Abstract
Objective
To compare gynecological cancer risk management between women with BRCA variants of unknown significance (VUS) to women with negative genetic testing.
Methods
Ninety-nine patients whose BRCA genetic testing yielded VUS were matched with 99 control patients with definitive negative BRCA results at a single institution. Demographics and risk management decisions were obtained through chart review. Primary outcome was the rate of risk-reducing bilateral salpingo-oophorectomy (RRBSO). Chi square tests, t-tests, and logistic regression were performed, with significance of p<0.05.
Results
VUS patients were more likely to be non-Caucasian (p=0.000) and of Ashkenazi-Jewish descent (p=0.000). There was no difference in gynecologic oncology referrals or recommendations to screen or undergo risk-reducing surgery for VUS vs. negative patients. Ultimately, 44 patients (22%) underwent RRBSO, with no significant difference in surgical rate based on the presence of VUS. Ashkenazi-Jewish descent was associated with a 4.5 times increased risk of RRBSO (OR=4.489; 95% CI=1.484–13.579) and family history of ovarian cancer was associated with a 2.6 times risk of RRBSO (OR=2.641; 95% CI=1.107–6.299).
Conclusion
In our institution, patients with VUS were surgically managed similarly to those with negative BRCA testing. The numbers of patients with VUS are likely to increase with the implementation of multi-gene panel testing. Our findings underscore the importance of genetic counseling and individualized screening and prevention strategies in the management of genetic testing results.
Keywords: Genetic Testing, Hereditary Breast and Ovarian Cancer Syndrome, Salpingo-oophorectomy, Risk Assessment
INTRODUCTION
BRCA mutations are implicated in 9%–24% of epithelial ovarian cancers and 4.5% of breast cancers. Women with BRCA 1 and 2 mutations have a 45-85% lifetime risk of developing breast cancer, and a 39%–46% chance of ovarian, fallopian, or primary peritoneal cancer for women with BRCA1, and 10%–27% probability with BRCA2 [1]. Five to 15% of BRCA mutations are classified as variants of unknown significance (VUS), with rates higher in African-Americans and Hispanics [2,3,4,5]. Over 6,000 unique VUS have been reported in over 13,000 families in 17 countries [6].
VUS results indicate that due to the rarity of the finding and the insufficient epidemiological evidence at the time of the test, there is not enough information to classify the mutation as definitively pathogenic or benign [7]. Counseling patients with VUS results is challenging because the test result alone cannot be used to quantify risk and guide management. VUS should be treated as a negative result, and risk assessment should be based on family history [8].
Prior studies have established the challenges of VUS results for both providers and patients. A survey of providers showed that fewer than 15% of practitioners recognized appropriate management for patients with VUS [9]. Another survey of genetic counselors showed that there is significant diversity in the interpretation of VUS results. Some counselors offered aggressive management given the “cancerphobia” of patients, whereas other counselors did not recommend prophylactic surgery even in “high risk” patients [10]. In addition, a Dutch study showed that 10 of 19 patients included in the study interpreted VUS as pathogenic and subsequently chose to undergo risk-reducing surgery [11]. Another study found that 30% of patients with VUS incorrectly believed that the result increased their cancer risk, and this was more likely in patients with only a high-school education [12].
Since patients with VUS should be managed similarly to patients with negative BRCA 1 or 2 results, we sought to compare the cancer risk management decisions of patients with VUS to those of patients with definitive negative genetic testing results. Our secondary aim was to identify potential factors associated with a decision to undergo risk-reducing bilateral salpingo-oophorectomy (RRBSO) following the identification of VUS.
MATERIALS AND METHODS
Women who underwent genetic testing for the BRCA1 and BRCA 2 genes at an urban academic medical center between January 2006 and December 2012 were included in this study. Women who had been identified by their physicians to be potentially at increased risk for BRCA mutations by personal and family history of cancers were seen by genetic counselors, who performed risk assessments and recommended testing based on the results of the assessment. Clinical management was performed by the primary physician, surgeon, or obstetrician/gynecologist. Prior to June 2013, and therefore for all patients in this study, all BRCA testing at our institution was performed by Myriad Genetics (Salt Lake City, UT, USA) as BRCA testing was proprietary by Myriad at the time of the study. The inclusion criteria for this study were women from our institution with VUS and negative BRCA results identified by Myriad Genetics' database who had at least one physician visit post genetic-testing to elucidate cancer risk management decision-making. The exclusion criteria for the study were all patients who were men, women below age 18 or above 90, women with deleterious mutations and patients who underwent bilateral salpingo-oophorectomies (BSO) prior to genetic testing. Patients with incomplete or missing paper or electronic chart documentation were also excluded from the study. This study was approved by our institutional review board.
