Summary
Pseudocapacitors hold great promise to provide high energy-storing capacity; however, their capacitances are still far below their theoretical values and they deliver much lower power than the traditional electric double-layer capacitors due to poor ionic accessibility. Here, we have engineered MoN nanoparticles as pseudocapacitive material on phosphorus-incorporated carbon fabric with enhanced ionic affinity and thermodynamic stability. This nanocomposite boosts surface redox kinetics, leading to pseudocapacitance of 400 mF/cm2 (2-fold higher than that of molybdenum nitride-based electrodes) with rapid charge-discharge rates. Density functional theory simulations are used to explain the origin of the good performance of MoN@P-CF in proton-based aqueous electrolytes. Finally, an all-pseudocapacitive solid-state asymmetric cell was assembled using MoN@P-CF and RuO2 (RuO2@CF) as negative and positive electrodes, respectively, which delivered good energy density with low relaxation time constant (τ0) of 13 ms (significantly lower than that of carbon-based supercapacitors).
Subject Areas: Chemistry, Inorganic Chemistry, Materials Science, Energy Materials
Graphical Abstract
Highlights
-
•
MoN nanocrystals coated on P-doped CF are used as high-performance pseudocapacitors
-
•
DFT simulations explain the origin of the good performance of MoN@P-CF electrode
-
•
MoN@P-CF and RuO2@CF serve as negative and positive electrodes, in asymmetric SC
-
•
All-pseudocapacitive ASC delivers high power density with long cycling stability
Chemistry; Inorganic Chemistry; Materials Science; Energy Materials
Introduction
Electrochemical energy storage (EES) devices such as supercapacitors (SCs) and Li-ion batteries are the primary choices for the majority of advanced applications ranging from implantable medical devices to electrical and hybrid vehicles (Dubal et al., 2015, Dubal et al., 2018, Ko et al., 2017, Nam et al., 2006). The energy and power densities of EES systems define their utility in commercial applications, and these parameters are associated with charge-storing mechanisms. For instance, battery system offers high energy density due to the reversible redox reactions associated with the intercalation of alkali metal ions in the bulk of electrode; however, it is limited by very slow discharge-charge rates (Nitta et al., 2015, Yuan et al., 2017). Conversely, SCs (mainly conventional electric double-layer capacitor [EDLC] based) provide high power density, robust lifetimes, and safe operation, but are restricted by the low energy storage capacity (Salanne et al., 2016, Borenstein et al., 2017). Apart from them, pseudocapacitors, a subclass of SCs can be considered as a promising device, which exhibit high energy and high power densities in a single system, thus bridging the gap between batteries and SCs (Augustyn et al., 2014, Eftekhari and Mohamedi, 2015). Principally, pseudocapacitors store charges through fast and reversible surface redox reactions or intercalation pseudocapacitance. Typically, transition metal oxide or hydroxides, sulfides, and conducting polymers show pseudocapacitive behavior. Nevertheless, the poor electrical conductivity and the cycling stability of current pseudocapacitive materials hamper their practical applications (Wang et al., 2012, Yu and Lou, 2018). Therefore new pseudocapacitive materials with new cell designs need to be explored to achieve high energy and high power densities with good cycling stability to satisfy the increasing demands of energy storage systems.
Transition metal nitrides have recently attracted great attention as a potential electrode material for SCs due to their pseudocapacitive behavior, high reversibility, metallic electrical conductivity, and high chemical stability (Balogun et al., 2017, Yang et al., 2016). For example, the electrical conductivity of titanium nitride is in the range of 103 to 104 S/cm, which is close to that of metal and thus, beneficial for SCs (Lu et al., 2012). In the past, different metal nitrides such as TiN, VN, Mo2N, and FeN have been explored as promising electrode materials for SCs (Balogun et al., 2015). Among these metal nitrides, molybdenum nitride (MoN) is one of the most promising pseudocapacitive material as it shows electrochemical features similar to those of ruthenium oxide (Liu et al., 1998). However, the smaller electrochemical potential window (∼0.6 V) and low capacitance of MoN-based pseudocapacitors limits their commercial utilization. Certainly, it is crucial to develop an adequate method to engineer MoN-based electrodes to achieve the desired electrochemical features.
An emerging strategy to extend the operational voltage of SCs is the fabrication of asymmetric SCs (ASCs), wherein the positive electrode is of a faradaic or pseudocapacitive nature and the negative electrode is made of non-faradaic materials (Chodankar et al., 2017, Dubal et al., 2017). Generally, carbonaceous material is utilized as the negative electrode with oxide- or conducting polymer-based positive electrode to engineer ASCs. However, the low capacitance of carbon-based negative electrode limits the overall performance of ASCs, which fosters the requirement of an alternative for carbon-based negative electrode. Molybdenum nitride (MoN) can provide high capacitance due to pseudocapacitive behavior and is able to work in the negative potential window, which makes it a promising alternative to carbon-based negative electrode. Thus by coupling MoN negative electrode with another pseudocapacitive positive electrode, a high-performance ASC can be assembled (Liu et al., 2016, Shah et al., 2014). To provide proof of concept, we have selected ruthenium oxide (RuO2) as the positive pseudocapacitive electrode because of its high capacitance and large overpotential for oxygen evolution in acidic electrolytes (Hu et al., 2006, Wang et al., 2014). So far, few reports are available on ASCs using RuO2 and carbon as positive and negative electrodes, respectively (Shen et al., 2016, Choi et al., 2012).
In the present work, we have engineered a solid-state asymmetric pseudocapacitor using RuO2 on carbon fabrics (RuO2@CF) as the positive electrode and MoN nanoparticles coated on phosphorous-doped carbon fabrics (MoN@P-CF) as the negative electrode in 1 M H2SO4 electrolyte. Briefly, ultra-small MoN particles were uniformly decorated on P-doped carbon fabric (MoN@P-CF) using phosphomolybdic acid (H3PMo12O40, PMo12) as a single source of Mo and P, followed by ammonia annealing at the desired temperature. On the other hand, pseudocapacitive RuO2 was grown on CF using a simple layer-by-layer (LBL) deposition method. Both the electrodes were initially tested in three-electrode configuration to evaluate their individual performances. The origin of improved electrochemical performances of MoN@P-CF in aqueous acidic electrolyte is unveiled by density functional theory (DFT) simulations.
Results
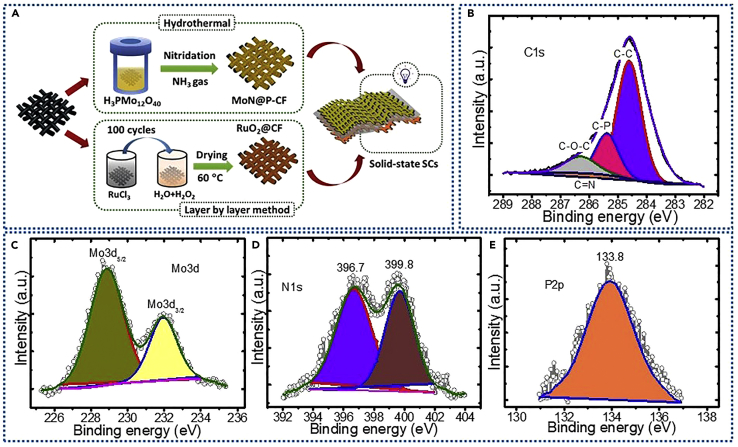
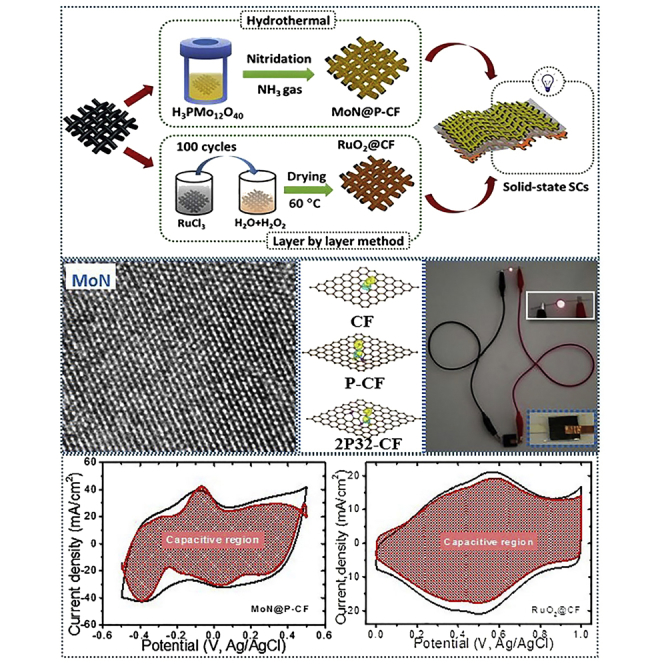
Figure 1A shows the steps involved in the fabrication of all-pseudocapacitive solid-state ASCs along with synthesis methods used to develop phosphorus-doped carbon fabric cloth decorated with MoN nanocrystals (MoN@P-CF) and RuO2 nanoparticles coated on flexible carbon fabric (RuO2@CF). To prepare MoN@P-CF, initially PMo12 nanoclusters (phosphomolybdic acid, PMo12) were anchored over the carbon fabric (CF) by the facile hydrothermal method, which will act as a single source of P and Mo. Later, PMo12-coated CF (PMo12@CF) undergoes heat treatment in ammonia (NH3) atmosphere at a desired temperature to obtain MoN@P-CF. On the other hand, RuO2 nanoparticles were directly grown over the CF using simple, low-cost, and scalable LBL method. Finally, using optimized MoN@P-CF as negative and RuO2@CF as positive electrodes, all-pseudocapacitive solid-state asymmetric cell was assembled as shown in Figure 1A.
