Abstract
Objective
Direct-acting antivirals (DAAs) for treating hepatitis C virus (HCV) infection exert a significantly high sustained viral response (SVR), and patients experience a rebound increase in low-density lipoprotein cholesterol (LDL) and total cholesterol levels. Carotid intima-media thickness (IMT) is a highly reproducible and non-invasive parameter for assessing the atherosclerotic process, and the small dense (sd) LDL level is useful for clinically evaluating the atherogenic risk.
Methods
A total of 48 patients with chronic HCV infection were treated with DAAs. All patients exhibited an SVR 24 weeks later. We compared the metabolic profiles of the patients, including the sdLDL and IMT values, at the start of DAA treatment with those after one year of treatment. We verified whether the HCV clearance after the administration of DAAs is associated with the development of atherosclerosis.
Results
The sdLDL, %sdLDL (sdLDL/LDL), and LDL values were exacerbated after a year of treatment; however, the triglyceride level, glycated hemoglobin level, insulin resistance, and body weight remained unaltered. The max-IMT was increased after a year compared to that at the start of treatment. Differences in the max-IMT (dmax-IMT) were greater in men than in women; however, no correlation was observed between the dmax-IMT and genotype, fibrosis, hypertension, hyperlipidemia, diabetes, obesity, and dialysis status. The %sdLDL at the start and a year later was positively correlated with the dmax-IMT. No correlation was observed among various factors including the LDL, triglyceride, body mass index, insulin resistance and dmax-IMT. In uni- and multivariate analyses, a significant correlation was observed between %sdLDL≥16% at the start of treatment and the sex and dmax-IMT.
Conclusion
Because the sdLDL and IMT values were exacerbated after a year of DAA treatment, atherosclerosis must be evaluated in patients achieving an SVR.
Keywords: hepatitis C virus, direct-acting antivirals, intima-media thickness, small dense low-density lipoprotein cholesterol
Introduction
The recent introduction of direct-acting antivirals (DAAs) has changed hepatitis C virus (HCV) infection treatment. Daclatasvir/asunaprevir (DCV/ASV) (1) and sofosbuvir/ledipasvir (SOF/LDV) (2) for HCV 1B infection and sofosbuvir/ribavirin (SOF/RBV) for HCV 2A/2B infection (3) are common DAA treatments in Japan. These treatments result in significantly high sustained viral response (SVR) rates (85-100%) after a short treatment course (12-24 weeks) without any severe adverse effects. As a result of DAA treatment for HCV infection, the rate of hepatocellular carcinoma occurrence or recurrence is not markedly different between patients achieving an SVR after receiving DAA and those achieving an SVR after receiving interferon-based therapy (4). In addition, the wait time for liver transplantation for HCV infection complicated by decompensated cirrhosis has decreased by over 30% due to the advent of DAA therapy (5).
It has been reported that SVR induced by interferon-based therapy resolves HCV-related extrahepatic disorders and reduces the overall mortality due to hepatic and non-hepatic pathologies as well as the risk of cardiovascular events and bacterial infections (6). SVR causes a two-thirds reduction in the risk of type 2 diabetes mellitus development in HCV-positive patients treated with interferon-based therapy (7). Interferon-based treatment is also associated with a lower risk of end-stage renal disease, acute coronary syndrome, and ischemic stroke (8). HCV-infected patients exhibit increased rates of insulin resistance, diabetes, and atherosclerosis, which may lead to increased cardiovascular morbidity and mortality (9).
The serum lipid profile is a prediction factor for SVR (10), and a significant proportion of patients successfully treated with interferons experience a rebound increase in low-density lipoprotein cholesterol (LDL) and total cholesterol to levels associated with an increased risk of coronary artery disease (10,11). Similarly, interferon-free DAA treatments increase serum LDL levels during and after DAA treatment (12-15). LDL is considered one of the most important risk factors for cardiovascular diseases (16). Therefore, monitoring the lipid levels in the body is important in patients receiving antiviral therapy (11).
