Abstract
Obesity is associated with high mortality and morbidity rates and low levels of quality of life among adults globally. It is critical to examine evidence-based practices for developing lifestyle behavioral changes such as physical movement and structured exercise training. The DoIT protocol, a high-intensity interval exercise training (HIIT) program, effectively reduces body mass, alters energy balance, and improves performance of obese adults with a high adherence rate. This study aims to determine the dose-response effects of the DoIT protocol on body composition, health, performance and quality of life in sedentary obese adults. This study will recruit 88 sedentary, obese males and females (BMI 25.0–34.9; 30–50 years) who will be randomly assigned to one of four groups: (i) control (n = 22), (ii) one session/week (n = 22), (iii) two sessions/week (n = 22) or (iv) three sessions/week (n = 22). DoIT will use a supervised, circuit-type (1–3 rounds), functional/neuromotor and progressive exercise program for 12 months. DoIT incorporates 8–12 multi-planar, fundamental and complex, whole body movements and uses bodyweight and alternative exercise modes as a resistance. DoIT utilizes prescribed work-to-rest ratios which will be varied every four weeks. Each session will last less than 30 min. DoIT will be implemented for a year and its effects on body mass and body composition, physical fitness, functional capacity, bone health, leptin, adiponectin, blood lipids, glycemic control, inflammation, oxidative stress and quality of life will be assessed. The outcomes of the proposed study will provide insight on optimal exercise prescription guidelines for such HIIT-type exercise protocols for overweight or obese individuals.
Keywords: Obesity, Body composition, High-intensity interval training, Metabolic health, Physical fitness, Enjoyment
1. Introduction
Obesity is a major global public health challenge associated with increased incidence of diabetes, metabolic syndrome, heart disease and other health issues [1]. In developed countries, two in three adults are overweight [2] while almost one in two adults is physically inactive [3]. It is well documented that lifestyle factors, i.e. lack of physical activity (PA) and poor nutrition, are the main drivers of obesity [4]. Exercise training is a crucial preventive and treatment anti-obesity strategy [5]. Weight loss programs using exercise usually target a modest (5–10%) reduction in body mass by promoting caloric expenditure over intake. Although, this rate may not normalize the weight of an obese individual, it will lower, however, the cardiometabolic risk [[6], [7]].
Obese adults have low cardiorespiratory fitness (CRF) that augments cardiometabolic risk and lowers functional capacity [[5], [8]]. Exercise guidelines for overweight/obese adults recommend the inclusion of cardiovascular, resistance, and flexibility training ideally targeting to 300–420 min/week aiming to improve all components of physical fitness [[9], [10]]. Continuous endurance training (CET), resistance exercise training (RT), high-intensity interval training (HIIT) are the main exercise modes used for effective weight management, improvement of skeletal muscle metabolism, increase of resting metabolic rate (RMR), physical performance, functional capacity, body composition and lowering of risk factors associated with obesity such as insulin resistance and lipid profile [11].
A major drawback of traditional training modalities, i.e. CET and RT, is their high attrition and low adherence rates when applied in previously inactive obese adults [12]. Lack of time is one of the most common personal barriers for exercise participation in adults [13]. Non-traditional, HIIT training protocols have recently become more popular among adults and improve health and physical performance in a more time-efficient manner [[14], [15], [16], [17]]. HIIT protocols appear to be equally effective in increasing CRF as traditional moderate-intensity CET protocols in adults with chronic cardiometabolic lifestyle diseases [18,19]. A limited number of studies revealed that 9-week [20] and 16-week [21,22] HIIT interventions can induce neuromuscular and metabolic adaptations in healthy untrained or recreationally active adults. Moreover, training programs that incorporate movements mimicking the activities of daily living challenge the neuromuscular system which is crucial for adults with impaired neuromuscular function as the obese [23].
We have recently shown [15] that an injury-free, HIIT-type protocol that incorporates integrated neuromuscular training (the DoIT protocol) using whole-body movements with alternative portable exercise equipment [24,25] and performed in a real-world setting, can provoke a considerably body mass and fat loss, increase RMR, strength and CRF in previously inactive overweight/obese women. Here we describe the proposed rationale, design, and methodology for a randomized clinical trial aiming to determine the dose-response effects of DoIT on body mass, body composition, health, performance and quality of life in sedentary male and female obese adults during a 1-year supervised training intervention. The results of this study will expand our understanding regarding the optimal volume and frequency of such protocols for promoting sustainable weight loss, favorable health adaptations, increased functionality and quality of daily life in otherwise-healthy, inactive, overweight/obese adults.
2. Methods
2.1. Overview
The methods, procedures, and ethics of the present study have been examined and approved by the Institutional Ethics Committee (ref. number 1386/6-6-2018). Procedures are in agreement with the 1975 Declaration of Helsinki as revised in 2000 and all data obtained will be confidential and only the researchers of the study will have access to database. The present study has been registered on the ClinicalTrials.gov website under the registry number NCT03759951.
2.2. Participant characteristics and eligibility
Participants will be contacted using advert fliers posted in the local community, social media and by word of mouth. Participants should meet the following inclusion criteria: a) inactivity (no participation in an exercise or PA program for ≥6 months prior to the study, a VO2max <30 ml·kg-1·min-1, <7,500 steps/day, and <30 min/day of moderate-to-vigorous PA based on accelerometry); b) age of 30–50 years; c) overweight or obese class 1 (BMI 25.0–34.9), d) body fat percentage ≥32% for women and ≥25% for men; d) medical clearance for strenuous exercise; e) no smoking for ≥6 months before the study; f) no diet intervention or usage of nutritional supplements/medications before (≥6 months) and during the study; g) no weight loss greater >10% of body mass before (≤6 months) the study; and h) no diagnosis or symptoms of cardiovascular, metabolic, pulmonary, renal, musculoskeletal or mental disorders. Moreover, participants will be excluded from the study if they: a) did not participate in ≥80% of total exercise sessions, b) adhered to a nutritional intervention, and c) modified their habitual PA levels during the study.
