Abstract
Lipoma is the most frequent benign soft-tissue tumor. It originates from fat cells. According to position, it is distinguished in superficial, typically subcutaneous lipoma, or deep, such as intramuscular lipoma. This latter form is infrequently and may resemble well-differentiated liposarcoma. For this reason, early radiological detection and characterization are necessary to obtain a wide complete resection and histopathologic evaluation to differentiate benign from malign lesions. We report an extremely rare case of an intrathoracic intramuscular lipoma of the chest wall detect and characterize with chest X-ray and computed tomography examinations, resected with thoracoscopic intervention and confirmed with histopathologic analysis.
Keywords: Intramuscular lipoma, Intrathoracic lipoma, Radiologic detection, Thoracoscopic resection
Introduction
Lipoma is a mesenchymal tumor originating from fat cells [1]. It can be superficial or deep. Superficial lipoma is common subcutaneous mass, usually benign and encapsulated, and composed almost entirely of fat [2]. Instead, deep lipoma and, especially, intramuscular lipoma (IL), deep-seated lipoma that originates within the muscle, is extremely rare [3], [4]. It should be assessed carefully, as it is more likely to be malignant and should be considered a well-differentiated liposarcoma until proven otherwise [5]. Indeed, Fletcher et al found that 83% presented infiltrative histopathologic features [6].
Computer tomography (CT) and magnetic resonance imaging (MRI) are the most useful diagnostic methods to identify IL characteristics, such as size, locations, and relationship with neighboring structures [1]. A wide excision is essential to reduce the risk of recurrence [7]. Moreover, surgical resection allows histologic diagnosis, necessary to differentiate benign from malign forms.
Herein we report an extremely rare case of an intrathoracic IL of the chest wall detect and characterize with chest X-ray and CT, resected with thoracoscopic intervention and confirmed with histopathologic analysis. This case underlines the importance to evaluate the imaging and pathology features to differentiate benign from malignant IL.
Case report
Patient's history
A 65-year-old Italian woman (weight 66.4 kg and height 158.4 cm) was referred to the Radiology Department of our Institution for mild dyspnea, discomfort during her domestic activities and slight pain at right lateral chest wall. She had a free previous medical history, not familiarity and not risk factors for pulmonary and cardio-vascular diseases.
Physical exam
Physical exam revealed no sign of pulmonary or cardiac pathology. The patient's temperature was 36°C, heart rate was 72 bpm, respiratory rate was 13 breaths per minute, blood pressure was 110/70 mmHg, and oxygen saturation in room air was 98%. Chest inspection and excursion were normal. Lung and cardiac auscultations were normal without any added noises.
Laboratory values
Blood analysis revealed normal hematocrit and platelet count. The blood biochemistries, as well as urine analysis were normal. Cardiac enzymes were not elevated. Liver enzymes were in the normal range. Creatinine value was 0.6 mg/dL and creatinine clearance was 98 mL/min (estimated with Cockcroft-Gault formula). Then, spirometry evaluation was assessed: forced expiratory volume during the first second was 122%, forced vital capacity was 144%, and diffusing capacity of the lung for carbon monoxide was 103%.
Imaging findings
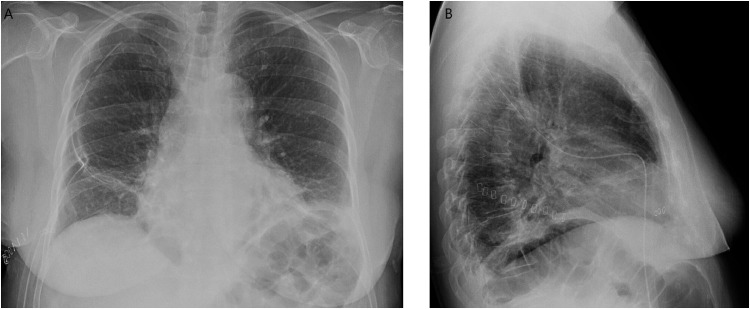
Patient performed a chest X-ray that showed a radio-opaque rounded area with regular margins in the middle field of the right lung near the lateral costal wall (Fig. 1).