The carotid intima-media thickness (IMT) is a highly reproducible and non-invasive parameter for assessing the atherosclerotic process (17). The measurement of small dense (sd) LDL by the current precipitation method is useful for evaluating the atherogenic risk and may be applicable in routine clinical examinations (18). The change in the sdLDL and IMT values in patients with SVR has not been evaluated. We compared the metabolic profiles of patients, including the sdLDL and IMT values, at the start of DAA treatment and one year into treatment and determined whether the HCV clearance after the administration of DAA is associated with the development of atherosclerosis.
Materials and Methods
Patients
A total of 48 patients with chronic infection with HCV genotypes 1B, 2A, and 2B along with chronic hepatitis and compensatory cirrhosis were treated with the DAAs DCV/ASV (Bristol-Myers Squibb, Tokyo, Japan) or SOF/LDV (Gilead Sciences, Tokyo, Japan) for 1B and SOF/RBV (Chugai Pharmaceutical, Tokyo, Japan) for 2A/2B at Nagasaki Harbor Medical Center between June 2014 and November 2016 (Table 1). Combination therapy with oral DCV/ASV was provided for 24 weeks (1), and SOF/LDV and SOF/RBV were orally administered for 12 weeks (2,3). During the treatment period, serum HCV-RNA was examined every 4 weeks; after the end of the treatment period, these measurements were performed every 12 weeks. SVR was determined at 24 weeks after the end of treatment. Data on concomitant medications for hypertension, hyperlipidemia, diabetes, obesity [body mass index (BMI) >25], and dialysis were obtained before starting the treatment.
Table 1.
Clinical Profiles of 48 Patients before DAA Treatment.
| Parameter | Number or Mean (SD) | |
|---|---|---|
| Women/Men | 28/20 | |
| FIB-4 (≥3.25/<3.25) | 21/27 | |
| FIB-4 | 3.63 (1.92) | |
| HCV genotype 1B/2A+2B | 40/8 | |
| DAA DCVASV/SOFLDV/SOFRBV | 4/36/8 | |
| Age (years) | 70.1 (11.08) | |
| HCV-RNA (log IU/mL) | 5.8 (1) | |
| ALT (U/L) | 46.8 (41.12) | |
| Albumin (g/dL) | 4.3 (0.5) | |
| AFP (ng/mL) | 9.4 (14.5) | |
| PIVKA-II (mAU/mL) | 19.8 (7.28) | |
| LDL (mg/dL) | 107.8 (34.8) | |
| sdLDL (mg/dL) | 19.8 (10.6) | |
| %sdLDL | 18 (7.2) | |
| HDL (mg/dL) | 60.9 (19.4) | |
| TG (mg/dL) | 107.8 (72.9) | |
| HbA1c (%) | 5.7 (0.7) | |
| Body weight (kg) | 53.9 (11) | |
| BMI (kg/m2) | 22 (3.419) | |
| HOMA-IR | 2.76 (1.72) | |
| Creatinine eGFR (mL/min/1.73 m2) | 64.9 (23.95) | |
| Cystatin eGFR (mL/min/1.73 m2) | 57.23 (24.1) | |
| Hypertension -/+ | 26/22 | |
| Hyperlipidemia -/+ | 43/6 | |
| Diabetes -/+ | 44/4 | |
| Obesity -/+ | 37/11 | |
| Dialysis -/+ | 44/4 |
Normal ranges of clinical parameters in fasting serum were as follows: ALT 5-40 U/L, albumin 3.8-5.2 g/dL, LDL 70-139 mg/dL, HDL men 40-86 mg/dL, HDL women 40-96 mg/dL, TG 50-149 mg/dL, and HbA1c 4.6-6.2%. All laboratory measurements were conducted after overnight fasting.
