Abstract
While the pathophysiology of transient global amnesia (TGA) is not understood, due to the specific nature of the clinical deficits, transient dysfunction in the medial temporal lobe, especially in the hippocampus, is assumed; however, concomitant disturbances in other brain regions and in executive function have been postulated. In this study, a cohort of 16 patients was prospectively recruited from the emergency department for resting-state functional MRI (fMRI) during the acute stage of TGA, as confirmed by a standardized neuropsychological assessment. Twenty age- and sex-matched controls, as well as twenty patients with a history of TGA, were recruited for comparison. Functional data were processed using independent component analysis (ICA), allowing the complete automatic (data-driven) identification of spontaneous network dynamics. We documented a severe disturbance in anterograde episodic long-term memory in all patients. Group-based ICA of resting-state data in acute TGA patients versus that of controls and patients with a past TGA episode demonstrated reduced FC mainly of structures belonging to the executive network (EN), but also the hippocampus, confirming its pathophysiological involvement in the disorder, as well as areas belonging to the salience network and other subcortical regions. No significant differences were found when comparing connectivity in patients with a history of TGA and controls. Our findings strengthen previous empirical and theoretical accounts of hippocampal and executive dysfunction in TGA. The disruption of frontal, parietal and insular control regions, together with disruption in the hippocampus, provides a new interpretation for the pathophysiology and neuropsychological profile of this neurological disorder on a large-scale network level
Keywords: Executive network, Hippocampus, Memory, Transient global amnesia, Resting-state functional connectivity
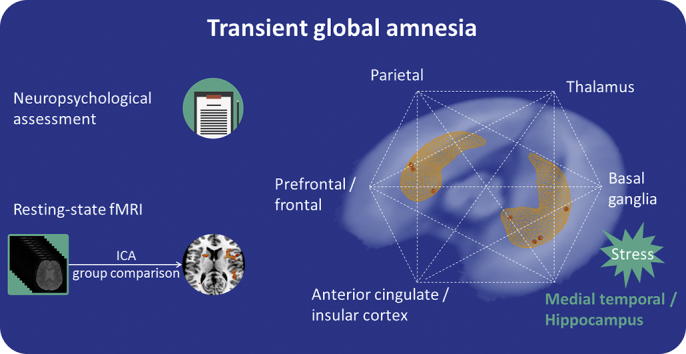
Graphical abstract
Highlights
-
•
During TGA connectivity is reduced in areas within and outside the executive network, including the hippocampus.
-
•
Relevant hubs within the salience network and subcortical regions are also involved.
-
•
The acute stage of TGA is interpreted on a large-scale network level.
1. Introduction
The most pronounced clinical finding during transient global amnesia (TGA) is a disturbance in anterograde episodic long-term memory; therefore, the transient dysfunction of the medial temporal lobe, especially of the hippocampus, is assumed to be one of the main pathophysiological mechanisms (Jager et al., 2009). Previous studies report the existence of additional, non-amnestic dysfunctions during TGA (Hodges, 1994). The results of a meta-analysis showed performance reductions in executive function with delayed recovery (Jager et al., 2009). The only structural finding consistently reproduced in TGA patients is that of small diffusion-weighted imaging (DWI) lesions that are located within the CA1-subfield of the hippocampus (Bartsch et al., 2006; Sedlaczek et al., 2004). In addition to these “core” lesions within the hippocampus, other brain regions, such as the thalamus and especially the prefrontal cortex, have been reported to be functionally disturbed during episodes of TGA in case studies using positron emission tomography (PET) assessment (Baron et al., 1994; Eustache et al., 1997; Guillery et al., 2002).
In this prospective study, we investigated functional connectivity (FC) in patients with acute TGA as documented by a standardized neuropsychological assessment in the emergency department (ED). We used independent component analysis (ICA) to determine group differences between acute TGA patients and controls, as well as between patients with a history of TGA. In addition to the previously reported neurocognitive features of TGA, we hypothesized that our analyses would demonstrate altered functional connectivity in networks involving the hippocampus and the executive network (EN) during TGA.
2. Methods and materials
2.1. Participants
Sixteen patients presenting with an acute episode of TGA to our ED were prospectively included in this study if they a) fulfilled the established criteria for TGA (Hodges and Warlow, 1990); b) were still in the acute episode, as demonstrated by a standardized neuropsychological assessment; and c) had no contraindications to undergo MRI. TGA was diagnosed according to the Hodges and Warlow criteria, indicating that clouding of consciousness, a focal neurological deficit, or a history of epilepsy or trauma was not present in any of the patients. In all cases, anterograde amnesia was documented by the ED neurologist or a capable witness. Patients and family were questioned regarding potential stressful events preceding the onset of TGA. These were documented and classified either as an immediately preceding physical or emotional stressor or as a remote or ongoing emotional stressor in the days or weeks before the episode. The study examinations were performed during the presentation of the patients to the ED. From our prospectively collected TGA database with over 400 cases that also fulfilled the abovementioned criteria, we included 20 right-handed TGA patients who had suffered an episode of TGA between 2010 and 2011 as a control group. We additionally recruited 20 healthy control subjects matched for age. All 40 control subjects received an MRI scan and a detailed neuropsychological evaluation. Datasets from one acute TGA patient and two healthy participants were excluded for technical reasons. The study was approved by the Ethics Committee of the Medical Faculty Mannheim, Heidelberg University and conformed to the Code of Ethics of the World Medical Association (Declaration of Helsinki, 6th revision, 2008). Written informed consent was obtained before inclusion and was repeated in acute TGA patients after symptom resolution.