Figure 1.
Schematic and XPS Analysis of MoN@P-CF
(A–E) (A) Schematic illustration of design and engineering of all-pseudocapacitive solid-state asymmetric supercapacitors. The steps involved in the synthesis of pseudocapacitive MoN@P-CF and RuO2@CF are presented. XPS analysis of MoN@P-CF-900 sample: core-level XPS spectra with deconvoluted peaks (B) C1s, (C) Mo3d, (D) N1s, and (E) P2p.
Design and Engineering of MoN@P-CF Negative Electrodes
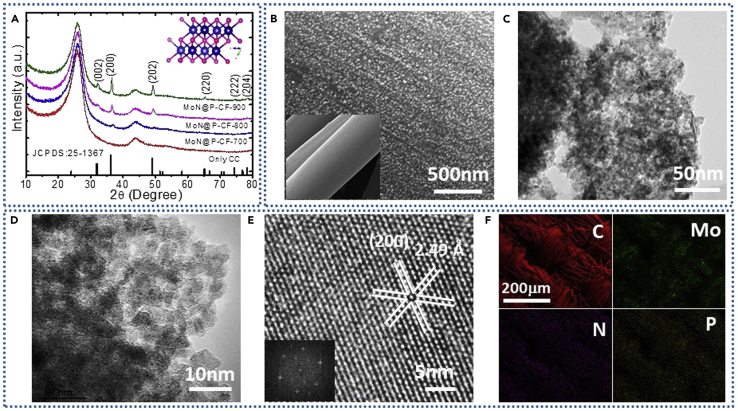
The full-range X-ray photoelectron spectroscopic (XPS) spectrum of MoN@P-CF confirms the presence of carbon, molybdenum, nitrogen, and phosphorous. The core-level XPS spectra for C1s can be deconvoluted into four peaks as shown in Figure 1B. The peak observed at a binding energy (BE) of 284.8 eV can be assigned to graphitic carbon with C-C, whereas the other low-intensity peaks at 285.2, 285.8, and 286.2 eV may correspond to C bonding with phosphorous (C-P), nitrogen (C-N), and oxygen (C-O-C), respectively (Yan et al., 2015, Zhang et al., 2014a). The presence of C-P bond confirms the incorporation of P (from PMo12) in carbon fabric. High-magnification XPS spectra of Mo3d exhibits a pair of Gaussian peaks at BEs of 228.8 and 232.2 eV (Figure 1C), which can be assigned to Mo3d3/2 and Mo3d5/2 of Mo-N bond (Yan et al., 2018). The N1s spectra shown in Figure 1D can be fitted in two major peaks where the peak at BE of 396.7 eV can be indexed to Mo-N bond from MoN, whereas the peak at 399.8 eV corresponds to the pyrrolic N (Zhang et al., 2014b). The high-resolution P2p spectrum shows a prominent peak at BE of 133.8 eV, which can be assigned to the boding between carbon and phosphorous, confirming the incorporation of P into CF (Figure 1E). The content of P in CF was determined to be 1.58 at %. Detailed analysis of samples unambiguously confirms the formation of P-doped CF coated with MoN nanoparticles (MoN@P-CF). Thus we have provided a single-step method to accommodate adatom (P) in carbon structure and deposit ultra-small MoN nanoparticles on carbon fabric, which is expected to significantly improve the electrochemical performances. Figure 2A presents X-ray diffraction (XRD) patterns for the MoN@P-CF samples prepared at different nitridation temperatures. The XRD patterns exhibit six prominent diffraction peaks at 31.8°, 36.2°, 49.1°, 65.1°, 74.3°, 78.3°, which can be indexed to (002), (200), (202), (220), (222), and (204) planes, respectively, suggesting the formation of hexagonal MoN (JCPDS: 25–1367). The increase in peak intensity with nitridation temperature suggests the improved crystallinity of MoN nanocrystals. Thus the high nitridation temperature is favorable for complete conversion of PMo12 clusters into MoN nanocrystals. The inset of Figure 2A shows the typical hexagonal crystal structure of the MoN with layered configuration where the Mo atoms are sandwiched between the nitrogen atoms in the center of individual monolayers (Xie et al., 2014). To get further insights about the chemical bonding in MoN@P-CF, Raman analysis was performed and presented in Figure S1. Two major bands are observed at 1,350 cm−1 and 1,572 cm−1 (known as D and G band, respectively), which can be associated to the characteristic carbon peaks originating from carbon fabric (CF). The intensity ratio of D and G bands (ID/IG) presents the disorder degree of carbon materials. A small increase in the ID/IG ratio was found with temperature (0.94–0.99), which can be partly associated with the decoration of MoN nanocrystals and partly to the incorporation of phosphorous (P) in carbon framework during the nitridation process.
Figure 2.
Characterizations of MoN@P-CF Samples
(A and B) (A) XRD patterns of MoN@P-CF samples prepared at different nitridation temperatures. Surface morphological analysis of MoN@P-CF-900 sample. (B) SEM images of bare carbon fabric (inset) and P-doped carbon fabric with ultra-small MoN particles.
(C–E) (C and D) TEM images and (E) HRTEM image with corresponding fast Fourier transform spectrum.
(F) Large-area SEM-EDS mapping images, confirming the presence of C, Mo, N, and P.
The scanning electron microscopic (SEM) analysis shows the uniform coating of MoN tiny nanoparticles on the surface of carbon fabric (Figure 2B). It is further observed that the size of MoN nanoparticles increases with annealing temperature (see Figure S1). The direct coating of MoN nanoparticles over the heteroatom-doped carbon fabric not only provides conducting scaffold but also avoids the use of unnecessary binders that will minimize the charge transfer resistance. The transmission electron microscopic (TEM) images revealed the formation of high-density ultra-small nanoparticles with sizes less than 10 nm (Figures 2C and 2D). Such tiny nanoparticles provide highly accessible surface for the electrochemical reactions and short diffusion lengths for ionic transportation. To investigate the crystallinity of ultra-small MoN nanocrystals, high-resolution (HR) TEM images are recorded and shown in Figure 2E. An HRTEM image and fast Fourier transform reveals the lattice fringes with a d spacing of 2.4 Å, corresponding to the (200) plane of MoN. Moreover, the selected area electron diffraction (SAED) pattern confirms the polycrystalline nature of the as-prepared MoN nanocrystals (Figure S2). The different spots in the SAED pattern can be assigned to the hexagonal MoN structure. Energy-dispersive X-ray spectroscopic (EDX) analysis revealed the uniform distribution of the C, Mo, N, and P over the CF surface (Figure 2F). Thus it should be emphasized that the uniform coating of MoN nanodots in this hybrid material is a model for electrode or electrolyte surface polarization, as pseudocapacitive energy storage is based on surface redox processes. Indeed, this unique MoN@P-CF hybrid combines a heteroatom-doped (P) conducting porous CF with pseudocapacitive MoN nanocrystals, thus providing an optimal starting point from a structural point of view for high energy storage.
The electrochemical performances of MoN@P-CF electrodes were evaluated in standard three-electrode setup with 1 M H2SO4 electrolyte. The comparative cyclic voltammetry (CV) and charge/discharge curves (CD) curves for the MoN@P-CF electrodes prepared at different nitridation temperatures are shown in Figure S3. It is revealed that the sample prepared at 900°C (abbreviated as MoN@P-CF-900) shows higher integrated area under the CV curves with well-defined redox peaks, representing the best pseudocapacitive features. Moreover, the redox peaks can be retained at a high scanning rate of 100 mV/s, representing good rate capability of the prepared MoN@P-CF-900 electrode (Figure 3A). The charge storage mechanism of the as-prepared MoN@P-CF electrode was uncovered using the previous literature (Wang et al., 2007). Typically, the total charge storage in the electrode is the sum of surface capacitive (Qs) and diffusion-controlled (Qd) contributions. The capacitive contribution corresponds to the electrostatic adsorption of the electrolyte ions as well as surface redox reactions (pseudocapacitance), whereas diffusion-controlled contributions are associated with the surface and bulk electrochemical reactions. To quantify these contributions, the CV curves for MoN@P-CF-900 were recorded at different scan rates. By using the power law, it is possible to determine the charge storage mechanism of the electrode.
| (Equation 1) |
where “a” and “b” are adjustable parameters having defined conditions. Here, b = 0.5 suggests that the maximum current is contributed by diffusion-controlled processes, whereas b = 1 suggests purely capacitive processes. Using Equation 1, one can estimate the value of b by plotting the graph of log (i) versus log (ν). Figure 3B shows the plot of b-values versus potentials, which lies between 0.8 and 0.95, suggesting the dominance of capacitive charge storage processes (Wang et al., 2007). The contribution from capacitive processes can be further quantified by assuming that the current response at the fixed potential is the combination of the capacitive (EDLC + pseudocapacitance) and diffusion-controlled processes as follows:
| (Equation 2) |
where Qt is the total charge storage of the electrode. By considering the semi-infinite linear diffusion, it is possible to derive the Qs (capacitive contribution) by plotting total charge (Qt) against the reciprocal of the square root of the scan rate (ν−1/2) according to the following equation:
| (Equation 3) |
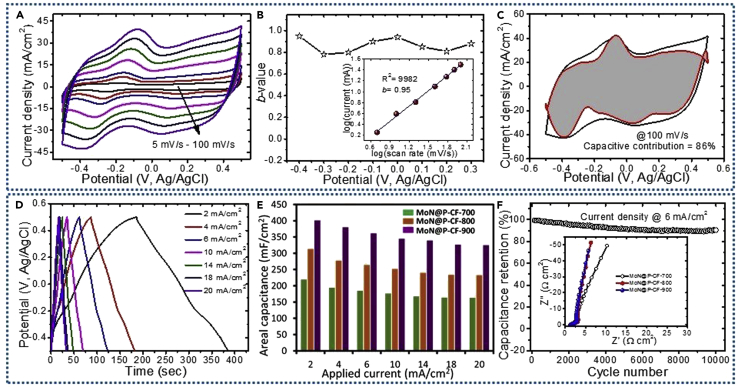
Figure 3.