A chart review of included patients was conducted to compare the cancer risk management decision-making of women with VUS results to those with negative BRCA results. VUS results included BRCA test results of “variants of unknown significance,” “favor polymorphism,” and “suspected deleterious.” To mitigate selection bias, patients with VUS results were matched 1:1 by age in years at the time of genetic testing and the closest date of BRCA test to patients with negative results. The primary outcome was the rate of risk-reducing gynecologic surgery for patients with VUS as compared to those with negative results. To identify factors that may influence cancer risk decision-making, demographic information and management decision were also collected. Demographic information included age at genetic testing, race, Ashkenazi-Jewish descent, insurance, and marital status. Personal and family history of breast, gynecologic, and other cancers, menopausal status, parity, oral contraceptive use, and hormonal therapy use were also examined. For patients with personal breast cancer history, hormone receptor status, hormone therapy use, and surgery were also included to elucidate potential indication for BSO for therapeutic and not necessarily risk-reducing reasons. For family history, up to three degrees of relatives were included. To elucidate the decision-making process, initial evaluating physician, referrals to gynecologic oncology, initial management recommendation of provider and final management decision were examined. For patients who underwent RRBSO, final pathology was also reviewed.
Demographic variables and clinical characteristics were examined in two sets of bivariate analysis. One bivariate analysis compared women with VUS to women with negative mutations, and another bivariate analysis compared women who underwent RRBSO vs. those that did not. Categorical variables were evaluated using χ2 tests and Fisher's exact tests for small cell sizes. Continuously measured variables were evaluated using means and two-sided t-tests. Univariate logistic regression was performed to evaluate factors that predicted RRBSO. A multivariate logistic regression analysis was performed using forward and backward stepwise likelihood ratio method to arrive at the most parsimonious model. The level of statistical significance for all analysis was p=0.05, with a confidence interval of 95%. All statistical analyses were performed by SPSS version 23 (IBM Corp., Armonk, NY, USA).
RESULTS
1. Demographics
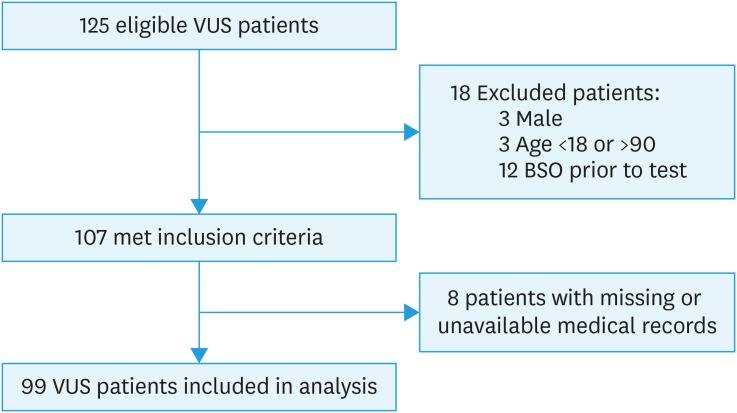
Approximately 2,812 patients underwent testing for BRCA1 and 2 through Myriad at our institution between January 1, 2006, and December 31, 2012. Of those, 125 (4.4%) had VUS results. A flowchart detailing the inclusion of patients is in Fig. 1. Of the 99 VUS patients who were ultimately included in the study, 79 (80%) were classified as “variants of uncertain significance,” 18 (18%) were “favor polymorphism,” and 2 (2%) were “suspected deleterious.” Ninety-nine patients with negative results were matched 1:1 with patients with VUS results. Demographic data are summarized in Table 1. The mean age at BRCA testing was 47 and the median age was 46 for both VUS and negative patients. VUS patients were more racially diverse than negative patients; 46% patients with VUS were non-Caucasian, compared to 15% of patients with negative results (p=0.000). VUS patients were more likely to be of Ashkenazi-Jewish descent (76% vs. 43%, p=0.000). Only 71% of patients with VUS were privately insured, compared to 92% of patients with negative results (p=0.000).
Fig. 1. Consolidated Standards of Reporting Trials diagram of included patients.
VUS, variants of unknown significance; BSO, bilateral salpingo-oophorectomy.
