Abstract
Background
Sub-Saharan Africa carries the highest HIV burden globally. It is important to understand how interventions cost-effectively fit within guidelines and implementation plans, especially in low- and middle-income settings. We reviewed the evidence from economic evaluations of HIV prevention interventions in sub-Saharan Africa to help inform the allocation of limited resources.
Methods
We searched PubMed, Web of Science, Econ-Lit, Embase, and African Index Medicus. We included studies published between January 2009 and December 2018 reporting cost-effectiveness estimates of HIV prevention interventions. We extracted health outcomes and cost-effectiveness ratios (CERs) and evaluated study quality using the CHEERS checklist.
Findings
60 studies met the full inclusion criteria. Prevention of mother-to-child transmission interventions had the lowest median CERs ($1144/HIV infection averted and $191/DALY averted), while pre-exposure prophylaxis interventions had the highest ($13,267/HIA and $799/DALY averted). Structural interventions (partner notification, cash transfer programs) have similar CERs ($3576/HIA and $392/DALY averted) to male circumcision ($2965/HIA) and were more favourable to treatment-as-prevention interventions ($7903/HIA and $890/DALY averted). Most interventions showed increased cost-effectiveness when prioritizing specific target groups based on age and risk.
Interpretation
The presented cost-effectiveness information can aid policy makers and other stakeholders as they develop guidelines and programming for HIV prevention plans in resource-constrained settings.
Research in context
Evidence Before This Study
There is an increasing interest in cost-effectiveness of HIV programming among stakeholders. The last systematic review on the cost-effectiveness of all HIV prevention interventions was published nearly ten years ago. At that time, cost-effectiveness studies were limited and often unavailable for specific interventions. A 2013 systematic review presented evidence from studies specifically on pre-exposure prophylaxis and concluded that the intervention's impact relied highly on contextual assumptions. In the past decade, an increasing number of cost-effectiveness studies in HIV prevention literature have become available, but there has not yet been a single review that synthesizes the evidence from all of these studies. We conducted a systematic review for cost-effectiveness studies on HIV prevention interventions. We searched PubMed/MEDLINE, Web of Science, Econ-Lit, Embase, and African Index Medicus, for studies published between January 1, 2009 and December 31, 2018. Search terms included “HIV”, “prevention” or “control”; “sub-Saharan Africa”; “cost” or “cost-effectiveness”.
Added Value of This Study
This is the first review that provides a comprehensive and update look at the cost-effectiveness of all HIV prevention interventions targeted towards HIV- individuals. Additionally, this review focuses solely on sub-Saharan Africa, the region that carries the vast majority of the global disease burden. We show that voluntary medical male circumcision (VMMC) and prevention of mother-to-child transmission (PMTCT) interventions are cost-effective in almost all contexts. We provide evidence of cost-effectiveness of other newer biomedical interventions, including pre-exposure prophylaxis (PrEP) and treatment as prevention (TasP). We hope that the evidence from this review will aid various stakeholders, including Ministries of Health, program implementers, and international donors, in their decision-making regarding resource allocation policy for HIV prevention.
Implications of All the Available Evidence
The number of studies included in this review reflects the increasing importance of considering cost-effectiveness when designing or implementing HIV prevention programs in sub-Saharan Africa. Numerous studies focused on new biomedical interventions, and many of these studies used mathematical modeling to provide evidence of these interventions' cost-effectiveness since they have not yet been scaled up in sub-Saharan Africa. However, this review shows that most interventions can be cost-effective in specific contexts. As such, we encourage others to use the results of this review with caution. Future economic and costing studies on HIV prevention should include more realistic scenarios so that these data are more accessible and relevant to policymakers and other stakeholders.
Alt-text: Unlabelled Box
1. Introduction
Sub-Saharan Africa (SSA) has experienced a large reduction in new HIV infections over the last decade, with the number of incident infections dropping over 30% since 2010 [1]. This decrease in burden reflects the accomplishment of a global effort focused on a region in which approximately 70% of all people living with HIV reside [2], [3]. Despite this success, the decline in incidence is slowing, and gaps in the scale-up of HIV prevention services persist throughout SSA [3].
US$4.5 billion was allocated for HIV prevention investments in 2016 by the international community; however, a recent UNAIDS report stated that an additional annual investment of US$7 billion is urgently needed to meet the 2030 Sustainable Development Goals targets [4], [5], [6]. To improve the efficiency of programming for HIV prevention, optimizing limited financial resources is crucial to scale up high-quality, cost-effective interventions to maximize HIV prevention [7].
In addition to evidence-based prevention tools such as voluntary medical male circumcision (VMMC) and prevention of mother-to-child transmission (PMTCT) strategies, new prevention methods such as HIV pre-exposure prophylaxis (PrEP) have been heralded for their remarkable clinical results in the reduction of HIV transmission. However, it is important for policy- and decision-makers to identify where and how such costly interventions fit within regional and national HIV implementation plans and budgets, particularly in resource-limited countries [8].
Ascertaining the cost-effectiveness of prevention interventions is necessary for optimal resource allocation and for identifying inefficiencies within prevention programs [7].
A systematic review of HIV prevention intervention cost-effectiveness was published in 2009 by Galarraga et al., which concluded that the number and quality of cost-effectiveness studies were insufficient and too limited at that time to aid decision making and policy recommendations [9], [10]. However, since 2009, many studies have been published on the cost-effectiveness of various prevention interventions, including newer PrEP technologies and treatment-as-prevention [8].
No systematic review to date has evaluated these newer prevention interventions with a focus on SSA. Such a review would provide important information on HIV prevention costs, outcomes, and effectiveness to support policies and decision-making [8], [9]. The purpose of this review is to systematically review published analyses of the cost-effectiveness of HIV prevention interventions in SSA settings. We aim to 1) review evidence from studies published in the last decade that have evaluated cost and outcome metrics for HIV prevention interventions, 2) compare the costs and effects of specific prevention interventions, and 3) understand the assumptions driving cost-effectiveness in order to inform allocation of limited HIV prevention resources.
2. Methods
2.1. Search Strategy and Selection Criteria
We conducted this systematic review in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [10]. We searched PubMed/MEDLINE, Web of Science, Econ-Lit, Embase, and African Index Medicus. Additionally, we reviewed reference lists of retrieved articles as well as governmental and organizational reports to complement our search. We limited studies published between January 1, 2009 and December 31, 2018. The following keywords were used: “HIV”; “prevention” or “control”; “cost” or “cost-analysis” or “cost-effectiveness”; “sub-Saharan Africa”. The full search strategy, including keywords for each database, can be found in the supplemental material.
Inclusion criteria included full articles that were peer-reviewed and published in English, and reported cost and outcome measures or analysed cost-effectiveness of an HIV prevention intervention. Interventions included, but were not limited to: VMMC, PMTCT, TasP, PrEP, behavioral interventions, vaccinations, and microbicides. As a multi-pronged strategy, two types of PMTCT interventions were considered: Prong II, interventions to prevent unintended pregnancies of HIV-positive women, and Prong III, interventions providing services to reduce HIV transmission from HIV-positive women to their infants. Geography was limited to country settings within SSA, as defined by the United Nations [11]. A full list of eligible country settings can be found in the supplemental material. Studies that focused on HIV treatment with no prevention aspect, systematic reviews, meta-analyses, conference abstracts, and guideline reports were excluded. Studies assessing cost-effectiveness of an intervention's combined impact for both HIV-positive and HIV-negative persons and studies that did not describe costing analyses and effectiveness measures were excluded. Two reviewers aggregated a list of articles produced by the database search and conducted independent screenings based on title and abstract. All discrepancies were resolved by a third reviewer.
2.2. Quality Assessment and Data Extraction
Two reviewers independently extracted data from each of the selected studies using a prepared data form, and an independent crosscheck by a third reviewer was conducted to identify and resolve any disagreements or uncertainties. We developed the data form using guidance from Emory colleagues and prior systematic reviews on similar topics. We assessed the quality of studies using the Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement, which contains a 24-point checklist to assess economic evaluation studies [12].
We extracted data on intervention type, study design or model type, geographic setting, HIV transmission method, population, intervention description, perspective, and time horizon. Additional extracted information included scenario descriptions, intervention effectiveness, cost-effectiveness metric results, and discounting rates for effects and costs. We categorized studies by prevention intervention type to compare intervention-specific results. The primary measures of interest were cost per HIV infection averted (HIA), cost per disability-adjusted life year (DALY) averted, cost per quality-adjusted life year (QALY) gained, and cost per life year gained (LYG). We converted study cost-effectiveness results to 2018 US$ using the Consumer Price Index (CPI) Inflation Calculator and compared them to the International Monetary Fund 2018 estimates of gross domestic product (GDP) per capita for each study setting [13], [14].
For each intervention type, we calculated median CERs. Separate medians were calculated for studies reporting cost per HIA estimates and studies reporting cost per DALY averted, QALY gained, or LYG. For studies that explored more than one geographic setting, we considered results from the different settings as individual estimates if they were reported as such within a single study; these results were considered separately when we calculated median CERs.
3. Results
We identified and screened 1115 articles, of which 146 met criteria to be assessed for eligibility. The 969 articles that were initially excluded were deemed ineligible based on the article title and abstract and did not meet either the geographic setting or intervention criteria. Out of the 146 articles, 60 met the full inclusion criteria (Fig. 1). These 60 peer-reviewed studies provided cost-effectiveness results for the following HIV prevention interventions: 14 studies on VMMC, 13 studies on PrEP, five studies on TasP, 15 studies on PMTCT, nine studies on other biomedical interventions, one study on behaviour change, and three studies on structural interventions [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58], [59], [60], [61], [62], [63], [64], [65], [66], [67], [68], [69], [70], [71], [72], [73], [74]. Among PMTCT studies, 14 considered Prong III strategies, while one focused on Prong II.
Fig. 1.
Flowchart diagram for study selection.
Table 1 describes characteristics of each study, including study design or model type, geographic setting, method of transmission, target population, time horizon, HIV prevalence of the target population, perspective, and description of the intervention assessed. Studies focused on heterosexual transmission among the general population except for studies exploring prevention of mother-to-child-transmission. Costs were predominately assessed through a healthcare payer perspective. Two studies included results from countries outside of SSA; non-SSA results were excluded from this review [56], [62].
Table 1.
Study design and setting overview.
| Reference | Study design | Setting | Population | Time horizon | HIV prevalencea | Perspectiveb | Intervention description |
|---|---|---|---|---|---|---|---|
| VMMC | |||||||
| Binagwaho et al. (2010) [15] | Deterministic compartmental simulation | Rwanda | 0-49 yoc, male population | Lifetime | 2.7% | Health care payer | Scale-up of VMMC to infants, adolescents, and adults |