2.2. Short neuropsychological evaluation in the emergency department
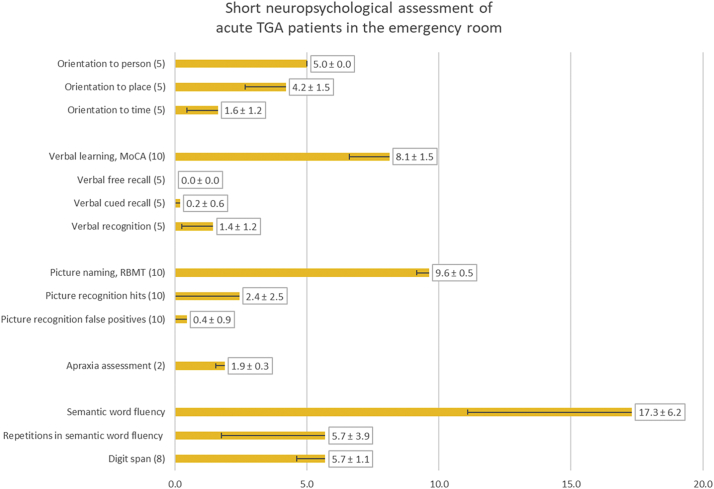
A standardized short neuropsychological assessment was performed at patient presentation to the ED to document ongoing episode of TGA (see Table 1). This included an assessment of orientation concerning time, place and person with 5 questions for each dimension (Folstein et al., 1975). Digit span was assessed, and word list learning with two learning trials for a list of 5 words, delayed free and cued recall as well as recognition (1 out of 3) was used to test episodic verbal memory (Härting et al., 2000; Nasreddine et al., 2005). Subtest “picture recognition – delayed recall” of the Rivermead Behavioral Memory Test was used to test naming and visual episodic memory (Wilson et al., 1985). Semantic word fluency was tested by naming of animals within 1 min (Morris et al., 1989). Apraxia was ruled out by imitation of two complex bimanual poses.
Table 1.
Neuropsychological assessment.
| Cognitive domain (test adaptation) | Test procedure/questions | Score |
|---|---|---|
| Orientation in person, time and space (MMSE) | Answer the following questions: “Name? Surname? Birthday? Address (Street/city)?” “Day of the week? Exact Date (Year/month/day)? Time?” “Country? State? City? Exact location? Floor?” |
1 point for each correct answer: maximum 15 points |
| Verbal memory item (MoCA) | Memorize and recall a list of words after a delay of 10 min: Face/Velvet/Church/Daisy/Red (2 learning trials) |
1 point for each word recalled in 2 learning trials: maximum 10 points; 1 point for each word recalled freely, with cue or recognized from a set of 3 choices: maximum 5 points in each trial |
| Picture recognition item (RBMT) | Name a set of 10 pictures and recognize them from a set of 20 pictures after 10 min | 1 point for each correctly named picture, 1 point each for correct delayed recognition: maximum 10 points; false positive scores counted: maximum 10 points |
| Apraxia | Imitation of 2 bimanual gestures
|
1 point for each correct gesture: maximum 2 points |
| Semantic word fluency (CERAD) | Name as many animals as possible in 60 s | 1 point for each correct word; repetitions counted |
| Verbal short-term memory (WMS-R) | Forward digit span of lists with lengths of 3–8 digits, with list length increasing after each correct trial and stopping after two incorrect trials of the same list length | Points for maximal list length successfully recalled: maximum 8 points |
MMSE: Mini-Mental State Examination; MoCA: Montreal Cognitive Assessment; RBMT: Rivermead Behavioral Memory Test, CERAD: Consortium to Establish a Registry for Alzheimer's Disease; WMS-R: Wechsler Memory Scale-Revised.
2.3. Acquisition of MRI data
The MRI session was performed on a 3 T MAGNETOM Skyra whole body MR scanner (Siemens Healthineers, Erlangen, Germany) using a 20-channel head/neck coil.
2.3.1. Structural imaging
We used a T1-weighted magnetization-prepared rapid gradient-echo (MP-RAGE) sequence (TR = 1900 ms, TE = 2.13 ms, flip angle = 9°, FoV = 240 × 240, matrix size = 256 × 256, voxel size = 0.9 × 0.9 × 0.9 mm3, slice oversampling = 16.7%, BW = 230 Hz/px, parallel acquisition technique GRAPPA acceleration factor 2). Additional diffusion-weighted sequences were performed perpendicularly to the long axis of the hippocampus.
2.3.2. Functional imaging
Blood oxygenation level-dependent (BOLD) whole-brain functional images were acquired using a T2*-weighted gradient-echo planar imaging (EPI) sequence (TR = 2210 ms, TE = 23 ms, FoV = 220 × 220 mm2, matrix size = 96 × 96, voxel size = 2.3 × 2.3 × 3.0 mm3, flip angle = 90°, bandwidth = 1270 Hz/px, parallel acquisition technique GRAPPA acceleration factor 2). For each image volume, 36 axial slices (slice thickness = 3.0 mm, no gap) were recorded in interleaved slice order, positioned along the anterior and posterior commissure (AC-PC orientation), measuring a total of 210 volumes. The participants were instructed to rest quietly with their eyes closed without sleeping during the resting-state fMRI measurement.
All patients received routine cranial MRI examinations after 24 h including a detailed DWI protocol with sequences parallel to the long axis of the hippocampus.
2.4. Analysis of resting-state fMRI data
Resting-state fMRI data were analysed using the multivariate exploratory linear optimized decomposition into independent components (MELODIC, v. 3.14) independent components analysis (ICA) from the FSL software package (v. 5.0.9) (Beckmann et al., 2005; Beckmann et al., 2009; Beckmann and Smith, 2004). The pre-processing of fMRI data included non-brain structure removal from the EPI volumes using the Brain Extraction Tool (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/BET), motion correction and high-pass temporal filtering (with a cut-off frequency of 0.01 Hz). The images were subsequently smoothed based on the full width at half maximum of the Gaussian kernel (5 mm). Functional MRI volumes were registered to the individual's structural scan and to MNI-152 standard space images using FMRIB's Linear Image Registration Tool (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/FLIRT). MELODIC ICA was applied using all fMRI scans (n = 53) to obtain robust group-ICA spatial maps that were estimated automatically.