FIB-4: fibrosis-4, DAA: direct acting anti-viral, DCV/ASV: Daclatasvir/asunaprevir, SPF/LDV: sofosbuvir/Ledipasvir, SOF/RBV: sofosbuvir/ribavirin, ALT: aspartate aminotransferase, AFP: α-fetoprotein, PIVKA: protein induced vitamin K ascent, BMI: body mass index, HOMA-IR: homeostasis model assessment insulin resistance
The identification of hypertension and hyperlipidemia affected the medical history and the use of orally medication to treat these diseases.
Cervical artery ultrasound imaging was performed at the start of treatment and a year into treatment. This study was performed by retrospective observations.
Informed consent was obtained from each patient included in the study, and the study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki, as evidenced by the approval of the study by the Human Research Ethics Committee of Nagasaki Harbor Medical Center.
Measurements
Laboratory data and anthropometric measurement data were obtained for each patient every 4 weeks during treatment and every 12 weeks after treatment. Laboratory examinations included the assessment of total protein, albumin, total bilirubin, alanine aminotransferase, γ glutamyl transpeptidase, cholinesterase, triglycerides (TG), total cholesterol, high density lipoprotein cholesterol (HDL), LDL, fasting plasma glucose, and glycated hemoglobin (HbA1c). The LDL/HDL ratio was calculated at the same time (i.e., at the start of treatment). Insulin resistance was evaluated by the homeostasis model assessment (HOMA-IR) method using the following formula: HOMA-IR = fasting glucose (mg/dL) ×9 fasting insulin (IU/mL)/405. sdLDL was assayed using a commercially available assay kit (sdLDL-EX; Denka Seiken, Tokyo, Japan) based on a previously established method (18). The %sdLDL was calculated based on the sdLDL/LDL determined at the same time. The extent of liver fibrosis was assessed based on the fibrosis-4 (FIB-4) status (19).
The carotid IMT was measured by B-mode ultrasonography with a 10-MHz probe [EZU-MT29-S1 (KE13191108); Avius HITACHI, Tokyo, Japan]. We examined the far walls of the left and right common carotid arteries. Examinations were made from three different longitudinal projections (i.e., anterior-oblique, lateral, and posterior-oblique). The IMT was assessed as the greatest thickness at any location on the far walls of carotid arteries, including atheromatous plaques on both sides. The greatest unilateral IMT value, which was higher than that of the other side, was defined as the max-IMT. All measurements (scans and image analyses) were performed by the same physician with the same equipment. Measurements of the mean-IMT were made at three levels of the lateral and medial walls 1-3 cm proximal to the carotid bifurcation.
Statistical analyses
Data were analyzed using the StatView 5.0 software program (SAS Institute, Cary, USA). Laboratory result variables were compared using a correlation analysis, t-tests, and χ2-square tests. Correlations were evaluated with coefficients of correlation. A multivariate analysis was performed with logistic regression tests. Values of p<0.05 were considered statistically significant.
Results
The clinical profiles of all patients at the start of DAA treatment are presented in Table 1. Regarding the HCV genotype, 28 patients had HCV 1B, and 20 patients had HCV 2A/2B infections. A total of 28 patients were women. The mean BMI was 22, and 11 patients were obese (BMI>25). The mean HOMA-IR was 2.76. On average, non-obese patients had insulin resistance at the start of treatment. Patients were not taking any antiplatelet agents or eicosapentaenoic acid agents orally, and statin was the only medication given for hyperlipidemia. All patients had achieved an SVR at 24 weeks after the end of treatment.
