Abstract
While manganese (Mn) is essential for proper central nervous system (CNS) development, excessive Mn exposure may lead to neurotoxicity. Mn preferentially accumulates in the basal ganglia and in adults it may cause Parkinson’s disease (PD)-like disorder. Compared to adults, younger individuals accumulate greater Mn levels in the CNS, and are more vulnerable to its toxicity. Moreover, the mechanisms mediating developmental Mn-induced neurotoxicity are not completely understood. The present study investigated the developmental neurotoxicity elicited by Mn exposure (5, 10 and 20 mg/kg; i.p.) from postnatal day 8 (PN8) to PN27 in rats. Neurochemical analyses were carried out on PN29, with a particular focus on striatal alterations in intracellular signaling pathways (MAPKs, AKT and DARPP-32), production of oxidative stress and cell death. Motor alterations were evaluated later in life at 3-, 4- and 5-weeks-of-age. Mn exposure (20 mg/kg) increased p38MAPK and AKT phosphorylation, but decreased DARPP-32-Thr-34 phosphorylation. Mn (10 and 20 mg/kg) increased caspase activity and F2-isoprostrane production (a biological marker of lipid peroxidation). Paralleling the changes in striatal biochemical parameters, Mn (20 mg/kg) also caused motor impairment, evidenced by increased falling latency in the rotarod test, and decreased distance travelled and the speed in the open-field test. Notably, the antioxidant Trolox™ reversed the Mn (20 mg/kg)-dependent augmentation in p38MAPK phosphorylation, and reduced the Mn (20 mg/kg)-induced caspase activity and F2-isoprostrane production. Trolox™ also reversed the Mn-induced motor coordination deficits. These findings are the first to show that long-term exposure to Mn during a critical period of neurodevelopment activates the striatal p38MAPK signaling pathway and caspase activity, and causes motor coordination dysfunction by a mechanism that is likely mediated by reactive oxygen species (ROS).
Keywords: manganese, developing brain, MAPK, DARPP-32, oxidative stress, behavior
INTRODUCTION
Manganese (Mn) is an essential ubiquitous trace element required for normal growth, development and cellular homeostasis (Aschner and Aschner 2005; Aschner et al. 2007). In humans and animals, Mn acts as an essential cofactor of several enzymes required for neuronal and glial cell function, as well as enzymes involved in neurotransmitter synthesis and metabolism (Aschner and Aschner 2005; Aschner et al. 2007). Despite its indispensable role in multiple metabolic functions, excessive Mn exposure may lead to increased metal accumulation in the brain and neurotoxicity in the basal ganglia, particularly globus pallidus, striatum and substantia nigra pars reticulata (Bowman et al. 2011; Dobson et al. 2004; Perl and Olanow 2007; Yamada et al. 1986). Increased brain Mn levels may cause a clinical disorder referred to as manganism, which is characterized by an extrapyramidal symptoms resembling idiopathic Parkinson’s disease (PD) (Menezes-Filho et al. 2009; Roth 2009). Moreover, Mn neurotoxicity has been linked to the development of neurodegenerative diseases, such as Alzheimer disease (AD), amyotrophic lateral sclerosis (Eschenko et al. 2010) and prion disease (Bowman et al. 2011).
Despite of the numerous studies on the neurotoxicity of Mn in adults, little is known on its effects on the developing central nervous system (CNS), which is the most susceptible period to toxic insult (Bondy and Campbell 2005; Grandjean and Landrigan 2006; Rodier 1995; Zheng et al. 2003). Furthermore, several lines of research suggest that developmental exposure to Mn may predispose individuals to later-life neurological disorders (Erikson et al. 2007; Hafeman et al. 2007; Moreno et al. 2009a; Moreno et al. 2009b). Notably, newborns retain greater Mn levels than adults (Hafeman et al. 2007), likely due to inefficient excretion, low physiological Fe levels as well as a permeable blood-brain barrier (Roth 2009), or an increased requirement for Mn immediately after birth.
Mn exposure is inherent to environmental and occupational conditions, given its ubiquitous use in water treatment, manufacturing of dry batteries, gasoline additives (as an antiknock agent; methylcyclopentadienyl manganese tricarbonyl, MMT) and fungicides (Burton and Guilarte 2009; Eschenko et al. 2010; Santamaria 2008). Moreover, Mn exposure may also result from administration of parenteral nutrition, where excessive Mn supplementation may predispose primarily children to neurological disorders (Erikson et al. 2007; Hardy 2009; Suzuki et al. 2003).
The late fetal and neonatal periods are characterized by significant changes in brain structure and function as well as cellular and intercellular signaling (Tkac et al. 2003). Substantial acceleration of RNA, DNA and protein synthesis, neuronal migration, growth of glial cells (particularly of astroglia, the main site for glutamate and metal uptake) and myelination of axons continues during the postnatal period (Gottlieb et al. 1977; Rice and Barone 2000; Rodier 1995). The rat brain between postnatal days 7–28 (PN7–28) represents an established model for studies on human brain development. From a neurodevelopmental point of view, the rat PN7 corresponds approximately to human gestational week 34, while rat PN28 corresponds to a child between 2 and 3-years-of-age (Dobbing 1990). Additionally, the pre-weaning and early post-weaning periods coincide with the development of dopaminergic pathways in brain regions such as the prefrontal cortex, nucleus accumbens and striatum, which are critical in regulating behavioral executive functions associated with learning, memory and attention, as well as motor functions (Broaddus and Bennett 1990a; Broaddus and Bennett 1990b; Leo et al. 2003; Lozoff and Georgieff 2006; Packard and Knowlton 2002). Given the sensitivity of dopaminergic systems to Mn, early exposure to this metal may result in significant neurological deficits in developing brain structures (Cordova et al. 2012; Kern et al. 2010).
The mechanisms that mediate Mn-induced neurotoxicity are not fully understood, but seem to be associated with mitochondrial dysfunction, leading to cell death via the formation of reactive oxygen species (ROS) and oxidative stress (Gunter et al. 2006; Roth and Garrick 2003; Tamm et al. 2008; Zhang et al. 2008). Higher rates of striatal oxidative stress have been noted in developmentally-induced Mn toxicity (Avila et al. 2008; Erikson et al. 2007). Furthermore, an association linking oxidative stress in the striatum with impairment in motor activity has been described (de Oliveira et al. 2007).
Specific signaling pathways coupled with programmed cell death are activated upon in vitro Mn exposure, including JNK, ERK, p38MAPK, PKC and caspases (Cai et al. 2011; Crittenden and Filipov 2011; Hirata et al. 2004; Ito et al. 2006; Jang 2009; Latchoumycandane et al. 2005; Park and Park 2010; Shin et al. 2010). While mostly derived from in vitro studies, recently, we have demonstrated the effects of short-term Mn exposure (PN8–12) on the modulation of ERK1/2, AKT, as well as dopamine and cAMP-regulated phosphoprotein of 32kD (DARPP-32) phosphorylation in the striatum of immature rats and its partial association with oxidative stress (Cordova et al. 2012). However, data on the relationship between such signaling pathways and Mn exposure upon longer periods during the early post-natal period have yet to be defined.
ERK1/2, JNK and p38MAPK kinases are the foremost studied mitogen activated protein kinase (MAPK) family members (Chang and Karin 2001; Thomas and Huganir 2004). These pathways are activated by growth factors, cytokines or cytotoxic insults and can regulate gene expression, embryogenesis, proliferation, cell death/survival and neuroplasticity (Chen et al. 2001; Thomas and Huganir 2004; Waetzig and Herdegen 2004). The PI3K/AKT (PKB) signaling pathway is activated by growth factors and plays a central role in controlling cell growth, proliferation, metabolism, cell survival and neuroplasticity (Brazil et al. 2004; Hanada et al. 2004).
DARPP-32 is a key regulator of signaling processing in the basal ganglia; it is highly expressed in medium-sized spiny neurons in the striatum (Qi et al. 2010; Walaas et al. 1983). The basal ganglia receive their main inputs through the striatum and coordinate vital behaviors, including movement, reward and motivational processes (Graybiel 2005). DARPP-32 function depends on its relative state of phosphorylation at two main regulatory sites, Thr-34 and Thr-75, which are phosphorylated by protein kinase A (PKA) or cdk5, respectively (Greengard et al. 1999). This combined phosphorylation state regulates transcription factors, ionotropic receptors and ion channels (Greengard et al. 1999; Svenningsson et al. 2004). DARPP-32 has important functions in regulating motor behavior (Botsakis et al. 2010; Lebel et al. 2010; Polissidis et al. 2010), and short-term Mn exposure has been shown to increase DARPP-Thr-34 phosphorylation (Cordova et al. 2012), establishing that striatal DARPP-32 is a critical target for Mn neurotoxicity.
The biological consequences of developmental Mn exposure may be severe and possibly with late onset, affecting neurogenesis, learning and memory. Furthermore, these early effects may predispose individuals to neurodegenerative disorders that unmask only decades later in life. We have recently demonstrated that developmental exposure of immature rats to Mn for a short period (five days; from PN8–12) caused marked motor deficits and altered intracellular signaling pathways associated with regulation of proliferation, plasticity and cell death (Cordova et al. 2012) in a manner partially dependent on oxidative stress. The relationship between these signaling pathways and more protracted Mn exposure during the early post-natal period has yet to be defined. Accordingly, the present study was carried out to evaluate alterations in striatal cell signaling pathways, production of cell death, induction of oxidative stress and motor deficits in a model of long-term developmental Mn exposure (twenty days, from PN8–27). Moreover, the role of a chain-breaking antioxidant (Trolox™) in modulating the effects of Mn on these biochemical and behavioral parameters was also evaluated in an attempt to elucidate possible mechanisms of neurotoxicity.
METHODS
Reagents
The primary antibodies anti-ERK1/2, anti-p38MAPK, anti-JNK1/2 and manganese chloride (MnCl2) were obtained from Sigma (St. Louis, MO, USA). The anti-phospho-CREB, anti-CREB, anti-phospho-ERK1/2, anti-phospho-p38MAPK, anti-phospho-JNK1/2, anti-AKT, anti-phospho-AKT, anti-DARPP-32, anti-phospho-DARPP-32-Thr-34 and anti-phospho-DARPP-32-Thr-75 antibodies were purchased from Cell Signaling (Beverly, MA, USA). Anti-β-actin was purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Acrylamide, bis-acrylamide, β-mercaptoethanol, Hybond™ nitrocellulose, Hyperfilm™ ECL, sodium dodecyl sulfate (SDS), Tris, secondary antibody (anti-rabbit IgG-horse radish peroxidase (HRP)-conjugated and ECL™ detection reagents were obtained from GE Healthcare Life Sciences (Piscataway, NJ, USA). 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox™) were obtained from Calbiochem (La Jolla, CA, USA). The N-acetyl-Asp-Glu-Val-Asp-7-amino-4-methylcoumarin (DEVD-AMC) was obtained from Biomol (Plymouth Meeting, PA, USA). All other reagents were of the highest analytical grade.
