Abstract
Objective
Patients with endometriosis are at increased risk of ovarian cancer. It has been suggested that atypical endometriosis is a precursor lesion of endometriosis-associated ovarian cancer (EAOC). The aim of this study is to evaluate if cytologic (cellular) atypia and architectural atypia (hyperplasia), histologic findings described as atypical endometriosis, play a different role in patients with EAOC.
Methods
A prospective study was conducted between January 2014 and April 2017 at our institution with patients undergoing surgery with a histologic diagnosis of endometriosis, ovarian cancer, or EAOC. The prevalence and immunohistologic study (Ki-67, BAF250a, COX-2) of cases of cellular and architectural atypia in endometriosis were analyzed.
Results
Two hundred and sixty-six patients were included: the diagnosis was endometriosis alone in 159 cases, ovarian cancer in 81, and EAOC in 26. Atypical endometriosis was reported in 23 cases (12.43%), 39.13% of them found in patients with EAOC. Endometriosis with cellular atypia was found mainly in patients without neoplasm (71.4%), and endometriosis with architectural atypia was seen in patients with ovarian cancer (88.9%) (p=0.009). Ki-67 was significantly higher in endometriosis patients with architectural atypia than those with cellular atypia.
Conclusion
The diagnosis of endometriosis with architectural atypia is important because it may be a precursor lesion of ovarian cancer; therefore, pathologists finding endometriosis should carefully examine the surgical specimen to identify any patients with hyperplasia-type endometriosis, as they may be at higher risk of developing EAOC.
Keywords: Endometriosis, Ovarian Cancer, Hyperplasia
INTRODUCTION
Ovarian cancer is the gynecologic tumor with the highest mortality, and the second most common of all malignant gynecologic neoplasms [1]. To date, no premalignant lesion has been identified in the natural course of this condition that would enhance effective prevention. Endometriosis is an estrogen-dependent inflammatory disease defined as the presence and growth of foci in the endometrial tissue (glands and stroma) outside the endometrial cavity [1]. The prevalence of endometriosis is 7% to 15% in women of childbearing potential [2]. Several studies have consistently shown that endometriosis is associated with a higher risk of epithelial ovarian cancer [3]. Additionally, the risk of neoplastic transformation of endometriosis has been estimated to be 0.5% to 1.0%. Atypical endometriosis has been proposed as a premalignant lesion strongly associated with endometriosis-associated ovarian cancer (EAOC) [4,5]. Atypical endometriosis also refers to two different histologic findings: cellular atypia, also known as cytologic atypia, and architectural atypia, commonly referred to as hyperplasia [2]. Cellular atypia describes the presence of atypia in the epithelial lining of endometrioid cysts (nuclear stratification, hyperchromatism, pleomorphism), whereas architectural atypia (hyperplasia) refers to the same spectrum of hyperplasia found in the endometrium (simple or complex, with or without cellular atypia) [6].
The aim of our study was to investigate the possible existence of an EAOC-specific precursor histologic lesion in the endometriosis spectrum by analyzing the presentation grade of the two histologic conditions included in atypical endometriosis (cellular atypia and architectural atypia or hyperplasia) in our series.
MATERIALS AND METHODS
1. Study population
A prospective, observational study was conducted in the Obstetrics and Gynecology Department at the ‘Virgen de la Arrixaca’ University Clinical Hospital (HCUVA) in Murcia, Spain, from January 2014 to April 2017. EAOC is diagnosed by anatomic pathology, hence the study population included patients undergoing surgery in our department in whom the definitive histologic diagnosis was endometriosis, ovarian cancer, or EAOC. Patients without histologic confirmation of endometriosis or ovarian cancer were excluded from the study. Informed consent was obtained from all patients, and the study was reviewed and approved by the Ethics Committee of the HCUVA before patients were recruited.
2. Histopathologic study
All surgical specimens were sent for pathologic examination as fresh specimens or fixed in 10% formaldehyde, and were examined independently by two pathologists from the department with extensive experience in benign and malignant ovarian conditions. A consensus was reached in cases of disagreement.
The specimens were evaluated after routine hematoxylin-eosin staining. A diagnosis of endometriosis was confirmed by the presence of tissue similar to the endometrium (glands and stroma) outside the endometrial cavity, although a diagnosis was also possible when there is a cystic cavity without epithelial lining but with stromal reaction; in the latter case, endometrial stroma had to be positive for CD10+ [6].