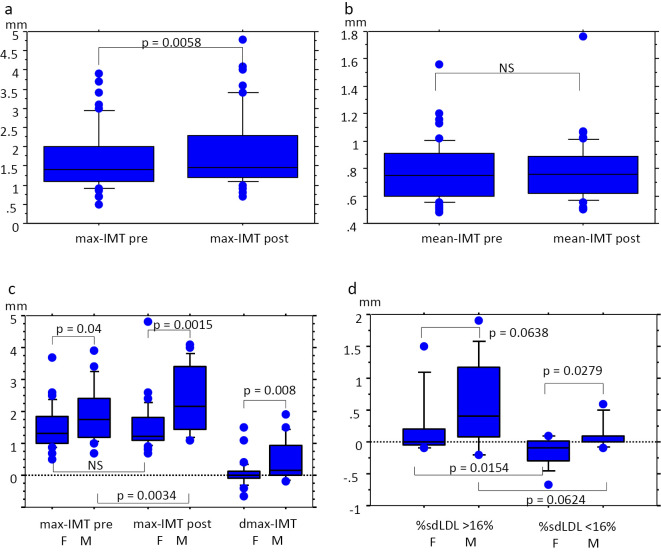
First, we selected factors related to metabolic disease and atherosclerosis, and these factors at the start of treatment were compared with those a year later (Table 2 and Figure). sdLDL, %sdLDL, LDL, and HDL were exacerbated after a year of treatment, but the HDL/LDL, TG, HbA1c, HOMA-IR, and body weight did not change markedly during the observation period. The max-IMT (median: 1.45; range: 0.7-4.8), not mean-IMT (0.755; 0.5-1.76), after a year was larger than that at the start of treatment (max-IMT: 1.4; 0.5-3.9, mean-IMT: 0.75; 1.08-1.56) (Figure a and b).
Table 2.
Changes in Metabolic Parameters from before DAA Treatment to One Year Later.
| Parameter | Before DAA | One year later | p value | |||
|---|---|---|---|---|---|---|
| sdLDL (mg/dL) | 19.8 (10.6) | 30.2 (15.6) | <0.0001 | |||
| %sdLDL | 18 (7.2) | 24.5 (9.9) | <0.0001 | |||
| LDL (mg/dL) | 107.5 (34.8) | 119.8 (36.2) | <0.0001 | |||
| HDL (mg/dL) | 60.9 (19.4) | 66.3 (22.4) | 0.002 | |||
| HDL/LDL | 0.618 (0.255) | 0.609 (0.38) | NS | |||
| TG (mg/dL) | 107.8 (72.9) | 114.9 (83.4) | NS | |||
| HbA1c (%) | 5.7 (0.7) | 5.8 (0.7) | NS | |||
| HOMA-IR | 2.76 (1.72) | 3.22 (2.8) | NS | |||
| Body weight (kg) | 54 (11) | 54.5 (11.2) | NS |
Values represent the mean (SD). Laboratory results were compared using t-tests.
Figure.
Relationship between the changes in the IMT and clinical factors. The changes in the IMT were calculated by subtracting the IMT (mm) before treatment with DAAs from that after a year of treatment. Changes in diameters by 1 unit (0.1 mm) or more were indicated. Error bars represent SD. Differences between pre- and post-treatment values were evaluated by a t-test, and values of p<0.05 were considered statistically significant. a: The max-IMT significantly increased a year after treatment compared to that at the start of treatment (p=0.0058). The Y-axis represents the max-IMT diameter (mm). b: The mean-IMT at the start of treatment did not differ markedly from that after a year. The Y-axis represents the mean-IMT diameter (mm). c: The max-IMT in men (M) was higher than that in women (F) at the start of treatment and a year later (p=0.04 and 0.0015, respectively), and the difference was significant when comparing values after a year with those at the start of treatment (p=0.0034). The difference in the max-IMT (dmax-IMT) was calculated as follows: dmax-IMT=max-IMT after a year - max-IMT at the start of treatment. The dmax-IMT was higher in men than in women (p=0.008). The Y-axis represents the max-IMT and dmax-IMT diameters (mm). d: The %sdLDL was calculated as follows: %sdLDL=sdLDL at the start of treatment/LDL at the start of treatment. The dmax-IMT in women with %sdLDL≥16% was higher than that in women with %sdLDL<16% (p=0.0154), and the dmax-IMT in men with %sdLDL<16% was higher than that in women with %sdLDL<16% (p=0.027). The numbers of women with %sdLDL≥16%, women with %sdLDL<16%, men with %sdLDL ≥16%, and men with %sdLDL<16% were 15, 13, 13, and 7, respectively. The Y-axis represents the dmax-IMT diameter (mm).