2.5. Identification of resting-state brain networks
MELODIC automatically estimated 93 independent components using a Laplace approximation (http://fsl.fmrib.ox.ac.uk/fsl) for all datasets of acute TGA patients and both control groups. We identified the traditional IC maps selected to represent intrinsically connected cortical networks by visual inspection based on their localized spatial connectivity patterns with reference to reported resting-state networks: visual networks, auditory network, default mode network (DMN), EN, right and left lateralized fronto-parietal networks, somatosensory network, as well as right and left hippocampal networks (Beckmann et al., 2005; Cole et al., 2010; Damoiseaux et al., 2006; Smith et al., 2013). For details please see Fig. 1 of the Supplementary material).
Fig. 1.
DWI lesions in acute TGA patients on day 1: 3D visualization of all 25 DWI lesions in MNI space. Hippocampus surface models were created from the Harvard-Oxford subcortical structural atlas.
Absolute (referenced to the middle time-point) and relative (compared with the previous time-point) estimations were used to quantify head motion. These estimates were taken as the root mean square values of translational and rotational movements. The group averages and group differences of the root mean square motion estimates were computed by FSL MCFLIRT. Absolute and relative motion parameters were compared between groups using one-way ANOVA.
2.6. Group comparisons
Along the ICA-dual regression approach, our main analyses consisted of comparing each network of interest (the EN, left and right hippocampal networks) between groups. The set of spatial maps from the group-average analysis was used to generate subject-specific versions of the spatial maps, and associated timeseries, using dual regression (Nickerson et al., 2017). First, for each subject, the group-average set of spatial maps was regressed (as spatial regressors in a multiple regression) into the subject's 4D space-time dataset. This resulted in a set of subject-specific timeseries, one per group-level spatial map. Next, those timeseries were regressed (as temporal regressors, again in a multiple regression) into the same 4D dataset, resulting in a set of subject-specific spatial maps, one per group-level spatial map. The comparison across the group of subjects was achieved using 5000 permutations in randomise (nonparametric statistical tool (Winkler et al., 2014) using unpaired t-test contrasts (FMRIB Software Library) with threshold-free cluster enhancement and family-wise error (p < 0.05) correction.
3. Results
3.1. Study population
Table 2 summarizes the characteristics of the study population. Six patients reported physical extortion, while 7 patients related on acute psychological stress immediately before symptom onset with sadness/grief being the most often reported emotion (n = 4). Additionally, 12 patients stated that they suffered from chronic emotional stress with sadness/grief being the most often named stressor (n = 4). Five patients had a history of psychiatric illness (1 anxiety disorder, 1 burn-out, 3 depression). In 6 patients the exact onset of symptoms was witnessed. In these patients, MRI was performed after a mean of 3.36 h since onset. In the remaining 10 patients, MRI was performed 5.16 h after the time they were last seen normal by relatives. MRI was performed immediately after the neuropsychological evaluation. Upon follow-up MRI, which was administered within 24 h after the initial measurement, all patients had developed hyperintense hippocampal DWI lesions (see Fig. 1). None of the subjects in the control groups showed abnormal findings in a detailed neuropsychological assessment – the results have been reported previously in detail (Griebe et al., 2015).
Table 2.
Characteristics of the study population.
| Acute TGA | Controls | History of TGA | |
|---|---|---|---|
| Number | 16 | 20 | 20 |
| Age, years; mean (SD) | 69.5 (±10.55) | 66.55 (±7.0) | 66.50 (±7.7) |
| Sex male; number | 8 | 8 | 8 |
| Psychiatric comorbiditiesa; number | 5 | 1 | 6 |
| MMSE | 28.64 (28–30) | 29.5 (28–30) | 30 (28–30) |
| Hippocampal DWI lesion | 16 | NA | 16b |
| Left | 5 | 4 | |
| Right | 6 | 2 | |
| Bilateral | 5 | 10 |
TGA: transient global amnesia, SD: standard deviation; MMSE: mini mental status examination; DWI: diffusion-weighted imaging; NA: not applicable.
Includes previously diagnosed depression in 3, anxiety disorder in 1, and burnout in 1 patient(s).
Indicates hippocampal lesions during acute phase of previous TGA.
3.2. Short neuropsychological evaluation in the emergency department
All patients were neuropsychologically tested in the ED. All of them were oriented to person, and the majority were oriented to place, whereas orientation to time was very poor overall. The naming of pictures was without pathological findings. Short-term memory functions, such as those involved in digit spans and learning 5 words, were normal, but none of the patients could freely recall any of the presented words. Cued recall was very poor, and recognition was in the range of chance. Picture recognition was below even what could be expected by chance because our patients tended to answer “no” to most presented recognition items. Semantic word fluency was normal, but patients showed a high rate of repetition due to memory dysfunction. None of the patients showed apraxia, and only two patients could not imitate one of two complex bimanual poses correctly. For details, see Fig. 2.
Fig. 2.
Results of neuropsychological testing in means plus standard deviation for performance of 16 patients with acute TGA in the emergency room. The numbers in brackets indicate the highest possible score.
3.3. Whole brain functional resting-state network comparison
The identified EN, as detected by group-independent ICA (encompassing all groups), is shown in Fig. 3. Compared with healthy controls as well as with patients with a history of TGA, patients with acute TGA showed a significant reduction of FC mainly - but not solely - in structures belonging to the EN. The areas showing significant between-group differences with the EN included frontal and prefrontal regions, such as the superior, inferior, and middle frontal gyri; the cingulate gyrus; and parietal and subcortical structures such as the insula and the striatum (caudate nucleus and putamen) in the basal ganglia, as well as the hippocampus (Fig. 4, Table 3). As a result of the exploratory analyses, no other resting-state networks showed significant changes in FC between groups. We found no significant differences in FC when comparing patients with a history of TGA and healthy controls. There was no significant difference between groups either in the relative or absolute motion parameters; relative motion was 0.153 mm for the healthy controls, 0.144 mm for subjects with a history of TGA and 0.172 mm for patients with acute TGA (F 2,50 = 0.601, p = 0.55), and absolute motion was 0.538 mm for healthy controls, 0.528 mm for subjects with a history of TGA and 0.643 mm for patients with acute TGA (F 2,50 = 0.520, p = 0.59).