| Njeuhmeli et al. (2011) [16] | Deterministic compartmental simulation | Sub-Saharan Africa | 15-49 yo, general population | Lifetime | 4.8% | Health care payer | Scale-up of VMMC |
| Uthman et al. (2011) [17] | Probabilistic decision analysis | Sub-Saharan Africa | 15 + yo, male population | Lifetime | 5.5% | Health care payer | Uptake of VMMC |
| Duffy et al. (2013) [18] | Cross-sectional descriptive cost-analysis | Uganda | 18 yo and older, male population | Lifetime | 5.9% | Health care payer | PrePex device for VMMC |
| Menon et al. (2014) [19] | Impact analysis | Tanzania | 10-49 yo, male population | Lifetime | 4.5% | Health care payer | Scale-up of VMMC |
| Awad et al. (2015) [20] | Deterministic compartmental simulation | Zimbabwe | 10-49 yo, male population | 15 years | 13.3% | Health care payer | Prioritisation of VMMC subpopulations by age, geographic location, sexual risk profile |
| Awad et al. (2015) [21] | Deterministic compartmental simulation | Zambia | 10-49 yo, male population | 15 yearsc | 11.5% | Health care payer | Prioritisation of VMMC subpopulations by age, geographic location, sexual risk profile |
| Haacker et al. (2016) [22] | Deterministic compartmental simulation | South Africa | 15-59, male population | Lifetime | 18.8% | Health care payer | Age prioritised VMMC scale up |
| Kripke et al. (2016) [23] | Deterministic compartmental simulation | Malawi | 10 + yo; male population | 15 years | 9.6% | Health care payer | Age prioritised VMMC scale up |
| Kripke et al. (2016) [24] | Deterministic compartmental simulation | Zimbabwe | 20-29 yo; male population | 15 years | 13.3% | Health care payer | Age prioritised VMMC scale up |
| Kripke et al. (2016) [25] | Deterministic compartmental simulation | Sub-Saharan Africa | 10-49 yo; male population | 15 years | 4.8% | Health care payer | Age prioritised VMMC scale up |
| Kripke et al. (2016) [26] | Deterministic compartmental simulation | Eswatini | 10-49 yo; male population | 15 years | 27.4% | Health care payer | Age prioritised VMMC scale up |
| Kripke et al. (2016) [27] | Deterministic compartmental simulation | Malawi, South Africa, Eswatini, Tanzania, Uganda | 10-49 yo; male population | 15 years | 9.6% (Malawi) 18.8% (South Africa) 27.4% (Eswatini) 4.5% (Tanzania) 5.9% (Uganda) |
Health care payer | Age prioritised VMMC scale up |
| Njeuhmeli et al. (2016) [28] | Deterministic compartmental simulation | Zimbabwe | Male infants | 36 years | 13.3% | Health care payer | Early infant male circumcision |
| PrEP | |||||||
| Pretorius et al. (2010) [29] | Deterministic compartmental simulation | South Africa | 15-49 yo, general population | 10 years | 18.8% | Health care payer | PrEP is scaled up to recruit all uninfected individuals |
| Hallett et al. (2011) [30] | Microsimulation | South Africa | HIV serodiscordant couples | Lifetime | 18.8% | Health care payer | PrEP for uninfected partner in serodiscordant relationships |
| Cremin et al. (2013) [31] | Deterministic compartmental simulation | KwaZulu-Natal, South Africa | 15-54 yo, general population | 10 years | 27.0% (KZNc)ref | Program | Combination prevention strategies of VMMC, early ART, and PrEP |
| Nichols et al. (2013) [32] | Deterministic compartmental simulation | Macha, Zambia | 12 + yo, general population | 10 years | 7.7% (Macha) | Health care payer | Prioritisation of PrEP |
| Verguet et al. (2013) [33] | Deterministic compartmental simulation | Sub-Saharan Africa | 15-49 yo, general population | 5 years | 4.8% | Health care payer | PrEP intervention to pre-existing levels of MC, ART, and condom use |
| Alistar et al. (2014) [34] | Dynamic compartmental simulation | South Africa | 15-49 yo, general population | 20 years | 18.8% | Health care payer | PrEP is scaled up to recruit all uninfected individuals |
| Nichols et al. (2014) [35] | Deterministic compartmental simulation | Macha, Zambia | 12 + yo, general population | 40 years | 7.7% (Macha) | Health care payer | Uptake of PrEP and TasP in combination |
| Cremin et al. (2015) [36] | Deterministic compartmental simulation | Nyanza province, Kenya | General population | 5 years | 13.9% (Nyanza) | Health care payer | Dynamic interaction between key determinants of PrEP impact and cost-effectiveness |
| Cremin et al. (2015) [37] | Deterministic compartmental simulation | Gaza province, Mozambique | Adult male mine workers | 5 years | 30.0% (female) 17.0% (male) |
Health care payer | Time-limited PrEP uptake among sexual partners of miners |
| Ying et al. (2015) [38] | Micro-costing analysis | Uganda | HIV serodiscordant couples | 10 years | 7.1% | Program | Targeted PrEP for serodiscordant couples |
| Glaubius et al. (2016) [39] | Deterministic compartmental simulation | South Africa | 15-54 yo, general population | 1) 10yrs 2) lifetime |
18.8% | Societal | Long-acting injective antiretrovirals used for PrEP |
| Walensky et al. (2016) [40] | Deterministic compartmental simulation | South Africa | 18-25 yo, high risk women | 5 years | Incidence: 5.0% (high risk women) | Program | Long-acting PrEP |
| Cremin et al. (2017) [41] | Deterministic compartmental simulation | Nairobi, Kenya | Key populations | 10 years | 4.8% | Health care payer | PrEP provided to FSW |
| TasP | |||||||
| Barnighausen et al. (2012) [42] | Discrete time mathematical model | South Africa | 15 + yo, general population | 10 years | 18.8% | Health care payer | Increased coverage of TasP, ART under the current WHO eligibility guidelines, and MMC |
| Granich et al. (2012) [43] | Deterministic compartmental simulation | South Africa | 15 + yo, general population | 1) 5 years 2) 40 years |
18.8% | Program | Enhanced combination prevention strategy |
| Smith et al. (2015) [44] | Individual-based simulation modelling study | KwaZulu-Natal, South Africa | 18 + yo, general population | 10 years | 27.0% (KZN)ref | Health care payer | Home HIV counselling and testing |
| Bershteyn et al. (2016) [45] | Individual-based simulation modelling study | South Africa | General population | 20 years | 18.8% | Health care payer | Age-targeting outreach with HIV treatment and prevention |
| Ying et al. (2016) [46] | Dynamic compartmental model | KwaZulu-Natal, South Africa | General population | 10 years | 27.0% (KZN)ref | Program | Home HIV testing and counselling |
| PMTCT | |||||||
| Halperin et al. (2009) [47] | Modelling analysis | Sub-Saharan Africa | Pregnant, HIV-infected women | 1 year | 4.8% | Service delivery | Antiretroviral prophylaxis programs and family planning programs |
| Nakakeeto et al. (2009) [48] | Forecasting model | Burkina Faso, Cameroon, Cote d’Ivoire, Malawi, Rwanda, Tanzania, and Zambia |
HIV-infected women, HIV-exposed infants | 8 years | 0.8% (Burkina Faso) 3.7% (Cameroon) 2.8% (Cote d’Ivoire) 9.6% (Malawi) 2.7% (Rwanda) 4.5% (Tanzania) 11.5% (Zambia) |
Health care payer | PMTCT package including: family planning, HIV testing and counselling, and provision of antiretroviral and cotrimoxazole prophylaxis |
| Orlando et al. (2010) [49] | Cost-effectiveness analysis | Malawi | Pregnant, HIV-infected women | 42 months | 16.9% (ANC) | Societal and Private | HAART-based intervention |
| Robberstad et al. (2010) [50] | Decision analysis | Tanzania | Pregnant, HIV-infected women | 18 months | 6.6% (ANC) | Health care payer | HAART-based intervention |
| Shah et al. (2011) [51] | Decision-based analytical model | Nigeria | Pregnant, HIV-infected women | 1 year | 2.8% | Health care payer | 2009 WHO PMTCT guidelines (long-course ART) |
| Kuznik et al. (2012) [52] | Cost-effectiveness analysis | Uganda | Pregnant, HIV-infected women | 19.3 years | 7.1% | Health care payer | Combination ART |
| Binagwaho et al. (2013) [53] | Cost-effectiveness analysis | Rwanda | HIV-infected pregnant women and their infants | Lifetime | 2.7% | Health care payer | Dual ARV and short course HAART prophylaxis with breastfeeding or replacement feeding |
| Fasawe et al. (2013) [54] | Decision analysis | Malawi | Pregnant, HIV-infected women | 10 years | 16.9% (ANC) | Health care payer | Implementation of Option B + |
| Maredza et al. (2013) [55] | Cost-effectiveness analysis | South Africa | Pregnant, HIV-infected women | 24 months | 28.0% (ANC) | Health care payer | HAART-based intervention |
| Gopalappa et al. (2014) [56] | Deterministic compartmental simulation | Kenya, South Africa, Zambia | 15-49 yo, female population | Lifetime | 5.9% (Kenya) 18.8% (South Africa) 11.5% (Zambia) |
Program | Implementation of Option B + |
| Ishikawa et al. (2014) [57] | Decision analysis | Zambia | Pregnant, HIV-infected women | 18 months | 11.5% | Health care payer | Comparison between Option A, Option B, and Option B + |
| Yu et al. (2014) [58] | Decision analysis | South Africa | Pregnant, HIV-infected women | 18 months | 28.0% (ANC) | Health care payer | 1) tested and treated promptly at any time during pregnancy (promptly treated cohort), 2) no testing or treatment until after delivery and appropriate standard treatments were offered (remedy treated cohort) |
| Zulliger et al. (2014) [59] | Cost-effectiveness analysis | South Africa | Pregnant, HIV-infected women | 1 year | 28.0% (ANC) | Health care payer | Expedited initiation onto lifelong ART in pregnant women who met South African ART eligibility criteria |
| Price et al. (2016) [60] | Decision analysis | Zambia | Pregnant women | Lifetime | 11.5% | Health care payer | Daily oral PrEP during pregnancy and breastfeeding |
| Tweya et al. (2016) [61] | Individual-based simulation modelling study | Malawi | Primigravida women | 50 years | 16.9% (ANC) | Health care payer | Option B vs. Option B + |
| Other biomedical | |||||||
| Verguet et al. (2010) [62] | Cost-effectiveness analysis | South Africa | 15-49 yo, female population | 1 year | 26.3% (Female) | Health care payer | Impact of microbicides distributed alongside condoms |
| Williams et al. (2011) [63] | Dynamic compartmental model | South Africa | General population | 20 years | 18.8% | Health care payer | Tenofovir gel uptake by sexually active women |
| Long et al. (2013) [64] | Dynamic compartmental simulation | South Africa | 15-49 yo, general population | 10 years | 18.8% | Health care payer | HIV screening and counselling, ART, VMMC, microbicides |
| Mbah et al. (2013) [65] | Dynamic compartmental simulation | Zimbabwe | 15-49 yo, female population | 10 years | 13.3% | Health care payer | Praziquantel as a preventive anthelminthic chemotherapy |
| Terris-Prestholt et al. (2014) [66] | Deterministic compartmental simulation | Gauteng Province, South Africa | 15-49 yo, general population + FSW and their partners |
15 years | 17.6% (Gauteng) | Health care payer | Uptake of tenofovir gel by women |
| Mvundura et al. (2015) [67] | Impact analysis | Sub-Saharan Africa | 15-49 yo, general population | 1 year | 4.8% | Health care payer | Distribution of 100,000 female condoms |
| Moodley et al. (2016) [68] | Semi-Markov simulation | South Africa | Adolescents enrolled in school | Lifetime | 10.2% (females 15-24) 3.9% (males 15-24) |
Health care payer | Hypothetical HIV vaccination provided to adolescent students |
| Moodley et al. (2016) [69] | Semi-Markov simulation | South Africa | Adolescents girls enrolled in school | Lifetime | 10.2% (females 15-24) 3.9% (males 15-24) |
Health care payer | National implementation of hypothetical HIV vaccination to adolescents |
| Wall et al. (2018) [70] | Cost-benefit analysis and cost-effectiveness analysis | Zambia | HIV serodiscordant couples | 5 years | 11.5% | Donor | Couples’ testing and counselling with TasP for seropositive partner |
| Behavior change | |||||||
| Enns et al. (2011) [71] | Stochastic network simulation | Eswatini, Tanzania, Uganda, Zambia | 15-49 yo, general population | 10 years | 27.4% (Eswatini) 4.7% (Tanzania) 7.1% (Uganda) 11.5% (Zambia) |
Program | Concurrency reduction campaigns focused on behaviour change scenario: 1) increased monogamy, 2) high-risk partnership reduction, 3) untargeted partnership reduction |
| Structural | |||||||
| Fieno et al. (2014) [72] | Cost simulation | South Africa | Women aged 15-20 yo, bottom quarter of income distribution | 6 years | 18.8% | Health care payer | Cash transfers |
| Remme et al. (2014) [73] | Cost-benefit analysis and cost-effectiveness analysis | Malawi | Adolescent girls attending school | 18 months | 9.6% | Health care payer | Cash transfers |
| Rutstein et al. (2014) [74] | Decision-tree model | Malawi | 15-49 yo, partners of STI clinic indexes | 1 year | 9.6% | Health care payer | Partner notification |
World Bank 2017 HIV prevalence estimates
Health care payer perspective refers to costs incurred or saved by the governmental healthcare system; Donor perspective refers to costs incurred of saved by international donors; Program and service delivery perspective refers to costs incurred by a stakeholders implementing HIV program; Societal perspective refers to all of society regardless of the payer; Private perspective takes into account the costs incurred by service providers
Abbreviations: ANC = antenatal care clinic; ARV = antiretrovirals; ART = antiretroviral therapy; FSW = female sex worker; HAART = highly active antiretroviral therapy; KZN = KwaZulu-Natal, South Africa; MC = male circumcision; MMC = medical male circumcision; PMTCT = prevention of mother-to-child transmission; PrEP = pre-exposure prophylaxis; TasP = treatment as prevention; VMMC = voluntary medical male circumcision; WHO = World Health Organization; yo = years old.