Animals and treatments
Wistar rats of both genders were obtained from the Federal University of Santa Catarina breeding colony. The animals were maintained in an air-conditioned room (22±1°C) on a 12 h light/dark cycle with water and food available ad libitum. They were treated, manipulated and euthanized according to the “Principles of Laboratory Animal Care” (NIH publication no. 80–23, revised 1996) and approved by the Committee on the Ethics of Animal Experiments of the Federal University of Santa Catarina (CEUA/UFSC; www.ceua.ufsc.br; Permit Number: PP00345). All efforts were made to minimize the number of animals used and animal suffering. At birth, the number of pups was randomly culled to eight pups per litter. The treatments began when the pups were eight days old (PN8). The litters were randomly assigned to each treatment group and the treatments were carried out at the same time every day (2:00 PM).
For the Mn exposure protocol, four to twelve groups from different litter were used. Each group with eight pups (8-days-old) of both sexes was divided into four treatments (control and MnCl2 5, 10 or 20 mg/kg) with two animals in each treatment group. The pups were treated for 20 consecutive days (8th to 27th postnatal day; PN8–27) with one daily intraperitoneal (i.p.) injection of saline (NaCl, 0.9%; control) or MnCl2 (5, 10 or 20 mg/kg, diluted in saline), as previously described (Cordova et al. 2012; Cordova et al. 2004; Rocha et al. 1995). Rats were euthanized on the 29th postnatal day (PN29) by decapitation, and the structures of interest were dissected out for neurochemical analyses (Cordova et al. 2012; Cordova et al. 2004). Briefly, the brain was carefully removed, the cerebral hemispheres were divided, and the hippocampi were isolated using two fine brushes (Cordova et al. 2012; Rodnight and Leal 1990). This procedure was followed by displacement of the cortex and striatum. The striatum was carefully dissected out and separated from nearby structures with a sharp spatula.
In addition, to verify the involvement of oxidative stress in Mn-induced neurotoxicity, pups were treated with the antioxidant 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox™) concomitantly with Mn. The same protocol as previously described was carried out with two pups in each treatment group: saline (0,9% NaCl; control), Trolox™ 1 mg/kg, MnCl2 20 mg/kg, MnCl2 20 mg/kg plus Trolox™ 1 mg/kg (administered 10 min before Mn) (Cordova et al. 2012). The pups’ body-weights were measured daily from PN8 to PN29 and the weight-gain (g) is reported as mean ± S.E.M.
Brain metal analyses
Tissue concentrations of Mn and iron (Fe) were analyzed by atomic absorption spectroscopy (Varian AA240™, Varian Inc., Palo Alto, CA, USA) (Fitsanakis et al. 2008). The striatum, hippocampus and cerebral cortex were digested in ultrapure nitric acid (1:10 wt/vol dilution) for 48–72 h in a sand bath (60 °C). One hundred microliters of digested tissue was brought to 1 ml of total volume with 2% nitric acid and analyzed for Mn and Fe content. The mixture was then centrifuged and the clear supernatant was used for analysis (100 μl aliquot brought up to a 1 ml volume with 2% nitric acid). Bovine liver (10 μg Mn/l) was digested in ultrapure nitric acid and used as an internal standard for analysis. The data are expressed as μg Mn or Fe/g tissue (mean ± S.E.M.)
Western blotting
The brain area of interest (striatum) was dissected and mechanically homogenized in 500 μl of sample buffer (200 mM Tris, 40 mM EDTA, 4% SDS, pH 6,8), and the homogenates were immediately boiled for 5 min. Sample dilution solution (1:4 vol/vol; 40% glicerol, 50 mM Tris and minimal bromophenol blue) and β-mercaptoethanol was added to each sample at a final concentration of 5%. Protein concentrations were estimated by the method described in Peterson (1977) based on a standard curve with bovine serum albumin. The samples (60 μg of total protein/lane) were separated by SDS-PAGE (miniVE Vertical Electrophoresis System™, GE Healthcare Life Sciences, Piscataway, NJ, USA) using 10% gels (Cordova et al. 2012; Cordova et al. 2004). The proteins were transferred to nitrocellulose membrane using a semi-dry blotting apparatus (TE 70 SemiPhor™ Unit, GE Healthcare Life Sciences, Piscataway, NJ, USA) (1.2 mA/cm2; 1.5 h) as described by Bjerrum and Heegaard (1988). The membranes were blocked (1 h) with 5% skim milk in TBS (10 mM Tris, 150 mM NaCl, pH 7.5). ERK1/2, JNK1/2, p38MAPK, AKT, CREB and DARPP-32 (total and phosphorylated forms) were detected with specific antibodies, diluted in TBS-T (10 mM Tris, 150 mM NaCl, 0,1% Tween-20, pH 7.5) containing 2.5% BSA, after overnight incubation. Final primary antibody dilutions were 1:1,000 (anti-phospho-CREB, anti-CREB, anti-AKT, anti-phospho-AKT-Ser-473, anti-DARPP-32, anti-phospho-DARPP-32-Thr-34, anti-phospho-DARPP-32-Thr-75, anti-phospho-JNK1/2 and anti-phospho-p38MAPK), 1:2,000 (anti-phospho-ERK1/2 and anti-β-actin), 1:10,000 (anti-JNK1/2 and anti-p38MAPK) or 1:40,000 (anti-ERK1/2). Next, the membranes were incubated for 1 h with anti-rabbit peroxidase-linked secondary antibody (1:4,000) and the reactions developed by chemiluminescence. All steps (blocking and antibody incubations) were followed by three washes (5 min) of the membranes with TBS-T. The optical density (O.D.) of the bands was quantified with Scion Image™ (Frederick, MD, USA). The phosphorylation level of each protein was determined as a ratio of the O.D. of the phosphorylated band/ O.D. of the total band. The data are expressed as percentage of the control (considered as 100%). Values are presented as mean ± S.E.M (Posser et al. 2007).
Quantitation of F2-IsoPs
The F2-isoprostranes (F2-IsoPs) are considered reliable biomarkers of oxidative stress (particularly, lipid peroxidation) in both in vitro and in vivo models (Milatovic et al. 2009). Total F2-IsoPs were determined with a stable isotope dilution method with detection by gas chromatography/mass spectrometry and selective ion monitoring as previously described (Milatovic et al. 2007; Morrow and Roberts 1999). Total F2-IsoPs were measured in the striatum dissected from the animals exposed in vivo to different doses of Mn and stored at −80 °C until analysis. Briefly, the striata were homogenized in Folch solution and lipids extracted from chloroform layer by evaporation (Milatovic and Aschner 2009) and then subjected to chemical saponification using 15% KOH to hydrolyze bound F2-IsoPs. The homogenates were adjusted to a pH of 3, followed by the addition of 0.1 ng of 15-F2α-IsoP-d4 internal standard. F2-IsoPs were subsequently purified by C18 and silica Sep-Pak extraction and by thin layer chromatography. They were then analyzed by pentafluorobenzyl ester, a trimethylsilyl ether derivative, via gas chromatography, negative ion chemical ionization-mass spectrometry.
Caspases activity
Caspase activity was monitored fluorimetrically by the production of fluorescent 7-amino-4-methylcoumarin (AMC) from DEVD-AMC fluorogenic substrate for caspase-3 and related cysteine proteases. Striatal homogenates were prepared (1:5, w/v) in a buffer containing 10 mM HEPES pH 7.4, 42 mM KCl, 5 mM MgCl2, 1 mM phenylmethylsulfonylfluoride (PMSF), 0.1 mM EDTA, 0.1 mM EGTA, 1 μg/ml, pepstatin A, 1 μg/ml leupeptin, 5 μg/ml aprotinin, 0.5% 3-[(3-cholamidopropyl)-dimethyl-ammonio]-1-propanesulfonate (CHAPS) and 1 mM dithiothreitol (DTT) at 4 °C. The reaction was carried out by mixing this homogenate (0.1 mg protein) with a buffer containing 25 mM HEPES pH 7.4, 0.1% CHAPS, 1 mM EDTA, 10% sucrose and 3 mM DTT. The reaction was started by the addition of 10μM caspase-3 fluorogenic substrate DEVD-AMC. Cleavage of the fluorogenic substrate was detected spectrofluorimetrically (Perkin Elmer LS55, Boston, MA, USA) after 2 h of incubation at 37 °C, using excitation and emission wavelengths of 380 and 460 nm, respectively (Zuse et al. 2007). Fluorescence of blank samples containing no fluorogenic substrate was subtracted from these values. Protein content was determined by Lowry’s method (Lowry et al. 1951), and the caspase activity was expressed as mean percent of control (100%) ± S.E.M.
Behavioral tests
The animals were kept until PN37. All animals were tested at 22, 29 and 36 days of age (3, 4 and 5 weeks old) on a rotarod and on PN37 on an open field task (Cordova et al. 2012). Animals were habituated to the test room for 1 h prior to the initiation of the behavior tests, which were carried out during the light phase of the cycle (10:00–17:00 h).
Rotarod analyses
The Rota-Rod system (Insight Equipamentos Científicos, Ribeirao Preto, Brazil) for locomotor assessment measures the time period an animal maintains its balance on a moving cylinder (Aguiar et al. 2009). Animals were first conditioned on a stationary rod for 30 seconds and during this time animals that fell off the cylinder were placed back on the rotarod. Next, the animals were conditioned at a constant speed of 5 rpm for a period of 90 seconds. Animals that failed the first conditioning were allowed two additional conditioning periods, and those that failed the third conditioning period were omitted from further testing. This assured that all the animals in all the treatment groups attained an analogous baseline (data not shown).
The same basic conditioning methodology was employed in testing treatment and control groups. Thirty minutes after the last conditioning, each animal was placed on the rotarod and its latency for falling determined. The starting speed was 5 rpm and it was increased by 0.1 revolutions per second.
Open field analyses
To evaluate Mn-induced motor changes, the animals were tested in the circular open field (Aguiar et al. 2009). The apparatus consists of acrylic chamber with 50 cm height x 60 cm diameter (Insight Equipamentos Científicos, Ribeirao Preto, Brazil) and is placed in a room with a ceiling-mounted video camera. Each animal was placed in the apparatus for 10 min and the distance, average speed, number of rearing and grooming were recorded. The data were evaluated with behavioral analysis software, ANY-maze™ (Stoelting, Wood Dale, IL, USA).
Statistical analyses
Statistical significance was assessed by one-way analysis of variance (ANOVA) followed by Duncan’s, or Newman-Keuls post-hoc test where appropriate. Kruskal-Wallis test followed by Dunn’s post-hoc was applied to the rotarod analysis. Analyses were performed with STATISTICA™ 5.1 ‘98 Edition (StatSoft, Tulsa, OK, USA). A value of p ≤ 0.05 was considered to be statistically significant.
RESULTS
Brain metal accumulation
Mn and Fe concentrations were analyzed in multiple brain regions in rats developmentally exposed to Mn for 20 days. Mn levels significantly increased, approximately five fold for the 10 mg Mn/kg (p < 0.01) dose and seven times for 20 mg Mn/kg (p < 0.001) dose in the striatum (Fig. 1A) relative to controls. Moreover, significant increase in Mn levels was also observed in the hippocampus (p < 0.01 for 10 mg Mn/kg; p < 0.001 for 20 mg Mn/kg; Fig. 1A) and cerebral cortex (p < 0.01 to 10 mg Mn/kg; p < 0.001 for 20 mg Mn/kg; Fig. 1A). Fe levels significantly increased (20 and 35%) only in the striatum of animals treated with 10 and 20 mg Mn/kg, respectively (p < 0.001; Fig 1B). At the termination of the study (PN29), no differences were noted in weight-gain (Table 1) for any of the groups when compared with controls.