2.3. Study design
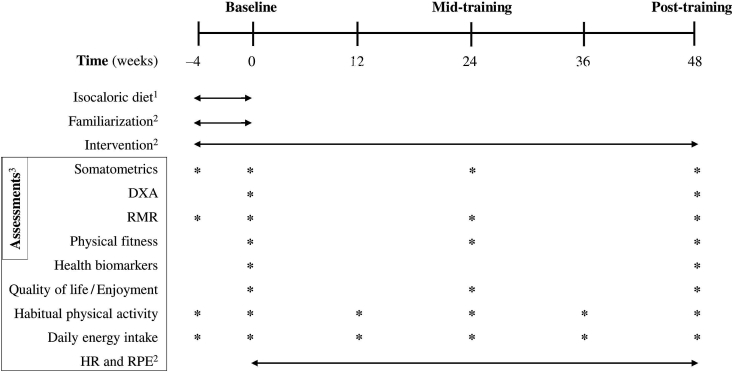
DoIT is a controlled, randomized, repeated-measures, 4-group, clinical trial. Fig. 1 shows the flow diagram of the study. Initially, participants will have their body mass and height, resting metabolic rate (RMR), habitual daily PA, and daily nutritional intake measured. Thereafter, a 4-week adaptive period will be implemented during which participants will adapt to an isocaloric diet (that will be designed by a trained clinical dietician), based on each participant's dietary analysis, daily PA and RMR. A dietitian will provide participants with instructions on how to adapt to a weight maintenance diet (55–60% carbohydrate, 15–20% protein, 20%–25% fat) during the adaptive period. The diet plan will be re-adjusted every three months to verify the accuracy of the assigned energy approach. During the adaptive period, participants will also be familiarized with exercises techniques and overload patterns that will be used throughout the 1-year study. At the end of the adaptive period, volunteers will undergo baseline testing (habitual PA, caloric intake, RMR, somatometrics, body composition, physical fitness, health biomarkers and quality of life) at University facilities.
Fig. 1.
Consolidated Standards of Reporting Trial (CONSORT) guidelines flow diagram of the DoIT study.
Following baseline testing, participants will be randomly assigned to the four groups: (i) a control group (C) during which no intervention will be implemented (only measurements will be performed); (ii) an exercise training group with one exercise session/week (DoIT-1); (iii) an exercise training group with two exercise sessions/week (DoIT-2); and (iv) an exercise training group with three exercise sessions/week (DoIT-3). All groups will be followed over a period of 12 months. Randomization will be achieved using a random-numbers table (allocation sequence conducted by an independent researcher and will be concealed until interventions will be assigned). A member of the research team will enroll and assign participants to interventions. Baseline testing will be repeated after 6 months (mid-training testing) and 12 months (post-training testing) of intervention at University facilities within 5 days after the completion of the last training session each time. Fig. 2 illustrates the experimental flowchart.
Fig. 2.
The experimental flowchart.
C, control group; DoIT-1, training group (1 session/week); DoIT-2, training group (2 sessions/week); DoIT-3, training group (3 sessions/week); DXA, dual energy X-ray absorptiometry; RMR, resting metabolic rate; HR, heart rate; RPE, rate of perceived exertion; 1for all groups (4-week adaptive period); 2only for DoIT-1, DoIT-2 and DoIT-3; 3for all groups.
2.4. Exercise intervention
A supervised exercise training protocol will be performed one (DoIT-1), two (DoIT-2) or three (DoIT-3) times/week for 12 consecutive months. All sessions will use an asynchronous music playing in the background and with no instruction to participants to perform exercises in synchronization with musical tempo [28]. The training protocol will be characterized by progression in both exercise intensity and volume. The exercises selection and work-to-rest ratio will be varied depending on the response of participants every 4 weeks. Specifically, the exercise will consist of 8–12 neuromotor exercise stations in circuit fashion (1–3 rounds) using a prescribed time of effort and passive recovery intervals according to specific work-to-rest ratios. The total length of each session will be less than 30 min excluding warm-up and cool down periods. Participants performed as many repetitions as possible at each station with proper form at a controlled, moderate speed.
All prescribed exercises will incorporate multi-planar, fundamental and complex movements (squat, hinge, lunge, push, pull, carry, rotation, and plank) using bodyweight [23,25] or adjunct portable modalities (suspension belts, balance balls, kettlebells, medicine balls, battle ropes, stability balls, speed ladders, foam rollers, and elastic bands) as resistance [29]. A 10-min warm-up with low-intensity cardiovascular exercise, dynamic stretching and movement preparation drills and a 5-min cool-down period of very low-intensity cardiovascular exercise and static stretching will be included in each session. Participants will be instructed to execute as many repetitions as possible at each exercise station with proper form. Each session will be consisted of alternate stations emphasizing on health- and motor-related fitness parameters including cardiovascular, resistance and neuromotor drills. To maintain a high-intensity stimulus, i.e. ≥60% of reserve oxygen consumption (VO2R), throughout each session according to the guidelines for exercise prescription in previously inactive obese adults [9], verbal encouragement will be provided to participants. The rate of perceived exertion (RPE) will be recorded at the end of each round and heart rate (Polar Team Solution, Polar Electro-Oy, Kempele, Finland) will be monitored throughout each session. Mean and maximal heart rates will be recorded and mean RPE will be calculated for each session. To maximize adherence, each session will have a maximum of 5–8 participants training simultaneously. Table 1 illustrates the configuration of the training protocol throughout the 1-year intervention.
Table 1.
