Abstract
Objective
To report hysteroscopic treatment combined with levonorgestrel-releasing intrauterine device (LNG-IUD) to treat women with early well differentiated endometrial cancer (EC) at high surgical risk.
Methods
Nine women diagnosed with stage IA, grade 1 endometrioid EC which was contraindicated or refused standard treatment with external beam radiation therapy with or without brachytherapy were enrolled in our prospective study. Endo-myometrial hysteroscopic resection of the whole uterine cavity and the placement of LNG-IUD for 5 years was performed. Response rate, perioperative complications, and recurrence of disease were evaluated.
Results
None had intra or post-operative complications and all were discharged no later than the third day of hospitalization. After 6 months from surgery, all the women showed a complete regression of the lesion. All the women completed the 5 years follow-up and in no case was detected sign of recurrence. Two women died for causes unrelated to the tumor or the ongoing therapy.
Conclusion
The alternative treatment with endo-myometrial hysteroscopic resection and LNG-IUD in women with stage IA, grade 1 endometrioid EC showed initial encouraging outcomes in terms of effectiveness and safety.
Keywords: Endometrial Cancer, Hysteroscopic Surgery, Obesity, High Surgical Risk
INTRODUCTION
Endometrial cancer (EC) is the most common malignancy of female genital tract in developed countries [1]. Because of the early onset of symptoms, bleeding, the majority of cases is diagnosed early (80% in stage I) [2] and with the standard surgery of total abdominal hysterectomy and bilateral salpingo-oophorectomy with lymph-node assessment a 5-year survival rate above 95% has been reported [3].
On the other hand, the increasing mean age of the population, the presence of various medical comorbidities and obesity are more and more frequently severe contraindications to standard surgical treatment [4,5]. In these inoperable women, EC treatment is still a challenge due to the lack of unequivocal management guidelines [6]. Number of therapeutic strategies have been adopted over the years, such as definitive radiation with brachytherapy alone or external beam radiation therapy (EBRT) combined with brachytherapy [7], the use of progestin therapy with high-dose medroxyprogesterone acetate (MPA) or megestrol acetate (MA) limited to pilot studies [8]. More recently, the additional benefit from a weight loss programme associated with metformin and levonorgestrel-releasing intrauterine device (LNG-IUD) was reported [9].
Recently some authors [1,10,11,12] adopted conservative therapeutic strategies with encouraging outcomes in women with EC of childbearing age and desiring pregnancies. In postmenopausal conditions, only 1 case was reported [13] about a woman with well differentiated EC that refusing hysterectomy was treated with hysteroscopic endometrial resection and did not present any recurrence at 5 years follow-up.
In this study, we adopted for the first time a new therapeutic strategy consisting of a hysteroscopic endo-myometrial resection of the uterine cavity and the positioning of adjuvant local hormone therapy for the treatment of a group of postmenopausal women with well differentiated EC who presented a high perioperative risk and who refused or were unable to undergo standard radiation therapy.
MATERIALS AND METHODS
1. Subjects
Between December 2009 and November 2015, 288 women with postmenopausal bleeding were evaluated at our tertiary center and diagnosed with an early stage well differentiated EC by subjecting them to a diagnostic hysteroscopy with a 5 mm hysteroscope with a 30° forward oblique lens (Office Hysteroscope; Karl Storz, Tuttlingen, Germany), and targeted multiple endometrial biopsies with a 5 Fr instruments. Forty women were assessed by experienced anesthesiologists and scheduled as “high-risk patient” according to Boyd and Jackson [14] definition and assigned to American Society of Anesthesiologists class III. These women were adequately counselled to standard radiotherapy treatment according to specific guidelines [3], but 9 of them were considered unsuitable not only for the standard surgical treatment but also for the standard radiotherapy. The clinical characteristics of the treated women and the risk conditions that have contraindicated the surgical treatment are summarized in Table 1. In these patients, the risk-benefit of the surgical procedure was carefully evaluated and those were taken into consideration such as possible post-operative complications, possible lengthening of hospitalization, and possible increase in health costs. Two women previously had radiotherapy for colorectal cancer and a second treatment was contraindicated [15], 1 had severe obesity (body mass index [BMI] >44) that radiotherapy could be ineffective at the expense of increased toxicity, and 6 refused the therapy.