Figure 1.
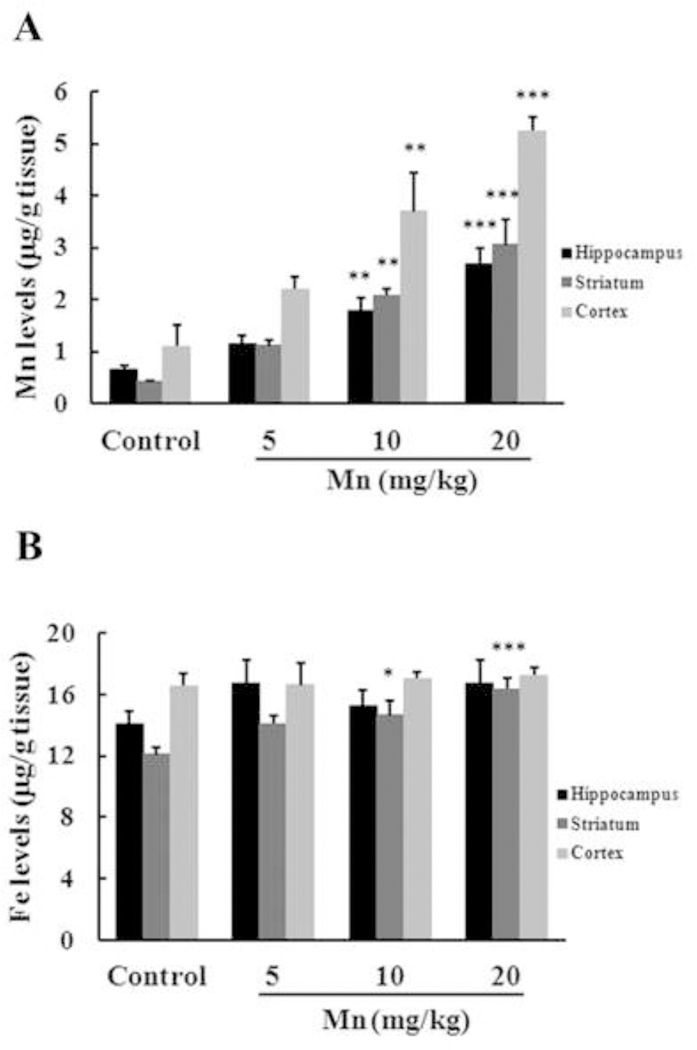
Effects of Mn exposure on metal accumulation in the hippocampus, striatum and cerebral cortex of immature rats. The panels show the accumulation of Mn (A) and Fe (B). Rat pups were treated with saline (control; NaCl 0.9%) or MnCl2 at doses of 5, 10 or 20 mg/kg for twenty days (PN8–27) and the brain metal concentrations were analyzed on PN29. Results represent mean ± S.E.M and are expressed in ĝ metal/g tissue. Statistical analysis was performed by ANOVA followed by Duncan’s test. n = 4; * p < 0.05, ** p < 0.01, *** p < 0.001 compared to control.
Table 1.
Weight gain in rats developmentally exposed to Mn.
| Body weight PN8 (g) | Body weight PN29 (g) | Weight gain (g) | |
|---|---|---|---|
| Control | 13.20 ± 0.78 | 61.88 ± 2.21 | 49.81 ± 1.52 |
| 5 mg Mn/kg | 14.51 ± 0.96 | 65.16 ± 1.91 | 50.65 ± 1.38 |
| 10 mg Mn/kg | 13.39 ± 0.73 | 62.58 ± 1.78 | 49.18 ± 1.21 |
| 20 mg Mn/kg | 13.90 ± 0.70 | 58.54 ± 2.08 | 46.26 ± 1.18 |
Immature rats were treated with saline (control; NaCl 0.9%) or MnCl2 at doses of 5, 10 or 20 mg/kg for twenty days (PN8–27). The animals’ body weights were recorded on PN8 and PN29. Results represent mean ± S.E.M and are expressed in grams (g). Statistical analysis was performed by ANOVA; n = 15.
Since the striatum is an important target for Mn neurotoxicity (Avila et al. 2008; Bowman et al. 2011; Cordova et al. 2012; Erikson et al. 2007) next we followed with neurochemical analyses in this structure.
Effects of Mn exposure on signaling pathways
Phosphorylation and expression of MAPKs, AKT, CREB and DARPP-32 were evaluated on PN29 in the striatum of rats exposed to Mn from PN8–27. The phosphorylation of p38MAPK (Fig. 2A) was increased in the 10 (21.25 ± 6.05%, p < 0.01) and 20 mg/kg (33.31 ± 8.14%, p < 0.001) treatment groups. AKT phosphorylation (Fig. 2B) was also significantly increased in the 20 mg/kg group (14.92 ± 2.94%, p < 0.001). Conversely, DARPP-32-Thr-34 phosphorylation (Fig. 2C) was significantly decreased in the 20 mg/kg Mn group (11.05 ± 2.34%, p < 0.01). The phosphorylation and/or expression of ERK1/2, JNK1/2 and CREB (Fig. 2D) were indistinguishable from controls upon exposure to Mn.
Figure 2.
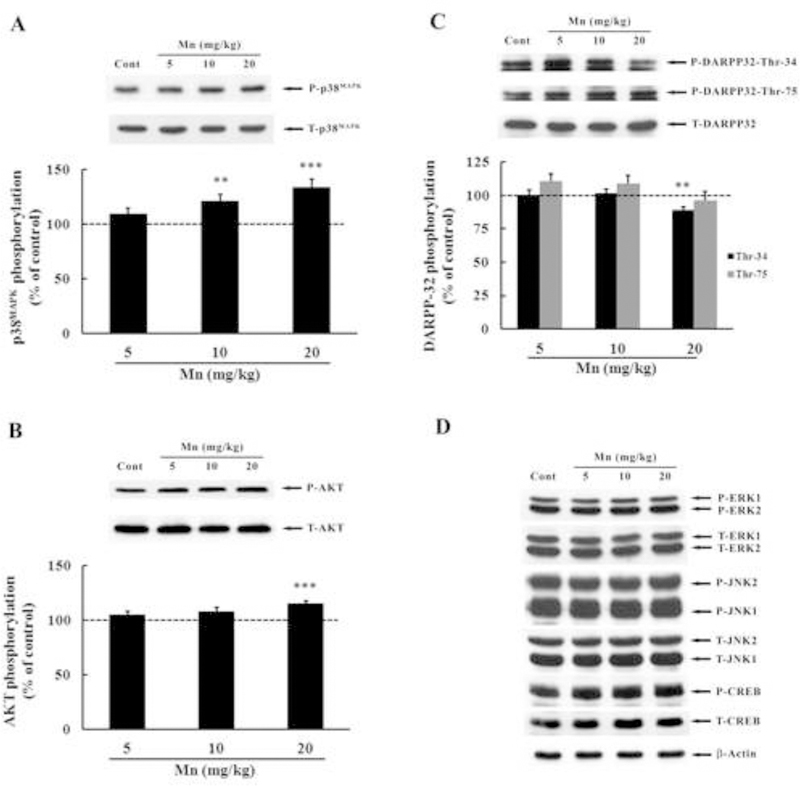
Effects of in vivo Mn exposure for twenty days on the phosphorylation of MAPK, AKT, CREB and DARPP-32 in the striatum of immature rats. The panels show representative immunoblots and quantification of phosphorylation of p38MAPK (A), AKT (B), DARPP-32-Thr-34 and-Thr-75 (C) and ERK1/2, JNK1/2, CREB and β-Actin (D) in striata of rats treated with saline (control; NaCl 0.9%) or MnCl2 at doses of 5, 10 or 20 mg/kg/day (PN8–27). The structures were analyzed on PN29. The total and phosphorylated form of each protein was detected with specific antibodies and the reaction was developed by chemiluminescence. The phosphorylation level of each protein was determined as the ratio of O.D. of the phosphorylated band/O.D. of the total band, and the data are expressed as percentage of the control (considered as 100%). Values are presented as mean ± S.E.M. Statistical analysis was performed by ANOVA followed by Duncan’s test. n = 12; ** p < 0.01, *** p < 0.001 compared to control.
Mn exposure may alter signaling pathways associated with cell death and cell survival. Accordingly, next we analyzed the enzymatic activity of caspase-3/7, which plays a key role in apoptosis. The analysis was carried out with DEVD cleavage fluorescent test in the striata of immature rats exposed to Mn for 20 days. As shown in Figure 3, Mn significantly increased (p < 0.001) caspase activity both in the 10 mg/kg (30.10 ± 6.23%) and 20 mg/kg (29.17 ± 6.51%) treatment groups compared with controls.
Figure 3.
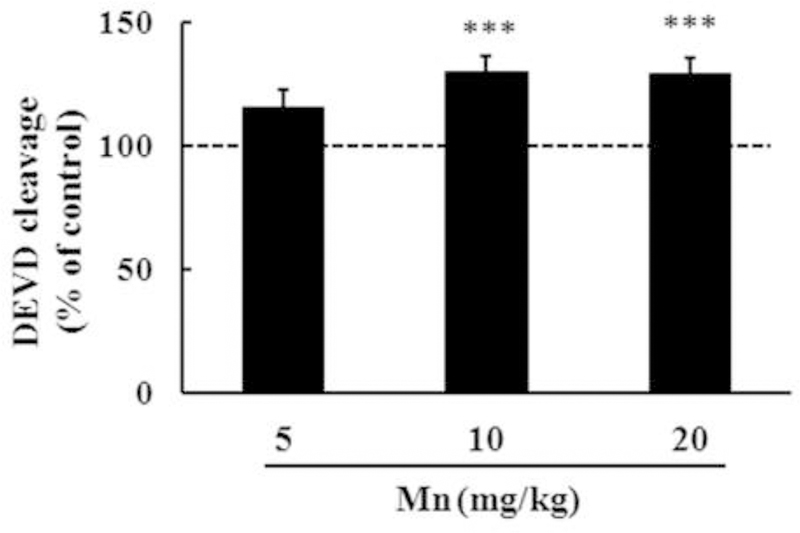
Caspase activity measured by the DEVD cleavage test in the striata of immature rats exposed in vivo to Mn for twenty days (PN8-PN-27). The panel shows the DEVD cleavage test from rats treated with saline (control; NaCl 0.9%) or MnCl2 at doses of 5, 10 or 20 mg/kg/day (PN8–27) and the structures analyzed at PN29. Results represent mean ± S.E.M and are expressed as percentage of control (100%). Statistical analysis was performed by ANOVA followed by Duncan’s test. n = 8; *** p < 0.001 compared to control.
Oxidative stress in Mn neurotoxicity
Recently, we demonstrated increased brain F2-IsoPs generation in a short-term model of Mn exposure (Cordova et al. 2012). Here, we also evaluated F2-IsoPs generation in rat striata upon a more protracted exposure to this metal. Consistent with our previous results, exposure to Mn at all doses led to a significant increase (p < 0.001; Fig. 4) in F2-IsoPs generation in the striata of developing rats.
