Abstract
22q11.2 heterozygous multigene deletions confer an increased risk of schizophrenia with marked impairment of cognition. We explored whether genes on 22q11.2 are associated with cognitive performance in patients with idiopathic schizophrenia. A total of 240 schizophrenia patients and 240 healthy controls underwent the Japanese-language version of the Brief Assessment of Cognition in Schizophrenia (BACS) and were genotyped for 115 tag single-nucleotide polymorphisms (tag SNPs) at the 22q11.2 region using the golden gate assay (Illumina®). Associations between z-scores of the BACS cognitive domains and SNPs and haplotypes were analyzed using linear regression in PLINK 1.07. An additional set of 149 patients with bipolar disorder were included for cognitive assessment and selected SNPs were genotyped using real-time PCR. Patients with schizophrenia and bipolar disorder showed qualitatively comparable profiles of cognitive impairment across BACS subdomains, as revealed by significant correlation between the two groups in the resulting cognitive effect sizes relative to controls. rs4819522 (TBX1) and rs2238769 (UFD1L) were significantly and nominally associated, respectively, with symbol coding in patients with schizophrenia. Haplotype analyses revealed that haplotypes containing the A allele at rs4819522 and G allele at rs2238769 showed significant negative associations with symbol coding in patients with schizophrenia. There was no effect of any haplotypes on cognition in patients with bipolar disorder. Our results have implications for the understanding of the role of haplotypes of UFD1L and TBX1 genes associated with symbol coding in patients with schizophrenia. Further replication studies in a cohort of newly diagnosed patients and other ethnicities are warranted.
Keywords: Schizophrenia, 22q11.2, TBX1, UFD1L, BACS, Symbol coding
1. Introduction
Growing evidence shows that a broad range of cognitive impairment is a hallmark of patients with schizophrenia. High heritability was found from studying the cognitive phenotypes of relatives of schizophrenia probands (Dickson et al., 2014; Scala et al., 2012; Schulze-Rauschenbach et al., 2015). Cognitive impairments, albeit less severe, are also present in a substantial number of patients with bipolar disorder in a euthymic state (Arts et al., 2008; Mann-Wrobel et al., 2011; Robinson et al., 2006). Patients with schizophrenia and bipolar disorder also show qualitatively similar patterns of cognitive impairment across different cognitive areas (Hill et al., 2013; Reichenberg et al., 2009; Sanchez-Morla et al., 2009; Schretlen et al., 2007; Zanelli et al., 2010), implying that the molecular underpinning of these traits may be shared by these two disorders. However, recent studies have suggested that multiple cognitive subgroups (almost intact, impaired in selective cognitive area, and globally impaired) are distributed among patients with bipolar disorder (Burdick et al., 2014; Russo et al., 2017) and even across diagnostic categories (Bora, 2016; Lewandowski et al., 2014).
Recent advances in genome-wide association studies (GWAS) have boosted our understanding of the molecular mechanisms behind the cognitive status of patients with schizophrenia and controls (Ohi et al., 2015). Here, we focus on the chromosomal segment spanning ~3.0 Mb or nested 1.5 Mb in size on the q11.2 band of chromosome 22, a region known as 22q11.2, with a tendency to undergo heterozygous multigene deletion, which in turn markedly increases the risk for neuropsychiatric disorders (Gur et al., 2017; Jonas et al., 2014). Several lines of linkage study suggesting the involvement of 22q11.2 region in psychosis (Hamshere et al., 2005; Williams et al., 2003) were preceded by studies in the early 1990s showing the link between 22q11.2 hemizygous deletion and schizophrenia (Driscoll et al., 1992; Pulver et al., 1994; Scambler et al., 1992; Shprintzen et al., 1992). A recent large-scale collaborative study showed that schizophrenia and a broad range of psychotic disorders were present in 30% and 41% of adults, respectively, among 1402 participants with 22q11.2 deletion (22q11.2 D) syndrome (Schneider et al., 2014). Moreover, a recent genome-wide study on the contribution of copy number variants to the risk has ranked 22q11.2D as one of the loci involved in the highest known genetic risk for the development of schizophrenia (Marshall et al., 2017). Regarding cognition, several studies have suggested that decline in full-scale intelligence quotient (IQ) (Vorstman et al., 2015) and cognitive functions, despite once being attained (Antshel et al., 2017), during childhood and adolescence are potential markers for the emergence of overt psychosis in carriers of 22q11.2D between late adolescence and early adulthood.
While 22q11.2 encompasses approximately 40–50 genes (Gur et al., 2017; Jonas et al., 2014), most clinical studies on the association between this genomic region and cognitive dysfunction in schizophrenia have been confined to catechol-O-methyltransferase gene (COMT) (Barnett et al., 2008; Bilder et al., 2002; Bruder et al., 2005; Dickerson et al., 2007; Egan et al., 2001; Lopez-Garcia et al., 2013). In contrast, specific genes mapped to the mouse ortholog of human 22q11.2 have been explored to elucidate the role of these genes in cognition and affective behaviors (Boku et al., 2018; Hiramoto et al., 2011; Hiroi, 2018; Hiroi et al., 2013; Hiroi et al., 2005; Paylor et al., 2006; Takahashi et al., 2016). We thus aim to determine whether the genetic architecture of cognitive dysfunction in schizophrenia may involve multiple adjacent genes on 22q11.2.
The Brief Assessment of Cognition in Schizophrenia (BACS) is a battery including six major cognitive domains (Keefe et al., 2004). It has been widely used as a portable and acceptable instrument with high reliability to assess the cognitive impairment of subjects with schizophrenia and affective disorder by other research groups (Bralet et al., 2007; Chianetta et al., 2008; Keefe et al., 2004) and ourselves (Akiyama et al., 2016; Kuratomi et al., 2013; Saito et al., 2017). The purpose of this study was to explore if genetic variants at 22q11.2 are associated with cognitive impairment in patients with schizophrenia and bipolar disorder with the latter being added to investigate the disease-specific aspects of our findings, in comparison with controls from the Japanese population.
2. Materials and methods
2.1. Sample collection of patients and control subjects
Participants consisted of 240 control subjects [143 males and 97 females; age: mean ± standard deviation (SD), 48.0 ± 13.0 years], 240 patients with schizophrenia (140 males and 100 females; age, 48.2 ± 12.6 years), and 149 patients with bipolar disorder (84 males and 65 females; age, 51.6 ± 11.6 years; Table 1). Patients with schizophrenia and bipolar disorder, diagnosed according to the Diagnostic and Statistical Manual of Mental Disorders fifth edition (American Psychiatric Association, 2013), were recruited from Dokkyo Medical University School of Medicine Hospital and affiliated hospitals. All patients had been clinically stable for at least 4 weeks before the present study. The control subjects were volunteers from among mainly nonprofessional university/hospital staff, unrelated to the patients, and were free of any mental disorders. All subjects lived in the Kanto region of Japan, and some of them had been included in our previous studies (Akiyama et al., 2016; Kuratomi et al., 2013; Saito et al., 2013; Saito et al., 2017). Exclusion criteria included a history of neurological disorder, significant head injury, or substance dependence or abuse with the exception of nicotine. Dosages of individual antipsychotics were gauged based on the equivalent milligram dosage of haloperidol as reported previously (Inada and Inagaki, 2015) and were summed to obtain cumulative dosages. The objective of the present study was clearly explained, and written informed consent was obtained from all subjects in accordance with the Declaration of Helsinki (http://www.wma.net). The whole study was formally approved by the Institutional Review Board of the Ethical Committees of Dokkyo Medical University School of Medicine and affiliated hospitals.
Table 1.
Demographic and clinical variables of healthy control subjects and patients with schizophrenia and bipolar disorder.
| Variables | Healthy control subjects (n = 240) |
Patients with schizophrenia (n = 240) |
Patients with bipolar disorder (n = 149) |
P-values⁎ |
|||||
|---|---|---|---|---|---|---|---|---|---|
| Mean (SD) | Median (25th–75th in %) | Mean (SD) | Median (25th–75th in %) | Mean (SD) | Median (25th–75th in %) | Ctrl vs Sch | Ctrl vs BP | Sch vs BP | |
| Male/female | 143/97 | 140/100 | 84/65 | P = 0.781 | P = 0.533 | P = 0.704 | |||
| Age (years) | 48.0 (13.0) | 50.0 (36.0–60.0) | 48.2 (12.6) | 49.0 (37.3–59.0) | 51.6 (11.6) | 52.0 (44.0–60.0) | P = 0.949 | P = 0.020 | P = 0.013 |
| JART-estimated premorbid IQ | 103.4 (10.7) | 104.8 (97.1–111.6) | 92.3 (10.4) | 90.4 (84.6–99.0) | 100.4 (11.1) | 101.9 (91.3–109.6) | P < 0.0001 | P = 0.011 | P < 0.0001 |
| Education duration (years) | 14.2 (2.3) | 15.0 (12.0–16.0) | 11.7 (2.1) | 12.0 (9.0–12.0) | 13.5 (2.3) | 13.0 (12.0–16.0) | P < 0.0001 | P = 0.001 | P < 0.0001 |
| Age at onset (years) | – | – | 24.8 (8.8) | 23.0 (19.0–28.0) | 33.3 (12.0) | 30.0 (24.0–42.0) | – | – | P < 0.0001 |
| PANSS positive | – | – | 14.1 (5.3) | 13.0 (10.0–17.8) | 8.9 (2.9) | 8.0 (7.0–9.0) | – | – | P < 0.0001 |
| Negative | – | – | 22.0 (6.7) | 22.0 (18.0–26.0) | 11.9 (5.9) | 9.0 (8.0–14.0) | – | – | P < 0.0001 |
| General psychopathology | – | – | 32.9 (9.5) | 32.0 (25.0–40.0) | 22.6 (7.6) | 20.0 (17.0–26.0) | – | – | P < 0.0001 |
| HAMD | – | – | – | – | 3.4 (4.7) | 2.0 (0–5.0) | – | – | – |
| YMRS | – | – | – | – | 1.2 (2.2) | 0 (0–2.0) | – | – | – |
| Total antipsychotics (mg/day)† | – | – | 12.6 (9.2) | 11.0 (6.0–16.6) | 5.1 (7.3) | 2.0 (0–7.3) | – | – | P < 0.0001 |
| Male/female (total) | – | – | 78/58 (136) | 84/65 (149) | – | – | P = 0.868 | ||
| Total SFS scores | – | – | 105.6 (28.5) | 127.3 (30.5) | – | – | P < 0.0001 | ||
Abbreviations: JART, Japanese Adult Reading Test; PANSS, Positive and Negative Syndrome Scale; HAM-D, Hamilton Rating Scale for Depression; YMRS, Young Mania Rating Scale; SFS, Social Functioning Scale.
