Abstract
Objective
In this study, we aimed to evaluate the clinicopathological features, obstetric, and oncological outcomes of patients diagnosed with a uterine smooth muscle tumors of uncertain malignant potential (STUMP).
Methods
A dual-institutional, database review was carried out to screen patients with STUMP who were treated with upfront surgery between January 2006 and December 2017. Data including age at the time of diagnosis, recurrence rate, disease-free survival, overall survival, and fertility outcomes were retrospectively analyzed.
Results
Fifty-seven patients with STUMPs were included in the study. The median age at the time of diagnosis was 42 (range, 16 to 75) years. The median follow-up was 57 (range, 16 to 125) months. Eight patients (14%) had recurrence during follow-up. Recurrent STUMPs were seen in seven patients and leiomyosarcoma after 14 months in one patient. Seven patients with a recurrent STUMP survived, while the remaining patient died. Recurrence rates were similar for women who underwent myomectomy and those who underwent hysterectomy. The presence of uterine localization of tumor (subserosal vs intramural-submucosal) statistically significantly affected recurrence rates (odds ratio=5.72; 95% confidence interval=1.349–24.290; p=0.018). Ten of 27 patients who underwent myomectomy for uterine myoma had fertility desire. Seven pregnancies were recorded.
Conclusions
Our study results suggest that fertility-sparing approaches are feasible in patients with STUMP, although recurrence may be seen.
Keywords: Smooth muscle tumors, uterine; Hysterectomy; Recurrence; Myomectomy
INTRODUCTION
Uterine smooth muscle tumors of uncertain malignant potential (STUMPs) represent a poorly defined subcategory of uterine smooth muscle tumors (SMTs). Uterine SMTs, which have a broad spectrum ranging from leiomyosarcomas (LMSs) to leiomyomas (LMs), can be distinguished based on histopathological features including the degree of cytologic atypia, mitotic count activity (mitotic index per 10 high-power fields [MIs/10 HPFs]), and presence of tumor cell necrosis [1,2]. However, in some SMT cases, these histopathological features may appear in an unusual combination which does not meet the diagnostic criteria of a LM or LMS, and these cases are classified as STUMPs [2].
Uterine smooth muscle tumors of uncertain malignant potential are a rare and interesting tumor group of which histological diagnosis, classification, treatment, follow-up, and prognosis have not been fully understood, yet, and the actual incidence is still unknown [3,4]. Patients with LMs, STUMPs, and LMSs may have similar symptoms including abnormal uterine bleeding, pelvic pain, and pressure, and therefore, preoperative diagnosis is challenging [5]. In addition, postoperative diagnosis of STUMP for a pathologist is also difficult due to the lack of standard diagnostic criteria. As a consequence, overdiagnosis of this type of tumors has been increased in years [6].
Treatment approaches and follow-up of these tumors have been still controversial, particularly in the reproductive age patients with fertility desire, due to the non-aggressive behavior and prolonged overall survival (OS) rate comparing to LMS. In the literature, there are only a few case series and some of these studies are lack of particular clinicopathological features and/or follow-up data [2,5,6].
In the present study, we aimed to evaluate the clinicopathological features, obstetric, and oncological outcomes of patients diagnosed with STUMPs in the light of literature data.
MATERIALS AND METHODS
A dual-institutional, retrospective database review was carried out to screen patients with STUMPs who were treated with upfront surgery between January 2006 and December 2017. A written informed consent was obtained from each patient. The study protocol was approved by the local Ethics Committee. The study was conducted in accordance with the principles of the Declaration of Helsinki.
The central pathology reviews were carried out by two pathologists specializing in gynecologic oncology. All pathological slides were reviewed by these two independent expert gynecological pathologists who were blinded to the patient outcomes. For the pathological examination, one section at least per cm was obtained from the patients in whom the tumor diameter was ≤10 cm, whereas two sections per cm were obtained from those in whom the tumor diameter was >10 cm. All slides were cut 4 mm in thickness and stained with hematoxylin and eosin. Histological features such as cellularity, cytological atypia, mitotic activity, and necrosis were analyzed using the criteria which were described in the study of Ip et al. [6]. The histopathological criteria for the definition of STUMPs were determined according to previous study conducted by Bell et al. [1] as follows (including one of these criteria): a tumor cell necrosis with <10 MIs/10 HPFs and without atypia or a diffuse atypia with <10 MIs/10 HPFs and without tumor cell necrosis or a >20 MIs/10 HPFs without atypia and tumor cell necrosis or a >4 MIs/10 HPFs with cellularity/hypercellularity or the tumor with irregular margins or vascular invasion. The tumor tissues of recurrent cases were re-evaluated by 2 independent gynecological pathologists to distinguish STUMP from LMS. Demographic characteristics, oncologic outcomes including disease-free survival (DFS), clinicopathological and fertility data were retrieved from medical records.
Statistical analysis
Statistical analysis was performed using the SPSS version 22.0 statistical software (IBM Corp., Armonk, NY, USA). The data were expressed in median and range for continuous variables. Binary variables were expressed in number and percentage. Categorical variables were evaluated using the χ2 test or Fisher's exact test as appropriate for the group size. Survival curves were generated using the Kaplan-Meier method and the differences between the survival curves were calculated using the log-rank test. To evaluate the prognostic factors for DFS and OS, a univariate Cox-regression model was used. A p-value of less than 0.05 was considered statistically significant.
