Abstract
Objective
Evidences from animal models seem to suggest that minimally invasive surgery may enhance cisplatin diffusion when the drug is administered in the context of post-operative hyperthermic intraperitoneal chemotherapy (HIPEC). The present study evaluates the cisplatin pharmacokinetic profile in a prospective series of women with platinum sensitive recurrent epithelial ovarian cancer treated with open secondary cytoreductive surgery (O-SCS) or minimally-invasive secondary cytoreductive surgery (MI-SCS).
Methods
Cisplatin levels were assessed at 0, 20, 40, 60, and 120 minutes in: 1) blood samples, 2) peritoneal perfusate, and 3) peritoneal biopsies at the end of HIPEC. Median Cmax has been used to identify women with high and low drug levels. Progression-free survival (PFS) was calculated as the time elapsed between SCS+HIPEC and secondary recurrence or last follow-up visit.
Results
Nine (45.0%) women received MI-SCS, and 11 (55.0%) O-SCS. At 60 minutes, median cisplatin Cmax in peritoneal tissue was higher in patients treated with MI-SCS compared to O-SCS (Cmax=8.262 µg/mL vs. Cmax=4.057 µg/mL). Furthermore, median cisplatin plasma Cmax was higher in patients treated with MI-SCS compared to O-SCS (Cmax=0.511 vs. Cmax=0.254 µg/mL; p-value=0.012) at 120 minutes. With a median follow-up time of 24 months, women with higher cisplatin peritoneal Cmax showed a longer PFS compared to women with low cisplatin peritoneal levels (2-years PFS=70% vs. 35%; p-value=0.054).
Conclusions
We demonstrate for the first time that minimally invasive route enhances cisplatin peritoneal tissue uptake during HIPEC, further evaluations are needed to confirm the correlation between peritoneal cisplatin levels after HIPEC and survival.
Trial Registration
ClinicalTrials.gov Identifier: NCT01539785
Keywords: Epithelial Ovarian Cancer; Cytoreduction Surgical Procedure; Injections, Intraperitoneal; Endoscopy
INTRODUCTION
In the last decade, relevant improvements have been achieved in the surgical and medical management of ovarian cancer. However, the majority of women still experience peritoneal relapse which represents the most common treatment failure, as well as the most frequent cause of death in patients with advanced ovarian cancer (AOC) [1,2]. For these reasons, locoregional treatments have been progressively improved, and hyperthermic intraperitoneal chemotherapy (HIPEC), administered after cytoreductive surgery, emerged as a promising approach to treat microscopic disease. Compelling evidences have demonstrated in case-control, retrospective series and randomized clinical trials the potential benefit of cisplatin-based HIPEC after cytoreductive surgery in patients with AOC [3,4,5,6,7,8,9,10]. However, despite this encouraging scenario, very few experiences have provided a complete pharmacokinetic evaluation of cisplatin levels in the blood and peritoneal tissue during and after cytoreductive surgery plus HIPEC in women with AOC [11,12,13].
Furthermore, the continuous effort to achieve the highest locoregional control with the lowest toxicity profile has resulted in the successful administration of cisplatin-based HIPEC after minimally invasive cytoreductive surgery in women with AOC [14,15]. Besides the advantages of minimally-invasive surgery itself, this approach appears an intriguing pharmacological strategy, given the evidences in pig models of an increased cisplatin diffusion using the endoscopic route compared with the traditional open approach [16,17]. However, these pharmacokinetic results have never been confirmed in humans.
In this context, we present here the first prospective evaluation of cisplatin pharmacokinetic profile in a homogeneous cohort of patients with platinum-sensitive recurrent epithelial ovarian cancer (REOC) receiving HIPEC after laparotomic or minimally-invasive secondary cytoreductive surgery (MI-SCS) in the context of the randomized HORSE trial (NCT01539785).
MATERIALS AND METHODS
1. Study patients
The study included a consecutive series of 20 women with platinum-sensitive REOC receiving SCS plus cisplatin-based HIPEC in the context of the HORSE study, a phase III randomized clinical trial currently on going in our institution (NCT01539785, IRB No. 4794/15). The following inclusion criteria were adopted to enroll women in the present study: age over 18 and under 70 years; patients affected by a first recurrence of ovarian cancer diagnosed after 6 months from primary treatment; Eastern Cooperative Oncology Group-performance status ≤2; disease limited to the abdominal cavity with or without extraperitoneal spread considered resectable at intraoperative evaluation; adequate respiratory, hepatic, cardiac, kidney, and bone marrow function (absolute neutrophil count >1,500/mm3, platelets >150,000/μL, creatinine clearance >60 mL/min according to Cockcroft formula); patient-compliant and psychologically able to follow the trial procedures. All women gave their written informed consent to be enrolled in the study, and for data and samples to be prospectively collected and analyzed.