Electrochemical Characterizations of MoN@P-CF-900 with Three-Electrode Cell Design
(A) CV profiles recorded at different scanning rates in 1 M H2SO4 electrolyte.
(B) Variation of b-values with anodic potential scan. Inset shows power law dependence of peak current density at scan rates from 5–100 mV/s.
(C) Voltammetric response at a scan rate of 100 mV/s. The capacitive contribution to the total current is shown by the shaded region (86% of total charge contribution).
(D) Galvanostatic charge-discharge curves measured at different current densities from 2 to 20 mA/cm2.
(E) Plot of areal capacitances as a function of current densities.
(F) Cycling stability was tested for 10,000 cycles at a current density of 6 mA/cm2. Inset shows Nyquist plots for MoN@P-CF samples prepared at different nitridation temperatures.
The surface capacitive and diffusion-controlled processes can be separated using Equation 3. Figure 3C shows the CV curves of calculated capacitive charges (shaded) and the experimental currents (solid line), suggesting that about 86% of the total current is contributed by capacitive storage at 100 mV/s. Thus the results demonstrate that the MoN@P-CF electrode is mainly pseudocapacitive in nature. The capacitive charge contribution increases with scan rates from 5 (56%) to 100 mV/s (86%) as shown in Figures S3 and S4, which can be explained by the fact that at a high scan rate the extrinsic surface effects due to both pseudocapacitive charging and electronic conduction at the interface contribute to surface capacitive processes (Liu et al., 1998), whereas at slow scan rates, large currents originate from diffusion-controlled reactions in the acidic electrolyte, probably due to the higher mobility of protons (H+). The high capacitive contribution at slow scan rates further suggests the good electrical conductivity of the electrode materials, which can be ascribed to P doping in CF and ultra-small MoN nanocrystals that offers super-highway and short diffusion for ion transportation.
To estimate the rate capability of as-prepared MoN@P-CF electrode, the galvanostatic CD measurements are carried out at different current densities (see Figure 3D). The linear CD curves without any potential drop even at high current density (20 mA/cm2) display good capacitive features of the MoN@P-CF-900 electrode. In addition, the charging and discharging parts are perfectly symmetric to each other, implying highly reversible redox reactions. The areal capacitances were calculated from CD curves and plotted in Figure 3E. The MoN@P-CF-900 electrode delivers a maximum areal capacitance of 400 mF/cm2 (598 F/g for mass loading of 1.4 mg/cm2) at current density of 2 mA/cm2, which decreases to 325 mF/cm2 (505 F/g) at 20 mA/cm2, retaining about 81% of initial capacitance. The MoN@P-CF900 electrode in the present investigation shows high gravimetric (areal) capacitance values (see Table S1), which can be attributed to good Ohmic contacts, ultra-small MoN nanocrystals, and P doping into CF. Nyquist plots for MoN@P-CF samples show linear dependency in the low-frequency region, indicating ideal capacitive behavior (inset of Figure 3F). Moreover, the low values of the equivalent series resistance (ESR) (1–1.2 Ω/cm2) and charge transfer resistance in the high-frequency region imply good electrical conductivity with facile electrochemical interaction between the active material and electrolyte ions. Finally, the phase angles for MoN@P-CF electrodes in Bode plot (Figure S5) are close to 90°, signifying that MoN@P-CF sample shows an ideal capacitive performance. The cycling stability was recorded (Figure 3F) at a current density of 6 mA/cm2, suggesting 91% capacitance retention over 10,000 cycles. The outperformance of MoN@P-CF can be credited to the uniform coating of ultra-small MoN nanocrystals on CF, which offer large electrochemically active sites and short diffusion paths for easy transportation of electrolyte ions. Moreover, the heteroatom (P)-doped carbon fabric matrix not only improves the electrical conductivity but also adds extra capacitive contribution in the charge storage.
To interpret the experimental results and link the atomic structure with the function of material, we have carried out DFT simulations. We have proposed following different models such as adsorption of H3O+ on CF, P-CF, MoN, and their nanocomposite interfaces (MoN@CF and MoN@P-CF), which are depicted in Figure S6. First, we discussed the adsorption energies of the doped systems and their interfaces by taking the adsorption energy on pristine CF as reference point (i.e., the adsorption energies of H3O+ are reported as the subtraction of adsorption energy on P-CF, MoN, MoN@CF, and MoN@P-CF from that on the pristine CF [ΔEads]). The simulations are performed for two atomic ratios (1 and 2), which generate six configurations by varying the number of bonds separating the two phosphorus atoms. In each configuration, multiple positions of H3O+ were optimized, and the best binder was selected for the discussion. The BE of H3O+ on CF and P-CF is negative, indicating that both pristine and P-doped CF systems have the ability to adsorb protons from the electrolyte solution. However, doping CF with one or two phosphorus atoms further enhances their adsorption capacity. The adsorption energies of P-CF and 2P-CF display much higher affinity toward H3O+ than pristine CF (ΔEads= −1.02 in case of P-CF and −1.44 eV in case of 2P-CF, see Table S2). In case of 2P-CF systems, H3O+ can be adsorbed on phosphorus or carbon atoms without significant preferences (note the difference between 2P1-CF and 2P32-CF). In 2P1-CF, H3O+ is adsorbed on phosphorus atom and both are out of the CF plane, whereas in 2P32-CF, H3O+ is adsorbed on carbon. To explain the trend of the adsorption energies and the nature of H3O+ bonding, bond charge density and Bader atomic charge analysis were performed. The bonding density is calculated as the difference between the density of the complex (H3O+@CF or P-CF) and density of the isolated fragments (CF or P-CF and H3O+). In case of CF and P-CF with 1 at % ratio, the insertion of n-type doping such as P enriches the electron density of the graphene fiber and induces polarized sites, which are beneficial for H3O+ binding (see Figure S7 and S8, and Table S3). The doping atom induces an electron transfer into the delocalized electron density over CF fiber because of electronegativity gradient between the P and C atoms (C: 2.55 and P: 2.19) (Figure S7).
It can be seen that an accumulated electron density on the adsorbed proton and the surrounding carbon atoms are higher in the doped fiber than in pristine. This indicates a charge transfer from H3O+ into CF and P-CF fibers where the bonding is ionic in nature. The gradient in electrostatic forces created between the adsorbed proton and P or C (the adsorption site) increases the complex stability. As seen in Figure 4, P-CF-I and II display higher electron density accumulated on or around the adsorption site than that for pristine CF. The difference in the adsorption ability of P-CF I and II can be rationalized by the electrostatic repulsion between the negative charge on the adsorbed proton (H3) and the negative charges on the surrounding carbons atoms (C1, C5, and C13) with the lone pair on the oxygen atom (see Table S3). In contrast, in case of P-CF I system, these electrostatic forces are attractive, which enhances more the stability of the adsorbed proton. Adding more phosphorus atoms into CF fibers enriches the electron density and increases the polarization sites compared with the pristine CF or P-CF at 1 at % doping ratio (see Figure S7). The adsorption energies reported in Figure S9, and Table S2, indicate that the adsorption on P can be as high as on C atoms. However, the adsorption on the carbon atoms near the doping sites has higher ability to bind protons. Again, the balance between the electrostatic forces between the charges of the adsorbed protons and the substrate atoms at the adsorption site tunes the proton's affinity of the P-CF (see Figure S10 and Table S4).