RESULTS
The medical records of a total of 57 patients were reviewed. The median age was 42 (range, 23–69) years. The median tumor size was 6 (range, 1–26) cm. Oncological and obstetric follow-up data were available for all patients. The median follow-up was 57 (range, 16–125) months. Of the patients, 27 patients underwent myomectomy and 30 patients underwent total hysterectomy. The anatomical localizations of STUMPs were evaluated by preoperative transvaginal ultrasonography or magnetic resonance imaging (MRI), and localization was confirmed intraoperatively. The anatomical localizations of STUMPs are shown in Table 1.
Table 1. Demographic and clinicopathological characteristics of patients (n=57).
| Characteristics | Values | |
|---|---|---|
| Age (yr), median | 42 (23–69) | |
| Gravida, median | 2 (0–6) | |
| Parity, median | 2 (0–4) | |
| Tumor size (cm), median | 6 (1–26) | |
| Serum CA-125 (U/mL), median | 21 (4–65) | |
| >35 | 10 (17.5%) | |
| <35 | 28 (49.1%) | |
| Unknown | 19 (33.3%) | |
| Smoking habit | ||
| Yes | 14 (24.6%) | |
| No | 39 (68.4%) | |
| Unknown | 4 (7%) | |
| Surgical type | ||
| TAH | 11 (19.3%) | |
| TAH+BSO | 14 (24.6%) | |
| TAH+USO | 4 (7%) | |
| VH | 1 (1.8%) | |
| Abdominal myomectomy | 26 (45.6%) | |
| Hysteroscopic myomectomy | 1 (1.8%) | |
| Uterine localization | ||
| Intramural | 38 (66.7%) | |
| Subserous | 12 (21.1%) | |
| Submucous | 7 (12.3%) | |
| Mitosis | ||
| 0–5 | 19 (33.3%) | |
| 5–10 | 32 (56.1%) | |
| ≥10 | 6 (10.5%) | |
| Cellularity | ||
| Moderate | 11 (19.3%) | |
| High | 46 (80.7%) | |
| Necrosis | ||
| Absent | 48 (84.2%) | |
| Multifocal | 9 (15.8%) | |
| Atypia | ||
| Mild | 14 (24.6%) | |
| Mild to moderate | 23 (40.4%) | |
| Moderate | 9 (15.8%) | |
| Moderate to severe | 11 (19.3%) | |
| Recurrence rate | 8 (14%) | |
| After hysterectomy | 2 (3.5%) | |
| After myomectomy | 6 (10.5%) | |
| Recurrent pathology | ||
| STUMP | 7 (12.3%) | |
| LMS | 1 (1.8%) | |
| Median follow-up (mo), (min-max) | 57 (16–125) | |
LMS, leiomyosarcoma; STUMP, uterine smooth muscle tumors of uncertain malignant potential; TAH+BSO, Total abdominal hysterectomy+bilateral salpingo-oophorectomy; TAH+USO, total abdominal hysterectomy+unilateral salpingo-oophorectomy; TAH, total abdominal hysterectomy; VH, vaginal hysterectomy.
When myomectomy and hysterectomy cases were compared, there was a significant difference between the two groups in terms of age, gravida, parity and tumor diameter (p<0.005). However, there was no significant difference in the median follow-up, serum CA-125 levels, and recurrence rates between the 2 groups (p>0.005) (Table 2).
Table 2. Differences between myomectomy and hysterectomy groups (n=57).
| Characteristics | Myomectomy (n=27) | Hysterectomy (n=30) | p-value |
|---|---|---|---|
| Age (yr) | 37 (23–52) | 46.5 (38–69) | <0.001 |
| Gravida | 0 (0–4) | 3 (1–6) | <0.001 |
| Parity | 0 (0–3) | 2 (1–4) | <0.001 |
| Tumor size (cm) | 8 (3–25) | 5 (1–26) | 0.036 |
| Median follow-up (mo) | 58 (16–125) | 50.5 (20–114) | 0.253 |
| Serum CA-125 (U/mL) | 22.5 (6–65) | 20 (4–60) | 0.769 |
| Recurrence rates, No. (%) | 6 (22.2%) | 2 (6.6%) | 0.091 |
Data shown are median (minimum–maximum) not otherwise specified.