Table 1. Characteristics associated with patients with BRCA negative and VUS mutations.
| Characteristics | Negative | VUS | p-value | |
|---|---|---|---|---|
| Age at testing, Median (Range) | 46 (26–79) | 46 (26–79) | ||
| VUS type | - | 99 | ||
| Unknown | - | 79 (80) | ||
| Favor polymorphism | - | 18 (18) | ||
| Suspect deleterious | - | 2 (2) | ||
| Race | 0.000 | |||
| Non-caucasian | 15 (15) | 45 (46) | ||
| Caucasian | 74 (75) | 44 (44) | ||
| Not reported | 10 (10) | 10 (10) | ||
| Ethnicity | 0.000 | |||
| Ashkenazi | 43 (43) | 75 (76) | ||
| Non-Ashkenazi | 37 (37) | 11 (11) | ||
| Not reported | 19 (19) | 13 (13) | ||
| Parity | 0.101 | |||
| 0 | 40 (40) | 29 (29) | ||
| ≥1 | 59 (60) | 70 (71) | ||
| Menopausal status | 0.155 | |||
| Premenopausal | 68 (69) | 54 (56) | ||
| Perimenopausal | 4 (4) | 10 (10) | ||
| Postmenopausal | 25 (25) | 32 (32) | ||
| Unknown | 2 (2) | 3 (3) | ||
| OCPs prior to RRBSO | 0.054 | |||
| Yes | 56 (57) | 39 (39) | ||
| No | 20 (20) | 28 (28) | ||
| Unknown | 23 (23) | 32 (32) | ||
| Personal history of breast cancer* | 74 (75) | 79 (80) | 0.396 | |
| Personal history of gynecologic cancer* | 1 (1) | 3 (3) | 0.621 | |
| Family history of breast cancer* | 65 (66) | 64 (68) | 0.878 | |
| Family history of all gynecologic cancers* | 20 (20) | 18 (19) | 0.312 | |
| Family history of ovarian cancer* | 19 (19) | 12 (12) | 0.171 | |
| RRBSO | 0.305 | |||
| No | 80 (81) | 74 (76) | ||
| Yes | 19 (19) | 25 (25) | ||
Values are presented as number (%).
Chi-square and Fisher's exact tests for small cells for categorical variables, and t-tests for continuous variables.
VUS, variants of unknown significance; OCP, oral contraceptive pills; RRBSO, risk-reducing bilateral salpingo-oophorectomy.
*Patients with unknown cancer history were excluded from this analysis.
There was no difference in personal breast cancer history between women with VUS and negative results, although this was the most common reason for genetic evaluation in our population for both VUS and negative patients. Fourteen percent of VUS patients had no personal history of cancer but were referred to genetic counseling due to a significant family history of cancer, compared to 21% of negative patients (p=0.192). There was no significant difference in family cancer history between patients with VUS and negative results. Breast cancer was the most common family history for both patients with VUS and negative results (68% vs. 66%, respectively, p=0.878). Nineteen percent of patients with VUS had a family history of gynecologic cancer (ovarian, tubal, uterine, cervical), compared to 20% of patients with negative results (p=0.312). Of those, 67% (12 of 18) of VUS patients and 95% (19 of 20) of negative patients had a family history of ovarian cancer.
2. Cancer risk management
Overall, out of the 198 patients, 44 patients (22%) underwent RRBSO after genetic and physician counseling. The presence of VUS results was not associated with an increased rate of risk-reducing gynecologic surgery in our patients (25% vs. 19%, p=0.305). Table 2 shows the characteristics associated with risk-reducing surgery. In a bivariate analysis comparing women who underwent risk-reducing surgery vs. no surgery, only Ashkenazi-Jewish descent was associated with the rate of RRBSO (p=0.023). In patients that underwent risk-reducing surgery, 16 out of 44 patients (36%) were premenopausal with a history of hormone receptor positive breast cancer (p=0.629). In women without a personal history of breast cancer, 3 of 20 women with VUS underwent RRBSO, compared to 5 of 25 women with negative results (p=0.716).
