Abstract
Objective
This study aims to evaluate the effects and pregnancy outcomes of gonadotropin-releasing hormone agonist (GnRH agonist) combined with aromatase inhibitor (AI) in preserving the fertility of obese women with grade 1 endometrial cancer (EC).
Methods
This study recruited obese EC patients who wished to preserve their fertility. The treatment regimen consisted of intramuscular GnRH agonist 3.75 mg every 4 weeks and oral AI 2.5 mg daily. The maintenance regimen was the same as the initial treatment regimen. Primary outcomes included response rate, time to complete response (CR), and time to recurrence; pregnancy outcomes included the time to pregnancy, pregnancy rate and live birth rate.
Results
Six obese patients with EC were included in this study, with the age (mean±standard deviation [SD]) of 30.5±3.3 years and body mass index (mean±SD) of 35.0±1.4 kg/m2. CR rate was 100%, and time to CR was 3–6 months. None of the patients had recurrence after a median follow-up of 4.0 years (range, 1.3–7.0 years). The most common side effects were menopause-like symptoms. Among these patients, no weight gain was observed during treatment. The pregnancy rate and live birth rate was 50.0% and 75.0%, respectively, with a median time to pregnancy of 2.4 years (range, 1.0–5.5 years).
Conclusion
The combination of GnRH agonist and AI demonstrated promising long-term effect in young obese EC patients who wished to preserve their fertility. No weight gain side effects were observed. Further studies with a larger sample size are needed to fully evaluate this novel treatment regimen.
Keywords: Endometrial Cancer, Obesity, Organ Sparing Treatments, Gonadotropin-Releasing Hormone, Aromatase Inhibitors
INTRODUCTION
Endometrial carcinomas (EC) that were diagnosed before the age 40 comprise approximately 5% of all endometrial carcinomas [1,2]. EC in young women is associated with unopposed estrogen exposure. Obesity, infertility, chronic anovulation, and polycystic ovarian syndrome (PCOS) are commonly seen in young women with EC [2]. As young EC patients may have the desire to preserve their fertility, conservative treatment with oral progesterone is sometimes provided. Several cohort studies have proved the clinical efficacy and safety of the oral progesterone approach, which includes medroxyprogesterone acetate (MPA) and megestrol acetate (MA) at various doses [3]. However, progesterone also had side effects including weight gain, incontrollable hyperglycemia, and compromised liver function. These side effect limited the application of high doses of progesterone, especially in obese patients [4]. Obesity has rapidly increased in developing countries including China in the past 2 decades [5]. Obesity is a strong risk factor for developing EC, and the mortality in obese EC patients is six times higher than in normal weight EC patients (risk ratio=6.25; 95% confidence interval [CI]=3.75–10.42). Furthermore, obese patients had lower pregnancy rate and longer time to conceive compared with their non-obese counterparts after fertility-sparing management and it also takes longer time for obese women to conceive than for normal weight women [6]. To shorten the time to complete response (CR) will allow more time to attempt pregnancy [7]. In addition, it is desirable if the treatment of EC do not lead to weight gain in obese patients.