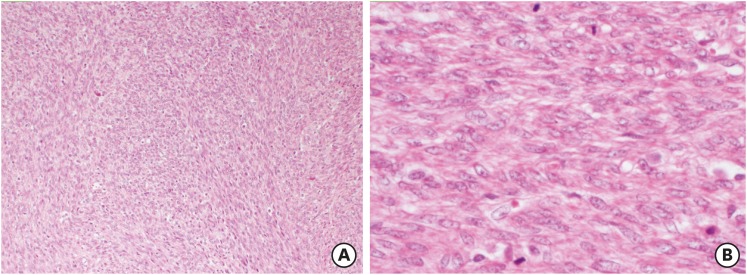
Recurrent disease was seen in eight patients (14%). Relapse occurred after hysterectomy in two patients (3.5%) and after myomectomy in six patients (10.5%). The recurrent pathology was still a STUMP in seven patients. In one patient with LMS, extensive abdominal masses in the pelvis and upper abdominal region developed at 14 months. Pathological specimens of this patient were retrospectively examined at the expert center and the initial diagnosis as a STUMP was confirmed. At the time of the first operation which was a myomectomy, the patient was 52 years old. The largest dimension of the myomectomy material was 20 cm and at least one section per 1 cm was obtained. Focal mild cytologic atypia and 6 MFs/10 HPFs were observed. However, a necrosis of undetermined etiology, whether it was tumor cell necrosis or infarct-type necrosis, was seen (Fig. 1). No immunoreactivity for WT-1, p16, and p53 was observed. The Ki-67 proliferation index was 10%. Nevertheless, recurrent tumor was a high-grade leiomyosarcoma showing diffuse, marked nuclear atypia and a mitotic rate exceeding 10 MFs/10 HPFs (Figs. 2 and 3). The tumor also exhibited tumor cell necrosis characterized by an abrupt transition from viable cells to necrotic cells, hyperchromatic nuclei of the necrotic cells, and perivascular viable tumor cells (Figs. 4 and 5). Gemcitabine + docetaxel chemotherapy was given to the patient following debulking surgery. Within two months, there were still massive masses covering the entire abdomen. Unfortunately, the patient died due to intestinal ileus and multiorgan failure at 16 months. Recurrences according to the localizations and treatment approaches are summarized in Table 3.
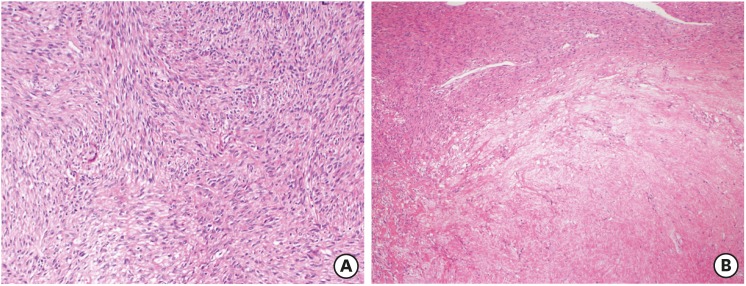
Fig. 1. A smooth muscle tumors of uncertain malignant potential case with recurrent leiomyosarcoma. A cellular smooth muscle tumor with mild atypia (A) and necrosis of uncertain type (B) (Hematoxylin and eosin stain, ×40).
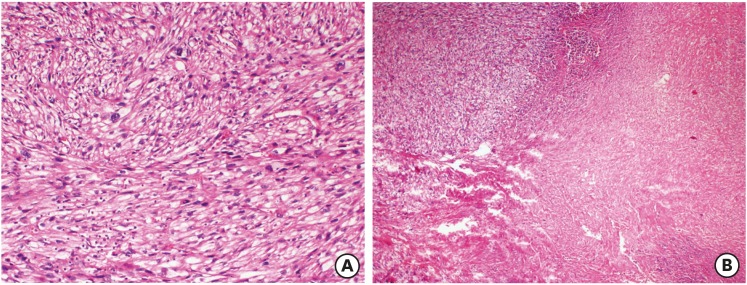
Fig. 2. Recurrent tumor as leiomyosarcoma with tumor cell necrosis (A) (H&E ×40) and severe atypia (B) (H&E ×200).
H&E, hematoxylin and eosin stain.
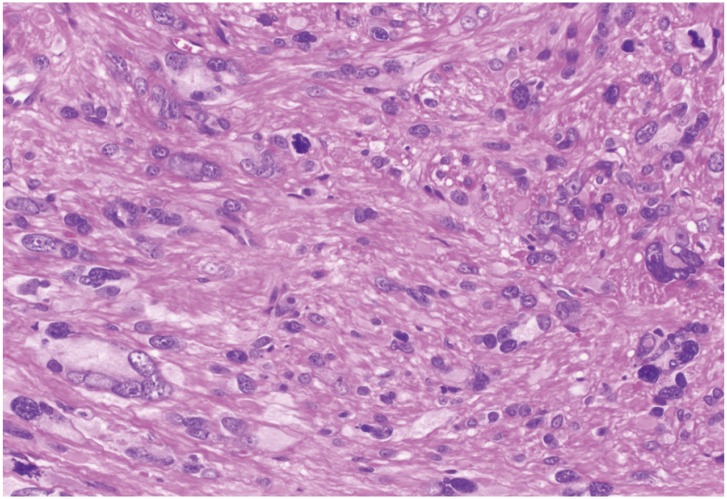
Fig. 3. An atypical smooth muscle tumor containing bizarre cells with hyperchromatic nuclei. Scattered mitotic figures and karyorrhectic nuclei were also seen (hematoxylin and eosin stain, ×200).
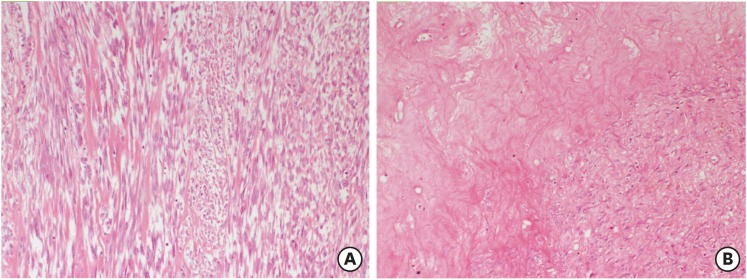
Fig. 4. A smooth muscle tumor with mild atypia (A) and necrosis of infarct-type (B) (hematoxylin and eosin stain, ×100).