Fig. 3.
Independent component maps representing the executive network detected by group-independent component analysis (Melodic FSL) in 53 subjects (acute TGA, controls, and controls with a history of TGA). Images show z statistics overlaid on the averaged high-resolution scan transformed into MNI-152 standard space.
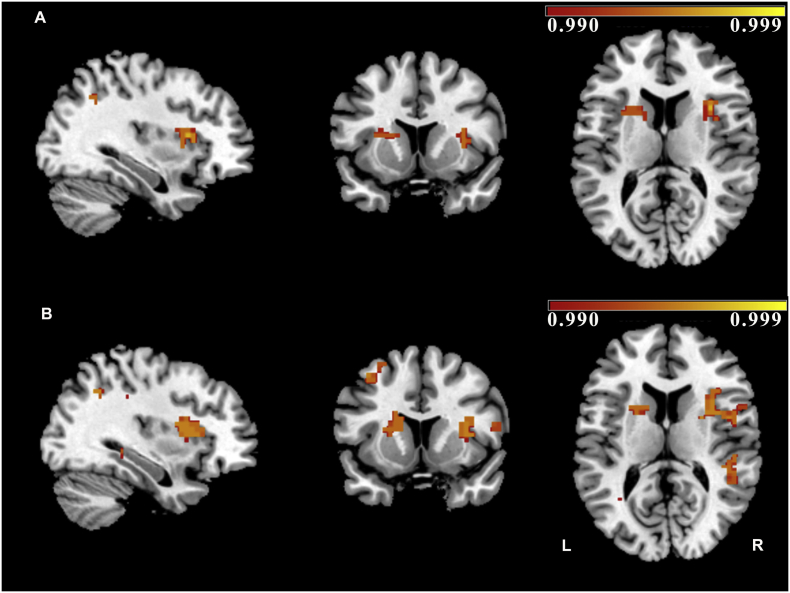
Fig. 4.
Results from dual regression analysis (whole brain between-subject comparison): (A) Healthy controls > acute transient global amnesia (TGA) (within the executive network) p < 0.005, slices are shown at [x = 34, y = 13, z = 12]; MNI-152 coordinates]). (B) Patients with a history of TGA > acute TGA (within the executive network) (p < 0.005; [x = 34, y = 13, z = 12]). Colour bars represent 1 minus P-value. (1.5-column fitting image).
Table 3.
Decreased functional connectivity clusters in acute transient global amnesia (TGA) compared to a) healthy controls and b) patients with a history of TGA (p-values are family-wise error (FWE)-corrected; Coordinate space: MNI, Montreal Neurological Institute).
| Cluster size (voxels) |
X (mm) |
Y (mm) |
Z (mm) |
p-values |
Brain location |
|---|---|---|---|---|---|
| a) | |||||
| 2618 | 34 | 14 | 12 | 0.003 | Insula, frontal lobe (frontal operculum cortex), basal ganglia (caudate, putamen), cingulate gyrus (anterior and posterior), superior-middle-inferior frontal gyrus, frontal pole, hippocampus |
| 46 | 70 | −22 | 16 | 0.01 | Parietal lobe (postcentral gyrus), temporal lobe (superior temporal gyrus) |
| 41 | −66 | −38 | −8 | 0.02 | Temporal lobe (middle temporal gyrus) |
| 27 | −38 | −26 | 40 | 0.04 | Parietal lobe (postcentral gyrus) |
| b) | |||||
| 2912 | 46 | −2 | 8 | 0.005 | Frontal lobe (central opercular cortex), insula, basal ganglia (caudate, putamen), cingulate gyrus (anterior and posterior), temporal lobe (superior-middle temporal gyrus), precuneus cortex; hippocampus superior-middle-inferior frontal gyrus, hippocampus |
| 13 | 22 | −6 | 52 | 0.03 | Frontal lobe (superior-middle frontal gyrus) |
| 12 | −51 | 46 | −12 | 0.04 | Frontal lobe |
| 10 | −46 | −30 | 40 | 0.05 | Parietal lobe (supramarginal Gyrus) |
4. Discussion
In this study, we performed whole-brain investigations of altered FC in acute TGA using resting-state fMRI. Regions with significantly reduced FC mainly constituted hubs of the EN. Additional regions that exhibited decreased FC with the EN included the hippocampus, confirming its pathophysiological involvement in the disorder, as well as regions that are known to be important hubs of the salience network and other subcortical regions. These results add to the understanding of cortico-cortical and subcortical interactions involved in the memory functions of the human brain and of the neuropsychological profile of TGA within a modern large-scale network view. In detail, the regions showing decreased connectivity with the EN included frontal and prefrontal regions, the cingulate gyrus, and parietal, insular and subcortical structures, as well as the hippocampus. As reported elsewhere, the chosen analysis method is sensitive enough to highlight regions between groups that are not also significantly included in the initial main group spatial map of the component, unmasking important differences not only in the connectivity within regions but also with the network of interest (Bos et al., 2014; Evangelisti et al., 2018; Voets et al., 2012).
To date, only one study has been published using resting-state fMRI in acute TGA (Peer et al., 2014). Peer et al. performed a literature-based and hippocampus-driven functional examination of ad hoc defined episodic memory networks during TGA and showed a significant reduction in its functional connectivity. They report that this reduction also involves regions other than the hippocampus (frontal regions, cingulate cortex and basal ganglia), was more pronounced in the hyperacute phase than in the postacute phase and was resolved on follow-up. In their cohort, only 3 patients were described as hyperacute, defined as disorientated in time. Our cohort consisted of 16 patients who all had a documented ongoing TGA episode with disturbed episodic long-term memory, as demonstrated by neuropsychological evaluation immediately before MRI scan. Furthermore, every patient in our cohort developed hippocampal DWI lesion on follow-up MRI on day 1. This is higher than reported in the literature and may partly be explained by the dedicated patient selection and thorough DWI assessment and indicates the quality of our patient selection (Bartsch et al., 2006). We chose to analyse our data using an ICA approach, thus without predefining the hippocampus as a specific seed region but instead allowing the complete automatic (data-driven) identification of spontaneous ongoing network dynamics. Although, to some extent, an overlap exists between our study and Peer's study, our approach specifically showed reduced FC of the EN in patients with acute TGA compared to patients with a history of TGA and healthy controls.