2. Treatment
All cases were submitted to complete blood work (blood count, chemistry, urine analysis, and cancer antigen 125 serum levels), fluoro-D-glucose integrated with computed tomography scan and staging-laparoscopy to exclude extra-abdominal disease and to assess the chances of optimal cytoreduction [18]. In particular, all women with involvement of extra-abdominal sites or showing liver metastases were not considered suitable for SCS. Regarding intraperitoneal disease spread, the presence of diffuse carcinomatosis in all abdominal quadrants, the presence of stomach or mesenteric roots involvement were also considered as criteria not to proceed with SCS. All the patients fulfilling above mentioned criteria underwent optimal SCS (removal of all macroscopically detectable disease or residual intraperitoneal lesions each less than 0.25 mm) followed by platinum-based HIPEC. The extension of peritoneal spread at the time of recurrence was classified according with the peritoneal cancer index (PCI) [19]. The type and site of recurrent disease has been also defined as previously reported [2,20].
SCS was performed through a standard open approach (O-SCS), or a minimally invasive route (MI-SCS). The choice to perform endoscopic SCS versus standard open debulking was based either on site and extension (isolated or localized vs. peritoneal carcinomatosis) of disease at relapse. In particular, MI-SCS was performed attempted only in women relapsing as single nodule in a single anatomic site, or with single nodules in different anatomic sites, while O-SCS was performed in all cases showing peritoneal carcinomatosis or diffuse relapse. Completeness of cytoreduction was defined at the end of surgery, and with abdomino-pelvic CT scan before starting planned systemic chemotherapy. Surgical complications were graded according to the Memorial Sloan Kettering Cancer Center grading system [21].
According with HORSE protocol, cisplatin-based chemotherapy was used as intraperitoneal drug. In particular, intraperitoneal cisplatin was used at a dosage of 75 mg/m2, with a temperature of 41.5°C for 60 minutes. The drug was administered in a perfusate of saline solution in a total volume of 2,000 mL/m2, with a perfusion speed of 600 mL/min. In all patients, closed HIPEC technique was employed, and after intraperitoneal drug delivery, the abdomen was carefully re-explored, with particular attention to hemostasis and integrity of bowel anastomoses. Systemic platinum-based chemotherapy was administered after SCS+HIPEC.
3. Samples collection
In all patients, blood samples were collected at the beginning of cisplatin-based HIPEC (T0), and at 20 (T20), 40 (T40), 60 (T60), and 120 (T120) minutes after starting HIPEC procedure. The blood taken into heparinized tubes directly from a peripheral vein was centrifuged, and plasma was transferred into cryovials. Similarly, peritoneal perfusate was retrieved at T0, T20, T40, and T60. Perfusate and plasma samples were stored at −20°C. Finally, at the end of perfusion a peritoneal biopsy was performed, and the tissue frozen in liquid nitrogen and stored at −80°C.
The entire material retrieved was finally shipped to the Cancer Pharmacology Laboratory at Mario Negri Institute for Pharmacological Research for experimental analysis respecting the frost chain.
4. Determination of platinum concentration in plasma, peritoneal fluids, and peritoneal tissue
To determine the concentration of cisplatin in the biological samples the amount of the platinum element was assayed by atomic absorption (AA) analysis using Analyst 600 (Perkin Elmer, Waltham, MA, USA) [22]. Aliquot of 200 µL of plasma or perfusate samples or 0.2 g of peritoneal tissue were digested overnight with 400 µL of HNO3: HCL, then added with 600 µL of bidistilled water, mixed and centrifuged 10 minutes at 13,000 rpm at 4°C. Aliquots of supernatant were injected into the AA instrument and assayed by means of a calibration curve made of platinum analytical standard (Sigma-Aldrich, St. Louis, MO, USA) prepared at concentrations in the range 2–200 ng/mL. The method has a limit of quantification of 2 ng/mL. The concentration of platinum was then expressed as the corresponding cisplatin concentration.
5. Statistical analysis
Differences between women receiving minimally invasive versus open SCS followed by HIPEC in terms of median cisplatin levels in blood, peritoneal perfusate, and tissues were analyzed using χ2, and Kruskal-Wallis test as appropriate. Follow-up time was calculated as the time interval between SCS and last follow-up contact. Progression-free survival (PFS) was calculated as the time elapsed from SCS+HIPEC and the date of progression or last follow up. Data are given as median and range. Categorical variables are reported as absolute values and percentage. Kaplan-Meyer method was used to estimate the survival distribution [23]. All statistical calculations were performed using STATA statistical software (Version 13.0; StataCorp, College Station, TX, USA).