Table 1. Characteristics of the 9 “high-risk” treated women.
| Case | Age (yr) | BMI (kg/m2) | Surgical high-risk factors | Contraindication to RT | Definitive histology |
|---|---|---|---|---|---|
| 1 | 85 | 33 | Previous myocardial infarction; AF | Refusal | EEA G1 with 2 mm MI |
| 2 | 57 | 30 | OSAS; RF | Refusal | EEA G1 |
| 3 | 74 | 29 | Mitochondrial degenerative disease | Prior pelvic RT (CRC) | EEA G1 |
| 4 | 69 | 34 | Previous stroke | Refusal | EEA G1 with 2 mm MI |
| 5 | 66 | 30 | Previous myocardial infarction; recurrent thromboembolism | Refusal | EEA G1 |
| 6 | 64 | 44 | COPD with RF | Severe obesity | EEA G1 |
| 7 | 58 | 31 | Cardiac shunt | Refusal | EEA G1 |
| 8 | 83 | 40 | CRF; DMII | Refusal | EEA G1 with 1 mm MI |
| 9 | 70 | 31 | Heart transplantation | Prior pelvic RT (CRC) | EEA G1 |
AF, atrial fibrillation; BMI, body mass index; CRC, colo-rectal cancer; COPD, chronic obstructive pulmonary disease; CRF, chronic renal failure; DMII, diabetes mellitus of type 2; EEA, endometrioid endometrial adenocarcinoma; G1, grade 1; MI, myometrial infiltration; OSAS, obstructive sleep apnea syndrome; RF, respiratory failure; RT, radiotherapy.
2. Diagnostic evaluation
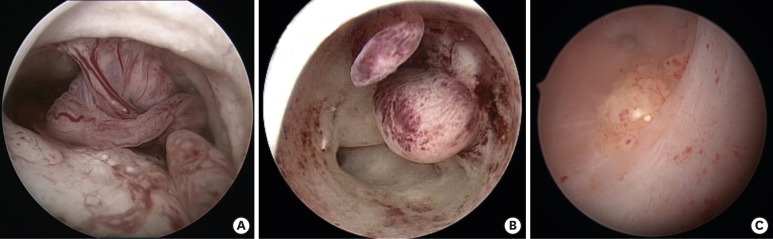
In 9 women who entered this pilot study, diagnostic hysteroscopy showed a uterine cavity completely coated by polypoid endometrium with atypical growth in 4 cases (Fig. 1A), 3 atypical polypoid lesions in a context of an irregular endometrium (Fig. 1B), and 2 atypical exophytic focal lesions in an atrophic endometrium context (Fig. 1C). All samples obtained from biopsy were sent to the same dedicated pathologist (G.C.), who revealed a well differentiated EC of type 1 not extended to isthmus or cervical area (International Federation of Gynecology and Obstetrics [FIGO] stage IA). The location of the suspected lesion, its relation with the uterine cavity, its characteristics and remaining endometrium were also evaluated. According to Colombo et al. [3], a specialized transvaginal ultrasound (TVS) was used to evaluate tumor size, exclude ovarian disease, assess myometrial invasion, and cervical stromal involvement. Enhanced pelvic magnetic resonance imaging (MRI) was then performed to complete evaluation about myometrial infiltration. TVS and MRI confirmed stage IA for each patient (no myometrial invasion, no cervical invasion). Furthermore, computed tomography scan revealed the absence of metastases to pelvic and/or para-aortic lymph nodes and distant metastases in all cases.
Fig. 1. (A) Increased endometrial thickness and evidence of diffuse polypoid endometrium with images of hyperplastic growth and widespread atypical vascularization. (B) Pleiomorphic polypoid lesions with friable surface and phenomena of angiogenesis in a context of irregular endometrium. (C) Polypoid area with pseudohyperplastic growth similar to a “seaweed pattern”: individual papillae and vascularization in each of them. The lesion is surrounded by hypo-atrophic endometrium.