4.1. The relevance of the executive network in TGA
The most prominent cognitive disturbance during TGA is a substantial reduction in anterograde episodic long-term memory, specifically regarding difficulty learning and subsequently recalling novel episodic information (Mazzucchi and Parma, 1990). This has led to the assumption that TGA is primarily an episodic memory and hippocampal formation disorder; however, it must be noted that making new episodic memories also necessarily requires executive functions. Whereas the hippocampus has been particularly implicated in aspects of episodic memory impairment related to long-term memory, frontal control regions, such as the dorsomedial, dorsolateral and ventromedial prefrontal cortex, have previously been associated with episodic memory, specifically with attentional/working memory and executive processes that involve inhibition, interference control processes and cognitive flexibility, all of which are well-known executive functions (Diamond, 2013; Eustache et al., 1997; LaBar et al., 2002; Seeley et al., 2007).
In particular, the medial prefrontal cortex may also play a role during the initial stages of memory consolidation, as well as in the recall of recent memories (Gonzalez et al., 2013). Although only a few studies have directly addressed executive functions during episodes of TGA, a recent meta-analysis was consistent with previous studies proposing subtle executive dysfunctions and reduced working-memory processes in TGA (Jager et al., 2009). For instance, when combined with possible frontal hypoperfusion, Stillhard et al. found that TGA patients performed relatively poorly in tests of prefrontal up to 22 days after the TGA episode (Stillhard et al., 1990). Eustache et al. noted hypometabolism in the left prefrontal cortex in a patient during the acute phase of TGA and suggested that this change may be associated with reductions in episodic memory retrieval and verbal fluency, functions in which the left prefrontal cortex is thought to be involved (Eustache et al., 1997). Similarly, Baron et al. suggested that TGA may be associated with a dysfunction in the lateral prefrontal cortex that could lead to both diminished episodic memory retrieval and executive (including working memory) dysfunction (Baron et al., 1994). Le Pira et al. suggested that TGA patients show poor planning skills, an ability that is thought to be mediated by the frontal lobe (Le Pira et al., 2005).
Moreover, most patients also exhibit a partial loss of retrograde episodic long-term memory with difficulty recalling episodic information that was learned (hours, days, or months, etc.) before the onset of the amnestic episode (Kritchevsky et al., 1997). According to the standard model of systems consolidation, during an initial stage of memory recall, the hippocampus connects distributed neocortical representations during the retrieval of recent memories (Alvarez and Squire, 1994). However, in a time-dependent manner, a hippocampal-cortical dialogue ultimately enables widespread cortical networks to mediate effortful recall and independently use cortically stored remote memories (Maviel et al., 2004). Therefore, one could argue that the difficulty many TGA patients have in recalling episodic information learned before the onset of the amnestic episode is already a sign of the involvement of structures outside of the temporal lobe.
4.2. Executive network and salience network hubs
Our results also extend previous findings by showing the critical role of the EN and related functions during TGA, as well as its relationship with other important brain hubs, such as its fronto-insular connectivity, including connections to the insula, the anterior cingulate cortex and the frontal operculum. These regions have been described as part of the salience network and as being important in the integration of highly processed sensory stimuli with visceral, autonomic, and emotional information, thus making these regions relevant for interoceptive-autonomic processing (Craig, 2002; Critchley, 2005; Mesulam, 1998). Interestingly, the executive and salience networks were reported to be strongly temporally associated with specific fronto-insular nodes, which suggested a transfer of information or the facilitation of a switch between the two networks (Seeley et al., 2007). Another study also found interactions between regions in the executive network (i.e., the bilateral dorsolateral prefrontal cortex) and salience network (i.e., the dorsal anterior cingulate cortex and left fronto-insular cortex) that are relevant for working memory accuracy (Fang et al., 2016). For example, the EN would allocate cognitive resources and divide or switch attention toward salient stimuli when necessary. General disturbances in executive functioning may hinder the integration of new relevant information into a pre-existing framework. In the TGA symptomatology, this deficit would highlight the critical role of executive functioning, not only specifically that of working memory. Following modern predictive coding theories, matching incoming sensory input with a priori stored information while constructing a percept is an essential part of selective attentional processes (Summerfield and Egner, 2009). Therefore, the findings of our study enable the visualization and estimation of real-time brain dysfunction and implicate widespread alterations during TGA.
4.3. Executive network and subcortical interactions
Interestingly, we also found differences in connectivity with different subcortical structures. This result is not surprising considering that the prefrontal cortex has also been shown to modulate subcortical structures and their connectivity (Passingham et al., 2013; Rae et al., 2015). Although a reduced functional connectivity of the basal ganglia was also reported by Peer et al. using their approach, our finding is novel at the whole brain level with respect to hippocampal impairment (Peer et al., 2014). As part of the memory system and in connection with parietal and hippocampal regions, the prefrontal cortex of healthy individuals has been described as a mediator between the dorsal attentional and hippocampal-cortical memory systems and as a trigger in the assimilation of new memories into pre-existing networks of knowledge (Preston and Eichenbaum, 2013; Vincent et al., 2008), thus creating a semantically meaningful multidimensional representation that is ready to be stored in temporal regions. This ability generally represents an important memory-related function, and in the context of TGA, it could be related to anterograde amnesia that is typically observed in these patients. A recent fMRI study using a fear-conditioning paradigm has proposed that the striatum is involved in implicit learning processes in TGA patients (Nees et al., 2016). Connectivity changes were also observed in the subdivisions of the striatum in the present study, specifically, in the central and dorsal striatum – including the caudate nucleus and the putamen – which have already been described as playing integral roles in cognition and executive function (Haber, 2016).