The characteristics of the exercise protocol throughout the 1-year intervention.
| Training Parameters | Months |
Yearly Mean | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | ||
| Session duration (min) | 13.5 | 15.7 | 20.7 | 30.3 | 33.3 | 33.5 | 36.5 | 39.5 | 33.8 | 36.8 | 34.0 | 37.0 | 30.4 |
| Net exercise time (min)a | 3.0 | 4.7 | 8.3 | 11.3 | 12.5 | 15.0 | 16.5 | 18.0 | 19.3 | 21.0 | 22.0 | 24.0 | 14.6 |
| Recovery time (min)b | 10.5 | 11.0 | 12.3 | 19.0 | 20.8 | 18.5 | 20.0 | 21.5 | 14.5 | 15.8 | 12.0 | 13.0 | 15.7 |
| Work-to-rest ratio | 1:3 | 1:2 | 1:2 | 1:1.4 | 1:1.4 | 1:1 | 1:1 | 1:1 | 1.4:1 | 1.4:1 | 2:1 | 2:1 | 1:1.1 |
| Work interval (sec) | 15.0 | 20.0 | 20.0 | 25.0 | 25.0 | 30.0 | 30.0 | 30.0 | 35.0 | 35.0 | 40.0 | 40.0 | 28.8 |
| Rest interval (sec) | 45.0 | 40.0 | 40.0 | 35.0 | 35.0 | 30.0 | 30.0 | 30.0 | 25.0 | 25.0 | 20.0 | 20.0 | 31.3 |
| Number of exercises | 6 | 7 | 8 | 9 | 10 | 10 | 11 | 12 | 11 | 12 | 11 | 12 | 10 |
| Rounds | 1–2 | 2 | 2 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 |
| Rest time/round (min) | 3.0 | 3.0 | 3.0 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 2.0 | 2.0 | 2.0 | 2.0 | 2.5 |
| Movement numberc | Max. | Max. | Max. | Max. | Max. | Max. | Max. | Max. | Max. | Max. | Max. | Max. | Max. |
Effort time is session duration minus recovery time.
Recovery time is session duration minus effort time.
maximal number of repetitions/station.
2.5. Measurements
2.5.1. Descriptor variables
2.5.1.1. Habitual physical activity measurements
Participants will be encouraged to maintain their usual daily PA throughout the study. Accelerometry (GT3X+, ActiGraph, Pensacola, FL, USA) will be used to determine habitual PA level every 3 months over a 7-day period as described [30]. Researchers will instruct participants on how to use the accelerometer, which will be placed into adjustable belts and will be over the right hip during the measurement apart from bathing, swimming and sleep. Collected data will be included in the analysis, if participants will have ≥4 days and ≥10 wear hours/day [31]. Non-wear time will be calculated [32] and daily activity levels and sedentary time will be expressed as steps per day and time spent at sedentary, light, moderate, vigorous, and moderate-to-vigorous PA [33]. ActiLife 6 software will be used for initializing the accelerometers and downloading data using a 60-s epoch length.
2.5.1.2. Dietary assessment
Caloric intake will be measured using 7-day diet recalls every three months to confirm the precision of the allocated energy approach. A clinical dietitian will train participants how to record food/fluid consumption. Participants will be asked to not modify their dietary behavior throughout the study. Diet recalls will be analyzed for energy and macronutrient intake using a nutrition analysis software (Science Fit Diet 200A, Science Technologies, Athens, Greece).
2.5.1.3. Exercise energy expenditure
will be measured in only 50% participants of each exercise group (randomly selected at baseline) due to time constraints. Exercise energy cost and excess post-exercise oxygen consumption (EPOC) will be measured using portable indirect calorimetry (VmaxST, Sensormedics, Yorba Linda, CA) as described [15,36]. Total energy expenditure of an exercise session will be estimated by summing a) the aerobic energy expenditure during exercise which will be estimated using a constant value of 21.14 kJ (5.05 kcal/)/liter oxygen [15,38], b) the anaerobic energy expenditure using resting and post-exercise blood lactate concentration measurements [15,39], and c) excess post-exercise oxygen consumption. Blood lactate will be measured using blood samples that will be collected pre-, mid- and post-exercise session (3 min post-exercise). In order to avoid any potential contamination due to interference with sweat, researchers will collect the blood sample after they will have thoroughly cleaned, disinfected and dried subjects' whole hands and the single finger that will be used for blood sampling. Thereafter, a lancet will be used to puncture participants’ skin at the finger, the first blood drop will be directed on the measurement strip and the blood lactate concentration will be analyzed using a hand-portable analyzer (Accutrend Plus, Roche Diagnostics, Switzerland) within a few seconds following collection [15].
2.5.2. Primary outcome measures
2.5.2.1. Somatometric measures
Somatometric measurements will be conducted at pre-, mid-, and post-training. Height and body mass will be measured to the nearest 0.1 cm and 0.1 kg, respectively, using a beam scale (Beam Balance-Stadiometer, SECA, Vogel & Halke, Hamburg, Germany) and body mass index (BMI) will be calculated as weight per height squared. Waist (WC) and hip circumferences (HC) will be measured three times using a Gullick II, the mean will be recorded while the waist-to-hip ratio (WHR) will be calculated [34]. Total and regional fat mass (FM), fat-free mass (FFM), bone mineral content (BMC) and bone density (BMD) will be determined utilizing a dual energy X-ray absorptiometry (DXA) scanner (GE Healthcare, Lunar DPX-NT) according to standard procedures as previously described [35]. DXA measurements will be performed pre- and post-training. The effective radiation dose will be < 0.3 mSv per whole-body scan. All analyses will be performed using the 12.2 GE enCORE software package.
2.5.2.2. Physical fitness measures
All physical fitness assessments will be conducted pre-, mid-, and post-training. CRF will be assessed using a low-risk, low-cost and single-stage submaximal treadmill walking test (Stex 8025T, South Korea) for estimating peak oxygen uptake (VO2peak). This test is considered a suitable for previously inactive, apparently healthy, and non-athletic adults with potential physical limitations [40]. According to the protocol, the walking speed for the test will be individually determined based on participant's gender, age, and fitness level using standard procedures [41].
Bilateral maximal isotonic strength (one repetition maximal, 1RM) will be assessed using standard procedures for novice and untrained individuals [42] in four upper body (seated chest press, lat pull-down, vertical row, seated shoulder press) and three lower body (horizontal leg press, lying leg curls, seated leg extension) exercises positioned on strength training equipment (Panatta Sport, Apiro, Italy). All 1RM tests will be performed following familiarization [42]. All attempts will be performed with no rest until muscle failure while verbal encouragement will be given to the participant. Muscular endurance will be assessed using timed tests for the abdominal (partial curl-ups), upper- (traditional push-ups for males and modified kneeling push-ups for females) and lower-body (modified chair squat) musculature. All tests require the participants to perform as many repetitions as possible within 60 s using standard procedures [42].