RESULTS
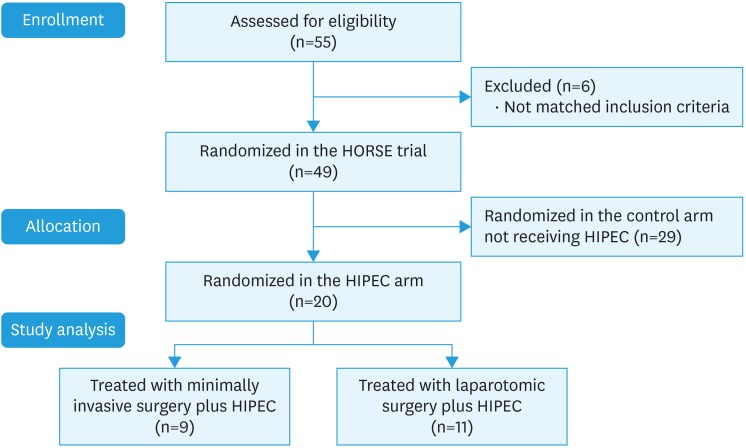
Between December 2013 and August 2016, 20 women with platinum sensitive REOC were enrolled in the study and received SCS plus HIPEC at the Department of Woman and Child Health of the Catholic University of Sacred Hearth of Rome. The study population has been described in the CONSORT diagram presented as Fig. 1. In particular, during the above-mentioned period 55 women with platinum-sensitive REOC were evaluated for inclusion in the HORSE trial, but only 49 matched inclusion criteria being finally enrolled in the trial. After randomization, 29 women were assigned to the control arm receiving surgery without HIPEC, and the remaining 20 patients were enrolled in the experimental arm including debulking surgery followed by HIPEC, and these patients represent the final population of the current pharmacokinetic study (Fig. 1). Among this group of women, in 9 (45.0%) patients SCS was successfully completed through a minimally invasive approach (MI-SCS), while the remaining 11 (55.0%) patients were submitted to the traditional O-SCS. In all cases complete cytoreduction with no gross residual disease has been achieved.
Fig. 1. CONSORT diagram describing the flow of patients through enrollment, and randomization to achieve final study population.
HIPEC, hyperthermic intraperitoneal chemotherapy.
The clinico-pathological characteristics of the study population have been presented in Table 1. The median age of the study population was 51 years (range, 30–66) without differences between the 2 groups, and all patients showed high-grade serous ovarian cancer. Similarly, no differences were observed in term of cisplatin dosage, and volume of perfusate administered during HIPEC between the 2 treatment arms. The median PCI was 3 (range, 2–12) in the O-SCS group compared with 2 (range, 2–5) in women receiving MI-SCS (p-value=0.119). A very favorable toxicity profile has been observed with the vast majority of women showing no complication (Table 1), and only 2 patients experiencing a grade 3 adverse event (pleural effusion requiring chest drainage placement, and acute renal failure due to post-operative hydronephrosis resolved with sequelae).
Table 1. Distribution of patients' clinical-pathological characteristics of the study population.
| Characteristics | All patients | O-SCS+HIPEC | MI-SCS+HIPEC | p-value* | |
|---|---|---|---|---|---|
| All cases | 20 | 11 (55.0) | 9 (45.0) | - | |
| Age (yr) | 51 (30–66) | 51 (30–66) | 49 (43–62) | 0.675 | |
| Site of recurrence | 0.065 | ||||
| Peritoneum alone | 12 (60.0) | 9 (81.8) | 3 (33.3) | ||
| Peritoneum+other | 8 (40.0) | 2 (18.2) | 6 (66.7) | ||
| PCI | 2 (2–12) | 3 (2–12) | 2 (2–5) | 0.119 | |
| Cisplatin dosage (mg) | 125 (122–142) | 125 (123–142) | 124 (122–130) | 0.220 | |
| Total perfusate volume (mL) | 3,320 (3,240–3,780) | 3,320 (3,280–3,780) | 3,300 (3,240–3,460) | 0.224 | |
| Early post-operative complications after SCS+HIPEC† | 0.728 | ||||
| None | 16 (80.0) | 8 (72.7) | 8 (88.9) | ||
| G1–2 | 2 (10.0) | 2 (18.2) | 0 | ||
| G3 | 2 (10.0) | 1 (9.1) | 1 (11.1) | ||
| PFI-1(mo)‡ | 21 (6–60) | 20 (6–60) | 24 (9–39) | 0.995 | |
| Secondary recurrence | 0.574 | ||||
| Yes | 6 (30.0) | 3 (27.3) | 3 (33.3) | ||
| No | 14 (70.0) | 8 (72.7) | 6 (66.7) | ||
| 3-yr PFS (CI)‡ | 60.5 (19.1–85.5) | 58.3 (15.7–85.4) | 70.6 (22.9–92.1) | 0.957 | |
| PFS>PFI-1 | 11 (55.0) | 7 (63.6) | 4 (44.4) | 0.342 | |
Values are presented as median (interquartile range) or number (%).CC, completeness of secondary cytoreduction; CI, confidence interval; HIPEC, hyperthermic intraperitoneal chemotherapy; MI-SCS, minimally invasive secondary cytoreductive surgery; O-SCS, open secondary cytoreductive surgery; PCI, peritoneal cancer index; PFI-1, primary platinum-free interval; PFS, progression free survival (time elapsed from SCS+HIPEC to disease progression or last follow-up); PRS, post-relapse survival (time elapsed from SCS+HIPEC to death or last follow-up).
*Calculated by χ2 test, and Kruskal-Wallis non-parametric test as appropriate; †Complications have been classified according to Memorial Sloan Kettering Cancer Center grading system; ‡Calculated according with Kaplan-Meier method.