Figure 4.
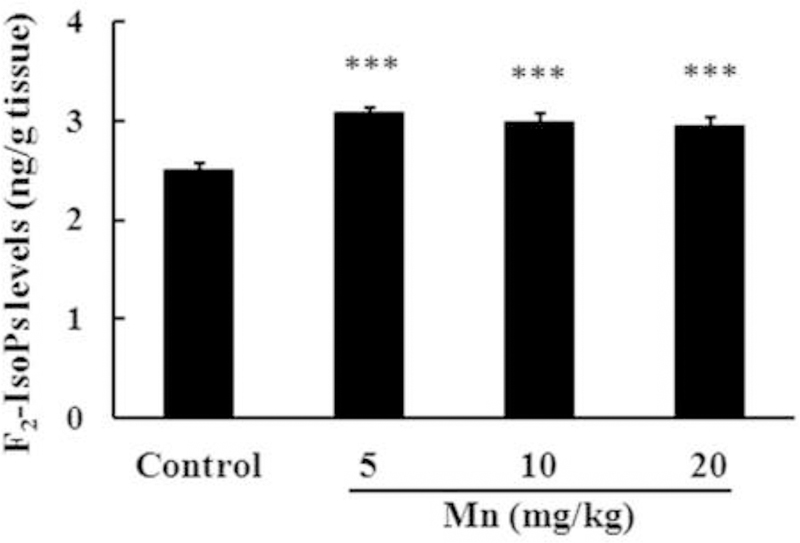
F2-IsoPs production in the striata of immature rats exposed in vivo to Mn for twenty days (PN8-PN27). The panel shows F2-IsoPs production in rats treated with saline (control; NaCl 0.9%) or MnCl2 at doses of 5, 10 or 20 mg/kg/day (PN8–27). Striata were analyzed on PN29. Results represent mean ± S.E.M and are expressed as ng F2-IsoPs/g tissue. Statistical analysis was performed by ANOVA followed by Duncan’s test. n = 4; *** p < 0.001 compared to control.
Effects of Mn exposure on motor activity
As shown in Fig. 5, overall performance on the rotarod test was indistinguishable between controls and rats treated with 5 or 10 mg Mn/kg. However, animals treated with 20 mg Mn/kg showed a significant decrease (p < 0.05) in the overall latency for falling off the rotarod vs. controls.
Figure 5.
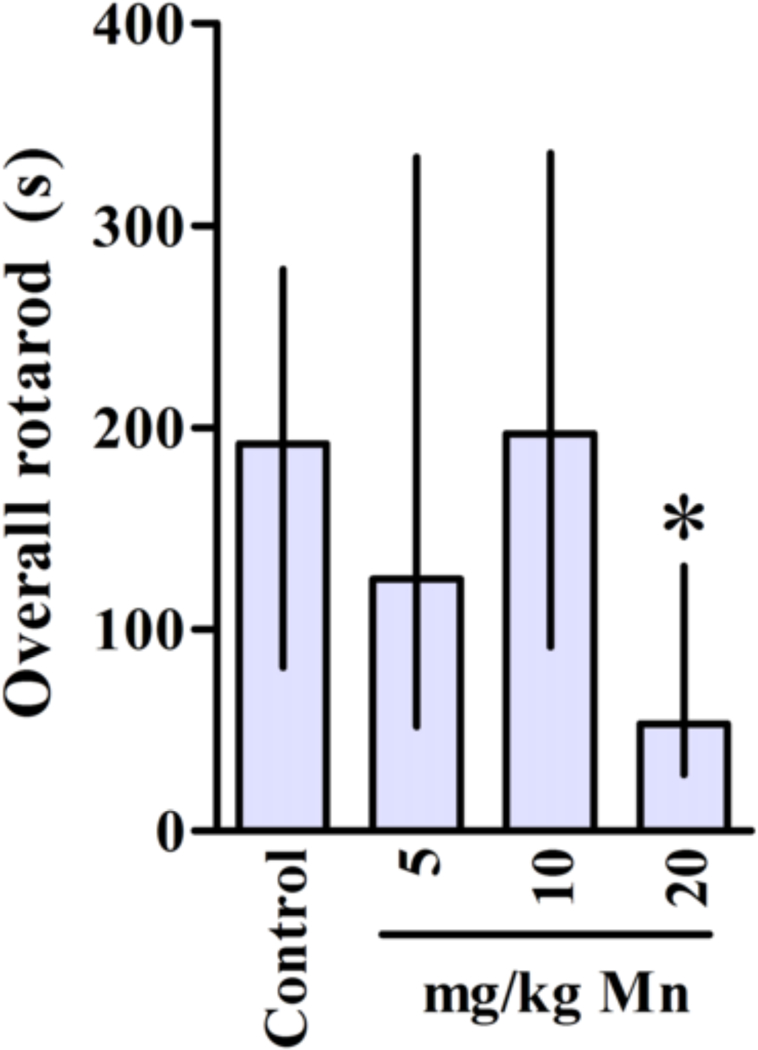
Motor coordination in rats exposed in vivo to Mn. Control and treated rats were tested on 22, 29 and 36 days of age (3, 4 and 5 week-old) on the rotarod task. The graphic shows the overall latency for falling in rats treated (PN8–27) with saline (control; NaCl 0.9%) or MnCl2 at doses of 5, 10 or 20 mg/kg/day. Results represent median ± interquartile range and are expressed in seconds (s) to latency for falling derived from twelve independent experiments. Statistical analysis was performed by Kruskal-Wallis followed by Dunn’s post-hoc test. * p < 0.05 compared to control.
Protective effects of Trolox™ on Mn-induced neurotoxicity
In order to gain a better understanding on the role of oxidative stress in mediating Mn-induced neurotoxicity, we co-treated animals with the chain-breaking antioxidant Trolox™ (1 mg/kg) and Mn (20 mg/kg). As shown in Fig. 6A, Trolox™ fully reversed the effect of Mn on p38MAPK phosphorylation, to levels indistinguishable from controls (p < 0.01). However, Trolox™ failed to block the Mn-induced increase in AKT phosphorylation (Fig. 6B). Interestingly, Trolox™ per se also increased AKT phosphorylation (35.55 ± 10.92%, p < 0.05; Fig. 6B). Furthermore, Trolox™ reversed the Mn-induced increase in caspase activity to levels indistinguishable from controls (p < 0.05 relative to Mn alone, Fig. 7) and decreased Mn-mediated F2-IsoPs production (p < 0.001 relative to Mn alone; Fig. 8). No changes in the rats’ weight were noted upon Trolox™ treatment (Table 2). Finally, as shown in Fig. 9, co-treatment with Mn and Trolox™ caused a significant (p < 0.05) improvement in motor coordination compared to Mn treated animals.
Figure 6.
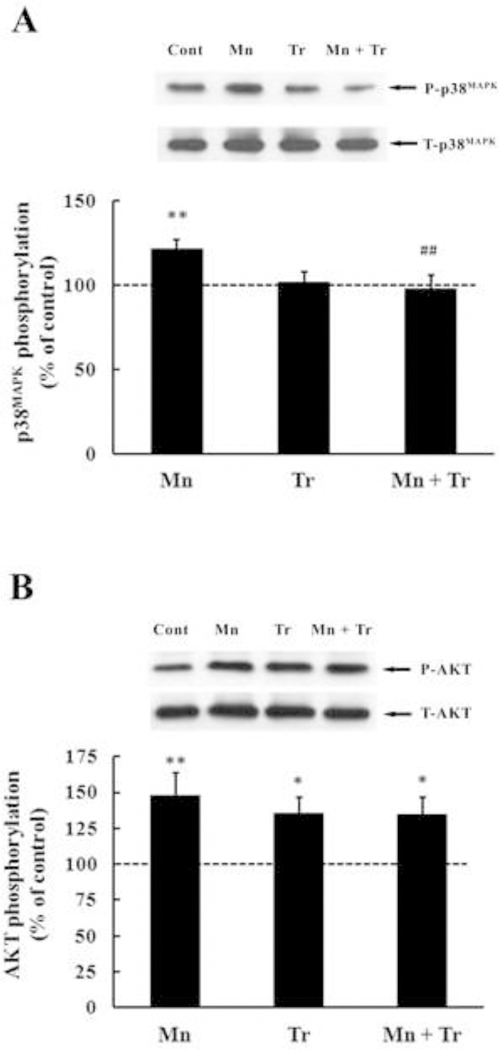
Effects of in vivo exposure to Mn and/or Trolox™ for twenty days (PN8-PN27) on phosphorylation of p38MAPK (A) and AKT (B) in the striatum of immature rats. The panels show representative immunoblots and quantification of p38MAPK and AKT phosphorylation from rats treated with saline (control; NaCl 0.9%), MnCl2 20 mg/kg (Mn), Trolox™ 1 mg/kg (Tr) or MnCl2 20 mg/kg plus Trolox™ 1 mg/kg (Mn + Tr) for twenty days (PN8–27). The structures were analyzed on PN29. The total and phosphorylated form of each protein was detected with specific antibodies and the reaction was developed by chemiluminescence. The phosphorylation level of each protein was determined as a ratio of O.D. of the phosphorylated band/O.D. of the total band and the data are expressed as percentage of the control (considered as 100%) and the values are presented as mean ± S.E.M. Statistical analysis was performed by ANOVA followed by Duncan’s test. n = 12; * p < 0.05, ** p < 0.01, compared to control; ## p < 0.01 compared to 20 mg Mn/kg group.
Figure 7.
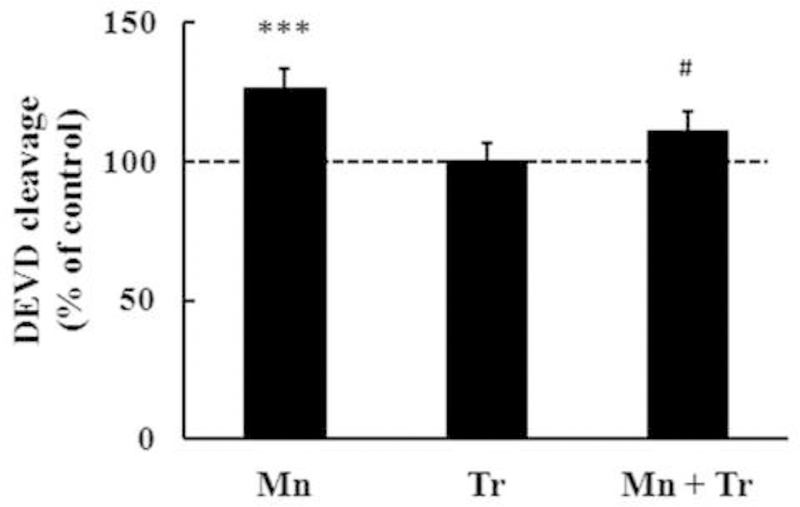
Effects of Trolox™ on caspase activity measured by DEVD cleavage test in striata from of immature rats exposed in vivo to Mn for twenty days (PN8-PN27). The panel shows the DEVD cleavage test from rats treated with saline (control; NaCl 0.9%), MnCl2 20 mg/kg (Mn), Trolox™ 1 mg/kg (Tr) or MnCl2 20 mg/kg plus Trolox™ 1 mg/kg (Mn + Tr) for twenty days (PN8–27). The structures were analyzed on PN29. Results represent mean ± S.E.M and are expressed as percentage of control (100%). Statistical analysis was performed by ANOVA followed by Duncan’s test. n = 8; *** p < 0.001 compared to control; # p < 0.05 compared to 20 mg Mn/kg group.
