ABSTRACT
Background
Household food insecurity (HFI) is a stressor that is associated with type 2 diabetes (T2D). However, little is known about HFI and the insulin resistance (IR) underlying T2D, and the mechanisms involved.
Objective
We examined the cross-sectional association between HFI and IR among low-income Latinos with T2D and tested whether inflammation and stress hormones mediated this association.
Methods
HFI was measured with the 6-item US Household Food Security Survey module. IR was calculated from fasting plasma blood glucose and serum insulin. Inflammation was indicated by high-sensitivity C-reactive protein (hsCRP), and stress hormones included urinary cortisol, metanephrine, and normetanephrine. To test for an indirect effect of HFI on homeostasis model assessment of IR, a parallel multiple mediation model was run with biological markers that significantly differed between food security status—entered as mediators in the model. We used 95% bias-corrected bootstrap CIs, with 10,000 bootstrap samples, to assess the significance of the indirect effects.
Results
The 121 participants with T2D were primarily Puerto Rican (85.8%), aged mean = 60.7 y, and 74% were female. Eighty-two (68%) were classified as food insecure. Compared with food-secure individuals, food-insecure individuals had a significantly higher IR [mean difference (Δ) = 7.21, P = 0.001], insulin (Δ = 9.7, P = 0.019), glucose (Δ = 41, P < 0.001), hsCRP (Δ = 0.8, P = 0.008), cortisol (Δ = 21, P = 0.045), and total cholesterol (Δ = 29, P = 0.004). Groups did not differ on other lipids, metanephrine, normetanephrine, or A1c. The mediation model showed a significant direct effect of HFI on hsCRP (P = 0.020) and on cortisol (P = 0.011). There was a direct effect of cortisol (P = 0.013), hsCRP (P = 0.044), and HFI on IR (P = 0.015). The total combined indirect effect of HFI through cortisol and hsCRP indicated partial mediation.
Conclusions
Among Latinos with T2D, HFI is associated with IR partially through inflammation and stress hormones. Interventions to ameliorate HFI and mitigate its effects on inflammation, stress, and IR are warranted. This trial was registered at clinicaltrials.gov as NCT01578096.
Keywords: household food insecurity, insulin resistance, fasting blood glucose, Latinos, diabetes
Introduction
Food insecurity is the limited or uncertain availability of nutritionally adequate and safe foods or the limited or uncertain ability to acquire such foods in socially acceptable ways (that is, without resorting to emergency food supplies, scavenging, stealing, or other coping strategies) (1). In 2017, 15 million people in the United States lived in food-insecure households (2) and the rates were higher for Latinos (18%) than for white non-Hispanic households (8.8%) (2). Several studies have demonstrated an association between household food insecurity (HFI) and the spectrum of metabolic disorders: from overweight and obesity (3, 4) to metabolic syndrome (5) and overt type 2 diabetes (T2D) (6), all of which disproportionately affect Latinos (7–11). Rates of T2D are 12.1% among Hispanics compared with 7.4% for non-Hispanic whites (12).
T2D is a disorder characterized by progressive insulin resistance (IR) and β-cell loss resulting in hyperglycemia (13). Recent data have demonstrated that among individuals with extant diabetes, HFI is related to greater hyperglycemia, i.e., poorer glycemic control (14, 15). However, glycemic control is an aggregated indicator reflecting the net combination of various disease management factors including access to medical care and culturally appropriate diabetes education, quality of the prescribed diabetes regimen, affordability of therapeutic medications, and the patient's own diabetes self-management behaviors. Alternatively, IR is the underlying hallmark of T2D and is a more strictly biological indicator of T2D progression that is relatively less confounded by diabetes treatment.
Food insecurity may affect IR through various putative biological mechanisms (16, 17) that have not been elucidated. First, HFI is associated with dietary-related adiposity, including central adiposity (18). Second, HFI is a psychosocial stressor (19). Circulating stress hormones may contribute to the pathogenesis of IR. Cortisol, for example, is a glucocorticoid, a stress hormone regulated by the hypothalamic–pituitary–adrenal axis. It directs fat deposits viscerally, thereby increasing central adiposity and IR (20, 21). Metanephrine and normetanephrine are metabolites of epinephrine (adrenaline) and norepinephrine, which are catecholamines. They are stress hormones produced by the adrenal gland that promote gluconeogenesis and hepatic glucose release (22). Third, food insecurity may be associated with systemic inflammation through associated psychosocial stress (23), adiposity (24), or a proinflammatory diet (25). High-sensitivity C-reactive protein (hsCRP) is an inflammatory marker associated with IR (26).
To test the association and putative mediators between HFI and IR, we leveraged baseline data from the CALMS-D (Community health educators Assisting Latinos Manage Stress and Diabetes) randomized controlled trial (27–29) that studied Latinos with T2D. We hypothesized that 1) HFI would be associated with IR and 2) stress hormones, hsCRP, and central adiposity would partially mediate this association.
Methods
Setting and sample
Data were drawn from a parent study, CALMS-D. CALMS-D was approved by the Institutional Review Boards of all institutions involved (29).