1. Pharmacokinetic results
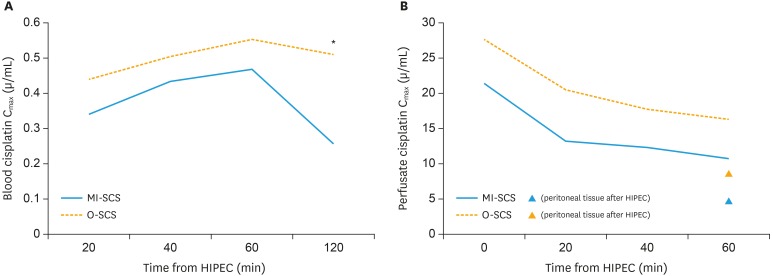
Table 2 describes the main pharmacokinetic results of our study. At all the time points, we documented a higher, cisplatin perfusate concentration in women receiving MI-SCS compared to patients treated with the open route (Table 2). Notably, at each of the time point monitored, the median perfusate cisplatin concentration was largely above the cytotoxic threshold (10 µg/mL). The higher perfusate concentration measured after MI-SCS generated also a superior drug exposure in the peritoneal tissue in this cohort of women, being median peritoneal concentration of cisplatin of 8.262 µg/mL, higher than 4.057 µg/mL measured in women receiving laparotomic surgery.
Table 2. Pharmacokinetic results of cisplatin according with surgical approach.
| Characteristics | All patients | O-SCS+HIPEC | MI-SCS+HIPEC | p-value* | |
|---|---|---|---|---|---|
| Cmax plasma (µg/mL) | |||||
| T0 | - | - | - | - | |
| T20 | 0.362 (0.026–1.41) | 0.332 (0.026–0.678) | 0.442 (0.098–1.410) | 0.119 | |
| T40 | 0.450 (0.15–1.71) | 0.411 (0.233–0.639) | 0.506 (0.15–1.710) | 0.270 | |
| T60 | 0.494 (0.176–1.338) | 0.446 (0.212–0.646) | 0.552 (0.176–1.338) | 0.305 | |
| T120 | 0.324 (0.175–1.225) | 0.254 (0.175–0.345) | 0.511 (0.257–1.225) | 0.012 | |
| Cmax perfusate (µg/mL) | |||||
| T0 | 22.572 (8.869–43.394) | 21.377 (13.352–43.394) | 27.665 (8.869–38.778) | 0.477 | |
| T20 | 20.058 (6.245–28.902) | 13.186 (6.245–28.902) | 20.492 (7.677–28.442) | 0.409 | |
| T40 | 16.341 (8.782–31.542) | 12.318 (8.782–27.000) | 17.738 (9.030–31.542) | 0.239 | |
| T60 | 12.309 (6.885–29.372) | 10.729 (6.885–18.415) | 16.274 (8.085–29.372) | 0.120 | |
| Cmax peritoneum (µg/mL) | 6.704 (1.477–27.411) | 4.057 (1.477–27.411) | 8.262 (1.970–22.149) | 0.386 | |
Values are presented as median (interquartile range).
HIPEC, hyperthermic intraperitoneal chemotherapy; MI-SCS, minimally invasive secondary cytoreductive surgery; O-SCS, open secondary cytoreductive surgery.
*Calculated by Kruskal-Wallis non-parametric test.
As concerning the systemic exposure of the HIPEC treatments, the median cisplatin plasma concentration, increased progressively during perfusion reaching the Cmax at 60 min after the beginning of HIPEC (T60=0.494 µg/mL). It is to note that comparing plasma concentration according with surgical approach, women receiving MI-SCS showed higher plasma exposure compared to patients treated with O-SCS; however, these differences reached statistical significance only at T120 (Fig. 2). In fact, 2 hours from the beginning of cisplatin-based HIPEC, the patients treated through a minimally invasive route showed double cisplatin plasma levels compared to women receiving the traditional laparotomic surgery (MI-SCS=0.511 vs. O-SCS=0.254 µg/mL; p-value=0.012).
Fig. 2. (A) Blood cisplatin Cmax at each time point during and after HIPEC in women receiving O-SCS (solid line), and MI-SCS (dashed line). Asterisk refers to the only time point (T120) showing a statistically significant difference (p-value=0.012). (B) Perfusate cisplatin Cmax at each time point during HIPEC in women receiving O-SCS (solid line), and MI-SCS (dashed line). Black triangle indicates cisplatin peritoneal levels after HIPEC in women receiving O-SCS. White triangle indicates cisplatin peritoneal levels after HIPEC in women receiving MI-SCS.
HIPEC, hyperthermic intraperitoneal chemotherapy; MI-SCS, minimally invasive secondary cyotreductive surgery; O-SCS, open secondary cytoreductive surgery.