Dashes (−) indicate that values are not present.
Equivalent milligram dosage of haloperidol.
P-values for the difference among the healthy control subjects (n = 240), patients with schizophrenia (n = 240), and those with bipolar disorder (n = 149) in demographic variables were determined using either the Mann–Whitney test or χ2 test. P-value for the difference between the patients with bipolar disorder (n = 149) and patients with schizophrenia (n = 136) undergoing SFS was determined using Student's t– test.
2.2. Assessments
2.2.1. Japanese version of the BACS
All participants were administered Version A of the Japanese version of the BACS, as per previous reports (Akiyama et al., 2016; Kaneda et al., 2007). This includes brief assessment of verbal memory (list learning), a digit sequencing task, a token motor task, a verbal fluency test (category instances and controlled oral word association test), symbol coding, and a Tower of London (TOL) task. The primary scores for each BACS subtest were transformed into z-scores whereby the mean for healthy control subjects was set to zero and the standard deviation (SD) to one.
2.2.2. Premorbid intelligence quotient assessment
All participants were assessed with the Japanese Adult Reading Test (JART) (Akiyama et al., 2016), a Japanese version of the National Adult Reading Test that provides a valid means of estimating premorbid intelligence in schizophrenia patients (Crawford et al., 2001).
2.2.3. Positive and negative symptom scale
Patients were assessed according to their clinical rating of symptomatology of schizophrenia using the Positive and Negative Symptom Scale (PANSS), a 30-item scale (Kay et al., 1987). Positive symptoms (items P1 to P7), negative symptoms (items N1 to N7), and general psychopathology (items G1 to G16) were evaluated with summed scores of each category being used as separate variables (subscales).
2.2.4. Assessment of euthymia
To ensure that we only selected bipolar patients with moods within the euthymic range, we primarily recruited patients who had scores of 10 points or less on the 17-item Hamilton Depression Rating Scale (HAM-D) and scores of 10 points or less on the Young Mania Rating Scale (YMRS), based on the findings of a previous meta-analysis (Robinson et al., 2006).
2.2.5. Assessment of the social functioning scale (SFS)
Patients (136 patients with schizophrenia and all patients with bipolar disorder) were assessed by the Japanese version of the SFS (Chino et al., 2009), a self-reported questionnaire. This included the following items: withdrawal, interpersonal communication, independence performance, recreational activities, pro-social performance, independence competence, and employment (Birchwood et al., 1990).
2.3. Tag SNP selection and quality control
We first consulted the existing HapMap genotyping database (http://www.hapmap.org/index.html.ja, release 27/phase II, population: Japanese) as a general map to select our tag SNPs to reduce the number of redundant markers (Crawford and Nickerson, 2005) and found SNPs mapped to 3 Mb corresponding to the 22q11.2 genomic region. The registered SNPs were categorized into the following four groups: class 1, non-synonymous exonic SNPs causing amino acid substitution; class 2, synonymous exonic SNPs, SNPs located in the untranslated regions (UTRs), SNPs located in the promoters up to 2 kb upstream, and intronic SNPs in close proximity to exon–intron boundaries of <50 bp; class 3, other intronic SNPs; and class 4, intergenic SNPs. Tag SNPs representing other SNPs in the same linkage disequilibrium (LD) were identified using the Tagger function implemented in Haploview (Barrett et al., 2005) with the criterion of an r2 threshold >0.8 in “pairwise tagging only” mode. SNPs were preferentially selected from those belonging to class 1 and class 2 when identified as tag SNPs. Even those that were assigned to class 3 and class 4 were selected unless there was a single class 1 or class 2 tag SNP within an LD. Additionally, clinically relevant SNPs, which were not included in the HapMap database, were manually included in accordance with the literatures. SNPs with a minor allele frequency of <0.01 were excluded. Validation of the selected SNPs was performed to check for consistency with the manufacturer's algorithm that defines eligibility for golden gate assay (Illumina®). The 131 SNPs were finally selected, with each being mapped per 17 kb on average over 1.95 Mb for genotyping for the controls and patients with schizophrenia.
2.4. Genotyping
We collected peripheral blood from all of the subjects, and extracted genomic DNA using a QIAamp DNA Blood Maxi Kit (Qiagen, Valencia, CA, USA). Genotyping was performed using golden gate assay (Illumina®) in Riken Genesis (Yokohama, Japan) for the controls and patients with schizophrenia. For patients with bipolar disorder, nine SNPs (8 SNPs shown in Table 4 and rs10160) were selected and genotyped using Taqman SNP Genotyping Assay (Applied Biosystems, Foster City, CA, USA).
Table 4.
Association between haplotypes mapping to 22q11.2 and cognitive performance on BACS in patients with bipolar disorder.
| Covariates* |
Sex/Age/education/Premorbid IQ |
Sex/Age/education/Premorbid IQ/PANSS |
||||
|---|---|---|---|---|---|---|
| Gene | Haplotype | t | P-value | t | P-value | |
| Digit sequence |
C22orf39 / UFD1L | rs11744–rs2238769 | 0.143 | 0.9806 | 0.294 | 0.947 |
| rs11744–2238769–rs5746742 | 4.56 | 0.3133 | 4.56 | 0.171 | ||
| UFD1L | rs2238769–rs5746742 | 3.99 | 0.2133 | 5.24 | 0.2437 | |
|
KLHL22 |
rs1771144–rs4821372 |
5.51 |
0.0645 |
4.73 |
0.1002 |
|
| Token motor |
TBX1 |
rs2238777–rs4819522–rs5746826 |
9.39 |
0.102 |
4.88 |
0.4565 |
| Symbol coding | C22orf39 / UFD1L | rs11744–rs2238769 | 5.07 | 0.1288 | 6.43 | 0.0762 |
| rs11744–rs2238769–rs5746742 | 4.51 | 0.315 | 4.2 | 0.1966 | ||
| UFD1L | rs2238769–rs5746742 | 2.16 | 0.4709 | 6.3 | 0.1619 | |
| TBX1 | rs2238777–rs4819522 | 1.1 | 0.7678 | 1.25 | 0.7147 | |
| rs4819522–rs5746826 | 0.336 | 0.9568 | 0.283 | 0.9641 | ||
| rs2238777–rs4819522–rs5746826 | 2.38 | 0.8413 | 4.12 | 0.564 | ||
Associations between haplotypes and the BACS z-scores were determined after adjustment for covariates using a linear regression model implemented in PLINK version 1.0.7. Sliding-window approach was adopted to generate two-SNP and three-SNPs. The associations were adjusted for two alternative sets of covariates, that is, the common set of covariates and the other composed of the common set and PANSS scores. Omnibus P-values were determined by running 10,000 permutations using the max (T) procedure to correct for multiple testing.
2.5. Statistical analyses
Statistical analyses involving factors other than genetics were implemented in SPSS (version 23.0; IBM Japan, Tokyo, Japan). The sex ratio between patients and healthy controls was analyzed using a chi-squared test. Age, JART-estimated premorbid IQ, education duration, and BACS z-scores were regarded as continuous variables and were assessed for a normal distribution using a Shapiro–Wilk test, and if appropriate, standardized and normalized across all of the subjects with a mean of zero and SD of one using rank transformation, as described previously (Kuratomi et al., 2013).
Deviations of each SNP from Hardy–Weinberg equilibrium were analyzed for each of the control and schizophrenia groups by the exact test implemented in PLINK version 1.0.7 (Purcell et al., 2007). SNPs with P values < 0.001 were considered to indicate departure from the Hardy–Weinberg equilibrium, as described by Saito et al. (Saito et al., 2013). The standard measures of pairwise LD, denoted as Dʹ, were estimated in Haploview 4.2 (Barrett et al., 2005) based on our genotype data from the controls and patients with schizophrenia.