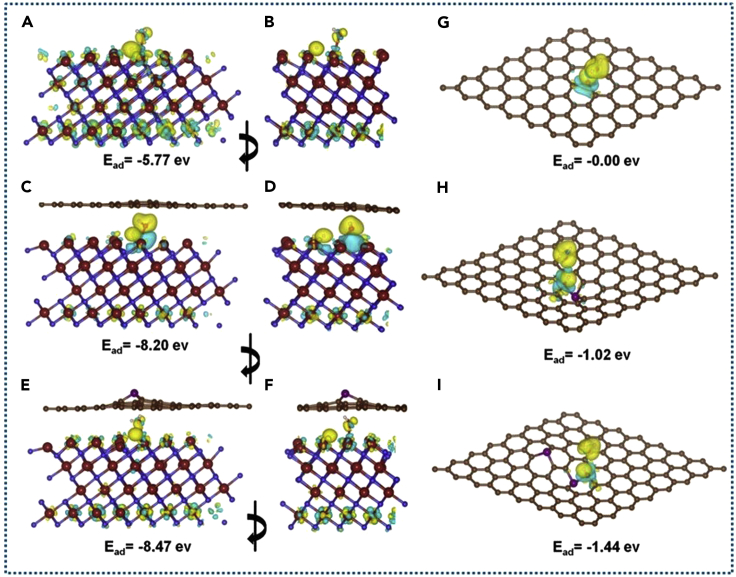
Figure 4.
DFT Simulations of MoN@P-CF
(A–I) Bonding charge density (or charge density difference of H3O+ adsorption) on MoN (A and B), MoN@CF (C and D), MoN@P-CF (E and F), CF (G), P-CF (H), and 2P32-CF (I). (B, D, and F) are rotated views of (A, C, and D). Yellow and blue represent electron density rich and depletion. Isosurfaces are at a resolution of 0.015 electron/bhor3; brown, dark blue, purple, plum, red, and white balls represent carbon, nitrogen, phosphorus, molybdenum, oxygen, and hydrogen atoms, respectively. H3O+ adsorption energies are reported in electron volts and are referenced to the adsorption on the pristine graphene (CF).
Adsorption of H3O+ on MoN nanoparticles and their interfaces with CF and P-CF is modeled by calculating the adsorption energy on MoN slab of the hexagonal crystal and its interfaces with CF and P-CF (see Figure S6). The proton's affinity of these nanocomposites is getting much higher than that of pristine CF or P-CF. Results shown in Table S2 highlighted an important effect of molybdenum nitride as an interesting material for H3O+ adsorption, as well as its composites with CF and P-CF. The nanocomposite between MoN, CF, and P-CF improves further the proton's affinity. Moreover, P-CF proved to be a better stabilizing factor of the nanocomposite morphology than CF (see Figure S11). Indeed, the BE per unit area of MoN with CF and P-CF are −62.0 and −84.0 meV, respectively (importantly, this is the interaction energy between MoN-slab and CF and P-CF sheets). The optimized structures of H3O+ on top of the MoN slab and at its interfaces with MoN@CF and MoN@P-CF showed that H3O+ dissociates into H+ and H2O similar to the adsorption on CF and P-CF. The adsorbed proton localized between three MoN atoms, whereas H2O molecules are directly coordinated to one (MoN@CF) or two Mo atoms (MoN@P-CF). Water adsorption might help to stabilize the adsorbed proton by screening the electrostatic repulsion on the metal surface inversely to the CF and P-CF cases where H2O molecules barely interact with the graphene atoms.
The binding charge density and atom in molecules (AIM) charges are presented in Figure 4 and Table S5. In case of H3O+@MoN complex, we observed that the surrounding Mo atoms are getting more positive and about 1.0 e is transferred into H3O+ (note charges on Mo114, Mo115, Mo118, and Mo122). The charge transfer from H3O+ is evidenced in binding charge density and atomic charges. The adsorbed proton undergoes a strong stabilizing interaction with positive charges on Mo atoms. This increases the polarization at adsorption site, which enhances the dipole-dipole, dipole-charge, and charge-charge interactions that are stabilizing adsorbed species (Figure 4). In case of MoN@CF and MoN@P-CF, more charge transfer from the metal surface into H3O+ (see Table S5) can be ascribed to the electron density flow coming from CF and P-CF into MoN surface. This strengthens the dipole moment created at adsorption site, which stabilizes further the adsorbed protons. The slight difference between P-CF and CF is due to the presence of positive charge at the phosphorus atom (+1.68e) that decreases the electrostatic repulsion between the adsorbed protons and the electron cloud delocalized over the graphene fiber (Figures 4 and S11).
Altogether, our DFT simulations enabled us to get deeper atomic insights into the n-type doping effect, which enhances the proton adsorption as well as the thermodynamic stability of the nanocomposite formed between the MoN nanoparticles and P-CF. Also, our results emphasize on the high affinity of proton of MoN nanoparticles, which is beneficial for the energy storage industry.
Fabrication of RuO2@CF Positive Electrode
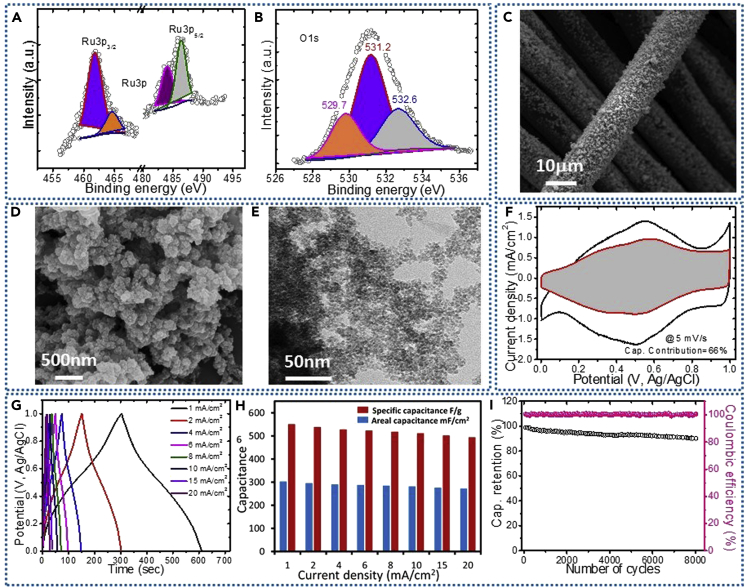
RuO2 nanoparticles were directly grown on carbon fabrics, for the first time using simple and scalable LBL method. The deposition kinetics of RuO2 nanoparticles is based on ion-by-ion growth mechanism, which involves the deposition of Ru species at the CF surface and then reaction with O species forming a nucleation center (RuO2 monolayer). XRD pattern for RuO2@CF shows two prominent peaks at 26.5° and 43.5° that are assigned to the carbon fabric (Figure S12). There is no peak found for RuO2 nanoparticles, suggesting the formation of amorphous phase. It is reported that the amorphous RuO2 shows better capacitive features than the crystalline one due to easy access to protons or ions for electrochemical reactions, and therefore whole electrode material can be utilized for the charge storage process (Dubal et al., 2013). The highly magnified XPS spectra for Ru3p of RuO2@CF sample shown in Figure 5A can be split into two peaks at BEs of 484.6 and 462.4 eV, suggesting the formation of RuO2 (Dubal et al., 2013, Hu et al., 2006). Moreover, the core-level XPS spectra for the O1s (Figure 5B) can be fitted into three major peaks at BEs of 529.7, 531.2, and 532.6 eV, which are ascribed to the metal (Ru)-oxygen bond, metal-OH bond, and the H-O-H bonding in residual water, respectively (Hu et al., 2006). It is further realized that the surface of the RuO2 is hydroxylated to form surface oxyhydroxide, which might increase surface wettability and improve the interactions between electrode material and electrolyte. Surface morphological analysis shows homogeneous distribution of RuO2 nanoparticles over the entire exposed surface of the CF. The single carbon fabric densely coated with RuO2 nanoparticles can be observed in Figure 5C. The highly magnified SEM image (Figure 5D) further confirms the formation of fractal-like agglomerates of fine RuO2 nanoparticles, leaving ample free space between them, facilitating ion transport processes. To get more insights about the nanostructure of RuO2, TEM analysis was carried out and shown in Figure 5E, which shows the formation of tiny nanoparticles with size in the range of 5–10 nm. These tiny RuO2 nanoparticles directly grown on carbon fabric can offer short ion-transport pathways and favor the direct contact of electrolytes to the interfacial electrochemically active species, consequently enhancing the supercapacitive performance. The uniform distribution of Ru and O on the carbon fabric (CF) can be further evidenced by EDX analysis, as shown in Figure S12.
Figure 5.
Physicochemical and Electrochemical Characterizations of RuO2@CF
(A and B) Magnified XPS spectra for Ru3p and O1s, respectively.
(C–F) (C and D) SEM images of RuO2 coated on carbon fabrics, (E) TEM image, showing uniform distribution of tiny nanoparticles without any aggregation. Electrochemical performance evaluation of RuO2@CF in three-electrode configuration: (F) CV curves at scan rate of 5 mV/s. The shaded area represents the capacitive contribution to the total current.
(G) Galvanostatic charge-discharge profiles at different current densities.
(H) Variation of areal and specific capacitances with current densities.
(I) Long-term cycling test with corresponding Coulombic efficiency measured at 10 mA/cm2 over 8,000 cycles.
