purification of the 6xHis-tagged DMP1 fusion protein using Ni-NTA affinity chromatography. The crude extract was subjected to a Ni-NTA column and analysed by SDS-PAGE under reducing conditions, followed by (A) blotting onto a nitrocellulose membrane and probing with HRP-conjugated goat anti-His antiserum or (B) staining with Coomassie brilliant blue. (A) Western blot analysis of purified DMP1 from E. coli probed with anti-histidine conjugated with HRP. (B) SDS-PAGE Lane 1: crude extract; Lane 2: flow through, Lane 3: wash, Lane 4: eluate.
3.3. Effect of DMP1 on cell viability
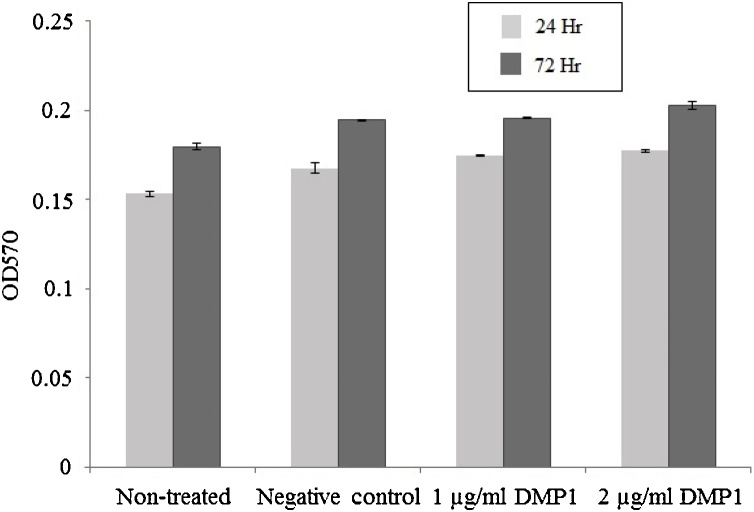
The effect of DMP1 on cell viability was investigated by culturing hPDL cells provided by 3 different donors with or without DMP1. The cell number was determined by the MTT assay after culturing for 24 and 72 h. The results showed that hPDL cells could attach and survive on all well surfaces. The viability of hPDL cells treated with 1 μg/ml and 2 μg/ml E. coli-produced DMP1 and negative E. coli protein was not significantly different (Fig. 5). Our data confirm that the DMP1 protein produced from E. coli does not affect the viability of hPDL cells, comparable to commercial DMP1 protein (R&D Systems, USA).
Fig. 5.
Effect of DMP1 on human periodontal ligament cell (hPDL) viability. The hPDL cell lines were cultured on dopamine-coated (9 ng/well) and E. coli-produced DMP1-coated surfaces (1 μg/well and 2 μg/well) for 24 h and 72 h. The experiments were performed in triplicate. Cell viability was evaluated by the MTT assay at 24 h and 72 h. Data represent the absorbance at 570 nm. Data represent the mean of 3 independent replicate samples ± SD. Cells treated with the proteins purified from E. coli without the DMP1 gene using the same protocol as DMP1 purification served as the negative control.
3.4. DMP1 produced in E. coli activates osteogenesis-related genes
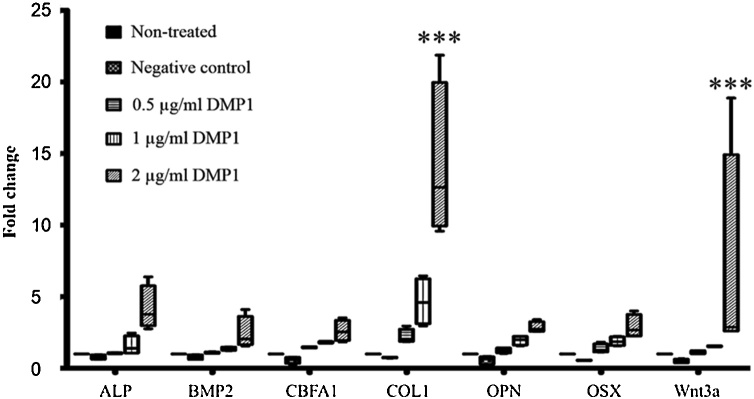
The cells were treated with 1 μg/ml and 2 μg/ml E. coli-produced DMP1 for 72 h. The mRNA was collected to investigate the expression of ALP, BMP2, CBFA1, COL1, OPN, OSX, and WNT3a genes by qRT-PCR. The results showed that the 2 μg/ml DMP1-coated surface could upregulate the expression of the ALP, BMP2, CBFA1, COL1, OPN, OSX, and WNT3a genes (Fig. 6).