Associations between genotypes and individual z-scores of the BACS cognitive domains were analyzed using a linear regression model implemented in PLINK version 1.0.7 (Purcell et al., 2007), as previously reported (Kuratomi et al., 2013). Sex and appropriately normalized values of age, years of education, and JART-estimated premorbid IQ were used as a common set of covariates in this linear regression for patients and controls. For the controls and patients with schizophrenia, associations between each SNP and cognitive domains were tested under the following three genetic models: dominant (comparing minor allele carriers with the major allele homozygotes), recessive (comparing minor allele homozygotes with major allele carriers), and additive (assuming an allelic dosage effect by coding none, one, and two copies of the minor allele as 0, 1, and 2, respectively).
Haplotype blocks were defined based on the pairwise values of Dʹ in accordance with the work of Gabriel et al. (Gabriel et al., 2002). Associations between haplotypes and the BACS z-scores were assessed using a linear regression model implemented in PLINK version 1.0.7 (Purcell et al., 2007), in which a sliding-window approach was adopted to generate two-SNP and three-SNP, and, if appropriate, four-SNP haplotypes, each shifted by one SNP within defined haplotype blocks. For the control, the haplotype association was calculated after adjustment for the common set of covariates (see above). For patients with schizophrenia and bipolar disorder, it was calculated after adjustment for two alternative sets of covariates, that is, the common set of covariates and the other composed of the common set and PANSS scores. When an omnibus P value for a constructed haplotype was significant (P < 0.00833, see below), estimated frequencies and β values for specific haplotypes were calculated. For both individual SNPs and haplotypes, P values were determined by running 10,000 permutations using the max (T) procedure adjusted for multiple tests. The significance levels were set at 0.00833 (0.05/6) to correct for the six BACS subdomains.
2.6. In silico analysis
The functional consequences of missense mutations were predicted by Polymorphism phenotyping (PolyPhen-2) (Adzhubei et al., 2010) (http://genetics.bwh.harvard.edu/pph2), and by Sorting Intolerant From Tolerant (SIFT) (Kumar et al., 2009) (http://sift.jcvi.org/).
2.7. Power analysis
A power analysis for linear regression was performed by G*Power 3.1.9 using linear multiple regression: Deviation of a Subset of Linear Regression Coefficients From Zero (Fixed Model) providing power analysis for testing the null hypothesis that a certain type of predictor does not increase the proportion of explained variance (Faul et al., 2009). According to Faul et al. (Faul et al., 2009), an effect size (f2) was calculated as follows: (R2 accounted for by A − R2 accounted for by B) divided by (1 − R2 accounted for by A), where B is the common set of covariates (see above, for patients, PANSS scores were added as an option) and A includes B plus genotypes of a relevant SNP. By using multiple regression analysis implemented in SPSS, R2 was calculated to ask for the extent to which z-scores of BACS subdomain of interest were explained by these covariates. Power (1–β) was calculated by assuming an α error of 0.0083, a sample size of 240 (for each of controls and patients with schizophrenia), and the obtained value of the f2.
3. Results
3.1. Demographic data
Descriptive data of the participants are shown in Table 1. Patients with schizophrenia and bipolar disorder had significantly (P < 0.0001, Mann−Whitney test) lower z-scores for all of the six BACS subdomains than controls. Further patients with schizophrenia had significantly lower z-scores for five BACS subdomains (P < 0.0001, Mann−Whitney test), with the exception of the TOL task (P = 0.295, Mann−Whitney test), than those with bipolar disorder (Fig. 1A). Cohen's d effect sizes of the performance difference among cases and controls were computed, which resulted in a significant correlation (r = 0.886, P = 0.019) being identified between the two case groups in cognitive effect sizes relative to controls (Fig. 1B).
Fig. 1.
Part A: The z-scores for six BACS subdomains for patients with schizophrenia (Sch, solid line) and those with bipolar disorder (BP, dotted line) relative to the healthy control subjects for which the mean and standard deviation were set to zero and one, respectively. Ⓐ,verbal memory (list learning); Ⓑ, digit sequencing task; Ⓒ, token motor task; Ⓓ, verbal fluency test; Ⓔ, symbol coding; Ⓕ, Tower of London (TOL) task.
Part B: Plots of Cohen's d effect sizes of the cognitive performance for patients with both disorders relative to that of controls (Ctrl). There was a significant correlation (r = 0.886, P = 0.019) between the two groups in the cognitive effect sizes across the BACS domains that are abbreviated as described above.
3.2. LD block constructed using SNP data of all of the controls and patients with schizophrenia
Sixteen SNPs were not called among the selected 131 SNPs. Subsequent analysis was performed for the remaining 115 SNPs, which were genotyped with a success rate over 99% across the 480 participants of controls and patients with schizophrenia. No hemizygosity was found for the used SNPs across the groups. Irrespective of diagnostic group (the controls, patients with schizophrenia and combined samples), all of the 115 SNPs were in Hardy–Weinberg equilibrium (all P > 0.001), and they had minor allele frequencies >3%. We completed the self-constructed LD map that ensures the capture of haplotypes for the population under study (van den Oord and Neale, 2004). The observed LD block length varied from 3 to 83 kb with an average of 28 kb.
3.3. Association between SNP and BACS cognitive subdomains in the controls and patients with schizophrenia
P values for the associations between SNPs and cognitive domains for the patients with schizophrenia and controls are shown in Fig. 2. In patients with schizophrenia, rs2238769 (UFD1L) was nominally (P < 0.041) associated with symbol coding under the additive model, and rs4819522 (TBX1), which is a missense variant, was significantly associated with symbol coding under the additive and dominant models (P < 0.0076 and P < 0.0075, respectively). In patients with schizophrenia, the calculated effect size (f2) of rs4819522 in explaining the z-scores of the symbol coding task was 0.0815, resulting in a power (1–β) of 0.960. When PANSS scores was included in covariates, this effect size (f2) of rs4819522 was 0.0743, resulting in a power (1–β) of 0.940 (Supplementary Table 1). Assessments with Polyphen-2 and SIFT showed that a missense mutation at rs4819522 induces an amino acid change (from threonine to methionine), which has a possibly damaging impact: a score of 0.736 for Polyphen-2 and 0.048 (cutoff = 0.05) for SIFT. In patients with schizophrenia, rs1771144 (KLHL22) was nominally (P < 0.0128) associated with digit symbol coding under the dominant model, and rs10160 (DGCR2) nominally (P < 0.01) with token motor task under the recessive model. The healthy controls did not show any association between single SNPs and BACS domains.
Fig. 2.
Part A: Association between 115 SNPs mapping to 22q11.2 and BACS cognitive subdomains in patients with schizophrenia.
Part B: Association between 115 SNPs mapping to 22q11.2 and BACS cognitive subdomains in controls.
Vertical lines represent P-values depicted on a logarithmic scale. SNPs were arranged on the horizontal lines in order of chromosomal position. P-values were determined by running 10,000 permutations using the max (T) procedure adjusted for multiple tests implemented in PLINK version 1.0.7. The significance level was set at 0.00833 (0.05/6) to correct for the six BACS subdomains. SNPs that showed at least a nominally significant level (P < 0.05) were denoted over P value peaks.
3.4. Haplotype association with BACS cognitive subdomains
The omnibus test scores for the associations of locus haplotypes with individual cognitive subdomains in the patients with schizophrenia and controls are shown in Table 2 and Supplementary Table 2 (for nominal significance only). Table 3 shows the association between cognitive subdomains and specific haplotypes only for haplotypes with P < 0.00833 in omnibus tests.
Table 2.
Omnibus tests for association between haplotypes mapping to 22q11.2 and cognitive performance on BACS in controls and patients with schizophrenia.
| Control |
Patients with schizophrenia |
|||||||
|---|---|---|---|---|---|---|---|---|
| Covariates | Sex/Age/Education/ Premorbid IQ | Sex/Age/Education/ Premorbid IQ | Sex/Age/Education/ Premorbid IQ/PANSS | |||||
| BACS subdomains | Gene | Haplotype | t | P-value | t | P-value | t | P-value |
| Digit sequence | CDC45 | rs5748239–rs5748240–rs2073734–rs2073733 | 4.3 | 0.74 | 21.7 | 0.0022 | 18.7 | 0.0067 |
| Token motor |
ESS2 | rs1052763–rs1052773 | 14.4 | 0.0047 | 5.34 | 0.251 | 5.08 | 0.2589 |
| rs3747052–rs1052763–rs1052773 | 18.5 | 0.003 | 5.33 | 0.4407 | 5.09 | 0.4675 | ||
| rs1223335–rs3747052–rs1052763–rs1052773 | 18.4 | 0.0062 | 5.22 | 0.6462 | 5.08 | 0.6601 | ||
|
TBX1 |
rs2238777–rs4819522–rs5746826 |
1.08 |
0.973 |
25.1 |
0.0003 |
18.1 |
0.0031 |
|
| Symbol coding | CLTCL1 | rs3761407–rs735369 | 0.323 | 0.8674 | 10.9 | 0.0044 | 9.56 | 0.0082 |
| HIRA | rs2238765–rs2283652 | 0.538 | 0.9691 | 10.4 | 0.0193 | 12.4 | 0.0082 | |
| UFD1L | rs2238769–rs5746742 | 1.5 | 0.842 | 13.3 | 0.0083 | 12.1 | 0.0156 | |
| TBX1 | rs2238777–rs4819522 | 7.68 | 0.0455 | 18.4 | 0.0007 | 13.8 | 0.0026 | |
| rs4819522–rs5746826 | 7.7 | 0.0453 | 16.6 | 0.0013 | 13.7 | 0.0029 | ||
| rs2238777–rs4819522–rs5746826 | 8.99 | 0.1154 | 26.5 | 0.0004 | 19.9 | 0.0015 | ||
Associations between haplotypes mapping to 22q11.2 and the BACS z-scores were determined after adjustment for covariates using a linear regression model implemented in PLINK version 1.0.7. Sliding-window approach was adopted to generate two-SNP and three-SNP haplotypes, and, if appropriate, four-SNP haplotypes. For the control, the associations were adjusted for the common set of covariates consisting of sex, age, years of education, and JART-estimated premorbid IQ. For patients with schizophrenia, the associations were adjusted for two alternative sets of covariates, i.e., the common set of covariates and the other composed of the common set and PANSS scores. Omnibus P-values were determined by running 10,000 permutations using the max (T) procedure to correct for multiple testing, and set at 0.00833 (0.05/6) as a significance level to correct for the six BACS subdomains. Statistically significant results are marked with bold letters.