Fig. 1.
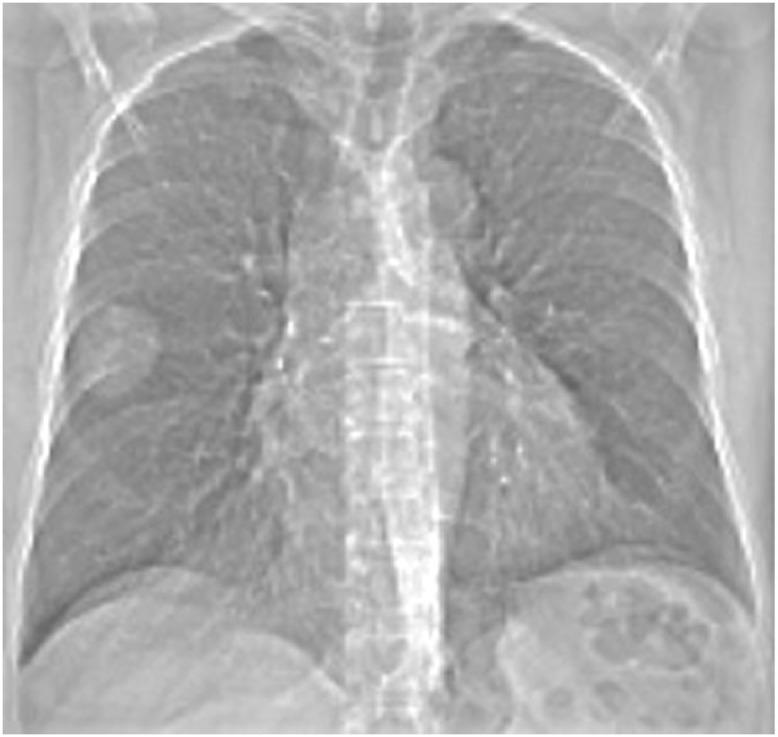
Detection with chest X-ray.
Chest X-ray in anterior-posterior projection showed a radio-opaque rounded area with regular margins in the middle field of the right lung near the lateral chest wall.
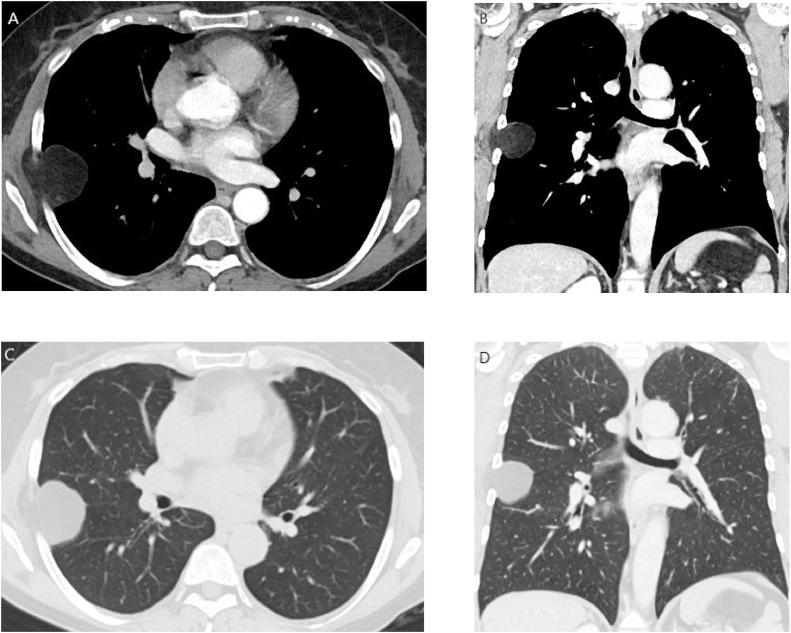
For this reason, she was scanned with third generation Dual Energy CT 192 × 2-sections (SOMATOM Force, Siemens, Germany). Patient received a nonionic low-osmolality contrast medium Visipaque 320 mgI/mL (iodixanol; GE Healthcare Life Sciences, Chalfont, UK), with a volume of 40 mL and flow injection rate of 3 mL/sec followed by 30 mL of saline solution injection at same velocity. The scanning area began from the upper limit of the lungs to 1 cm below the diaphragm with cranio-caudal direction. The parameters used for the exam were 70 kV of tube voltage A; 140 kV of tube B voltage; automatic tube current modulation technique (CARE Dose, Siemens). Then the raw data were postprocessed and were included coronal reconstruction. The use of dual energy with the opportunity to produce virtual noncontrast images, allowed avoiding unenhanced acquisition. CT confirmed a deep circumscribed homogeneous mass, that measured 3.3 cm × 3.8 cm × 4.1 cm with the presence of individual voxels in the image that have fat attenuation values (Hounsfield Unit < 0), sited in intercostal muscles, between the fifth and sixth right rib, with compression of lung parenchyma and without postcontrast enhancement (Fig. 2A-D). Then an elective surgical wide excision was planned.
Fig. 2.
Computer tomography for surgery planning.