Fig. 6.
E. coli-produced DMP1 increased the mRNA expression of osteogenic markers. The hPDL cells were treated with 0.5 μg/ml, 1 μg/ml and 2 μg/ml E. coli-produced DMP1 for 72 h. Total RNA was extracted, and real-time PCR was performed using primer sets for human ALP, BMP2, CBFA1, COL1, OPN, OSX, and WNT3a genes. The values obtained for nontreated cells were set at 1 for subsequent fold change calculation. Cells treated with the proteins purified from E. coli without the DMP1 gene using the same protocol as DMP1 purification served as the negative control. The data are shown as the mean ± SD, derived from triplicate experiments. Means with three asterisks (***) are significantly different (P < 0.01).
3.5. DMP1 activates calcification
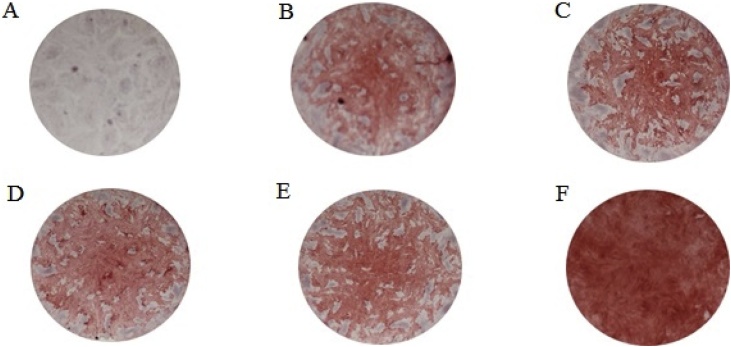
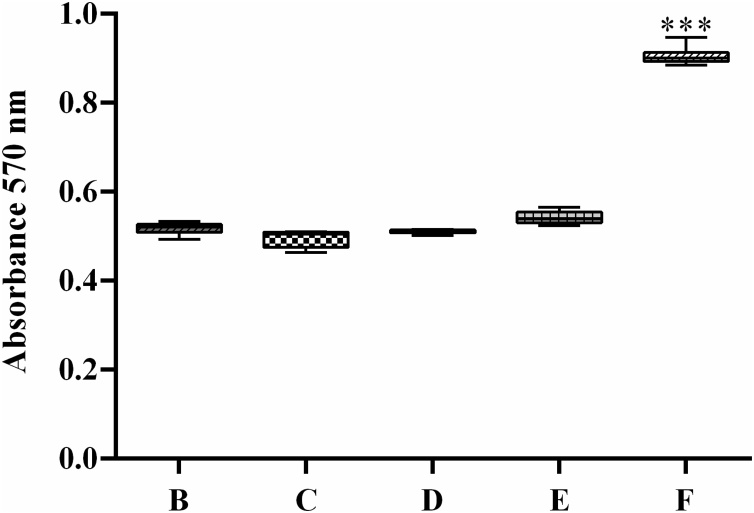
The hPDL cells were grown in general medium and osteogenic medium for 14 days. The hPDL cells were stained with Alizarin red S at the end of the experiment. The hPDL cells cultured in general media were not red (Fig. 7A), but the cells were light red when they were cultured in osteogenic medium (Fig. 7B). When the cells treated with the negative control (contaminated proteins from E. coli purified with the same protocol as DMP1) (Fig. 7C) or with 0.5 μg/ml and 1 μg/ml DMP1 protein (Fig. 7D and 7E, respectively) were grown in osteogenic medium, the cells were also light red, similar to the nontreated cells cultured in osteogenic medium. However, when hPDL cells were treated with 2 μg/ml DMP1 protein cultured in osteogenic medium, the cells were bright red (Fig. 7F), indicating calcium deposition in these cells. The quantification of calcification is reported in Fig. 8. The results confirmed that the cells treated with 2 μg/ml DMP1 protein (Group F) exhibited significantly higher calcium deposition than the other groups.