Table 3.
Association between specific haplotypes mapping to 22q11.2 and cognitive performance on BACS in patients with schizophrenia.
| Covariates |
Sex/Age/education/premorbid IQ |
Sex/Age/education/premorbid IQ /PANSS |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Gene | Haplotype | Specific Haplotype | Freq | β | t | P-value | Freq | β | t | P-value | |
| Digit sequence |
CDC45 |
rs5748239–rs5748240–rs2073734–rs2073733 |
A–G–C–A | 0.215 | 0.0506 | 0.597 | 0.978 | 0.215 | 0.0451 | 0.528 | 0.9868 |
| A–G–G–G | 0.0771 | 0.267 | 6.6 | 0.1173 | 0.0771 | 0.265 | 7.27 | 0.08379 | |||
| G–A–C–G | 0.25 | 0.103 | 2.7 | 0.5887 | 0.25 | 0.0724 | 1.48 | 0.8513 | |||
| G–G–C–G | 0.132 | 0.0362 | 0.216 | 0.9986 | 0.132 | 0.0308 | 0.176 | 0.9994 | |||
| A–G–C–G |
0.325 |
–0.244 |
17.3 |
0.0009 |
0.325 |
–0.208 |
13.8 |
0.0029 |
|||
| Token motor |
TBX1 |
rs2238777–rs4819522–rs5746826 |
A–G–C | 0.416 | –0.131 | 3.26 | 0.3236 | 0.416 | –0.109 | 2.84 | 0.3807 |
| G–G–C | 0.0467 | 0.783 | 21.3 | 0.0002 | 0.0467 | 0.594 | 14.7 | 0.0008 | |||
| G–A–A | 0.0854 | –0.195 | 2.33 | 0.491 | 0.0854 | –0.0964 | 0.705 | 0.8994 | |||
| A–G–A | 0.0592 | 0.0884 | 0.334 | 0.9699 | 0.0592 | 0.149 | 1.16 | 0.7797 | |||
| G–G–A |
0.393 |
0.0435 |
0.333 |
0.9703 |
0.393 |
0.00837 |
0.0155 |
1 |
|||
| Symbol coding |
CLTCL1 |
rs3761407–rs735369 |
A–G | 0.314 | –0.17 | 11.4 | 0.0107 | 0.314 | –0.139 | 9.38 | 0.0262 |
| C–C |
0.682 |
0.166 |
10.9 |
0.0134 |
0.682 |
0.14 |
9.56 |
0.0245 |
|||
|
HIRA |
rs2238765–rs2283652 |
A–A | 0.343 | 0.114 | 5.38 | 0.1716 | 0.343 | 0.11 | 6.16 | 0.1168 | |
| G–G | 0.109 | –0.213 | 7.65 | 0.0596 | 0.109 | –0.209 | 9.28 | 0.0274 | |||
| A–G |
0.548 |
–0.0268 |
0.309 |
0.9937 |
0.548 |
–0.0224 |
0.267 |
0.9961 |
|||
|
UFD1L |
rs2238769–rs5746742 |
C–A | 0.294 | 0.113 | 4.86 | 0.2555 | 0.294 | 0.116 | 6.34 | 0.1306 | |
| G–G | 0.317 | –0.174 | 12.7 | 0.0068 | 0.317 | –0.142 | 10.4 | 0.02 | |||
| C–G |
0.39 |
0.0602 |
1.63 |
0.8215 |
0.39 |
0.0307 |
0.522 |
0.9877 |
|||
| TBX1 | rs2238777–rs4819522 |
G–A | 0.0854 | –0.339 | 16 | 0.0006 | 0.0854 | –0.277 | 13.1 | 0.0034 | |
| A–G | 0.475 | –0.00777 | 0.0259 | 0.9999 | 0.475 | 0.0157 | 0.133 | 0.9944 | |||
| G–G |
0.44 |
0.116 |
5.66 |
0.1012 |
0.44 |
0.0718 |
2.64 |
0.4438 |
|||
| rs4819522–rs5746826 |
G–C | 0.462 | 0.0238 | 0.233 | 0.9864 | 0.462 | 0.0167 | 0.143 | 0.9938 | ||
| A–A | 0.0854 | –0.339 | 16 | 0.0006 | 0.0854 | –0.277 | 13.1 | 0.0034 | |||
| G–A |
0.452 |
0.087 |
3.04 |
0.3561 |
0.452 |
0.0729 |
2.68 |
0.4368 |
|||
| rs2238777–rs4819522–rs5746826 | A–G–C | 0.416 | –0.037 | 0.553 | 0.9333 | 0.416 | –0.0209 | 0.22 | 0.9863 | ||
| G–G–C | 0.0467 | 0.36 | 9.22 | 0.0167 | 0.0467 | 0.226 | 4.39 | 0.184 | |||
| G–A–A | 0.0854 | –0.339 | 16 | 0.0006 | 0.0854 | –0.277 | 13.1 | 0.0034 | |||
| A–G–A | 0.0592 | 0.125 | 1.46 | 0.7067 | 0.0592 | 0.17 | 3.27 | 0.3295 | |||
| G–G–A | 0.393 | 0.0607 | 1.4 | 0.7235 | 0.393 | 0.0366 | 0.637 | 0.9138 | |||
Estimated frequencies, β values and P-values for specific haplotypes were calculated, when an omnibus P-value for a constructed haplotype was significant (P<0.00833). Associations between specific haplotypes and the BACS z-scores were assessed after adjustment for two alternative sets of covariates using a linear regression model implemented in PLINK version 1.0.7. P-values < 0.0083 (0.05/6) were considered as statistically significant to correct for the number of BACS-J subtests. Statistically significant results are marked with bold letters.
First, haplotypes formed on the UFD1L (rs2238769–rs5746742) gene and the CDC45 (rs5748239–rs5748240–rs2073734–rs2073733) gene were significantly associated with symbol coding and digit sequence task in omnibus tests, respectively, in patients with schizophrenia. After adjustment for covariates, including PANSS scores, the association involving rs5748239–rs5748240–rs2073734–rs2073733 with digit sequence was attenuated, whereas that of rs2238769–rs5746742 was no longer under the significant threshold. The specific haplotype A–G–C–G at rs5748239–rs5748240–rs2073734–rs2073733 had significant negative effects on the digit sequence task in patients with schizophrenia when adjusted for both types of covariate set. For rs2238769–rs5746742, its specific haplotype G–G had a significant negative effect on symbol coding in patients with schizophrenia after adjusting for covariates minus PANSS scores only.
Haplotypes formed at the TBX1 gene were significantly associated with token motor and symbol coding task in patients with schizophrenia in omnibus tests after adjustment for both types of covariate sets, although this was attenuated by adjustment for covariates including PANSS. For rs2238777–rs4819522, rs4819522–rs5746826 and rs2238777–rs4819522–rs5746826, their specific haplotypes G–A, A–A, and G–A–A, respectively, had significant negative effects on symbol coding task in patents with schizophrenia.