Participants were Hartford residents, age ≥18 y, Latino or Hispanic, Spanish-speaking, ambulatory, with T2D for ≥6 mo, and with a most recent past year glycated hemoglobin (HbA1c) score >8.0. Participants were excluded for major medical conditions; and uncontrolled, or recent changes to the treatment of, psychiatric disorders, as described in detail elsewhere (29, 30).
Procedures
As previously reported (19, 29), participants were recruited from an urban outpatient clinic at Hartford Hospital, serving primarily low-income Latinos. Self-reported information was entered into REDCap databases (31). Using standard urine collection supplies and technique, participants collected and kept refrigerated all their urine over a 24-h period. Blood draws were performed by a community phlebotomist in participants’ homes. Urine and blood samples were delivered immediately to the UConn Health Center for processing and analysis. Participants were paid $10 for completing surveys and $10 for the blood draw.
Measures
Descriptive demographic, socioeconomic, and clinical characteristics
Demographic information included age, gender, and marital status. Socioeconomic information included income and education. Serum blood lipids were assayed with spectrophotometry at the UConn John Dempsey Hospital Laboratory. HbA1c was measured in the UConn clinical laboratory using HPLC. In this laboratory, HbA1c shows the following CVs for normal and high values: Level 1 mean = 5.51%, CV = 3.3 based on n = 320; and Level 2 mean = 9.01%, CV = 3.2 based on n = 304.
Independent variable: food security status
Food security status was assessed using the 6-item US Household Food Security Survey module, short form (32, 33). The sum of affirmative responses produces a scale score (0–6). Higher scores indicate greater food insecurity. Recent evidence indicates people in households with “marginal food security,” usually classified as food secure in the US government's prevalence estimates, may also face an increased likelihood of impaired health and nutrition (34). For this reason, we defined scores = 0 as food secure and scores ≥1 as food insecure, the latter including marginal, low, and very low food security levels. In our sample, Cronbach's α for the food security scale was 0.89.
Dependent variable: IR
Log-transformed HOMA-IR (logHOMA-IR) was calculated from fasting plasma glucose and serum insulin values, according to the method described by Matthews et al. (35). Higher HOMA-IR values indicate higher IR. Fasting plasma glucose was analyzed at the UConn John Dempsey Hospital Laboratory using the LXI R system by Beckman Coulter. Fasting serum insulin was analyzed by the UConn Clinical Research Center laboratory using a solid-phase chemiluminescent immunometric assay using the Immulite 1000 Analyzer by Siemens Healthcare Diagnostics. The insulin assay has a median range of 8.9 uIU/mL, sensitivity of 2 uIU/mL, intra-assay CV of 3.6%, and interassay CV of 2.5%.
Potential mediators
Stress hormones
Urinary cortisol, metanephrine, and normetanephrine were measured from 24-h urine samples. The UConn Clinical Research Center laboratory used kits from Alpco Diagnostics. The cortisol assay has a normal range of 50–190 μg/24 h, sensitivity of 2.0 ng/mL, intra-assay CV of 7.4%, and interassay CV of 7.8%. The metanephrine assay has a normal range of <350 μg/d, sensitivity of 13 ng/mL, intra-assay CV of 8.9%, and interassay CV of 12%. The normetanephrine assay has a normal range of <600 μg/d, sensitivity of 23 ng/mL, intra-assay CV of 17.5%, and interassay CV of 20.4%.
hsCRP, an acute-phase reactant that reflects acute and chronic inflammatory processes, was analyzed by a solid-phase chemiluminescent immunometric assay using the Immulite 1000 Analyzer by Siemens Healthcare Diagnostics at the UConn Clinical Research Center laboratory. The hsCRP assay has a normal range of <3 mg/L, sensitivity of 0.1 mg/L, intra-assay CV of 3.3%, and interassay CV of 4.5%.
Anthropometrics
Following recommended procedures (36), waist-to-hip ratio (WHR) was calculated as waist in centimeters divided by hip in centimeters, measured by trained study staff to the nearest centimeter with calibrated equipment, with tape parallel to the floor. Waist circumference was measured at the midpoint between the lower margin of the last palpable rib and the top of the iliac crest. Hip circumference was measured at the widest portion of the buttocks. All measurements were taken twice and averaged.
Statistical analyses
An independent-samples t test was used to compare food-secure with food-insecure groups on the biological markers. For markers showing excessive positive skew (>2), a log transformation was performed. Any outliers (z >3) after the transformation were removed and then the t test was conducted on the log-transformed variable. This resulted in 3 participants being removed owing to excessive values on 2 cortisol readings and 1 hsCRP reading. To test for an indirect effect of food insecurity on HOMA-IR, a parallel multiple mediation model was run with the relevant biological markers that significantly differed between food-secure and -insecure participants entered as mediators in the model. A parallel mediation model includes >1 mediator in the model and simultaneously tests each indirect effect associated with each mediator. Because the sampling distribution for an indirect effect is nonnormally distributed (37), 95% bias-corrected bootstrap CIs, with 10,000 bootstrap samples, were used to assess the significance of the indirect effects. Descriptive statistics were performed in IBM SPSS Statistics for Windows, Version 24 and the mediation model was run in Mplus version 7.3 (38). A power analysis was performed for the parent study, the CALMS-D randomized controlled trial; however, no a priori power analysis was conducted for the secondary data analysis reported here (29).