Fig. 7.
Calcification in hPDL cells seeded at 50,000 cells/well and cultured in osteogenic medium for 14 days. The hPDL colonies were stained with Alizarin red S solution at the end of the experiment. (a) Control cells growing in general medium, (b) control cells growing in osteogenic medium, (c) cells treated with the proteins purified from E. coli without the DMP1 gene using the same protocol as DMP1 purification in osteogenic medium, (d) cells treated with 0.5 μg/ml DMP1 in osteogenic medium, (e) cells treated with 1 μg/ml DMP1 in osteogenic medium and (f) cells treated with 2 μg/ml DMP1 in osteogenic medium.
Fig. 8.
The quantification of Alizarin red S staining. The hPDL cells stained with Alizarin red S solution were dissolved with 10% cetylpyridinium chloride in aqueous 10 mM sodium phosphate solution at pH 7. The solution was measured at an absorbance of 570 nm. (b) Control cells growing in osteogenic medium, (c) cells treated with the proteins purified from E. coli without the DMP1 gene using the same protocol as DMP1 purification in osteogenic medium, (d) cells treated with 0.5 μg /ml DMP1 in osteogenic medium, (e) cells treated with 1 μg /ml DMP1 in osteogenic medium and (f) cells treated with 2 μg /ml DMP1 in osteogenic medium. The data are shown as the mean ± SD, derived from triplicate experiments. Means with three asterisks (***) are significantly different (P < 0.01).
4. Discussion
Bone tissue engineering is one of the prominent fields in tissue engineering and regenerative medicine. The accomplishment of this technology depends on three different components: cells, scaffolds, and signalling molecules. These three elements can be linked by protein molecules. Protein signalling is crucial to regulating cell phenotype and engineered tissue structure and function. However, there are several limitations in protein availability. Therefore, recombinant protein technology can be used to design proteins with targeted properties.
Previous reports have described the generation of recombinant proteins for bone tissue engineering, such as osteopontin (OPN) [9], Jagged1 [10], BMP-2 [11] and DMP1 [12]. DMP1 is one target for bone tissue engineering. DMP1 activates FAK-mediated MAPK signalling which coordinates the extracellular environment of osteolytic lacunae and bone metabolism [13]. DMP1 plays an important role in bone regeneration.
E. coli was used as a host to produce DMP1 protein due to several advantages, including its rapid growth rate, good genetic characterization, high protein expression level, low cost, and easy genetic manipulation and transformation. The organism itself is generally recognized as safe (GRAS) [[14], [15], [16]]. In this study, DMP1 was cloned and expressed in E. coli. E. coli was previously used to produce few recombinant proteins for tissue engineering, such as human BMP-2 [17] and osteocalcin [18].
A previous report on the expression of DMP1 in E. coli reported a size of 90–95 kDa [19]. The DMP1 protein was shown to undergo extensive dimerization [20]. In our study, pET22b-DMP1 was transformed and expressed in E. coli. The predicted size of DMP1 is approximately 55 kDa. Under reducing conditions, the Western blot confirmed that the size of DMP1 expressed in our study was approximately 60 kDa and 120 kDa, similar to commercial DMP1 protein (Fig. 2). The upper and lower bands correspond to the dimer and monomer forms of DMP1, respectively. Moreover, the results from mass spectrometry also confirmed that the proteins at 60 kDa and 120 kDa are human DMP1 (data not shown). Due to the extensive dimerization of DMP1 [20], the dimer form remained and was detected under reducing conditions (Fig. 2).
The optimization of protein expression in E. coli is necessary because different recombinant proteins require different conditions for the best expression. In this study, IPTG concentration, induction time, and induction temperature were optimized. Different concentrations of IPTG were tested, including 0.2 mM, 0.5 mM and 1 mM IPTG. The induction time also varied (2 h, 4 h, and 6 h). IPTG induction was performed at 28 °C and 37 °C. Our results confirmed that the DMP1 expression level was highest when E. coli was induced with 0.5 mM IPTG for 6 h at 37 °C.