A diagnostic-therapeutic pathway by endo-myometrial resectoscopic ablation and application of LNG-IUD (Mirena®; Bayer Healthcare Pharmaceuticals, Pittsburgh, PA, USA) was proposed, and the women agreed to perform a regular and long-term follow-up based on TVS and diagnostic hysteroscopy with biopsies during follow-up. Full ethics approval was obtained from our local Ethics Committee. Appropriate informed consent for surgery and all other investigations was therefore obtained by specifying the lack of scientifically validated data on alternative treatments and the importance of close follow up. Inclusion criteria for hysteroscopic treatment in surgical high-risk women with EC are listed in Table 2.
Table 2. Inclusion criteria for hysteroscopic treatment in high-risk women with EC.
| EC G1 |
|---|
| No evidence of metastatic disease |
| No evidence of suspicious adnexal mass |
| Absence of lymphadenopathy |
| No history of HNPCC/Lynch II syndrome |
| No contraindication to medical treatment |
| Absence of LVSI at post-operative histology |
| No evidence of myometrial invasion (more than 3 mm) at post-operative histology |
EC, endometrial cancer; G1, grade 1; HNPCC, hereditary non-polyposis colorectal cancer; LVSI, lymph-vascular space invasion.
3. Surgery
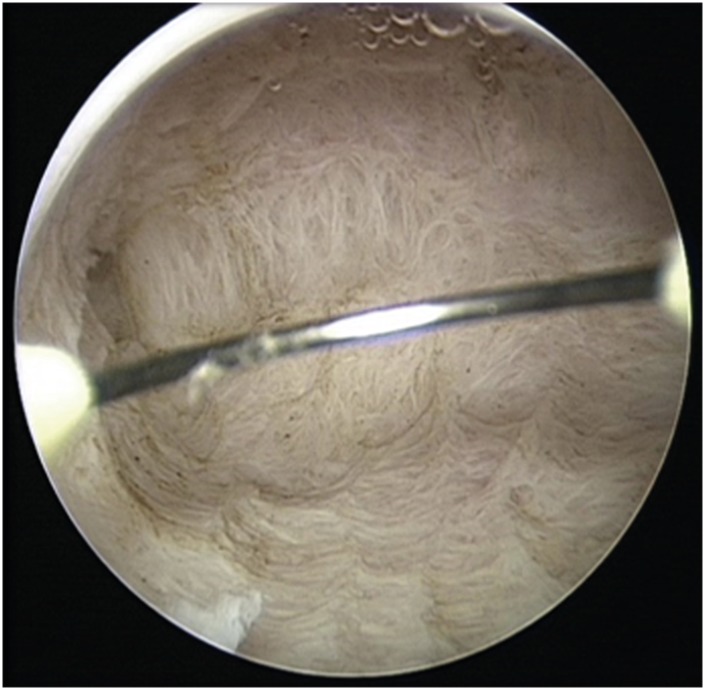
An operative hysteroscopy was performed in all cases by the same surgeon who performed the diagnostic one. The women underwent spinal anesthesia; a 10 mm cervical dilatation was performed with Hegar dilators, and a 26 Fr resectoscope (Karl Storz) with a 0° lens was introduced. The uterus was distended with Mannitol 0.54%–Sorbitol 2.7% 3,000 mL Urologic Irrigating (Baxter Healthcare Corporation, Rome, Italy). Intrauterine pressure was automatically controlled (80–100 mmHg) by an electronic irrigation and suction device (Hamou Endomat Irrigation Suction Pump; Karl Storz). The entire endometrium (uterine walls, tubal ostia, and uterine fundus) and the first 5 mm of the underling myometrium were cautiously resected with a 5-mm cutting loop electrode and 100 W of pure cutting output power by minimally invasive techniques (Fig. 2). The lining epithelium of the cervical canal was spared by resection in order to make the uterine cavity explorable during the hysteroscopic follow-up. Additional myometrial biopsies were taken from the endocervix. No cases of intravasation have been recorded. At the end of the hysteroscopic procedure, a LNG-IUD was inserted.