We extracted and converted each study's reported cost-effectiveness measure and converted them to 2018 US$. Table 2 describes these measures. Most studies provided discounted results, with discounting ranging from 0%–5% for the base case scenario, as is standard in cost-effectiveness literature [37]. Outcome measures were presented as number of HIV infections averted (HIA) for a specific scenario, with fewer studies reporting quality-adjusted life years (QALYs) gained or disability-adjusted life years (DALYs) averted. A number of studies did not provide numerical values for cost-effectiveness measures but rather stated whether an intervention was a dominant (cost-savings with better outcomes) or dominated (costlier with poorer outcomes) strategy [55], [58], [67]. The most cost-effective interventions included -$8356 per HIA for a microbicide intervention in South Africa, −$312 per HIA for a PMTCT intervention in Malawi, and $470 per HIA for a VMMC intervention in Uganda [18], [49], [62].
Table 2.
Intervention cost and output results.
| Reference | Scenario | Outcome measure | Cost-effectiveness measure reported in publication (US$) | Cost-effectiveness measure (US$ 2018) | Discount rate | Country GDP per capita (current US$), 2018a[76] |
|---|---|---|---|---|---|---|
| VMMC | ||||||
| Binagwaho et al. (2010) [15] | Infants | 1288 HIAl | Cost-saving | -- | 3% | Rwanda: $800 ⋅ 21 |
| Adolescents | 1283 HIA | CERl = $3,932/HIA | $4,698/HIA | |||
| Adults | 859 HIA | CER = $4,949/HIA | $5,914/HIA | |||
| Njeuhmeli et al. (2011) [16] | 80% VMMC coverage in 13 countries | 9 VMMCs/1 HIA | $809/HIA | $927/HIA | NR | SSA: $1,620 ⋅ 00 |
| Uthman et al. (2011) [17] | All adult males | 15 ⋅ 5 DALYl averted/HIA | $-325/DALY averted (cost savings) | $-388/DALY averted | 3% | SSA: $1,620 ⋅ 00 |
| Duffy et al. (2013) [18] | Surgical circumcision method | NRm | $430/HIA | $470/HIA | NR | Uganda: $717 ⋅ 50 |
| PrePex circumcision method | NR | $580/HIA | $634/HIA | |||
| Menon et al. (2014) [19] | Scale-up and maintenance of 80% VMMC coverage | NR | $3,200/HIA | $3,668/HIA | 3% | Tanzania: $1,090 ⋅ 00 |
| Awad et al. (2015) [20] | Current VMMC scale-up program | 326,000 HIA 11 VMMCs/1 HIA (2010-2025) |
$1,010/HIA | $1,072/HIA | 3% | Zimbabwe: $1,270 ⋅ 00 |
| VMMC program with subpopulation prioritization | 10-53 VMMCs/1 HIA | $811-$5,518/HIA | $861-$5,861/HIA | |||
| Awad et al. (2015) [21] | Current VMMC scale-up program | 306,000 HIA 23 VMMCs/1 HIA (2010-2017) 12 VMMCs/1 HIA (2017-2025) |
$1,089/HIA | $1,156/HIA | 3% | Zambia: $1,145 ⋅ 00 |
| VMMC program with subpopulation prioritization | 11-36 VMMCs/1 HIA | $888-$3300/HIA | $943-$3505/HIA | |||
| Haacker et al. (2016) [22] | VMMC at 0 yo | 4 ⋅ 2 VMMCs/HIA | $859/HIA | $919/HIA | 5% | South Africa: $6,560 ⋅ 00 |
| VMMC at 20 yo | 4 ⋅ 4 VMMCs/HIA | $659/HIA | $705/HIA | |||
| VMMC at 55 yo | 214 ⋅ 2 VMMCs/HIA | $24,157/HIA | $25,846/HIA | |||
| Kripke et al. (2016) [23] | 60% coverage among 10-29 yo | 79 HIA | $5,100/HIA | $5,307/HIA | 3% | Malawi: $349 ⋅ 13 |
| 60% coverage among 10–34 yo | 92 HIA | $4,600/HIA | $4,786/HIA | |||
| 60% coverage among 10–49 yo | 106 HIA | $4,600/HIA | $4,786/HIA | |||
| 60% coverage among 15–49 yo | 104 HIA | $3,600/HIA | $3,746/HIA | |||
| 80% coverage among 15–49 yo | 148 HIA | $3,500/HIA | $3,642/HIA | |||
| Kripke et al. (2016) [24] | 80% Scenario: Scale up to 80% among 10-29 yo | 87,000 HIA | $4,800/HIA | $4,994/HIA | 3% | Zimbabwe: $1,270 ⋅ 00 |
| Base Scenario: Scale up to 80% among 10-19 yo | 63,000 HIA | $6,000/HIA | $6,243/HIA | |||
| Scenario A: 80% Scenario with 2x unit cost for 20-29 yo | 78,000 HIA | $6,600/HIA | $6,867/HIA | |||
| Scenario B: 80% Scenario with 2x unit costs for 20-24 yo and 3x unit costs for 25-29 yo | 83,000 HIA | $7,200/HIA | $7,492/HIA | |||
| Kripke et al. (2016) [25] | Actual VMMC performance through 2014 | 240,000 HIA (229,000, 572,000) | $4,400/HIA (median over 14 countries) | $4,578/HIA | 3% (costs only) | SSA: $1,620 ⋅ 00 |
| 80% coverage among 15-49 yo | 1,082,000 HIA (744,000, 1,839,000) | NR | -- | |||
| Kripke et al. (2016) [26] | 50% EIMC coverage/80% coverage among 10-24 yo | 20,000 HIA (14,000, 24,000) | $1,500/HIA ($1,100, $1,900) | $1,560/HIA ($1,144, $1,977) | 3% | Eswatini: $4,090 ⋅ 00 |
| 50% EIMC coverage/80% coverage among 10-29 yo | 27,000 HIA (19,000, 34,000) | $1,300/HIA ($900, $1,600) | $1,352/HIA ($936, $1,664) | |||
| 50% EIMC coverage/80% coverage among 10-34 yo | 29,000 HIA (21,000, 38,000) | $1,200/HIA ($900, $1,600) | $1,248/HIA ($936, $1,664) | |||
| Kripke et al. (2016) [27] | 80% coverage among 10-49 yo | Malawi: 149,000 HIA | $4,600/HIA | $4,600/HIA | Malawi: $349 ⋅ 13 South Africa: $6,560 ⋅ 00 Eswatini: $4,090 ⋅ 00 Tanzania: $1,090 ⋅ 00 Uganda: $717 ⋅ 50 |
|
| South Africa: 375,000 HIA | $2,700/HIA | $2,700/HIA | ||||
| Eswatini: 31,500 HIA | $1,200/HIA | $1,200/HIA | ||||
| Tanzania: 53,400 HIA | $5,800/HIA | $5,800/HIA | ||||
| Uganda: 486,000 HIA | $1,500/HIA | $1,500/HIA | ||||
| 80% coverage among 15-49 yo | Malawi: 148,000 HIA | $3,500/HIA | $3,500/HIA | |||
| South Africa: 372,000 HIA | $2,200/HIA | $2,200/HIA | ||||
| Eswatini: 32,200 HIA | $900/HIA | $900/HIA | ||||
| Tanzania: 50,500 HIA | $4,100/HIA | $4,266/HIA | ||||
| Uganda: 475,000 HIA | $1,100/HIA | $1,144/HIA | ||||
| 80% coverage among 15-24 yo | Malawi: 82,000 HIA | $4,300/HIA | $4,474/HIA | |||
| South Africa: 182,000 HIA | $2,500/HIA | $2,601/HIA | ||||
| Eswatini: 18,900 HIA | $1,000/HIA | $1,040/HIA | ||||
| Tanzania: 28,300 HIA | $4,900/HIA | $5,098/HIA | ||||
| Uganda: 241,000 HIA | $1,400/HIA | $1,456/HIA | ||||
| 80% coverage among 15-29 yo | Malawi: 109,000 HIA | $3,700/HIA | $3,850/HIA | |||
| South Africa: 246,000 HIA | $2,200/HIA | $2,289/HIA | ||||
| Eswatini: 25,700 HIA | $900/HIA | $936/HIA | ||||
| Tanzania: 36,200 HIA | $4,300/HIA | $4,474/HIA | ||||
| Uganda: 324,000 HIA | $1,200/HIA | $1,248/HIA | ||||
| 80% coverage among 15-34 yo | Malawi: 128,000 HIA | $3,500/HIA | $3,642/HIA | |||
| South Africa: 303,000 HIA | $2,100/HIA | $2,185/HIA | ||||
| Eswatini: 29,700 HIA | $900/HIA | $936/HIA | ||||
| Tanzania: 43,200 HIA | $4,000/HIA | $4,162/HIA | ||||
| Uganda: 388,000 HIA | $1,100/HIA | $1,144/HIA | ||||
| 80% coverage among 10-24 yo | Malawi: 83,000 HIA | $6,100/HIA | $6,347/HIA | |||
| South Africa: 190,000 HIA | $3,600/HIA | $3,746/HIA | ||||
| Eswatini: 19,600 HIA | $1,400/HIA | $1,456/HIA | ||||
| Tanzania: 31,300 HIA | $7,800/HIA | $8,116/HIA | ||||
| Uganda: 256,000 HIA | $2,100/HIA | $2,185/HIA | ||||
| 80% coverage among 10-29 yo | Malawi: 110,000 HIA | $5,100/HIA | $5,307/HIA | |||
| South Africa: 250,000 HIA | $3,000/HIA | $3,121/HIA | ||||
| Eswatini: 26,300 HIA | $1,200/HIA | $1,248/HIA | ||||
| Tanzania: 38,700 HIA | $6,800/HIA | $7,076/HIA | ||||
| Uganda: 337,000 HIA | $1,700/HIA | $1,769/HIA | ||||
| Njeuhmeli et al. (2016) [28] | Scale up of VMMC among adolescents | 266,000 HIA | $4,127/HIA | $4,415/HIA | 3% | Zimbabwe: $1,270 ⋅ 00 |
| Introduction of EIMC into existing VMMC program | 268,000 HIA | $5,256/HIA | $5,623/HIA | |||
| PrEP | ||||||
| Pretorius et al. (2010) [29] | Targeted PrEP for 25-35 yo women | NR | $12,500 - $20,000/HIA | $14,328 - $22,924/HIA | NR | South Africa: $6,560 ⋅ 00 |
| Hallett et al. (2011) [30] | PrEP always used after HIV diagnosis in serodiscordant couple | 15% - 52% HIA | $0 - $26,000/HIA | $0 - $28,944/HIA | 3% | South Africa: $6,560 ⋅ 00 |
| PrEP used up through ART initiation for HIV infected partner | 11% - 36% HIA | $-2,200 - $21,000/HIA | $-2,449 - $26,025/HIA | |||
| PrEP used only during periods of trying to conceive a pregnancy and during pregnancy | 1% - 2% HIA | $-6,000 - $8,000/HIA | $-6,679 - $8,906/HIA | |||
| Cremin et al. (2013) [31] | PrEP provided to 7.3% of uninfected 15-24 yo | 3 ⋅ 2% HIA | $10,540/HIA | $11,362/HIA | 3% | South Africa: $6,560 ⋅ 00 |
| PrEP provided to 4.4% of uninfected 15-54 yo | 3 ⋅ 6% HIA | $9,390/HIA | $10,122/HIA | |||
| Nichols et al. (2013) [32] | Non-prioritized PrEP | 2,333 HIA; 23,571 QALYsl gained |
$1,843/QALY gained | $2,051/QALY gained | 3% | Zambia: $1,145 ⋅ 00 |
| Prioritized PrEP | 3,200 HIA; 36,216 QALYs gained |
$323/QALY gained | $359/QALY gained | |||
| Verguet et al. (2013) [33] | PrEP intervention | 200 - 94,100 HIA 3,300 - 1,266,000 DALYs averted |
$550 - $44,600/DALY averted | $612 - $49,651/DALY averted | NR | SSA: $1,620 ⋅ 00 |
| Alistar et al. (2014) [34] | 10% Guidelines ART, 50% Focused PrEP | 1,837,744 HIA | CER = cost saving | CER = cost saving | 3% | South Africa: $6,560 ⋅ 00 |
| 10% Guidelines ART, 100% Focused PrEP | 3,084,508 HIA | CER = cost saving | CER = cost saving | |||
| 50% Guidelines ART, 100% General PrEP | 3,642,543 HIA | $163/QALY gained | $174/QALY gained | |||
| 100% Guidelines ART, 100% Focused PrEP | 3,840,111 HIA | $229/QALY gained | $245/QALY gained | |||
| 50% Universal ART, 100% Focused PrEP | 4,468,827 HIA | $276/QALY gained | $295/QALY gained | |||
| 100% Universal ART, 100% Focused PrEP | 4,663,411 HIA | $302/QALY gained | $323/QALY gained | |||
| 10% Guidelines ART, 50% General PrEP | 2,998,344 HIA | $1,172/QALY gained | $1,253/QALY gained | |||
| 10% Guidelines ART, 100% General PrEP | 3,381,214 HIA | $1,158/QALY gained | $1,239/QALY gained | |||
| Nichols et al. (2014) [35] | Treatment available at CD4 < 500 cells/μL | 3388 HIA; 40,643 QALYs gained |
CER = $62/QALY gained ($46–$75) ICER = $62/QALY gained ($46–$75) |
CER = $69/QALY gained ($51–$83) ICER = $69/QALY gained ($51–$83) |
3% | Zambia: $1,145 ⋅ 00 |
| Prioritized PrEP (most sexually active) | 1502 HIA; 13,611 QALYs gained |
CER = $4,103/QALY gained ($2,890–$5,803) ICERl = dominated |
CER = $4,567/QALY gained ($3,217 – $6,460) ICER = dominated |
|||
| Prioritized PrEP (mostly sexually active and treatment available at CD4 < 500 cells/μL) | 4494 HIA; 50,936 QALYs gained |
CER = $1,153/QALY gained ($686–$1,756) ICER = dominated |
CER = $1,283/QALY gained ($763–$1,954) ICER = dominated |
|||
| Non-prioritized PrEP (randomly distributed) | 4053 HIA; 40,318 QALYs gained |
CER = $3,730/QALY gained ($2,454–$5,691) ICER = dominated |
CER = $4,152/QALY gained ($2,731–$6,335) ICER = dominated |
|||
| Non-prioritized PrEP (randomly distributed and treatment available at CD4 < 500 cells/μL) | 5894 HIA; 67,835 QALYs gained |
CER = $2,253/QALY gained ($1,672–$3,188) ICER = dominated |
CER = $2,508/QALY gained ($1,861–$3,549) ICER = dominated |
|||
| Cremin et al. (2015) [36] | Standard PrEP intervention ($20 million budget) | 24,603 (~ 11%) HIA (3,750 - 49,450) |
$2,060 - $36,360/HIA | $2,293 - $40,478/HIA | 3% | South Africa: $6,560 ⋅ 00 |
| Cremin et al. (2015) [37] | All uninfected women eligible to receive PrEP | NR | $15,647/HIA | $17,419/HIA | 3% | Mozambique: $481 ⋅ 25 |
| Providing PrEP only to partners of miners | NR | $71,374/HIA | $79,458/HIA | |||
| Providing PrEP only to partners of miners and only during the last six weeks of the year | NR | $9,538/HIA | $10,618/HIA | |||
| Ying et al. (2015) [38] | 40% overall ART coverageb; 10% coverage for persons with CD4 350-500 cells/μL | 94,000 HIA | Ref. | -- | 3% | Uganda: $717 ⋅ 50 |
| Increase ART Coverage (50% coverage for persons with CD4 350-500 cells/μL) | 104,000 HIA | Dominated | -- | |||
| Targeted PrEP and ART to 90% serodiscordant couples | 120,000 HIA | $1,340/HIA | $1,466/HIA | |||
| Glaubius et al. (2016) [39] | Optimistic scenario, Non-prioritized PrEP |
1 ⋅ 6% - 9 ⋅ 1% HIA | $20,905 - $22,022/HIA $176,755 - $181,734/LYG |
$22,874 - $24,096/HIA $192,313 - $198,856/LYG |
3% | South Africa: $6,560 ⋅ 00 |
| Optimistic scenario, Age-prioritized PrEP |
2 ⋅ 9% - 17 ⋅ 2% HIA | $10,880 - $11,094/HIA $84,418 - $85,105/LYG |
$11,905 - $12,139/HIA $92,371 - $93,123/LYG |
|||
| Optimistic scenario, Risk-prioritized PrEP |
8 ⋅ 1% HIA | $11,094/HIA $85,105/LYG |
$12,139/HIA $93,123/LYG |
|||
| Conservative scenario, Non-prioritized PrEP |
1 ⋅ 0 - 5 ⋅ 5% HIA | $35,090 - $37,137/HIA $276,605 - $284,781/LYG |
$38,396 - $40,635/HIA $302,665 - $311,611/LYG |
|||
| Conservative scenario, Age-prioritized PrEP |
1 ⋅ 8 - 10 ⋅ 3% HIA | $18,429 - $19,213/HIA $133,428 - $135,695/LYG |
$20,165 - $21,023/HIA $145,999 - $148,479/LYG |
|||
| Conservative scenario, Risk-prioritized PrEP |
4 ⋅ 4% HIA | $1,242/HIA $11,568/LYG |
$1,359/HIA $12,657/LYG |
|||
| Walensky et al. (2016) [40] | Standard PrEP | 127 HIA | $10,100/HIA Cost saving (vs. no PrEP) |
$10,806/HIA | 3% | South Africa: $6,560 ⋅ 00 |
| Long-acting PrEP | 156 HIA | $12,400/HIA Cost saving (vs. no PrEP) |
$13,267/HIA | |||
| Cremin et al. (2017) [41] | 50% PrEP coverage to all FSW | NR | $65,160/HIA (95% CI: $43,520 - $95,250) | $66,404/HIA (95% CI: $44,351 - $97,069) | 0% | Kenya: $1,870 ⋅ 00 |
| 50% PrEP coverage to high-risk FSW | NR | $10,920/HIA (95% CI: $4,700 - $51,560) | $11,128/HIA (95% CI: $4,789 - $52,544) | |||
| TasP | ||||||
| Barnighausen et al. (2012) [42] | Coverage: 70% ART, 20% TasP, 45% MMCl | 650,000 HIA (compared to 50% ART and 45% MMC) | $7,157/HIA | $7,813/HIA | 3% | South Africa: $6,560 ⋅ 00 |
| Coverage: 80% ART, 40% TasP, 45% MMC | 1,000,000 HIA | $7,482/HIA | $8,186/HIA | |||
| Coverage: 80% ART, 60% TasP, 45% MMC | 1,100,000 HIA | $7,937/HIA | $8,684/HIA | |||
| Coverage: 80% ART, 80% TasP, 45% MMC | 1,260,000 HIA | $8,370/HIA | $9,158/HIA | |||