CT in soft tissues window (width 400; level 50) in axial (A) and coronal reconstruction (B) and in lung window (width 1500; level −600) in axial (C) and coronal reconstruction (D). The figure showed deep-seated circumscribed homogeneous intramuscular mass, with the presence of individual voxels in the image that have fat attenuation values (Hounsfield Unit < 0), sited in the lateral chest wall, between the fifth and sixth right rib, with compression of near lung parenchyma. The shape of the mass was ovoid.
Surgical results
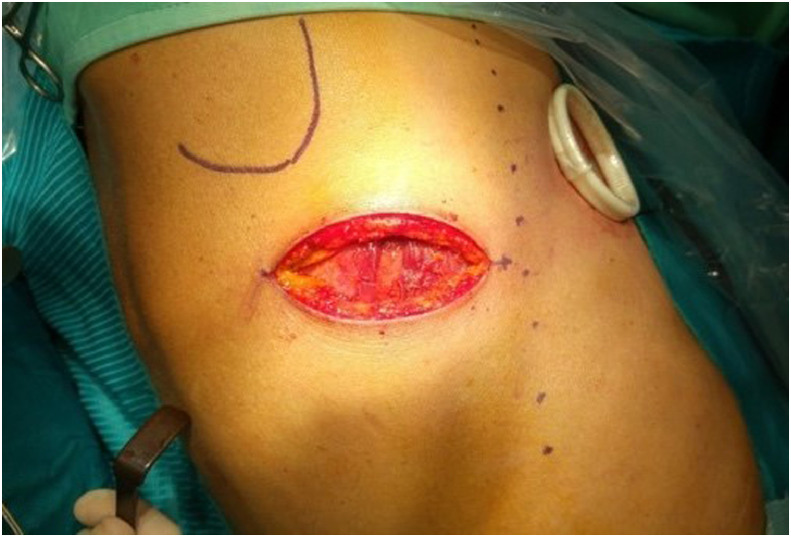
A 3 cm single-port incision was taken on the fifth intercostal space next to anterior axillary line (Fig. 3). The lesion appeared well capsulated and fixed on the chest wall. After excluding any lung infiltration, a 6 cm incision was made just above the lesion on the lateral arc of the fifth rib. After coming off latissimus dorsi and anterior serratus muscles, the lesion appeared attached to the intercostal muscle then was resected with en-bloc exportation (Fig. 4). Margins analyzed during intraoperative frozen biopsy section were free of disease. Intercostal nerve block was made with Naropina 7.5% 20 mL on the fourth-fifth-sixth intercostal spaces and a chest tube of 24CH was placed into pleural cavity. The mass was peripheral to the visceral pleura and was adherent to the parietal pleura. The wide excision extend into the pleural space and a portion of peripheral pleural was resected. Clinical postsurgery course was regular without any functional problems and the pain was well controlled. During the first postoperative day, the patient performed a chest X-ray that showed good lung expansion, no signs of pneumothorax and slight bilateral pleural effusion (Fig. 5A and B). Chest drainage was removed during the second postoperative day. The patient was discharged in forth postoperative day. After 8 days, she repeated a chest X-ray exam confirmed good lung expansion and no signs of pneumothorax.