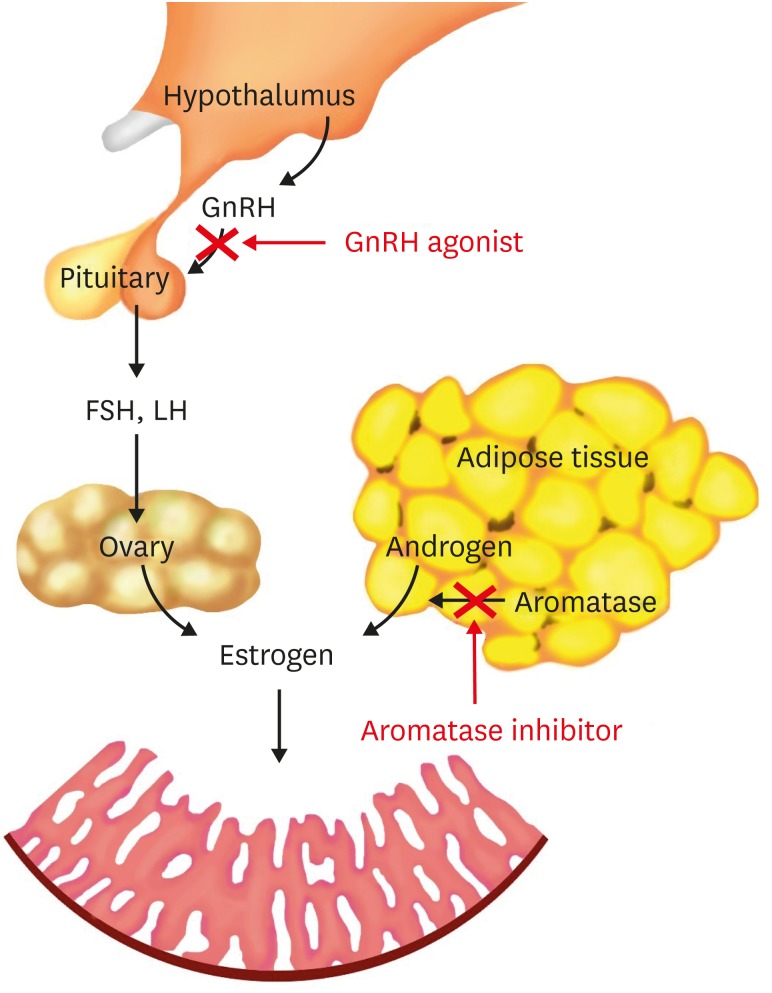
In obese patients with endometrial cancer, estrogens are either synthesized by ovaries or are converted from androgens peripherally. Suppressing the production of estrogen from both ovary and peripheral tissue should be effective for treating EC in obese patients (Fig. 1). Gonadotropin-releasing hormone agonist (GnRH agonist) helps to maintain a low level of estrogen over time by suppressing the secretion of follicle-stimulating hormone and luteinizing hormone, and has been increasingly used in EC recently [4,8,9]. The peripheral conversion of androgens to estrogens is the major source of excess estrogens in obese EC patients [10]. The aromatase, which is a cytochrome P450 enzyme and plays an important role in the conversion of androstenedione and testosterone to estrone and estradiol, was found in the adipose tissue [11]. The aromatase inhibitors (AIs) decrease the peripheral conversion of androgens to estrogens, thus decrease the level of circulating estrogens [10] (Fig. 1). It is reasonable to hypothesize that GnRH agonist combined with AI would be effective in obese EC patients who wish to preserve their fertility. This pilot study provided preliminary results of obese EC patients with the conservative treatment of GnRH agonist and AI.
Fig. 1. The main origin of estrogen in obese patients with EC and the possible pathological mechanism of the combined treatment of GnRH agonist and AI.
AI, aromatase inhibitor; EC, endometrial carcinoma; GnRH agonist, gonadotropin-releasing hormone agonist.
MATERIALS AND METHODS
This study recruited young obese EC patients that were admitted between 2010 and 2015 at the Department of Obstetrics and Gynecology, Peking Union Medical College Hospital. The patients had been followed up till May 2018. This study has been approved by the Internal Review Board of Peking Union Medical College Hospital (No. S-K656).
The inclusion criteria were 1) the pathological diagnosis of grade 1 endometrial carcinoma was confirmed independently by 2 pathologists; 2) patient were younger than 40 years old [6,9] and had strong desire to preserve her fertility; 3) trans-vaginal ultrasound and magnetic resonance imaging (MRI) excluded the possibility of myometrium invasion; 4) patient's body mass index (BMI) was greater than 30 kg/m2; and 5) patient consented to the treatment of GnRH agonist with AI.
1. The treatment regimen of GnRH agonist and AI
All participants received treatment regimen included intramuscular injection of 3.75 mg of GnRH agonist (triptorelin) every 4 weeks and oral AI (letrozole) 2.5 mg daily. The efficacy of treatment was evaluated by trans-vaginal ultrasound every three months and endometrial sampling via dilatation and curettage (D&C) every 3 months.