Table 2. Characteristics associated with patients undergoing RRBSO.
| Characteristics | No RRBSO (n=154) | RRBSO (n=44) | p-value | |
|---|---|---|---|---|
| Age at testing, Median (Range) | 46 (26–79) | 47.5 (30–67) | ||
| Variant type | 0.603 | |||
| Negative | 80 (52) | 19 (43) | ||
| Unknown | 59 (38) | 20 (46) | ||
| Favor polymorphism | 14 (9) | 4 (9) | ||
| Suspect deleterious | 1 (1) | 1 (2) | ||
| Race | 0.095 | |||
| Non-caucasian | 98 (64) | 20 (46) | ||
| Caucasian | 42 (27) | 18 (41) | ||
| Not reported | 14 (9) | 6 (14) | ||
| Ethnicity | 0.023 | |||
| Ashkenazi | 88 (57) | 30 (68) | ||
| Non-Ashkenazi | 44 (29) | 4 (9) | ||
| Not reported | 22 (14) | 10 (23) | ||
| Parity | 0.403 | |||
| 0 | 56 (36) | 13 (30) | ||
| ≥1 | 98 (64) | 31 (71) | ||
| Menopausal status | 0.216 | |||
| Premenopausal | 99 (64) | 23 (52) | ||
| Perimenopausal | 10 (7) | 4 (9) | ||
| Postmenopausal | 40 (26) | 17 (39) | ||
| Unknown | 5 (3) | 0 (0) | ||
| OCPs prior to RRBSO | 0.520 | |||
| Yes | 77 (50) | 18 (41) | ||
| No | 35 (23) | 13 (30) | ||
| Unknown | 42 (27) | 13 (30) | ||
| Personal history of breast cancer* | 117 (76) | 36 (82) | 0.415 | |
| Personal history of gynecologic cancer* | 3 (1) | 1 (2) | 1.000 | |
| Family history of breast cancer* | 100 (67) | 29 (67) | 0.924 | |
| Family history of all gynecologic cancers* | 26 (17.3) | 12 (28) | 0.124 | |
| Family history of ovarian cancer* | 20 (13) | 11 (25) | 0.053 | |
Values are presented as number (%).
Chi-square and Fisher's exact tests for small cells for categorical variables, and t-tests for continuous variables. For family history, patients with unknown family histories were excluded.
RRBSO, risk-reducing bilateral salpingo-oophorectomy; OCP, oral contraceptive pills.
*Patients with unknown cancer history were excluded from this analysis.
There was no significant difference in patients with VUS vs. negative results in being referred to gynecologic oncologists (57% vs. 68%, p=0.107). However, 82% of patients who underwent risk-reducing surgery were referred to gynecologic oncologists, compared to 18% who did not undergo surgery (p=0.000). There was no difference in treatment recommendations of observation vs. surgery on the basis of VUS results (p=0.333).
A univariate logistic regression model was completed using potential clinical predictive factors for risk-reducing surgery, including VUS status, race, ethnicity, menopausal status, parity, and personal and family history of breast and ovarian cancer (Table 3). All predictive factors were used in a stepwise selection to provide the final multivariate model (Table 4). Women of Ashkenazi-Jewish descent were 4.5 times more likely than women not from an Ashkenazi-Jewish descent to have a RRBSO (OR=4.489, 95% CI=1.484–13.579) controlling for family history of ovarian cancer. In addition, women who had a family history of ovarian cancer were 2.6 times more likely than women without an ovarian cancer family history to undergo RRBSO (OR=2.641, 95% CI=1.107–6.299) controlling for Ashkenazi-Jewish descent. Age, parity, race, menopausal status, and personal and family history of breast cancer were not significant predictors of risk-reducing surgery and were excluded from the final model.
Table 3. Univariate logistic regression analysis of predictive factors associated with the decision to undergo risk-reducing surgery.
| Predictors | OR | 95% CI | p-value |
|---|---|---|---|
| Age | 1.010 | (0.976–1.045) | 0.561 |
| Parity ≥1 | 1.363 | (0.659–2.817) | 0.404 |
| Non-caucasian* | 2.100 | (1.066–4.138) | 0.032 |
| Ashkenazi Jewish descent* | 4.000 | (1.351–11.845) | 0.012 |
| Peri or postmenopausal status | 1.643 | (0.835–3.235) | 0.150 |
| Personal history of breast cancer | 0.703 | (0.300–1.645) | 0.416 |
| HR+, premenopausal breast cancer | 1.189 | (0.590–2.395) | 0.629 |
| Family history of ovarian cancer | 2.233 | (0.975–5.114) | 0.057 |
| Family history of breast cancer | 0.958 | (0.473–1.940) | 0.905 |
| VUS result | 1.422 | (0.724–2.794) | 0.306 |
Univariate logistic regression was performed.
OR, odds ratio; CI, confidence interval; HR, hormone receptor; VUS, variants of unknown significance.
*Indicates significance.