Fig. 3.
Thoracoscopic intervention.
A mini-invasive 3 cm single-port incision was taken on the fifth intercostal space next to anterior axillary line.
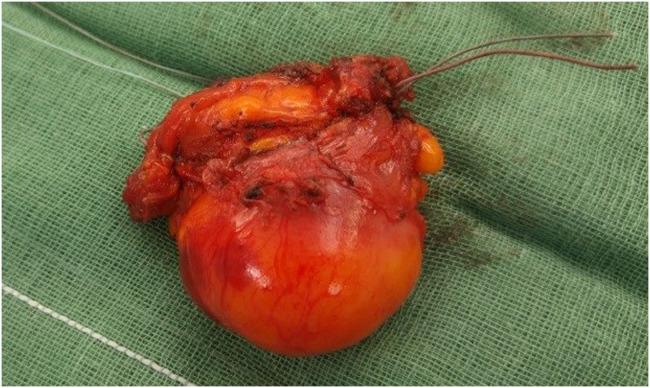
Fig. 4.
Intramuscular lesion.
The lesion appeared soft, well capsulated without lung infiltration. Margins analyzed during intraoperative frozen biopsy section were free of disease.
Fig. 5.
Postsurgical chest X-ray.
Postsurgical chest X-ray in anterior-posterior (A) and latero-lateral (B) projections showed good lung expansion, no signs of pneumothorax and slight bilateral pleural effusion.
Pathology findings
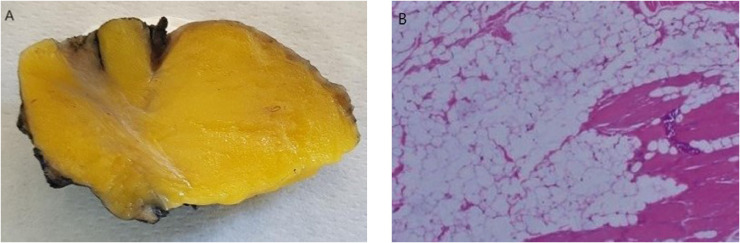
Histopathologic analysis confirmed intrathoracic IL of the chest wall diagnosis with no signs of malignancy. Macroscopically appeared as a yellow soft mass, encapsulated, with some fibrous septa (Fig. 6A). Microscopically was composed by mature adipocytes with no cellular atypia, a small amount of muscle and fibers cells, without areas of fat necrosis and blood vessels (Fig. 6B).
Fig. 6.
Pathology analysis.
On gross specimen lesion appeared a yellow soft mass, encapsulated, with some fibrous septa inside fat tissue (A). Microscopically (H&E stain, ×100) was composed by mature adipocytes with no cellular atypia, a small amount of muscle and fibers cells, without areas of fat necrosis and blood vessels (B). Color version of figure is available online.