The electrochemical properties of the as-prepared RuO2@CF was investigated using three-electrode cell in 1 M H2SO4 electrolyte. The CV curves for RuO2@CF recorded at different scan rates exhibits semi-rectangular shapes over a wide potential range of 0–1 V (versus Ag/AgCl) (see Figure S12). The shapes of CV curves can be retained even at a high scan rate of 100 mV/s, indicating highly reversible redox transitions of RuO2@CF electrode, which meets the high-power requirement of SCs. These worthy electrochemical properties might be attributed to the amorphous and tiny nanoparticulate structure of hydrous RuO2@CF that offers facile transport pathways for both protons and electrons. The energy storage or delivery process within hydrous RuO2 generally obeys the following equation:
| (Equation 4) |
To provide evidence of the pseudocapacitive nature of RuO2@CF, b-value was calculated using Equation 1, which was found to be 0.92 as shown in Figure S13. It can be further seen from Figure 5F that about 66% of the total current of RuO2@CF electrode is contributed by the capacitive mechanism at 5 mV/s. The capacitive charge contribution increases with scan rates from 5 to 100 mV/s, reaching 93% at 100 mV/s as shown in Figure S14. The galvanostatic charge/discharge (GCD) measurements for RuO2@CF electrode were performed at different current densities within the potential range of 0–1 V (versus Ag/AgCl), as shown in Figure 5G. All charge curves are symmetric to their discharging counterparts, implying fast and reversible electrochemical features. The maximum areal capacitance for RuO2@CF electrode was found to be 303 mF/cm2 (551 F/g) at a current density of 1 mA/cm2, which was decreased to 272 mF/cm2 (494 F/g) at 20 mA/cm2, retaining about 88% of initial capacitance (Figure 5H). The cycling performance was tested at a current density of 10 mA/cm2 over 8,000 cycles. As can be seen from Figure 5I, the RuO2@CF electrode exhibited good cycling stability of 87% retention of initial capacitance after 8,000 cycles. Moreover, RuO2@CF electrode shows good Coulombic efficiency (almost 100%) over 8,000 cycles, which is beneficial for the high-power applications. The decrease in capacitance by a small amount with cycles can be ascribed to the loss of active material caused by the dissolution or detachment, during the early charging or discharging cycles in the electrolyte. The overall resistance contributions including ESR, charge transfer resistance, and current collectors was determined to be 2.2 Ω, as seen from the inset of Figure S13. Moreover, the relaxation time constant was determined to be τ0 = 22 ms, suggesting fast response of the RuO2@CF electrode.
All-Pseudocapacitive Solid-State Asymmetric Supercapacitor (MoN@P-CF//RuO2@CF)
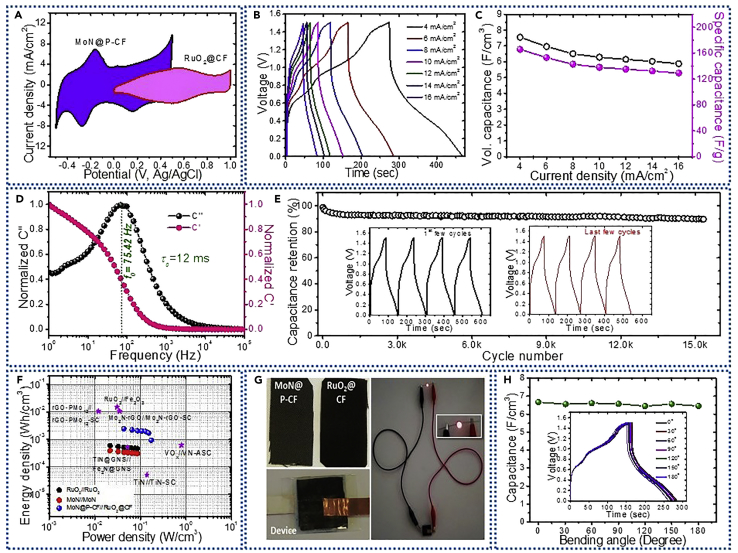
To demonstrate the commercial feasibility of MoN@P-CF hybrid electrode, all-pseudocapacitive asymmetric solid-state SC was assembled using MoN@P-CF and RuO2@CF as negative and positive electrodes, respectively, with PVA-H2SO4 gel electrolyte. It is important to note that to achieve the highest cell voltage, the charges stored in both electrodes must be balanced, which can be done by adjusting the mass loading in each electrode. The calculated mass ratio from MoN to RuO2 electrode is 1:2 (see Methods section) with a total mass loading of 3.4 mg/cm2. The CV curves for MoN@P-CF and RuO2@CF electrodes suggest that both electrodes can work in different potential windows, proposing the cell voltage of 1.5 V for MoN@P-CF//RuO2@CF cell (Figure 6A). The CV curves recorded at different scan rates (see Figure S15) show a prominent redox pair, confirming the dominance of Faradaic charge storage mechanism. These redox peaks can be retained at high scan rates of 100 mV/s, implying good reversibility of the electrode materials in device. The CD profiles of MoN@P-CF//RuO2@CF asymmetric cell show non-linear shapes as observed in CV results, which can be assigned to the pseudocapacitive nature of electrodes (Figure 6B). The cell delivers a maximum capacitance of 7.54 F/cm3 at a current density of 4 mA/cm2, which can be retained to 5.88 F/cm3 when the current density was increased to 16 mA/cm2 (78% retention), as shown in Figure 6C. The good rate capability of the cell can be attributed to the fact that all the active sites of electrode materials are easily reachable for the electrolyte ions even at high current density. In addition, the rapid redox reactions, kinetic balance, and capacity matching of both electrodes can also be responsible for high capacitance and good rate performance. The obtained capacitance for MoN@P-CF//RuO2@CF cell is the highest value reported so far; for example, MnO2//WON (tungsten oxynitride) (2.73 F/cm3) (Yu et al., 2015), rGO-PW12//rGO-PMo12 (3.91 F/cm3) (Dubal et al., 2017), Fe2O3//MnO2 (1.2 F/cm3) (Lu et al., 2014).
Figure 6.
Electrochemical Testing of All-Pseudocapacitive Solid-State Asymmetric Cell, MoN@P-CF//RuO2@CF
(A) CV curves for the MoN@P-CF and RuO2@CF electrodes at constant scan rate of 10 mV/s in different working voltage windows.
(B) CD profiles recorded at different current densities.
(C) Variation of specific and volumetric capacitances with the current density.
(D) Plot of real and imaginary capacitances as a function of frequency.
(E) The plot of capacitance retention with cycle number over 15,000 cycles measured at 12 mA/cm2; inset shows the first and last charge-discharge cycles.
(F) Ragone plot with comparison of energy and power density values with the literature.
(G) Practical demonstration of MoN@P-CF//RuO2@CF cell by powering a red light-emitting diode during the discharge state.
(H) The graph of variation of capacitance with bending angle of device; inset shows CD curves of asymmetric cell at different bending angles.
The high rate performance of MoN@P-CF//PPy@CF asymmetric cell was studied by electrochemical impedance analysis within the frequency range of 100 kHz to 1 Hz with an amplitude of 5 mV. Nyquist plot for the asymmetric cell shows an ESR of only 1.2 Ω/cm2, manifesting good electrical conductivity of both the electrode materials as well as strong binding between the active electrode material and current collector (Figure S15). The low charge transfer resistance (small semicircle) can be attributed to the good interaction between electrode material and electrolyte. In the low-frequency region, our device exhibits typical capacitive behavior, where a vertical line nearly parallel to the y axis is observed. The capacitive characteristics was further approved by the Bode plot where a phase is close to 90° (Figure S15). The real (C′) and imaginary capacitances (C″) at different frequencies for MoN@P-CF//PPy@CF cell are calculated and shown in Figure 6D. The plot shows common relaxation-type dispersions where the real capacitance C′ decreases with frequency, whereas C″ shows a maximum. The relaxation time constant (τ0) is the minimum time needed to discharge all the energy with efficiency greater than 50% and can be calculated using τ0 = 1/f0 (where f0 is characteristic frequency). The τ0 for MoN@P-CF//RuO2@CF device was calculated to be 12 ms (for the corresponding frequency of 82.87 Hz and 13 ms from Bode plot at 11.5 Hz) as displayed in Figure 6D. Such a small relaxation time (τ0) signifies that this all-pseudocapacitive asymmetric cell exhibits ultra-fast power output. This small τ0 value for the MoN@P-CF//RuO2@CF cell is promising than the values reported for fast-charging SCs in the literature: RuO2//Ti3C2Tx asymmetric cell (740 ms) (Jiang et al., 2018) and symmetric cells based on titanium nitride (TiN, 12.5–25 ms) (Yu et al., 2015), carbon anions (26 ms) (Pech et al., 2010), and laser-scribed graphene (19 ms) (El-Kady and Kaner, 2013). The extremely small relaxation time constant further confirms the ultra-fast charge-discharge ability of our asymmetric cell, which makes it promising for advanced portable electronic devices. The cycling stability for our asymmetric cell was performed at constant current density of 12 mA/cm2 over 15,000 cycles as shown in Figure 6E. The device exhibited a capacitance retention of 89% over 15,000 cycles. The energy and power densities define the real-world applications of energy storage devices. From CD curves, we have estimated the energy and power densities of MoN@P-CF//RuO2@CF cell and compared with the literature values. The cell delivers energy density of 2.36 mWh/cm3 (at power density of 43 mW/cm3), which is comparable with that of carbon-based micro-SCs and lithium thin-film batteries (10−3 -10−2 Wh/cm3) and is significantly higher than those of previously reported flexible metal nitride SCs (0.05 mWh/cm3) (Lu et al., 2012). Even at a high power density of 174 mW/cm3 our MoN@P-CF//RuO2@CF device maintains an energy density of 1.8 mWh/cm3, confirming the capacity of device to provide high energy and high power in one system (Figure 6F). The summary of comparison of literature values is provided in Table S6 and Figure S15 (Lu et al., 2012, Lu et al., 2013, Jiang et al., 2018, Zhu et al., 2015, Ma et al., 2015, Tan et al., 2018), for example, VOx//VN (0.61 mW/cm3) (Lu et al., 2013), TiN@GNS//Fe2N@GNS (0.51 mW/cm3) (Zhu et al., 2015), and TiN//TiN (0.05 mW/cm3) (Lu et al., 2012). The energy efficiency (Eeff) of the asymmetric cell was determined to be 73.2% at 4 mA/cm2 and 81.1% at 12 mA/cm2 with an average value of above 70%, which is slightly poor than the previous reports (Javed et al., 2018) as shown in Figure S15. The practical applicability of cell is presented using a demonstration with red light-emitting diodes as shown in Figure 6G. The bending test was further executed consecutively, and angles were altered manually, as indicated in Figure 6H. The corresponding CD curves recorded at different bending angles are shown in the inset of Figure 6H. The negligible change in capacitance and shapes of the CD curves even at bending angle of 180° assures its good flexibility and mechanical stability. These good electrochemical features can be credited to interactive contributions of both electrode materials and the all-pseudocapacitive asymmetric cell design, which are summarized as follows. (1) Binderless nanostructured electrode materials avoid unnecessary binders and minimize the ESR. Moreover, heteroatom (phosphorus) doping in CF provides additional pseudocapacitance. (2) Tiny nanoparticles of MoN and RuO2 electrodes provide easy access to electrochemical sites and short diffusion paths for ionic transportation. (3) The complementary potential windows of the RuO2 and MoN electrodes in H2SO4 electrolyte allow to develop new high-voltage all-pseudocapacitive asymmetric cell with improved energy density.