Although previous studies do not suggest clinical or morphological consequences of an episode TGA, we chose to use not only a group of healthy controls but also a group of controls with a history of TGA to study the presence or a lack of persisting functional connectivity changes after the disorder. Even though, we did not find statistically significant differences between the two control groups, a cluster in the lateral temporal lobes is impaired in TGA compared to healthy controls, but not compared to patients with past TGA. This result could possibly indicate a persisting change of temporal lobe activity in the patients with a history of TGA, that future studies should further investigate in more detail.
Finally, from an aetiological point of view, our findings are also in line with the recent hypothesis that TGA might be caused by a transient stress-related inhibition of memory formation in the hippocampus, as physically or emotionally stressful episodes have been reported to be a precipitating event in up to 89% of TGA cases (Quinette et al., 2006). Conditions of psychological stress cause the impairment of the prefrontal cortex via amygdala activation and impair higher-order prefrontal cortex abilities such as working memory and attention regulation (Arnsten, 2009). Using fMRI in humans, Oei et al. showed that acute cortisol elevation is associated with decreased brain activity in the prefrontal cortex and hippocampus during declarative memory retrieval (Oei et al., 2007).
5. Conclusions
In summary, our findings indicate that in TGA, significant changes in whole brain networks occur that function outside the specific and direct control of the hippocampus. These changes may exacerbate anterograde amnesia, which is the most stable and most often reported memory deficit during acute TGA episodes, and thus may go beyond the mere impairment of long-term memory systems. The impaired functioning of prefrontal and parietal control regions of the executive and salience networks may strongly contribute to the creation and storage of a unitary representation through the involvement of temporo-medial structures. The inability to create new memories, which is initiated by hippocampal impairment, may also be due to reduced connectivity between the executive and salience networks that mediate the reduced identification of relevant new information to be integrated during an amnesic episode. The present data add information regarding neuroplasticity following hippocampal impairment and help strengthen, extend and integrate previous knowledge to develop an advanced neuroscientific model of hippocampal impairment in TGA.
This study has some limitations. The combined rarity of the disorder and short duration of the episode posed a challenge in recruiting a more desirable number of TGA patients in the acute stage, compared to the 20 or more participants that are typically suggested for traditional fMRI (Thirion et al., 2007). Although the chosen methodological approach, ICA combined with dual regression, has been described as being a very powerful approach for reducing noise sources and is more sensitive than other approaches in highlighting interindividual differences, further studies with larger cohorts are advisable (Smith et al., 2014). Moreover, future studies should include follow-up fMRI analysis in TGA. Finally, other factors associated with TGA (e.g. patient emotional state and stress during the acute condition) could also contribute to differences in functional connectivity between patients and healthy controls.
Funding
This work was supported by the German Research Foundation (Deutsche Forschungsgemeinschaft DFG; SFB636/C07 to F.N. and K.S.).
Declaration of competing interests
The authors report no competing interests.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2019.101869.
Contributor Information
Francesca Zidda, Email: francesca.zidda@zi-mannheim.de.
Martin Griebe, Email: martin.griebe@medma.uni-heidelberg.de.
Anne Ebert, Email: anne.ebert@umm.de.
Michaela Ruttorf, Email: michaela.ruttorf@medma.uni-heidelberg.de.
Christina Roßmanith, Email: christina.rossmanith@medma.uni-heidelberg.de.
Achim Gass, Email: achim.gass@medma.uni-heidelberg.de.
Jamila Andoh, Email: jamila.andoh@zi-mannheim.de.
Frauke Nees, Email: frauke.nees@zi-mannheim.de.
Kristina Szabo, Email: kristina.szabo@umm.de.
Appendix A. Supplementary data
Supplementary material
References
- Alvarez P., Squire L.R. Memory consolidation and the medial temporal lobe: a simple network model. Proc. Natl. Acad. Sci. U. S. A. 1994;91:7041–7045. doi: 10.1073/pnas.91.15.7041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten A.F. Stress signalling pathways that impair prefrontal cortex structure and function. Nat. Rev. Neurosci. 2009;10:410–422. doi: 10.1038/nrn2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron J.C., Petit-Taboue M.C., Le Doze F., Desgranges B., Ravenel N., Marchal G. Right frontal cortex hypometabolism in transient global amnesia. A PET study. Brain J. Neurol. 1994;117:545–552. doi: 10.1093/brain/117.3.545. Pt 3. [DOI] [PubMed] [Google Scholar]
- Bartsch T., Alfke K., Stingele R., Rohr A., Freitag-Wolf S., Jansen O., Deuschl G. Selective affection of hippocampal CA-1 neurons in patients with transient global amnesia without long-term sequelae. Brain J. Neurol. 2006;129:2874–2884. doi: 10.1093/brain/awl248. [DOI] [PubMed] [Google Scholar]
- Beckmann C.F., Smith S.M. Probabilistic independent component analysis for functional magnetic resonance imaging. IEEE Trans. Med. Imaging. 2004;23:137–152. doi: 10.1109/TMI.2003.822821. [DOI] [PubMed] [Google Scholar]