Table 4. Multivariate logistic regression analysis of predictive factors associated with the decision to undergo risk-reducing surgery.
| Predictors | OR | 95% CI | p-value |
|---|---|---|---|
| Ashkenazi Jewish descent | 4.489 | (1.484–13.579) | 0.008 |
| Family history of ovarian cancer | 2.641 | (1.107–6.299) | 0.029 |
Multivariate logistic regression analysis of final covariates included after stepwise analysis; age, parity, race, menopausal status, personal and family history of breast cancer removed from final model.
OR, odds ratio; CI, confidence interval.
3. Final pathology
The majority of patients in our study that underwent RRBSO following genetic testing had benign pathology; however, four (of 44, or 9.0%) RRBSO patients did have a gynecologic malignancy. No serous tubal intraepithelial carcinoma lesions were observed. One patient with a “favor polymorphism” result was found to have a malignancy of the uterus on final pathology. Among patients with documented negative genetic results, three patients were found to have malignancy on final pathology: one uterine, and two ovarian carcinomas (Table 5).
Table 5. Patients with malignant pathology following RRBSO.
| Patient | Genetic test result | Age at testing | Cancer history | Surgery performed | Final pathology |
|---|---|---|---|---|---|
| Patient 84 | Negative | 41 | Cervical cancer | TAH BSO | Ovarian malignancy |
| Patient 112 | Negative | 42 | HR+ breast cancer | BSO | Ovarian/tubal malignancy |
| Patient 218 | Negative | 49 | HR+ breast cancer | TAH BSO | Uterine malignancy |
| Patient 343 | Favor polymorphism | 67 | HR+ breast cancer | TAH BSO | Uterine malignancy |
RRBSO, risk-reducing bilateral salpingo-oophorectomy; TAH, total abdominal hysterectomy; BSO, bilateral salpingo-oophorectomy; HR, hormone receptor.
DISCUSSION
In this study at a single institution comparing the cancer risk management of patients with VUS to those with negative results, VUS results were not associated with an increased rate of RRBSO. Patients with VUS were more likely to be racially diverse, which is consistent with prior studies [7]. VUS patients were also more likely to be of Ashkenazi-Jewish descent, and this was associated with an increased rate of RRBSO. Our RRBSO rate of 22% was consistent with previous studies [3,13]. Referral to a gynecologic oncologist was not associated with the presence of VUS but was associated with a higher rate of RRBSO. Ashkenazi-Jewish descent and a family history of ovarian cancer were predictive of the rate of RRBSO.
Current guidelines suggest that for women with BRCA 1 or 2 mutations, RRBSO should be offered by age 35–40 or at the completion of childbearing to reduce the risk of breast cancer and ovarian cancer most effectively [14,15]. Given the limitations in screening for ovarian cancer, risk-reducing surgery has been shown to be effective in decreasing overall mortality in women with BRCA mutations. Prospective studies have shown a 70% to 85% reduction in ovarian cancer and a 37% to 54% reduction in breast cancer [16,17]. For women with population risks of ovarian cancer, however, RRBSO does not have a beneficial effect on overall health and has many deleterious effects. RRBSO is a major surgical procedure and is not without potential risks including the risk of bowel injury, bleeding, infection, transfusion, and prolonged hospital stay. The surgical menopause induced by RRBSO has an overall negative impact on cardiovascular, neurologic, and bone health, especially in the absence of estrogen replacement [18]. In addition, RRBSO is associated with an increased mortality from all-cause death in women who have never used estrogen therapy and is not associated with increased survival at any age except in cases of risk of death from ovarian and breast cancer [19]. Thus, RRBSO should not be recommended lightly for women with population risks of breast and ovarian cancer.
Breast and ovarian cancer risks are dependent on a multitude of factors. While deleterious BRCA 1 and 2 mutations confer the strongest risk for breast and ovarian cancers, other risk factors include a family history of ovarian cancer, breast cancer diagnosis, and Ashkenazi Jewish ethnicity, and these risk factors should be used to inform decision-making in the context of negative genetic testing. While Ashkenazi Jewish women are up to 10 times more likely than the general population to have a BRCA mutation, only 24% to 41% of ovarian cancer is attributed to known founder BRCA mutations [20,21]. Even for families with mutations in the same gene, there is variability in the risk of cancer. In addition, with the advancement of panel testing, 64.1% to 86.5% of people with a suspected hereditary predisposition to cancer have yet to find a pathogenic mutation [22].