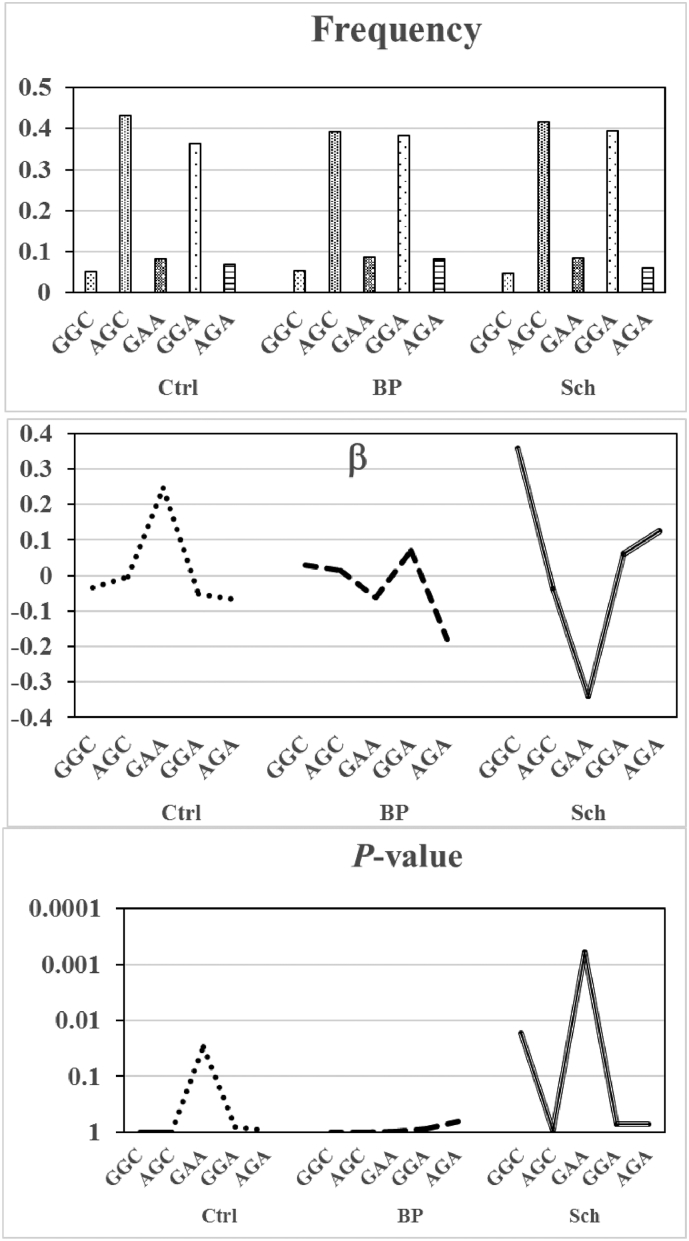
No haplotype was associated with any cognitive subdomains for patients with bipolar disorder (Table 4). Subsequently, between-group comparisons were made regarding frequency, β, and P values for the association between TBX1 haplotypes and symbol coding performance (Fig. 3). G–A–A, which is a missense-carrying specific haplotype at rs2238777–rs4819522–rs5746826, is present at a comparably low frequency among the three groups. However, β representing its effects on symbol coding task takes positive (β = 0.246, P = 0.0298) and negative (β = −0.339, P = 0.0006) values in the controls and patients with schizophrenia, respectively, suggesting the inversion of effects of missense-carrying haplotypes by both groups. Meanwhile, it had no effects (β = −0.0626, P = 0.987) on this task in patents with bipolar disorder. Similar findings were noted for G–A at rs2238777–rs4819522 and A–A at rs4819522–rs5746826 (data not shown).
Fig. 3.
Between-group comparisons regarding frequency, β, and P-values for the association between TBX1 rs2238777–rs4819522–rs5746826 TBX1 haplotypes and symbol coding performance. Ctrl, Controls; BP, Patients with bipolar disorder; Sch, Patients with schizophrenia. Note that summed frequencies of haplotypes closely approximate one.
4. Discussion
To our knowledge, this is the first study investigating an association between genetic variants at 22q11.2 and the cognitive impairment of patients with schizophrenia and bipolar disorder assessed using BACS. There are three main strengths to this study: 1) The two disease groups had similar profile patterns of cognitive performance on BACS subdomains, as revealed by a significant correlation between the two groups in their cognitive effect sizes. 2) Patients with schizophrenia, but not those with bipolar disorder or controls, had significant associations between symbol coding and haplotypes at UFD1L and TBX1. 3) Haplotypes including a missense allele carried at rs4819522 of TBX1 had differential effects on symbol coding among the controls, patients with schizophrenia, and patients with bipolar disorder.
The effect sizes for cognitive performance for patients with schizophrenia and bipolar disorder (Fig. 1) are almost consistent with those in previous studies (Hill et al., 2013; Reichenberg et al., 2009; Sanchez-Morla et al., 2009; Schretlen et al., 2007; Zanelli et al., 2010). This supports the utility of BACS as a reliable instrument to measure cognitive performance for bipolar disorder as well as schizophrenia.
UFD1L encodes the human homolog of the yeast ubiquitin fusion degradation 1 protein, which plays a role in the ubiquitin-dependent proteolytic pathway in the stress response in endoplasmic reticulum-associated degradation (Chen et al., 2011). A number of studies have reported that rs5992403 located upstream of the first exon of UFD1L is associated with schizophrenia (De Luca et al., 2001), and with early onset of this disorder (Ota et al., 2010). Xie et al. reported that haplotypes consisting of four SNPs, among which rs2238769, rs1547931, and rs5992403 were included in our study, were significantly associated with schizophrenia in Chinese family trios (Xie et al., 2008). Moreover, UFD1L gene expression was reported to be upregulated in the blood cells of ultra-high-risk subjects (Santoro et al., 2015). As for a role in cognition, only one study reported that rs5992403 AA genotype carriers had higher preservation error scores in the Wisconsin Card Sorting test (Ota et al., 2013). CDC45, the human homolog of a yeast cell cycle protein, is located in a genomic region telomeric and adjacent to UFD1L (McKie et al., 1998). Although the functional roles of the haplotypes of UFD1L and CDD45 that were significantly associated with cognitive impairment in schizophrenia remain unknown, further studies on the potential contribution of these genes to compromised neural networks in schizophrenia are warranted.
TBX1 and its mouse ortholog Tbx1 encode a transcription factor of the T-box family and induce physical phenotypes mimicking 22q11.2 deletion syndrome (22q11.2 DS) when mutated (Jerome and Papaioannou, 2001; Ogata et al., 2014; Yagi et al., 2003). The effect of this gene on neural function has been explored using mice heterozygous deletion for Tbx1 (Hiramoto et al., 2011; Hiroi et al., 2013; Paylor et al., 2006; Takahashi et al., 2016) and mice having copy number elevations of Tbx1 (Boku et al., 2018). These studies elucidate the pleiotropic effects of Tbx1 on behavioral phenotypes, especially in terms of social dimension (Hiramoto et al., 2011; Takahashi et al., 2016) and working memory (Boku et al., 2018; Hiramoto et al., 2011). A possibly damaging effect of a substitution at rs4819522 (from coding reference G to missense A allele) implied by Polyphen-2 and SIFT can contribute to significant negative impacts of specific haplotypes including this missense allele at the TBX1 gene on symbol coding performance in schizophrenia patients. The discrepant effects of this haplotype on symbol coding among the three groups may be interpreted as a qualitative interaction or crossover effects, meaning that environmental exposures can change the association in the opposite direction depending on the particular diagnostic group (Dorak, 2017). Cognitive reserve may be such an environmental factor that yields differential effects. The concept of cognitive reserve refers to the capacity to overcome pathophysiology and to cope with challenging difficulties in everyday life, and involves educational attainment, premorbid IQ and social activities (Anaya et al., 2016; Grande et al., 2017). In the present study, in accordance with previous studies (Hellvin et al., 2010; Trotta et al., 2015), patients with schizophrenia had shorter education duration, and lower JART-estimated premorbid IQ and total SFS scores than those with bipolar disorder (Table 1). We found that the effect sizes (f2) of SFS, in conjunction with sets of covariates (age, sex, education, premorbid IQ, and PANSS scores), in explaining z-scores of symbol coding as a dependent variable of rs4819522 were 0.056 (power = 0.532) and 0 for patients with schizophrenia and those with bipolar disorders, respectively (Supplementary Table 3). Another possibility is that haplotype effects might have been obscured by within-group cognitive heterogeneity in bipolar disorder, meaning that the cognitive ability in patients with bipolar disorder is divergent and may be classified into three different clusters: globally impaired, selectively impaired, and intact (Burdick et al., 2014; Russo et al., 2017).
Analysis of digit symbol coding reveals more severe impairment in patients with schizophrenia than other widely used cognitive instruments (Dickinson et al., 2007). This task was traditionally classified as a measure of “processing speed”, which is separated from classical cognitive domains, and as such included in the Measurement and Treatment Research to Improve Cognition in Schizophrenia Consensus Cognitive Battery for this purpose (Nuechterlein et al., 2008). However, recent studies suggested that other general cognitive domains (e.g., working memory and executive function) contribute to the level of performance of digit symbol coding that schizophrenia patients cannot coordinate (Bachman et al., 2010; Knowles et al., 2015). Taking these findings together, the neurocognitive coordination between processing speed and other multiple cognitive domains may play a key role in engaging in updated cognitive demands. Poor performance at symbol coding in patients with schizophrenia may be attributable to impairment of this coordination to the extent that they become incapable of engaging in the executive function that control subjects use, and have to rely on an alternative cognitive pathway (Knowles et al., 2015).
CLTCL1 gene and HIRA gene were found to have haplotypes significantly associated with symbol coding in patients with schizophrenia in omnibus tests (Table 2), but not in specific tests (Table 3). Little is known about the role of CLTCL1 and HIRA in cognition or psychosis, except for a study reporting the occurrence of rare variants in the HIRA gene in cases of intellectual disability (Anazi et al., 2017).
Control subjects did not show any haplotype–cognitive association like that of patients with schizophrenia. However, in controls but not in patients with schizophrenia, there was a significant association between haplotypes constructed by 3′-UTR SNPs in ESS2 and the token motor task in omnibus haplotype analysis (Table 2). Our controls were volunteers from nonprofessional university/hospital staff who are regarded as convenient source of samples and might have caused the healthy worker effect.