Results
Of the 121 participants, 85.8% were Puerto Rican, 90 (74%) were female, and the mean ± SD age of all participants was 60.7 ± 11.6 y. Most (77%) had less than a high school education, did not work (90%), lived with a partner (69%), and had a monthly income <$1000 (66%). The sample is described by food security status in detail elsewhere (19).
Figure 1 shows the frequency distribution of the 6-item food security scale. Thirty-nine (32%) of the 121 participants responded “no” to all 6 questions and were classified as food secure. The remaining 82 (68%) responded “yes” to ≥1 of the questions and were classified as food insecure. Of these, 23 (28% among those affirming the item, or 19% of the total sample) responded yes to all 6 questions.
FIGURE 1.
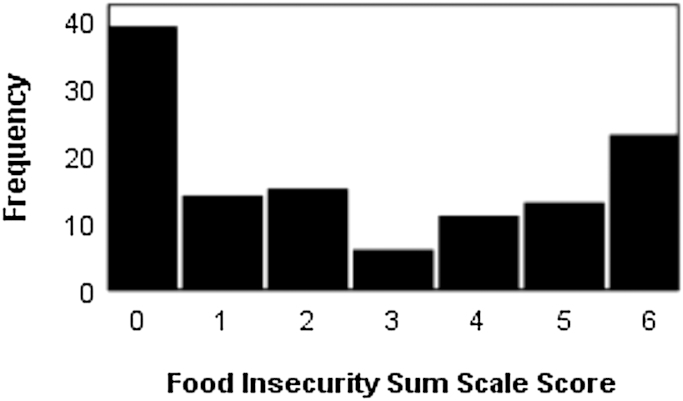
Frequency distribution of endorsement of the 6 food insecurity questions by Latinos with type 2 diabetes.
Table 1 shows the mean ± SD for the biological markers by food security group. Compared with food-secure individuals, those who were food insecure had significantly higher concentrations of total cholesterol (P = 0.004), hsCRP (P = 0.008), cortisol (P = 0.045), insulin (P = 0.019), and glucose (P < 0.001), and significantly higher HOMA-IR (P = 0.001). WHR tended to be greater in the food-insecure group than in the food-secure group (P = 0.058), whereas they did not differ on HbA1c or metanephrine, normetanephrine, HDL cholesterol, LDL cholesterol, or TGs.
TABLE 1.
Biological markers by food security status in Latinos with type 2 diabetes1
| Outcome | Food secure (n = 39) | Food insecure (n = 82) | P |
|---|---|---|---|
| Urinary metanephrine, μg/d* | 68.4 ± 39.8 | 87.9 ± 58.4 | 0.107 |
| Urinary normetanephrine, μg/d* | 312 ± 174 | 372 ± 209 | 0.190 |
| Serum total cholesterol, mg/dL | 159 ± 42 | 188 ± 53 | 0.004 |
| Serum HDL cholesterol, mg/dL | 42.5 ± 10.0 | 45.3 ± 12.8 | 0.318 |
| Serum LDL cholesterol, mg/dL | 90.0 ± 33.1 | 101.3 ± 31.1 | 0.134 |
| TGs, mg/dL* | 147 ± 94 | 202 ± 169 | 0.148 |
| Serum hsCRP, mg/dL* | 9.7 ± 26.1 | 10.5 ± 12.9 | 0.008 |
| Urinary cortisol, μg/d | 88.8 ± 39.4 | 109.8 ± 57.2 | 0.045 |
| Waist-to-hip ratio | 0.94 ± 0.07 | 0.98 ± 0.10 | 0.058 |
| Serum insulin, uIU/mL* | 21.4 ± 18.0 | 32.1 ± 25.2 | 0.019 |
| Plasma glucose, mg/dL | 143 ± 48 | 184 ± 61 | <0.001 |
| HOMA-IR* | 8.2 ± 10.7 | 15.3 ± 14.5 | 0.001 |
| HbA1c, % | 8.46 ± 1.90 | 8.69 ± 1.65 | 0.498 |
1Values are means ± SD or %. *An independent-samples t test was used to compare food-secure with food-insecure groups on the biological markers. t Test on log-transformed variable. HbA1c, glycated hemoglobin.
Figure 2 shows the standardized path diagram of the multiple mediation model. There was a significant direct effect of food insecurity on cortisol (P = 0.011) and hsCRP (P = 0.020). Further, there were direct effects of cortisol on HOMA-IR (P = 0.013), hsCRP on HOMA-IR (P = 0.044), WHR on HOMA-IR (P = 0.039), and food insecurity on HOMA-IR (P = 0.015). The 95% bootstrap CI for the mediation effect of cortisol did not contain zero (0.005, 0.103) and likewise the 95% bootstrap CI for the mediation effect of hsCRP did not include zero (0.001, 0.116). Thus, there was evidence for parallel mediation. The lower limit of the 95% bootstrap CI for the mediation effect of WHR included zero (0.000, 0.094). The estimate of the total combined indirect effect of food insecurity through cortisol, hsCRP, and WHR was 0.11 and smaller than the direct effect of food insecurity (B = 0.19), which indicates partial mediation rather than full mediation.
FIGURE 2.