Discussion
Lipoma represents the most common soft-tissue tumor. The prevalence has been estimated at 2.1 per 100 people. According to position, lipoma can be classified as superficial or deep. Superficial lipoma is subcutaneous, and extraordinarily common. IL, deep-seated lipoma that originates within the muscle, is extremely rare [8]. Superficial lipoma is capsulated, composed by fat cells and small amount of nonadipose components, such as fibrous septa, areas of necrosis, blood vessels, and interposed muscle fibers [9]. Instead, IL commonly lacks of capsule and tends to invade adjacent tissue [10]. Usually, it is a slowly growing asymptomatic mass. Pain is a late and uncommon symptom, usually in deep and very large IL due to expansion of adjacent soft tissues or compression of the adjacent peripheral nerve [11]. CT and MRI are the most useful diagnostic methods to identify and mark out this mass and to reveal IL characteristics, such as size, locations, and relationship with neighboring structures to choose the best treatment. CT scan appearance of ILs reveals a hypodense mass situated within the muscle with Hounsfield values in the negative range. Attenuation is similar to that of fat tissue. The shape of the mass may vary but is usually ovoid or fusiform. The mass may be well circumscribed or have poorly defined margins. Thick and thin soft tissue density streaks are commonly found inside the lesion. The thickness of the streaks varies and they are interrupted occasionally. MRI is very useful in distinguishing fat-containing tumors from other soft tissue tumors. MRI is also an excellent imaging modality to distinguish among lipomatous masses. The fatty tissue in the ILs demonstrates high signal intensity on both T1- and T2-weighted images. Fat-suppressed sequences demonstrate signal suppression similar to normal fat. ILs can be homogeneous with intensity similar to subcutaneous fat or heterogeneous with intermingled muscle fibers and other types of tumor tissue [12], [13]. Ultrasonography instead is important to define the fatty nature of the lesion although it does not offer sufficient knowledge about relationship with adjacent structures [1], [14], [15]. Early surgical excision is better than radiographic observation. Only surgical excision allows histopathologic analysis necessary to differentiate benign from malign forms [6]. On gross examination, the majority of ILs are seemingly circumscribed, masses of uniform, yellowish adipose tissue with mottled tan areas, and a soft consistency. Histologically, ILs have relatively uniform appearance characterized by mature univacuolated adipocytes of fairly uniform size and shape, which irregularly infiltrate between muscle fibers and, in many places, completely replacing the muscle bundles. They do not display nuclear atypia and there is no increased mitosis, hyperchromasia, pleomorphism, or multinucleation of fat cells [16]. Hyun Ho Han et al described a case of liposarcoma diagnosed at histopathologic analysis that was valued as lipoma with CT and MRI examinations before surgery [17]. Moreover, a surgical wide excision is essential because of recurrence rate risk. Some ILs have the tendency to recur and the recurrence rate is from 3.0% to 62.5%, ranging from several months to more than 10 years [6]. Bancroft et al in their review described different treatments based on lipoma features, such as symptoms, location, growth, and histology. Observation and radiologic follow-up are required in small and asymptomatic subcutaneous lipoma. Growing up lipoma may be taken off by a marginal resection if capsulated. Treatment for deep-seated lipoma is surgical intervention that allows a complete resection [18]. Herein we report an extremely rare case of 65-year-old woman with an intrathoracic IL of the chest wall detect and characterize with chest X-ray and CT examinations, resected with a wide thoracoscopic intervention and confirmed with histopathologic analysis. Tokitsu et al described 2 cases of intrathoracic lipomas arising from the chest wall. The first one was a 55-year-old male, and the other one was a 70-year-old female detect by CT, and removed by thoracotomy. In both cases, the diagnosis of ILs was confirmed postoperatively by histopathologic examination [19]. Ono et al reported a case of a 43-year-old woman with pleural tumor on chest X-ray examination for healthy check-up. CT showed that it had an intrathoracic IL. The tumor was excised along with pleura, periostea, and intercostal muscle approximately 1 cm apart from it. Microscopically it was found to be an infiltrative IL [20]. Lee et al described, in a 40-year-old Korean man, a well-circumscribed IL removed by thoracoscopic surgery [21]. Finally, Jinwook et al reported a case of an IL in the intercostal muscle. In their case, the tumor was resected by wide excision to reduce recurrence rate risk [22].
Conclusion
In conclusion, intrathoracic IL is an extremely rare form of lipoma. Early radiological detection and characterization are necessary for surgical planning, to obtain a wide complete resection, less invasive as possible, to reduce recurrence rate risk. Histopathologic diagnosis is essential to differentiate benign forms from liposarcoma.