The modified sit-and-reach test was used to assess the flexibility of lower back and hamstrings and goniometry (Lafayette 01135 Gollehon Extendable Goniometer, Lafayette Instrument Inc., Lafayette, IN, USA) to measure the range of motion (ROM) of the ankles, knees, hips, elbows, and shoulders as previously described [43]. Static balance will be assessed using the modified Romberg test as previously described [44]. Functional mobility, postural stability and movement behavior in different settings without locomotion will be evaluated using a quick, noninvasive, low-cost and movement-based screening tool called Functional Movement Screening (FMS). The FMS will attempt to assess seven movement tasks that each will be scored from 0 to 3 points (0 = pain with pattern regardless of quality, 1 = unable to perform pattern, 2 = able to perform pattern with compensation/imperfection, 3 = able to perform pattern as directed) and their sum will provide the total score ranging from 0 to 21 points. The FMS is considered a simple and quantifiable method of evaluating basic movement abilities while identifying muscle imbalances and asymmetries during physical exercise and ADL [45].
2.5.2.3. Physiological measures
Resting metabolic rate measurement (RMR) will be assessed pre-, mid, and post-training using resting VO2/CO2 measurements in the morning (07.00–09.00) after an overnight fast utilizing an open-circuit indirect calorimeter with a ventilated hood system (Vmax Encore 29, BEBJO296, Yorba Linda, CA, USA) as described [36] and the 24-h RMR will be calculated using the Weir equation [37]. RMR assessments will be performed in only 50% participants of each group (randomly selected at baseline) due to time constraints.
2.5.2.4. Measures of wellbeing
Health-related quality of life (HRQoL) will be assessed pre-, mid-, and post-training using the physical and mental component subscales of the Greek 36-Item Short-Form Health Survey (SF-36), which has been shown to be valid and reliable [46]. The SF-36 consists of eight subscales (vitality, physical functioning, bodily pain, general health perceptions, physical role functioning, emotional role functioning, social role functioning, and mental health) and its scores on both component subscales of the SF-36 will range from 0 to 100, with higher scores indicating better health status while the minimal clinically important difference will be 2 points. Exercise enjoyment will be assessed using the Exercise Enjoyment Scale (EES), which is a single-item 7-point scale to assess enjoyment pre-, during, and post-exercise ranging from “not at all” at 1 to “extremely” at 7 [47].
2.5.2.5. Biochemical measures
Resting blood samples will be collected pre- and post-training at 07:00–09:00 a.m. following an overnight fasting (10–12 h). Participants will be abstained from any type of strenuous physical activity or structured exercise for the last 72 h before testing. A physician will collect all blood samples. Venous blood samples (∼10 mL) will be drawn at a seated position from the antecubital arm vein by venipuncture with a 20-gauge disposable needle equipped with a Vacutainer tube holder (Becton Dickinson, Franklin Lakes, NJ, USA). Blood will be collected in tubes containing ethylenediaminetetraacetic acid (EDTA) or SST-Gel/clot activator for plasma and serum preparation, respectively. Plasma or serum will be obtained by centrifugation (1370 g, 4 °C, 10 min for plasma separation; 1500 g, 4 °C, 15 min for serum separation). For serum separation, blood will be allowed to clot at room temperature first. Serum and plasma samples will be stored in multiple aliquots at −80 °C until assayed (in duplicate).
2.5.2.5.1. Cardiometabolic measures
Serum leptin will be determined by using a commercially available ELISA kit and plasma adiponectin will be analyzed by using a commercially available RIA (Linco Research, Inc., St. Charles, MO, USA) [36]. Serum cortisol will be measured using an electrochemiluminescence immunoassay (ECLIA) (Cobas e 411 Analyzer, Roche Diagnostics GmbH, Mannheim, Germany). Serum insulin will be measured photometrically with an immunoassay and plasma glucose will be determined spectrophotometrically using the glucose oxidase method [36]. The homeostatic model assessment for insulin resistance (HOMA-IR) will be calculated according to the equation: glucose (mM) X insulin (μIU/ml)/22,5 [49]. Triglycerides (TG), total cholesterol (TC) and high-density lipoprotein cholesterol (HDL) will be measured photometrically using a biochemical analyzer [50]. Low-density lipoprotein (LDL) cholesterol will be calculated using the Friedwald equation [51]. Atherogenic index will be calculated as TC/HDL.
2.5.2.5.2. Inflammatory measures
C-reactive protein (CRP) will be measured as an inflammatory marker with a clinical chemistry analyzer [52]. Reduced glutathione (GSH), oxidized glutathione (GSSG), thiobarbituric acid-reactive substances (TBARS), protein carbonyls (PC), and catalase activity (CAT) in erythrocyte lysates, serum total antioxidant capacity (TAC), and catalase activity (CAT) will be analyzed spectrophotometrically as previously described [53].
2.5.2.5.3. Measures of appetite regulation
Possible changes in appetite-regulating hormones including cholecystokinin (CKK), pancreatic polypeptide (PP), peptide YY (PYY), oxyntomodulin (OXM), ghrelin and glucagon-like peptide-1 (GLP-1) will be measured as previously described [54]. Changes in appetite will be assessed pre-, mid, and post-training using the Visual Analog Scale (VAS) to measure perceived hunger, satiety, and individual's own interpretation of their hunger sensations. VAS is a straight horizontal line of fixed length, usually 100 mm. The ends are defined as the extreme limits of the parameter to be measured orientated from the left (worst) to the right (best) [48].