| Granich et al. (2012) [43] | ART initiation at CD4 count ≤ 350 cells/μL vs. ≤ 200 cells/μL | 200,000-1,400,000 HIA | NR | -- | 3% | South Africa: $6,560 ⋅ 00 |
| ART initiation at CD4 count < 500 cells/mm3 vs. ≤ 350 cells/μL | 200,000-1,500,000 HIA | $182/DALY averted | $199/DALY averted | |||
| ART initiation at all CD4 levels vs. CD4 count ≤ 500 cells/μL | 300,000-1,400,000 HIA | $1,381/DALY averted | $1,510/DALY averted | |||
| Smith et al. (2015) [44] | High ART cost | Low ART cost | 3% | South Africa: $6,560 ⋅ 00 | |||
| ART initiation at ≤ 200 cells/μL (vs. status quo) | 2,000 DALYs averted | $22,300/HIA | $12,900/HIA $1,230/DALY averted | $414/DALY averted |
$24,400/HIA | $14,115/HIA $1,345/DALY averted | $453/DALY averted |
|||
| ART initiation at ≤ 350 cells/μL | 3,100 DALYs averted | $10,400/HIA | $4,210/HIA $1,020/DALY averted | $788/DALY averted |
$11,379/HIA | $4,606/HIA $1,116/DALY averted | $851/DALY averted |
|||
| ART initiation at < 500 cells/μL | 3,300 DALYs averted | $8,910/HIA | $2,780/HIA $1,090/DALY averted | $342/DALY averted |
$9,749/HIA | $3,041/HIA $1,192/DALY averted | $374/DALY averted |
|||
| Universal ART | 3,300 DALYs averted | $8,190/HIA | $1,960/HIA $1,300/DALY averted | $310/DALY averted |
$8,961/HIA | $2,144/HIA $1,422/DALY averted | $339/DALY averted |
|||
| Bershteyn et al. (2016) [45] | Targeting 10-30 yo | NR | $6,238/HIA | $6,491/HIA | 3% | South Africa: $6,560 ⋅ 00 |
| Targeting 20-30 yo | NR | $5,031/HIA | $5,235/HIA | |||
| Targeting 22-27 yo | NR | $4,279/HIA | $4,452/HIA | |||
| Targeting 25-27 yo | NR | $3,967/HIA | $4,128/HIA | |||
| Targeting to full population | NR | $10,812/HIA | $11,250/HIA | |||
| Ying et al. (2016) [46] | Base case (36% of HIV-infected people achieving viral suppression) | Ref. | Ref. | -- | 3% | South Africa: $6,560 ⋅ 00 |
| Home HTC (48% of HIV-infected people achieving viral suppression) | 152,000 HIA | $3,290/HIA | $3,546/HIA | |||
| Home HTC + High Viral Load (60% ART uptake if CD4 > 350 cells/μL and VL > 10,000 copies/mL) | 183,000 HIA | $3,320/HIA | $3,579/HIA | |||
| Home HTC + CD4 (60% ART uptake if CD4 350–500 cells/μL) | 195,000 HIA | $2,960/HIA | $3,190/HIA | |||
| PMTCT | ||||||
| Halperin et al. (2009) [47] | Perinatal HIV transmission prevention program | 241,596 HIA | $543/HIA | $631/HIA by perinatal infection | NR | SSA: $1,620 ⋅ 00 |
| Services to prevent unintended pregnancies | 72,000 HIA | $359/HIA | $417/HIA by unintended pregnancy | |||
| Nakakeeto et al. (2009) [48] | Meeting UNGASSl targets for PMTCT by 2010 | NR | Burkina Faso: $2,292/HIA | $2,741/HIA | 3% | Burkina Faso: $734.03 Cameroon: $1,540 ⋅ 00 Cote d’Ivoire: $1,790 ⋅ 00 Malawi: $349 ⋅ 13 Rwanda: $800 ⋅ 21 Tanzania: $1,090 ⋅ 00 Zambia: $1,145 ⋅ 00 |
| Cameroon: $1,366/HIA | $1,633/HIA | |||||
| Cote d’Ivoire: $1,391/HIA | $1,663/HIA | |||||
| Malawi: $965/HIA | $1,154/HIA | |||||
| Rwanda: $1,085/HIA | $1,297/HIA | |||||
| Tanzania: $1,068/HIA | $1,277/HIA | |||||
| Zambia: $829/HIA | $991/HIA | |||||
| Orlando et al. (2010) [49] | PMTCT program with VCT, HAART, treatment of malnutrition, TB, malaria, STDs (private perspective) | 370 HIA 10,449 DALYs averted |
$998/HIA $35 ⋅ 36/DALY averted |
$1,193/HIA $42 ⋅ 30/DALY averted |
3% | Malawi: $349 ⋅ 13 |
| PMTCT program with VCT, HAART, treatment of malnutrition, TB, malaria, STDs (public perspective) | 370 HIA 10,449 DALYs averted |
$-261/HIA $-16 ⋅ 55/DALY averted |
$-312/HIA $-19 ⋅ 80/DALY averted |
|||
| Robberstad et al. (2010) [50] | Single-dose NVPl | 0 ⋅ 00051 HIA (per pregnancy) 0 ⋅ 0129 DALYs averted |
$26,826/HIA $1,071/DALY averted |
$20,749/HIA $1,227/DALY averted |
NR | Tanzania: $1,090 ⋅ 00 |
| PMTCT Plusc | 0 ⋅ 00267 HIA (per pregnancy) 0 ⋅ 067 DALYs averted |
$7,204/HIA $287/DALY averted |
$8,257/HIA $328/DALY averted |
|||
| Shah et al. (2011) [51] | Current PMTCT Coverage (10% of all HIV-infected women) | 1400 HIA | $3,620/HIA | $4,149/HIA | 3% | Nigeria: $2,050 ⋅ 00 |
| Current ANC Coverage (58% of HIV-infected women) | 7680 HIA | $3,203/HIA | $3,671/HIA | |||
| Full PMTCT Coverage (100% of HIV-infected women) | 14400 HIA | $3,167/HIA | $3,630/HIA | |||
| Kuznik et al. (2012) [52] | 18 months ART vs. sdNVPl | 5 ⋅ 21 DALYs averted | $46/DALY averted | $51/DALY averted | 3% | Uganda: $717 ⋅ 50 |
| 18 months ART vs. DTl | 3 ⋅ 22 DALYs averted | $99/DALY averted | $110/DALY averted | |||
| 18 months ART vs. no treatment | 8 ⋅ 58 DALYs averted | $34/DALY averted | $37/DALY averted | |||
| Lifetime ART vs. sdNVP | 19 ⋅ 2 DALYs averted | $205/DALY averted | $228/DALY averted | |||
| Lifetime ART vs. DT | 11 ⋅ 87 DALYs averted | $354/DALY averted | $394/DALY averted | |||
| Lifetime ART vs. no treatment | 31 ⋅ 6 DALYs averted | $172/DALY averted | $191/DALY averted | |||
| Binagwaho et al. (2013) [53] | Dual ARV + breastfeeding | NR | Dominated | -- | 3% | Rwanda: $800 ⋅ 21 |
| Dual ARV + replacement feeding | NR | Dominated | -- | |||
| Sc-HAART + 6 mo. breastfeeding | NR | Dominated | -- | |||
| Sc-HAART + 12 mo. breastfeeding | 9,837 HIV uninfected children still alive | -- | -- | |||
| Sc-HAART + 18 mo. breastfeeding | 9,292 HIV uninfected children still alive | ICER = $11,882/HIA (compared to 12 mo.) | $12,882/HIA | |||
| Sc-HAART + replacement feeding | NR | Dominated | -- | |||
| Fasawe et al. (2013) [54] | Current Practice | 4,503 HIA | $816/HIA $37/QALY gained |
$935/HIA $42/QALY gained |
3% | Malawi: $349 ⋅ 13 |
| Option A | 15,606 HIA | $844/HIA $37/QALY gained |
$967/HIA $42/QALY gained |
|||
| Option B | 15,997 HIA | $1,331/HIA $60/QALY gained |
$1,525/HIA $68/QALY gained |
|||
| Option B + | 15,997 HIA | $1,265/HIA $57/QALY gained |
$1,450/HIA $65/QALY gained |
|||
| Maredza et al. (2013) [55] | Increase coverage of extended NVP to infants (rural) | 220 DALYs averted | Dominant | Dominant | 3% | South Africa: $6,560 ⋅ 00 |
| Promote formula feeding (rural) | 420 DALYs averted | $1,300/DALY averted | $1,490/DALY averted | |||
| Promote breastfeeding (rural) | 160 DALYs averted | Dominant | -- | |||
| Increase coverage of extended NVP to infants (urban) | 90 DALYs averted | Dominant | -- | |||
| Promote formula feeding (urban) | 160 DALYs averted | Dominant | -- | |||
| Promote breastfeeding (urban) | -240 DALYs avertedd | $3,200/DALY averted | $3,667/DALY averted | |||
| Gopalappa et al. (2014) [56] | Option B + vs. Option A | NRe | Kenya: $6,015/ HIA South Africa: $22,987/HIA Zambia: $6,778/HIA |
Kenya: $6,763/HIA South Africa: $25,590/HIA Zambia: $7,545/HIA |
3% | Kenya: $1,870 ⋅ 00 South Africa: $6,560 ⋅ 00 Zambia: $1,145 ⋅ 00 |
| Ishikawa et al. (2014) [57] | Option B | 7,176 HIA | $1,023/HIA | $1,094/HIA | 3% | Zambia: $1,145 ⋅ 00 |
| Option B + | 7,318 HIA | $1,254/HIA | $1,341/HIA | |||
| Yu et al. (2014) [58] | Remedy cohortf | 110 infant HIA | Extended dominatedg | -- | 3% | South Africa: $6,560 ⋅ 00 |
| Remedy cohort, breastfeed | 421 infant HIA | Extended dominated | -- | |||
| Remedy cohort, replacement feed | 11 infant HIA | Extended dominated | -- | |||
| Promptly treated cohorth | 698 infant HIA | Undominatedi | -- | |||
| Promptly treated cohort, breastfeed | 360 infant HIA | Extended dominated | -- | |||
| Promptly treated cohort, replacement feed | 883 infant HIA | Undominated | -- | |||
| Zulliger et al. (2014) [59] | Rapid initiation of ART in Pregnancy pilot program | 16.88 QALYs saved | $1,160/QALY gained | $1,291/QALY gained | 3% | South Africa: $6,560 ⋅ 00 |
| Price et al. (2016) [60] | Oral PrEP at first ANC visit with HIV- test and end with breastfeeding cessation | 381 HIA | $965/DALY averted | $1,025/DALY averted | 3% | Zambia: $1,145 ⋅ 00 |
| Tweya et al. (2016) [61] | Option B + vs. Option B | 133 DALYs averted | $841/DALY averted | $875/DALY averted | 3% | Malawi: $349 ⋅ 13 |
| Other biomedical | ||||||
| Verguet et al. (2010) [62] | Access to condoms and microbicide effective at 55% | 1,908 HIA | $-6,712/HIA | $-8,356/HIA | NR | South Africa: $6,560 ⋅ 00 |
| Williams et al. (2011) [63] | Tenofovir 25% Coverage | 250,000 HIA (20,000 – 380,000) | $2,392/HIA ($562-$4,222) | $2,662/HIA ($625-$4,700) | 3% | South Africa: $6,560 ⋅ 00 |
| Tenofovir 90% Coverage | 1,100,000 HIA (60,000 – 2,040,000) | $1,701/HIA ($420-$2,982) | $1,893/HIA ($467-$3,319) | |||
| Long et al. (2013) [64] | Scale-up of VMMC to 75% of all men | 12 ⋅ 1% HIA | Cost-saving | -- | NR | South Africa: $6,560 ⋅ 00 |
| Tenofovir gel used by 50% of women | 14 ⋅ 1% HIA | $526/QALY gained | $602/QALY gained | |||
| Use of PrEP by 50% of all uninfected persons | 28 ⋅ 4% HIA | $9,009/QALY gained | $10,326/QALY gained | |||
| VMMC, microbicide, and PrEP | 43 ⋅ 5% HIA | $5,739/QALY gained | $6,578/QALY gained | |||
| Mbah et al. (2013) [65] | Praziquantel treatment received during childhood | 21,120 HIA | $259/HIA | $314/HIA | 3% | Zimbabwe: $1,270 ⋅ 00 |
| Praziquantel treatment received during childhood and FGSl prevalence is reduced relative to those who did not receive treatment | 41,500 HIA | $132/HIA | $174/HIA | |||
| Terris-Prestholt et al. (2014) [66] | 72% microbicide gel use consistency and 54% HIV efficacy | 55,366 HIA | $297/DALY averted | $392/DALY averted | 3% | South Africa: $6,560 ⋅ 00 |
| Mvundura et al. (2015) [67] | Distribution of 100,000 female condoms | 273 HIA | Lower Bound: Cost Savingsj Higher Bound: $154/DALY avertedk |
-- Higher Bound: $168/DALY averted |
NR | SSA: $1,620 ⋅ 00 |
| Moodley et al. (2016) [68] | HIV vaccine intervention for school-based adolescents | 4 ⋅ 36 QALYs gained in lifetime | $43/QALY gained | $47/QALY gained | 3% | South Africa: $6,560 ⋅ 00 |
| Moodley et al. (2016) [69] | 60% coverage at $12 per vaccine dose | NR | $4 ⋅ 98/LYG (95%: $2 ⋅ 77–$11 ⋅ 61) | $5 ⋅ 45/LYG (95%: $3 ⋅ 03–$12 ⋅ 70) | 3% | South Africa: $6,560 ⋅ 00 |
| Wall et al. (2018) [70] | Nationwide CVCT | 166,153 HIA | $394/HIA | $394/HIA | 0% | Zambia: $1,145 ⋅ 00 |
| TasP for serodiscordant couples identified by CVCT | 9,656 HIA | $7,930/HIA | $7,930/HIA | |||
| Population TasP for all HIV + cohabitating men and women identified by individual HTC | 17,872 HIA | $12,891/HIA | $12,891/HIA | |||
| Behaviour change | ||||||
| Enns et al. (2011) [71] | Increased monogamy | 77 (8 ⋅ 7%) HIA | NR | -- | 3% | Eswatini: $4,090 ⋅ 00 Tanzania: $1,090 ⋅ 00 Uganda: $717 ⋅ 50 Zambia: $1,145 ⋅ 00 |
| High-risk partnership reduction | 115 (11 ⋅ 7%) HIA | NR | -- | |||
| Untargeted partnership reduction | 76 (8 ⋅ 9%) HIA | NR | -- | |||
| Structural | ||||||
| Fieno et al. (2014) [72] | Cash transfer at $5 monthly benefit | 3,400 HIA | $1,650/HIA | $1,919/HIA | NR | South Africa: $6,560 ⋅ 00 |
| Cash transfer at $10 monthly benefit | 4,250 HIA | $2,640/HIA | $3,071/HIA | |||
| Cash transfer at $20 monthly benefit | 5,100 HIA | $4,400/HIA | $5,118/HIA | |||
| Remme et al. (2014) [73] | Long-term benefits of 18-month cash transfer trial | 93,600 HIV DALYs averted | $297/HIV DALY averted | $345/HIV DALY averted | NR | Malawi: $349 ⋅ 13 |
| Rutstein et al. (2014) [74] | Passive Referral | Ref. | Ref. | -- | NR | Malawi: $349 ⋅ 13 |
| Provider Notification | 27 ⋅ 5 HIA | ICER = $3,560/HIA | $4,080/HIA | |||
| Contract Notification | 0 ⋅ 4 HIA | ICER = $51,421/HIA | $58,941/HIA | |||
Country GDP estimates retrieved from International Monetary Fund, World Economic Outlook.
ART coverage means HIV treatment for people with CD4 < 350 cells/μL and TasP coverage means HIV treatment for people with CD4 ≥ 350 cells/μL.
PMTCT Plus refers to a HAART intervention for all HIV infected women during pregnancy and lactation, regardless of CD4 count, according to 2009 WHO guidelines.
Negative value indicates an intervention was less effective than base case.
Not reported for infant only infections averted.
Women in remedy cohort received HIV testing and standard treatment only after delivery.
Extended dominated excludes any intervention that has a higher ICER than more effective interventions.
Women in the promptly treated cohort received HIV testing and treatment at some point during pregnancy.
Undominated refers to strategies that are more cost-effective.
The intervention was cost-saving in the following countries: Botswana, South Africa, Eswatini, Zambia, Zimbabwe.
Cost($)/DALY averted for other included countries: Cameroon (43), Kenya (110), Lesotho (9), Malawi (114), Mozambique (154), Namibia (9), Tanzania (73), Uganda (25).
Abbreviations: DT = dual therapy (zidovudine and lamivudine); ANC = antenatal care clinic; ARV = antiretrovirals; ART = antiretroviral therapy; CI = confidence intervals; DALY = disability-adjusted life year; EIMC = early infant male circumcision; FGS = female genital schistosomiasis; FSW = female sex worker; HAART = highly-active antiretroviral therapy; HIA = HIV infections averted; LYG = life years gained; NVP = nevirapine; PMTCT = prevention of mother-to-child transmission; PrEP = pre-exposure prophylaxis; QALY = quality-adjusted life year; Sc-HAART = short-course highly-active antiretroviral therapy; sdNVP = single dose nevirapine; SSA = sub-Saharan Africa; STD = sexually transmitted disease; TB = tuberculosis; UNGASS = UN General Assembly Special Session on AIDS; VCT = voluntary counselling and testing; VMMC = voluntary medical male circumcision; yo = years old.
Abbreviations: NR = not reported; in certain instances, studies may have 1) reported cost-effectiveness measure without stating an effectiveness measure or 2) presented visualized cost-effectiveness results without stating the numeric value of the cost-effectiveness measure. These instances would lead to an ‘NR’.