Figure 8.
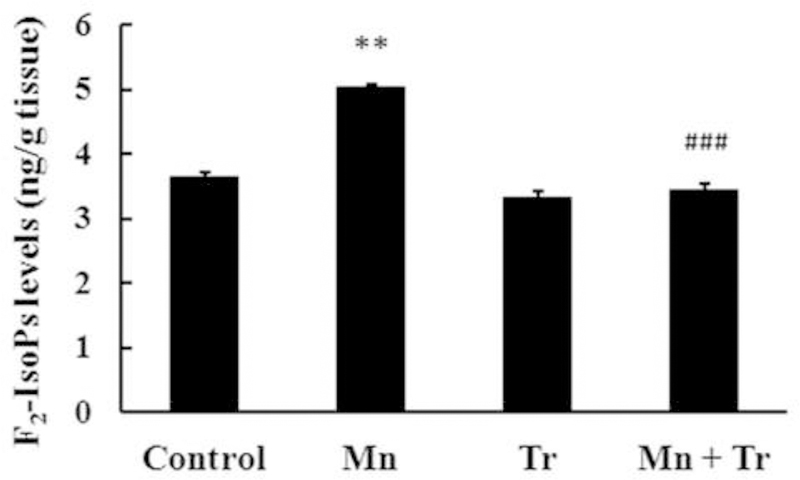
F2-IsoPs production in striata from immature rats exposed in vivo to Mn and/or Trolox™ for twenty days (PN8-PN27). The panel shows F2-IsoPs production from rats treated with saline (control; NaCl 0.9%), MnCl2 20 mg/kg (Mn), Trolox™ 1 mg/kg (Tr) or MnCl2 20 mg/kg plus Trolox™ 1 mg/kg (Mn + Tr) for twenty days (PN8–27). The structures were analyzed on PN29. Results represent mean ± S.E.M and are expressed as ng F2-IsoPs/g tissue. Statistical analysis was performed by ANOVA followed by Duncan’s test. n = 4; ** p < 0.01 compared to control; ### p < 0.001 compared to 20 mg Mn/kg group.
Table 2.
Effects of Mn and/or Trolox™ on body weight gain in immature rats exposed in vivo.
| Body weight PN8 (g) | Body weight PN29 (g) | Weight gain (g) | |
|---|---|---|---|
| Control | 13.79 ± 0.68 | 65.74 ± 2.91 | 51.94 ± 2.37 |
| Mn | 13.19 ± 0.70 | 60.08 ± 2.59 | 46.89 ± 2.03 |
| Tr | 14.08 ± 1.21 | 64.71 ± 3.85 | 50.64 ± 2.85 |
| Mn + Tr | 14.02 ± 0.72 | 64.22 ± 2.68 | 50.20 ± 2.13 |
Immature rats were treated with saline (control; NaCl 0.9%), MnCl2 20 mg/kg (Mn), Trolox™ 1 mg/kg (Tr) or MnCl2 20 mg/kg plus Trolox™ 1 mg/kg (Mn + Tr) for twenty days (PN8–27). The animals’ body weights were recorded on PN8 and PN29. Results represent mean ± S.E.M and are expressed in grams (g). Statistical analysis was performed by ANOVA; n = 8.
Figure 9.
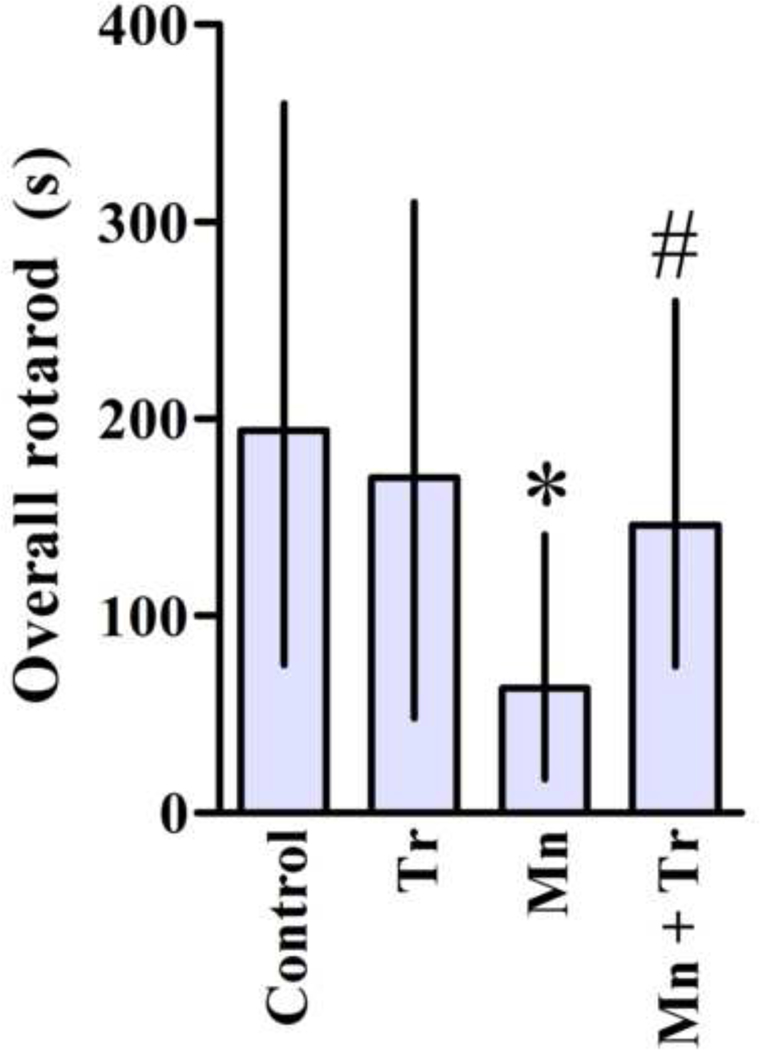
Effects of Trolox™ on motor coordination of immature rats exposed to Mn. Rats were treated for twenty days (PN8–27) with saline (control; NaCl 0.9%), MnCl2 20 mg/kg (Mn), Trolox™ 1 mg/kg (Tr) or MnCl2 20 mg/kg plus Trolox™ 1 mg/kg (Mn + Tr), and tested on 22, 29 and 36 days of age (3, 4 and 5 week old) on the rotarod task. The graphic shows the overall latency for falling and results represent mean ± interquartile range and are expressed as seconds (s) to latency for falling derived from twelve independent experiments. Statistical analysis was performed by Kruskal-Wallis followed by Dunn’s post-hoc test. * p < 0.05 compared to control., # p < 0.05 compared to 20 mg Mn/kg group.
In the open field test, animals treated with the highest Mn dose (20 mg/kg) showed a significant (p < 0.05) reduction in the distance traveled (Fig. 10A) and speed (Fig. 10B) vs. controls. Co-treatment with Trolox™ treatment did not block these deficits (Fig. 10). Mn treatment did not alter grooming (Fig. 10C) or rearing (Fig. 10D) behaviors.
Figure 10.
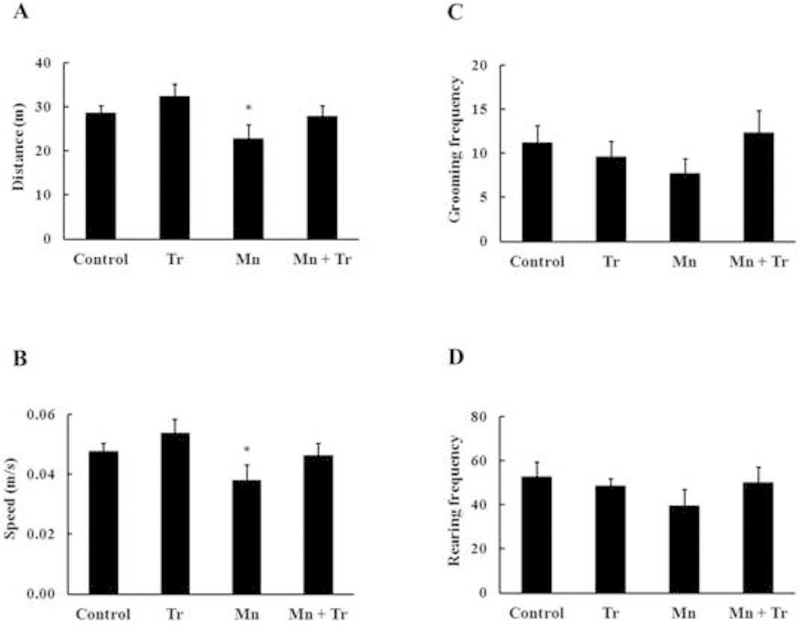
Evaluation of motor changes induced by Mn and/or Trolox™ exposure of immature rats in the circular open field task. Rats were treated with saline (control; NaCl 0.9%), MnCl2 20 mg/kg (Mn), Trolox™ 1 mg/kg (Tr) or MnCl2 20 mg/kg plus Trolox™ 1 mg/kg (Mn + Tr) for twenty days (PN8–27). The panels show the distance (m) (A), speed (m/s) (B), grooming frequency (C) and rearing frequency (D). Results represent mean ± S.E.M. Statistical analysis was performed by ANOVA followed by Newman-Keuls test. n = 12; * p < 0.05 compared to control.
DISCUSSION
Mn is an essential metal for living organisms, but excessive levels may cause pathological processes characterized by irreversible CNS damage (Lazrishvili et al. 2009). Furthermore, exposure to elevated levels of Mn may play a role in several neurodegenerative disorders, including PD (Menezes-Filho et al. 2009), Huntington’s disease (Guilarte et al. 2006), AD and amyotrophic lateral sclerosis (Eschenko et al. 2010; Roth 2009). Despite the burgeoning knowledge on Mn-induced neurodegeneration in adult animals, there is a dearth of information about its neurotoxicity in the developing brain. Few in vivo studies have addressed the potential for Mn to alter intracellular signaling pathways and/or its behavioral effects in developing animals. Recently, we showed increased oxidative stress and modulation of ERK1/2, AKT and DARPP-32 in PN14 rats upon in vivo exposure to Mn (Cordova et al. 2012). However, given the importance of the 2nd to 4th post-natal weeks in maintaing optimal neurodevelopment, here we tested the effects of protracted Mn exposure (PN8-PN29) to allow for better understanding on the role of oxidative stress in mediating the effects of this metal on immature rat brain development.