2.6. Statistical analyses
A Kolmogorov-Smirnov analysis will be used to determine data normality. Parametric statistics will be applied if data is normally distributed. Initially, a one-way ANOVA will be utilized for baseline comparisons of the four groups on all primary outcome variables. Based on study's design, the primary independent endpoints are the treatment groups (four groups based on training frequency) time (three time points, i.e. baseline, mid-training, post-training). To examine the interaction between the independent endpoints, a two-way ANOVA repeated measures will be applied on each primary outcome measure. If an interaction exists, the simple effect and pairwise comparisons will be used to detect differences between groups at each time point. If no interaction exists, an analysis for main effect on each independent factor will be performed. If a main effect is detected, a post-hoc analysis (Bonferroni) will be performed to detect the differences between groups or repeated measures for each primary outcome. If data will not be normally distributed, non-parametric tests will be applied. Baseline comparisons will be performed using the Kruskal–Wallis test. Time-effects in each trial will be determined using the Friedman test followed by the Wilcoxon signed-rank test for pairwise comparisons. Between trials differences will be examined via the Kruskal–Wallis test followed by the Mann-Whitney test for pairwise comparisons. Statistical significance will be accepted at p < 0.05. Effect sizes (ES) and confidence intervals (CI) will be calculated on results of all dependent variables using the Hedge's g method, corrected for bias. ES will be interpreted as none, small, medium-sized, and large for values 0.00–0.19, 0.20–0.49, 0.50–0.79, and ≥0.8, respectively.
The G*Power program (G*Power 3.1.9.2) was utilized to perform power analysis [26] to calculate the sample size needed for the interaction between the independent endpoints (four groups and three measurements points) and main effects for group or time in the main dependent endpoint measures (body mass, body fat mass, lean body mass) using an effect size of >0.55, a probability error of 0.05, and a two-tailed alpha level and power of 0.80. The results indicated that a number of 16 participants/group is needed to detect meaningful interaction between endpoints, 12 for main effect on the independent endpoint time and 16 for main effect on the independent endpoint group. Accepting a potential drop-out rate of 20% and using the formula: n’ = n/(1-p) [27], it was estimated that the number of participants to be recruited would be 12.8–20 per group. Therefore, a number of 22 participants/group will be recruited, resulting in a total sample size of 88.
3. Discussion
Approximately one in three adults demonstrates insufficient physical activity [3] and more than one in two adults are overweight or obese worldwide [2]. It has been estimated that the global cost of obesity-related illness is truly alarming, and therefore, evidence-based and cost-effective treatments are high priorities for the public healthcare systems worldwide [55]. Extensive research findings support the positive impact of organized exercise in morbidity and mortality rates of the sedentary obese adult population [56,57]. Exercise alone can be a beneficial strategy for reducing obesity-related medical risks by improving a variety of risk factors that systematically decrease life expectancy and quality of life [9]. Lack of time [13], pleasure, enjoyment and adherence have been reported as key factors for preventing exercise engagement in obese adults [58].
Several investigations have been conducted to induce cardiovascular, neuromuscular and metabolic adaptations while improving well-being and enhancing health benefits through traditional and nontraditional exercise programs among obese adults [20,26]. Although CET, RT, HIIT, and various combinations of these exercise modalities appear to provoke weight and fat loss and improve performance in untrained, obese adults [11], the majority of studies in this field were of limited length of time, used traditional exercise protocols and demonstrated low adherence and high attrition rates [12,13]. Emerging research supports that HIIT-type exercise protocols elicit beneficial cardiometabolic adaptations in both healthy and clinical populations as CET but with a shorter time commitment [59,60]. Recently published data demonstrate that HIIT in the real world can be applied as an appropriate exercise training option for overweight or obese individuals [61]. However, unsupervised 12-month HIIT exhibited a low adherence rate in overweight or obese adults [61]. On the other hand, RT offers positive neuromuscular adaptations and health benefits when applied in type 2 diabetes and overweight/obese adults with metabolic syndrome and increased cardiovascular disease risk factors [62,63].
Recently, implementation of a new of HIIT-type, integrated neuromuscular training modality that uses resistance exercises that incorporate whole-body movements in overweight/obese adult women, induced a ∼6% loss of body mass and fat (∼0.6% loss per month), which was associated with an increase of fat free mass (∼3.5%), RMR (∼10%), strength (∼22%), and CRF (∼24%) [15]. These beneficial adaptations were obtained using a metabolic overload of only 5–12 MET-hours/week and total exercise time/week of ∼100 min/week [15]. These training-induced gains were not attenuated by a 5-month detraining [15]. Furthermore, this program demonstrated a high adherence rate (94%) [15] corroborating reports suggesting that nontraditional, HIIT-type training modalities using bodyweight resistance exercises and alternative resistance equipment using movements that mimic everyday life movement patterns are very attractive and popular [14,64,65]. This is the first longitudinal study that attempts to assess the dose-response effects of this HIIT-type, integrated neuromuscular training on body mass, energy balance, health status, performance and well-being of inactive overweight/obese adults.
Current guidelines for weight loss developed by respected organizations such as the World Health Organization suggest organized PA of at least 150 min/week. HIIT-type protocols seem to be more time efficient, inducing considerable health gains in shorter time [9,11,15]. The DoIT protocol requires a total session duration of less than 30 min while the net exercise time being less than 15 min/session [15]. This trial will not only determine the efficacy of HIIT-type, integrated neuromuscular training on weight and fat loss of inactive overweight/obese adults of both sexes but it will allow us to understand the optimal workload and frequency associated with beneficial changes in health and quality of life of this cohort. Results of this study will provide insight in our effort to provide more accurate exercise guidelines for HIIT-type protocols by identifying the frequency threshold capable of promoting sustained body mass and fat loss, health gains and changes in the quality of life in a high-risk population.
4. Conclusions
The DoIT trial is the first randomized controlled trial that investigates in-depth the long-term efficacy and the dose-response effects on health, performance and quality of life of a new HIIT-type exercise intervention incorporating integrated neuromuscular, whole-body resistance exercises in sedentary, obese adults of both sexes. Results of this study will provide more detailed data associated with a novel, supervised and time-efficient exercise protocol in order to be considered as an evidence-based and applicable anti-obesity strategy in the real world. Results will aid the formulation of more solid guidelines of exercise prescription for the implementation of this type of protocols in previously inactive overweight/obese adults. Specifically, the outcomes of this study will provide information about the optimal volume and frequency associated with favorable changes in body mass, body composition, daily energy balance, health, performance, and quality of life of overweight/obese adults.