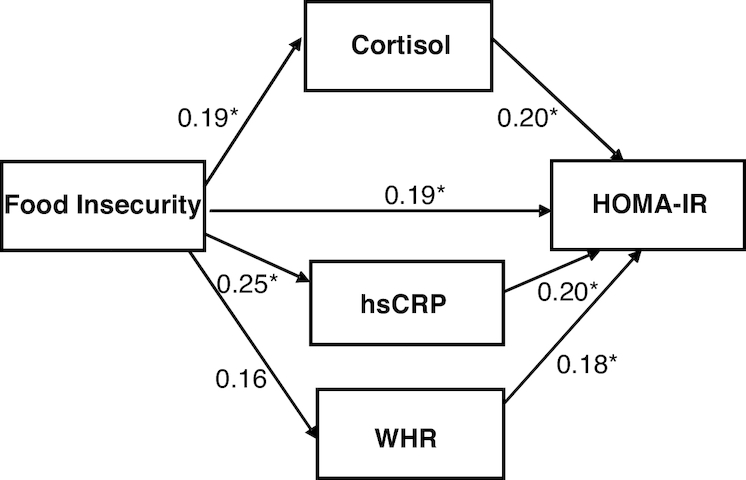
Standardized path diagram of parallel multiple mediation model between food insecurity and insulin resistance in Latinos with type 2 diabetes. *P < 0.05. hsCRP, high-sensitivity C-reactive protein; WHR, waist-to-hip ratio.
Discussion
The main findings from this study of Latinos with T2D are that 1) food insecurity is associated with elevated IR, the basic pathology that is the hallmark of T2D; 2) the association between food insecurity and IR is partially mediated by inflammation and stress hormones; and 3) central adiposity may also help explain the association between HFI and IR, although findings for WHR as a mediator were only marginally significant.
Individuals who lived in food-insecure households in the past year had higher IR than those who were food secure. This finding shows an association between HFI and underlying T2D disease progression and extends our understanding of reports that food insecurity is tied to worse glycemic control as measured by HbA1c (14, 15). This sample did not show difference in A1c by food insecurity status. Our pattern of findings, i.e., associations with IR but not A1c, underscores that IR is less confounded by treatment than is A1c. The participants in this study were all recruited from the same clinic that serves low-income, inner-city Latinos, so were likely exposed to a similar quality of diabetes treatment, which may help to explain the lack of findings for A1c.
Various complex mechanisms might partially explain the association between HFI and IR. Inflammation is one such putative mechanism. Consistent with existing literature (39), food-insecure individuals had higher hsCRP concentrations than their food-secure counterparts. Further, this inflammatory marker mediated the association with IR. It is well documented that persons with T2D have elevated concentrations of hsCRP (40). Our findings indicate that, despite their already proinflammatory state by virtue of having T2D, individuals with T2D experiencing food insecurity show more chronic, low-grade inflammation.
The stress hormone cortisol was also a significant mediator of the HFI–IR association. Cortisol reflects sympathetically modulated hypothalamic–pituitary–adrenal axis activity. It binds to receptors on the fat cells, liver, and pancreas, affecting digestion and growth. Cortisol is implicated in chronic and psychosocial distress. Hormones associated with stress protect the body in the short run and promote adaptation to environmental challenges. But chronic or repeated exposure can compromise metabolic, cardiovascular, neural, and immunological systems (41). Food insecurity is a powerful psychosocial stressor (42) that interferes with other physiological functions such as sleep; we have previously reported that this sample showed a strong association between food insecurity and psychological distress (19). Higher concentrations of cortisol have been shown to be associated with progression of IR among Latinos (43).
Whereas our results point to cortisol as a mediating stress hormone, no such evidence was found for metanephrine or normetanephrine. Metanephrine and normetanephrine are metabolites of adrenaline and noradrenaline which reflect sympathetically modulated adrenal activity. Adrenaline and noradrenaline primarily bind to receptors on the heart and heart vessels increasing heart rate, force of muscle contraction, and respiration (44), but may have relatively weaker effects on metabolic indicators. Also, compared with cortisol, epinephrine and norepinephrine are implicated in more acute, rather than chronic, stress reactions.
Analyses testing our hypothesized association among HFI, WHR, and IR yielded only marginally significant associations. This contrasts with previous studies that have found this association (45). One potential explanation for our marginal findings is that we used WHR as the indicator of central adiposity and other studies have used visceral abdominal tissue or subcutaneous abdominal tissue. Their measures may have been more sensitive and may also be less prone to measurement error than is WHR. However, they are expensive and require sophisticated, laboratory-based imaging, making them less well suited for community-based research (46). Measures of adipokines could also help delineate subcutaneous from visceral fat. Another explanation is variance sharing, i.e., intercorrelation, among all the mediators. Central adiposity is associated with hsCRP, hypercortisolemia, and disruption of the normal cortisol circadian rhythm (47, 48). Thus, it is possible that our other mediators accounted for variance in IR that might otherwise be explained by WHR.
The differences by food security status for total cholesterol add to existing literature (6). Specifically, we found differences for total cholesterol but not for specific lipid fractions. We interpret these findings to mean that the nonsignificantly higher LDL cholesterol (P = 0.134) and TGs (P = 0.148) in the food-insecure group reached statistical significance only when combined into a total lipid panel. Food-insecure individuals may eat diets characterized by energy-dense and high-glycemic foods (49) that promote dyslipidemia. We did not specifically test for lipids as a mediator because of lack of theoretical justification, but include them as another indicator of metabolic health that varies by food security status.