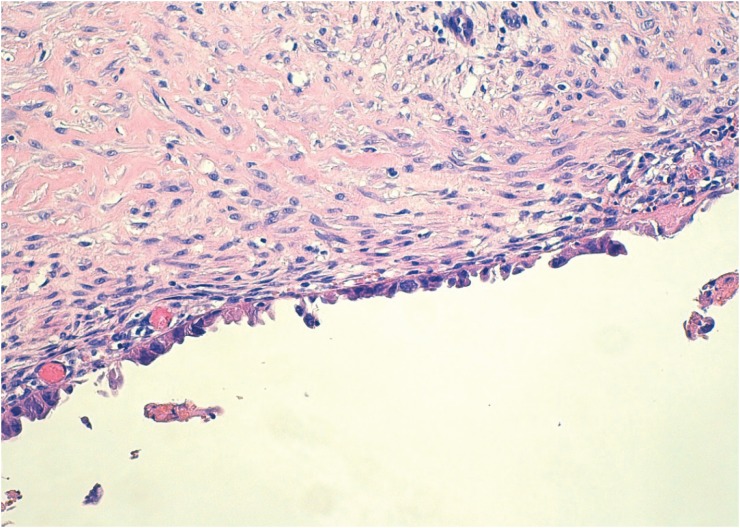
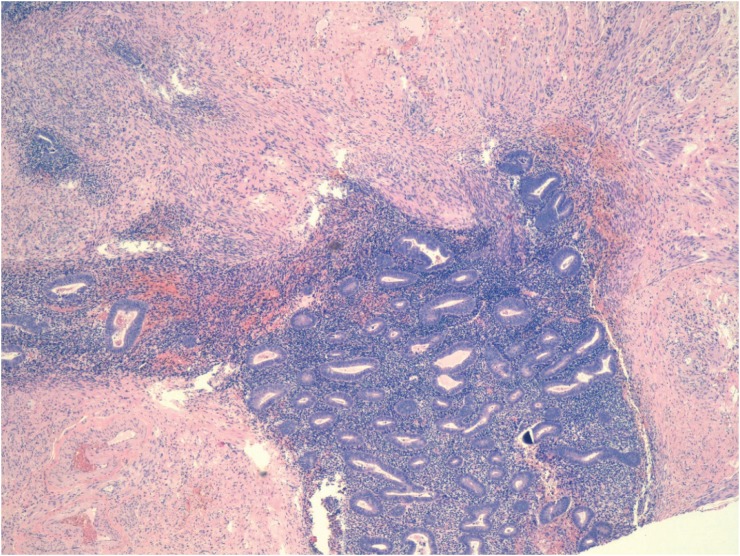
To diagnose atypical endometriosis, we assessed the presence of cellular atypia and/or hyperplasia in the endometrioid cyst and in transition areas between endometrial tissue and tissue with evidence of malignancy in cases of EAOC. Cases of atypical endometriosis were classified into two subtypes, based on histologic findings: endometriosis with cytologic (cellular) atypia (ECA) and endometriosis with architectural atypia (EAA) [2], with ECA corresponding to atypia in the epithelial lining of endometrial cysts (nuclear stratification, hyperchromatism, pleomorphism) (Fig. 1) and EAA referring to the same spectrum of hyperplasia (simple or complex, with or without cellular atypia) found in the endometrium [6] (Fig. 2). Cases with an overlapping diagnosis of EAA and ECA were classified as EAA.
Fig. 1. Endometriosis with cellular atypia: nucleomegaly, hyperchromatism, and nuclear pleomorphism (hematoxylin-eosin, original magnification, ×100).
Fig. 2. Endometriosis with architectural atypia: hyperplasia, stratification, cribriform images, and cell stacking (hematoxylin-eosin, original magnification, ×40).
In addition, the degree of cell proliferation was studied by immunohistochemical staining for Ki-67 and measured by evaluating the percentage of Ki-67 with nuclear staining of cells [7] (Supplementary Figs.1 and 2). We analyzed COX-2 (cyclooxygenase-2) as an inflammatory marker [8] and BAF250a (Brahma associated factor [BRG]-associated factor 250a) as a surrogate marker of the ARID1A (AT-rich interactive domain 1A) gene mutation [9].
Ovarian cancers were classified according to the World Health Organization criteria [10] and staged using the International Federation of Gynecology and Obstetrics guidelines [11].
All EAOCs were classified using Sampson [12] and Scott's criteria [13], and all EAOC cases were divided into three categories (A, B, and C) according to Van Gorp's criteria [14]: (A) ovarian cancers with histologic proof of areas of transition between endometriosis, atypical endometriosis, and endometriosis-associated carcinoma according to Sampson and Scott's definition; (B) ovarian cancers with endometriosis in the same ovary, but without histologic proof of transition; (C) ovarian cancers with endometriosis at any location in the pelvis.
3. Statistical study
This study is part of a research project to establish the prevalence of atypical endometriosis and its histological subtypes: cellular atypia and atypia or architectural hyperplasia in patients with endometriosis and EAOC. With an estimated proportion of 8% [14] at a 95% confidence level, a sample size of 231 is needed to achieve a 3.5% margin of error for the proportion of atypical endometriosis. Quantitative variables are expressed as the mean, median, and quartiles, whereas qualitative variables are expressed as frequencies and percentages. The quantitative variables were compared by the Student t test or the Mann-Whitney U test (ANOVA or Kruskal-Wallis for more than two comparison groups), and the qualitative variables were compared by the chi-square test. Normal distribution was confirmed by the Kolmogorov-Smirnov test. Two-tail tests were used, and a p value of 0.05 was considered significant. All analyses were performed using IBM SPSS 19.0 (IBM Corporation, Armonk, New York, NY, USA).