Conflicts of interest
None.
Disclosures
None.
Funding source
The study will be supported by departmental funding and by a Ph.D. scholarship given by the department's Graduate program.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.conctc.2019.100386.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Zheng Y., Manson J.E., Yuan C., Liang M.H., Grodstein F., Stampfer M.J. Associations of weight gain from early to middle adulthood with major health outcomes later in life. J. Am. Med. Assoc. Jul. 18 2017;318(3):255–269. doi: 10.1001/jama.2017.7092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization . 2018. Noncommunicable Diseases Country Profiles 2018.https://www.who.int/nmh/publications/ncd-profiles-2018/en/ [Google Scholar]
- 3.Guthold R., Stevens G.A., Riley L.M., Bull F.C. Worldwide trends in insufficient physical activity from 2001 to 2016: a pooled analysis of 358 population-based surveys with 1·9 million participants. The Lancet. Oct. 2018;6(10):1077–1086. doi: 10.1016/S2214-109X(18)30357-7. [DOI] [PubMed] [Google Scholar]
- 4.Romieu I., Dossus L., Barquera S., Blottière H.M., Franks P.W., Gunter M. Energy balance and obesity: what are the main drivers? Cancer Causes Control. Mar. 2017;28(3):247–258. doi: 10.1007/s10552-017-0869-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Do K., Brown R.E., Wharton S., Ardern C.I., Kuk J.L. Association between cardiorespiratory fitness and metabolic risk factors in a population with mild to severe obesity. BMC Obes. 2018;5(5) doi: 10.1186/s40608-018-0183-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Riou M.È., Jomphe-Tremblay S., Lamothe G., Stacey D., Szczotka A., Doucet É. Predictors of energy compensation during exercise interventions: a systematic review. Nutrients. 2015;7(5):3677–3704. doi: 10.3390/nu7053677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wadden T.A., Butryn M.L., Wilson C. Lifestyle modification for the management of obesity. Gastroenterology. 2007;132(6):2226–2238. doi: 10.1053/j.gastro.2007.03.051. [DOI] [PubMed] [Google Scholar]
- 8.Pataky Z., Armand S., Müller-Pinget S., Golay A., Allet L. Effects of obesity on functional capacity. Obesity (Silver Spring) Jan 22 2014;22(1):56–62. doi: 10.1002/oby.20514. [DOI] [PubMed] [Google Scholar]
- 9.American College of Sports Medicine . tenth ed. Wolters Kluwer Health/Lippincott Williams & Wilkins; Philadelphia: 2017. ACSM's Guidelines for Exercise Testing and Prescription. [Google Scholar]
- 10.American Heart Association AHA/ACC/TOS guideline for the management of overweight and obesity in adults. Circulation. 2014;129(Suppl 2):S102–S138. doi: 10.1161/01.cir.0000437739.71477.ee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Petridou A., Siopi A., Mougios V. Exercise in the management of obesity. Metabolism. 2019;92:163–169. doi: 10.1016/j.metabol.2018.10.009. [DOI] [PubMed] [Google Scholar]
- 12.Burgess E., Hassmén P., Welvaer M., Pumpa K.L. Behavioural treatment strategies improve adherence to lifestyle intervention programmes in adults with obesity: a systematic review and meta-analysis. Clin. Obes. 2017;7(2):105–114. doi: 10.1111/cob.12180. [DOI] [PubMed] [Google Scholar]
- 13.Godin G., Desharnais R., Valois P., Lepage L., Jobin J., Bradet R. Differences in perceived barriers to exercise between high and low intenders: observations among different populations. Am. J. Health Promot. 1994;8:279–285. [Google Scholar]
- 14.Thompson W. Worldwide survey reveals fitness trends for 2019. ACSM's Health & Fit. J. 2018;22(6):10–17. [Google Scholar]
- 15.Batrakoulis A., Jamurtas A.Z., Georgakouli K., Draganidis D., Deli C.K., Papanikolaou K. High intensity, circuit-type integrated neuromuscular training alters energy balance and reduces body mass and fat in obese women: a 10-month training-detraining randomized controlled trial. PLoS One. 2018;13(8) doi: 10.1371/journal.pone.0202390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kilpatrick M.W., Jung M.E., Little J.P. High-intensity interval training: a review of physiological and psychological responses. ACSM's Health & Fit. J. 2014;18(5):11–16. [Google Scholar]
- 17.Nybo L., Sundstrup E., Jakobsen M.D., Mohr M., Hornstrup T., Simonsen L. High-intensity training versus traditional exercise interventions for promoting health. Med. Sci. Sport. Exerc. 2010;42(10):1951–1958. doi: 10.1249/MSS.0b013e3181d99203. [DOI] [PubMed] [Google Scholar]
- 18.Batacan R.B., Jr., J Duncan M., Dalbo V.J., Tucker P.S., Fenning A.S. Effects of high-intensity interval training on cardiometabolic health: a systematic review and meta-analysis of intervention studies. Br. J. Sports Med. 2017;51:494–503. doi: 10.1136/bjsports-2015-095841. [DOI] [PubMed] [Google Scholar]
- 19.Weston K.S., Wisløff U., Coombes J.S. High-intensity interval training in patients with lifestyle-induced cardiometabolic disease: a systematic review and meta-analysis. Br. J. Sports Med. Aug. 2014;48(16):1227–1234. doi: 10.1136/bjsports-2013-092576. [DOI] [PubMed] [Google Scholar]