A significant increase in Mn levels was noted in several brain regions (Fig. 1A) of PN29 animals. Consistent with earlier observations (Normandin et al. 2002; Roth 2009; Yamada et al. 1986), we noted increased Mn levels in the striatum of rats dosed with 10 and 20 mg Mn/kg (Figure 1A). However, a significant increase in Mn levels was also noted in the hippocampus and cerebral cortex, corroborating previous studies showing the propensity of Mn to accumulate throughout the CNS, both in young and adult animals (Dorman et al. 2000; Morello et al. 2008; Moreno et al. 2009a; Normandin et al. 2002; Schneider et al. 2009). In addition, we observed a discrete increase in Fe levels in the striatum of PN29 animals treated with 10 and 20 mg Mn/kg (Fig. 1B), but not in animals treated with a lower dose (5 mg Mn/kg). Notably, increased Fe levels in the absence of significant changes in the level of other metals were previously observed in the striatum of juvenile mice exposed to Mn (Erikson et al. 2007; Hafeman et al. 2007; Moreno et al. 2009a; Moreno et al. 2009b). Therefore, it is possible that Mn exerts its effects on the immature striatum, at least in part, secondary to altered Fe metabolism. It is noteworthy that the increment in Fe levels approached 20–35%, while Mn levels increased 400–650% at 10 and 20 mg Mn/kg, respectively. Moreover, significant elevation in F2-IsoPs concentration was observed with all the Mn doses, including the 5 mg Mn/kg in which the striatal Fe level remained indistinguishable from controls (Fig. 1A). In contrast, other studies reported decreased brain Fe levels in animals exposed to excessive Mn (Fitsanakis et al. 2010; Garcia et al. 2006; Guilarte and Chen 2007; Guilarte et al. 2006; Hansen et al. 2009) paralleled by increased transferrin (Tf) plasma levels, and divalent metal 1 transporter (DMT-1) and Tf receptor (TfR) expression (Garcia et al. 2006). While these changes may possibly explain the increased levels of Fe observed in our study, additional studies will be required to determine expression and/or activity of these transporters in the treatment protocol described herein.
Consistent with our previous study (Cordova et al. 2012), Mn exposure led to increased striatal oxidative stress, characterized by significant elevation in F2-IsoPs concentrations (Fig. 4). F2-IsoPs are prostaglandin-like molecules produced by the free radical-mediated peroxidation of arachidonic acid (Milatovic and Aschner 2009), which can be efficiently reduced by antioxidants treatment (Milatovic et al. 2011). Several studies have shown oxidative stress to be a major mechanism for Mn-induced neurotoxicity both in in vitro and in vivo models (Benedetto et al. 2009; Gunter et al. 2006; Milatovic et al. 2009; Roth and Garrick 2003; Tamm et al. 2008; Zhang et al. 2008). Mn-induced ROS generation (Avila et al. 2008; Park and Park 2010; Roth 2009) is triggered by dysfunctional mitochondria, resulting in both apoptotic and necrotic cell death (Roth and Garrick 2003; Tamm et al. 2008; Yin et al. 2008), triggering the opening of the permeability transition pore (Roth 2009; Zhang et al. 2004). Consistent with these observations, we show that Mn induced an increase in striatal caspase activity (Fig. 3) and that Trolox™ attenuated the Mn (20 mg/kg)-induced increase in F2-IsoPs generation (Fig. 8), with caspase activity returning to control levels (Fig. 7).
In addition to oxidative stress generation and activation of caspases, many classic intracellular signaling pathways associated with programmed cell death are altered by Mn treatment in vitro. Among them are the activation of MAPKs and AKT (Gonzalez et al. 2008; Hirata et al. 2004; Ito et al. 2006; Li et al. 2010; Park and Park 2010; Prabhakaran et al. 2008; Yin et al. 2008). Herein, we observed p38MAPK and AKT activation in the striatum of immature rats exposed for 20 days to Mn (Fig. 2A and 2B). Interestingly, co-treatment with Trolox™ led to a significant reduction in p38MAPK phosphorylation to levels indistinguishable from controls, strongly suggesting that Mn-mediated p38MAPK activation may be occurring by a ROS-dependent mechanism (Fig. 6A). Conversely, the activation of AKT by Mn (20mg/kg; Fig 2B) appeared independent of oxidative stress, since Trolox™ was ineffective in reversing the Mn-dependent AKT activation (Fig 6B). It is noteworthy that Trolox™ itself also increased AKT phosphorylation. Although the current evidences point to the activation of AKT pathway as an important event mediating Mn-induced neurotoxicity (present study; Cordova et al., 2012; McDougall et al. 2011), the understanding on the mechanisms involved in AKT activation by Mn remains elusive. However, compounds that induce oxidative stress are known to inhibit, rather than activate, the PI3K/AKT pathway. Moreover, antioxidants including Trolox™ may restore or increase AKT phosphorylation levels, thus affording neuroprotection (Choi et al. 2012; Sun et al. 2012). Accordingly, it is possible that other mechanisms that unrelated to ROS production, such as neurotransmitter imbalances, might be involved in the AKT activation in response to Mn. Nonetheless, additional studies are necessary to confirm such hypothesis.
DARPP-32 phosphorylation was also altered by 20 days Mn exposure (Fig. 2C). DARPP-32 is highly expressed in striatal medium spiny neurons, and in rodents its level increases at birth and reaches adult level approximately three weeks postnatally. The early and defined appearance of DARPP-32 suggests that it may influence specific aspects of neuronal differentiation and synaptogenesis (Svenningsson et al. 2004). Regulation of the state of DARPP-32 phosphorylation provides a mechanism for integrating information arriving at dopaminoceptive neurons in striatal medium spiny neurons via a variety of neurotransmitters. As an example, activation of D1 dopaminergic pathways stimulates DARPP-32 phosphorylation at Thr-34 (via cAMP/PKA) and thereby converts DARPP-32 into a potent inhibitor of protein phosphatase 1 (PP1). Conversely, DARPP-32 is also phosphorylated at Thr-75 by glutamatergic neurotransmission (via Cdk5 activation), which converts DARPP-32 into an inhibitor of PKA. Therefore, DARPP-32 has the unique property of being a dual-function protein, acting either as an inhibitor of PP1 or of PKA (Svenningsson et al. 2004). In this way, DARPP-32 is implicated in modulation of motor responses (Polissidis et al. 2010). In the striatum of mice, PKA-dependent phosphorylation of DARPP-32 at Thr-34 might be related to suppressive effects on motor activity (Andersson et al. 2005; Borgkvist et al. 2008). In PD and/or L-DOPA treated animal models, increased DARPP-32-Thr-34 phosphorylation is associated with behavioral disturbances (Botsakis et al. 2010; Lebel et al. 2010; Santini et al. 2007). Recently, we demonstrated the modulation of DARPP-32 by Mn in a short-term exposure protocol (Cordova et al. 2012), noting increased phosphorylation of DARPP-32 at Thr-34 in PN14 rats treated with 5 and 10 mg Mn/kg. Herein, we noted a slight decrease in the phosphorylation of DARPP-32-Thr-34 and no changes at Thr-75 in PN29 rats exposed to 20 mg Mn/kg (Fig. 2C). These differences potentially reflect changes in striatal dopaminergic signaling, which alter the availability of dopamine and/or dopamine receptors (Guilarte et al. 2008).
In addition to the cell signaling alterations, we also noted significant behavioral changes in developing rats exposed to Mn (20 mg/kg) for 20 days, characterized by a significant decline in motor coordination (Fig. 5). Co-treatment with Mn and Trolox™ significantly improved the animals’ performance in the rotarod task (Fig. 9). Mn (20 mg/kg) also decreased the distance and speed travelled in the open field task (Fig. 10), but in this case Trolox™ failed to mitigate the Mn-induced effects. Altered movement has been previously shown to be associated with increased oxidative stress, mainly in the striatum (de Oliveira et al. 2007). Furthermore, an in vivo study conducted in nonhuman primates showed a direct correlation between the levels of Mn in the caudate nucleus, putamen and globus pallidus, and the severity of the behavioral deficits (Schneider et al. 2009). Thus, the significant accumulation of Mn in the striatum, the generation of oxidative stress, apoptotic cell death and disturbed cell signaling in response to Mn exposure might directly or indirectly trigger these behavioral deficits.
Mn has a relatively long half-life in the CNS, consistent with a slow elimination rate (Morello et al. 2008). Exposure of developing individuals to Mn levels that exceed the homeostatic capacity may result in an overload condition, with increased risk for neurodegenerative diseases, such as PD at later-life stages (Lucchini and Zimmerman 2009). Additionally, the involvement of important intracellular signaling pathways in Mn neurotoxicity, such as MAPKs, may lead to cell death, reduced survival and plasticity, which are unmasked during later life stages. Our study shows that Mn-induced oxidative stress in vivo perturbs cell signaling pathways and triggers behavioral changes. Accordingly, the possibility that the aberrant effects of developmental Mn exposure may be attenuated by therapeutic modalities that reduce oxidative stress should be further explored.
Acknowledgements
This work was supported by the National Council for Scientific and Technological Development (CNPq) Brazil (#305194/2010–0); Brazilian Institute for Neuroscience (IBN).Net/CNPq; Coordination for the Training and Improvement of Higher Education Personnel (CAPES); National Institute of Science and Technology (INCT) for Excitotoxicity and Neuroprotection; Santa Catarina Research Fundation (FAPESC) and FAPESC/PRONEX Program -NENASC project; National Institute of Environmental Health Sciences (NIEHS) R01 ES10563 (MA) and P30 ES000267 (MA). RBL, AL and RDSP are recipients of CNPq fellowships. The funding agencies had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Conflict of Interest statement: None of the authors has a known or perceived conflict of interest with the contents of this manuscript.