RESULTS
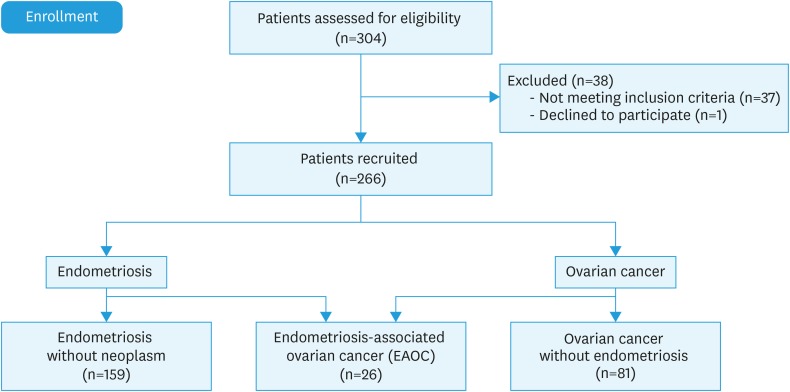
Among patients who had undergone surgery with a preoperative diagnosis of endometriosis and/or ovarian cancer during the study period, 266 had histologic confirmation of the diagnosis of endometriosis, ovarian cancer, or EAOC (Fig. 3). In the 185 patients with endometriosis, the characteristic findings of atypical endometriosis was reported by the pathologist in 23 cases (12.43%), of which 14 (60.86%) had endometriosis alone (ie, no associated neoplasm) and the other 9 (39.13%) had EAOC. The 14 cases of atypical endometriosis found in endometriosis alone accounted for 8.8% of all patients with endometriosis. An analysis of the 23 cases of atypical endometriosis found that 11 (47.8%) patients had ECA and 12 (52.2%) had EAA. In addition, we observed that ECA was found mainly in patients without neoplasm (71.4%) and that EAA was seen in patients with ovarian cancer (88.9%) (p=0.009) (Table 1). Using the Yates continuity correction, the power of the test is 71.9%. Furthermore, among the 9 cases of EAOC with atypical endometriosis, 8 were EAA and 1 was ECA.
Fig. 3. Study population.
Table 1. Comparison of ECA or EAA between patients with EN alone and patients with EAOC.
| Groups | ECA | EAA | p-value* |
|---|---|---|---|
| EN (n=14) | 10 (71.4%) | 4 (28.6%) | 0.009 |
| EAOC (n=9) | 1 (11.1%) | 8 (88.9%) |
EAA, endometriosis with architectural atypia; EAOC, endometriosis-associated ovarian cancer; ECA, endometriosis with cellular atypia; EN, endometriosis.
*Fisher's exact test.
The Ki-67 cell proliferation index was higher in patients with atypical endometriosis than in those with typical endometriosis (p<0.001) and was higher in patients with EAA than in those with ECA (p=0.004).
We found a higher proportion of staining expression for COX-2 in typical endometriosis than in atypical endometriosis (p<0.001). When we compared both subtypes of atypical endometriosis, COX-2 was positive in 80% of ECA cases versus 20% of EAA cases (p=0.089).
We observed that higher loss of BAF250a expression in the atypical versus typical endometriosis group (23.8% vs. 3%) (p=0.004). When we compared BAF250a expression between ECA versus EAA, loss of BAF250a was 9.1% for ECA versus 40% for EAA (p=0.149). The immunohistologic study is shown in Table 2.
Table 2. Immunohistologic study.
| Immunohistologic finding | Typical endometriosis | Atypical endometriosis | p-value | ECA | EAA | p-value |
|---|---|---|---|---|---|---|
| Ki-67 | 2.36% | 14.61% | <0.001* | 5.93%‡ | 22.58%‡ | 0.004* |
| BAF250a negative | 3% | 23.8% | 0.004† | 9.1% | 40% | 0.149† |
| COX-2 positive | 96.8% | 60% | <0.001† | 80% | 20% | 0.089† |
EAA, endometriosis with architectural atypia; ECA, endometriosis with cellular atypia.
*Mann-Whitney U test; †Fisher's exact test; ‡Average value of the Ki-67 index.
Histologic type was serous in 53.1% of patients with non-EAOC and 15.4% in patients with EAOC. Additionally, 23.1% of EAOC cases were clear-cell adenocarcinoma (CCA) and 42.3% were endometrioid adenocarcinoma (EA), compared with 6.2% and 14.8%, respectively, of cases observed in non-EAOC (p<0.001) (Table 3). The 8 cases of EAOC with EAA corresponded to 4 cases of EA, 2 of CCA, 1 of mucinous carcinoma, and 1 of high-grade sarcoma. The only case of EAOC with ECA was found in a patient with a high-grade, stage IIIb serous tumor. In this case, ECA was found in the same ovary as the cancer, but without direct continuity (Van Gorp type B).
Table 3. Comparison between EAOC and non-EAOC: histology and tumor stage.
| Histology and tumor stage | EAOC | Non-EAOC | p-value* | |
|---|---|---|---|---|
| Tumor histology | <0.001 | |||
| Serous carcinoma | 4 (15.4%) | 43 (53.1%)† | ||
| Mucinous carcinoma | 4 (15.4%) | 9 (11.1%) | ||
| Clear-cell adenocarcinoma | 6 (23.1%)† | 5 (6.2%) | ||
| Endometrioid adenocarcinoma | 11 (42.3%)† | 12 (14.8%) | ||
| Other | 1 (3.8%) | 12 (14.8%) | ||
| FIGO tumor stage | <0.001 | |||
| I | 20 (76.9%)† | 36 (44.4%) | ||
| II | 0 (0%) | 17 (21%) | ||
| III | 6 (23.1%) | 22 (27.2%) | ||
| IV | 0 (0%) | 6 (7.4%) | ||
EAOC, endometriosis-associated ovarian cancer, FIGO, International Federation of Gynecology and Obstetrics.