- 20.Sperlich B., Wallmann-Sperlich B., Zinner C., Von Stauffenberg V., Losert H., Holmberg H.C. Functional high-intensity circuit training improves body composition, peak oxygen uptake, strength, and alters certain dimensions of quality of life in overweight women. Front. Physiol. 2017;8:172. doi: 10.3389/fphys.2017.00172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Feito Y., Hoffstetter W., Serafini P., Mangine G. Changes in body composition, bone metabolism, strength, and skill-specific performance resulting from 16-weeks of HIFT. PLoS One. 2018;13(6) doi: 10.1371/journal.pone.0198324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Greenlee T.A., Greene D.R., Ward N.J., Reeser G.E., Allen C.M., Baumgartner N.W. Effectiveness of A 16-week high-intensity cardio-resistance training (HICRT) program in adults. J. Strength Cond. Res. 2017;31(9):2528–2541. doi: 10.1519/JSC.0000000000001976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.King M., Stanforth D. The movement-based programming method for select populations. ACSM's Health & Fit. J. 2014;19(1):17–22. [Google Scholar]
- 24.Haff G.G., Beminger D., Caulfield S. Exercise technique for alternative modes and nontraditional implement training. In: Haff G.G., Triplett N., editors. Essentials of Strength Training and Conditioning. fourth ed. Human Kinetics; Champaign: 2016. pp. 417–421. [Google Scholar]
- 25.Stanforth D., Brumitt J., Ratamess N., Atkins W., Keteyian S. Training toys … bells, ropes, and balls – oh my! ACSM's Health & Fit. J. 2015;19(4):5–11. [Google Scholar]
- 26.Faul F., Erdfelder E., Buchner A., Lang A.G. Statistical power analyses using G*power 3.1: tests for correlation and regression analyses. Behav. Res. Methods. 2009;41(4):1149–1160. doi: 10.3758/BRM.41.4.1149. [DOI] [PubMed] [Google Scholar]
- 27.Sakpal T.V. Sample size estimation in clinical trial. Perspect. Clin. Res. 2010;1(2):67–69. [PMC free article] [PubMed] [Google Scholar]
- 28.Karageorghis C.I., Terry P.C., Lane A.M., Bishop D.T., Priest D.L. The BASES Expert Statement on use of music in exercise. J. Sport. Sci. 2012;30(9):953–956. doi: 10.1080/02640414.2012.676665. [DOI] [PubMed] [Google Scholar]
- 29.Klika B., Jordan C. High-intensity circuit training using body weight: maximum results with minimal investment. ACSM's Health & Fit. J. 2013;17(3):8–13. [Google Scholar]
- 30.Watson K.B., Carlson S., Carroll D.D., Fulton J. Comparison of accelerometer cut points to estimate physical activity in U.S. adults. J. Sport. Sci. 2014;32(7):660–669. doi: 10.1080/02640414.2013.847278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gorman E., Hanson H.M., Yang P.H., Khan K.M., Liu-Ambrose T., Ashe M.C. Accelerometry analysis of physical activity and sedentary behavior in older adults: a systematic review and data analysis. Eur. Rev. Aging Phys. Act. 2014;11:35–49. doi: 10.1007/s11556-013-0132-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Choi L., Ward S.C., Schnelle J.F., Buchowski M.S. Assessment of wear/nonwear time classification algorithms for triaxial accelerometer. Med. Sci. Sport. Exerc. 2012;44(10):2009–2016. doi: 10.1249/MSS.0b013e318258cb36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Keadle S.K., Shiroma E.J., Freedson P.S., Lee I.M. Impact of accelerometer data processing decisions on the sample size, wear time and physical activity level of a large cohort study. BMC Public Health. 2014;14(Nov. 24):1210. doi: 10.1186/1471-2458-14-1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.World Health Organization . 2008. Waist Circumference and Waist-Hip Ratio: Report of a WHO External Consultation.http://apps.who.int/iris/bitstream/10665/44583/1/9789241501491_eng.pdf [Google Scholar]
- 35.Rothney M.P., J Brychta R., Schaefer E.V., Chen K.Y., Skarulis M.C. Body composition measured by dual-energy X-ray absorptiometry half-body scans in obese adults. Obesity (Silver Spring) 2009;17(6):1281–1286. doi: 10.1038/oby.2009.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fatouros I.G., Tournis S., Leontsini D., Jamurtas A.Z., Sxina M., Thomakos P. Leptin and adiponectin responses in overweight inactive elderly following resistance training and detraining are intensity-related. J. Clin. Endocrinol. Metab. 2005;90(11):5970–5977. doi: 10.1210/jc.2005-0261. [DOI] [PubMed] [Google Scholar]
- 37.Weir J.B. New methods for calculating metabolic rate with special reference to protein. J. Physiol. 1949;109:1–9. doi: 10.1113/jphysiol.1949.sp004363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.di Prampero P.E., Ferretti G. The energetics of anaerobic muscle metabolism: a reappraisal of older and more recent concepts. Respir. Physiol. 1999;118:103–115. doi: 10.1016/s0034-5687(99)00083-3. [DOI] [PubMed] [Google Scholar]
- 39.Baldari C., Bonavolontà V., Emerenziani G.P., Gallotta M.C., Silva A.J., Guidetti L. Accuracy, reliability, linearity of Accutrend and Lactate Pro versus EBIO plus analyzer. Eur. J. Appl. Physiol. 2009;107(1):105–111. doi: 10.1007/s00421-009-1107-5. [DOI] [PubMed] [Google Scholar]
- 40.Sartor F., Vernillo G., de Morree H.M., Bonomi A.G., La Torre A., Kubis H.P. Estimation of maximal oxygen uptake via submaximal exercise testing in sports, clinical, and home settings. Sports Med. 2013;43(9):865–873. doi: 10.1007/s40279-013-0068-3. [DOI] [PubMed] [Google Scholar]
- 41.Ebbeling C.B., Ward A., Puleo E.M., Widrick J., Rippe J.M. Development of a single-stage submaximal treadmill walking test. Med. Sci. Sport. Exerc. Aug. 1991;23(8):966–973. [PubMed] [Google Scholar]
- 42.American College of Sports Medicine . fifth ed. Wolters Kluwer Health/Lippincott Williams & Wilkins; Philadelphia: 2017. ACSM's Health-Related Physical Fitness Assessment Manual. [Google Scholar]