DMP1 was previously shown to induce the proliferation of different cell types [[21], [22], [23]]. In this study, DMP1 produced from E. coli was tested for toxicity on hPDL cells using the MTT assay. Our results showed that there was no significant difference among the viability of hPDL cells treated with 1 μg/ml DMP1, 2 μg/ml DMP1, and without DMP1 at 24 h and 72 h (Fig. 5). These data confirm that DMP1 is not toxic to hPDL cells.
DMP1 was previously shown to play an important role in osteogenic differentiation in dental follicle stem cells [24]. Therefore, DMP1 was further studied for the induction of osteogenic-related genes in human PDL cells. Human PDL stem cells are able to develop into cementoblast-like cells, adipocytes or collagen fibres [25]. PDL cells also have the potential to form calcified deposits with other mesenchymal stem cells [26]. DMP1 was previously expressed in E. coli and stimulated the Runx2 gene in mouse calvarial preosteoblast MC3T3-E1 cells [[27], [28], [29]]. DMP1 in the human body is found in both phosphorylated and nonphosphorylated forms [30]. The different functions of each DMP1 form have not yet been studied.
In this study, we first showed that 2 μg/ml DMP1 was biologically active in vitro in human PDL cells by inducing the ALP, BMP2, CBFA, COL1, OPN, OSX, and Wnt3a genes. These osteogenic-related genes were significantly increased in human PDL cells after 72 h of treatment (Fig. 6). Not only were higher mRNA levels of the osteogenic-related genes observed but also more calcium deposition on the PDL cells treated with DMP1 was found (Fig. 7, Fig. 8). This result suggests that DMP1 can strongly induce osteoblast differentiation in human PDL cells.
In conclusion, recombinant DMP1 does not affect cell viability but strongly induces osteogenic differentiation and calcium deposition in human PDL cells. Therefore, the DMP1 protein has the potential to serve as a target protein used in dental tissue engineering for tooth repair and regeneration.
Conflict of interest
None.
Acknowledgements
This study was supported by the Thailand Research Fund (IRN59W001 and MRG5980087) and The Chulalongkorn Academic Advancement Into Its 2nd Century Project. Aktsar Roskiana Ahmad was supported by a Scholarship Program for ASEAN Countries and the 90th Anniversary of Chulalongkorn University Fund (Ratchadaphiseksomphot Endowment Fund) from Chulalongkorn University. Kaewta Rattanapisit was supported by the Ratchadaphiseksomphot Fund, Chulalongkorn University.
References
- 1.Ravindran S., George A. Multifunctional ECM proteins in bone and teeth. Exp. Cell Res. 2014;325:148–154. doi: 10.1016/j.yexcr.2014.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Narayanan K., Ramachandran A., Hao J., He G., Park K.W., Cho M., George A. Dual functional roles of dentin matrix protein 1. Implications in biomineralization and gene transcription by activation of intracellular Ca2+ store. J. Bio. Chem. 2003;278:17500–17508. doi: 10.1074/jbc.M212700200. [DOI] [PubMed] [Google Scholar]
- 3.Feng J.Q., Ward L.M., Liu S., Lu Y., Xie Y., Yuan B., Yu X., Rauch F., Davis S.I., Zhang S., Rios H., Drezner M.K., Quarles L.D., Bonewald L.F., White K.E. Loss of DMP1 causes rickets and osteomalacia and identifies a role for osteocytes in mineral metabolism. Nat. Genet. 2006;38:1310–1315. doi: 10.1038/ng1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ruoslahti E. RGD and other recognition sequences for integrins. Annu. Rev. Cell Dev. Biol. 1996;12:697–715. doi: 10.1146/annurev.cellbio.12.1.697. [DOI] [PubMed] [Google Scholar]
- 5.Kulkarni G.V., Chen B., Malone J.P., Narayanan A.S., George A. Promotion of selective cell attachment by the RGD sequence in dentine matrix protein 1. Arch. Oral Biol. 2000;45:475–484. doi: 10.1016/s0003-9969(00)00010-8. [DOI] [PubMed] [Google Scholar]