Significant findings in this study were derived mainly from haplotype-based linear regression analyses, confirming the utility of haplotype to detect the loci involved in specific phenotypes of interest (van den Oord and Neale, 2004). In general, haplotypes are associated with phenotypes more strongly than individual SNPs. This may be attributable to a concealed SNP that is in high LD with SNPs that make up the investigated haplotypes and might influence the association with phenotype (Dorak, 2017). This assumption appears to hold for CDC45, HIRA and ESS2 in the current study.
The G*power analysis indicated that the sample sizes in the current study, albeit small, approved statistical power to detect the association between genetic variants and cognition. There are several limitations to this study. The first involves the chronicity of patients who had been exposed to prolonged effects of psychosocial and other environmental factors. Future study warrants a cohort of newly diagnosed patients. The second limitation involves ethnicity; namely, the participants in the present study were all Japanese, suggesting that replications are necessary in other ethnicities. Third, the relationship between SNP density and haplotype block varied by studies in an average of SNP/17 kb or a median of SNP/5.5 kb (van den Oord and Neale, 2004). This aspect represents a compromise between attaining the high SNP density and avoiding redundant markers. Fourth, we analyzed only limited SNPs for patients with bipolar disorder. This was intended to preferentially explore whether the effects of several haplotypes at UFD1L and TBX1 genes on symbol coding would be replicated in bipolar disorder akin to the findings in schizophrenia. The comparison of schizophrenia with bipolar disorder regarding the effect of a large number of genetic variants on cognitive areas is important, but requires consideration of the statistical interaction between genetics and the environment. Fifth, the effect of missense substitution at rs4819522 had not been expected a priori, warranting further study on its function.
In conclusion, we showed that, among multiple tag SNPs, only a few of the haplotypes at the CDC45, UFD1L, and TBX1 genes were significantly associated with poor performance of symbol coding and digit sequence task in patients with schizophrenia. Depending on the selection of tag SNPs, this finding suggests that genetic variants relevant to cognitive impairment associated with schizophrenia may not be ubiquitously distributed across 22q11.2. Meanwhile, their effects on cognition may be at a moderate level, which needs to be adjusted for cognitive reserve, environmental factors and gene–gene interaction. Further replication studies in a cohort of newly diagnosed patients- and those from other ethnicities are warranted.
The following are the supplementary data related to this article.
Effect sizes (f2) and power (1-β) of rs4819522 for controls and patients with schizophrenia in accounting for the z-scores of the symbol coding.
Omnibus tests for nominal association between haplotypes mapping to 22q11.2 and cognitive performance on BACS in controls and patients with schizophrenia.
Effect sizes (f2) and power (1-β) of total SFS scores in accounting for z-scores of the symbol coding in patients with schizophrenia and bipolar disorder.
Contributors
Author KA designed the study, performed the statistical analysis and drafted the manuscript. Authors KA and SS performed the BACS. Author AS designed the study and selected tag SNPs. Authors YO, TW, KF and KS contributed to the case recruitment and reviewed the draft. All authors contributed to the discussion and interpretation of the results and approved the final manuscript.
Funding body agreement and politics
Funding for this study was provided by a Grant-in-Aid for Scientific Research (C) (KAKENHI 23591681) (to Kazufumi Akiyama) from the Japan Society for the Promotion of Science.
Conflict of interests
Author KA has received consulting honoraria from Taisho Toyama Pharmaceutical Co., Ltd. This consultancy had no further role in the study design, the collection, analysis and interpretation of data, the writing of the report, or the decision to submit the paper for publication. Author KS has received research support from Novartis Pharma K.K., Dainippon Sumitomo Pharma Co., Astellas Pharma Inc., Meiji Seika Pharma Co., Ltd., Eisai Co., Ltd., Pfizer Inc., Otsuka Pharmaceutical Co., Ltd., Daiichi Sankyo Co., and Takeda Pharmaceutical Co., Ltd., and honoraria from Eisai Co., Ltd., Mitsubishi Tanabe Pharma Corporation, Takeda Pharmaceutical Co., Ltd., Meiji Seika Pharma Co., Ltd., Janssen Pharmaceutical K.K., Shionogi & Co., Ltd., Dainippon Sumitomo Pharma Co., Daiichi Sankyo Co., and Pfizer Inc. None of the remaining authors declare any potential conflicts of interest.
Acknowledgements
The authors thank Enago (www.enago.jp) for the English language review and Ms. Mikiko Ishikawa for her excellent technical support.
References
- Adzhubei I.A., Schmidt S., Peshkin L. A method and server for predicting damaging missense mutations. Nat. Methods. 2010;7:248–249. doi: 10.1038/nmeth0410-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama K., Saito S., Saito A. Predictive value of premorbid IQ, negative symptoms, and age for cognitive and social functions in Japanese patients with schizophrenia: A study using the Japanese version of the Brief Assessment of Cognition in Schizophrenia. Psychiatry Res. 2016;246:663–671. doi: 10.1016/j.psychres.2016.10.070. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association . VA. American Psychiatric Publishing; USA: 2013. Diagnostic and Statistical Manual of Mental Disorders, fifth ed., Arlington. [Google Scholar]
- Anaya C., Torrent C., Caballero F.F. Cognitive reserve in bipolar disorder: relation to cognition, psychosocial functioning and quality of life. Acta Psychiatr. Scand. 2016;133:386–398. doi: 10.1111/acps.12535. [DOI] [PubMed] [Google Scholar]
- Anazi S., Maddirevula S., Faqeih E. Clinical genomics expands the morbid genome of intellectual disability and offers a high diagnostic yield. Mol. Psychiatry. 2017;22:615–624. doi: 10.1038/mp.2016.113. [DOI] [PubMed] [Google Scholar]
- Antshel K.M., Fremont W., Ramanathan S., Kates W.R. Predicting Cognition and Psychosis in Young Adults with 22q11.2 Deletion Syndrome. Schizophr. Bull. 2017;43:833–842. doi: 10.1093/schbul/sbw135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arts B., Jabben N., Krabbendam L., Van Os J. Meta-analyses of cognitive functioning in euthymic bipolar patients and their first-degree relatives. Psychol. Med. 2008;38:771–785. doi: 10.1017/S0033291707001675. [DOI] [PubMed] [Google Scholar]
- Bachman P., Reichenberg A., Rice P. Deconstructing processing speed deficits in schizophrenia: application of a parametric digit symbol coding test. Schizophr. Res. 2010;118:6–11. doi: 10.1016/j.schres.2010.02.1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett J.H., Scoriels L., Munafo M.R. Meta-analysis of the cognitive effects of the catechol-O-methyltransferase gene Val158/108Met polymorphism. Biol. Psychiatry. 2008;64:137–144. doi: 10.1016/j.biopsych.2008.01.005. [DOI] [PubMed] [Google Scholar]
- Barrett J.C., Fry B., Maller J., Daly M.J. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21:263–265. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- Bilder R.M., Volavka J., Czobor P. Neurocognitive correlates of the COMT Val(158)Met polymorphism in chronic schizophrenia. Biol. Psychiatry. 2002;52:701–707. doi: 10.1016/s0006-3223(02)01416-6. [DOI] [PubMed] [Google Scholar]
- Birchwood M., Smith J., Cochrane R., Wetton S., Copestake S. The Social Functioning Scale. The development and validation of a new scale of social adjustment for use in family intervention programmes with schizophrenic patients. Br. J. Psychiatry. 1990;157:853–859. doi: 10.1192/bjp.157.6.853. [DOI] [PubMed] [Google Scholar]
- Boku S., Izumi T., Abe S. Copy number elevation of 22q11.2 genes arrests the developmental maturation of working memory capacity and adult hippocampal neurogenesis. Mol. Psychiatry. 2018;23:985–992. doi: 10.1038/mp.2017.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bora E. Differences in cognitive impairment between schizophrenia and bipolar disorder: Considering the role of heterogeneity. Psychiatry Clin. Neurosci. 2016;70:424–433. doi: 10.1111/pcn.12410. [DOI] [PubMed] [Google Scholar]
- Bralet M.C., Falissard B., Neveu X., Lucas-Ross M., Eskenazi A.M., Keefe R.S. Validation of the French version of the BACS (the brief assessment of cognition in schizophrenia) among 50 French schizophrenic patients. Eur. Psychiatry. 2007;22:365–370. doi: 10.1016/j.eurpsy.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Bruder G.E., Keilp J.G., Xu H. Catechol-O-methyltransferase (COMT) genotypes and working memory: associations with differing cognitive operations. Biol. Psychiatry. 2005;58:901–907. doi: 10.1016/j.biopsych.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Burdick K.E., Russo M., Frangou S. Empirical evidence for discrete neurocognitive subgroups in bipolar disorder: clinical implications. Psychol. Med. 2014;44:3083–3096. doi: 10.1017/S0033291714000439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M., Gutierrez G.J., Ronai Z.A. Ubiquitin-recognition protein Ufd1 couples the endoplasmic reticulum (ER) stress response to cell cycle control. Proc. Natl. Acad. Sci. U. S. A. 2011;108:9119–9124. doi: 10.1073/pnas.1100028108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chianetta J.M., Lefebvre M., Leblanc R., Grignon S. Comparative psychometric properties of the BACS and RBANS in patients with schizophrenia and schizoaffective disorder. Schizophr. Res. 2008;105:86–94. doi: 10.1016/j.schres.2008.05.024. [DOI] [PubMed] [Google Scholar]
- Chino B., Nemoto T., Fujii C., Mizuno M. Subjective assessments of the quality of life, well-being and self-efficacy in patients with schizophrenia. Psychiatry Clin. Neurosci. 2009;63:521–528. doi: 10.1111/j.1440-1819.2009.01995.x. [DOI] [PubMed] [Google Scholar]
- Crawford D.C., Nickerson D.A. Definition and clinical importance of haplotypes. Annu. Rev. Med. 2005;56:303–320. doi: 10.1146/annurev.med.56.082103.104540. [DOI] [PubMed] [Google Scholar]
- Crawford J.R., Deary I.J., Starr J., Whalley L.J. The NART as an index of prior intellectual functioning: a retrospective validity study covering a 66-year interval. Psychol. Med. 2001;31:451–458. doi: 10.1017/s0033291701003634. [DOI] [PubMed] [Google Scholar]
- De Luca A., Pasini A., Amati F. Association study of a promoter polymorphism of UFD1L gene with schizophrenia. Am. J. Med. Genet. 2001;105:529–533. doi: 10.1002/ajmg.1489. [DOI] [PubMed] [Google Scholar]
- Dickerson F.B., Boronow J.J., Stallings C., Origoni A.E., Sullens A., Yolken R.H. The catechol O-methyltransferase Val158Met polymorphism is not associated with broad-based cognitive functioning in schizophrenia. Schizophr. Res. 2007;96:87–92. doi: 10.1016/j.schres.2007.05.021. [DOI] [PubMed] [Google Scholar]
- Dickinson D., Ramsey M.E., Gold J.M. Overlooking the obvious: a meta-analytic comparison of digit symbol coding tasks and other cognitive measures in schizophrenia. Arch. Gen. Psychiatry. 2007;64:532–542. doi: 10.1001/archpsyc.64.5.532. [DOI] [PubMed] [Google Scholar]
- Dickson H., Cullen A.E., Reichenberg A. Cognitive impairment among children at-risk for schizophrenia. J. Psychiatr. Res. 2014;50:92–99. doi: 10.1016/j.jpsychires.2013.12.003. [DOI] [PubMed] [Google Scholar]
- Dorak, M. T. 2017. Genetic Association Studies: Backgraound, Conduct, Analysis, Interpretation, London Garland Science.