Fig. 2. Appearance of endo-myometrial resection of the whole uterine cavity at the end of the hysteroscopic procedure. There should be no areas of residual endometrium.
The different samples from cancer, contralateral walls and endocervix were collected separately and sent to the same dedicated pathologist. Definitive histology confirmed biopsies and revealed a very focal superficial myometrial invasion in 3 cases (2 mm in cases 1 and 4, 1 mm in case 8). The contralateral resected endometrium/myometrium and endocervical biopsies were negative for malignancy in all cases.
RESULTS
The women had a mean±standard deviation (SD) age of 69±9 years and BMI of 33±5 kg/m2. All were discharged no later than the third of hospitalization with no side-effects and complications. Median duration of hospitalization was 2 days (range, 1–3 days). The women were followed for the first year every 3 to 9 months with diagnostic hysteroscopy with targeted biopsies to verify endometrial features and the correct position of the LNG-IUD, then with TVS and hysteroscopy with biopsies every 6 months up to the third year and annually in the last 2 years. Five (55.6%) women showed a complete response to hormone therapy after 3 months while the others (44.4%) showed a partial response at 3 months and a complete response at 6 months. All of them completed follow-up and none had side effects due to local hormone therapy. Surveillance by TVS and hysteroscopy were negative for recurrences for all women during the entire follow-up. None of the women complained abnormal uterine bleeding. The LNG-IUD was replaced at the end of follow-up and currently 7 women (77.8%) are examined annually with TVS while 2 women (22.2%) died from their comorbidity. One died of pancreatic cancer after 2 more years without signs of recurrence in uterine histology. Another woman died of chronic renal failure unrelated to the EC.
DISCUSSION
Despite today's advances in surgical techniques, anesthesiology and perioperative management, a significant fraction (10%) of women newly diagnosed with EC is unable to undergo standard surgery not only for age but also for their own medical comorbidities such as high BMI, cardiovascular disease, obesity-hypoventilation syndrome, and diabetes-related organ damage [4,16]. Frequent perioperative complications are obstructive sleep apnea, arrhythmias, acute cardiac events, and venous thrombotic events. Therefore, these women require more detailed preoperative evaluation and postoperative intensive care according to Royal College of Obstetricians and Gynaecologists [17]. In such cases, a benefit-risk assessment of surgery may lead to an individualized decision to perform a non-standard surgery or a limited staging procedure. Alternatively, these cases can still be managed surgically by vaginal hysterectomy, definitive radiation therapy, combining EBRT and brachytherapy, or hormonal treatment [3].
EC is radiosensitive, and radiotherapy can be used as the sole treatment modality. Radiotherapy in surgically inoperable women reported 5-year survival rate of 87% for cases with stage I [18], although recurrence rates are 18% in obese women [16]. Furthermore, radiotherapy is often not applicable, especially in obese women, due to material and technical reasons and dosimetric difficulties [6].
Regarding the use of oral progestin in EC, few studies conducted on younger women wishing to preserve fertility reported a response rate of 50% to 70% in women treated with high-dose progesterone as primary therapy, but a remarkable rate of recurrence (>25%) was observed [8]. LNG-IUD has also been used as part of a fertility-sparing regimen for both precancerous and grade 1 EC [19]. MPA, MA, and LNG-IUD are the progestins most commonly used; nevertheless, the doses and duration of the treatment are not yet standardized [20]. Some studies also reported the use of LNG-IUD in selected cases of inoperable EC with contradictory outcomes, histologic regression from 25% [21] to 75% [22] after 6/12 months therapy. A recent meta-analysis [23] did not take a definitive position in the non-operable EC conservative management as there are no randomised controlled trials on the use of this therapy.