Overall, our findings add to the literature supporting the plausibility of links between food insecurity and poor health. We advocate for incorporating these findings into the design of equitable food and nutrition policies rooted in the socio-ecological framework (50). Within such a framework, resources should be redirected toward ending or decreasing food insecurity, a powerful social determinant of health. Until such policies are developed and implemented, efforts to further mitigate the deleterious effects of food insecurity on health outcomes including T2D are needed. Policies to decrease food insecurity such as 1) modified disbursement of Supplemental Nutrition Assistance Program benefits could yield consequent benefits for diabetes control; 2) increasing access to minimally processed foods (e.g., fruits, vegetables, whole grains) holds promise through local store-based and non–store-based (e.g., community or home gardening) venues (51); and 3) interventions implementing food resource management education (i.e., Cooking Matters for Adults) (52–54) may also help to partially mitigate the negative effects of HFI (55). Food pantries that offer affordable diabetes-appropriate foods may improve diabetes control (56). Lastly, programs such as the CALMS-D intervention (29) can promote healthy stress management skills to reduce the psychosocial distress associated with food insecurity.
Limitations and future directions
Findings should be considered in light of study limitations. First, we were unable to test whether diet mediates the association between food insecurity and IR. This is a major limitation because dietary intake is likely one of the most important behavioral mediators between HFI and IR. For example, individuals in food-insecure households eat fewer fruits and vegetables (57, 58), resulting in higher consumption of highly palatable foods and sweets (7, 59), which may contribute to central adiposity and inflammation (60, 61). This hypothesis will need to be tested through future studies that collect valid data on usual dietary intakes taking into account portion sizes.
Second, we employed secondary analysis of cross-sectional data and thus we cannot establish temporal precedence or causality. Prospective studies would further this line of research in 2 important ways. First, they would better describe the temporal sequencing of HFI, metabolic perturbations, and IR. Second, they would allow for testing mechanisms underlying associations between HFI (and the strategies used and extent to which respondents coped with it) and IR. Ideally, microlongitudinal designs would be employed that would track the temporal unfolding of food cycles of food abundance and scarcity relative to health outcomes. It is possible that food insecurity causes a stress response and inflammation which in turn leads to IR, or, alternatively, food insecurity may lead to both simultaneously. It is also possible, although perhaps less likely, that IR is a causative factor in food insecurity via poor health and associated low socioeconomic status. Finally, a shared genetic predisposition to appetitive behaviors and components of the metabolic syndrome cannot be ruled out, especially through interactions with environmental factors such as diet, physical activity, and stress.
The sample size was relatively small, especially for testing the effects of WHR which ended up being marginally significant. Studies of larger samples that carefully distinguish between visceral and subcutaneous fat would be informative. Future studies should test additional alternative explanations for our findings. For example, individuals from food-insecure households may receive poorer diabetes medical management (62) and show lower overall diabetes self-management behaviors (63, 64).
These limitations are generally outweighed by the study's strengths including a novel and important research question along with the unique combination of social and biological variables. A considerable strength is also that we focused on a highly vulnerable and hard-to-reach clinical sample of low-income Latinos who are often excluded from health research owing to challenges of transportation, literacy and numeracy, language, and mistrust.
Acknowledgments
The authors’ responsibilities were as follows—RP-E and JAW: designed the research; JC: coordinated recruitment; SS-P and GD: conducted the research; RSF: analyzed the data; JAW and AB-M: drafted the manuscript; JAW, AB-M, RSF, SS-P, and RP-E: critically revised the manuscript; AB-M: had primary responsibility for the final content; JAW and RP-E: secured funding for the study; and all authors: read and approved the final manuscript.
Notes
Supported by National Institute on Minority Health and Health Disparities grant R01MD005879 (to JAW and RP-E) and American Diabetes Association grant 7-13-TS-31 (to JAW).
Author disclosures: AB-M, JAW, RSF, SS-P, GD, JC, and RP-E, no conflicts of interest.
Abbreviations used: CALMS-D, Community health educators Assisting Latinos Manage Stress and Diabetes; HbA1c, glycated hemoglobin; HFI, household food insecurity; hsCRP, high-sensitivity C-reactive protein; IR, insulin resistance; T2D, type 2 diabetes; WHR, waist-to-hip ratio.