2. Outcomes
A complete response (CR) was defined as EC regression to benign endometrium. A partial response (PR) was defined as the regression of EC to hyperplasia or atypia. Disease persistence (DPer) was used to describe grade 1 EC persistence. Disease progression (Dpro) was defined as that during treatment, the pathology developed from grade 1 adenocarcinoma to grade 2 or 3 adenocarcinoma, or that trans-vaginal ultrasound and MRI revealed myometrium invasion. Disease recurrence (DR) was defined as that pathological results showed atypical hyperplasia (AH) or EC after remission had been achieved. The primary outcomes included the response rate, the time to CR, and the time to recurrence.
3. Maintenance treatment after CR
For patients with CR, maintenance treatment of GnRH agonist and AI was used for 3 to 6 months. During maintenance treatment, trans-vaginal ultrasound was performed every 3 months and endometrial sampling was performed every 3 months via D&C.
4. Other response
Patients with DPer were advised to receive surgery. Patients that rejected surgery either continued GnRH agonist and AI treatment or received oral MA/MPA 320 mg daily. The outcomes were assessed after another 3 months. Patients who had DPer or Dpro after 12 months of hormone therapy were considered to have failed fertility-preserving treatment [13]. Patients who failed fertility-preserving treatments underwent standard staging surgery for endometrial cancer.
5. Follow up of the patients
During the treatment, patients were asked to come to the clinic every 3 months for a follow up evaluation. During each follow up, the efficacy of treatment was evaluated by trans-vaginal ultrasound as well as endometrial sampling via D&C. Other measurements and tests include body weight, blood pressure, complete blood cell count, CA125, liver function and kidney function, and fasting blood glucose in diabetic patients. Patients were also inquired if they had abnormal vaginal bleeding and menopause-like symptoms. During maintenance treatment and after the termination of treatment, patients were also followed up every 3 months with the assessment of trans-vaginal ultrasound.
6. Pregnancy outcomes
During the treatment and maintenance period, each patient was recommended for life style intervention to lose weight. After maintenance, the patients who achieved CR were encouraged to attempt conception. To facilitate conception, patients were referred to see a reproductive specialist. Time to pregnancy, pregnancy and live birth rate were used as pregnancy outcomes.
RESULTS
From 2010 to 2015, 6 obese EC patients participated in this study. The characteristics of the participants were shown in Table 1. The mean and standard deviation of age was 30.5±3.3 years and the BMI was 35.0±1.4 kg/m2.
Table 1. Patient characteristics.
| Characteristics | Values | |
|---|---|---|
| Age (yr) | 30.5±3.3 | |
| BMI (kg/m2) | 35.0±1.4 | |
| Nulliparity | 5 | |
| Comorbidity | 0 | |
| Diabetic mellitus | 2 | |
| Hypertension | 1 | |
| Polycystic ovary syndrome | 4 | |
| History of progesterone treatment | 4 | |
Values are expressed as mean±standard deviation or number.
1. Clinical efficacy
The detailed treatments and outcomes of enrolled patients were shown in Table 2. Patients #1–3 were diagnosed with endometrial adenocarcinoma, and patients #4–6 were diagnosed with severe atypical hyperplasia with localized endometrial adenocarcinoma. Four out of the six patients had histories of progesterone treatment before the initiation of GnRH agonist and AI treatments. These patients' comorbidities were also shown in Table 2.
Table 2. Treatments and outcomes of the patients.
| Patient No. | BMI (kg/m2) | Past treatment | Adverse effects of past treatment/comorbidities | Pathology before triptorelin+letrozole | Evaluation at 3 months | Time to CR (mo) | Length of maintenance (mo) | Method of maintenance | Disease progression | Follow-up length (yr)* | Recurrence |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 33.6 | MPA 2 years 9 months 500 mg qd, progress from AH to EC after more than 2 years of MPA | Elevated ALT, PCOS | EC | CR | 3 | 6 | GnRHa and AI | No | 6.0 | No |
| 2 | 37.0 | - | Diabetes mellitus, PCOS | EC | CR | 3 | 6 | GnRHa and AI | No | 5.0 | No |
| 3 | 33.2 | MA 3 months 160 mg bid, Dper after 3 months of MA | - | EC | DPer | 6 | 3 | GnRHa and AI | No | 3.0 | No |
| 4 | 35.7 | MA 1 month 160 mg qd | PCOS | AH+EC | CR | 3 | 6 | GnRHa and AI | No | 2.5 | No |
| 5 | 35.0 | MA 3 months 320 mg qd | Hyperglycemia after 3 months of MA | AH+EC | CR | 3 | 6 | GnRHa and AI | No | 2.0 | No |
| PR after 3 months of MA | |||||||||||
| 6 | 35.7 | - | Hypertension, PCOS | AH+EC | CR | 3 | 6 | GnRHa and AI | No | 1.0 | No |
AH, atypical hyperplasia; AI, aromatase inhibitor; ALT, alanine aminotransferase; bid, twice a day; BMI, body mass index; CR, complete response; Dper, disease persistence; EC, endometrial carcinoma; GnRHa, Gonadotropin-releasing hormone agonist; MA, megestrol acetate; MPA, medroxyprogesterone acetate; PCOS, polycystic ovarian syndrome; PR, partial response; qd, every day.