Fig. 5. A cellular smooth muscle tumor (A) (H&E ×100) with brisk mitotic activity (B) (H&E ×400).
H&E, hematoxylin and eosin stain.
Table 3. Clinical and pathological characteristics and outcome of patients with recurrence disease (n=8).
| Patients | Age (yr) | Parity | Tumor size (cm) | Initial surgery | Recurrence location | Recurrence treatment | Recurrence pathology | Outcome |
|---|---|---|---|---|---|---|---|---|
| 1 | 35 | 3 | 16 | Myomectomy | Uterus | TAH | STUMP | ANED |
| 2 | 47 | 0 | 5 | Myomectomy | Uterus | TAH+BSO | STUMP | ANED |
| 3 | 38 | 0 | 9 | Myomectomy | Uterus | Myomectomy | STUMP | ANED |
| 4 | 45 | 2 | 13 | TAH+BSO | Pelvic RP mass | Mass excision | STUMP | ANED |
| 5 | 35 | 0 | 2 | Hysteroscopic myomectomy | Uterus | Hysteroscopic myomectomy | STUMP | ANED |
| 6 | 52 | 0 | 20 | Myomectomy | Pelvic+ upper abdominal mass | Debulking+ chemotherapy | LMS | DOD |
| 7 | 30 | 1 | 4 | Myomectomy | Uterus | TAH | STUMP | ANED |
| 8 | 39 | 2 | 11 | TAH+USO | Pelvic RP mass | Mass excision | STUMP | ANED |
STUMP, uterine smooth muscle tumors of uncertain malignant potential; TAH+BSO, total abdominal hysterectomy+bilateral salpingo-oophorectomy; TAH+USO, total abdominal hysterectomy+unilateral salpingo-oophorectomy; ANED, alive with no evidence of disease; RP, retroperitoneal; CT, chemotherapy; DOD, dead of disease; LMS, leiomyosarcoma.
The 5-year DFS was 86.3% with a mean DFS of 98.7 month. There was no significant difference in terms of age, parity, tumor diameter, smoking, CA-125, type of surgery, mitotic index, necrosis, atypia, and cellularity between the patients with and without recurrence (Table 4). In the Cox regression analysis, there was no significant effect of cigarette smoking (yes vs. no), surgical procedure (hysterectomy vs. myomectomy), age (<42 years vs. ≥42 years), parity (<2 vs. ≥2 mitotic index, Necrosis, atypia and cellularity on recurrences.
Table 4. Differences between non-recurrence and recurrence groups (n=57).
| Characteristics | Non-recurrence group (n=49) | Recurrence group (n=8) | p-value | |
|---|---|---|---|---|
| Age (yr), median | 42 (23–69) | 38.5 (30–52) | 0.418 | |
| Gravida, median | 2 (0–4) | 1 (0–4) | 0.228 | |
| Parity, median | 2 (0–3) | 0.5 (0–3) | 0.159 | |
| Tumor size (cm), median | 6 (1–26) | 10 (3–20) | 0.160 | |
| Median follow-up (mo) | 56 (25–114) | 64 (16–125) | 0.512 | |
| Serum CA-125 (U/mL), median | 20 (4–60) | 28 (17–65) | 0.167 | |
| Smoking, No. (%) | 12 (24.5%) | 3 (37.5%) | 0.531 | |
| Type of surgery, No. (%) | 0.353 | |||
| TAH | 11 (22.4%) | 0 | ||
| TAH+BSO | 13 (26.5%) | 1 (12.5%) | ||
| TAH+USO | 3 (6.1%) | 1 (12.5%) | ||
| VH | 1 (2%) | 0 | ||
| Myomectomy | 21 (42.9%) | 6 (75%) | ||
| Uterine localization, No. (%) | 0.002 | |||
| Intramural | 37 (75.5%) | 1 (12.5%) | ||
| Subserous | 7 (14.3%) | 5 (62.5%) | ||
| Submucous | 5 (10.2%) | 2 (25%) | ||
| Mitosis, No. (%) | 0.399 | |||
| 0–5 | 18 (36.7%) | 1 (12.5%) | ||
| 5–10 | 26 (53.1%) | 6 (75%) | ||
| ≥10 | 5 (10.2%) | 1 (12.5%) | ||
| Cellularity | 0.659 | |||
| Moderate | 9 (18.4%) | 2 (25%) | ||
| High | 40 (81.6%) | 6 (75%) | ||
| Atypia | 0.379 | |||
| Mild | 11 (22.4%) | 3 (37.5%) | ||
| Mild to moderate | 22 (44.9%) | 1 (12.5%) | ||
| Moderate | 7 (14.3%) | 2 (25%) | ||
| Moderate to severe | 9 (18.4%) | 2 (25%) | ||
| Necrosis | 0.783 | |||
| Absent | 41 (84.2%) | 7 (87.5%) | ||
| Multifocal | 8 (15.8%) | 1 (12.5%) | ||
TAH+BSO, total abdominal hysterectomy+bilateral salpingo-oophorectomy; TAH+USO, total abdominal hysterectomy+unilateral salpingo-oophorectomy; TAH, total abdominal hysterectomy; VH, vaginal hysterectomy; STUMP, uterine smooth muscle tumors of uncertain malignant potential.