References
- 1. Anderson SE. Core indicators of nutritional state for difficult-to-sample populations. J Nutr. 1990;120(Suppl 11):1555–600. [DOI] [PubMed] [Google Scholar]
- 2. Coleman-Jensen A, Rabbitt MP, Gregory CA, Singh A. 2018 Household food insecurity in the United States in 2017. ERR-256 Washington (DC): US Department of Agriculture, Economic Research Service; 2017. [Google Scholar]
- 3. Hernandez DC, Reesor LM, Murillo R. Food insecurity and adult overweight/obesity: gender and race/ethnic disparities. Appetite. 2017;117:373–8. [DOI] [PubMed] [Google Scholar]
- 4. del Carmen Morales-Ruán M, Méndez-Gómez Humarán I, Shamah-Levy T, Valderrama-Álvarez Z, Melgar-Quiñónez H. Food insecurity is associated with obesity in adult women of Mexico. Salud Publica Mex. 2014;56(Suppl 1):s54–61. [PubMed] [Google Scholar]
- 5. Parker ED, Widome R, Nettleton JA, Pereira MA. Food security and metabolic syndrome in U.S. adults and adolescents: findings from the National Health and Nutrition Examination Survey, 1999–2006. Ann Epidemiol. 2010;20:364–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Berkowitz SA, Baggett TP, Wexler DJ, Huskey KW, Wee CC. Food insecurity and metabolic control among U.S. adults with diabetes. Diabetes Care. 2013;36:3093–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Laraia BA. Food insecurity and chronic disease. Adv Nutr. 2013;4:203–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pérez-Escamilla R, Ferris AM, Drake L, Haldeman L, Peranick J, Campbell M, Peng YK, Burke G, Bernstein B. Food stamps are associated with food security and dietary intake of inner-city preschoolers from Hartford, Connecticut. J Nutr. 2000;130:2711–7. [DOI] [PubMed] [Google Scholar]
- 9. Jordan ML, Perez-Escamilla R, Desai MM, Shamah-Levy T. Household food insecurity and sleep patterns among Mexican adults: results from ENSANUT-2012. J Immigr Minor Health. 2016;18(5):1093–103. [DOI] [PubMed] [Google Scholar]
- 10. Gonzalez AB, Salas D, Umpierrez GE. Special considerations on the management of Latino patients with type 2 diabetes mellitus. Curr Med Res Opin. 2011;27:969–79. [DOI] [PubMed] [Google Scholar]
- 11. Yracheta JM, Alfonso J, Lanaspa MA, Roncal-Jimenez C, Johnson SB, Sánchez-Lozada LG, Johnson RJ. Hispanic Americans living in the United States and their risk for obesity, diabetes and kidney disease: genetic and environmental considerations. Postgrad Med. 2015;127:503–10. [DOI] [PubMed] [Google Scholar]
- 12. Centers for Disease Control and Prevention. National diabetes statistics report, 2017. Atlanta (GA): Centers for Disease Control and Prevention, US Department of Health and Human Services; 2017. [Google Scholar]
- 13. Fonseca VA. Defining and characterizing the progression of type 2 diabetes. Diabetes Care. 2009;32(Suppl 2):S151–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Berkowitz SA, Gao X, Tucker KL. Food-insecure dietary patterns are associated with poor longitudinal glycemic control in diabetes: results from the Boston Puerto Rican Health study. Diabetes Care. 2014;37:2587–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bawadi HA, Ammari F, Abu-Jamous D, Khader YS, Bataineh S, Tayyem RF. Food insecurity is related to glycemic control deterioration in patients with type 2 diabetes. Clin Nutr. 2012;31:250–4. [DOI] [PubMed] [Google Scholar]
- 16. Franklin B, Jones A, Love D, Puckett S, Macklin J, White-Means S. Exploring mediators of food insecurity and obesity: a review of recent literature. J Community Health. 2012;37:253–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Willis DE, Fitzpatrick KM. Psychosocial factors as mediators of food insecurity and weight status among middle school students. Appetite. 2016;103:236–43. [DOI] [PubMed] [Google Scholar]
- 18. Salinas JJ, Shropshire W, Nino A, Parra-Medina D. Food insecurity, not stress is associated with three measures of obesity in low-income, Mexican-American women in south Texas. Food Public Health. 2016;6:149. [PMC free article] [PubMed] [Google Scholar]
- 19. Bermúdez-Millán A, Pérez-Escamilla R, Segura-Peréz S, Damio G, Chhabra J, Osborn CY, Wagner J. Psychological distress mediates the association between food insecurity and suboptimal sleep quality in Latinos with type 2 diabetes mellitus. J Nutr. 2016;146:2051–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Joseph JJ, Wang X, Spanakis E, Seeman T, Wand G, Needham B, Golden SH. Diurnal salivary cortisol, glycemia and insulin resistance: the multi-ethnic study of atherosclerosis. Psychoneuroendocrinology. 2015;62:327–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Champaneri S, Xu X, Carnethon MR, Bertoni AG, Seeman T, DeSantis AS, Diez Roux A, Shrager S, Golden SH. Diurnal salivary cortisol is associated with body mass index and waist circumference: the Multiethnic Study of Atherosclerosis. Obesity (Silver Spring). 2013;21:E56–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Brealey D, Singer M. Hyperglycemia in critical illness: a review. J Diabetes Sci Technol. 2009;3:1250–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rohleder N. Stimulation of systemic low-grade inflammation by psychosocial stress. Psychosom Med. 2014;76:181–9. [DOI] [PubMed] [Google Scholar]