2. Survival evaluation
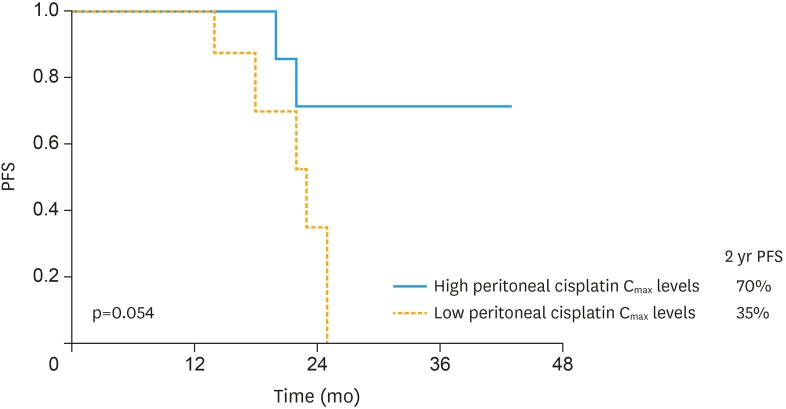
At December 2017, the median follow-up time of our study population (calculated from the date of HIPEC administration to last follow-up) was 24 months (ranging from 14 to 43 months). As reported in Table 1, 3-years PFS was 60.5% in the overall series, with 6 women experiencing secondary recurrence (30.0%) without differences according with surgical route of SCS. Interestingly, 55% of women included in the study showed a PFS longer than primary platinum-free interval (PFI-1) (Table 1). To assess whether a correlation may exist between pharmacokinetic variables and PFS, we used the median value of each parameter as the threshold to identify women with high, and low levels. As presented in Table 3, peritoneal cisplatin Cmax significantly correlated with duration of PFS. In particular women with higher cisplatin peritoneal Cmax showed a longer PFS compared to women with low peritoneal levels of the drug (2-years PFS=70% vs. 35%; p-value=0.054; Fig. 3).
Table 3. Correlation between pharmacokinetic parameters and PFS.
| Characteristics | 2-years PFS (%) | p-value* | |
|---|---|---|---|
| Cmax plasma T20 | 0.628 | ||
| High | 55 | ||
| Low | 50 | ||
| Cmax plasma T40 | 0.547 | ||
| High | 54 | ||
| Low | 55 | ||
| Cmax plasma T60 | 0.342 | ||
| High | 66 | ||
| Low | 43 | ||
| Cmax plasma T120 | 0.589 | ||
| High | 63 | ||
| Low | 63 | ||
| Cmax perfusate T20 | 0.940 | ||
| High | 58 | ||
| Low | 50 | ||
| Cmax perfusate T40 | 0.611 | ||
| High | 58 | ||
| Low | 26 | ||
| Cmax perfusate T60 | 0.908 | ||
| High | 56 | ||
| Low | 50 | ||
| Cmax peritoneum | 0.054 | ||
| High | 70 | ||
| Low | 35 | ||
PFS, progression-free survival.
*Calculated with log-rank test.
Fig. 3. Comparison of PFS between patients with high (solid line), and low (dashed line) peritoneal cisplatin Cmax.
PFS, progression-free survival.
Discussion
In the effort to improve locoregional control, the administration of cisplatin-based HIPEC has been progressively recognized as a potential useful strategy; furthermore, the results of a recently published randomized clinical trial have provided the first high level evidence supporting the use of HIPEC in ovarian cancer [5]. However, the risk of increased toxicity still represents the main limitation to the introduction of HIPEC into routine clinical practice. In this context, even if our study was focused on cisplatin pharmacokinetic profile, the clinical results appear encouraging. In fact, only 20% of women receiving SCS+HIPEC showed early post-operative complications, and no grade 4–5 adverse events were recorded. Our data are in line with the interim analysis of a recently published phase II trial showing no deaths or grade 4 morbidities in a population of 30 AOC patients receiving extensive cytoreductive plus HIPEC [24]. Moreover, we confirmed a very favorable prognosis (3-years PFS=60.5%) [3,4,5,6,7,8,9,10], with a PFS>PFI-1 in more than 50% of patients [4], and a superimposable clinical outcome when comparing open, and minimally invasive surgical route [14,15].
Focusing on the primary aim of our study, we demonstrate that women receiving HIPEC through a minimally invasive approach reach double cisplatin peritoneal tissue levels compared to patients submitted to O-SCS. Furthermore, we observed a statistically significant higher plasma concentration of the drug 2 hours after HIPEC beginning in the MI-SCS compared with the O-SCS group. However, the higher blood cisplatin levels (T60=0.553 µg/mL) observed in the MI-SCS group were below the threshold of drug cytotoxicity (10 µg/mL) [25]. Therefore, our data suggest that the minimally invasive route, even increasing drug absorption, does not modify the systemic cisplatin toxicity profile, but it allows at the same time to reach very high intraperitoneal drug concentrations (perfusate Cmax in MI-SCS group ranging from 16.274 to 27.665 µg/mL), thus improving the overall therapeutic index of cisplatin during intraperitoneal administration. In this context, it could be inferred that the described pharmacokinetic results may be related to a higher initial cisplatin dosage in women receiving MI-SCS. However, the lack of differences in terms of drug concentration, and perfusate volume between the 2 groups (Table 1) further supports the hypothesis that the surgical approach (endoscopy versus laparotomy) may influence cisplatin pharmacokinetic profile in women receiving SCS plus HIPEC.