However, we found a statistically significant effect of uterine localization of tumor (subserosal vs. intramural-submucosal) on recurrence rates (odds ratio=5.72; confidence interval 95%=1.349–24.290; p=0.018). Univariate analysis showed that the risk of recurrence was 5.7-fold higher for subserosal location (Table 5).
Table 5. Univariate analysis of recurrence risk factors.
| Variables | p-value | Univariate analysis | ||
|---|---|---|---|---|
| OR | 95% CI | |||
| Age (yr) | 0.432 | 0.56 | 0.133–2.367 | |
| <42 | ||||
| ≥42 | ||||
| Parity | 0.184 | 0.37 | 0.089–1.591 | |
| <2 | ||||
| ≥2 | ||||
| Tumor size (cm) | 0.501 | 0.60 | 0.139–2.621 | |
| <6 | ||||
| ≥6 | ||||
| Smoking | 0.818 | 0.83 | 0.180–3.883 | |
| Yes | ||||
| No | ||||
| Surgical type | 0.157 | 0.31 | 0.063–1.563 | |
| Hysterectomy | ||||
| Myomectomy | ||||
| Uterine localization | 0.018 | 5.72 | 1.349–24.290 | |
| Intramural-submucous | ||||
| Subserous | ||||
| Necrosis | 0.784 | 0.73 | 0.079–6.795 | |
| Absent | ||||
| Multifocal | ||||
| Cellularity | 0.661 | 0.67 | 0.117–3.908 | |
| Moderate | ||||
| High | ||||
| Mitosis | 0.446 | 0.24 | 0.027–2.174 | |
| 0–5 | ||||
| 5–10 | ||||
| ≥10 | ||||
| Necrosis | 0.784 | 0.73 | 0.079–6.795 | |
| Absent | ||||
| Multifocal | ||||
| Atypia | 0.468 | 0.81 | 0.111–5.987 | |
| Mild | ||||
| Mild to moderate | ||||
| Moderate | ||||
| Moderate to severe | ||||
Of 27 patients who underwent myomectomy for uterine myoma, 10 had fertility desire. Seven pregnancies were recorded. One of them occurred with assisted reproductive technologies (intracytoplasmic sperm injection) (cases 5, 6, and 7) and the other four pregnancies were spontaneous. Six full-term infants and one 35-week of birth were alive. One of the relapses occurred as uterus intramural and the other as uterine submucosal. In 2 patients with recurrence and hysterectomy, STUMP was confirmed as the final diagnosis. Other clinicopathological features are listed in Table 6.
Table 6. Clinical and pathological characteristics of patients with obstetrics outcomes (n=7).
| Patient | Age (yr) | Gestational age (wk) | Parity | Tumor size (cm) | Initial surgery | Recurrence location | Recurrence treatment | Recurrence pathology | Birth type | Fertility outcome |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 35 | 38 | 3 | 16 | Myomectomy | Uterus | TAH | STUMP | C/S | 2,800 g, live birth |
| 2 | 36 | 35 | 0 | 5 | Myomectomy | - | - | - | C/S | 2,200 g, live birth |
| 3 | 25 | 39 | 0 | 6 | Myomectomy | - | - | - | C/S | 3,450 g, live birth |
| 4 | 23 | 39 | 0 | 8 | Myomectomy | - | - | - | C/S | 3,150 g, live birth |
| 5 | 26 | 39 | 1 | 10 | Myomectomy | - | - | - | C/S | 3,600 g, live birth |
| 6 | 38 | 38 | 0 | 11 | Myomectomy | - | - | - | C/S | 3,100 g, live birth |
| 7 | 30 | 38 | 1 | 3 | Hysteroscopic | Uterus | TAH | STUMP | VB | 3,200 g, live birth |
| Myomectomy |
STUMP, uterine smooth muscle tumors of uncertain malignant potential; TAH, total abdominal hysterectomy; VB, vaginal birth; C/S, cesarean section.
DISCUSSION
In this study, we evaluated the clinicopathological features and the obstetric and oncological outcomes of patients diagnosed with STUMPs. In the literature, there is a limited number of large series of studies with STUMPs and, therefore, the clinical behavior of these tumors still remains to be elucidated. To the best of our knowledge, this is the largest study evaluating clinicopathological factors, recurrence rates, long-term outcomes, and fertility outcomes in patients with uterine STUMPs. Therefore, our study is important for this topic and we believe that this study provides additional information to the body of knowledge on this topic.