- Beckmann C.F., DeLuca M., Devlin J.T., Smith S.M. Investigations into resting-state connectivity using independent component analysis. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2005;360:1001–1013. doi: 10.1098/rstb.2005.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann C.F., Mackay C.E., Filippini N., Smith S.M. OHBM; 2009. Group Comparison of Resting-State FMRI Data Using Multi-Subject ICA and Dual Regression. [Google Scholar]
- Bos D.J., van Raalten T.R., Oranje B., Smits A.R., Kobussen N.A., Belle J., Rombouts S.A., Durston S. Developmental differences in higher-order resting-state networks in autism Spectrum disorder. Neuroimage Clin. 2014;4:820–827. doi: 10.1016/j.nicl.2014.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole D.M., Smith S.M., Beckmann C.F. Advances and pitfalls in the analysis and interpretation of resting-state FMRI data. Front. Syst. Neurosci. 2010;4:8. doi: 10.3389/fnsys.2010.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig A.D. How do you feel? Interoception: the sense of the physiological condition of the body. Nat. Rev. Neurosci. 2002;3:655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- Critchley H.D. Neural mechanisms of autonomic, affective, and cognitive integration. J. Comp. Neurol. 2005;493:154–166. doi: 10.1002/cne.20749. [DOI] [PubMed] [Google Scholar]
- Damoiseaux J.S., Rombouts S.A., Barkhof F., Scheltens P., Stam C.J., Smith S.M., Beckmann C.F. Consistent resting-state networks across healthy subjects. Proc. Natl. Acad. Sci. U. S. A. 2006;103:13848–13853. doi: 10.1073/pnas.0601417103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond A. Executive functions. Annu. Rev. Psychol. 2013;64:135–168. doi: 10.1146/annurev-psych-113011-143750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eustache F., Desgranges B., Petit-Taboue M.C., de la Sayette V., Piot V., Sable C., Marchal G., Baron J.C. Transient global amnesia: implicit/explicit memory dissociation and PET assessment of brain perfusion and oxygen metabolism in the acute stage. J. Neurol. Neurosurg. Psychiatry. 1997;63:357–367. doi: 10.1136/jnnp.63.3.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evangelisti S., Testa C., Ferri L., Gramegna L.L., Manners D.N., Rizzo G., Remondini D., Castellani G., Naldi I., Bisulli F., Tonon C., Tinuper P., Lodi R. Brain functional connectivity in sleep-related hypermotor epilepsy. Neuroimage Clin. 2018;17:873–881. doi: 10.1016/j.nicl.2017.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang X., Zhang Y., Zhou Y., Cheng L., Li J., Wang Y., Friston K.J., Jiang T. Resting-state coupling between Core regions within the central-executive and salience networks contributes to working memory performance. Front. Behav. Neurosci. 2016;10:27. doi: 10.3389/fnbeh.2016.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein M.F., Folstein S.E., McHugh P.R. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Gonzalez C., Kramar C., Garagoli F., Rossato J.I., Weisstaub N., Cammarota M., Medina J.H. Medial prefrontal cortex is a crucial node of a rapid learning system that retrieves recent and remote memories. Neurobiol. Learn. Mem. 2013;103:19–25. doi: 10.1016/j.nlm.2013.04.006. [DOI] [PubMed] [Google Scholar]
- Griebe M., Nees F., Gerber B., Ebert A., Flor H., Wolf O.T., Gass A., Hennerici M.G., Szabo K. Stronger pharmacological cortisol suppression and anticipatory cortisol stress response in transient global amnesia. Front. Behav. Neurosci. 2015;9:63. doi: 10.3389/fnbeh.2015.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillery B., Desgranges B., de la Sayette V., Landeau B., Eustache F., Baron J.C. Transient global amnesia: concomitant episodic memory and positron emission tomography assessment in two additional patients. Neurosci. Lett. 2002;325:62–66. doi: 10.1016/s0304-3940(02)00233-1. [DOI] [PubMed] [Google Scholar]
- Haber S.N. Corticostriatal circuitry. Dialogues Clin. Neurosci. 2016;18:7–21. doi: 10.31887/DCNS.2016.18.1/shaber. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Härting C., Markowitsch H.J., Neufeld H., Calabrese P., Deisinger K., Kessler J. Hans Huber, Bern. 2000. German version of the Wechsler memory scale – revised. [Google Scholar]
- Hodges J.R. Semantic memory and frontal executive function during transient global amnesia. J. Neurol. Neurosurg. Psychiatry. 1994;57:605–608. doi: 10.1136/jnnp.57.5.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges J.R., Warlow C.P. The aetiology of transient global amnesia. A case-control study of 114 cases with prospective follow-up. Brain J. Neurol. 1990;113:639–657. doi: 10.1093/brain/113.3.639. Pt 3. [DOI] [PubMed] [Google Scholar]
- Jager T., Bazner H., Kliegel M., Szabo K., Hennerici M.G. The transience and nature of cognitive impairments in transient global amnesia: a meta-analysis. J. Clin. Exp. Neuropsychol. 2009;31:8–19. doi: 10.1080/13803390801955193. [DOI] [PubMed] [Google Scholar]
- Kritchevsky M., Zouzounis J., Squire L.R. Transient global amnesia and functional retrograde amnesia: contrasting examples of episodic memory loss. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 1997;352:1747–1754. doi: 10.1098/rstb.1997.0157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaBar K.S., Gitelman D.R., Parrish T.B., Mesulam M.M. Functional changes in temporal lobe activity during transient global amnesia. Neurology. 2002;58:638–641. doi: 10.1212/wnl.58.4.638. [DOI] [PubMed] [Google Scholar]
- Le Pira F., Giuffrida S., Maci T., Reggio E., Zappala G., Perciavalle V. Cognitive findings after transient global amnesia: role of prefrontal cortex. Appl. Neuropsychol. 2005;12:212–217. doi: 10.1207/s15324826an1204_5. [DOI] [PubMed] [Google Scholar]
- Maviel T., Durkin T.P., Menzaghi F., Bontempi B. Sites of neocortical reorganization critical for remote spatial memory. Science. 2004;305:96–99. doi: 10.1126/science.1098180. [DOI] [PubMed] [Google Scholar]
- Mazzucchi A., Parma M. Neuropsychological testing of transient global amnesia during attack and during follow-up. In: Markowitsch H.J., editor. Transient Global Amnesia and Related Disorders. Hogrefe & Huber Publishers; Toronto: 1990. pp. 152–167. [Google Scholar]