*The follow-up length is the interval from termination of treatment till the last outpatient follow-up.
The response rate was 100% and all of the patients achieved CR within 3–6 months. Five (83.3%) patients achieved CR after 3 months, another patient (#3) achieved CR at 6 months. The pathological change of the responded patients was mainly glandular atrophy, cytoplasmic changes including squamous, and eosinophilic metaplasia and normal endometrium tissue.
During the 3–6 months' maintenance treatment period, no progression of the disease was detected in any of the six patients. After termination of treatment, dydrogesterone 10 mg twice a day was used till the patients started the treatment of ovulation induction. No patients experienced recurrence during a median follow-up of 4.0 years (range, 1.3–7.0 years).
2. Conception outcomes
As shown in Table 3, 3 (50%) of the patients with remission conceived a total of 4 times, after a median time to pregnancy of 2.4 years (range, 1.0–5.5 years). Patient #1 had 2 live births, with 1 conceived 2 months after ovulation induction, and the other 1 conceived 4 months after ovulation induction. Patient #2 had 1 live birth, which was conceived naturally after attempting pregnancy for 2 months. Patient #2 also had a history of 4 sessions of failed in vitro fertilization and embryo transfer (IVF-ET). Patient #4 conceived after IVF-ET, but finally had a miscarriage. Therefore, the live birth rate was 75% (3 out of 4 pregnancies). At the end of the follow up, patient #2 was planning for a second child and refused to have surgery; patient #1 was in her lactation period.
Table 3. The pregnancy outcome of the patients who had complete response.
| Patients' No. | Viable pregnancy | IVF-ET | Time to pregnancy (yr) | Fertilization method for the pregnancy | Outcome |
|---|---|---|---|---|---|
| 1 | Yes | No | 1.0 and 5.5 | Ovulation induction | Full term CS of both pregnancies |
| 2 | Yes | Failed 4 times | 3.3 | Natural conception | Full term CS |
| 4 | Yes | One time | 1.5 | IVF-ET | Miscarriage |
Time to pregnancy: the interval from termination of treatment to date of last menstrual period of the pregnancy.
CS, cesarean section; IVF-ET, in vitro fertilization and embryo transfer.
3. Adverse effects
All patients experienced menopause-like symptoms such as hot flashes and mood swings. Estradiol valerate of 1 mg every other day or Remifemin® (Schaper & Brummer, GmBH, Salzgitter, Germany) of 0.28 g twice a day was provided to relieve these discomforts. All patients had good tolerance of the menopause-like symptoms. No patient had weight gain or compromised liver function during the treatment.
DISCUSSION
The preliminary results of this pilot study indicate that a combination of GnRH agonist and AI is effective in young obese EC patients with a 100% response rate. The time to CR was 3–6 months and there was no recurrence during a median follow-up of 4 years. The pregnancy rate and live birth rate was 50% and 75%, respectively, with a median time to pregnancy of 2.4 years.