The accumulation of data about STUMPs is difficult due to the difficulty of clinicopathological diagnosis of this tumor and the majority of published reports include small sample sizes, although they are multi-center studies [7,8,9,10,11,12,13]. The largest study was a series of 41 patients conducted in the M.D. Anderson Cancer Center in 2009 as reported by Guntupalli et al. [3]. In the aforementioned study, the mean age was 43 (range, 25–75) years. Three patients (7.3%) had recurrence after a mean follow-up period of 45 months, and recurrence rates were similar in myomectomy and hysterectomy groups. In one of the recurrent cases, LMS was seen, while all three patients were alive with a DFS of 121 months. In another study by Basaran et al. [14], there were 22 patients with an initial diagnosis of a STUMP and 21 with a final diagnosis of a STUMP and one LMS. Recurrence was seen in four of these patients (19.0%). Of these, three patients (75%) were diagnosed with a LMS, and one patient (4.8%) with recurrence succumbed to disease recurrent tumors were LMS in 3 patients (75%). Recurrence rates were also similar in myomectomy and hysterectomy groups. In our study, recurrence was seen in eight (14%) patients. Two of them (3.5%) occurred after hysterectomy, and six (10.5%) after myomectomy. Of these, one patient (1.8%) was diagnosed with a LMS, and this patient died due to recurrent disease.
Unclear prognostic factors are the major problems in the management and follow-up of patients with STUMPs. In the literature, there are few studies about prognostic factors of these tumors. These studies have shown that age of the patient at the time of diagnosis is highly important in the subsequent recurrences of STUMPs [3,4,6]. Patients with recurrent STUMPs are often younger than with those without recurrence [4]. In the study of Ip et al. [6], patients diagnosed with a recurrent STUMP were three-year younger than those without recurrence. In another study, patients with recurrence were on average 10 years younger than patients without recurrence [3]. Unlike these, Basaran et al. [14] found no prognostic factors associated with recurrence, such as the type of the initial surgery or age of the patient. Guntupalli et al. [3] found that three patients (7.3%) recurred at a mean follow-up of 45 months. The authors concluded that ethnicity, tobacco use, or type of initial surgery was not predictor of recurrence. In our study, similarly, cigarette smoking or the type of surgery was not predictor of recurrence. We found no significant differences in the pathological characteristics of recurrent and non-recurrent tumors including mitosis, cellularity, atypia, and necrosis. In addition, in our study, we found that uterine localization of the tumor (intramural-submucosal vs. subserosal) had a statistically significant effect on recurrence and the risk of recurrence was 5.7-fold higher for subserosal location. However, of 8 patients with recurrence, 5 patients had subserosal, 2 patients had submucosal, and only 1 patient had intramural recurrence. In the literature, there is no study investigating the relationship between the anatomical localization of the tumor and recurrence. We, therefore, recommend further large-scale studies to establish a conclusion on the relationship between the localization of the tumor and recurrence.
Recent studies have suggested the use of immunohistochemical stains, including epithelial growth factor receptor, galectin-3, p16, p16INK4a, p53, MIB-1, Twist, BCL-2, estrogen, and progesterone receptors to identify uterine smooth muscle tumors with a higher risk of recurrence [15,16,17,18,19]. Although recurrent tumors regarded as STUMPs are associated with diffuse immunoreactivity for p16 and p53 [20,21], the number of cases in these studies are small and further studies are needed to confirm the reliability of such markers.
On the other hand, some recent studies have shown that high genomic indices in the array-based comparative genomic hybridization can be used in the differential diagnosis of LM, STUMP, and LMS [11,22]. In particular, high genomic indices are associated with recurrence and survival. In a study, STUMPs with a relatively higher genomic index were found to be associated with higher recurrences and unfavorable outcomes [11,22]. Although genomic index profiling of STUMPs seems promising in the management of STUMPs, this study has some drawbacks, as mentioned previously. In the aforementioned study, most of the recurrent STUMPs with a high genomic index proved to be LMS after slide review and some STUMPs had low genomic index, while some of the benign leiomyomas had significantly high genomic indices, despite their benign clinical courses [11,22].
According to the literature data, STUMP is a slowly growing tumor which recurs after a mean of 51 months following the initial diagnosis with a mean life of 61 months and a 5-year OS of 92% [23,24]. Due to these characteristics of the disease, we consider that fertility-sparing approaches can be applied to those patients who wish to have fertility desire. However, the oncological outcomes of STUMP patients with fertility desire after myomectomy are still unknown due to limited data. Therefore, the major strength of our study is that we presented our data about the results of fertility-sparing approaches. To the best of our knowledge, there are few case reports and case series about fertility desire in patients with STUMPs in the literature [23,25,26,27,28]. In a single-center study including 19 patients, Ha et al. [27] reported recurrence in 2 (10.5%) patients during a 47-month follow-up period. Similar to our study, one of these patients experienced LMS. In the aforementioned study, fertility outcomes revealed that seven patients underwent fertility-sparing surgery and three of 5 patients (60%) with fertility desire gave live births. In our study, 8 patients (14%) had recurrence during a 57-month follow-up period including recurrent STUMP in seven and LMS in one patient. In addition, in our study, 10 of 27 patients who underwent myomectomy for uterine myoma had fertility desire. Seven of these patients achieved pregnancy: three with in vitro fertilization (cases 5, 6, and 7) and four with spontaneously. Recurrence occurred in 2 of 7 patients. One of the relapses occurred as uterus intramural, the other as uterine submucosal. In 2 cases of recurrence and hysterectomy, STUMP was confirmed as the final diagnosis, as assessed by pathological examination. In addition, in all patients, 5-year DFS was 86.3% with a mean DFS of 98.7 months. Therefore, we speculate that fertility-sparing approaches are feasible in this patient group; however, patients should be informed about the prognosis of recurrent STUMPs. If patients choose fertility-sparing approaches, complementary surgery should be performed after successful pregnancy to minimize the risk of poor prognosis of recurrent STUMPs.