Since there is no standardized treatment in EC cases with surgical high risk, several authors have tried alternative treatments. Laframboise et al. [6] described a case of a woman with EC of 49 years medically severely compromised treated with a complete hysteroscopic resection and high-dose-rate intracavitary radiation. Hawkes et al. [9] investigated the effectiveness of LNG-IUD with or without metformin and with or without weight loss in obese women with early stage EC. They did not find a better outcome than standard treatment but demonstrated reduction in health system costs and lower incidence of adverse events. Vilos et al. [13] reported a case of a 54-year-old woman with well-differentiated EC and superficial myometrial invasion that strongly refused hormonal therapy and standard surgery without surgical contraindications. She was treated only with hysteroscopic resection and showed no recurrence after 5 years follow-up.
In this pilot study, we reported for the first time a case series on hysteroscopic treatment combined with hormonal therapy in a group of 9 women with primary EC with or without minimal myometrial invasion at high surgical risk with standard surgical treatment. This treatment is based on the favorable prognosis of grade 1 mini-invasive tumors and on the fact that no residual cancer was found in post-hysterectomy specimens after incidental diagnosis of EC by hysteroscopic resection [24]. In spite of limitations of case series, we demonstrated that hysteroscopic resection is a safe and effective procedure in these women since none of them had acute or delayed complications, the lesions were completely removed and the margins were disease-free in all cases. Women were extensively counselled about pros and cons of different treatments (radiotherapy/progesterone therapy), lack of data, risk of recurrence and need for close follow up. The outcomes we have had supported our view. Currently 7 women are negative for recurrence after 5-year follow up. Two women died of unrelated cancer diseases. Therefore, we believe that the hysteroscopic resection is the best procedure to evaluate tumor extension and status of endometrium. It provides with certainty of tumor staging and involvement of the myometrium and allows an optimal cytoreduction facilitating the therapeutic effect of progestin. This procedure is safe and effective if performed by surgeons experienced in minimally invasive techniques. Our preliminary outcomes are comparable to those of radiotherapy but with a lower or absent complication rate [18].
Concerning to the increased risk of cell dissemination within the peritoneal cavity after hysteroscopic procedure, several studies demonstrated that a positive peritoneal cytology does not modify the prognosis of the disease [25]. Other authors [26,27] observed that the clinical significance of positive peritoneal cytology could reflect other adverse prognostic factors (i.e. high-risk EC or presence of other metastases) and that positive peritoneal cytology did not influence survival in case of stage I EC.
Despite limited number of cases and short follow-up, we can advance the hypothesis that hysteroscopic resection and LNG-IUD therapy can be considered an effective alternative to traditional treatment for women at high surgical risk with stage IA EC. Further evaluation is needed before using this procedure in the routine practice, and prospective, and multicenter studies with a longer follow-up are requested to validate this therapeutic option.
ACKNOWLEDGMENTS
We would like to thank Dr. Giacomo Caprara and the Department of Pathology (S. Orsola Hospital) for the kind collaboration
Footnotes
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
- Conceptualization: C.P.
- Data curation: T.M.R., P.R.
- Investigation: G.F., T.M.R.
- Methodology: G.F.
- Project administration: S.R.
- Resources: M.G.
- Software: M.C.
- Supervision: S.R.
- Validation: C.P., P.R., S.R.
- Writing - original draft: T.M.R.
- Writing - review & editing: T.M.R., P.R.