- 43.Norkin C.C., White D.J. fourth ed. F.A. Davis Company; 2009. Measurement of Joint Motion: A Guide to Goniometry. [Google Scholar]
- 44.Newton R. Review of tests of standing balance abilities. Brain Inj. Oct.-Dec. 1989;3(4):335–343. doi: 10.3109/02699058909004558. [DOI] [PubMed] [Google Scholar]
- 45.Kraus K., Schütz E., Taylor W.R., Doyscher R. Efficacy of the functional movement screen: a review. J. Strength Cond. Res. Dec. 2014;28(12):3571–3584. doi: 10.1519/JSC.0000000000000556. [DOI] [PubMed] [Google Scholar]
- 46.Pappa E., Kontodimopoulos N., Niakas D. Validating and norming of the Greek SF-36 health survey. Qual. Life Res. 2005;14:1433–1438. doi: 10.1007/s11136-004-6014-y. [DOI] [PubMed] [Google Scholar]
- 47.Stanley D.M., Williams S.E., Cumming J. Preliminary validation of a single-item measure of exercise enjoyment: the exercise enjoyment scale. J. Sport Exerc. Psychol. 2009;31:S138–S139. [Google Scholar]
- 48.Flint A., Raben A., Blundell J.E., Astrup A. Reproducibility, power and validity of visual analogue scales in assessment of appetite sensations in single test meal studies. Int. J. Obes. Relat. Metab. Disord. 2000;24:38–48. doi: 10.1038/sj.ijo.0801083. [DOI] [PubMed] [Google Scholar]
- 49.Matthews D.R., Hosker J.P., Rudenski A.S., Naylor B.A., Treacher D.F., Turner R.C. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 50.Yfanti C., Tsiokanos A., Fatouros I.G., Theodorou A.A., Deli C.K., Koutedakis Y. Chronic eccentric exercise and antioxidant supplementation: effects on lipid profile and insulin sensitivity. J. Sport. Sci. Med. Aug 8 2017;16(3):375–382. [PMC free article] [PubMed] [Google Scholar]
- 51.Friedewald W.T., Levy R.I., Fredrickson D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 1972;18(6):499–502. [PubMed] [Google Scholar]
- 52.Mohr Μ., Draganidis D., Chatzinikolaou A., Barbero-Álvarez J.C., Castagna C., Douroudos I. Muscle damage, inflammatory, immune and performance responses to three football games in 1 week in competitive male players. Eur. J. Appl. Physiol. 2016;116(1):179–193. doi: 10.1007/s00421-015-3245-2. [DOI] [PubMed] [Google Scholar]
- 53.Theodorou A.A., Nikolaidis M.G., Paschalis V., Sakellariou G.K., Fatouros I.G., Koutedakis Y., Jamurtas A.J. Comparison between glucose-6-phosphate dehydrogenase-deficient and normal individuals after eccentric exercise. Med. Sci. Sport. Exerc. 2010;42(6):1113–1121. doi: 10.1249/MSS.0b013e3181c67ecd. [DOI] [PubMed] [Google Scholar]
- 54.Sim A.Y., Wallman K.E., Fairchild T.J., Guelfi K.J. Effects of high-intensity intermittent exercise training on appetite regulation. Med. Sci. Sport. Exerc. 2015;47(11):2441–2449. doi: 10.1249/MSS.0000000000000687. [DOI] [PubMed] [Google Scholar]
- 55.Dee A., Kearns K., O'Neill C., Sharp L., Staines A., O'Dwyer V. The direct and indirect costs of both overweight and obesity: a systematic review. BMC Res. Notes. 2014;7:242. doi: 10.1186/1756-0500-7-242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Blair S. Physical inactivity: the biggest public health problem of the 21st century. Br. J. Sports Med. 2009;43:1–2. [PubMed] [Google Scholar]
- 57.Hainer V., Toplak H., Stich V. Fat or Fit: what is more important? Diabetes Care. Nov. 2009;32(Suppl 2):S392–S397. doi: 10.2337/dc09-S346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ekkekakis P., Vazou S., Bixby W.R., Georgiadis E. The mysterious case of the public health guideline that is (almost) entirely ignored: call for a research agenda on the causes of the extreme avoidance of physical activity in obesity. Obes. Rev. 2016;17(4):313–329. doi: 10.1111/obr.12369. [DOI] [PubMed] [Google Scholar]
- 59.Álvarez C., Ramírez-Campillo R., Ramírez-Vélez R., Izquierdo M. Effects and prevalence of nonresponders after 12 weeks of high-intensity interval or resistance training in women with insulin resistance: a randomized trial. J. Appl. Physiol. 2017;122(4):985–996. doi: 10.1152/japplphysiol.01037.2016. [DOI] [PubMed] [Google Scholar]
- 60.Gibala M.J., Heisz J.J., Nelson A.J. Interval training for cardiometabolic and brain health. ACSM's Health & Fit. J. 2018;22(6):30–34. [Google Scholar]
- 61.Roy M., Williams S.M., Brown R.C., Meredith-Jones K.A., Osborne H., Jospe M. High-intensity interval training in the real world: outcomes from a 12-month intervention in overweight Adults. Med. Sci. Sport. Exerc. Sep. 2018;50(9):1818–1826. doi: 10.1249/MSS.0000000000001642. [DOI] [PubMed] [Google Scholar]
- 62.Clark J.E., Goon D.T. The role of resistance training for treatment of obesity related health issues and for changing health status of the individual who is overfat or obese: a review. J. Sport. Med. Phys. Fit. Mar. 2015;55(3):205–222. [PubMed] [Google Scholar]
- 63.Sword D.O. Exercise as a management strategy for the overweight and obese: where does resistance exercise Fit in? Strength Condit. J. Oct. 2012;34(5):47–55. [Google Scholar]
- 64.Stenger L. What is functional/neuromotor fitness. ACSM's Health & Fit. J. 2018;22(6):35–43. [Google Scholar]
- 65.Machado A.F., Evangelista A.L., Miranda J.M.Q., La Scala Teixeira C.V., Rica R.L., Lopes C.R. Description of training loads using whole-body exercise during high-intensity interval training. Clinics (Sao Paulo) 2018;73:e516. doi: 10.6061/clinics/2018/e516. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.