Nonetheless, there are some limitations to this study including relatively small sample size with STUMPs, relatively short median follow-up, its retrospective design. Despite these limitations, however, our study provides additional information to the body of knowledge on this topic.
In conclusion, the difficulties of these tumors at the time of diagnosis and unnecessary interventions resulting from misdiagnosis may lead to unnecessary follow-up and treatment anxiety. Therefore, consultation with experienced centers of these patients should be the first step in the management of STUMPs. Furthermore, it should be kept in mind that these tumors are fatal, and recurrence may be seen, although the recurrence rate is not extremely high, and that these patients should be closely monitored for possible recurrences.
Footnotes
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
- Conceptualization: Ş.H., A.A., O.M.A.
- Formal analysis: Ş.H., C.G., Ö.Ö., E.Ö.
- Funding acquisition:
- Investigation: Ş.H., C.G., Ö.Ö., E.Ö.
- Methodology: Ş.H., A.A., Ö.Ö.
- Validation: Ş.H., K.F., C.G.
- Writing - original draft: Ş.H.
- Writing - review & editing: E.Ö, O.M.A., A.A.
References
- 1.Bell SW, Kempson RL, Hendrickson MR. Problematic uterine smooth muscle neoplasms. A clinicopathologic study of 213 cases. Am J Surg Pathol. 1994;18:535–558. [PubMed] [Google Scholar]
- 2.Amant F, Moerman P, Vergote I. Report of an unusual problematic uterine smooth muscle neoplasm, emphasizing the prognostic importance of coagulative tumor cell necrosis. Int J Gynecol Cancer. 2005;15:1210–1212. doi: 10.1111/j.1525-1438.2005.00183.x. [DOI] [PubMed] [Google Scholar]
- 3.Guntupalli SR, Ramirez PT, Anderson ML, Milam MR, Bodurka DC, Malpica A. Uterine smooth muscle tumor of uncertain malignant potential: a retrospective analysis. Gynecol Oncol. 2009;113:324–326. doi: 10.1016/j.ygyno.2009.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kalogiannidis I, Stavrakis T, Dagklis T, Petousis S, Nikolaidou C, Venizelos I, et al. A clinicopathological study of atypical leiomyomas: benign variant leiomyoma or smooth-muscle tumor of uncertain malignant potential. Oncol Lett. 2016;11:1425–1428. doi: 10.3892/ol.2015.4062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mowers EL, Skinner B, McLean K, Reynolds RK. Effects of morcellation of uterine smooth muscle tumor of uncertain malignant potential and endometrial stromal sarcoma: case series and recommendations for clinical practice. J Minim Invasive Gynecol. 2015;22:601–606. doi: 10.1016/j.jmig.2015.01.007. [DOI] [PubMed] [Google Scholar]
- 6.Ip PP, Cheung AN, Clement PB. Uterine smooth muscle tumors of uncertain malignant potential (STUMP): a clinicopathologic analysis of 16 cases. Am J Surg Pathol. 2009;33:992–1005. doi: 10.1097/PAS.0b013e3181a02d1c. [DOI] [PubMed] [Google Scholar]
- 7.Shapiro A, Ferenczy A, Turcotte R, Bruchim I, Gotlieb WH. Uterine smooth-muscle tumor of uncertain malignant potential metastasizing to the humerus as a high-grade leiomyosarcoma. Gynecol Oncol. 2004;94:818–820. doi: 10.1016/j.ygyno.2004.05.049. [DOI] [PubMed] [Google Scholar]
- 8.Ng JS, Han A, Chew SH, Low J. A clinicopathologic study of uterine smooth muscle tumours of uncertain malignant potential (STUMP) Ann Acad Med Singapore. 2010;39:625–628. [PubMed] [Google Scholar]
- 9.Croce S, Young RH, Oliva E. Uterine leiomyomas with bizarre nuclei: a clinicopathologic study of 59 cases. Am J Surg Pathol. 2014;38:1330–1339. doi: 10.1097/PAS.0000000000000249. [DOI] [PubMed] [Google Scholar]
- 10.Dall'Asta A, Gizzo S, Musarò A, Quaranta M, Noventa M, Migliavacca C, et al. Uterine smooth muscle tumors of uncertain malignant potential (STUMP): pathology, follow-up and recurrence. Int J Clin Exp Pathol. 2014;7:8136–8142. [PMC free article] [PubMed] [Google Scholar]
- 11.Croce S, Ribeiro A, Brulard C, Noel JC, Amant F, Stoeckle E, et al. Uterine smooth muscle tumor analysis by comparative genomic hybridization: a useful diagnostic tool in challenging lesions. Mod Pathol. 2015;28:1001–1010. doi: 10.1038/modpathol.2015.3. [DOI] [PubMed] [Google Scholar]
- 12.Bacanakgil BH, Deveci M, Karabuk E, Soyman Z. Uterine smooth muscle tumor of uncertain malignant potential: clinicopathologic-sonographic characteristics, follow-up and recurrence. World J Oncol. 2017;8:76–80. doi: 10.14740/wjon1031w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maltese G, Fontanella C, Lepori S, Scaffa C, Fucà G, Bogani G, et al. Atypical uterine smooth muscle tumors: a retrospective evaluation of clinical and pathologic features. Oncology. 2018;94:1–6. doi: 10.1159/000479818. [DOI] [PubMed] [Google Scholar]