- 24. Yuan G, Zhou L, Tang J, Yang Y, Gu W, Li F, Hong J, Gu Y, Li X, Ning G. Serum CRP levels are equally elevated in newly diagnosed type 2 diabetes and impaired glucose tolerance and related to adiponectin levels and insulin sensitivity. Diabetes Res Clin Pract. 2006;72:244–50. [DOI] [PubMed] [Google Scholar]
- 25. Bergmans RS, Palta M, Robert SA, Berger LM, Ehrenthal DB, Malecki KM. Associations between food security status and dietary inflammatory potential within lower-income adults from the United States National Health and Nutrition Examination Survey, cycles 2007 to 2014. J Acad Nutr Diet. 2018;118:994–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Calle MC, Fernandez ML. Inflammation and type 2 diabetes. Diabetes Metab. 2012;38:183–91. [DOI] [PubMed] [Google Scholar]
- 27. Pérez-Escamilla R, Bermúdez-Millán A, Segura-Pérez S, Damio G, Chhabra J, Wagner J. Household food insecurity and higher blood glucose levels among Latinos with type 2 diabetes. 142nd APHA Annual Meeting and Exposition, November 18th, 2014; New Orleans, LA. 2014. [Google Scholar]
- 28. Wagner J, Bermúdez-Millán A, Segura-Pérez S, Damio G, Chhabra J, Pérez-Escamilla R. Group stress management delivered by community health workers: effects on emotional functioning among Latinos with type 2 diabetes. 142nd APHA Annual Meeting and Exposition, November 18th, 2014; New Orleans, LA. 2014. [Google Scholar]
- 29. Wagner JA, Bermúdez-Millán A, Damio G, Segura-Pérez S, Chhabra J, Vergara C, Feinn R, Pérez-Escamilla R. A randomized, controlled trial of a stress management intervention for Latinos with type 2 diabetes delivered by community health workers: outcomes for psychological wellbeing, glycemic control, and cortisol. Diabetes Res Clin Pract. 2016;120:162–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wagner J, Bermúdez-Millán A, Damio G, Segura-Pérez S, Chhabra J, Vergara C, Pérez-Escamilla R. Community health workers assisting Latinos manage stress and diabetes (CALMS-D): rationale, intervention design, implementation, and process outcomes. Transl Behav Med. 2015;5:415–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bickel G, Nord M, Price C, Hamilton W, Cook J. Guide to measuring household food security, revised 2000. Alexandria (VA): US Department of Agriculture, Food and Nutrition Service; 2000. [Google Scholar]
- 33. Blumberg SJ, Bialostosky K, Hamilton WL, Briefel RR. The effectiveness of a short form of the Household Food Security Scale. Am J Public Health. 1999;89:1231–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cook JT, Black M, Chilton M, Cutts D, Ettinger de Cuba S, Heeren TC, Rose-Jacobs R, Sandel M, Casey PH, Coleman S et al.. Are food insecurity's health impacts underestimated in the U.S. population? Marginal food security also predicts adverse health outcomes in young U.S. children and mothers. Adv Nutr. 2013;4:51–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–9. [DOI] [PubMed] [Google Scholar]
- 36. World Health Organization (WHO). Waist circumference and waist–hip ratio: report of a WHO expert consultation: Geneva, 8–11 December 2008. Geneva: WHO; 2011. [Google Scholar]
- 37. Mackinnon DP, Lockwood CM, Williams J. Confidence limits for the indirect effect: distribution of the product and resampling methods. Multivariate Behav Res. 2004;39:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Muthén LK, Muthén BO. Mplus user's guide. 8th ed. Los Angeles (CA): Muthén & Muthén; 2017. [Google Scholar]
- 39. Gowda C, Hadley C, Aiello AE. The association between food insecurity and inflammation in the US adult population. Am J Public Health. 2012;102:1579–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Marques‐Vidal P, Bastardot F, von Känel R, Paccaud F, Preisig M, Waeber G, Vollenweider P. Association between circulating cytokine levels, diabetes and insulin resistance in a population‐based sample (CoLaus study). Clin Endocrinol (Oxf). 2013;78:232–41. [DOI] [PubMed] [Google Scholar]
- 41. McEwen BS. Protective and damaging effects of stress mediators. N Engl J Med. 1998;338:171–9. [DOI] [PubMed] [Google Scholar]
- 42. Bermúdez-Millán A, Damio G, Cruz J, D'Angelo K, Segura-Pérez S, Hromi-Fiedler A, Pérez-Escamilla R. Stress and the social determinants of maternal health among Puerto Rican women: a CBPR approach. J Health Care Poor Underserved. 2011;22:1315–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Adam TC, Hasson RE, Ventura EE, Toledo-Corral C, Le K, Mahurkar S, Lane CJ, Weigensberg MJ, Goran MI. Cortisol is negatively associated with insulin sensitivity in overweight Latino youth. J Clin Endocrinol Metab. 2010;95:4729–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lundberg U. Stress hormones in health and illness: the roles of work and gender. Psychoneuroendocrinology. 2005;30:1017–21. [DOI] [PubMed] [Google Scholar]