REFERENCES
- Aguiar-Jr AS, Araujo AL, da-Cunha TR, et al. (2009) Physical exercise improves motor and short-term social memory deficits in reserpinized rats. Brain Research Bulletin 79(6):452–457 [DOI] [PubMed] [Google Scholar]
- Alaimo A, Gorojod RM, Kotler ML (2011) The extrinsic and intrinsic apoptotic pathways are involved in manganese toxicity in rat astrocytoma C6 cells. Neurochemistry International 59(2):297–308 [DOI] [PubMed] [Google Scholar]
- Andersson M, Usiello A, Borgkvist A, et al. (2005) Cannabinoid action depends on phosphorylation of dopamine-and cAMP-regulated phosphoprotein of 32 kDa at the protein kinase A site in striatal projection neurons. The Journal of Neuroscience 25(37):8432–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aschner JL, Aschner M (2005) Nutritional aspects of manganese homeostasis. Molecular Aspects of Medicine 26(4–5):353–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aschner M, Guilarte TR, Schneider JS, Zheng W (2007) Manganese: recent advances in understanding its transport and neurotoxicity. Toxicology and Applied Pharmacology 221(2): 131–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avila DS, Gubert P, Fachinetto R, et al. (2008) Involvement of striatal lipid peroxidation and inhibition of calcium influx into brain slices in neurobehavioral alterations in a rat model of short-term oral exposure to manganese. Neurotoxicology 29(6):1062–8 [DOI] [PubMed] [Google Scholar]
- Benedetto A, Au C, Aschner M (2009) Manganese-induced dopaminergic neurodegeneration: insights into mechanisms and genetics shared with Parkinson’s disease. Chemical Reviews 109(10):4862–84. [DOI] [PubMed] [Google Scholar]
- Bjerrum OJ, Heegaard NHH (1988) CRC Handbook of Immunoblotting of Proteins, vol I, CRC Press [Google Scholar]
- Bondy SC, Campbell A (2005) Developmental neurotoxicology. Journal of Neuroscience Research 81(5):605–12 [DOI] [PubMed] [Google Scholar]
- Borgkvist A, Marcellino D, Fuxe K, Greengard P, Fisone G (2008) Regulation of DARPP-32 phosphorylation by Delta9-tetrahydrocannabinol. Neuropharmacology 54(1):31–5 [DOI] [PubMed] [Google Scholar]
- Botsakis K, Pavlou O, Poulou PD, Matsokis N, Angelatou F (2010) Blockade of adenosine A2A receptors downregulates DARPP-32 but increases ERK1/2 activity in striatum of dopamine deficient “weaver” mouse. Neurochemistry International 56(2):245–9 [DOI] [PubMed] [Google Scholar]
- Bowman AB, Kwakye GF, Hernandez EH, Aschner M (2011) Role of manganese in neurodegenerative diseases. J Trace Elem Med Biol 25(4):191–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brazil DP, Yang ZZ, Hemmings BA (2004) Advances in protein kinase B signalling: AKTion on multiple fronts. Trends in Biochemical Sciences 29(5):233–42 [DOI] [PubMed] [Google Scholar]
- Broaddus WC, Bennett JP Jr. (1990a) Postnatal development of striatal dopamine function. I. An examination of D1 and D2 receptors, adenylate cyclase regulation and presynaptic dopamine markers. Brain Research Developmental Brain Research 52(1–2):265–71 [DOI] [PubMed] [Google Scholar]
- Broaddus WC, Bennett JP Jr. (1990b) Postnatal development of striatal dopamine function. II. Effects of neonatal 6-hydroxydopamine treatments on D1 and D2 receptors, adenylate cyclase activity and presynaptic dopamine function. Brain Research Developmental Brain Research 52(1–2):273–7 [DOI] [PubMed] [Google Scholar]
- Burton NC, Guilarte TR (2009) Manganese neurotoxicity: lessons learned from longitudinal studies in nonhuman primates. Environmental Health Perspectives 117(3):325–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai T, Che H, Yao T, et al. (2011) Manganese induces tau hyperphosphorylation through the activation of ERK MAPK pathway in PC12 cells. Toxicological Sciences 119(1):169–77 [DOI] [PubMed] [Google Scholar]
- Chang L, Karin M (2001) Mammalian MAP kinase signalling cascades. Nature 410(6824):37–40 [DOI] [PubMed] [Google Scholar]
- Chen Z, Gibson TB, Robinson F, et al. (2001) MAP kinases. Chemical Reviews 101(8):2449–76 [DOI] [PubMed] [Google Scholar]
- Choi H, Park HH, Koh SH, et al. (2012) Coenzyme Q10 protects against amyloid beta-induced neuronal cell death by inhibiting oxidative stress and activating the P13K pathway. Neurotoxicology 33(1):85–90 [DOI] [PubMed] [Google Scholar]
- Cordova FM, Aguiar AS Jr., Peres TV, et al. (2012) In vivo manganese exposure modulates erk, akt and darpp-32 in the striatum of developing rats, and impairs their motor function. PloS One 7(3):e33057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordova FM, Rodrigues AL, Giacomelli MB, et al. (2004) Lead stimulates ERK1/2 and p38MAPK phosphorylation in the hippocampus of immature rats. Brain Research 998(1):65–72 [DOI] [PubMed] [Google Scholar]
- Crittenden PL, Filipov NM (2011) Manganese modulation of MAPK pathways: effects on upstream mitogen activated protein kinase kinases and mitogen activated kinase phosphatase-1 in microglial cells. Journal of Applied Toxicology 31(1): 1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Oliveira MR, de Bittencourt Pasquali MA, Silvestrin RB, Mello EST, Moreira JC (2007) Vitamin A supplementation induces a prooxidative state in the striatum and impairs locomotory and exploratory activity of adult rats. Brain Research 1169:112–9 [DOI] [PubMed] [Google Scholar]
- Dobbing J (1990) Brain, behaviour, and iron in the infant diet. Springer-Verlag [Google Scholar]
- Dobson AW, Erikson KM, Aschner M (2004) Manganese neurotoxicity. Annals of the New York Academy of Sciences 1012:115–28 [DOI] [PubMed] [Google Scholar]
- Dorman DC, Struve MF, Vitarella D, Byerly FL, Goetz J, Miller R (2000) Neurotoxicity of manganese chloride in neonatal and adult CD rats following subchronic (21-day) high-dose oral exposure. Journal of Applied Toxicology 20(3):179–87 [DOI] [PubMed] [Google Scholar]
- Erikson KM, Suber RL, Aschner M (2002) Glutamate/aspartate transporter (GLAST), taurine transporter and metallothionein mRNA levels are differentially altered in astrocytes exposed to manganese chloride, manganese phosphate or manganese sulfate. Neurotoxicology 23(3):281–8 [DOI] [PubMed] [Google Scholar]
- Erikson KM, Thompson K, Aschner J, Aschner M (2007) Manganese neurotoxicity: a focus on the neonate. Pharmacology & Therapeutics 113(2):369–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eschenko O, Canals S, Simanova I, Logothetis NK (2010) Behavioral, electrophysiological and histopathological consequences of systemic manganese administration in MEMRI. Magnetic Resonance Imaging 28(8):1165–74 [DOI] [PubMed] [Google Scholar]
- Fitsanakis VA, Zhang N, Anderson JG, et al. (2008) Measuring brain manganese and iron accumulation in rats following 14 weeks of low-dose manganese treatment using atomic absorption spectroscopy and magnetic resonance imaging. Toxicological Sciences 103(1):116–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitsanakis VA, Zhang N, Garcia S, Aschner M (2010) Manganese (Mn) and iron (Fe): interdependency of transport and regulation. Neurotoxicity Research 18(2): 124–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia SJ, Gellein K, Syversen T, Aschner M (2006) A manganese-enhanced diet alters brain metals and transporters in the developing rat. Toxicological Sciences 92(2):516–25 [DOI] [PubMed] [Google Scholar]
- Gonzalez LE, Juknat AA, Venosa AJ, Verrengia N, Kotler ML (2008) Manganese activates the mitochondrial apoptotic pathway in rat astrocytes by modulating the expression of proteins of the Bcl-2 family. Neurochemistry International 53(6–8):408–15 [DOI] [PubMed] [Google Scholar]
- Gottlieb A, Keydar I, Epstein HT (1977) Rodent brain growth stages: an analytical review. Biology of the Neonate 32(3–4):166–76 [DOI] [PubMed] [Google Scholar]
- Grandjean P, Landrigan PJ (2006) Developmental neurotoxicity of industrial chemicals. Lancet 368(9553):2167–78 [DOI] [PubMed] [Google Scholar]
- Graybiel AM (2005) The basal ganglia: learning new tricks and loving it. Current Opinion in Neurobiology 15(6):638–44 [DOI] [PubMed] [Google Scholar]
- Greengard P, Allen PB, Nairn AC (1999) Beyond the dopamine receptor: the DARPP-32/protein phosphatase-1 cascade. Neuron 23(3):435–47 [DOI] [PubMed] [Google Scholar]
- Guilarte TR, Burton NC, McGlothan JL, et al. (2008) Impairment of nigrostriatal dopamine neurotransmission by manganese is mediated by pre-synaptic mechanism(s): implications to manganese-induced parkinsonism. Journal of Neurochemistry 107(5):1236–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilarte TR, Chen MK (2007) Manganese inhibits NMDA receptor channel function: implications to psychiatric and cognitive effects. Neurotoxicology 28(6):1147–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilarte TR, Chen MK, McGlothan JL, et al. (2006) Nigrostriatal dopamine system dysfunction and subtle motor deficits in manganese-exposed non-human primates. Experimental Neurology 202(2):381–90 [DOI] [PubMed] [Google Scholar]
- Gunter TE, Gavin CE, Aschner M, Gunter KK (2006) Speciation of manganese in cells and mitochondria: a search for the proximal cause of manganese neurotoxicity. Neurotoxicology 27(5):765–76 [DOI] [PubMed] [Google Scholar]
- Hafeman D, Factor-Litvak P, Cheng Z, van Geen A, Ahsan H (2007) Association between manganese exposure through drinking water and infant mortality in Bangladesh. Environmental Health Perspectives 115(7): 1107–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanada M, Feng J, Hemmings BA (2004) Structure, regulation and function of PKB/AKT--a major therapeutic target. Biochimica et Biophysica Acta 1697(1–2):3–16 [DOI] [PubMed] [Google Scholar]
- Hansen SL, Trakooljul N, Liu HC, Moeser AJ, Spears JW (2009) Iron transporters are differentially regulated by dietary iron, and modifications are associated with changes in manganese metabolism in young pigs. The Journal of Nutrition 139(8):1474–9 [DOI] [PubMed] [Google Scholar]
- Hardy G (2009) Manganese in parenteral nutrition: who, when, and why should we supplement? Gastroenterology 137(5 Suppl):S29–35 [DOI] [PubMed] [Google Scholar]
- Hirata Y, Furuta K, Miyazaki S, Suzuki M, Kiuchi K (2004) Anti-apoptotic and pro-apoptotic effect of NEPP11 on manganese-induced apoptosis and JNK pathway activation in PC12 cells. Brain Research 1021(2):241–7 [DOI] [PubMed] [Google Scholar]
- Ito Y, Oh-Hashi K, Kiuchi K, Hirata Y (2006) p44/42 MAP kinase and c-Jun N-terminal kinase contribute to the up-regulation of caspase-3 in manganese-induced apoptosis in PC12 cells. Brain Research 1099(1):1–7 [DOI] [PubMed] [Google Scholar]
- Jang BC (2009) Induction of COX-2 in human airway cells by manganese: role of PI3K/PKB, p38 MAPK, PKCs, Src, and glutathione depletion. Toxicology In Vitro 23(1):120–6 [DOI] [PubMed] [Google Scholar]
- Kern CH, Stanwood GD, Smith DR (2010) Preweaning manganese exposure causes hyperactivity, disinhibition, and spatial learning and memory deficits associated with altered dopamine receptor and transporter levels. Synapse 64(5):363–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latchoumycandane C, Anantharam V, Kitazawa M, Yang Y, Kanthasamy A, Kanthasamy AG (2005) Protein kinase Cdelta is a key downstream mediator of manganese-induced apoptosis in dopaminergic neuronal cells. The Journal of Pharmacology and Experimental Therapeutics 313(1):46–55 [DOI] [PubMed] [Google Scholar]