For obese EC patients who wish to preserve their fertility, it is important to shorten their time to achieve remission. A shorter time to CR will lead to a higher probability to conceive, especially for EC patients who are approaching advanced age or wish to have a second child [10]. In our study, it took only 3 months for 5 of the 6 patients to achieve CR, and 6 months for another 1 patient to achieve CR. In the literature, most EC studies reported the conservative treatments of progesterone regimen. The time to obtain a CR after progesterone regimen was 6 months [14]. A histologic study also indicated that evaluation of the responses to progesterone therapy should be performed at least 6 months after the therapy [15]. The overall reported response rate to progesterone therapy was only 65% [16]. The response rate of the GnRH agonist and AI regimen was surprisingly high (i.e. 100%) in the present study. No recurrence was observed in our study following the treatments of GnRH agonist and AI during a median follow-up length of 4 years; this allows more time for patients to conceive. On the contrary, the recurrence rate of progesterone treatment was as high as 20%–46% [10,17]. In addition, the application of progesterone treatment is always limited to its adverse effects such as weight gain, especially in obese patients [4]. The result of this study indicated that the combination of GnRH agonist and AI did not increase body weight and this is also beneficial for future pregnancy.
Compared with previous studies that adopted progesterone regimen in young obese EC patients, the treatment regimen of GnRH agonist and AI might have shorter interval to CR, higher response rate. However, with the small sample size of the present study, it is premature to conclude that the best intervention for the fertility-sparing management of obese EC patients is the regimen of GnRH agonist and AI. More studies on this novel regimen need to be conducted.
According to the result of this study, by suppressing the production of estrogen from both ovary and peripheral tissue, the endometria mainly showed atrophic or metaplastic changes. While the histologic changes after progesterone treatment included a decreased gland-to-stroma ratio, stromal hyperplasia with decidual transformation [16,18]. These changes were not seen in our responded patients. More studies were needed to learn the pathological feature after the treatment of GnRH agonist and AI and its difference from that of progesterone regimen.
Regarding pregnancy rate, it was not always easy to know how many women were actively trying to conceive after remission. The reported pregnancy rate after conservative treatment of EC varies widely, ranging from 7%–42% [3,19]. The live birth rate was reported to be 11%–28%. [17,20] It was believed that the majority of successful pregnancies were the results of assisted reproductive technology [3]. The pregnancy/live birth rate was higher in this study than in previous studies that adopted progesterone treatment. In addition, none of the live births in this study are the results of IVF-ET. This might be partially explained by the relative small number of patients included in the present study. In addition, previous progesterone studies reported weight gain side effects. As obesity has a negative impact on fertility (i.e. a lower pregnancy rate and a longer time to pregnancy), our high pregnancy rate may also be partially explained by the lack of weight gain in our cohort [6,7]. The ultimate goal of fertility-sparing treatment of EC is for patients to have live births. As conservative management only provides patients a relatively short window to conceive [3], it is reasonable for patients to accept any facilitation to conceive after CR. Currently conservative treatment is not recommended for patients who expect to achieve CR but do not have the desire to conceive.
The impact of obesity on the effectiveness of conservative treatment of EC and fertility has rarely been assessed. Gonthier et al. [6] included 40 EC/AH patients in a retrospective study and demonstrated similar response/recurrence rates (the rate were insignificantly lower in obese patients) and a lower rate of pregnancy in obese patients after progesterone treatment (1 patient combined progesterone with GnRH agonist, another patient combined with Levonorgestrel IUD). The pregnancy rate at year 1 and year 3 was 10%–25% [6]. Burnett et al. [10] reported that two obese EC patients received the treatment of AI and medroxyprogesterone acetate. It took 3 to 6 months for the patients to achieve CR. The present study for the first time provided preliminary evidence that inhibiting estrogen from both peripheral and ovary origin might be more effective than anti-estrogen treatment alone in obese EC patients.
This combined regimen could be utilized either as a primary treatment for obese EC patients or as a secondary treatment after utilizing oral progesterone. The time to CR was similar for patients with and without a history of progesterone treatment according to our results. We recommended 3–6 months of maintenance treatment to the patients, and most of them accepted 6 months of maintenance treatment. The maintenance regimen was recommended to be the same as the initial treatment. In future studies, we would like to explore that after a 3-month CR, whether the 3-month interval of D&C could be extended to 6 months in the combined treatment. As the endothelium was extremely precious for future conception, invasive evaluation should be performed minimally during the treatment. It would be ideal if we could increase the interval of D&C evaluation and minimize the times of D&C evaluation without compromising the safety. Other methods that may increase live birth rates may also need to be investigated and evaluated.