It should be emphasized that our study confirms, for the first time in humans, the results previously observed in animal models. In fact, Gesson-Paute et al. [16,17] reported an increased oxaliplatin amount crossing through the peritoneal barrier, with a higher drug diffusion in the omentum, peritoneum, and liver in pigs receiving HIPEC through the minimally invasive approach compared to animals submitted to laparotomic HIPEC. A potential explanation to our results could be found in experimental data suggesting that the increase of intra-abdominal pressure enhances drug penetration, and blood absorption in rat models [26,27]. Therefore, it is reasonable to hypothesize that the integrity of the abdominal wall during HIPEC after MI-SCS allows to reach a higher intraabdominal pressure compared to the traditional laparotomic procedure, thus enhancing cisplatin crossing though the peritoneal/plasma barrier. The clinical implications of our findings have not to be underestimated, since the demonstration that endoscopy enhances the cisplatin blood absorption in REOC patients gives a strong rationale to actively test, and further develop novel techniques of intraperitoneal drug administration, such as pressurized intraperitoneal chemotherapy [28,29,30], able to provide at the same time intraperitoneal pressure modulation, hyperthermia, and drug perfusion.
Another relevant finding of our study is the observation of a longer PFS in REOC patients showing higher peritoneal cisplatin levels after SCS+HIPEC. Looking carefully at experimental data, it is well known, since its first preclinical development, that the cytotoxic effect of cisplatin depends on its concentration and on the length of cancer cells exposure to the drug [31]. Furthermore, more recently, it has been demonstrated a longer survival in mice with peritoneal disseminated gastric cancer receiving intraperitoneal pegylated cisplatin [32], thus confirming in animal models that an increased penetration and exposure of cancer cells to cisplatin may ensure a survival benefit. In this context, it should be acknowledged that the small sample size, and the short duration of median follow-up time in our series do not allow drawing definitive conclusions regarding oncological outcome. However, it appears reasonable to hypothesize that microscopic tumor foci have been more effectively controlled in those patients showing higher cisplatin peritoneal levels after HIPEC (threshold of 6.704 µg/mL corresponding to the median value of our series), thus ultimately resulting in a prolonged PFS (2-years PFS 70% vs. 35%; p-value=0.054). In this context, our data offer potential explanations to contrasting results obtained from RCTs on the role of dose-dense chemotherapy in AOC [33].
In conclusion, we acknowledge that the results of our manuscript need to be confirmed in further studies expanding sample size and improving reliability of our results. On the other hand, our study demonstrates for the first time that the minimally invasive route enhances cisplatin blood absorption in women receiving HIPEC, thus providing a strong rationale to further develop novel strategies of endoscopic intraperitoneal drug administration in REOC patients with locoregional disease. If further confirmed the observed borderline correlation between peritoneal cisplatin levels after HIPEC and survival may open the route for the development of novel therapeutic strategies.
Footnotes
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
- Conceptualization: P.M., Z.M., C.S., M.L., R.C., C.A., D.M., S.G., F.A.
- Formal analysis: P.M., Z.M., C.S., M.L., R.C., C.A.
- Investigation: P.M., Z.M., C.S., M.L., R.C., C.A.
- Methodology: P.M., Z.M., M.L., R.C., C.A., D.M.
- Supervision: R.C., C.A., D.M., S.G., F.A.
- Validation: P.M., Z.M., C.S., M.L., R.C., C.A., D.M., S.G., F.A.
- Writing - original draft: P.M., Z.M., C.S., M.L., R.C., C.A., D.M., S.G., F.A.
- Writing - review & editing: P.M., Z.M., D.M., S.G., F.A.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 2.Ferrandina G, Legge F, Salutari V, Paglia A, Testa A, Scambia G. Impact of pattern of recurrence on clinical outcome of ovarian cancer patients: clinical considerations. Eur J Cancer. 2006;42:2296–2302. doi: 10.1016/j.ejca.2006.03.025. [DOI] [PubMed] [Google Scholar]
- 3.Ceelen WP, Van Nieuwenhove Y, Van Belle S, Denys H, Pattyn P. Cytoreduction and hyperthermic intraperitoneal chemoperfusion in women with heavily pretreated recurrent ovarian cancer. Ann Surg Oncol. 2012;19:2352–2359. doi: 10.1245/s10434-009-0878-6. [DOI] [PubMed] [Google Scholar]