For women with personal or family histories suspicious for hereditary cancer syndromes without documented pathogenic BRCA 1 or 2 mutations, management is certainly less clear, and this ambiguity is even more pronounced when the genetic testing result is so inconclusive as to be labeled “unknown,” as in VUS results. Whether appropriate or not, a negative genetic testing result offers a patient information that is often used when determining cancer risk management; in deciding to undergo genetic testing, a patient acknowledges how the results will influence her screening and prevention decisions. Because all counseling includes discussions about negative results not being definitive except in the case of there being a known mutation in a family, negative results are less confusing to patients than VUS results [23]. In contrast, Culver et al. [5] showed that because of the ambiguous nature of the VUS result, patients with VUS results found genetic counseling to be less informative and reassuring. Whereas VUS results indicate that there is not enough information to classify the result as deleterious or benign, patients and some clinicians often interpret VUS results as not negative [23]. Ready et al. [24] showed that 35% patients with a personal history of breast cancer and VUS underwent risk-reducing strategies that would not otherwise have been recommended based on family history alone. Because many patients may assume that VUS results are pathogenic unless the potential for an inconclusive result has been discussed prior to genetic testing, improved genetic literacy among clinical professionals must be prioritized, in addition to a close relationship between genetic counselors and clinicians [6].
While there are tools that can be used to estimate cancer risk regardless of gene status including Breast and Ovarian Analysis of Disease Incidence (BOADICEA), BRCAPro, and the International Breast Cancer Intervention Study for genetic counselors, ultimately, it is physicians that make management recommendations to patients, taking into account various factors that inform a patient's individual risk for cancer [22]. Our study was conducted as genetic testing was becoming influential in counseling and management of patients when the standard of care for VUS patients was not necessarily known. Today, as panel testing provides additional uncertainty for cancer risk management decisions for patients, this study describes issues that providers may face moving forward.
Strengths of this study include the larger sample size of VUS results than previously published studies that examine VUS management strategies specifically. This is also the first study to compare VUS results with BRCA negative patients. Although this study's population is from a single institution, unique to our institution is the affiliation with both a private university hospital and a public hospital where many patients are uninsured or underinsured. Both hospitals are affiliated with the same medical school, and faculty and residents serve both institutions. As genetic testing becomes more widely available to include diverse populations, more studies will need to capture racially diverse populations in assessing cancer risk.
A major limitation of the study is the descriptive nature of the study; the data collected was from physician chart and genetic counseling notes, which does not capture the depth, complexity or the nuances of the patient-provider discussions on genetic testing. In addition, the patient population at our institution has a high percentage of Ashkenazi Jews, so the results may not be generalizable across other patient populations and also represents an ascertainment bias. BRCA testing at the time of the study was proprietary to Myriad, and as a result, genetic testing was limited to patients with high risk. In addition, given its proprietary nature, the available genetic testing information (e.g., the specific genetic testing results, the methodology in classifying variants and its annotation, possible reclassification) was limited and not part of our analysis. Finally, because our study was focused on the decision-making by gynecological oncologists, decision-making regarding risk-reducing mastectomy was not examined in this study. Though bilateral salpingo-oophorectomy can be potentially indicated in women for therapeutic indications, as in the case for premenopausal women with hormone receptor positive breast cancer, this distinction was not adequately captured in the documentation and thus was not included in this study. Recently, Morgan et al. [25] studied the risk management decisions of women in regards to risk reducing mastectomy for women with VUS, and found no difference in the rates of surgery when comparing pathologic BRCA mutations to VUS mutations.
In conclusion, VUS results should be interpreted as non-informative and should not influence cancer management; rather, risk management decisions should be guided by personal and family history. Additionally, prevention and screening strategies should be individualized based on history and test results. As the numbers of patients with VUS results increase with the implementation of multi-gene testing, providers and genetic counselors must correctly counsel patients regarding VUS results to avoid misinterpretation, unnecessary procedures, and anxiety. Given the complexity of decision-making for women with VUS results, psychological support should be provided as well. Qualitative survey studies of patients with VUS results may offer insight into patient and providers' decision-making process in cancer risk management.
ACKNOWLEDGEMENTS
We would like to acknowledge the staff, genetic counselors, physicians, and the patients at New York University School of Medicine.
Footnotes
Presentation: Poster presented at American Society of Clinical Oncology Annual Meeting, May 2015 (Chicago, IL, USA). Poster presented at Hereditary Breast and Ovarian Cancer Symposium, May 2018 (Montreal, Quebec, Canada).
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
- Conceptualization: L.S.S., B.S.V.