*Pearson's χ2; †Standardized residual >1.96.
According to the categories proposed by Van Gorp et al., among the 26 cases in our study with EAOC, 6 (23.1%) were A (ie, contiguous typical/atypical endometriosis and ovarian cancer were identified in the same ovary), 9 (34.6%) cases were B, and 11 (42.3%) were C. In addition to the 8 patients with EAOC who also had EAA, 6 corresponded to the 6 cases of type A mentioned above (4 with EA and 2 with CCA, all stage I). In the other 2, 1 was B (high-grade sarcoma with stage IIIb neuroectodermal differentiation) and 1 was C (stage I mucinous carcinoma).
According to EAOC staging, 76.9% patients of EAOC cases and 44.45% of non-EAOC were stage I (p<0.001) (Table 3).
Of the 11 cases with a histologic finding of ECA, 5 were found in specimens from hysterectomy with double adnexectomy and 6 were from fertility-sparing surgery, whereas all 12 cases of EAA were from hysterectomy with double adnexectomy.
DISCUSSION
Endometriosis is a common chronic inflammatory disease in women of childbearing age defined as the presence of tissue similar to the endometrium (glands and stroma) outside the endometrial cavity [6,15]. The prevalence of this condition in the general population varies from 5% to 15% according to the study, but is 30% to 50% among women with pelvic pain and/or infertility [16]. Endometriosis has different clinical presentations [17], and has been related to various chronic inflammatory diseases as well as to several types of cancer, such as melanoma and breast or ovarian cancer [18,19].
As in eutopic endometrium, the presence of hyperplasia and/or cellular atypia has been histologically documented in ectopic endometrium present in endometriosis [2], findings classified by some authors as atypical endometriosis [20,21]. Approximately 8% of endometriosis contain atypical endometriosis [14]. In addition, atypia has been more frequently found in endometriosis accompanied by ovarian cancer than in benign endometriosis cysts [22,23]. Most of the reports on atypical endometriosis show a direct continuity with the EAOC [5]. The spatial and chronologic association of atypical endometriosis associated with ovarian cancer suggests that this is a precancerous lesion similar to atypical endometrial hyperplasia [22]. This fact is supported by the histologic evidence of transition between endometriosis, atypical endometriosis, and EAOC [5].
In our series, the prevalence of atypical endometriosis in neoplasm-free endometriosis is 8.8%, but rises to 34.6% in the case of EAOC (p<0.001). The literature reports an incidence of 1.7% [24] to 32.3% [20] for atypical endometriosis in neoplasm-free endometriosis and 4.4% [24] to 22.8% [22] for atypical endometriosis with neoplasm.
The term “atypical endometriosis” has been used in the literature to refer to two different histologic findings [6]. One of them, called cytologic or cellular atypia, corresponds to the presence of cytologic atypia within the lining of endometriotic cysts, while the other, called architectural atypia or hyperplasia, refers to the same spectrum of hyperplasia (simple or complex, with or without cytologic atypia) found in the endometrium. Most studies have used “atypical endometriosis” to refer collectively to both cytologic atypia and hyperplasia, but an attempt should be made to distinguish them because they almost certainly differ in their clinical significance [6] and, therefore, we have considered them separately.
Actually, ECA with mild-to-moderate atypia and within endometriotic cysts is a common finding [6]. Its reported frequency obviously depends on the diagnostic threshold for this diagnosis. Czernobilsky and Morris [25]found mild atypia in 22% of their cases of ovarian endometriosis, whereas severe atypia was present in only 3.6% of cases. Seidman [20] reported that atypia, if present, is most commonly in an endometriotic cyst at one or more sites, with the cyst lined by enlarged polygonal epithelial cells; dense eosinophilic cytoplasm may be scant but is usually abundant. Most typically, no endometriotic glandular component is associated with this atypia. Moreover, this author reports considerable differences in the size and shape of the nuclei of such atypical cells, which are variably hyperchromatic and often exhibit smudged chromatin. Stromal and/or epithelial neutrophilic infiltrate is frequently present. He also found prominent inflammation along with prominent nucleoli in most of these cases, which means that it is a reactive process. In their series, Czernobilsky and Morris [25] found that the changes described as moderate atypia are probably reactive to severe local inflammation and/or superficial ulceration with regenerative activity. In most cases, cytologic atypia in endometriotic cysts is likely a reactive or degenerative change [6,20].