- 4.Fagotti A, Costantini B, Petrillo M, Vizzielli G, Fanfani F, Margariti PA, et al. Cytoreductive surgery plus HIPEC in platinum-sensitive recurrent ovarian cancer patients: a case-control study on survival in patients with two year follow-up. Gynecol Oncol. 2012;127:502–505. doi: 10.1016/j.ygyno.2012.09.020. [DOI] [PubMed] [Google Scholar]
- 5.van Driel WJ, Koole SN, Sikorska K, Schagen van Leeuwen JH, Schreuder HW, Hermans RH, et al. Hyperthermic intraperitoneal chemotherapy in ovarian cancer. N Engl J Med. 2018;378:230–240. doi: 10.1056/NEJMoa1708618. [DOI] [PubMed] [Google Scholar]
- 6.Le Brun JF, Campion L, Berton-Rigaud D, Lorimier G, Marchal F, Ferron G, et al. Survival benefit of hyperthermic intraperitoneal chemotherapy for recurrent ovarian cancer: a multi-institutional case control study. Ann Surg Oncol. 2014;21:3621–3627. doi: 10.1245/s10434-014-3693-7. [DOI] [PubMed] [Google Scholar]
- 7.Di Giorgio A, De Iaco P, De Simone M, Garofalo A, Scambia G, Pinna AD, et al. Cytoreduction (peritonectomy procedures) combined with hyperthermic intraperitoneal chemotherapy (HIPEC) in advanced ovarian cancer: retrospective italian multicenter observational study of 511 cases. Ann Surg Oncol. 2017;24:914–922. doi: 10.1245/s10434-016-5686-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Petrillo M, De Iaco P, Cianci S, Perrone M, Costantini B, Ronsini C, et al. Long-term survival for platinum-sensitive recurrent ovarian cancer patients treated with secondary cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy (HIPEC) Ann Surg Oncol. 2016;23:1660–1665. doi: 10.1245/s10434-015-5050-x. [DOI] [PubMed] [Google Scholar]
- 9.Van Driel W, Sikorska K, Schagen van Leeuwen J, Schreuder H, Hermans R, de Hingh I, et al. A phase 3 trial of hyperthermic intraperitoneal chemotherapy (HIPEC) for ovarian cancer; ASCO Annual Meeting; 2017 Jun 2–6; Chicago, IL: American Society of Clinical Oncology; 2017. p. Abstract. [Google Scholar]
- 10.Cheol Lim M, Chang S, Jong Yoo H, Nam B, Bristow R, Park S. Randomized trial of hyperthermic intraperitoneal chemotherapy (HIPEC) in women with primary advanced peritoneal, ovarian, and tubal cancer; ASCO Annual Meeting; 2017 Jun 2–6; Chicago, IL: American Society of Clinical Oncology; 2017. p. Abstract. [Google Scholar]
- 11.Zivanovic O, Abramian A, Kullmann M, Fuhrmann C, Coch C, Hoeller T, et al. HIPEC ROC I: a phase I study of cisplatin administered as hyperthermic intraoperative intraperitoneal chemoperfusion followed by postoperative intravenous platinum-based chemotherapy in patients with platinum-sensitive recurrent epithelial ovarian cancer. Int J Cancer. 2015;136:699–708. doi: 10.1002/ijc.29011. [DOI] [PubMed] [Google Scholar]
- 12.Ansaloni L, Coccolini F, Morosi L, Ballerini A, Ceresoli M, Grosso G, et al. Pharmacokinetics of concomitant cisplatin and paclitaxel administered by hyperthermic intraperitoneal chemotherapy to patients with peritoneal carcinomatosis from epithelial ovarian cancer. Br J Cancer. 2015;112:306–312. doi: 10.1038/bjc.2014.602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Löffler MW, Schuster H, Zeck A, Quilitz N, Weinreich J, Tolios A, et al. Pharmacodynamics of oxaliplatin-derived platinum compounds during hyperthermic intraperitoneal chemotherapy (HIPEC): an emerging aspect supporting the rational design of treatment protocols. Ann Surg Oncol. 2017;24:1650–1657. doi: 10.1245/s10434-017-5790-x. [DOI] [PubMed] [Google Scholar]
- 14.Fagotti A, Costantini B, Gallotta V, Cianci S, Ronsini C, Petrillo M, et al. Minimally invasive secondary cytoreduction plus HIPEC versus open surgery plus HIPEC in isolated relapse from ovarian cancer: a retrospective cohort study on perioperative outcomes. J Minim Invasive Gynecol. 2015;22:428–432. doi: 10.1016/j.jmig.2014.11.008. [DOI] [PubMed] [Google Scholar]
- 15.Fagotti A, Petrillo M, Costantini B, Fanfani F, Gallotta V, Chiantera V, et al. Minimally invasive secondary cytoreduction plus HIPEC for recurrent ovarian cancer: a case series. Gynecol Oncol. 2014;132:303–306. doi: 10.1016/j.ygyno.2013.12.028. [DOI] [PubMed] [Google Scholar]
- 16.Gesson-Paute A, Ferron G, Thomas F, de Lara EC, Chatelut E, Querleu D. Pharmacokinetics of oxaliplatin during open versus laparoscopically assisted heated intraoperative intraperitoneal chemotherapy (HIPEC): an experimental study. Ann Surg Oncol. 2008;15:339–344. doi: 10.1245/s10434-007-9571-9. [DOI] [PubMed] [Google Scholar]
- 17.Thomas F, Ferron G, Gesson-Paute A, Hristova M, Lochon I, Chatelut E. Increased tissue diffusion of oxaliplatin during laparoscopically assisted versus open heated intraoperative intraperitoneal chemotherapy (HIPEC) Ann Surg Oncol. 2008;15:3623–3624. doi: 10.1245/s10434-008-0115-8. [DOI] [PubMed] [Google Scholar]