- Driscoll D.A., Spinner N.B., Budarf M.L. Deletions and microdeletions of 22q11.2 in velo-cardio-facial syndrome. Am. J. Med. Genet. 1992;44:261–268. doi: 10.1002/ajmg.1320440237. [DOI] [PubMed] [Google Scholar]
- Egan M.F., Goldberg T.E., Kolachana B.S. Effect of COMT Val108/158 Met genotype on frontal lobe function and risk for schizophrenia. Proc. Natl. Acad. Sci. U. S. A. 2001;98:6917–6922. doi: 10.1073/pnas.111134598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faul F., Erdfelder E., Buchner A., Lang A.G. Statistical power analyses using G*Power 3.1: tests for correlation and regression analyses. Behav. Res. Methods. 2009;41:1149–1160. doi: 10.3758/BRM.41.4.1149. [DOI] [PubMed] [Google Scholar]
- Gabriel S.B., Schaffner S.F., Nguyen H. The structure of haplotype blocks in the human genome. Science. 2002;296:2225–2229. doi: 10.1126/science.1069424. [DOI] [PubMed] [Google Scholar]
- Grande I., Sanchez-Moreno J., Sole B. High cognitive reserve in bipolar disorders as a moderator of neurocognitive impairment. J. Affect. Disord. 2017;208:621–627. doi: 10.1016/j.jad.2016.10.012. [DOI] [PubMed] [Google Scholar]
- Gur R.E., Bassett A.S., Mcdonald-Mcginn D.M. A neurogenetic model for the study of schizophrenia spectrum disorders: the International 22q11.2 Deletion Syndrome Brain Behavior Consortium. Mol. Psychiatry. 2017;22:1664–1672. doi: 10.1038/mp.2017.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamshere M.L., Bennett P., Williams N. Genomewide linkage scan in schizoaffective disorder: significant evidence for linkage at 1q42 close to DISC1, and suggestive evidence at 22q11 and 19p13. Arch. Gen. Psychiatry. 2005;62:1081–1088. doi: 10.1001/archpsyc.62.10.1081. [DOI] [PubMed] [Google Scholar]
- Hellvin T., Sundet K., Vaskinn A. Validation of the Norwegian version of the Social Functioning Scale (SFS) for schizophrenia and bipolar disorder. Scand. J. Psychol. 2010;51:525–533. doi: 10.1111/j.1467-9450.2010.00839.x. [DOI] [PubMed] [Google Scholar]
- Hill S.K., Reilly J.L., Keefe R.S. Neuropsychological impairments in schizophrenia and psychotic bipolar disorder: findings from the Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP) study. Am. J. Psychiatry. 2013;170:1275–1284. doi: 10.1176/appi.ajp.2013.12101298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiramoto T., Kang G., Suzuki G. Tbx1: identification of a 22q11.2 gene as a risk factor for autism spectrum disorder in a mouse model. Hum. Mol. Genet. 2011;20:4775–4785. doi: 10.1093/hmg/ddr404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiroi N. Critical reappraisal of mechanistic links of copy number variants to dimensional constructs of neuropsychiatric disorders in mouse models. Psychiatry Clin. Neurosci. 2018;72:301–321. doi: 10.1111/pcn.12641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiroi N., Zhu H., Lee M. A 200-kb region of human chromosome 22q11.2 confers antipsychotic-responsive behavioral abnormalities in mice. Proc. Natl. Acad. Sci. U. S. A. 2005;102:19132–19137. doi: 10.1073/pnas.0509635102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiroi N., Takahashi T., Hishimoto A., Izumi T., Boku S., Hiramoto T. Copy number variation at 22q11.2: from rare variants to common mechanisms of developmental neuropsychiatric disorders. Mol. Psychiatry. 2013;18:1153–1165. doi: 10.1038/mp.2013.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inada T., Inagaki A. Psychotropic dose equivalence in Japan. Psychiatry Clin. Neurosci. 2015;69:440–447. doi: 10.1111/pcn.12275. [DOI] [PubMed] [Google Scholar]
- Jerome L.A., Papaioannou V.E. DiGeorge syndrome phenotype in mice mutant for the T-box gene, Tbx1. Nat. Genet. 2001;27:286–291. doi: 10.1038/85845. [DOI] [PubMed] [Google Scholar]
- Jonas R.K., Montojo C.A., Bearden C.E. The 22q11.2 deletion syndrome as a window into complex neuropsychiatric disorders over the lifespan. Biol. Psychiatry. 2014;75:351–360. doi: 10.1016/j.biopsych.2013.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneda Y., Sumiyoshi T., Keefe R., Ishimoto Y., Numata S., Ohmori T. Brief assessment of cognition in schizophrenia: validation of the Japanese version. Psychiatry Clin. Neurosci. 2007;61:602–609. doi: 10.1111/j.1440-1819.2007.01725.x. [DOI] [PubMed] [Google Scholar]
- Kay S.R., Fiszbein A., Opler L.A. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr. Bull. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- Keefe R.S., Goldberg T.E., Harvey P.D., Gold J.M., Poe M.P., Coughenour L. The Brief Assessment of Cognition in Schizophrenia: reliability, sensitivity, and comparison with a standard neurocognitive battery. Schizophr. Res. 2004;68:283–297. doi: 10.1016/j.schres.2003.09.011. [DOI] [PubMed] [Google Scholar]
- Knowles E.E., Weiser M., David A.S., Glahn D.C., Davidson M., Reichenberg A. The puzzle of processing speed, memory, and executive function impairments in schizophrenia: fitting the pieces together. Biol. Psychiatry. 2015;78:786–793. doi: 10.1016/j.biopsych.2015.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar P., Henikoff S., Ng P.C. Predicting the effects of coding non-synonymous variants on protein function using the SIFT algorithm. Nat. Protoc. 2009;4:1073–1081. doi: 10.1038/nprot.2009.86. [DOI] [PubMed] [Google Scholar]
- Kuratomi G., Saito A., Ozeki Y. Association of the Hermansky-Pudlak syndrome type 4 (HPS4) gene variants with cognitive function in patients with schizophrenia and healthy subjects. BMC Psychiatry. 2013;13:276. doi: 10.1186/1471-244X-13-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewandowski K.E., Sperry S.H., Cohen B.M., Ongur D. Cognitive variability in psychotic disorders: a cross-diagnostic cluster analysis. Psychol. Med. 2014;44:3239–3248. doi: 10.1017/S0033291714000774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Garcia P., Young Espinoza L., Molero Santos P., Marin J., Ortuno Sanchez-Pedreno F. Impact of COMT genotype on cognition in schizophrenia spectrum patients and their relatives. Psychiatry Res. 2013;208:118–124. doi: 10.1016/j.psychres.2012.09.043. [DOI] [PubMed] [Google Scholar]
- Mann-Wrobel M.C., Carreno J.T., Dickinson D. Meta-analysis of neuropsychological functioning in euthymic bipolar disorder: an update and investigation of moderator variables. Bipolar Disord. 2011;13:334–342. doi: 10.1111/j.1399-5618.2011.00935.x. [DOI] [PubMed] [Google Scholar]
- Marshall C.R., Howrigan D.P., Merico D. Contribution of copy number variants to schizophrenia from a genome-wide study of 41,321 subjects. Nat. Genet. 2017;49:27–35. doi: 10.1038/ng.3725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mckie J.M., Wadey R.B., Sutherland H.F., Taylor C.L., Scambler P.J. Direct selection of conserved cDNAs from the DiGeorge critical region: isolation of a novel CDC45-like gene. Genome Res. 1998;8:834–841. doi: 10.1101/gr.8.8.834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuechterlein K.H., Green M.F., Kern R.S. The MATRICS Consensus Cognitive Battery, part 1: test selection, reliability, and validity. Am. J. Psychiatry. 2008;165:203–213. doi: 10.1176/appi.ajp.2007.07010042. [DOI] [PubMed] [Google Scholar]