References
- 1.Mazzon I, Corrado G, Masciullo V, Morricone D, Ferrandina G, Scambia G. Conservative surgical management of stage IA endometrial carcinoma for fertility preservation. Fertil Steril. 2010;93:1286–1289. doi: 10.1016/j.fertnstert.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 2.Creasman W. Revised FIGO staging for carcinoma of the endometrium. Int J Gynaecol Obstet. 2009;105:109. doi: 10.1016/j.ijgo.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 3.Colombo N, Creutzberg C, Amant F, Bosse T, González-Martín A, Ledermann J, et al. ESMO-ESGO-ESTRO consensus conference on endometrial cancer: diagnosis, treatment and follow-up. Ann Oncol. 2016;27:16–41. doi: 10.1093/annonc/mdv484. [DOI] [PubMed] [Google Scholar]
- 4.Schwarz JK, Beriwal S, Esthappan J, Erickson B, Feltmate C, Fyles A, et al. Consensus statement for brachytherapy for the treatment of medically inoperable endometrial cancer. Brachytherapy. 2015;14:587–599. doi: 10.1016/j.brachy.2015.06.002. [DOI] [PubMed] [Google Scholar]
- 5.Modesitt SC, van Nagell JR., Jr The impact of obesity on the incidence and treatment of gynecologic cancers: a review. Obstet Gynecol Surv. 2005;60:683–692. doi: 10.1097/01.ogx.0000180866.62409.01. [DOI] [PubMed] [Google Scholar]
- 6.Laframboise S, Milosevic M, Leyland N. Hysteroscopic endometrial resection and high-dose-rate brachytherapy: treatment of endometrial cancer in a medically compromised patient. Gynecol Oncol. 1999;75:149–151. doi: 10.1006/gyno.1999.5521. [DOI] [PubMed] [Google Scholar]
- 7.Coon D, Beriwal S, Heron DE, Kelley JL, Edwards RP, Sukumvanich P, et al. High-dose-rate Rotte “Y” applicator brachytherapy for definitive treatment of medically inoperable endometrial cancer: 10-year results. Int J Radiat Oncol Biol Phys. 2008;71:779–783. doi: 10.1016/j.ijrobp.2007.10.026. [DOI] [PubMed] [Google Scholar]
- 8.Kim JJ, Chapman-Davis E. Role of progesterone in endometrial cancer. Semin Reprod Med. 2010;28:81–90. doi: 10.1055/s-0029-1242998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hawkes AL, Quinn M, Gebski V, Armes J, Brennan D, Janda M, et al. Improving treatment for obese women with early stage cancer of the uterus: rationale and design of the levonorgestrel intrauterine device ± metformin ± weight loss in endometrial cancer (feMME) trial. Contemp Clin Trials. 2014;39:14–21. doi: 10.1016/j.cct.2014.06.014. [DOI] [PubMed] [Google Scholar]
- 10.Falcone F, Laurelli G, Losito S, Di Napoli M, Granata V, Greggi S. Fertility preserving treatment with hysteroscopic resection followed by progestin therapy in young women with early endometrial cancer. J Gynecol Oncol. 2017;28:e2. doi: 10.3802/jgo.2017.28.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Casadio P, Guasina F, Paradisi R, Leggieri C, Caprara G, Seracchioli R. Fertility-sparing treatment of endometrial cancer with initial infiltration of myometrium by resectoscopic surgery: a pilot study. Oncologist. 2018;23:478–480. doi: 10.1634/theoncologist.2017-0285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Giampaolino P, Di Spiezio Sardo A, Mollo A, Raffone A, Travaglino A, Boccellino A, et al. Hysteroscopic endometrial focal resection followed by levonorgestrel intrauterine device insertion as a fertility-sparing treatment of atypical endometrial hyperplasia and early endometrial cancer: a retrospective study. J Minim Invasive Gynecol. 2018 doi: 10.1016/j.jmig.2018.07.001. Forthcoming. [DOI] [PubMed] [Google Scholar]