According to Clement [6], EAA is defined by a variety of hyperplastic changes, with or without cytologic atypia, similar to these findings in the endometrium. These changes are less common than the pure cytologic atypia described in the preceding section. The infrequency of hyperplasia in endometriosis complicates the assessment of its premalignant potential, but it is likely similar to that of endometrial hyperplasia in view of its occasional association with a synchronous or metachronous neoplasm at the same site. There is a significant association between the presence of a carcinoma and synchronous complex hyperplasia within the endometriosis [6,23]. Hyperplasia with severe atypia cannot be considered a response to inflammation because none of the cases described by other authors had inflammation or ulceration in the vicinity of hyperplasia [22,25].
In our study, we decided to determine if these histologic entities have potential for differing courses and, therefore, differing clinical and prognostic significance in the pathogenesis of EAOC. Consequently, the study has attempted to discern which patients with atypical endometriosis are at higher risk of ovarian cancer. To do so, we classified patients with atypical endometriosis into two subgroups: patients with a histologic finding of ECA and patients with EAA. We observed that ECA (71.4%) was found mainly in patients without neoplasm and that EAA was seen in patients with ovarian cancer (88.9%) (p=0.009).
The premalignant potential of EAA is not known with certainty, but there is a high association in the presence of carcinoma and synchronous complex hyperplasia within the endometriosis [5,6,23]. To clarify the possible reactive origin or the premalignant potential of each type of atypical endometriosis, we applied the immunohistochemical study COX-2, Ki-67, and BAF250a.
COX-2 would have utility as an inflammatory marker and also as a potential prognostic marker of ovarian cancer [8]. In our study, we found a higher proportion of staining expression for COX-2 in typical versus atypical endometriosis (p<0.001), which could confirm a higher degree of inflammation in typical endometriosis. When we compared both subtypes of atypical endometriosis, COX-2 was positive in the 80% of the ECA versus 20% of the EAA. Although the data are insufficient to draw significant conclusions, the difference in percentage suggests that ECA could be a reactive change as other authors postulated [5,6,20,25].
It is also known that Ki-67 is a nuclear protein observed in proliferating cells and that the percentage of cells with nuclear stain positive for Ki-67 indicates the cell proliferation index or mitotic index. In aggressive tumors, this percentage is high and indicates a poor prognosis [26]. In our series, the Ki-67 cell proliferation index was significantly higher in EAA than in ECA (22.58% vs. 5.93%), and Ki-67 was also higher in atypical versus typical endometriosis (14.61% vs. 2.36%). These results are consistent with those obtained by Yamamoto [7], who found higher Ki-67 in atypical endometriosis and CCA than in endometriosis without neoplasm, and similar to the results of Ogawa [22], who found higher Ki-67 in atypical endometriosis than in typical endometriosis but lower than that in ovarian carcinoma.
One of the most important aims in current research on the pathogenesis of EAOC is to identify the initial genetic alterations responsible for the development of EAOC from endometriosis. In this regard, we undertook an immunohistochemical study of BAF250a as a surrogate marker for a mutation in ARID1A, a tumor-suppressor gene that encodes the BAF250a protein. BAF250a is an important component of the SWI/SNF multiprotein. This protein complex is involved in regulating a number of cell processes, among them, development, differentiation, proliferation, DNA repair and tumor suppression [9]. Wiegand confirmed a decrease in BAF250a protein expression that correlated with ARID1A mutations, but, more importantly, in two patients, ARID1A mutations and decreased BAF250a expression were evident in the tumor and in the atypical endometriosis contiguous to the tumor, but not in the endometriotic lesions distant from the tumor [9]. ARID1A mutation expressing as a loss of BAF250a seems to be an important early event in the malignant transformation of endometriosis to ovarian cancer [9,27]. We observed that the loss of BAF250a expression is significantly higher in the atypical versus typical endometriosis group (23.8% vs. 3%). When we compared the expression of BAF250a between ECA and EAA, we found that the loss of BAF250a is 9.1% for ECA versus 40% for EAA. We cannot draw significant conclusions despite the difference in percentages, but these data are consistent with those of Ki-67, as both coincide with the fact that EAA could be an important step in the development of endometriosis to EAOC.
The data collected in this study show that EAA can be considered a differentiated group of histologic lesions in atypical endometriosis with different characteristics and behavior from those of ECA. The infrequency of hyperplasia or architectural atypia (EAA) in endometriosis complicates the assessment of its premalignant potential, but it is likely similar to that of endometrial hyperplasia in view of its occasional association with a synchronous or metachronous neoplasm at the same site [20]. In our series, 6 (23.1%) of the 26 EAOC cases were Van Gorp type A, ie, contiguous (also known as histologic transition) typical/atypical endometriosis, and ovarian cancer identified in the same ovary, fulfilling the criteria of Sampson [12] and Scott [13]. Apart from these 6 cases of type A, the atypical endometriosis found was of hyperplasia type. Histologic findings of transition between endometriosis, atypical endometriosis, and EAOC is important evidence in favor of the association of atypical endometriosis and EAOC [5,12,13].
The literature contains highly variable prevalences for EAOC [27,28,29,30], and in fact a recent meta-analysis reported considerable heterogeneity (3.4%–52.6%) [31]. In our series, the prevalence of EAOC was 24.29%, in contrast with 5.4% in a previous study we conducted [32]. Although both figures are within the range reported in the literature [31], we believe that this difference is due to the retrospective nature of the previous study and, consequently, due to a possible bias arising because the pathologist reported only the main diagnosis (ie, ovarian cancer) and did not mention endometriosis when the patient had both conditions. In our new prospective study, the pathologist examined and reported on the presence of endometriotic and/or ovarian cancer tissue in a single tissue specimen and stated whether the endometriosis was typical or atypical, and in the latter case, if possible, identified histologic findings of ECA or EAA.