- Data curation: L.S.S., C.J.Y., B.S.V.
- Formal analysis: L.S.S., C.J.Y., F.M.K., L.J., B.S.V.
- Funding acquisition: B.S.V.
- Investigation: L.S.S., C.J.Y., F.M.K., L.J., B.S.V.
- Methodology: L.S.S., C.J.Y., B.S.V.
- Project administration: B.S.V.
- Resources: L.S.S., C.J.Y., F.M.K., B.S.V.
- Software: L.S.S., C.J.Y., F.M.K., B.S.V.
- Supervision: B.S.V.
- Validation: L.S.S., C.J.Y., F.M.K., B.S.V.
- Visualization: L.S.S., C.J.Y., F.M.K., L.J., B.S.V.
- Writing - original draft: L.S.S., C.J.Y., B.S.V.
- Writing - review & editing: L.S.S., C.J.Y., F.M.K., L.J., B.S.V.
References
- 1.Committee on Practice Bulletins-Gynecology. Committee on Genetics Society of Gynecgologic Oncology: Practice Bulletin No 182: Hereditary breast and ovarian cancer syndrome. Obstet Gynecol. 2017;130:657–659. [Google Scholar]
- 2.Frank TS, Deffenbaugh AM, Reid JE, Hulick M, Ward BE, Lingenfelter B, et al. Clinical characteristics of individuals with germline mutations in BRCA1 and BRCA2: analysis of 10,000 individuals. J Clin Oncol. 2002;20:1480–1490. doi: 10.1200/JCO.2002.20.6.1480. [DOI] [PubMed] [Google Scholar]
- 3.Murray ML, Cerrato F, Bennett RL, Jarvik GP. Follow-up of carriers of BRCA1 and BRCA2 variants of unknown significance: variant reclassification and surgical decisions. Genet Med. 2011;13:998–1005. doi: 10.1097/GIM.0b013e318226fc15. [DOI] [PubMed] [Google Scholar]
- 4.Calò V, Bruno L, La Paglia L, Perez M, Margarese N, Di Gaudio F, et al. The clinical significance of unknown sequence variants in BRCA genes. Cancers (Basel) 2010;2:1644–1660. doi: 10.3390/cancers2031644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Culver JO, Brinkerhoff CD, Clague J, Yang K, Singh KE, Sand SR, et al. Variants of uncertain significance in BRCA testing: evaluation of surgical decisions, risk perception, and cancer distress. Clin Genet. 2013;84:464–472. doi: 10.1111/cge.12097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eccles DM, Mitchell G, Monteiro AN, Schmutzler R, Couch FJ, Spurdle AB, et al. BRCA1 and BRCA2 genetic testing-pitfalls and recommendations for managing variants of uncertain clinical significance. Ann Oncol. 2015;26:2057–2065. doi: 10.1093/annonc/mdv278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lindor NM, Goldgar DE, Tavtigian SV, Plon SE, Couch FJ. BRCA1/2 sequence variants of uncertain significance: a primer for providers to assist in discussions and in medical management. Oncologist. 2013;18:518–524. doi: 10.1634/theoncologist.2012-0452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Radice P, De Summa S, Caleca L, Tommasi S. Unclassified variants in BRCA genes: guidelines for interpretation. Ann Oncol. 2011;22(Suppl 1):i18–23. doi: 10.1093/annonc/mdq661. [DOI] [PubMed] [Google Scholar]
- 9.Pal T, Cragun D, Lewis C, Doty A, Rodriguez M, Radford C, et al. A statewide survey of practitioners to assess knowledge and clinical practices regarding hereditary breast and ovarian cancer. Genet Test Mol Biomarkers. 2013;17:367–375. doi: 10.1089/gtmb.2012.0381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Petrucelli N, Lazebnik N, Huelsman KM, Lazebnik RS. Clinical interpretation and recommendations for patients with a variant of uncertain significance in BRCA1 or BRCA2: a survey of genetic counseling practice. Genet Test. 2002;6:107–113. doi: 10.1089/10906570260199357. [DOI] [PubMed] [Google Scholar]
- 11.Vos J, Otten W, van Asperen C, Jansen A, Menko F, Tibben A. The counsellees' view of an unclassified variant in BRCA1/2: recall, interpretation, and impact on life. Psychooncology. 2008;17:822–830. doi: 10.1002/pon.1311. [DOI] [PubMed] [Google Scholar]