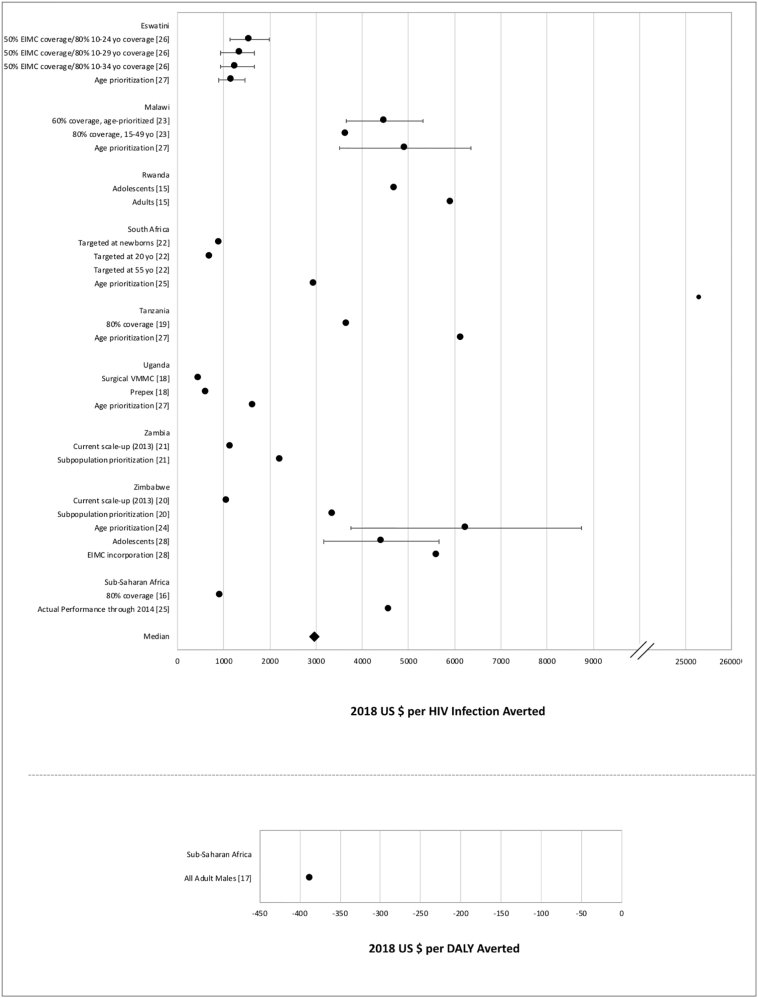
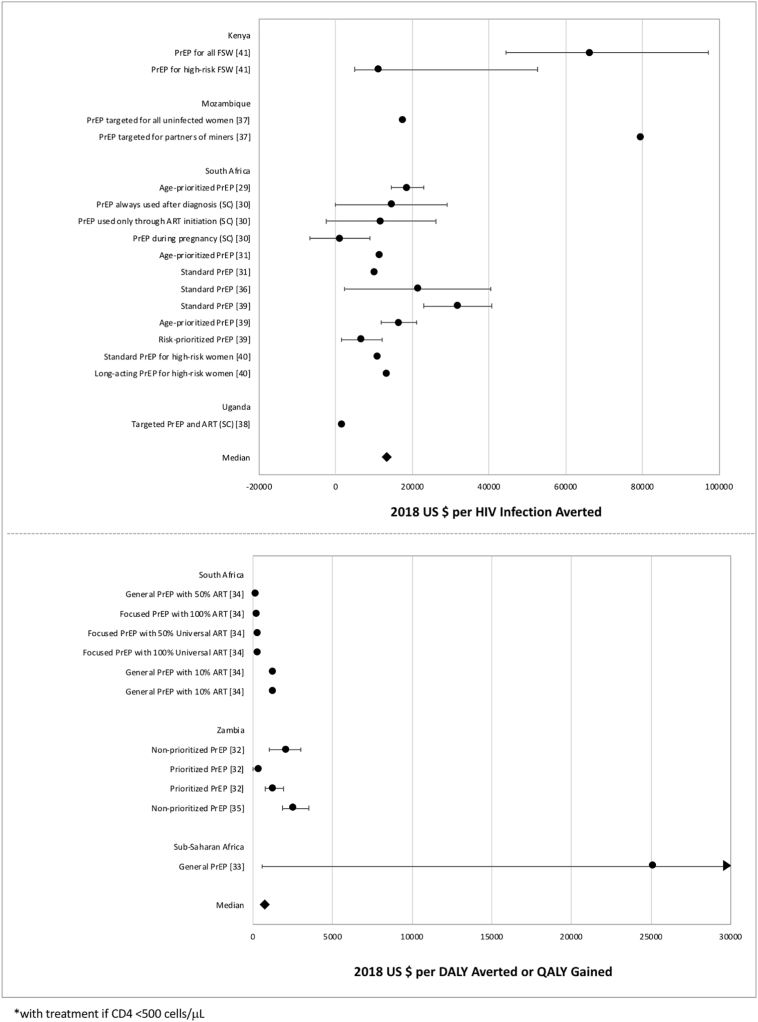
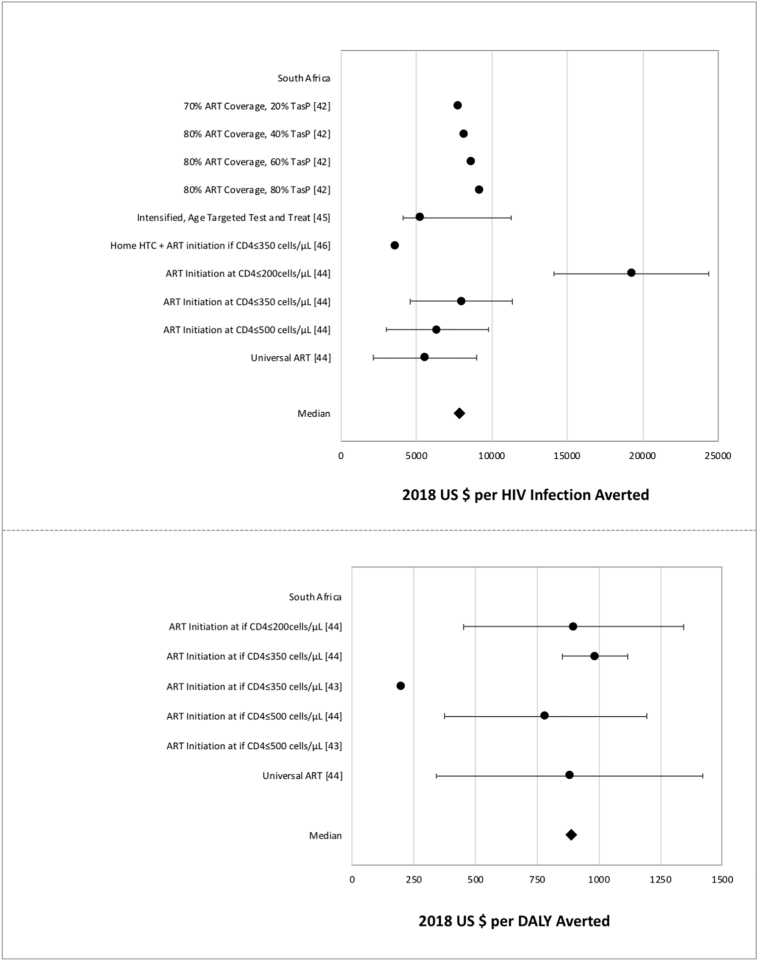
The median CERs for each intervention type were as follows: $2967 per HIA and $-388/DALY averted for VMMC, $13,267 per HIA and $799 per QALY gained for PrEP, $7903 per HIA and $890 per DALY averted for TasP, $1421 per HIA and $191 per DALY averted or QALY gained for PMTCT, $1143 per HIA and $392/DALY averted for other biomedical interventions (microbicides, vaccination, praziquantel treatment, combination prevention, condom distribution), and $3575/HIA and $345/DALY averted for structural interventions (partner notification, cash transfer programs). For several of the intervention types, scenarios that prioritized specific sub-populations based on age and/or risk factors were more cost-effective than scenarios that targeted the general population (Fig. 2, Fig. 3, Fig. 4, Fig. 5, Fig. 6, Fig. 7).
Fig. 2.
Cost-effectiveness measures of VMMC interventions.
Data points reflect the measures from VMMC studies reporting cost per HIV infection averted (above) and cost per DALY averted (below). Points represent study-specific cost-effectiveness estimates; error bars represent estimate ranges, if provided in study results.
Fig. 3.
Cost-effectiveness measures of PrEP interventions.
Data points reflect the measures from PrEP studies reporting cost per HIV infection averted (above) and cost per DALY averted or QALY gained (below). Points represent study-specific cost-effectiveness estimates; error bars represent estimate ranges, if provided in study results.
Fig. 4.
Cost-effectiveness measures of TasP interventions.
Data points reflect the measures from TasP studies reporting cost per HIV infection averted (above) and cost per DALY averted (below). Points represent study-specific cost-effectiveness estimates; error bars represent estimate ranges, if provided in study results.
Fig. 5.
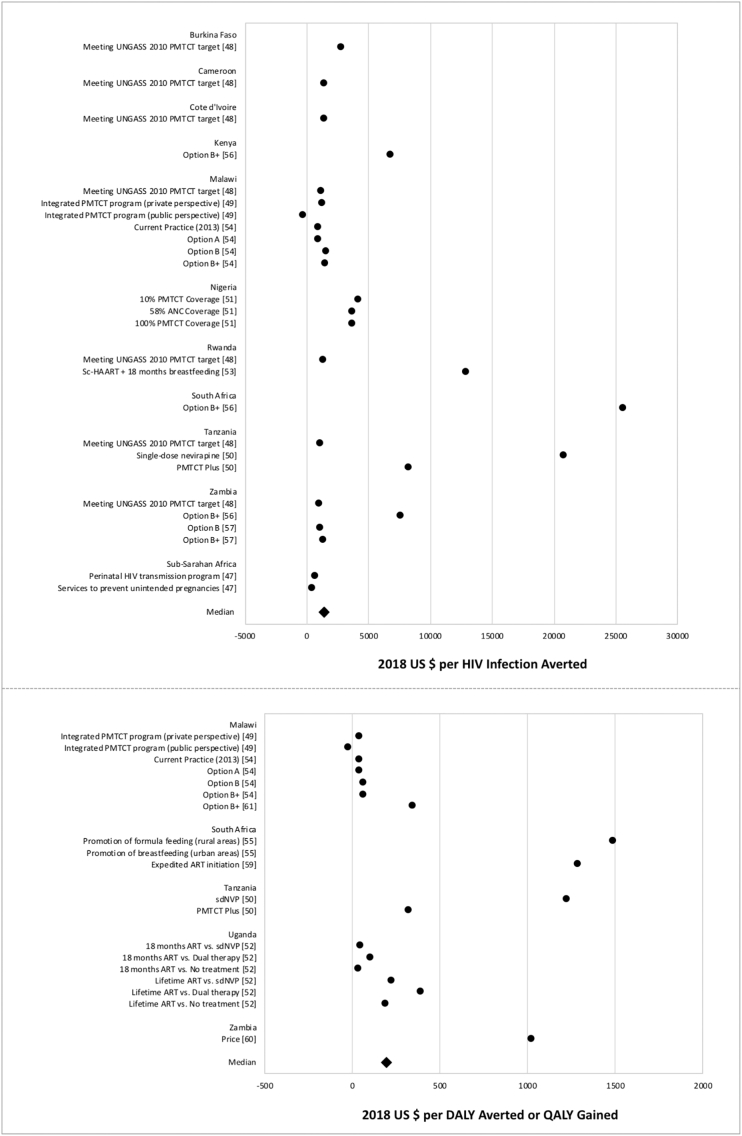
Cost-effectiveness measures of PMTCT interventions.
Data points reflect the measures from PMTCT studies reporting cost per HIV infection averted (above) and cost per DALY averted or QALY gained (below). Points represent study-specific cost-effectiveness estimates.
Fig. 6.
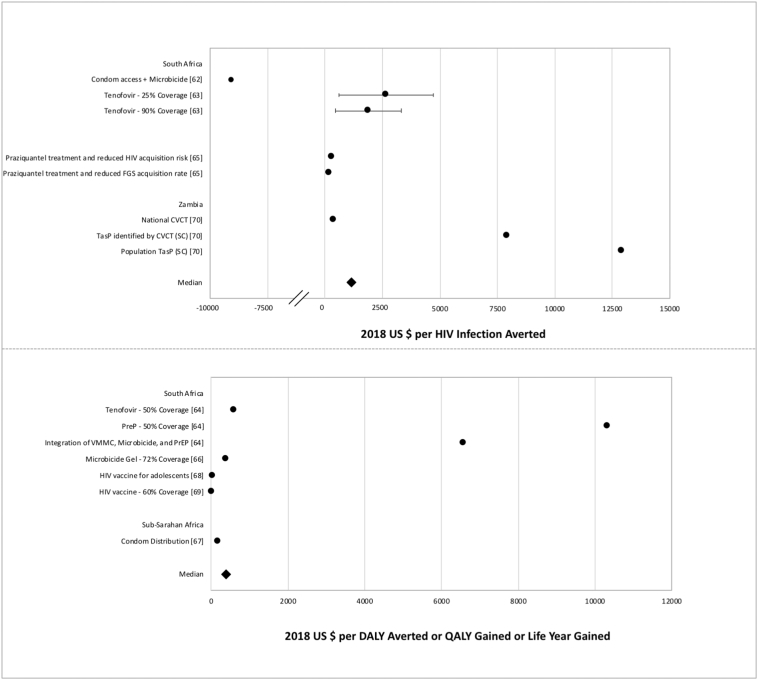
Cost-effectiveness measures of biomedical interventions.
Data points reflect the measures from miscellaneous biomedical studies reporting cost per HIV infection averted (above) and cost per DALY averted or QALY gained (below). Points represent study-specific cost-effectiveness estimates; error bars represent estimate ranges, if provided in study results.
Fig. 7.
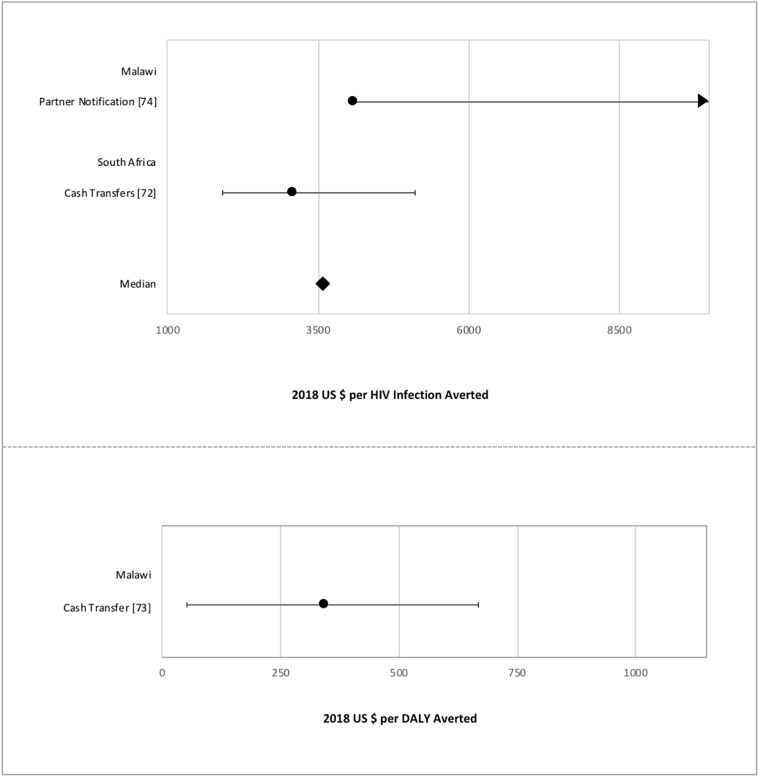
Cost-effectiveness measures of structural interventions.
Data points reflect the measures from structural intervention studies reporting cost per HIV infection averted (above) and cost per DALY averted (below). Points represent study-specific cost-effectiveness estimates; error bars represent estimate ranges, if provided in study results.
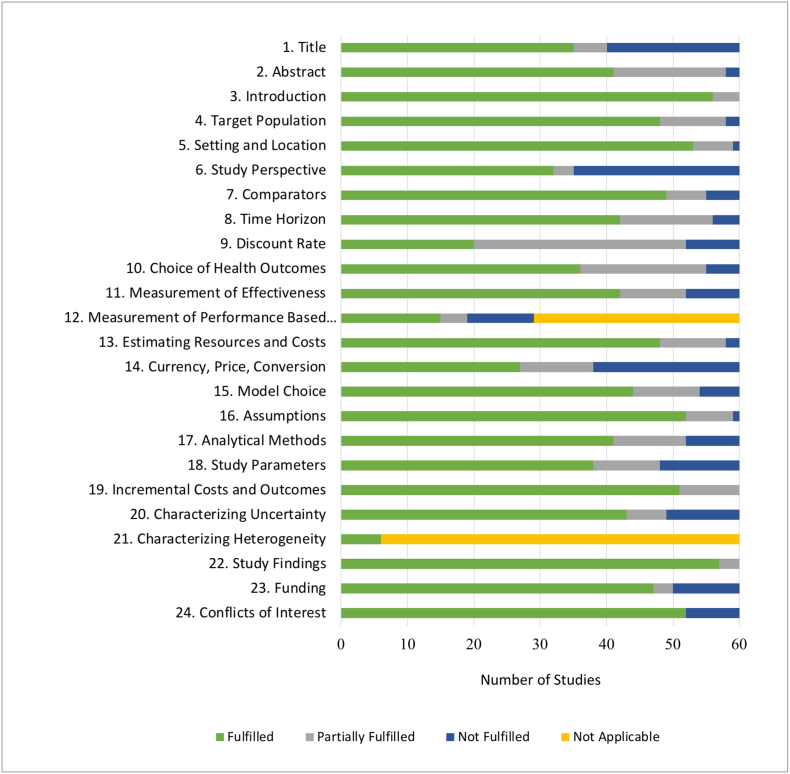
Table 3 and Fig. 8 provide the results of the quality assessment of each study using the CHEERS checklist.