Author statement
Ethical standards
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the Helsinki Declaration of 1975, as revised in 1983. Informed consent was obtained from all individual participants included in the study.
Informed consent
Informed written consent was obtained from the patient for publication of this report and any accompanying images.
Acknowledgments
None.
Footnotes
Competing interests: The authors declare that they have no conflict of interest.
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.radcr.2019.05.020.
Appendix. Supplementary materials
References
- 1.Mc Tighe S., Chernev I. Intramuscular lipoma: a review of the literature. Orthop Rev. 2014;6(4):5618. doi: 10.4081/or.2014.5618. [DOI] [PMC free article] [PubMed] [Google Scholar]; Mc Tighe S., Chernev I.Intramuscular lipoma: a review of the literature. Orthop Rev. 2014; 6(4):5618. [DOI] [PMC free article] [PubMed]
- 2.Fletcher C. Diagnostic Histopathology of Tumors. 4th ed. Elsevier; 2014. Tumors of soft tissue. [Google Scholar]; Fletcher C.Tumors of soft tisssuetissue. In:Diagnostic Histopathology of Tumors. 2014; 4th edAU: Please provide publisher name for refs. 2 and 4..
- 3.Murphey M., Carroll J.F., Flemming D.J., Pope T.L., Gannon F.H., Kransdorf M.J. From the archives of the AFIP: benign musculoskeletal lipomatous lesions. Radiographics. 2004;24(5):1433–1466. doi: 10.1148/rg.245045120. [DOI] [PubMed] [Google Scholar]; Murphey M., Carroll J.F., Flemming D.J., Pope T.L., Gannon F.H., Kransdorf M.J.From the archives of the AFIP: benign musculoskeletal lipomatous lesions. Radiographics. 2004; 24(5):1433–66. [DOI] [PubMed]
- 4.Di Subba R.D., Suhny A., Jonathan H.C. 1st edition. Elsevier; 2019. Problem solving in chest imaging. [Google Scholar]; Di Subba R.D., Suhny A., Jonathan H.C.Problem solving in chest imaging. 2019; 1st edition.
- 5.Nishida J., Morita T., Ogose A., Okada K., Kakizaki H., Tajino T. Imaging characteristics of deep-seated lipomatous tumors: intramuscular lipoma, intermuscular lipoma, and lipoma-like liposarcoma. J Orthop Sci. 2007;12(6):533–541. doi: 10.1007/s00776-007-1177-3. [DOI] [PubMed] [Google Scholar]; Nishida J., Morita T., Ogose A., et al. Imaging characteristics of deep-seated lipomatous tumors: intramuscular lipoma, intermuscular lipoma, and lipoma-like liposarcoma. J Orthop Sci. 2007; 12(6):533–41. [DOI] [PubMed]
- 6.Fletcher C.D., Martin-Bates E. Intramuscular and intermuscular lipoma: neglected diagnoses. Histopathology. 1998;12(3):275–287. doi: 10.1111/j.1365-2559.1988.tb01942.x. [DOI] [PubMed] [Google Scholar]; Fletcher C.D., Martin-Bates E.Intramuscular and intermuscular lipoma: neglected diagnoses. Histopathology. 1998; 12(3):275–87. [DOI] [PubMed]
- 7.Su C., Hung J., Chang I. Surgical treatment of intramuscular, infiltrating lipoma. Int Surg. 2001;96(1):56–59. doi: 10.9738/1396.1. [DOI] [PubMed] [Google Scholar]; Su C., Hung J., Chang I.Surgical treatment of intramuscular, infiltrating lipoma. Int Surg. 2001; 96(1):56–9. [DOI] [PubMed]