- 14.Basaran D, Usubutun A, Salman MC, Narin MA, Boyraz G, Turkmen O, et al. The clinicopathological study of 21 cases with uterine smooth muscle tumors of uncertain malignant potential: centralized review can purify the diagnosis. Int J Gynecol Cancer. 2018;28:233–240. doi: 10.1097/IGC.0000000000001178. [DOI] [PubMed] [Google Scholar]
- 15.Soltan MM, Albasry AM, Eldosouky MK, Abdelhamid HS. Immunoexpression of progesterone receptor, epithelial growth factor receptor and galectin-3 in uterine smooth muscle tumors. Cell Mol Biol. 2018;64:7–12. [PubMed] [Google Scholar]
- 16.Conconi D, Chiappa V, Perego P, Redaelli S, Bovo G, Lavitrano M, et al. Potential role of BCL2 in the recurrence of uterine smooth muscle tumors of uncertain malignant potential. Oncol Rep. 2017;37:41–47. doi: 10.3892/or.2016.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cao HY, Yang S, Wang S, Deng LY, Lou JY. Is differential expression of p16INK4a based on the classification of uterine smooth muscle tumors associated with a different prognosis? A meta-analysis. Genet Mol Res. 2017;16:16. doi: 10.4238/gmr16019481. [DOI] [PubMed] [Google Scholar]
- 18.Hewedi IH, Radwan NA, Shash LS. Diagnostic value of progesterone receptor and p53 expression in uterine smooth muscle tumors. Diagn Pathol. 2012;7:1. doi: 10.1186/1746-1596-7-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O'Neill CJ, McBride HA, Connolly LE, McCluggage WG. Uterine leiomyosarcomas are characterized by high p16, p53 and MIB1 expression in comparison with usual leiomyomas, leiomyoma variants and smooth muscle tumours of uncertain malignant potential. Histopathology. 2007;50:851–858. doi: 10.1111/j.1365-2559.2007.02699.x. [DOI] [PubMed] [Google Scholar]
- 20.Atkins KA, Arronte N, Darus CJ, Rice LW. The Use of p16 in enhancing the histologic classification of uterine smooth muscle tumors. Am J Surg Pathol. 2008;32:98–102. doi: 10.1097/PAS.0b013e3181574d1e. [DOI] [PubMed] [Google Scholar]
- 21.Ünver NU, Acikalin MF, Öner Ü, Ciftci E, Ozalp SS, Colak E. Differential expression of P16 and P21 in benign and malignant uterine smooth muscle tumors. Arch Gynecol Obstet. 2011;284:483–490. doi: 10.1007/s00404-010-1690-z. [DOI] [PubMed] [Google Scholar]
- 22.Croce S, Ducoulombier A, Ribeiro A, Lesluyes T, Noel JC, Amant F, et al. Genome profiling is an efficient tool to avoid the STUMP classification of uterine smooth muscle lesions: a comprehensive array-genomic hybridization analysis of 77 tumors. Mod Pathol. 2018;31:816–828. doi: 10.1038/modpathol.2017.185. [DOI] [PubMed] [Google Scholar]
- 23.Dgani R, Piura B, Ben-Baruch G, Open M, Glezerman M, Nass D, et al. Clinical-pathological study of uterine leiomyomas with high mitotic activity. Acta Obstet Gynecol Scand. 1998;77:74–77. doi: 10.1034/j.1600-0412.1998.770116.x. [DOI] [PubMed] [Google Scholar]
- 24.Vilos GA, Marks J, Ettler HC, Vilos AG, Prefontaine M, Abu-Rafea B. Uterine smooth muscle tumors of uncertain malignant potential: diagnostic challenges and therapeutic dilemmas. Report of 2 cases and review of the literature. J Minim Invasive Gynecol. 2012;19:288–295. doi: 10.1016/j.jmig.2011.12.025. [DOI] [PubMed] [Google Scholar]
- 25.Takeda A, Imoto S, Mori M, Nakamura H. Successful pregnancy outcome after laparoscopic-assisted excision of a bizarre leiomyoma: a case report. J Med Case Reports. 2011;5:344. doi: 10.1186/1752-1947-5-344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Campbell JE, Knudtson JF, Valente PT, Robinson RD, Kost ER. Successful pregnancy following myomectomy for uterine smooth muscle tumor of uncertain malignant potential: a case report and review of the literature. Gynecol Oncol Rep. 2015;15:1–3. doi: 10.1016/j.gore.2015.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ha HI, Choi MC, Heo JH, Kim KA, Jung SG, Park H, et al. A clinicopathologic review and obstetric outcome of uterine smooth muscle tumor of uncertain malignant potential (STUMP) in a single institution. Eur J Obstet Gynecol Reprod Biol. 2018;228:1–5. doi: 10.1016/j.ejogrb.2018.06.003. [DOI] [PubMed] [Google Scholar]
- 28.Hughes L, Roex A, Parange A. STUMP, a surprise finding in a large fibroid uterus in a 20-year-old woman. Int J Womens Health. 2018;10:211–214. doi: 10.2147/IJWH.S153838. [DOI] [PMC free article] [PubMed] [Google Scholar]