Table 3.
CHEERS quality assessment, by intervention type.
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11a | 11b | 12 | 13a | 13b | 14 | 15 | 16 | 17 | 18 | 19 | 20a | 20b | 21 | 22 | 23 | 24 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Binagwaho et al. (2010) [15] | Y⁎ | Y | Y | Y | Y | Y | Y | Y | Y | N⁎ | Y | N/A⁎ | N/A | N/A | Y | N | P⁎ | Y | N | Y | Y | N/A | Y | N/A | Y | N | Y |
| Njeuhmeli et al. (2011) [16] | N | Y | Y | Y | Y | Y | N | Y | Y | Y | N/A | Y | N/A | N/A | Y | N | Y | Y | N | Y | Y | N/A | Y | N/A | Y | Y | Y |
| Uthman et al. (2011) [17] | Y | Y | Y | Y | Y | Y | Y | Y | P | Y | N/A | N | N/A | N/A | Y | Y | Y | Y | Y | Y | Y | N/A | Y | N/A | Y | N | N |
| Duffy et al. (2013) [18] | Y | Y | Y | Y | Y | Y | N | Y | Y | N | P | N/A | N/A | Y | N/A | Y | N | Y | P | P | Y | N/A | N/A | N/A | Y | N | Y |
| Menon et al. (2014) [19] | N | Y | Y | Y | Y | N | N | P | Y | P | N | N/A | N/A | Y | N/A | Y | Y | Y | N | Y | Y | N | N/A | N/A | Y | Y | Y |
| Awad et al. (2015) [20] | N | Y | Y | Y | Y | N | Y | Y | Y | Y | N/A | Y | N/A | N/A | Y | N | Y | Y | Y | N | Y | N/A | Y | N/A | Y | Y | Y |
| Awad et al. (2015) [21] | N | Y | Y | Y | Y | Y | Y | Y | Y | Y | N/A | Y | N/A | N/A | Y | N | Y | Y | Y | Y | Y | N/A | Y | N/A | Y | Y | Y |
| Haacker et al. (2016) [22] | Y | Y | Y | Y | Y | N | Y | N | Y | P | N/A | Y | N/A | Y | Y | Y | Y | Y | Y | N | Y | N/A | Y | N/A | Y | Y | Y |
| Kripke et al. (2016) [23] | P | P | Y | Y | Y | P | Y | Y | P | N | N/A | N | P | N/A | Y | N | Y | P | N | N | Y | N/A | P | Y | Y | Y | Y |
| Kripke et al. (2016) [24] | Y | P | Y | Y | Y | N | Y | Y | P | Y | N/A | Y | N/A | N/A | Y | P | P | P | P | P | P | N/A | Y | N/A | Y | Y | Y |
| Kripke et al. (2016) [25] | N | P | Y | P | P | N | N | P | P | P | N/A | N | N/A | N/A | Y | P | Y | P | P | N | Y | N/A | Y | Y | Y | Y | Y |
| Kripke et al. (2016) [26] | N | P | Y | Y | Y | N | P | Y | P | Y | N/A | N | N/A | N/A | Y | P | Y | Y | N | N | P | N/A | Y | Y | Y | Y | Y |
| Kripke et al. (2016) [27] | N | P | P | Y | Y | N | N | Y | P | Y | N/A | P | N/A | N/A | Y | Y | P | P | Y | N | Y | N/A | Y | Y | Y | Y | Y |
| Njeuhmeli et al. (2016) [28] | P | P | Y | P | Y | N | Y | Y | P | Y | N/A | P | N/A | N/A | Y | P | Y | P | P | N | Y | N/A | P | N/A | Y | Y | Y |
| PrEP | |||||||||||||||||||||||||||
| Pretorius et al. (2010) [29] | Y | Y | Y | Y | Y | N | Y | P | N | P | Y | N/A | N | N/A | P | N | Y | Y | Y | Y | P | N/A | Y | N/A | Y | Y | Y |
| Hallett et al. (2011) [30] | N | Y | Y | Y | P | N | Y | N | P | Y | N/A | Y | N | N/A | P | N | Y | Y | Y | Y | Y | N/A | P | N/A | Y | Y | Y |
| Cremin et al. (2013) [31] | N | P | Y | Y | Y | Y | Y | Y | P | P | N/A | Y | N/A | N/A | Y | Y | Y | Y | Y | Y | P | N/A | Y | N/A | Y | Y | Y |
| Nichols et al. (2013) [32] | Y | Y | Y | Y | Y | P | Y | P | P | P | N/A | P | N | N/A | P | N | Y | Y | Y | Y | Y | N/A | Y | N/A | Y | Y | Y |
| Verguet et al. (2013) [33] | N | Y | Y | P | Y | N | Y | Y | N | P | N/A | Y | Y | N/A | P | N | Y | Y | Y | Y | Y | N/A | Y | N/A | Y | N | Y |
| Alistar et al. (2014) [34] | Y | Y | Y | P | Y | N | Y | Y | P | P | N/A | Y | N | N/A | Y | N | Y | Y | Y | Y | Y | N/A | Y | N/A | Y | Y | Y |
| Nichols et al. (2014) [35] | Y | Y | Y | Y | Y | N | Y | Y | P | P | N/A | Y | N | N/A | P | N | P | Y | Y | P | Y | N/A | P | N/A | Y | Y | Y |
| Cremin et al. (2015a) [36] | N | N | Y | Y | Y | Y | Y | Y | P | P | Y | N/A | N/A | N/A | Y | N | Y | Y | P | P | Y | N/A | N | N/A | P | Y | Y |
| Cremin et al. (2015b) [37] | Y | Y | Y | Y | Y | Y | Y | Y | P | P | N/A | Y | N/A | N/A | P | N | Y | Y | N | Y | Y | N/A | N | N/A | P | Y | Y |
| Ying et al. (2015) [38] | Y | Y | Y | Y | Y | Y | Y | Y | P | P | Y | N/A | N | N/A | Y | Y | Y | N | P | N | P | N/A | N | N/A | Y | Y | Y |
| Glaubius et al. (2016) [39] | Y | Y | Y | Y | Y | Y | Y | Y | P | P | N/A | Y | N/A | N/A | Y | Y | P | Y | Y | Y | Y | N/A | Y | N/A | Y | Y | Y |
| Walensky et al. (2016) [40] | Y | Y | Y | Y | Y | Y | Y | Y | P | P | N/A | P | N/A | N/A | Y | Y | Y | Y | Y | Y | Y | N/A | Y | N/A | Y | Y | Y |
| Cremin et al. (2017) [41] | N | Y | Y | Y | Y | Y | Y | Y | P | P | N/A | Y | N/A | N/A | Y | P | Y | Y | Y | P | P | N/A | P | N/A | Y | Y | Y |
| TasP | |||||||||||||||||||||||||||
| Barnighausen et al. (2012) [42] | P | P | Y | P | Y | N | Y | Y | Y | P | N/A | Y | N/A | N/A | P | N | Y | Y | Y | N | Y | N/A | Y | N/A | Y | N | Y |
| Granich et al. (2012) [43] | Y | Y | Y | Y | Y | P | Y | Y | Y | Y | N/A | P | Y | N/A | Y | Y | Y | Y | Y | Y | Y | N/A | Y | N/A | Y | Y | Y |
| Smith et al. (2015) [44] | Y | Y | Y | Y | Y | Y | Y | Y | P | Y | N/A | Y | P | Y | N/A | P | Y | Y | Y | P | Y | N/A | Y | N/A | Y | Y | Y |
| Bershteyn et al. (2016) [45] | N | Y | Y | P | Y | N | P | P | P | N | N/A | N | N | N/A | P | N | Y | Y | Y | N | Y | N/A | Y | Y | Y | Y | Y |
| Ying et al. (2016) [46] | N | P | Y | Y | Y | Y | Y | P | P | P | Y | N/A | N | Y | N/A | Y | P | Y | Y | N | Y | Y | N/A | N/A | Y | Y | Y |
| PMTCT | |||||||||||||||||||||||||||
| Halperin et al. (2009) [47] | N | Y | Y | P | P | Y | P | N | N | Y | N/A | Y | N/A | N/A | Y | N | N | Y | Y | Y | Y | N/A | N | N/A | Y | Y | Y |
| Nakakeeto et al. (2009) [48] | P | P | P | P | P | Y | Y | P | P | Y | N/A | Y | N/A | N/A | Y | Y | Y | Y | N | Y | P | N/A | P | N/A | Y | Y | N |
| Orlando et al. (2010) [49] | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | N/A | Y | N/A | Y | Y | N | P | P | P | Y | N | N/A | N/A | Y | P | N |
| Robberstad et al. (2010) [50] | Y | Y | Y | Y | Y | N | Y | N | P | Y | N | N/A | Y | N/A | N | N | P | Y | P | P | P | Y | N/A | N/A | Y | Y | N |
| Shah et al. (2011) [51] | Y | P | Y | p | Y | Y | Y | Y | Y | N/A | N/A | Y | Y | N/A | Y | Y | Y | Y | Y | Y | Y | N/A | Y | N/A | Y | Y | Y |
| Kuznik et al. (2012) [52] | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | N/A | N | Y | N/A | Y | Y | Y | Y | Y | Y | Y | Y | N/A | N/A | Y | N | Y |
| Binagwaho et al. (2013) [53] | Y | P | Y | Y | Y | Y | Y | Y | Y | Y | N/A | Y | N/A | N/A | Y | P | N | Y | Y | Y | P | N/A | Y | Y | Y | N | N |
| Fasawe et al. (2013) [54] | Y | Y | Y | Y | Y | Y | Y | Y | P | Y | N/A | Y | P | N/A | Y | P | Y | Y | Y | Y | Y | N/A | Y | N/A | Y | P | N |
| Maredza et al. (2013) [55] | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | N/A | Y | Y | N/A | Y | Y | P | Y | Y | Y | Y | N/A | Y | N/A | Y | N | N |
| Gopalappa et al. (2014) [56] | N | Y | Y | Y | P | N | Y | Y | P | P | N/A | Y | N/A | N/A | Y | P | Y | Y | P | Y | Y | N/A | N | N/A | Y | Y | Y |
| Ishikawa et al. (2014) [57] | P | Y | Y | Y | Y | Y | Y | Y | P | Y | N/A | P | Y | N/A | Y | N | Y | Y | Y | Y | Y | N/A | Y | N/A | Y | Y | Y |
| Yu et al. (2014) [58] | Y | Y | Y | Y | Y | N | Y | Y | P | Y | N/A | Y | Y | N/A | Y | Y | Y | Y | Y | Y | Y | N/A | Y | N/A | Y | Y | Y |
| Zulliger et al. (2014) [59] | Y | Y | P | Y | Y | N | Y | Y | Y | Y | Y | N/A | Y | Y | N/A | Y | N | Y | P | Y | Y | Y | N/A | N/A | Y | Y | Y |
| Price et al. (2016) [60] | Y | Y | Y | Y | Y | Y | Y | P | P | Y | N/A | Y | Y | N/A | Y | Y | P | Y | Y | Y | Y | N/A | Y | N/A | Y | Y | Y |
| Tweya et al. (2016) [61] | Y | Y | Y | Y | Y | N | Y | P | P | Y | N/A | Y | N | N/A | P | P | Y | Y | Y | Y | Y | N/A | Y | N/A | Y | Y | Y |
| Other biomedical | |||||||||||||||||||||||||||
| Verguet et al. (2010) [62] | Y | Y | Y | Y | P | N | Y | Y | Y | Y | N/A | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | N/A | Y | N/A | Y | N | Y |
| Williams et al. (2011) [63] | N | P | Y | N | N | N | P | P | N | Y | Y | N/A | P | N/A | Y | N | Y | Y | Y | N | Y | N/A | N | N/A | Y | Y | Y |
| Long et al. (2013) [64] | Y | Y | Y | Y | Y | N | P | Y | N | Y | N/A | Y | Y | Y | N | P | Y | Y | Y | Y | Y | N/A | Y | N/A | Y | Y | Y |
| Mbah et al. (2013) [65] | Y | P | Y | Y | Y | Y | Y | P | Y | Y | N/A | Y | N/A | Y | Y | Y | Y | Y | Y | Y | Y | N/A | Y | N/A | P | Y | Y |
| Terris-Prestholt et al. (2014) [ 66] | Y | Y | Y | Y | Y | Y | Y | Y | P | Y | N/A | Y | N | Y | Y | Y | Y | Y | Y | Y | Y | N/A | Y | N/A | Y | Y | Y |
| Mvundura et al. (2015) [67] | Y | Y | Y | N | Y | N | Y | Y | N | Y | N/A | P | N/A | N/A | Y | Y | Y | Y | N | P | Y | N/A | N | N/A | Y | P | Y |
| Moodley et al. (2016) [68] | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | N/A | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | N/A | Y | N/A | Y | Y | Y |
| Moodley et al. (2016) [69] | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | N/A | N/A | N/A | Y | Y | Y | Y | Y | Y | Y | N/A | Y | N/A | Y | Y | Y |
| Wall et al. (2018) [70] | N | P | Y | P | Y | Y | Y | Y | Y | Y | P | N/A | N/A | Y | N/A | N | N | P | Y | P | Y | Y | Y | N/A | Y | Y | Y |
| Behaviour Change | |||||||||||||||||||||||||||
| Enns et al. (2011) [71] | Y | Y | Y | Y | Y | Y | Y | Y | P | P | N/A | Y | N/A | N/A | P | N | Y | Y | Y | Y | Y | N/A | Y | N/A | Y | Y | Y |
| Structural | |||||||||||||||||||||||||||
| Fieno et al. (2014) [72] | N | N | P | Y | Y | N | P | P | N | Y | Y | N/A | N/A | Y | N/A | N | P | Y | P | Y | Y | N | N/A | N/A | Y | N | Y |
| Remme et al. (2014) [73] | N | P | Y | Y | Y | Y | Y | P | P | Y | P | N/A | Y | Y | N/A | Y | Y | Y | Y | Y | Y | Y | N/A | N/A | Y | Y | Y |
| Rutstein et al. (2014) [74] | Y | P | Y | Y | Y | Y | Y | P | N | Y | Y | N/A | N/A | Y | N/A | Y | Y | Y | Y | Y | Y | Y | N/A | N/A | Y | Y | N |
Abbreviations: Y = item completely fulfilled; P = item partially fulfilled; N = item not fulfilled; N/A = item not applicable to the study
Item Checklist: 1. Title; 2. Abstract; 3. Introduction 4. Target Population; 5. Setting and Location; 6. Study Perspective; 7. Comparators; 8. Time Horizon; 9. Discount Rate; 10. Choice of health outcomes; 11a. Measurement of effectiveness (single study-based estimates); 11b. Measurement of effectiveness (synthesis-based estimates); 12. Measurement of performance based outcomes; 13a. Estimating Resources and Costs (single study-based economic evaluation); 13b. Estimating Resources and Costs (model-based economic evaluation); 14. Currency, Price, Conversion; 15. Model Choice; 16. Assumptions; 17. Analytical Methods; 18. Study Parameters; 19. Incremental Costs and Outcomes; 20a. Characterizing Uncertainty (single study-based economic evaluation); 20b. Characterizing Uncertainty (model-based economic evaluation); 21. Heterogeneity; 22. Study Findings; 23. Funding; 24. Conflicts of Interest
Fig. 8.
Visual representation of CHEERS checklist evaluation.
Green bars represent the number of studies that completely fulfilled the corresponding item of the CHEERS checklist. Blue bars represent the number of studies that did not fulfill an applicable item. Gray bars represent the number of studies that partially, but did not completely, fulfilled the CHEERS checklist item. Yellow bars represent number of studies for which the item was not applicable.
4. Discussion
This review summarizes the evidence to date on recent studies of the cost-effectiveness of HIV prevention interventions and serves as an SSA-specific update to the 2009 review by Galarraga et al. [9] Results from this review illustrate that established interventions, such as VMMC and PMTCT, remain cost-effective, as previously found in the 2009 review. For newer prevention strategies, such as PrEP and TasP, many of the studies relied on various assumptions and scenarios that may not reflect reality.