- 18.Fagotti A, Fanfani F, Rossitto C, Lorusso D, De Gaetano AM, Giordano A, et al. A treatment selection protocol for recurrent ovarian cancer patients: the role of FDG-PET/CT and staging laparoscopy. Oncology. 2008;75:152–158. doi: 10.1159/000159266. [DOI] [PubMed] [Google Scholar]
- 19.Sugarbaker PH, Jablonski KA. Prognostic features of 51 colorectal and 130 appendiceal cancer patients with peritoneal carcinomatosis treated by cytoreductive surgery and intraperitoneal chemotherapy. Ann Surg. 1995;221:124–132. doi: 10.1097/00000658-199502000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Petrillo M, Fagotti A, Ferrandina G, Fanfani F, Costantini B, Vizzielli G, et al. Ovarian cancer patients with localized relapse: clinical outcome and prognostic factors. Gynecol Oncol. 2013;131:36–41. doi: 10.1016/j.ygyno.2013.06.020. [DOI] [PubMed] [Google Scholar]
- 21.Chi DS, Franklin CC, Levine DA, Akselrod F, Sabbatini P, Jarnagin WR, et al. Improved optimal cytoreduction rates for stages IIIC and IV epithelial ovarian, fallopian tube, and primary peritoneal cancer: a change in surgical approach. Gynecol Oncol. 2004;94:650–654. doi: 10.1016/j.ygyno.2004.01.029. [DOI] [PubMed] [Google Scholar]
- 22.Canta A, Chiorazzi A, Carozzi V, Meregalli C, Oggioni N, Sala B, et al. In vivo comparative study of the cytotoxicity of a liposomal formulation of cisplatin (lipoplatin™) Cancer Chemother Pharmacol. 2011;68:1001–1008. doi: 10.1007/s00280-011-1574-3. [DOI] [PubMed] [Google Scholar]
- 23.Kaplan EL, Meyer P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 24.Lim MC, Kang S, Choi J, Song YJ, Park S, Seo SS, et al. Hyperthermic intraperitoneal chemotherapy after extensive cytoreductive surgery in patients with primary advanced epithelial ovarian cancer: interim analysis of a phase II study. Ann Surg Oncol. 2009;16:993–1000. doi: 10.1245/s10434-008-0299-y. [DOI] [PubMed] [Google Scholar]
- 25.Royer B, Guardiola E, Polycarpe E, Hoizey G, Delroeux D, Combe M, et al. Serum and intraperitoneal pharmacokinetics of cisplatin within intraoperative intraperitoneal chemotherapy: influence of protein binding. Anticancer Drugs. 2005;16:1009–1016. doi: 10.1097/01.cad.0000176505.94175.d4. [DOI] [PubMed] [Google Scholar]
- 26.Esquis P, Consolo D, Magnin G, Pointaire P, Moretto P, Ynsa MD, et al. High intra-abdominal pressure enhances the penetration and antitumor effect of intraperitoneal cisplatin on experimental peritoneal carcinomatosis. Ann Surg. 2006;244:106–112. doi: 10.1097/01.sla.0000218089.61635.5f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jacquet P, Stuart OA, Chang D, Sugarbaker PH. Effects of intra-abdominal pressure on pharmacokinetics and tissue distribution of doxorubicin after intraperitoneal administration. Anticancer Drugs. 1996;7:596–603. doi: 10.1097/00001813-199607000-00016. [DOI] [PubMed] [Google Scholar]
- 28.Alyami M, Gagniere J, Sgarbura O, Cabelguenne D, Villeneuve L, Pezet D, et al. Multicentric initial experience with the use of the pressurized intraperitoneal aerosol chemotherapy (PIPAC) in the management of unresectable peritoneal carcinomatosis. Eur J Surg Oncol. 2017;43:2178–2183. doi: 10.1016/j.ejso.2017.09.010. [DOI] [PubMed] [Google Scholar]
- 29.Tempfer CB, Winnekendonk G, Solass W, Horvat R, Giger-Pabst U, Zieren J, et al. Pressurized intraperitoneal aerosol chemotherapy in women with recurrent ovarian cancer: A phase 2 study. Gynecol Oncol. 2015;137:223–228. doi: 10.1016/j.ygyno.2015.02.009. [DOI] [PubMed] [Google Scholar]
- 30.Solass W, Kerb R, Mürdter T, Giger-Pabst U, Strumberg D, Tempfer C, et al. Intraperitoneal chemotherapy of peritoneal carcinomatosis using pressurized aerosol as an alternative to liquid solution: first evidence for efficacy. Ann Surg Oncol. 2014;21:553–559. doi: 10.1245/s10434-013-3213-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Drewinko B, Brown BW, Gottlieb JA. The effect of cis-diamminedichloroplatinum (II) on cultured human lymphoma cells and its therapeutic implications. Cancer Res. 1973;33:3091–3095. [PubMed] [Google Scholar]
- 32.Iinuma H, Maruyama K, Okinaga K, Sasaki K, Sekine T, Ishida O, et al. Intracellular targeting therapy of cisplatin-encapsulated transferrin-polyethylene glycol liposome on peritoneal dissemination of gastric cancer. Int J Cancer. 2002;99:130–137. doi: 10.1002/ijc.10242. [DOI] [PubMed] [Google Scholar]
- 33.Katsumata N, Yasuda M, Takahashi F, Isonishi S, Jobo T, Aoki D, et al. Dose-dense paclitaxel once a week in combination with carboplatin every 3 weeks for advanced ovarian cancer: a phase 3, open-label, randomised controlled trial. Lancet. 2009;374:1331–1338. doi: 10.1016/S0140-6736(09)61157-0. [DOI] [PubMed] [Google Scholar]