- Mesulam M.M. From sensation to cognition. Brain. 1998;121(Pt 6):1013–1052. doi: 10.1093/brain/121.6.1013. [DOI] [PubMed] [Google Scholar]
- Morris J.C., Heyman A., Mohs R.C., Hughes J.P., van Belle G., Fillenbaum G., Mellits E.D., Clark C. The consortium to establish a registry for Alzheimer's disease (CERAD). Part I. clinical and neuropsychological assessment of Alzheimer's disease. Neurology. 1989;39:1159–1165. doi: 10.1212/wnl.39.9.1159. [DOI] [PubMed] [Google Scholar]
- Nasreddine Z.S., Phillips N.A., Bedirian V., Charbonneau S., Whitehead V., Collin I., Cummings J.L., Chertkow H. The Montreal cognitive assessment, MoCA: a brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 2005;53:695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- Nees F., Griebe M., Ebert A., Ruttorf M., Gerber B., Wolf O.T., Schad L.R., Gass A., Szabo K. Implicit learning in transient global amnesia and the role of stress. Front. Behav. Neurosci. 2016;10:222. doi: 10.3389/fnbeh.2016.00222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickerson L.D., Smith S.M., Ongur D., Beckmann C.F. Using dual regression to investigate network shape and amplitude in functional connectivity analyses. Front. Neurosci. 2017;11:115. doi: 10.3389/fnins.2017.00115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oei N.Y., Elzinga B.M., Wolf O.T., de Ruiter M.B., Damoiseaux J.S., Kuijer J.P., Veltman D.J., Scheltens P., Rombouts S.A. Glucocorticoids decrease hippocampal and prefrontal activation during declarative memory retrieval in young men. Brain Imaging Behav. 2007;1:31–41. doi: 10.1007/s11682-007-9003-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passingham R.E., Rowe J.B., Sakai K. Has brain imaging discovered anything new about how the brain works? Neuroimage. 2013;66:142–150. doi: 10.1016/j.neuroimage.2012.10.079. [DOI] [PubMed] [Google Scholar]
- Peer M., Nitzan M., Goldberg I., Katz J., Gomori J.M., Ben-Hur T., Arzy S. Reversible functional connectivity disturbances during transient global amnesia. Ann. Neurol. 2014;75:634–643. doi: 10.1002/ana.24137. [DOI] [PubMed] [Google Scholar]
- Preston A.R., Eichenbaum H. Interplay of hippocampus and prefrontal cortex in memory. Curr. Biol. 2013;23:R764–R773. doi: 10.1016/j.cub.2013.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinette P., Guillery-Girard B., Dayan J., de la Sayette V., Marquis S., Viader F., Desgranges B., Eustache F. What does transient global amnesia really mean? Review of the literature and thorough study of 142 cases. Brain J. Neurol. 2006;129:1640–1658. doi: 10.1093/brain/awl105. [DOI] [PubMed] [Google Scholar]
- Rae C.L., Hughes L.E., Anderson M.C., Rowe J.B. The prefrontal cortex achieves inhibitory control by facilitating subcortical motor pathway connectivity. J. Neurosci. 2015;35:786–794. doi: 10.1523/JNEUROSCI.3093-13.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedlaczek O., Hirsch J.G., Grips E., Peters C.N., Gass A., Wohrle J., Hennerici M. Detection of delayed focal MR changes in the lateral hippocampus in transient global amnesia. Neurology. 2004;62:2165–2170. doi: 10.1212/01.wnl.0000130504.88404.c9. [DOI] [PubMed] [Google Scholar]
- Seeley W.W., Menon V., Schatzberg A.F., Keller J., Glover G.H., Kenna H., Reiss A.L., Greicius M.D. Dissociable intrinsic connectivity networks for salience processing and executive control. J. Neurosci. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S.M., Vidaurre D., Beckmann C.F., Glasser M.F., Jenkinson M., Miller K.L., Nichols T.E., Robinson E.C., Salimi-Khorshidi G., Woolrich M.W., Barch D.M., Ugurbil K., Van Essen D.C. Functional connectomics from resting-state fMRI. Trends Cogn. Sci. 2013;17:666–682. doi: 10.1016/j.tics.2013.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D.V., Utevsky A.V., Bland A.R., Clement N., Clithero J.A., Harsch A.E., McKell Carter R., Huettel S.A. Characterizing individual differences in functional connectivity using dual-regression and seed-based approaches. Neuroimage. 2014;95:1–12. doi: 10.1016/j.neuroimage.2014.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stillhard G., Landis T., Schiess R., Regard M., Sialer G. Bitemporal hypoperfusion in transient global amnesia: 99m-Tc-HM-PAO SPECT and neuropsychological findings during and after an attack. J. Neurol. Neurosurg. Psychiatry. 1990;53:339–342. doi: 10.1136/jnnp.53.4.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summerfield C., Egner T. Expectation (and attention) in visual cognition. Trends Cogn. Sci. 2009;13:403–409. doi: 10.1016/j.tics.2009.06.003. [DOI] [PubMed] [Google Scholar]
- Thirion B., Pinel P., Meriaux S., Roche A., Dehaene S., Poline J.B. Analysis of a large fMRI cohort: statistical and methodological issues for group analyses. Neuroimage. 2007;35:105–120. doi: 10.1016/j.neuroimage.2006.11.054. [DOI] [PubMed] [Google Scholar]
- Vincent J.L., Kahn I., Snyder A.Z., Raichle M.E., Buckner R.L. Evidence for a frontoparietal control system revealed by intrinsic functional connectivity. J. Neurophysiol. 2008;100:3328–3342. doi: 10.1152/jn.90355.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voets N.L., Beckmann C.F., Cole D.M., Hong S., Bernasconi A., Bernasconi N. Structural substrates for resting network disruption in temporal lobe epilepsy. Brain J. Neurol. 2012;135:2350–2357. doi: 10.1093/brain/aws137. [DOI] [PubMed] [Google Scholar]
- Wilson B.A., Cockburn J., Baddeley A.D. 1985. The Rivermead Behavioural Memory Test. (Bury St. Edmunds, UK) [Google Scholar]
- Winkler A.M., Ridgway G.R., Webster M.A., Smith S.M., Nichols T.E. Permutation inference for the general linear model. Neuroimage. 2014;92:381–397. doi: 10.1016/j.neuroimage.2014.01.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material