- 8.Myhre-Jensen O. A consecutive 7-year series of 1331 benign soft tissue tumors. Clinicopathologic data. Comparison with sarcomas. Acta Orthop Scand. 1981;52(3):287–293. doi: 10.3109/17453678109050105. [DOI] [PubMed] [Google Scholar]; Myhre-Jensen O.A consecutive 7-year series of 1331 benign soft tissue tumors. Clinicopathologic data. Comparison with sarcomas. Acta Orthop Scand. 1981; 52(3):287–93. [DOI] [PubMed]
- 9.Johnson C., Ha A., Chen E., Davidson D. Lipomatous soft-tissue tumors. J Am Acad Orthop Surg. 2018;26(22):779–788. doi: 10.5435/JAAOS-D-17-00045. [DOI] [PubMed] [Google Scholar]; Johnson C., Ha A., Chen E., Davidson D.Lipomatous soft-tissue tumors. J Am Acad Orthop Surg. 2018; 26(22):779–88. [DOI] [PubMed]
- 10.Ramos P., Alonso L., Santos S., Ferrández P. Intramuscular lipoma of the deltoid mimicking a sarcoma. A case report. Chir Organi Mov. 2001;86(2):153–157. [PubMed] [Google Scholar]; Ramos P., Alonso L., Santos S., Ferrández P.Intramuscular lipoma of the deltoid mimicking a sarcoma. A case report. Chir Organi Mov. 2001; 86(2):153–7. [PubMed]
- 11.Ferrari L., Haynes P., Mack J., Di Felice G.S. Intramuscular lipoma of the supraspinatus causing impingement syndrome. Orthopedics. 2009;32(8) doi: 10.3928/01477447-20090624-24. [DOI] [PubMed] [Google Scholar]; Ferrari L., Haynes P., Mack J., Di Felice G.S.Intramuscular lipoma of the supraspinatus causing impingement syndrome. Orthopedics. 2009; 32(8).AU: Please provide page range for ref. 11. [DOI] [PubMed]
- 12.Nishida J., Kakizaki H., Tajino T., Hatori M., Orui H., Ehara S. Imaging characteristics of deep-seated lipomatous tumors: intramuscular lipoma, intermuscular lipoma, and lipoma-like liposarcoma. J Orthop Sci. 2007;12(6):533–541. doi: 10.1007/s00776-007-1177-3. [DOI] [PubMed] [Google Scholar]; Nishida J., et al. Imaging characteristics of deep-seated lipomatous tumors: intramuscular lipoma, intermuscular lipoma, and lipoma-like liposarcoma. J Orthop Sci. 2007; 12(6):533–41.AU: The names of all authors should be given unless there are more than 6, in which case the names of the first 6 authors are used, followed by “et al." Pease provide the names of first six authors for refs. 5, 12, 15, and 19. [DOI] [PubMed]
- 13.Matsumoto K., Hukuda S., Ishizawa M., Chano T., Okabe H. MRI findings in intramuscular lipomas. Skeletal Radiol. 1999;28(3):145–152. doi: 10.1007/s002560050491. [DOI] [PubMed] [Google Scholar]; Matsumoto K., Hukuda S., Ishizawa M., Chano T., Okabe H.MRI findings in intramuscular lipomas. Skeletal Radiol. 1999; 28(3):145–52. [DOI] [PubMed]
- 14.Berquist H., Ehman R., King B., Hodgman C., Ilstrup D. Value of MR imaging in differentiating benign from malignant soft-tissue masses: study of 95 lesions. Am J Roentgenol. 1990;155(6):1251–1255. doi: 10.2214/ajr.155.6.2122675. [DOI] [PubMed] [Google Scholar]; Berquist H., Ehman R., King B., Hodgman C., Ilstrup D.Value of MR imaging in differentiating benign from malignant soft-tissue masses: study of 95 lesions. The American Journal of Roentgenology. 1990; 155(6):1251–5. [DOI] [PubMed]