Discussion
In summary, we have designed and engineered ultra-fast all-pseudocapacitive solid-state ASC by coupling ultra-small MoN particles coated on P-doped CF (MoN@P-CF) negative electrode with RuO2@CF positive electrode. Our DFT simulations showed that the adsorption is further enhanced on MoN surface as well as at its interfaces with P-CF, which will provide guidelines to develop nitride-based pseudocapacitors. Owing to the excellent pseudocapacitive properties of both electrodes, MoN@P-CF//RuO2@CF asymmetric cell delivered a high capacitance (7.74 F/cm3), high energy (2.4 mWh/cm3), and power densities (174 mW/cm3) with good cycling stability (89%) over 15,000 cycles. Moreover, the device demonstrated extremely small relaxation time (τ0) of 12 ms, significantly lower than that for conventional carbon-based (EDLC) SCs, which can be directly applied in high-power microelectronics and in many diverse flexible and wearable electronics.
Limitations of the Study
For currently used polyoxometalates (POM) precursor, it is difficult to alter the nanoparticulate morphology of MoN and control the concentration of P-doping in CF, which limits further enhancement of its electrochemical performances. It is anticipated that by employing other suitable precursors, the nanostructure of MoN and the amount of P-doping can be further optimized, achieving higher performance of the asymmetric pseudocapacitors.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
D.P.D. acknowledges Queensland University of Technology and Australian Research Council (ARC) for the Future Fellowship (FT180100058). S.A.-A. acknowledges the computational resource of the college of Engineering and Geosciences (CPG) (Alfahd supercomputer) employed for the DFT simulations.
Author Contributions
D.P.D. and Y.-K.H. designed the experiments, analyzed the data, and wrote the manuscript. D.P.D. carried out synthesis and characterization of electrode materials. S.A.-A. performed all the DFT calculations and interpreted the results. D.P.D. and N.R.C. designed and carried out electrochemical measurements of electrodes and devices. All authors contributed equally to the preparation and reviewing manuscript.
Declaration of Interests
The authors declares no competing interest.
Published: June 28, 2019
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2019.05.018.
Supplemental Information
References
- Augustyn V., Simon P., Dunn B. Pseudocapacitive oxide materials for high-rate electrochemical energy storage. Energy Environ. Sci. 2014;7:1597–1614. [Google Scholar]; Augustyn, V., Simon, P., Dunn, B.. (2014). Pseudocapacitive oxide materials for high-rate electrochemical energy storage. Energy Environ. Sci. 7, 1597-1614.
- Balogun M.S., Qiu W., Wang W., Fang P., Lu X., Tong Y. Recent advances in metal nitrides as high-performance electrode materials for energy storage devices. J. Mater. Chem. A. 2015;3:1364–1387. [Google Scholar]; Balogun, M.S., Qiu, W., Wang, W., Fang, P., Lu, X., Tong, Y.. (2015). Recent advances in metal nitrides as high-performance electrode materials for energy storage devices. J. Mater. Chem. A 3, 1364-1387.
- Balogun M.S., Huang Y., Qiu W., Yang H., Ji H., Tong Y. Updates on the development of nanostructured transition metal nitrides for electrochemical energy storage and water splitting. Mater. Today. 2017;20:425–451. [Google Scholar]; Balogun, M.S., Huang, Y., Qiu, W., Yang, H., Ji, H., Tong, Y.. (2017). Updates on the development of nanostructured transition metal nitrides for electrochemical energy storage and water splitting. Mater. Today 20, 425-451.
- Borenstein A., Hanna O., Attias R., Luski S., Brousse T., Aurbach D. Carbon-based composite materials for supercapacitor electrodes: a review. J. Mater. Chem. A. 2017;5:12653–12672. [Google Scholar]; Borenstein, A., Hanna, O., Attias, R., Luski, S., Brousse, T., Aurbach, D.. (2017). Carbon-based composite materials for supercapacitor electrodes: a review. J. Mater. Chem. A 5, 12653-12672.
- Choi B.G., Chang S., Kang H., Park C., Kim H., Hong W., Lee S., Huh Y. High performance of a solid-state flexible asymmetric supercapacitor based on graphene films. Nanoscale. 2012;4:4983–4988. doi: 10.1039/c2nr30991b. [DOI] [PubMed] [Google Scholar]; Choi, B.G., Chang, S., Kang, H., Park, C., Kim, H., Hong, W., Lee, S., Huh, Y.. (2012). High performance of a solid-state flexible asymmetric supercapacitor based on graphene films. Nanoscale 4, 4983-4988. [DOI] [PubMed]
- Chodankar N.R., Dubal D.P., Kwon Y., Kim D.H. Direct growth of FeCo2O4 nanowire arrays on flexible stainless steel mesh for high-performance asymmetric supercapacitor. NPG Asia Mater. 2017;9:e419. [Google Scholar]; Chodankar, N.R., Dubal, D.P., Kwon, Y., Kim, D.H.. (2017). Direct growth of FeCo2O4 nanowire arrays on flexible stainless steel mesh for high-performance asymmetric supercapacitor. NPG Asia Mater.. 9, e419.
- Dubal D.P., Gund G.S., Holze R., Jadhav H.S., Lokhande C.D., Park C. Solution-based binder-free synthetic approach of RuO2 thin films for all solid state supercapacitors. Electrochim. Acta. 2013;103:103–109. [Google Scholar]; Dubal, D.P., Gund, G.S., Holze, R., Jadhav, H.S., Lokhande, C.D., Park, C.. (2013). Solution-based binder-free synthetic approach of RuO2 thin films for all solid state supercapacitors. Electrochim. Acta 103, 103-109.
- Dubal D.P., Ayyad O., Ruiz V., Gomez-Romero P. Hybrid energy storage: the merging of battery and supercapacitor chemistries. Chem. Soc. Rev. 2015;44:1777–1790. doi: 10.1039/c4cs00266k. [DOI] [PubMed] [Google Scholar]; Dubal, D.P., Ayyad, O., Ruiz, V., Gomez-Romero, P.. (2015). Hybrid energy storage: the merging of battery and supercapacitor chemistries. Chem. Soc. Rev. 44, 1777-1790. [DOI] [PubMed]
- Dubal D.P., Chodankar N.R., Vinu A., Kim D.H., Gomez-Romero P. Asymmetric supercapacitors based on reduced graphene oxide with different polyoxometalates as positive and negative electrodes. ChemSusChem. 2017;10:2742–2750. doi: 10.1002/cssc.201700792. [DOI] [PubMed] [Google Scholar]; Dubal, D.P., Chodankar, N.R., Vinu, A., Kim, D.H., Gomez-Romero, P.. (2017). Asymmetric supercapacitors based on reduced graphene oxide with different polyoxometalates as positive and negative electrodes. ChemSusChem 10, 2742-2750. [DOI] [PubMed]
- Dubal D.P., Chodankar N.R., Kim D.H., Gomez-Romero P. Towards flexible solid-state supercapacitors for smart and wearable electronics. Chem. Soc. Rev. 2018;47:2065–2129. doi: 10.1039/c7cs00505a. [DOI] [PubMed] [Google Scholar]; Dubal, D.P., Chodankar, N.R., Kim, D.H., Gomez-Romero, P.. (2018). Towards flexible solid-state supercapacitors for smart and wearable electronics Chem. Soc. Rev. 47, 2065-2129. [DOI] [PubMed]
- El-Kady M.F., Kaner R.B. Scalable fabrication of high-power graphene micro-supercapacitors for flexible and on-chip energy storage. Nat. Commun. 2013;4:1475. doi: 10.1038/ncomms2446. [DOI] [PubMed] [Google Scholar]; El-Kady, M.F., Kaner, R.B.. (2013). Scalable fabrication of high-power graphene micro-supercapacitors for flexible and on-chip energy storage. Nat. Commun. 4, 1475. [DOI] [PubMed]
- Eftekhari A., Mohamedi M. Tailoring pseudocapacitive materials from a mechanistic perspective. Mater. Today. 2015;18:252–264. [Google Scholar]; Eftekhari, A., Mohamedi, M.. (2015). Tailoring pseudocapacitive materials from a mechanistic perspective. Mater. Today 18, 252-264.