- Lazrishvili IL, Shukakidze AA, Chkhartishvili NN, Bikashvili TZ (2009) Morphological changes and manganese content in the brains of rat pups subjected to subchronic poisoning with manganese chloride. Neuroscience and Behavioral Physiology 39(1):7–12 [DOI] [PubMed] [Google Scholar]
- Lebel M, Chagniel L, Bureau G, Cyr M (2010) Striatal inhibition of PKA prevents levodopa-induced behavioural and molecular changes in the hemiparkinsonian rat. Neurobiology of Disease 38(1):59–67 [DOI] [PubMed] [Google Scholar]
- Leo D, Sorrentino E, Volpicelli F, et al. (2003) Altered midbrain dopaminergic neurotransmission during development in an animal model of ADHD. Neuroscience and Biobehavioral Reviews 27(7):661–9 [DOI] [PubMed] [Google Scholar]
- Li Y, Sun L, Cai T, et al. (2010) alpha-Synuclein overexpression during manganese-induced apoptosis in SH-SY5Y neuroblastoma cells. Brain Research Bulletin 81(4–5):428–33 [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. The Journal of Biological Chemistry 193(1):265–75 [PubMed] [Google Scholar]
- Lozoff B, Georgieff MK (2006) Iron deficiency and brain development. Seminars in Pediatric Neurology 13(3): 158–65 [DOI] [PubMed] [Google Scholar]
- Lucchini R, Zimmerman N (2009) Lifetime cumulative exposure as a threat for neurodegeneration: need for prevention strategies on a global scale. Neurotoxicology 30(6):1144–8 [DOI] [PubMed] [Google Scholar]
- McDougall SA, Der-Ghazarian T, Britt CE, Varela FA, Crawford CA (2011) Postnatal manganese exposure alters the expression of D2L and D2S receptor isoforms: relationship to PKA activity and Akt levels. Synapse 65(7):583–91 [DOI] [PubMed] [Google Scholar]
- Menezes-Filho JA, Bouchard M, Sarcinelli Pde N, Moreira JC (2009) Manganese exposure and the neuropsychological effect on children and adolescents: a review. Pan American Journal of Public Health 26(6):541–8 [DOI] [PubMed] [Google Scholar]
- Milatovic D, Aschner M (2009) Measurement of isoprostanes as markers of oxidative stress in neuronal tissue. Curr Protoc Toxicol Chapter 12:Unit12 14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milatovic D, Gupta RC, Yu Y, Zaja-Milatovic S, Aschner M (2011) Protective effects of antioxidants and anti-inflammatory agents against manganese-induced oxidative damage and neuronal injury. Toxicology and Applied Pharmacology 256(3):219–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milatovic D, Yin Z, Gupta RC, et al. (2007) Manganese induces oxidative impairment in cultured rat astrocytes. Toxicological Sciences 98(1):198–205 [DOI] [PubMed] [Google Scholar]
- Milatovic D, Zaja-Milatovic S, Gupta RC, Yu Y, Aschner M (2009) Oxidative damage and neurodegeneration in manganese-induced neurotoxicity. Toxicology and Applied Pharmacology 240(2):219–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morello M, Canini A, Mattioli P, et al. (2008) Sub-cellular localization of manganese in the basal ganglia of normal and manganese-treated rats An electron spectroscopy imaging and electron energy-loss spectroscopy study. Neurotoxicology 29(1):60–72 [DOI] [PubMed] [Google Scholar]
- Moreno JA, Streifel KM, Sullivan KA, Legare ME, Tjalkens RB (2009a) Developmental exposure to manganese increases adult susceptibility to inflammatory activation of glia and neuronal protein nitration. Toxicological Sciences 112(2):405–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno JA, Yeomans EC, Streifel KM, Brattin BL, Taylor RJ, Tjalkens RB (2009b) Age-dependent susceptibility to manganese-induced neurological dysfunction. Toxicological Sciences 112(2):394–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow JD, Roberts LJ 2nd. (1999) Mass spectrometric quantification of F2-isoprostanes in biological fluids and tissues as measure of oxidant stress. Methods in Enzymology 300:3–12 [DOI] [PubMed] [Google Scholar]
- Normandin L, Carrier G, Gardiner PF, et al. (2002) Assessment of bioaccumulation, neuropathology, and neurobehavior following subchronic (90 days) inhalation in Sprague-Dawley rats exposed to manganese phosphate. Toxicology and Applied Pharmacology 183(2): 135–45 [PubMed] [Google Scholar]
- Packard MG, Knowlton BJ (2002) Learning and memory functions of the Basal Ganglia. Annual Review of Neuroscience 25:563–93 [DOI] [PubMed] [Google Scholar]
- Park EJ, Park K (2010) Induction of oxidative stress and inflammatory cytokines by manganese chloride in cultured T98G cells, human brain glioblastoma cell line. Toxicology In Vitro 24(2):472–9 [DOI] [PubMed] [Google Scholar]
- Perl DP, Olanow CW (2007) The neuropathology of manganese-induced Parkinsonism. Journal of Neuropathology and Experimental Neurology 66(8):675–82 [DOI] [PubMed] [Google Scholar]
- Peterson GL (1977) A simplification of the protein assay method of Lowry et al. which is more generally applicable. Analytical Biochemistry 83(2):346–56 [DOI] [PubMed] [Google Scholar]
- Polissidis A, Chouliara O, Galanopoulos A, et al. (2010) Individual differences in the effects of cannabinoids on motor activity, dopaminergic activity and DARPP-32 phosphorylation in distinct regions of the brain. Int J Neuropsychopharmacol 13(9): 1175–91 [DOI] [PubMed] [Google Scholar]
- Posser T, de Aguiar CB, Garcez RC, et al. (2007) Exposure of C6 glioma cells to Pb(II) increases the phosphorylation of p38(MAPK) and JNK1/2 but not of ERK1/2. Archives of Toxicology 81(6):407–14 [DOI] [PubMed] [Google Scholar]
- Prabhakaran K, Ghosh D, Chapman GD, Gunasekar PG (2008) Molecular mechanism of manganese exposure-induced dopaminergic toxicity. Brain Research Bulletin 76(4):361–7 [DOI] [PubMed] [Google Scholar]
- Qi Z, Miller GW, Voit EO (2010) The internal state of medium spiny neurons varies in response to different input signals. BMC Systems Biology 4:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice D, Barone S Jr., (2000) Critical periods of vulnerability for the developing nervous system: evidence from humans and animal models. Environmental Health Perspectives 108 Suppl 3:511–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha JB, Pereira ME, Emanuelli T, Christofari RS, Souza DO (1995) Effect of treatment with mercury chloride and lead acetate during the second stage of rapid postnatal brain growth on delta-aminolevulinic acid dehydratase (ALA-D) activity in brain, liver, kidney and blood of suckling rats. Toxicology 100(1–3):27–37 [DOI] [PubMed] [Google Scholar]
- Rodier PM (1995) Developing brain as a target of toxicity. Environmental Health Perspectives 103 Suppl 6:73–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodnight R, Leal R (1990) Regional variations in protein phosphorylating activity in rat brain studied in micro-slices labeled with [32P]phosphate. Journal of Molecular Neuroscience 2(2):115–22 [DOI] [PubMed] [Google Scholar]
- Roth JA (2009) Are there common biochemical and molecular mechanisms controlling manganism and parkisonism. Neuromolecular Medicine 11(4):281–96 [DOI] [PubMed] [Google Scholar]
- Roth JA, Garrick MD (2003) Iron interactions and other biological reactions mediating the physiological and toxic actions of manganese. Biochemical Pharmacology 66(1):1–13 [DOI] [PubMed] [Google Scholar]
- Santamaria AB (2008) Manganese exposure, essentiality & toxicity. The Indian Journal of Medical Research 128(4):484–500 [PubMed] [Google Scholar]
- Santini E, Valjent E, Usiello A, et al. (2007) Critical involvement of cAMP/DARPP-32 and extracellular signal-regulated protein kinase signaling in L-DOPA-induced dyskinesia. The Journal of Neuroscience 27(26):6995–7005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider JS, Decamp E, Clark K, Bouquio C, Syversen T, Guilarte TR (2009) Effects of chronic manganese exposure on working memory in non-human primates. Brain Research 1258:86–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin HJ, Choi MS, Ryoo NH, et al. (2010) Manganese-mediated up-regulation of HIF-1alpha protein in Hep2 human laryngeal epithelial cells via activation of the family of MAPKs. Toxicology In Vitro 24(4):1208–14 [DOI] [PubMed] [Google Scholar]
- Sun C, Wang D, Zheng W (2012) Hydrogen peroxide attenuates the prosurvival signaling of insulin-like growth factor-1 through two pathways. Neuroreport 23(13):768–73 [DOI] [PubMed] [Google Scholar]
- Suzuki H, Takanashi J, Saeki N, Kohno Y (2003) Temporal parenteral nutrition in children causing t1 shortening in the anterior pituitary gland and globus pallidus. Neuropediatrics 34(4):200–4 [DOI] [PubMed] [Google Scholar]
- Svenningsson P, Nishi A, Fisone G, Girault JA, Nairn AC, Greengard P (2004) DARPP-32: an integrator of neurotransmission. Annual Review of Pharmacology and Toxicology 44:269–96 [DOI] [PubMed] [Google Scholar]
- Tamm C, Sabri F, Ceccatelli S (2008) Mitochondrial-mediated apoptosis in neural stem cells exposed to manganese. Toxicological Sciences 101(2):310–20 [DOI] [PubMed] [Google Scholar]
- Thomas GM, Huganir RL (2004) MAPK cascade signalling and synaptic plasticity. Nature Reviews Neuroscience 5(3):173–83 [DOI] [PubMed] [Google Scholar]
- Tkac I, Rao R, Georgieff MK, Gruetter R (2003) Developmental and regional changes in the neurochemical profile of the rat brain determined by in vivo 1H NMR spectroscopy. Magnetic Resonance in Medicine 50(1):24–32 [DOI] [PubMed] [Google Scholar]
- Waetzig V, Herdegen T (2004) Neurodegenerative and physiological actions of c-Jun N-terminal kinases in the mammalian brain. Neuroscience Letters 361(1–3):64–7 [DOI] [PubMed] [Google Scholar]
- Walaas SI, Aswad DW, Greengard P (1983) A dopamine-and cyclic AMP-regulated phosphoprotein enriched in dopamine-innervated brain regions. Nature 301(5895):69–71 [DOI] [PubMed] [Google Scholar]
- Yamada M, Ohno S, Okayasu I, et al. (1986) Chronic manganese poisoning: a neuropathological study with determination of manganese distribution in the brain. Acta Neuropathologica 70(3– 4):273–8 [DOI] [PubMed] [Google Scholar]
- Yin Z, Aschner JL, dos Santos AP, Aschner M (2008) Mitochondrial-dependent manganese neurotoxicity in rat primary astrocyte cultures. Brain Research 1203:1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Xu Z, Gao J, Xu B, Deng Y (2008) In vitro effect of manganese chloride exposure on energy metabolism and oxidative damage of mitochondria isolated from rat brain. Environmental Toxicology and Pharmacology 26(2):232–6 [DOI] [PubMed] [Google Scholar]
- Zhang S, Fu J, Zhou Z (2004) In vitro effect of manganese chloride exposure on reactive oxygen species generation and respiratory chain complexes activities of mitochondria isolated from rat brain. Toxicology In Vitro 18(1):71–7 [DOI] [PubMed] [Google Scholar]
- Zheng W, Aschner M, Ghersi-Egea JF (2003) Brain barrier systems: a new frontier in metal neurotoxicological research. Toxicology and Applied Pharmacology 192(1):1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuse A, Prinz H, Muller K, et al. (2007) 9-Benzylidene-naphtho[2,3-b]thiophen-4-ones and benzylidene-9(10H)-anthracenones as novel tubulin interacting agents with high apoptosis-inducing activity. European Journal of Pharmacology 575(1–3):34–45 [DOI] [PubMed] [Google Scholar]