- 12.Richter S, Haroun I, Graham TC, Eisen A, Kiss A, Warner E. Variants of unknown significance in BRCA testing: impact on risk perception, worry, prevention and counseling. Ann Oncol. 2013;24(Suppl 8):i69. doi: 10.1093/annonc/mdt312. [DOI] [PubMed] [Google Scholar]
- 13.Garcia C, Lyon L, Littell RD, Powell CB. Comparison of risk management strategies between women testing positive for a BRCA variant of unknown significance and women with known BRCA deleterious mutations. Genet Med. 2014;16:896–902. doi: 10.1038/gim.2014.48. [DOI] [PubMed] [Google Scholar]
- 14.Finch AP, Lubinski J, Møller P, Singer CF, Karlan B, Senter L, et al. Impact of oophorectomy on cancer incidence and mortality in women with a BRCA1 or BRCA2 mutation. J Clin Oncol. 2014;32:1547–1553. doi: 10.1200/JCO.2013.53.2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.American Cancer Society. Ovarian cancer detailed guide [Internet] Atlanta, GA: American Cancer Society; c2016. [cited 2017 Feb 20]. Available from: https://www.cancer.org/cancer/ovarian-cancer/causes-risks-prevention/prevention.html. [Google Scholar]
- 16.American College of Obstetricians and Gynecologists; ACOG Committee on Practice Bulletins--Gynecology; ACOG Committee on Genetics; Society of Gynecologic Oncologists. ACOG Practice Bulletin No. 103: Hereditary breast and ovarian cancer syndrome. Obstet Gynecol. 2009;113:957–966. doi: 10.1097/AOG.0b013e3181a106d4. [DOI] [PubMed] [Google Scholar]
- 17.Walker JL, Powell CB, Chen LM, Carter J, Bae Jump VL, Parker LP, et al. Society of Gynecologic Oncology recommendations for the prevention of ovarian cancer. Cancer. 2015;121:2108–2120. doi: 10.1002/cncr.29321. [DOI] [PubMed] [Google Scholar]
- 18.Berek JS, Chalas E, Edelson M, Moore DH, Burke WM, Cliby WA, et al. Prophylactic and risk-reducing bilateral salpingo-oophorectomy: recommendations based on risk of ovarian cancer. Obstet Gynecol. 2010;116:733–743. doi: 10.1097/AOG.0b013e3181ec5fc1. [DOI] [PubMed] [Google Scholar]
- 19.Parker WH, Feskanich D, Broder MS, Chang E, Shoupe D, Farquhar CM, et al. Long-term mortality associated with oophorectomy compared with ovarian conservation in the nurses' health study. Obstet Gynecol. 2013;121:709–716. doi: 10.1097/AOG.0b013e3182864350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weiderpass E, Tyczynski JE. Epidemiology of patients with ovarian cancer with and without a BRCA1/2 mutation. Mol Diagn Ther. 2015;19:351–364. doi: 10.1007/s40291-015-0168-x. [DOI] [PubMed] [Google Scholar]
- 21.Robles-Díaz L, Goldfrank DJ, Kauff ND, Robson M, Offit K. Hereditary ovarian cancer in Ashkenazi Jews. Fam Cancer. 2004;3:259–264. doi: 10.1007/s10689-004-9552-0. [DOI] [PubMed] [Google Scholar]
- 22.Hartmann LC, Lindor NM. The role of risk-reducing surgery in hereditary breast and ovarian cancer. N Engl J Med. 2016;374:454–468. doi: 10.1056/NEJMra1503523. [DOI] [PubMed] [Google Scholar]
- 23.Plon SE, Eccles DM, Easton D, Foulkes WD, Genuardi M, Greenblatt MS, et al. Sequence variant classification and reporting: recommendations for improving the interpretation of cancer susceptibility genetic test results. Hum Mutat. 2008;29:1282–1291. doi: 10.1002/humu.20880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ready K, Gutierrez-Barrera AM, Amos C, Meric-Bernstam F, Lu K, Hortobagyi G, et al. Cancer risk management decisions of women with BRCA1 or BRCA2 variants of uncertain significance. Breast J. 2011;17:210–212. doi: 10.1111/j.1524-4741.2010.01055.x. [DOI] [PubMed] [Google Scholar]
- 25.Morgan R, Brown A, Hamman KJ, Sampson J, Naik A, Massimino K. Risk management decisions in women with BRCA1 and BRCA2 mutations. Am J Surg. 2018;215:899–903. doi: 10.1016/j.amjsurg.2018.02.010. [DOI] [PubMed] [Google Scholar]