One limitation of the study is that we did not evaluate bone mineral density during the treatment of GnRH. Although measures were taken to alleviate patients' menopause-like symptoms, it is crucial to assess the risk of osteoporosis during a long-term treatment of GnRH agonist or AI. This study is also limited by the small sample size. Due to the anticipated small number of young obese EC patients, a control group was not included. However, the long-term preliminary results of the combination regimen were encouraging with good clinical outcomes and acceptable adverse effects. Furthermore, there is a rationale to use a combination of GnRH agonist and AI in treating EC. The result of this study indicated that this combined treatment might be promising as a conservative treatment for young EC obese patients. More research on this combined treatment should be conducted in a large sample of obese EC patients and in randomized clinical trials.
In conclusion, this pilot study showed promising preliminary results of a novel conservative treatment of GnRH agonist and AI in young obese patients with EC. Comparing to previous studies that used other treatment regimen, this novel treatment seemed to lead a shorter time to CR (i.e. 3 months in 83% patients) and a higher pregnancy rate (i.e. 50%) and a higher live birth rate (i.e. 75%). This novel regimen need to be further evaluated in future randomized clinical trials.
Footnotes
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
- Conceptualization: H.H.
- Data curation: H.H., Z.Z.
- Formal analysis: Z.Z., C.N.
- Investigation: C.N.
- Methodology: H.H., C.N.
- Project administration: C.N.
- Resources: H.H., F.F., W.J., C.N.
- Writing - original draft: Z.Z., C.N.
- Writing - review & editing: Z.Z., W.J., C.N.
References
- 1.Creasman WT, Odicino F, Maisonneuve P, Beller U, Benedet JL, Heintz AP, et al. Carcinoma of the corpus uteri. J Epidemiol Biostat. 2001;6:47–86. [PubMed] [Google Scholar]
- 2.Navarria I, Usel M, Rapiti E, Neyroud-Caspar I, Pelte MF, Bouchardy C, et al. Young patients with endometrial cancer: how many could be eligible for fertility-sparing treatment? Gynecol Oncol. 2009;114:448–451. doi: 10.1016/j.ygyno.2009.05.038. [DOI] [PubMed] [Google Scholar]
- 3.Simpson AN, Feigenberg T, Clarke BA, Gien LT, Ismiil N, Laframboise S, et al. Fertility sparing treatment of complex atypical hyperplasia and low grade endometrial cancer using oral progestin. Gynecol Oncol. 2014;133:229–233. doi: 10.1016/j.ygyno.2014.02.020. [DOI] [PubMed] [Google Scholar]
- 4.Zhou H, Cao D, Yang J, Shen K, Lang J. Gonadotropin-releasing hormone agonist combined with a levonorgestrel-releasing intrauterine system or letrozole for fertility-preserving treatment of endometrial carcinoma and complex atypical hyperplasia in young women. Int J Gynecol Cancer. 2017;27:1178–1182. doi: 10.1097/IGC.0000000000001008. [DOI] [PubMed] [Google Scholar]
- 5.Sun M, Feng W, Wang F, Li P, Li Z, Li M, et al. Meta-analysis on shift work and risks of specific obesity types. Obes Rev. 2018;19:28–40. doi: 10.1111/obr.12621. [DOI] [PubMed] [Google Scholar]
- 6.Gonthier C, Walker F, Luton D, Yazbeck C, Madelenat P, Koskas M. Impact of obesity on the results of fertility-sparing management for atypical hyperplasia and grade 1 endometrial cancer. Gynecol Oncol. 2014;133:33–37. doi: 10.1016/j.ygyno.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 7.Wise LA, Rothman KJ, Mikkelsen EM, Sørensen HT, Riis A, Hatch EE. An internet-based prospective study of body size and time-to-pregnancy. Hum Reprod. 2010;25:253–264. doi: 10.1093/humrep/dep360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moroni RM, Martins WP, Ferriani RA, Vieira CS, Nastri CO, Candido Dos Reis FJ, et al. Add-back therapy with GnRH analogues for uterine fibroids. Cochrane Database Syst Rev. 2015:CD010854. doi: 10.1002/14651858.CD010854.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Minig L, Franchi D, Boveri S, Casadio C, Bocciolone L, Sideri M. Progestin intrauterine device and GnRH analogue for uterus-sparing treatment of endometrial precancers and well-differentiated early endometrial carcinoma in young women. Ann Oncol. 2011;22:643–649. doi: 10.1093/annonc/mdq463. [DOI] [PubMed] [Google Scholar]