The review found that PMTCT and VMMC interventions were the most cost-effective. Studies on PMTCT interventions, including HAART, infant feeding methods, expedited ART, and Option B + suggest that these strategies are very cost-effective [47], [49], [50], [54], [56], [57], [59]. These studies provide evidence supporting WHO guidelines of transitioning from Option A and of recommending PMTCT Option B and Option B +. When WHO began the policy transition from Option B to Option B + in 2013, the agency conducted a preliminary cost analysis to estimate the incremental cost of switching to the new policy [75]. The authors argued that researchers should develop additional cost-effectiveness models to appropriately evaluate the cost of the policy with programmatic data. A number of studies have since provided evidence supporting the policy decisions around Option B + [56], [57]. However, stakeholders should be mindful that implementation of strategies like Option B + raises concerns since many of these studies do not take into account initial costs and upfront investment required to scale up PMTCT programs to a level that can be considered cost-effective over an extended time period [61], [75]. Additionally, while the majority of PMTCT studies included in this review focused on Prong III, only one study addressed PMTCT Prong II by studying the expansion of family planning services as a cost-effective method to avert HIV infections through the prevention of unintended pregnancies [47]. This focus may reflect recent programmatic shifts towards PMTCT Prong III and treatment of HIV infected women, even though family planning is effective in reducing MTCT.
The VMMC studies included in this review agreed that the intervention was cost-effective. Seven different studies developed models that estimated cost effectiveness of VMMC at 80% coverage, which is a common target for many HIV prevention programs; however, achieving this level of coverage is often not feasible in many settings [16], [19], [23], [24], [25], [26], [27]. Additional studies exploring cost effectiveness at various levels of VMMC coverage may help inform decision makers in areas where 80% coverage would be difficult to attain. Multiple studies explored scenarios targeting VMMC at different age groups, with a consensus that prioritizing younger males is more favourable and cost-saving compared to targeting the general male adult population [15], [22], [24].
Similarly, a common conclusion was that PrEP strategies targeting specific risk groups were more cost-effective than general PrEP strategies [29], [32], [34], [35], [39]. Four studies found that PrEP was most cost-effective when using a prioritization strategy aimed at young individuals who are most at-risk, including having more than four partners and reporting low condom use [32], [34], [39], [41]. The majority of included PrEP studies were set in South Africa, a country that could perhaps better absorb the higher costs of PrEP implementation compared with others in the region. However, three studies in Zambia and Mozambique agreed that prioritizing high-risk individuals would create the most effective scenario for PrEP implementation, adding to the evidence that a targeted PrEP strategy could be feasible across country settings [32], [35], [36].
The assumption of 100% PrEP coverage considered in many studies may be difficult to implement [11], [14]. This scenario implies that every eligible individual would receive PrEP, which may not be realistic in settings where universal treatment has not even been realized. Many studies point out that achieving such a high level of PrEP coverage would be less cost-effective than simply increasing ART coverage. Accordingly, WHO issued recommendations in 2015 to provide PrEP as a prevention option to individuals at substantial risk of acquiring HIV in settings with high HIV incidence [76]. Although studies have shown that PrEP can be cost-effective when targeted towards high-risk groups and when assuming high adherence, it remains a challenging intervention due to high costs, ethical issues, and inequitable distribution [8].
The five studies included in this review were not in agreement with regard to the cost-effectiveness or the feasibility of TasP strategy; one study concluded that TasP was less cost-effective than a combination of VMMC and ART, which is already the standard practice in many sub-Saharan African settings [42]. From this review, it is unclear whether or not TasP would be more cost-effective in certain settings over others. Despite this uncertainty, many countries have already developed and implemented guidelines for TasP and universal test-and-treat (UTT) [77]. Healthcare investment to provide UTT services successfully is substantial, especially in extensive resource-constrained settings [78].
This review also included studies that explored cost-effectiveness of methods that are still in development and not currently available on the market, including long-acting PrEP injections, HIV vaccines, and microbicide gels. The findings from these studies suggest that these interventions would be cost-effective once accessible [62], [63], [64], [66], [68], [69]. Only one study included in this review considered the reduction of HIV incidence by estimating the intervention effect of schistosomiasis treatment. Mbah et al. showed that mass praziquantel administration would be a cost-effective approach to reduce HIV transmission. In addition to its affordability, praziquantel treatment is very safe, well tolerated, and easily administered, but it has not been explicitly considered as a HIV prevention intervention, as the link between HIV acquisition and schistosomiasis remains unclear [65].
The vast majority of the included studies determined cost-effectiveness based on the WHO-CHOICE guidance that considers interventions to cost-effective if the cost per DALY averted is between one and three times the study country's GDP per capita [79]. This threshold is becoming increasingly contested, as many experts believe that it does not consider governments' ability to generate the appropriate resources or willingness to pay [80], [81]. Some studies have translated HIA to DALYs; we did not find a standard conversion that would be applicable to the various country settings [17]. Moreover, the usefulness of this type of threshold is especially important when discussing high cost interventions, such as PrEP and TasP. Although these prevention strategies may be considered cost-effective under certain assumptions, this may not always translate into feasible implementation. The GDP-based threshold is unrelated to national and donor HIV budgets, both of which are needed to understand an intervention's affordability. Thus, more information is need on whether many SSA countries would be able to implement a large-scale PrEP program, although its use as a main prevention strategy has been heavily emphasized in policy discussion [8]. Many countries are already struggling to provide universal ART, and adding a high-cost strategy may apply further pressure on resource limited prevention programs.
In the 2009 review, Galarraga et al. concluded that not enough information regarding cost-effectiveness of many prevention strategies existed for decision-making or policy change [9]. Their review included many cost-effectiveness studies on interventions for behaviour change, intravenous drug use (IDU) harm reduction, and information, education, and communication. The present review found only two studies on behaviour change and structural interventions, with most recently published studies focusing on biomedical interventions. This shift represents a reflection of changing priorities of the international donor community and emerging technology available from pharmaceutical companies. The authors mentioned the lack of cost-effectiveness studies on vulnerable groups, such as men who have sex with men (MSM) and female sex workers (FSW). Similarly, the current review found only one study focusing on FSWs, although there are published studies on these populations in settings outside of Africa [66], [82]. The continuing dearth of studies on these vulnerable populations in sub-Saharan Africa ought to be addressed by future research, as costing studies can inform policymaking.
Several limitations were recognized while conducting this review. First, behavioral and structural interventions, like partner concurrency reduction and condom use, have historically been included in HIV prevention programs [71]. Although the studies in this paper suggest that these strategies are cost-effective, most analyses do not separate the effect of behaviour change on HIV incidence from other interventions, thus not allowing us to understand the effectiveness of these interventions in isolation [67], [71]. Second, comparability across studies was difficult since parameters, settings, and assumptions vary. Unless studies present cost-effectiveness estimates using the same assumptions, base year, time horizon, and discount rate, we should take caution when comparing study estimates.
Third, many of the cost-effectiveness studies offer evidence for a specific intervention in a number of scenarios, but few address the potential effects of an intervention in scenarios outside the scope of the study. This makes it difficult to generalize a study's results to other country settings, creating an obstacle for policy makers in determining how and when a single intervention is the most cost-effective for a specific country. The limited geographic coverage among the studies additionally does not allow for broad generalizability. South Africa was the setting in 24 of the 60 studies (40%) and just three countries (South Africa, Zambia, and, Malawi) comprise over 60% of the studies.
Lastly, this review is not immune to publication bias. Studies that do not demonstrate interventions as cost-effective are less likely to be submitted to peer-reviewed journals or to be published by journals [83]. It is possible that some cost-effectiveness results of current HIV interventions are not available to key policy makers, which poses a large problem. Despite the aforementioned limitations, this review included studies of good quality, highlighting the strength of the available evidence.
The large number of studies included in this review reflects the increasing importance of considering cost-effectiveness as a factor in implementing HIV prevention interventions in sub-Saharan Africa. The studies demonstrated intervention cost-effectiveness under a variety of scenarios and emphasized interventions targeting high-risk populations. In contrast to the 2009 Galarraga review, which concluded that sufficient cost-effectiveness data did not exist to inform large-scale decision making, the results from emergent, more robust and varied costing studies may serve as an aid to inform evidence-based decisions. Key stakeholders, such as international donors and government agencies, should consider cost-effectiveness results and affordability when developing national guidelines and protocols for HIV prevention to maximize prevention impact under resource constraints. However, important gaps in the research persist: a lack of focus on vulnerable populations remains an important concern in this region, and additional studies that discuss the cost-effectiveness of different combinations of interventions are needed to reflect the reality of HIV programs in this region [84].
Role of Funding Source
The contents are the responsibility of the authors and do not necessarily reflect the views of sponsors, who had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The corresponding author had full access to all of the data in the study and had final responsibility for the decision to submit for publication.
Contributors
KW conceived the study. SS and SE conducted the literature search. PK, SE, and SC conducted the data extraction. SS wrote the first draft, and KW, PC, SE, SC, and PK reviewed and provided feedback. All authors approved the final paper.
Declaration of Interests
The authors declare that they do not have any competing interests.
Acknowledgements
Funding: National Institutes of Health (K01 MH107320), Laney Graduate School at Emory University, and Emory Center for AIDS Research (P30AI050409).
Footnotes
Supplementary material to this article can be found online at https://doi.org/10.1016/j.eclinm.2019.04.006.
Appendix A. Supplementary Material
Supplementary material
References
- 1.AIDSInfo UN joint programme on HIV/AIDS (UNAIDS). Trends of new HIV infections. http://aidsinfo.unaids.org/ Available from:
- 2.Programmatic update - antiretroviral treatment as prevention (TASP) of HIV and TB. HIV/AIDS programme. World Health Organization; 2012. [Google Scholar]
- 3.Prevention gap report: UN joint Programme on HIV/AIDS (UNAIDS) 2016. [Google Scholar]
- 4.HIV Investments . 2016. UN joint Programme on HIV/AIDS (UNAIDS) [Google Scholar]
- 5.Fast-track update on investments needed in the AIDS response: UN joint programme on HIV/AIDS (UNAIDS) 2016. [Google Scholar]
- 6.Get on the fast-track: The life-cycle approach to HIV: UN joint Programme on HIV/AIDS (UNAIDS) 2016. [Google Scholar]
- 7.Bautista-Arredondo S., Sosa-Rubi S.G., Opuni M. Assessing cost and technical efficiency of HIV prevention interventions in sub-Saharan Africa: the ORPHEA study design and methods. BMC Health Serv Res. 2014;14:599. doi: 10.1186/s12913-014-0599-9. [published Online First: 2014/01/01] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gomez G.B., Borquez A., Case K.K. The cost and impact of scaling up pre-exposure prophylaxis for HIV prevention: a systematic review of cost-effectiveness modelling studies. PLoS Med. 2013;10(3) doi: 10.1371/journal.pmed.1001401. [published Online First: 2013/04/05] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Galarraga O., Colchero M.A., Wamai R.G. HIV prevention cost-effectiveness: a systematic review. BMC Public Health. 2009;9:14. doi: 10.1186/1471-2458-9-S1-S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moher D., Liberati A., Tetzlaff J., Altman D.G., the PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8:336–341. doi: 10.1016/j.ijsu.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 11.United Nations Development Programme About Sub-Saharan Africa; Africa at a Turning Point. 2019. http://www.africa.undp.org/content/rba/en/home/regioninfo.html Available from:
- 12.Husereau D., Drummond M., Petrou S. Consolidated Health Economic Evaluation Reporting Standards (CHEERS)—explanation and elaboration: a report of the ISPOR Health Economic Evaluation Publication Guidelines Good Reporting Practices Task Force. Value Health. 2013;16(2):231–250. doi: 10.1016/j.jval.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 13.Statistics USBoL Consumer Price index. https://www.bls.gov/cpi/ updated June 29, 2016. Available from.