- 6.Chaussain C., Eapen A.S., Huet E., Floris C., Ravindran S., Hao J., Menashi S., George A. MMP2-cleavage of DMP1 generates a bioactive peptide promoting differentiation of dental pulp stem/progenitor cell. Eur. Cell. Mater. 2009;18:84–95. doi: 10.22203/ecm.v018a08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ravindran S., George A. Dentin matrix proteins in bone tissue engineering. Adv. Exp. Med. Biol. 2015;881:129–142. doi: 10.1007/978-3-319-22345-2_8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xia L., Zhang Z., Chen L., Zhang W., Zeng D., Zhang X., Chang J., Jiang X. Proliferation and osteogenic differentiation of human periodontal ligament cells on akermanite and beta-TCP bioceramics. Eur. Cell. Mater. 2011;22:68–82. doi: 10.22203/ecm.v022a06. [DOI] [PubMed] [Google Scholar]
- 9.Rattanapisit K., Abdulheem S., Chaikeawkaew D., Kubera A., Mason H.S., Ma J.K., Pavasant P., Phoolcharoen W. Recombinant human osteopontin expressed in Nicotiana benthamiana stimulates osteogenesis related genes in human periodontal ligament cells. Sci. Rep. 2017;7:17358. doi: 10.1038/s41598-017-17666-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Manokawinchoke J., Nattasit P., Thongngam T., Pavasant P., Tompkins K.A., Egusa H., Osathanon T. Indirect immobilized Jagged1 suppresses cell cycle progression and induces odonto/osteogenic differentiation in human dental pulp cells. Sci. Rep. 2017;7:10124. doi: 10.1038/s41598-017-10638-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ansari S., Freire M.O., Pang E.K., Abdelhamid A.I., Almohaimeed M., Zadeh H.H. Immobilization of murine anti-BMP-2 monoclonal antibody on various biomaterials for bone tissue engineering. Biomed Res. Int. 2014;2014 doi: 10.1155/2014/940860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tabatabaei F.S., Ai J., Jafarzadeh Kashi T.S., Khazaei M., Kajbafzadeh A.M., Ghanbari Z. Effect of dentine matrix proteins on human endometrial adult stem-like cells: in vitro regeneration of odontoblasts cells. Arch. Oral Biol. 2013;58:871–879. doi: 10.1016/j.archoralbio.2013.01.013. [DOI] [PubMed] [Google Scholar]
- 13.Lee J.W., Yamaguchi A., Iimura T. Functional heterogeneity of osteocytes in FGF23 production: the possible involvement of DMP1 as a direct negative regulator. Bonekey Rep. 2014;3:543. doi: 10.1038/bonekey.2014.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gaciarz A., Khatri N.K., Velez-Suberbie M.L., Saaranen M.J., Uchida Y., Keshavarz-Moore E., Ruddock L.W. Efficient soluble expression of disulfide bonded proteins in the cytoplasm of Escherichia coli in fed-batch fermentations on chemically defined minimal media. Microb. Cell Fact. 2017;16:108. doi: 10.1186/s12934-017-0721-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosano G.L., Ceccarelli E.A. Recombinant protein expression in Escherichia coli: advances and challenges. Front. Microbiol. 2014;5:172. doi: 10.3389/fmicb.2014.00172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ahmad Z.A., Yeap S.K., Ali A.M., Ho W.Y., Alitheen N.B., Hamid M. scFv antibody: principles and clinical application. Clin. Dev. Immunol. 2012;2012 doi: 10.1155/2012/980250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim I.S., Lee E.N., Cho T.H., Song Y.M., Hwang S.J., Oh J.H., Park E.K., Koo T.Y., Seo Y.K. Promising efficacy of Escherichia coli recombinant human bone morphogenetic protein-2 in collagen sponge for ectopic and orthotopic bone formation and comparison with mammalian cell recombinant human bone morphogenetic protein-2. Tissue Eng. Part A. 2011;17:337–348. doi: 10.1089/ten.TEA.2010.0408. [DOI] [PubMed] [Google Scholar]