- Hu C.C., Chang K.H., Lin M.C., Wu Y.T. Design and tailoring of the nanotubular arrayed architecture of hydrous RuO2 for next generation supercapacitors. Nano Lett. 2006;6:2690–2695. doi: 10.1021/nl061576a. [DOI] [PubMed] [Google Scholar]; Hu, C.C., Chang, K.H., Lin, M.C., Wu, Y.T.. (2006). Design and tailoring of the nanotubular arrayed architecture of hydrous RuO2 for next generation supercapacitors. Nano Lett. 6, 2690-2695. [DOI] [PubMed]
- Javed M.S., Shah H.U., Shaheen N., Lin R., Qiu M., Xie J., Li J., Raz R., Mai W., Hu C. High energy density hybrid supercapacitor based on 3D mesoporous cuboidal Mn2O3 and MOF-derived porous carbon polyhedrons. Electrochim. Acta. 2018;282:1–9. [Google Scholar]; Javed, M.S., Shah, H.U., Shaheen, N., Lin, R., Qiu, M., Xie, J., Li, J., Raz, R., Mai, W., Hu, C.. (2018). High energy density hybrid supercapacitor based on 3D mesoporous cuboidal Mn2O3 and MOF-derived porous carbon polyhedrons. Electrochim. Acta 282, 1-9.
- Jiang Q., Kurra N., Alhabeb M., Gogotsi Y., Alshareef H.N. All pseudocapacitive MXene-RuO2 asymmetric supercapacitors. Adv. Energy Mater. 2018;8:1703043. [Google Scholar]; Jiang, Q., Kurra, N., Alhabeb, M., Gogotsi, Y., Alshareef, H.N.. (2018). All pseudocapacitive MXene-RuO2 asymmetric supercapacitors. Adv. Energy Mater.. 8, 1703043.
- Ko Y., Kwon M., Bae W., Lee B., Lee S., Cho J. Flexible supercapacitor electrodes based on real metal-like cellulose papers. Nat. Commun. 2017;8:536. doi: 10.1038/s41467-017-00550-3. [DOI] [PMC free article] [PubMed] [Google Scholar]; Ko, Y., Kwon, M., Bae, W., Lee, B., Lee, S., Cho, J.. (2017). Flexible supercapacitor electrodes based on real metal-like cellulose papers. Nat. Commun.. 8, 536. [DOI] [PMC free article] [PubMed]
- Liu T.C., Pell W.G., Conway B.E., Roberson S.L. Behavior of molybdenum nitrides as materials for electrochemical capacitors comparison with ruthenium oxide. J. Electrochem. Soc. 1998;145:1882–1888. [Google Scholar]; Liu, T.C., Pell, W.G., Conway, B.E., Roberson, S.L.. (1998). Behavior of molybdenum nitrides as materials for electrochemical capacitors comparison with ruthenium oxide. J. Electrochem. Soc. 145, 1882-1888.
- Liu J., Huang K., Tang H.L., Lei M. Porous and single-crystalline-like molybdenum nitride nanobelts as a non-noble electrocatalyst for alkaline fuel cells and electrode materials for supercapacitors. Int. J. Hydrogen Energy. 2016;41:996–1001. [Google Scholar]; Liu, J., Huang, K., Tang, H.L., Lei, M.. (2016). Porous and single-crystalline-like molybdenum nitride nanobelts as a non-noble electrocatalyst for alkaline fuel cells and electrode materials for supercapacitors. Int. J. Hydrogen Energy 41, 996-1001.
- Lu X., Wang G., Zhai T., Yu M., Xie S., Ling Y., Liang C., Tong Y., Li Y. Stabilized TiN nanowire arrays for high-performance and flexible supercapacitors. Nano Lett. 2012;12:5376–5381. doi: 10.1021/nl302761z. [DOI] [PubMed] [Google Scholar]; Lu, X., Wang, G., Zhai, T., Yu, M., Xie, S., Ling, Y., Liang, C., Tong, Y., Li, Y.. (2012). Stabilized TiN nanowire arrays for high-performance and flexible supercapacitors. Nano Lett. 12, 5376-5381. [DOI] [PubMed]
- Lu X., Zeng Y., Yu M., Zhai T., Liang C., Xie S., Balogun M., Tong Y. Oxygen-deficient hematite nanorods as high-performance and novel negative electrodes for flexible asymmetric supercapacitors. Adv. Mater. 2014;26:3148–3155. doi: 10.1002/adma.201305851. [DOI] [PubMed] [Google Scholar]; Lu, X., Zeng, Y., Yu, M., Zhai, T., Liang, C., Xie, S., Balogun, M., Tong, Y.. (2014). Oxygen-deficient hematite nanorods as high-performance and novel negative electrodes for flexible asymmetric supercapacitors. Adv. Mater. 26, 3148-3155. [DOI] [PubMed]
- Lu X., Yu M., Zhai T., Wang G., Xie X., Liu T., Liang C., Tong Y., Li Y. High energy density asymmetric quasi-solid-state supercapacitor based on porous vanadium nitride nanowire anode. Nano Lett. 2013;13:2628–2633. doi: 10.1021/nl400760a. [DOI] [PubMed] [Google Scholar]; Lu, X., Yu, M., Zhai, T., Wang, G., Xie, X., Liu, T., Liang, C., Tong, Y., Li, Y.. (2013). High energy density asymmetric quasi-solid-state supercapacitor based on porous vanadium nitride nanowire anode. Nano Lett. 13, 2628-2633. [DOI] [PubMed]
- Ma G., Wang Z., Gao B., Ding T., Zhong Q., Peng X., Su J., Hu B., Yuan L., Chu P. Multilayered paper-like electrodes composed of alternating stacked mesoporous Mo2N nanobelts and reduced graphene oxide for flexible all-solid-state supercapacitors. J. Mater. Chem. A. 2015;3:14617–14624. [Google Scholar]; Ma, G., Wang, Z., Gao, B., Ding, T., Zhong, Q., Peng, X., Su, J., Hu, B., Yuan, L., Chu, P., et al. (2015). Multilayered paper-like electrodes composed of alternating stacked mesoporous Mo2N nanobelts and reduced graphene oxide for flexible all-solid-state supercapacitors. J. Mater. Chem. A, 3, 14617-14624.
- Nam K., Kim D., Yoo P., Chiang C., Meethong N., Hammond P., Chiang Y., Belcher A. Virus-enabled synthesis and assembly of nanowires for lithium ion battery electrodes. Science. 2006;312:885–888. doi: 10.1126/science.1122716. [DOI] [PubMed] [Google Scholar]; Nam, K., Kim, D., Yoo, P., Chiang, C., Meethong, N., Hammond, P., Chiang, Y., Belcher, A.., Virus-enabled synthesis and assembly of nanowires for lithium ion battery electrodes. Science 312, 885-888 (2006) [DOI] [PubMed]
- Nitta N., Wu F., Lee J.T., Yushin G. Li-ion battery materials: present and future. Mater. Today. 2015;18:252–264. [Google Scholar]; Nitta, N., Wu, F., Lee, J.T., Yushin, G.. (2015). Li-ion battery materials: present and future. Mater. Today 18, 252-264.
- Pech D., Brunet M., Durou H., Huang P., Mochalin V., Gogotsi Y., Taberna P., Simon P. Ultrahigh-power micrometre-sized supercapacitors based on onion-like carbon. Nat. Nanotechnol. 2010;5:651–654. doi: 10.1038/nnano.2010.162. [DOI] [PubMed] [Google Scholar]; Pech, D., Brunet, M., Durou, H., Huang, P., Mochalin, V., Gogotsi, Y., Taberna, P., Simon, P.. (2010). Ultrahigh-power micrometre-sized supercapacitors based on onion-like carbon, Nat. Nanotechnol. 5, 651-654. [DOI] [PubMed]
- Shah S.I.U., Hector A.L., Owen J.R. Redox supercapacitor performance of nanocrystalline molybdenum nitrides obtained by ammonolysis of chloride- and amide-derived precursors. J. Power Sources. 2014;266:456–463. [Google Scholar]; Shah, S.I.U., Hector, A.L., Owen, J.R.. (2014). Redox supercapacitor performance of nanocrystalline molybdenum nitrides obtained by ammonolysis of chloride- and amide-derived precursors. J. Power Sources 266, 456-463.