- Ogata T., Niihori T., Tanaka N. TBX1 mutation identified by exome sequencing in a Japanese family with 22q11.2 deletion syndrome-like craniofacial features and hypocalcemia. PLoS One. 2014;9 doi: 10.1371/journal.pone.0091598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohi K., Hashimoto R., Ikeda M. Glutamate Networks Implicate Cognitive Impairments in Schizophrenia: Genome-Wide Association Studies of 52 Cognitive Phenotypes. Schizophr. Bull. 2015;41:909–918. doi: 10.1093/schbul/sbu171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ota V.K., Belangero S.I., Gadelha A. The UFD1L rs5992403 polymorphism is associated with age at onset of schizophrenia. J. Psychiatr. Res. 2010;44:1113–1115. doi: 10.1016/j.jpsychires.2010.04.008. [DOI] [PubMed] [Google Scholar]
- Ota V.K., Berberian A.A., Gadelha A. Polymorphisms in schizophrenia candidate gene UFD1L may contribute to cognitive deficits. Psychiatry Res. 2013;209:110–113. doi: 10.1016/j.psychres.2013.03.035. [DOI] [PubMed] [Google Scholar]
- Paylor R., Glaser B., Mupo A. Tbx1 haploinsufficiency is linked to behavioral disorders in mice and humans: implications for 22q11 deletion syndrome. Proc. Natl. Acad. Sci. U. S. A. 2006;103:7729–7734. doi: 10.1073/pnas.0600206103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulver A.E., Nestadt G., Goldberg R. Psychotic illness in patients diagnosed with velo-cardio-facial syndrome and their relatives. J. Nerv. Ment. Dis. 1994;182:476–478. doi: 10.1097/00005053-199408000-00010. [DOI] [PubMed] [Google Scholar]
- Purcell S., Neale B., Todd-Brown K. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet. 2007;81:559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichenberg A., Harvey P.D., Bowie C.R. Neuropsychological function and dysfunction in schizophrenia and psychotic affective disorders. Schizophr. Bull. 2009;35:1022–1029. doi: 10.1093/schbul/sbn044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson L.J., Thompson J.M., Gallagher P. A meta-analysis of cognitive deficits in euthymic patients with bipolar disorder. J. Affect. Disord. 2006;93:105–115. doi: 10.1016/j.jad.2006.02.016. [DOI] [PubMed] [Google Scholar]
- Russo M., Van Rheenen T.E., Shanahan M. Neurocognitive subtypes in patients with bipolar disorder and their unaffected siblings. Psychol. Med. 2017;47:2892–2905. doi: 10.1017/S003329171700143X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito A., Kuratomi G., Ito C. An association study of the Hermansky-Pudlak syndrome type 4 gene in schizophrenic patients. Psychiatr. Genet. 2013;23:163–173. doi: 10.1097/YPG.0b013e32836130a9. [DOI] [PubMed] [Google Scholar]
- Saito S., Fujii K., Ozeki Y. Cognitive function, treatment response to lithium, and social functioning in Japanese patients with bipolar disorder. Bipolar Disord. 2017;19:552–562. doi: 10.1111/bdi.12521. [DOI] [PubMed] [Google Scholar]
- Sanchez-Morla E.M., Barabash A., Martinez-Vizcaino V. Comparative study of neurocognitive function in euthymic bipolar patients and stabilized schizophrenic patients. Psychiatry Res. 2009;169:220–228. doi: 10.1016/j.psychres.2008.06.032. [DOI] [PubMed] [Google Scholar]
- Santoro M.L., Gadelha A., Ota V.K. Gene expression analysis in blood of ultra-high risk subjects compared to first-episode of psychosis patients and controls. World J. Biol. Psychiatry. 2015;16:441–446. doi: 10.3109/15622975.2015.1048724. [DOI] [PubMed] [Google Scholar]
- Scala S., Lasalvia A., Cristofalo D., Bonetto C., Ruggeri M. Neurocognitive profile and its association with psychopathology in first-degree relatives of patients with schizophrenia. a case-control study. Psychiatry Res. 2012;200:137–143. doi: 10.1016/j.psychres.2012.05.006. [DOI] [PubMed] [Google Scholar]
- Scambler P.J., Kelly D., Lindsay E. Velo-cardio-facial syndrome associated with chromosome 22 deletions encompassing the DiGeorge locus. Lancet. 1992;339:1138–1139. doi: 10.1016/0140-6736(92)90734-k. [DOI] [PubMed] [Google Scholar]
- Schneider M., Debbane M., Bassett A.S. Psychiatric disorders from childhood to adulthood in 22q11.2 deletion syndrome: results from the International Consortium on Brain and Behavior in 22q11.2 Deletion Syndrome. Am. J. Psychiatry. 2014;171:627–639. doi: 10.1176/appi.ajp.2013.13070864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schretlen D.J., Cascella N.G., Meyer S.M. Neuropsychological functioning in bipolar disorder and schizophrenia. Biol. Psychiatry. 2007;62:179–186. doi: 10.1016/j.biopsych.2006.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze-Rauschenbach S., Lennertz L., Ruhrmann S. Neurocognitive functioning in parents of schizophrenia patients: Attentional and executive performance vary with genetic loading. Psychiatry Res. 2015;230:885–891. doi: 10.1016/j.psychres.2015.11.031. [DOI] [PubMed] [Google Scholar]
- Shprintzen R.J., Goldberg R., Golding-Kushner K.J., Marion R.W. Late-onset psychosis in the velo-cardio-facial syndrome. Am. J. Med. Genet. 1992;42:141–142. doi: 10.1002/ajmg.1320420131. [DOI] [PubMed] [Google Scholar]
- Takahashi T., Okabe S., Broin P.O. Structure and function of neonatal social communication in a genetic mouse model of autism. Mol. Psychiatry. 2016;21:1208–1214. doi: 10.1038/mp.2015.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trotta A., Murray R.M., Maccabe J.H. Do premorbid and post-onset cognitive functioning differ between schizophrenia and bipolar disorder? A systematic review and meta-analysis. Psychol. Med. 2015;45:381–394. doi: 10.1017/S0033291714001512. [DOI] [PubMed] [Google Scholar]
- Van Den Oord E.J., Neale B.M. Will haplotype maps be useful for finding genes? Mol. Psychiatry. 2004;9:227–236. doi: 10.1038/sj.mp.4001449. [DOI] [PubMed] [Google Scholar]
- Vorstman J.A., Breetvelt E.J., Duijff S.N. Cognitive decline preceding the onset of psychosis in patients with 22q11.2 deletion syndrome. JAMA Psychiat. 2015;72:377–385. doi: 10.1001/jamapsychiatry.2014.2671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams N.M., Norton N., Williams H. A systematic genomewide linkage study in 353 sib pairs with schizophrenia. Am. J. Hum. Genet. 2003;73:1355–1367. doi: 10.1086/380206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie L., Ye L., Ju G. A family- and population-based study of the UFD1L gene for schizophrenia. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2008;147B:1076–1079. doi: 10.1002/ajmg.b.30719. [DOI] [PubMed] [Google Scholar]
- Yagi H., Furutani Y., Hamada H. Role of TBX1 in human del22q11.2 syndrome. Lancet. 2003;362:1366–1373. doi: 10.1016/s0140-6736(03)14632-6. [DOI] [PubMed] [Google Scholar]
- Zanelli J., Reichenberg A., Morgan K. Specific and generalized neuropsychological deficits: a comparison of patients with various first-episode psychosis presentations. Am. J. Psychiatry. 2010;167:78–85. doi: 10.1176/appi.ajp.2009.09010118. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Effect sizes (f2) and power (1-β) of rs4819522 for controls and patients with schizophrenia in accounting for the z-scores of the symbol coding.
Omnibus tests for nominal association between haplotypes mapping to 22q11.2 and cognitive performance on BACS in controls and patients with schizophrenia.
Effect sizes (f2) and power (1-β) of total SFS scores in accounting for z-scores of the symbol coding in patients with schizophrenia and bipolar disorder.