- 13.Vilos GA, Ettler HC, Edris F, Hollett-Caines J, Abu-Rafea B. Endometrioid adenocarcinoma treated by hysteroscopic endomyometrial resection. J Minim Invasive Gynecol. 2007;14:119–122. doi: 10.1016/j.jmig.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 14.Boyd O, Jackson N. How is risk defined in high-risk surgical patient management? Crit Care. 2005;9:390–396. doi: 10.1186/cc3057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murray LJ, Lilley J, Hawkins MA, Henry AM, Dickinson P, Sebag-Montefiore D. Pelvic re-irradiation using stereotactic ablative radiotherapy (SABR): a systematic review. Radiother Oncol. 2017;125:213–222. doi: 10.1016/j.radonc.2017.09.030. [DOI] [PubMed] [Google Scholar]
- 16.Podzielinski I, Randall ME, Breheny PJ, Escobar PF, Cohn DE, Quick AM, et al. Primary radiation therapy for medically inoperable patients with clinical stage I and II endometrial carcinoma. Gynecol Oncol. 2012;124:36–41. doi: 10.1016/j.ygyno.2011.09.022. [DOI] [PubMed] [Google Scholar]
- 17.Royal College of Obstetricians & Gynaecologists (UK) Endometrial cancer in obese women (scientific impact paper No. 32) [Internet] London: Royal College of Obstetricians & Gynaecologists; c2012. [cited 2012 May]. Available from: https://www.rcog.org.uk/en/guidelines-research-services/guidelines/sip32. [Google Scholar]
- 18.van der Steen-Banasik E. Primary brachytherapy as a radical treatment for endometrial carcinoma. J Contemp Brachytherapy. 2014;6:106–112. doi: 10.5114/jcb.2014.42028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Orbo A, Vereide A, Arnes M, Pettersen I, Straume B. Levonorgestrel-impregnated intrauterine device as treatment for endometrial hyperplasia: a national multicentre randomised trial. BJOG. 2014;121:477–486. doi: 10.1111/1471-0528.12499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vitale SG, Rossetti D, Tropea A, Biondi A, Laganà AS. Fertility sparing surgery for stage IA type I and G2 endometrial cancer in reproductive-aged patients: evidence-based approach and future perspectives. Updates Surg. 2017;69:29–34. doi: 10.1007/s13304-017-0419-y. [DOI] [PubMed] [Google Scholar]
- 21.Dhar KK, NeedhiRajan T, Koslowski M, Woolas RP. Is levonorgestrel intrauterine system effective for treatment of early endometrial cancer? Report of four cases and review of the literature. Gynecol Oncol. 2005;97:924–927. doi: 10.1016/j.ygyno.2004.10.031. [DOI] [PubMed] [Google Scholar]
- 22.Montz FJ, Bristow RE, Bovicelli A, Tomacruz R, Kurman RJ. Intrauterine progesterone treatment of early endometrial cancer. Am J Obstet Gynecol. 2002;186:651–657. doi: 10.1067/mob.2002.122130. [DOI] [PubMed] [Google Scholar]
- 23.Baker J, Obermair A, Gebski V, Janda M. Efficacy of oral or intrauterine device-delivered progestin in patients with complex endometrial hyperplasia with atypia or early endometrial adenocarcinoma: a meta-analysis and systematic review of the literature. Gynecol Oncol. 2012;125:263–270. doi: 10.1016/j.ygyno.2011.11.043. [DOI] [PubMed] [Google Scholar]
- 24.Vilos GA, Edris F, Al-Mubarak A, Ettler HC, Hollett-Caines J, Abu-Rafea B. Hysteroscopic surgery does not adversely affect the long-term prognosis of women with endometrial adenocarcinoma. J Minim Invasive Gynecol. 2007;14:205–210. doi: 10.1016/j.jmig.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 25.Revel A, Tsafrir A, Anteby SO, Shushan A. Does hysteroscopy produce intraperitoneal spread of endometrial cancer cells? Obstet Gynecol Surv. 2004;59:280–284. doi: 10.1097/01.ogx.0000120173.09136.4a. [DOI] [PubMed] [Google Scholar]
- 26.Lurain JR, Rumsey NK, Schink JC, Wallemark CB, Chmiel JS. Prognostic significance of positive peritoneal cytology in clinical stage I adenocarcinoma of the endometrium. Obstet Gynecol. 1989;74:175–179. [PubMed] [Google Scholar]
- 27.Kadar N, Homesley HD, Malfetano JH. Positive peritoneal cytology is an adverse factor in endometrial carcinoma only if there is other evidence of extrauterine disease. Gynecol Oncol. 1992;46:145–149. doi: 10.1016/0090-8258(92)90246-f. [DOI] [PubMed] [Google Scholar]