- 15.Inampudi P., Jacobson J.A., Fessell D.P., Carlos R.C., Patel S.V., Delaney-Sathy L.O. Soft-tissue lipomas: accuracy of sonography in diagnosis with pathologic correlation. Radiology. 2004;233(3):763–767. doi: 10.1148/radiol.2333031410. [DOI] [PubMed] [Google Scholar]; Inampudi M., et al. Soft-tissue lipomas: accuracy of sonography in diagnosis with pathologic correlation. Radiology. 2004; 233(3):763–7. [DOI] [PubMed]
- 16.Chernev I. Intramuscular lipoma: infiltrating vs. well-circumscribed variant. Pan Afr Med J. 2014;17:170. doi: 10.11604/pamj.2014.17.170.3578. [DOI] [PMC free article] [PubMed] [Google Scholar]; Chernev I.Intramuscular lipoma: infiltrating vs. well-circumscribed variant. Pan Afr Med J. 2014; 17:170. [DOI] [PMC free article] [PubMed]
- 17.Han H., Choi J., Seo B., Moon S., Oh D., Ahn S. Treatment for intramuscular lipoma frequently confused with sarcoma: a 6-year restrospective study and literature review. Biomed Res Int. 2014;(8):67–69. doi: 10.1155/2014/867689. [DOI] [PMC free article] [PubMed] [Google Scholar]; Han H., Choi J., Seo B., Moon S., Oh D., Ahn S., et al. Treatment for intramuscular lipoma frequently confused with sarcoma: a 6-year restrospective study and literature review. Biomed Res Int. 2014.AU: Please provide volume and page range for ref. 17. [DOI] [PMC free article] [PubMed]
- 18.Bancroft L., Kransdorf M., Peterson J., O'Connor M. Benign fatty tumors: classification, clinical course, imaging appearance, and treatment. Skeletal Radiol. 2006;35(10):719–733. doi: 10.1007/s00256-006-0189-y. [DOI] [PubMed] [Google Scholar]; Bancroft L., Kransdorf M., Peterson J., O'Connor M.Benign fatty tumors: classification, clinical course, imaging appearance, and treatment. Skeletal Radiol. 2006; 35(10):719–33. [DOI] [PubMed]
- 19.Tokitsu K., Tachibana S., Kawakami M., Nakao K., Morita T., Hashimoto T. Two surgical cases of intrathoracic lipoma. Kyobu Geka. 1999;52(3):251–253. [PubMed] [Google Scholar]; Tokitsu K., et al. Two surgical cases of intrathoracic lipoma. Kyobu Geka. 1999; 52(3):251–3. [PubMed]
- 20.Ono M., Tsuda K., Sasagawa N., Satoh M., Ikeda K., Sudoh K. An hourglass type intrathoracic lipoma-a case report of surgical treatment. Nihon Kyobu Geka Gakkai Zasshi. 1993;41(9):1556–1561. [PubMed] [Google Scholar]; Ono M., Tsuda K., Sasagawa N., Satoh M., Ikeda K., Sudoh K.An hourglass type intrathoracic lipoma-a case report of surgical treatment. Nihon Kyobu Geka Gakkai Zasshi. 1993; 41(9):1556–61. [PubMed]
- 21.Lee J.H., Do H.D., Lee J.C. Well-circumscribed type of intramuscular lipoma in the chest wall. J Cardiothorac Surg. 2013;8:181. doi: 10.1186/1749-8090-8-181. [DOI] [PMC free article] [PubMed] [Google Scholar]; Lee J.H., Do H.D., Lee J.C.Well-circumscribed type of intramuscular lipoma in the chest wall. J Cardiothorac Surg. 2013; 8:181. [DOI] [PMC free article] [PubMed]
- 22.Jinwook H., Won-Min J., Byoung-Ju M., Jae S. Deep-seated intramuscular lipoma penetrates the intercostal muscle. J Thorac Dis. 2015;7(10):E493–E495. doi: 10.3978/j.issn.2072-1439.2015.10.29. [DOI] [PMC free article] [PubMed] [Google Scholar]; Jinwook H., Won-Min J., Byoung-Ju M., Jae S.Deep-seated intramuscular lipoma penetrates the intercostal muscle. J Thorac Dis. 2015; 7(10):E493–5. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.