- 10.Burnett AF, Bahador A, Amezcua C. Anastrozole, an aromatase inhibitor, and medroxyprogesterone acetate therapy in premenopausal obese women with endometrial cancer: a report of two cases successfully treated without hysterectomy. Gynecol Oncol. 2004;94:832–834. doi: 10.1016/j.ygyno.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 11.Gao C, Wang Y, Tian W, Zhu Y, Xue F. The therapeutic significance of aromatase inhibitors in endometrial carcinoma. Gynecol Oncol. 2014;134:190–195. doi: 10.1016/j.ygyno.2014.04.060. [DOI] [PubMed] [Google Scholar]
- 12.Barker LC, Brand IR, Crawford SM. Sustained effect of the aromatase inhibitors anastrozole and letrozole on endometrial thickness in patients with endometrial hyperplasia and endometrial carcinoma. Curr Med Res Opin. 2009;25:1105–1109. doi: 10.1185/03007990902860549. [DOI] [PubMed] [Google Scholar]
- 13.Ushijima K, Yahata H, Yoshikawa H, Konishi I, Yasugi T, Saito T, et al. Multicenter phase II study of fertility-sparing treatment with medroxyprogesterone acetate for endometrial carcinoma and atypical hyperplasia in young women. J Clin Oncol. 2007;25:2798–2803. doi: 10.1200/JCO.2006.08.8344. [DOI] [PubMed] [Google Scholar]
- 14.Imai M, Jobo T, Sato R, Kawaguchi M, Kuramoto H. Medroxyprogesterone acetate therapy for patients with adenocarcinoma of the endometrium who wish to preserve the uterus-usefulness and limitations. Eur J Gynaecol Oncol. 2001;22:217–220. [PubMed] [Google Scholar]
- 15.Wheeler DT, Bristow RE, Kurman RJ. Histologic alterations in endometrial hyperplasia and well-differentiated carcinoma treated with progestins. Am J Surg Pathol. 2007;31:988–998. doi: 10.1097/PAS.0b013e31802d68ce. [DOI] [PubMed] [Google Scholar]
- 16.Gunderson CC, Dutta S, Fader AN, Maniar KP, Nasseri-Nik N, Bristow RE, et al. Pathologic features associated with resolution of complex atypical hyperplasia and grade 1 endometrial adenocarcinoma after progestin therapy. Gynecol Oncol. 2014;132:33–37. doi: 10.1016/j.ygyno.2013.11.033. [DOI] [PubMed] [Google Scholar]
- 17.Gallos ID, Yap J, Rajkhowa M, Luesley DM, Coomarasamy A, Gupta JK. Regression, relapse, and live birth rates with fertility-sparing therapy for endometrial cancer and atypical complex endometrial hyperplasia: a systematic review and metaanalysis. Am J Obstet Gynecol. 2012;207:266.e1–266.12. doi: 10.1016/j.ajog.2012.08.011. [DOI] [PubMed] [Google Scholar]
- 18.Deligdisch L. Hormonal pathology of the endometrium. Mod Pathol. 2000;13:285–294. doi: 10.1038/modpathol.3880050. [DOI] [PubMed] [Google Scholar]
- 19.Dursun P, Erkanli S, Güzel AB, Gultekin M, Tarhan NC, Altundag O, et al. A Turkish Gynecologic Oncology Group study of fertility-sparing treatment for early-stage endometrial cancer. Int J Gynaecol Obstet. 2012;119:270–273. doi: 10.1016/j.ijgo.2012.06.010. [DOI] [PubMed] [Google Scholar]
- 20.Kim MK, Yoon BS, Park H, Seong SJ, Chung HH, Kim JW, et al. Conservative treatment with medroxyprogesterone acetate plus levonorgestrel intrauterine system for early-stage endometrial cancer in young women: pilot study. Int J Gynecol Cancer. 2011;21:673–677. doi: 10.1111/IGC.0b013e3181fd9a06. [DOI] [PubMed] [Google Scholar]