- 45. Karter AJ, Mayer-Davis EJ, Selby JV, D'Agostino RB Jr, Haffner SM, Sholinsky P, Bergman R, Saad MF, Hamman RF. Insulin sensitivity and abdominal obesity in African-American, Hispanic, and non-Hispanic white men and women. The Insulin Resistance and Atherosclerosis Study. Diabetes. 1996;45:1547–55. [DOI] [PubMed] [Google Scholar]
- 46. Wagenknecht LE, Langefeld CD, Scherzinger AL, Norris JM, Haffner SM, Saad MF, Bergman RN. Insulin sensitivity, insulin secretion, and abdominal fat: the Insulin Resistance Atherosclerosis Study (IRAS) Family Study. Diabetes. 2003;52:2490–6. [DOI] [PubMed] [Google Scholar]
- 47. Donoho CJ, Weigensberg MJ, Emken BA, Hsu J, Spruijt‐Metz D. Stress and abdominal fat: preliminary evidence of moderation by the cortisol awakening response in Hispanic peripubertal girls. Obesity. 2011;19:946–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wee CC, Mukamal KJ, Huang A, Davis RB, McCarthy EP, Mittleman MA. Obesity and C‐reactive protein levels among white, black, and Hispanic US adults. Obesity. 2008;16:875–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Sharkey JR, Nalty C, Johnson CM, Dean WR. Children's very low food security is associated with increased dietary intakes in energy, fat, and added sugar among Mexican-origin children (6–11 y) in Texas border Colonias. BMC Pediatr. 2012;12:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Walter B. What political framework is necessary to reduce malnutrition? A civil society perspective. World Rev Nutr Diet. 2016;115:203–10. [DOI] [PubMed] [Google Scholar]
- 51. Ayala GX, Baquero B, Pickrel JL, Mayer J, Belch G, Rock CL, Linnan L, Gittelsohn J, Sanchez-Flack J, Elder JP. A store-based intervention to increase fruit and vegetable consumption: the El Valor de Nuestra Salud cluster randomized controlled trial. Contemp Clin Trials. 2015;42:228–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kaiser L, Chaidez V, Algert S, Horowitz M, Martin A, Mendoza C, Neelon M, Ginsburg DC. Food resource management education with SNAP participation improves food security. J Nutr Educ Behav. 2015;47:374–8..e1. [DOI] [PubMed] [Google Scholar]
- 53. Pooler JA, Morgan RE, Wong K, Wilkin MK, Blitstein JL. Cooking Matters for Adults improves food resource management skills and self-confidence among low-income participants. J Nutr Educ Behav. 2017;49:545–53..e1. [DOI] [PubMed] [Google Scholar]
- 54. Lohse B, Belue R, Smith S, Wamboldt P, Cunningham-Sabo L. About Eating: an online program with evidence of increased food resource management skills for low-income women. J Nutr Educ Behav. 2015;47:265–72. [DOI] [PubMed] [Google Scholar]
- 55. Rivera RL, Maulding MK, Abbott AR, Craig BA, Eicher-Miller HA. SNAP-Ed (Supplemental Nutrition Assistance Program–Education) increases long-term food security among Indiana households with children in a randomized controlled study. J Nutr. 2016;146:2375–82. [DOI] [PubMed] [Google Scholar]
- 56. Seligman HK, Lyles C, Marshall MB, Prendergast K, Smith MC, Headings A, Bradshaw G, Rosenmoss S, Waxman E. A pilot food bank intervention featuring diabetes-appropriate food improved glycemic control among clients in three states. Health Aff. 2015;34:1956–63. [DOI] [PubMed] [Google Scholar]
- 57. Asfour L, Natale R, Uhlhorn S, Arheart KL, Haney K, Messiah SE. Ethnicity, household food security, and nutrition and activity patterns in families with preschool children. J Nutr Educ Behav. 2015;47:498–505..e1. [DOI] [PubMed] [Google Scholar]
- 58. Dave JM, Evans AE, Saunders RP, Watkins KW, Pfeiffer KA. Associations among food insecurity, acculturation, demographic factors, and fruit and vegetable intake at home in Hispanic children. J Am Diet Assoc. 2009;109:697–701. [DOI] [PubMed] [Google Scholar]
- 59. Leung CW, Epel ES, Ritchie LD, Crawford PB, Laraia BA. Food insecurity is inversely associated with diet quality of lower-income adults. J Acad Nutr Diet. 2014;114:1943–53..e2. [DOI] [PubMed] [Google Scholar]
- 60. DeClercq V, Cui Y, Forbes C, Grandy SA, Keats M, Parker L, Sweeney E, Yu ZM, Dummer TJ. Association between diet quality and adiposity in the Atlantic PATH cohort. Nutrients. 2017;9:1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Gao X, Bermudez OI, Tucker KL. Plasma C-reactive protein and homocysteine concentrations are related to frequent fruit and vegetable intake in Hispanic and non-Hispanic white elders. J Nutr. 2004;134:913–8. [DOI] [PubMed] [Google Scholar]
- 62. Moreno G, Morales LS, Isiordia M, de Jaimes FN, Tseng CH, Noguera C, Mangione CM. Latinos with diabetes and food insecurity in an agricultural community. Med Care. 2015;53:423–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Barnard LS, Wexler DJ, DeWalt D, Berkowitz SA. Material need support interventions for diabetes prevention and control: a systematic review. Curr Diab Rep. 2015;15:574. [DOI] [PubMed] [Google Scholar]
- 64. Essien UR, Shahid NN, Berkowitz SA. Food insecurity and diabetes in developed societies. Curr Diab Rep. 2016;16:79. [DOI] [PubMed] [Google Scholar]