There is agreement that endometriosis is associated with two specific histologic types of ovarian cancer: CCA and EA [2,33,34,35,36]. Our study also confirms this significant association of endometriosis with Kurman [37] type I ovarian cancer (ie, with CCA and EA, 42.3% and 23.1%, respectively). On the other hand, endometriosis is rarely (<5%) associated with high-degree Kurman type II serous carcinomas (p<0.001).
Furthermore, as widely reported previously [30,35,36,38], our series also confirms that EAOC is usually diagnosed earlier than non-EAOC (Table 3). However, it is not clear if CCA and EA have a better prognosis when associated with endometriosis than when not, because this earlier diagnosis may be due to the more abundant symptoms seen in patients with endometriosis compared with the few symptoms found in patients with non-EAOC [39].
The limitations of this study and the difficulties encountered in research in this field mainly stem from the low prevalence of atypical endometriosis, most particularly of the ECA and EAA subgroups.
In conclusion, our results suggest that atypical endometriosis is a premalignant lesion for ovarian cancer, as occurs in endometrial cancer. What is new about this study is the prospective design and the findings consistent with other authors [20,24,25], who have reported two types of histologic findings defined as cellular atypia and hyperplasia included in the concept of atypical endometriosis and with differing significance in terms of role and prognosis in the pathogenesis of EAOC. Our findings indicate a significant association of EAA with EAOC, which also exhibits more pronounced proliferative activity than in the case of ECA, as reflected by a higher Ki-67 index in addition to greater loss of BAF250a expression.
We believe that the findings of this study are important, although further research is needed before clinical decisions can be proposed. In a condition as prevalent as endometriosis, it would be extremely useful to define a subgroup of patients actually at higher risk of EAOC. Consequently, based on our results and those reported by other authors [20,24,25], we consider that a pathologist who observes endometriosis should also look beyond the diagnosis of this condition to examine the specimen for the presence of histologic lesions that could be described as atypical endometriosis, particularly EAA, and if found, carefully rule out the presence of malignancy in the rest of the histologic specimens [20]. Like Prefumo [23], we believe that patients with this histologic finding should receive closer gynecologic follow-up.
Footnotes
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
- Conceptualization: Ñ.S.I., M.L.F., M.S.M.P., A.J.J., T.A.
- Data curation: Ñ.S.I.
- Formal analysis: Ñ.S.I., M.L.F., M.S.M.P., A.J.J., T.A.
- Funding acquisition: M.L.F., M.S.M.P., T.A., N.D.A., S.F.M.L.
- Investigation: Ñ.S.I., M.L.F., M.S.M.P., A.J.J., T.A.
- Methodology: Ñ.S.I., M.L.F., A.J.J., T.A.
- Project administration: M.L.F.
- Resources: M.L.F., M.S.M.P., A.J.J., T.A., N.D.A., S.F.M.L.
- Software: A.J.J.
- Supervision: M.L.F., M.S.M.P., A.J.J., T.A., N.D.A., S.F.M.L.
- Validation: Ñ.S.I., M.L.F., M.S.M.P., A.J.J., T.A.
- Visualization: Ñ.S.I., M.L.F., M.S.M.P., A.J.J., T.A., N.D.A.
- Writing - original draft: Ñ.S.I., M.L.F., M.S.M.P., N.D.A., S.F.M.L.
- Writing - review & editing: M.L.F., M.S.M.P., A.J.J., T.A., N.D.A., S.F.M.L.
SUPPLEMENTARY MATERIALS
Endometriosis characterized by cellular atypia with low Ki-67 (<3%) (Ki-67, original magnification, ×100).
Endometriosis with architectural atypia with moderate-to-high Ki-67 (20%–30%) (Ki-67, original magnification, ×200).