- Salanne M., Rotenberg B., Naoi K., Kaneko K., Taberna P., Grey C., Dunn B., Simon P. Efficient storage mechanisms for building better supercapacitors. Nat. Energy. 2016;1:16070. [Google Scholar]; Salanne, M., Rotenberg, B., Naoi, K., Kaneko, K., Taberna, P., Grey, C., Dunn, B., Simon, P.. (2016). Efficient storage mechanisms for building better supercapacitors. Nat. Energy, 1, 16070.
- Shen B., Zhang X., Guo R., Lang J., Chen J., Yan X. Carbon encapsulated RuO2 nano-dots anchoring on graphene as an electrode for asymmetric supercapacitors with ultralong cycle life in an ionic liquid electrolyte. J. Mater. Chem. A. 2016;4:8180–8189. [Google Scholar]; Shen, B., Zhang, X., Guo, R., Lang, J., Chen, J., Yan, X.. (2016). Carbon encapsulated RuO2 nano-dots anchoring on graphene as an electrode for asymmetric supercapacitors with ultralong cycle life in an ionic liquid electrolyte. J. Mater. Chem. A 4, 8180-8189.
- Tan Y., Meng L., Wang Y., Dong W., Kong L., Kang L., Ran F. Negative electrode materials of molybdenum nitride/N-doped carbon nano-fiber via electrospinning method for high-performance supercapacitors. Electrochim. Acta. 2018;277:41–49. [Google Scholar]; Tan, Y., Meng, L., Wang, Y., Dong, W., Kong, L., Kang, L., Ran, F.. (2018). Negative electrode materials of molybdenum nitride/N-doped carbon nano-fiber via electrospinning method for high-performance supercapacitors. Electrochim. Acta 277, 41-49.
- Wang G., Zhang L., Zhang J. A review of electrode materials for electrochemical supercapacitors. Chem. Soc. Rev. 2012;41:797–828. doi: 10.1039/c1cs15060j. [DOI] [PubMed] [Google Scholar]; Wang, G., Zhang, L., Zhang, J.. (2012). A review of electrode materials for electrochemical supercapacitors. Chem. Soc. Rev., 41, 797-828. [DOI] [PubMed]
- Wang W., Guo S., Lee I., Ahmed K., Zhong J., Favors Z., Zaera F., Ozkan M., Ozkan C. Hydrous ruthenium oxide nanoparticles anchored to graphene and carbon nanotube hybrid foam for supercapacitors. Sci. Rep. 2014;4:4452. doi: 10.1038/srep04452. [DOI] [PMC free article] [PubMed] [Google Scholar]; Wang, W., Guo, S., Lee, I., Ahmed, K., Zhong, J., Favors, Z., Zaera, F., Ozkan, M., Ozkan, C.. (2014). Hydrous ruthenium oxide nanoparticles anchored to graphene and carbon nanotube hybrid foam for supercapacitors. Sci. Rep. 4, 4452. [DOI] [PMC free article] [PubMed]
- Wang J., Polleux J., Lim J., Dunn B. Pseudocapacitive contributions to electrochemical energy storage in TiO2 (Anatase) nanoparticles. J. Phys. Chem. C. 2007;111:14925–14931. [Google Scholar]; Wang, J., Polleux, J., Lim, J., Dunn, B.. (2007). Pseudocapacitive contributions to electrochemical energy storage in TiO2 (Anatase) nanoparticles. J. Phys. Chem. C 111, 14925-14931.
- Xie J., Li S., Zhang X., Zhang J., Wang R., Zhang H., Pan B., Xie Y. Atomically-thin molybdenum nitride nanosheets exposing active surface sites for efficient hydrogen evolution. Chem. Sci. 2014;5:4615–4620. [Google Scholar]; Xie, J., Li, S., Zhang, X., Zhang, J., Wang, R., Zhang, H., Pan, B., Xie, Y.. (2014). Atomically-thin molybdenum nitride nanosheets exposing active surface sites for efficient hydrogen evolution. Chem. Sci., 5, 4615-4620.
- Yan H., Tian C., Wang L., Wu A., Meng M., Zhao L., Fu H. Phosphorus-modified tungsten nitride/reduced graphene oxide as a high-performance, non-noble-metal electrocatalyst for the hydrogen evolution reaction. Angew. Chem. Int. Ed. 2015;54:6325–6329. doi: 10.1002/anie.201501419. [DOI] [PubMed] [Google Scholar]; Yan, H., Tian, C., Wang, L., Wu, A., Meng, M., Zhao, L., Fu, H.. (2015) Phosphorus-modified tungsten nitride/reduced graphene oxide as a high-performance, non-noble-metal electrocatalyst for the hydrogen evolution reaction. Angew. Chem. Int. Ed. 54, 6325-6329. [DOI] [PubMed]
- Yan H., Jiao Y., Wu A., Tian C., Wang L., Zhang X., Fu H. Synergism of molybdenum nitride and palladium for high-efficient formic acid electrooxidation. J. Mater. Chem. A. 2018;6:7623–7630. [Google Scholar]; Yan, H., Jiao, Y., Wu, A., Tian, C., Wang, L., Zhang, X., Fu, H.. (2018). Synergism of molybdenum nitride and palladium for high-efficient formic acid electrooxidation. J. Mater. Chem. A, 6, 7623-7630.
- Yang P., Chao D., Zhu C., Xia X., Zhang Y., Wang X., Sun P., Tay B., Shen Z., Mai W., Fan H. Ultrafast-charging supercapacitors based on corn-like titanium nitride nanostructures. Adv. Sci. 2016;3:1500299. doi: 10.1002/advs.201500299. [DOI] [PMC free article] [PubMed] [Google Scholar]; Yang, P., Chao, D., Zhu, C., Xia, X., Zhang, Y., Wang, X., Sun, P., Tay, B., Shen, Z., Mai, W., Fan, H.. (2016). Ultrafast-charging supercapacitors based on corn-like titanium nitride nanostructures. Adv. Sci. 3, 1500299. [DOI] [PMC free article] [PubMed]
- Yuan Y., Amine K., Lu J., Shahbazian-Yassar R. Understanding materials challenges for rechargeable ion batteries with in situ transmission electron microscopy. Nat. Commun. 2017;8:15806. [Google Scholar]; Yuan, Y., Amine, K., Lu, J., Shahbazian-Yassar, R.. (2017). Understanding materials challenges for rechargeable ion batteries with in situ transmission electron microscopy. Nat. Commun. 8, 15806.
- Yu M., Han Y., Cheng X., Hu L., Zeng Y., Chen M., Cheng F., Lu X., Tong Y. Holey tungsten oxynitride nanowires: novel anodes efficiently integrate microbial chemical energy conversion and electrochemical energy storage. Adv. Mater. 2015;15:3085–3091. doi: 10.1002/adma.201500493. [DOI] [PubMed] [Google Scholar]; Yu, M., Han, Y., Cheng, X., Hu, L., Zeng, Y., Chen, M., Cheng, F., Lu, X., Tong, Y.. (2015). Holey tungsten oxynitride nanowires: novel anodes efficiently integrate microbial chemical energy conversion and electrochemical energy storage. Adv. Mater., 15, 3085-3091. [DOI] [PubMed]
- Yu X.Y., Lou X.W. Mixed metal sulfides for electrochemical energy storage and conversion. Adv. Energy Mater. 2018;8:1701592. [Google Scholar]; Yu, X.Y., Lou, X.W.. (2018). Mixed metal sulfides for electrochemical energy storage and conversion. Adv. Energy Mater. 8, 1701592.
- Zhang B., Cui G., Zhang K., Zhang L., Han P., Dong S. Molybdenum nitride/nitrogen-doped graphene hybrid material for lithium storage in lithium ion batteries. Electrochim. Acta. 2014;150:15–22. [Google Scholar]; Zhang, B., Cui, G., Zhang, K., Zhang, L., Han, P., Dong, S.. (2014a). Molybdenum nitride/nitrogen-doped graphene hybrid material for lithium storage in lithium ion batteries. Electrochim. Acta 150, 15-22.
- Zhang W., Wu Z.Y., Jiang H.L., Yu S.H. Nanowire-directed templating synthesis of metal–organic framework nanofibers and their derived porous doped carbon nanofibers for enhanced electrocatalysis. J. Am. Chem. Soc. 2014;136:14385–14388. doi: 10.1021/ja5084128. [DOI] [PubMed] [Google Scholar]; Zhang, W., Wu, Z.Y., Jiang, H.L., Yu, S.H.. (2014b). Nanowire-directed templating synthesis of metal-organic framework nanofibers and their derived porous doped carbon nanofibers for enhanced electrocatalysis. J. Am. Chem. Soc. 136, 14385-14388. [DOI] [PubMed]
- Zhu C., Yang P., Chao D., Wang X., Zhang X., Chen S., Tay B., Huang H., Zhang H., Mai W., Fan H. All metal nitrides solid-state asymmetric supercapacitors. Adv. Mater. 2015;27:4566–4571. doi: 10.1002/adma.201501838. [DOI] [PubMed] [Google Scholar]; Zhu, C., Yang, P., Chao, D., Wang, X., Zhang, X., Chen, S., Tay, B., Huang, H., Zhang, H., Mai, W., Fan, H.. (2015). All metal nitrides solid-state asymmetric supercapacitors. Adv. Mater., 27, 4566-4571. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.