- 18.Kang W., Kim T.I., Yun Y., Kim H.W., Jang J.H. Engineering of a multi-functional extracellular matrix protein for immobilization to bone mineral hydroxyapatite. Biotechnol. Let. 2011;33:199–204. doi: 10.1007/s10529-010-0412-8. [DOI] [PubMed] [Google Scholar]
- 19.Srinivasan R., Chen B., Gorski J.P., George A. Recombinant expression and characterization of dentin matrix protein 1. Connect. Tissue Res. 1999;40:251–258. doi: 10.3109/03008209909000703. [DOI] [PubMed] [Google Scholar]
- 20.He G., Gajjeraman S., Schultz D., Cookson D., Qin C., Butler W., Hao J., George A. Spatially and temporally controlled biomineralization is facilitated by interaction between self-assembled dentin matrix protein 1 and calcium phosphate nuclei in solution. Biochemistry. 2015;44:16140–16148. doi: 10.1021/bi051045l. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hase N., Ozeki N., Hiyama T., Yamaguchi H., Kawai R., Kondo A., Nakata K., Mogi M. Products of dentin matrix protein-1 degradation by interleukin-1beta-induced matrix metalloproteinase-3 promote proliferation of odontoblastic cells. Biosci. Trends. 2015;9:228–236. doi: 10.5582/bst.2015.01092. [DOI] [PubMed] [Google Scholar]
- 22.Ye L., Mishina Y., Chen D., Huang H., Dallas S.L., Dallas M.R., Sivakumar P., Kunieda T., Tsutsui T.W., Boskey A., Bonewald L.F., Feng J.Q. Dmp1-deficient mice display severe defects in cartilage formation responsible for a chondrodysplasia-like phenotype. J. Biol. Chem. 2005;280:6197–6203. doi: 10.1074/jbc.M412911200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maglic D., Stovall D.B., Cline J.M., Fry E.A., Mallakin A., Taneja P., Caudell D.L., Willingham M.C., Sui G., Inoue K. DMP1beta, a splice isoform of the tumour suppressor DMP1 locus, induces proliferation and progression of breast cancer. J. Pathol. 2015;236:90–102. doi: 10.1002/path.4504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rezai Rad M., Liu D., He H., Brooks H., Xiao M., Wise G.E., Yao S. The role of dentin matrix protein 1 (DMP1) in regulation of osteogenic differentiation of rat dental follicle stem cells (DFSCs) Arch. Oral Biol. 2015;60:546–556. doi: 10.1016/j.archoralbio.2014.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brent A.E., Schweitzer R., Tabin C.J. A somitic compartment of tendon progenitors. Cell. 2003;113:235–248. doi: 10.1016/s0092-8674(03)00268-x. [DOI] [PubMed] [Google Scholar]
- 26.Seo B.M., Miura M., Gronthos S., Bartold P.M., Batouli S., Brahim J., Young M., Robey P.G., Wang C.Y., Shi S. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet. 2004;364:149–155. doi: 10.1016/S0140-6736(04)16627-0. [DOI] [PubMed] [Google Scholar]
- 27.Srinivasan R., Chen B., Gorski J.P., George A. Recombinant expression and characterization of dentin matrix protein 1. Connect. Tissue Res. 1999;40:251–258. doi: 10.3109/03008209909000703. [DOI] [PubMed] [Google Scholar]
- 28.Eapen A., Sundivakkam P., Song Y., Ravindran S., Ramachandran A., Tiruppathi C., George A. Calcium-mediated stress kinase activation by DMP1 promotes osteoblast differentiation. J. Biol. Chem. 2010;285:36339–36351. doi: 10.1074/jbc.M110.145607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eapen A., Ramachandran A., Pratap J., George A. Activation of the ERK1/2 mitogen-activated protein kinase cscde by dentin matrix protein 1 promotes osteoblast differentiation. Cell. Tissues. Organs. 2011;194:255–260. doi: 10.1159/000324258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Deshpande A.S., Fang P., Zhang X., Jayaraman T., Sfeir C., Beniash E. Primary structure and phosphorylation of Dentin Matrix Protein 1 (DMP1) and Dentin Phophophoryn (DPP) uniquely determine their role in biomineralization. Biomacromolecules. 2011;12:2933–2945. doi: 10.1021/bm2005214. [DOI] [PMC free article] [PubMed] [Google Scholar]