References
- 1.Guo SW. Endometriosis and ovarian cancer: potential benefits and harms of screening and risk-reducing surgery. Fertil Steril. 2015;104:813–830. doi: 10.1016/j.fertnstert.2015.08.006. [DOI] [PubMed] [Google Scholar]
- 2.Mikami Y. Endometriosis-related ovarian neoplasms: pathogenesis and histopathologic features. Diagn Histopathol. 2014;20:357–363. [Google Scholar]
- 3.Vercellini P, Viganò P, Somigliana E, Fedele L. Endometriosis: pathogenesis and treatment. Nat Rev Endocrinol. 2014;10:261–275. doi: 10.1038/nrendo.2013.255. [DOI] [PubMed] [Google Scholar]
- 4.Munksgaard PS, Blaakaer J. The association between endometriosis and ovarian cancer: a review of histological, genetic and molecular alterations. Gynecol Oncol. 2012;124:164–169. doi: 10.1016/j.ygyno.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 5.LaGrenade A, Silverberg SG. Ovarian tumors associated with atypical endometriosis. Hum Pathol. 1988;19:1080–1084. doi: 10.1016/s0046-8177(88)80090-x. [DOI] [PubMed] [Google Scholar]
- 6.Clement PB. The pathology of endometriosis: a survey of the many faces of a common disease emphasizing diagnostic pitfalls and unusual and newly appreciated aspects. Adv Anat Pathol. 2007;14:241–260. doi: 10.1097/PAP.0b013e3180ca7d7b. [DOI] [PubMed] [Google Scholar]
- 7.Yamamoto S, Tsuda H, Miyai K, Takano M, Tamai S, Matsubara O. Cumulative alterations of p27-related cell-cycle regulators in the development of endometriosis-associated ovarian clear cell adenocarcinoma. Histopathology. 2010;56:740–749. doi: 10.1111/j.1365-2559.2010.03551.x. [DOI] [PubMed] [Google Scholar]
- 8.Grimstad FW, Decherney A. A review of the epigenetic contributions to endometriosis. Clin Obstet Gynecol. 2017;60:467–476. doi: 10.1097/GRF.0000000000000298. [DOI] [PubMed] [Google Scholar]
- 9.Wiegand KC, Shah SP, Al-Agha OM, Zhao Y, Tse K, Zeng T, et al. ARID1A mutations in endometriosis-associated ovarian carcinomas. N Engl J Med. 2010;363:1532–1543. doi: 10.1056/NEJMoa1008433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meinhold-Heerlein I, Fotopoulou C, Harter P, Kurzeder C, Mustea A, Wimberger P, et al. The new WHO classification of ovarian, fallopian tube, and primary peritoneal cancer and its clinical implications. Arch Gynecol Obstet. 2016;293:695–700. doi: 10.1007/s00404-016-4035-8. [DOI] [PubMed] [Google Scholar]
- 11.Prat J FIGO Committee on Gynecologic Oncology. FIGO's staging classification for cancer of the ovary, fallopian tube, and peritoneum: abridged republication. J Gynecol Oncol. 2015;26:87–89. doi: 10.3802/jgo.2015.26.2.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sampson JA. Endometrial carcinoma of the ovary, arising in endometrial tissue in that organ. Arch Surg. 1925;10:1–72. [Google Scholar]
- 13.Scott RB. Malignant changes in endometriosis. Obstet Gynecol. 1953;2:283–289. [PubMed] [Google Scholar]
- 14.Van Gorp T, Amant F, Neven P, Vergote I, Moerman P. Endometriosis and the development of malignant tumours of the pelvis. A review of literature. Best Pract Res Clin Obstet Gynaecol. 2004;18:349–371. doi: 10.1016/j.bpobgyn.2003.03.001. [DOI] [PubMed] [Google Scholar]
- 15.Machado-Linde F, Pelegrin P, Sanchez-Ferrer ML, Leon J, Cascales P, Parrilla JJ. 2-methoxyestradiol in the pathophysiology of endometriosis: focus on angiogenesis and therapeutic potential. Reprod Sci. 2012;19:1018–1029. doi: 10.1177/1933719112446080. [DOI] [PubMed] [Google Scholar]
- 16.Zafrakas M, Grimbizis G, Timologou A, Tarlatzis BC. Endometriosis and ovarian cancer risk: a systematic review of epidemiological studies. Front Surg. 2014;1:14. doi: 10.3389/fsurg.2014.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garry R. Is insulin resistance an essential component of PCOS?: the endometriosis syndromes: a clinical classification in the presence of aetiological confusion and therapeutic anarchy. Hum Reprod. 2004;19:760–768. doi: 10.1093/humrep/deh147. [DOI] [PubMed] [Google Scholar]
- 18.Dunselman GA, Vermeulen N, Becker C, Calhaz-Jorge C, D'Hooghe T, De Bie B, et al. ESHRE guideline: management of women with endometriosis. Hum Reprod. 2014;29:400–412. doi: 10.1093/humrep/det457. [DOI] [PubMed] [Google Scholar]
- 19.Kvaskoff M, Mu F, Terry KL, Harris HR, Poole EM, Farland L, et al. Endometriosis: a high-risk population for major chronic diseases? Hum Reprod Update. 2015;21:500–516. doi: 10.1093/humupd/dmv013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seidman JD. Prognostic importance of hyperplasia and atypia in endometriosis. Int J Gynecol Pathol. 1996;15:1–9. doi: 10.1097/00004347-199601000-00001. [DOI] [PubMed] [Google Scholar]