- 14.International Monetary Fund World economic outlook databases. 2018. https://www.imf.org/external/datamapper/ Available from:
- 15.Binagwaho A., Pegurri E., Muita J. Male circumcision at different ages in Rwanda: a cost-effectiveness study. PLoS Med. 2010;7(1) doi: 10.1371/journal.pmed.1000211. [published Online First: 2010/01/26] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Njeuhmeli E, Forsythe S, Reed J, et al. Voluntary medical male circumcision: modeling the impact and cost of expanding male circumcision for HIV prevention in eastern and southern Africa. PLoS Med 2011;8(11):e1001132. doi: 10.1371/journal.pmed.1001132 [[published Online First: 2011/12/06]]. [DOI] [PMC free article] [PubMed]
- 17.Uthman O.A., Popoola T.A., Yahaya I. The cost-utility analysis of adult male circumcision for prevention of heterosexual acquisition of HIV in men in sub-Saharan Africa: a probabilistic decision model. Value Health. 2011;14(1):70–79. doi: 10.1016/j.jval.2010.10.011. [DOI] [PubMed] [Google Scholar]
- 18.Duffy K., Galukande M., Wooding N. Reach and cost-effectiveness of the PrePex device for safe male circumcision in Uganda. PLOS ONE. 2013;8(5) doi: 10.1371/journal.pone.0063134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Menon V., Gold E., Godbole R. Costs and impacts of scaling up voluntary medical male circumcision in Tanzania. PLoS One. 2014;9(5) doi: 10.1371/journal.pone.0083925. [published Online First: 2014/05/08] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Awad S.F., Sgaier S.K., Ncube G. A reevaluation of the voluntary medical male circumcision scale-up plan in Zimbabwe. PLoS One. 2015;10(11) doi: 10.1371/journal.pone.0140818. [published Online First: 2015/11/04] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Awad S.F., Sgaier S.K., Tambatamba B.C. Investigating voluntary medical male circumcision program efficiency gains through subpopulation prioritization: insights from application to Zambia. PLoS One. 2015;10(12) doi: 10.1371/journal.pone.0145729. [published Online First: 2015/12/31] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haacker M., Fraser-Hurt N., Gorgens M. Effectiveness of and financial returns to voluntary medical male circumcision for HIV prevention in South Africa: an incremental cost-effectiveness analysis. PLoS Med. 2016;13(5):19. doi: 10.1371/journal.pmed.1002012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kripke K., Chimbwandira F., Mwandi Z., Matchere F., Schnure M., Reed J. Voluntary medical male circumcision for HIV prevention in Malawi: modeling the impact and cost of focusing the program by client age and geography. PLoS ONE. 2016;11(7):e0156521. doi: 10.1371/journal.pone.0156521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kripke K., Hatzold K., Mugurungi O., Ncube G., Xaba S., Gold E. Modeling impact and cost-effectiveness of increased efforts to attract voluntary medical male circumcision clients ages 20–29 in Zimbabwe. PLoS ONE. 2016;11(10):e0164144. doi: 10.1371/journal.pone.0164144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kripke K., Bock N., Dalal S., Hankins C., Stegman P., Samuelson J. Assessing progress, impact, and next steps in rolling out voluntary medical male circumcision for HIV prevention in 14 priority countries in eastern and southern Africa through 2014. PLOS ONE. 2016;11(7):e0158767. doi: 10.1371/journal.pone.0158767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kripke K., Okello V., Maziya V., Benzerga W., Mirira M., Gold E. Voluntary medical male circumcision for HIV prevention in Swaziland: modeling the impact of age targeting. PLoS ONE. 2016;11(7):e0156776. doi: 10.1371/journal.pone.0156776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kripke K., Opuni M., Schnure M., Sgaier S., Castor D., Reed J. Age targeting of voluntary medical male circumcision programs using the decision makers' program planning toolkit (DMPPT) 2.0. PLoS ONE. 2016;12(3):e0174466. doi: 10.1371/journal.pone.0156909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Njeuhmeli E., Stegman P., Kripke K., Mugurungi O., Ncube G., Xaba S. Modeling costs and impacts of introducing early infant male circumcision for long-term sustainability of the voluntary medical male circumcision program. PLOS ONE. 2016;11(7):e0159167. doi: 10.1371/journal.pone.0159167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pretorius C., Stover J., Bollinger L. Evaluating the cost-effectiveness of pre-exposure prophylaxis (PrEP) and its impact on HIV-1 transmission in South Africa. PLoS One. 2010;5(11) doi: 10.1371/journal.pone.0013646. [published Online First: 2010/11/17] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hallett TB, Baeten JM, Heffron R, et al. Optimal uses of antiretrovirals for prevention in HIV-1 serodiscordant heterosexual couples in South Africa: a modelling study. PLoS Med 2011;8(11):e1001123. doi: 10.1371/journal.pmed.1001123 [[published Online First: 2011/11/24]]. [DOI] [PMC free article] [PubMed]
- 31.Cremin I., Alsallaq R., Dybul M. The new role of antiretrovirals in combination HIV prevention: a mathematical modelling analysis. Aids. 2013;27(3):447–458. doi: 10.1097/QAD.0b013e32835ca2dd. [published Online First: 2013/01/09] [DOI] [PubMed] [Google Scholar]
- 32.Nichols B.E., Boucher C.A., van Dijk J.H. Cost-effectiveness of pre-exposure prophylaxis (PrEP) in preventing HIV-1 infections in rural Zambia: a modeling study. PLoS One. 2013;8(3) doi: 10.1371/journal.pone.0059549. [published Online First: 2013/03/26] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Verguet S., Stalcup M., Walsh J.A. Where to deploy pre-exposure prophylaxis(prep) in sub-saharan Africa. Sex Transm Infect. 2013;89(8):628–634. doi: 10.1136/sextrans-2012-050891. [DOI] [PubMed] [Google Scholar]
- 34.Alistar S.S., Grant P.M., Bendavid E. Comparative effectiveness and cost-effectiveness of antiretroviral therapy and pre-exposure prophylaxis for HIV prevention in South Africa. BMC Med. 2014;12:46. doi: 10.1186/1741-7015-12-46. [published Online First: 2014/03/19] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nichols B.E., Baltussen R., van Dijk J.H. Cost-effectiveness of PrEP in HIV/AIDS control in Zambia: a stochastic league approach. J Acquir Immune Defic Syndr. 2014;66(2):221–228. doi: 10.1097/QAI.0000000000000145. [published Online First: 2014/04/04] [DOI] [PubMed] [Google Scholar]
- 36.Cremin I., Morales F., Jewell B.L. Seasonal PrEP for partners of migrant miners in southern Mozambique: a highly focused PrEP intervention. J Int AIDS Soc. 2015;18(4 Suppl 3):19946. doi: 10.7448/IAS.18.4.19946. [published Online First: 2015/07/23] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cremin I., Hallett T.B. Estimating the range of potential epidemiological impact of pre-exposure prophylaxis: run-away success or run-away failure? Aids. 2015;29(6):733–738. doi: 10.1097/QAD.0000000000000591. [DOI] [PubMed] [Google Scholar]
- 38.Ying R., Sharma M., Heffron R., Celum C.L., Baeten J.M., Katabira E. Cost-effectiveness of pre-exposure prophylaxis targeted to high-risk serodiscordant couples as a bridge to sustained ART use in Kampala, Uganda. J Int AIDS Soc. 2015;18(4 Suppl 3):20013. doi: 10.7448/IAS.18.4.20013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Glaubius R.L., Hood G., Penrose K.J. Cost-effectiveness of injectable preexposure prophylaxis for HIV prevention in South Africa. Clin Infect Dis. 2016;63(4):539–547. doi: 10.1093/cid/ciw321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Walensky R.P., Jacobsen M.M., Bekker L.G. Potential clinical and economic value of Long-acting preexposure prophylaxis for south African women at high-risk for HIV infection. J Infect Dis. 2016;213(10):1523–1531. doi: 10.1093/infdis/jiv523. [published Online First: 2015/12/19] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cremin I., McKinnon L., Kimani J., Cherutich P., Gakii G., Muriuki F. PrEP for key populations in combination HIV prevention in Nairobi: a mathematical modelling study. The Lancet HIV. 2017;4(5):e214–e222. doi: 10.1016/S2352-3018(17)30021-8. [DOI] [PubMed] [Google Scholar]
- 42.Barnighausen T, Eyal N, Wikler D. HIV treatment-as-prevention research at a crossroads. PLoS Med 2014;11(6) doi: ARTN e1001654 10.1371/journal.pmed.1001654. [DOI] [PMC free article] [PubMed]
- 43.Granich R., Kahn J.G., Bennett R. Expanding ART for treatment and prevention of HIV in South Africa: estimated cost and cost-effectiveness 2011–2050. PLoS One. 2012;7(2) doi: 10.1371/journal.pone.0030216. [published Online First: 2012/02/22] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Smith J.A., Sharma M., Levin C. Cost-effectiveness of community-based strategies to strengthen the continuum of HIV care in rural South Africa: a health economic modelling analysis. The lancet HIV. 2015;2(4):e159–e168. doi: 10.1016/S2352-3018(15)00016-8. [published Online First: 2015/04/07] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bershteyn A., Klein D.J., Eckhoff P.A. Age-targeted HIV treatment and primary prevention as a 'ring fence' to efficiently interrupt the age patterns of transmission in generalized epidemic settings in South Africa. Int Health. 2016;8(4):277–285. doi: 10.1093/inthealth/ihw010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ying R., Sharma M., Celum C., Baeten J.M., van Rooyen H., Hughes J.P. Home testing and counselling to reduce HIV incidence in a generalised epidemic setting: a mathematical modelling analysis. The lancet HIV. 2016;3(6) doi: 10.1016/S2352-3018(16)30009-1. (E275-E82) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Halperin D.T., Stover J., Reynolds H.W. Benefits and costs of expanding access to family planning programs to women living with HIV. Aids. 2009;23 doi: 10.1097/01.aids.0000363785.73450.5a. (S123-S30) [DOI] [PubMed] [Google Scholar]
- 48.Nakakeeto O.N., Umaranayake L. The global strategy to eliminate HIV infection in infants and young children: a seven-country assessment of costs and feasibility. Aids. 2009;23(8):987–995. doi: 10.1097/qad.0b013e32832a17e9. [DOI] [PubMed] [Google Scholar]
- 49.Orlando S., Marazzi M.C., Mancinelli S. Cost-effectiveness of using HAART in prevention of mother-to-child transmission in the DREAM-project Malawi. Jaids. 2010;55(5):631–634. doi: 10.1097/QAI.0b013e3181f9f9f5. [DOI] [PubMed] [Google Scholar]
- 50.Robberstad B., Evjen-Olsen B. Preventing mother to child transmission of HIV with highly active antiretroviral treatment in Tanzania—a prospective cost-effectiveness study. J Acquir Immune Defic Syndr. 2010;55(3):397–403. doi: 10.1097/QAI.0b013e3181eef4d3. [published Online First: 2010/08/27] [DOI] [PubMed] [Google Scholar]
- 51.Shah M., Johns B., Abimiku A., Walker D.G. Cost-effectiveness of new WHO recommendations for prevention of mother-to-child transmission of HIV in a resource-limited setting. Aids. 2011;25(8):1093–1102. doi: 10.1097/QAD.0b013e32834670b9. [DOI] [PubMed] [Google Scholar]
- 52.Kuznik A., Lamorde M., Hermans S. Evaluating the cost-effectiveness of combination antiretroviral therapy for the prevention of mother-to-child transmission of HIV in Uganda. Bull World Health Organ. 2012;90(8):595–603. doi: 10.2471/BLT.11.095430. [published Online First: 2012/08/16] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Binagwaho A., Pegurri E., Drobac P.C., Mugwaneza P., Stulac S.N., Wagner C.M. Prevention of mother-to-child transmission of HIV: cost-effectiveness of antiretroviral regimens and feeding options in Rwanda. Plos One. 2013;8(2):12. doi: 10.1371/journal.pone.0054180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fasawe O., Avila C., Shaffer N. Cost-effectiveness analysis of Option B + for HIV prevention and treatment of mothers and children in Malawi. PLoS One. 2013;8(3) doi: 10.1371/journal.pone.0057778. [published Online First: 2013/04/05] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Maredza M., Bertram M.Y., Saloojee H. Cost-effectiveness analysis of infant feeding strategies to prevent mother-to-child transmission of HIV in South Africa. Afr J AIDS Res. 2013;12(3):151–160. doi: 10.2989/16085906.2013.863215. [DOI] [PubMed] [Google Scholar]
- 56.Gopalappa C., Stover J., Shaffer N. The costs and benefits of Option B + for the prevention of mother-to-child transmission of HIV. AIDS. 2014;28:S5–S14. doi: 10.1097/QAD.0000000000000083. [DOI] [PubMed] [Google Scholar]
- 57.Ishikawa N., Shimbo T., Miyano S. Health outcomes and cost impact of the new WHO 2013 guidelines on prevention of mother-to-child transmission of HIV in Zambia. PLoS One. 2014;9(3) doi: 10.1371/journal.pone.0090991. [published Online First: 2014/03/08] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yu W., Li C., Fu X. The cost-effectiveness of different feeding patterns combined with prompt treatments for preventing mother-to-child HIV transmission in South Africa: estimates from simulation modeling. PLoS One. 2014;9(7) doi: 10.1371/journal.pone.0102872. [published Online First: 2014/07/24] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zulliger R., Black S., Holtgrave D.R. Cost-effectiveness of a package of interventions for expedited antiretroviral therapy initiation during pregnancy in Cape Town, South Africa. AIDS Behav. 2014;18(4):697–705. doi: 10.1007/s10461-013-0641-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Price J.T., Wheeler S.B., Stranix-Chibanda L. Cost-effectiveness of pre-exposure HIV prophylaxis during pregnancy and breastfeeding in Sub-Saharan Africa. J Acquir Immune Defic Syndr. 2016;72 doi: 10.1097/QAI.0000000000001063. (S145-S53) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tweya H., Keiser O., Haas A.D. Comparative cost-effectiveness of Option B + for prevention of mother-to-child transmission of HIV in Malawi. AIDS. 2016;30(6):953–962. doi: 10.1097/QAD.0000000000001009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Verguet S., Walsh J.A. Vaginal microbicides save money: a model of cost-effectiveness in South Africa and the USA. Sex Transm Infect. 2010;86(3):212–216. doi: 10.1136/sti.2009.037176. [published Online First: 2010/06/05] [DOI] [PubMed] [Google Scholar]
- 63.Williams B.G., Abdool Karim S.S., Karim Q.A., Gouws E. Epidemiological impact of tenofovir gel on the HIV epidemic in South Africa. J Acquir Immune Defic Syndr. 2011;58(2):207–210. doi: 10.1097/QAI.0b013e3182253c19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Long E.F., Stavert R.R. Portfolios of biomedical HIV interventions in South Africa: a cost-effectiveness analysis. J Gen Intern Med. 2013;28(10):1294–1301. doi: 10.1007/s11606-013-2417-1. [published Online First: 2013/04/17] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mbah M.L.N., Poolman E.M., Atkins K.E. Potential cost-effectiveness of schistosomiasis treatment for reducing HIV transmission in Africa - the case of Zimbabwean women. PLoS Negl Trop Dis. 2013;7(8):9. doi: 10.1371/journal.pntd.0002346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Terris-Prestholt F., Foss A.M., Cox A.P. Cost-effectiveness of tenofovir gel in urban South Africa: model projections of HIV impact and threshold product prices. BMC Infect Dis. 2014;14:14. doi: 10.1186/1471-2334-14-14. [published Online First: 2014/01/11] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mvundura M., Nundy N., Kilbourne-Brook M. Estimating the hypothetical dual health impact and cost-effectiveness of the Woman's Condom in selected sub-Saharan African countries. Int J Womens Health. 2015;7:271–277. doi: 10.2147/IJWH.S75040. [published Online First: 2015/03/19] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Moodley N., Gray G., Bertram M. Projected economic evaluation of the national implementation of a hypothetical HIV vaccination program among adolescents in South Africa, 2012. BMC Public Health. 2016;16(1):330. doi: 10.1186/s12889-016-2959-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Moodley N, Gray G, Bertram M. The case for adolescent HIV vaccination in South Africa: a cost-effectiveness analysis. Medicine 2016;95(4):e2528. doi: 10.1097/md.0000000000002528 [[published Online First: 2016/01/31]]. [DOI] [PMC free article] [PubMed]
- 70.Wall K., Inambao M., Kilembe W. HIV testing and counselling couples together for affordable HIV prevention in Africa. Int J Epidemiol. 2018;48(1):217–227. doi: 10.1093/ije/dyy203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Enns E.A., Brandeau M.L., Igeme T.K. Assessing effectiveness and cost-effectiveness of concurrency reduction for HIV prevention. International Journal of STD and AIDS. 2011;22(10):558–567. doi: 10.1258/ijsa.2011.010322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fieno J., Leclerc-Madlala S. The promise and limitations of cash transfer programs for HIV prevention. African journal of AIDS research: AJAR. 2014;13(2):153–160. doi: 10.2989/16085906.2014.943251. [DOI] [PubMed] [Google Scholar]
- 73.Remme M., Vassall A., Lutz B., Luna J., Watts C. Financing structural interventions: going beyond HIV-only value for money assessments. AIDS. 2014;28(3):425-34. doi: 10.1097/QAD.0000000000000076. [DOI] [PubMed] [Google Scholar]
- 74.Rutstein S.E., Brown L.B., Biddle A.K., Wheeler S.B., Kamanga G., Mmodzi P. Cost-effectiveness of provider-based HIV partner notification in urban Malawi. Health Policy Plan. 2014;29(1):115–126. doi: 10.1093/heapol/czs140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.O'Brien L., Shaffer N., Sangrujee N. The incremental cost of switching from Option B to Option B + for the prevention of mother-to-child transmission of HIV. Bull World Health Organ. 2014;92(3):162–170. doi: 10.2471/BLT.13.122523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.McGillen J.B., Anderson S.J., Hallett T.B. PrEP as a feature in the optimal landscape of combination HIV prevention in sub-Saharan Africa. J Int AIDS Soc. 2016;19(7(Suppl 6)):21104. doi: 10.7448/IAS.19.7.21104. [published Online First: 2016/10/21] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.World Health Organization Progress report 2016 - prevent HIV, test and treat all; WHO support for country impact. 2016. https://www.who.int/hiv/pub/progressreports/2016-progress-report/en/ Available from.
- 78.Iwuji C, Newell ML. Towards control of the global HIV epidemic: addressing the middle-90 challenge in the UNAIDS 90–90–90 target. PLoS Med 14(5): e1002293. 10.1371/journal.pmed.1002293. [DOI] [PMC free article] [PubMed]
- 79.World Health Organization Cost effectiveness thresholds. November 27 2013. https://www.who.int/choice/cost-effectiveness/en/ Available from.
- 80.Bertram M.Y., Lauer J.A., De Joncheere K. Cost-effectiveness thresholds: pros and cons. Bull World Health Organ. 2016;94(12):925–930. doi: 10.2471/BLT.15.164418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Woods B., Revill P., Sculpher M., Claxton K. Country-level cost-effectiveness thresholds: initial estimates and the need for further research. Value Health. 2016;19(8):929–935. doi: 10.1016/j.jval.2016.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rinaldi G., Kiadaliri A.A., Haghparast-Bidgoli H. Cost effectiveness of HIV and sexual reproductive health interventions targeting sex workers: a systematic review. Cost Eff Resour Alloc. 2018;16:63. doi: 10.1186/s12962-018-0165-0. 2018 Dec 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bell C.M., Urbach D.R., Ray J.G. Bias in published cost effectiveness studies: systematic review. BMJ. 2006;332(7543):699–703. doi: 10.1136/bmj.38737.607558.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Meyer-Rath G., van Rensburg C., Larson B., Jamieson L., Rosen S. Revealed willingness-to-pay versus standard cost-effectiveness thresholds: evidence from the South African HIV Investment Case. PLOS ONE. 2017;12(10) doi: 10.1371/journal.pone.0186496. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material