- 21.Mandai M, Yamaguchi K, Matsumura N, Baba T, Konishi I. Ovarian cancer in endometriosis: molecular biology, pathology, and clinical management. Int J Clin Oncol. 2009;14:383–391. doi: 10.1007/s10147-009-0935-y. [DOI] [PubMed] [Google Scholar]
- 22.Ogawa S, Kaku T, Amada S, Kobayashi H, Hirakawa T, Ariyoshi K, et al. Ovarian endometriosis associated with ovarian carcinoma: a clinicopathological and immunohistochemical study. Gynecol Oncol. 2000;77:298–304. doi: 10.1006/gyno.2000.5765. [DOI] [PubMed] [Google Scholar]
- 23.Prefumo F, Todeschini F, Fulcheri E, Venturini PL. Epithelial abnormalities in cystic ovarian endometriosis. Gynecol Oncol. 2002;84:280–284. doi: 10.1006/gyno.2001.6529. [DOI] [PubMed] [Google Scholar]
- 24.Oral E, Ilvan S, Tustas E, Korbeyli B, Bese T, Demirkiran F, et al. Prevalence of endometriosis in malignant epithelial ovary tumours. Eur J Obstet Gynecol Reprod Biol. 2003;109:97–101. doi: 10.1016/s0301-2115(03)00047-2. [DOI] [PubMed] [Google Scholar]
- 25.Czernobilsky B, Morris WJ. A histologic study of ovarian endometriosis with emphasis on hyperplastic and atypical changes. Obstet Gynecol. 1979;53:318–323. [PubMed] [Google Scholar]
- 26.Yalcin SE, Ocal I, Yalcin Y, Selim HS, Caltekin MD, Aydogmus H, et al. Evaluation of the Ki-67 proliferation index and urocortin expression in women with ovarian endometriomas. Eurasian J Med. 2017;49:107–112. doi: 10.5152/eurasianjmed.2017.17070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fukunaga M, Nomura K, Ishikawa E, Ushigome S. Ovarian atypical endometriosis: its close association with malignant epithelial tumours. Histopathology. 1997;30:249–255. doi: 10.1046/j.1365-2559.1997.d01-592.x. [DOI] [PubMed] [Google Scholar]
- 28.Garrett LA, Growdon WB, Goodman A, Boruta DM, Schorge JO, del Carmen MG. Endometriosis-associated ovarian malignancy: a retrospective analysis of presentation, treatment, and outcome. J Reprod Med. 2013;58:469–476. [PubMed] [Google Scholar]
- 29.Wilbur MA, Shih IM, Segars JH, Fader AN. Cancer implications for patients with endometriosis. Semin Reprod Med. 2017;35:110–116. doi: 10.1055/s-0036-1597120. [DOI] [PubMed] [Google Scholar]
- 30.Bounous VE, Ferrero A, Fuso L, Ravarino N, Ceccaroni M, Menato G, et al. Endometriosis-associated ovarian cancer: a distinct clinical entity? Anticancer Res. 2016;36:3445–3449. [PubMed] [Google Scholar]
- 31.Heidemann LN, Hartwell D, Heidemann CH, Jochumsen KM. The relation between endometriosis and ovarian cancer - a review. Acta Obstet Gynecol Scand. 2014;93:20–31. doi: 10.1111/aogs.12255. [DOI] [PubMed] [Google Scholar]
- 32.Machado-Linde F, Sánchez-Ferrer ML, Cascales P, Torroba A, Orozco R, Silva Sánchez Y, et al. Prevalence of endometriosis in epithelial ovarian cancer. Analysis of the associated clinical features and study on molecular mechanisms involved in the possible causality. Eur J Gynaecol Oncol. 2015;36:21–24. [PubMed] [Google Scholar]
- 33.Thomas EJ, Campbell IG. Molecular genetic defects in endometriosis. Gynecol Obstet Invest. 2000;50(Suppl 1):44–50. doi: 10.1159/000052878. [DOI] [PubMed] [Google Scholar]
- 34.Brilhante AV, Augusto KL, Portela MC, Sucupira LC, Oliveira LA, Pouchaim AJ, et al. Endometriosis and ovarian cancer: an integrative review (endometriosis and ovarian cancer) Asian Pac J Cancer Prev. 2017;18:11–16. doi: 10.22034/APJCP.2017.18.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pearce CL, Templeman C, Rossing MA, Lee A, Near AM, Webb PM, et al. Association between endometriosis and risk of histological subtypes of ovarian cancer: a pooled analysis of case-control studies. Lancet Oncol. 2012;13:385–394. doi: 10.1016/S1470-2045(11)70404-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ruderman R, Pavone ME. Ovarian cancer in endometriosis: an update on the clinical and molecular aspects. Minerva Ginecol. 2017;69:286–294. doi: 10.23736/S0026-4784.17.04042-4. [DOI] [PubMed] [Google Scholar]
- 37.Kurman RJ, Shih IM. Molecular pathogenesis and extraovarian origin of epithelial ovarian cancer--shifting the paradigm. Hum Pathol. 2011;42:918–931. doi: 10.1016/j.humpath.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Acién P, Velasco I, Acién M, Capello C, Vela P. Epithelial ovarian cancers and endometriosis. Gynecol Obstet Invest. 2015;79:126–135. doi: 10.1159/000367597. [DOI] [PubMed] [Google Scholar]
- 39.Kumar S, Munkarah A, Arabi H, Bandyopadhyay S, Semaan A, Hayek K, et al. Prognostic analysis of ovarian cancer associated with endometriosis. Am J Obstet Gynecol. 2011;204:63.e1–63.e7. doi: 10.1016/j.ajog.2010.08.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Endometriosis characterized by cellular atypia with low Ki-67 (<3%) (Ki-67, original magnification, ×100).
Endometriosis with architectural atypia with moderate-to-high Ki-67 (20%–30%) (Ki-67, original magnification, ×200).