Second, we evaluated the relationship between changes in the max-IMT and clinical factors (Table 3 and 4 and Figure). Differences in the max-IMT (dmax-IMT) were calculated as follows: dmax-IMT=max-IMT at the start of treatment - max-IMT after a year. Changes in the factor value (dfactor) were calculated as follows: dfactor=factor value after a year (factor post) - factor value at the start of treatment (factor pre). The dmax-IMT in men was larger than that in women (Table 3 and Figure c). The max-IMT (2.15; 1.1-4.1) in men after a year was higher than that at the start of treatment (1.75; 0.7-3.9), but the max-IMT in women did not change markedly during the observation period (pre: 1.3; 0.5-3.7, post: 1.215; 0.7-4.8) (Figure c). The HCV genotype, FIB-4, hypertension, hyperlipidemia, diabetes, obesity, and dialysis did not significantly affect the dmax-IMT. In addition, the dsdLDL in SOF/LDV [mean (standard deviation; SD): 10.86 (9.22)] was not significantly different from that in SOF/RBV [9.27 (4.78)] and DCV/ASV [1.87 (0.92)]. The dsdLDL [mean (SD): 7.32 (5.53)] in patients with hyperlipidemia who used statins did not differ markedly from that in patients without hyperlipidemia [10.39 (8.92)]. A negative correlation was observed between the dHbA1c and dmax-IMT, but the HbA1c values at the start of treatment and a year later did not differ markedly. A positive correlation was observed between the %sdLDL at the start and a year later and the dmax-IMT, but the sdLDL at the start and a year later was only weakly correlated with the dmax-IMT (Table 4). The LDL, HDL, HDL/LDL, TG, BMI, and HOMA-IR values were not correlated with the dmax-IMT.
Table 3.
Association between the Dmax-IMT and Each Clinical Factor.
| Factors | Mean (SD) | p value | ||
|---|---|---|---|---|
| Women/Men | 0.057 (0.412)/0.465 (0.623) | 0.0089 | ||
| Genotype 1B/2A+2B | 0.182 (0.5)/0.455 (0.723) | NS | ||
| FIB-4 (≥3.5/<3.5) | 0.116 (0.53)/0.314 (0.556) | NS | ||
| Hypertension -/+ | 0.219 (0.538)/0.236 (0.563) | NS | ||
| Hyperlipidemia -/+ | 0.189 (0.527)/0.492 (0.636) | NS | ||
| Diabetes -/+ | 0.227 (0.547)/0.225 (0.591) | NS | ||
| Obesity -/+ | 0.216 (0.545)/0.264 (0.563) | NS | ||
| Dialysis -/+ | 0.23 (0.555)/0.22 (0.22) | NS |
dmax-IMT was calculated as follows: max-IMT at the start of treatment - max-IMT after a year. Laboratory results were compared using t-tests.
Table 4.
The Relationship between the dmax-IMT and Clinical Factors.
| Factors | R | p value | ||
|---|---|---|---|---|
| FIB-4 | -0.207 | NS | ||
| LDLpre (mg/dL) | 0.123 | NS | ||
| LDLpost (mg/dL) | 0.078 | NS | ||
| dLDL (mg/dL) | -0.79 | NS | ||
| HDLpre (mg/dL) | 0.067 | NS | ||
| HDLpost (mg/dL) | 0.115 | NS | ||
| dHDL (mg/dL) | 0.107 | NS | ||
| HDL/LDLpre | -0.028 | NS | ||
| HDL/LDLpost | 0.04 | NS | ||
| dHDL/LDL | -0.131 | NS | ||
| TGpre (mg/dL) | 0.067 | NS | ||
| TGpost (mg/dL) | -0.005 | NS | ||
| dTG (mg/dL) | -0.077 | NS | ||
| HbA1cpre (%) | 0.24 | NS | ||
| HbA1cpost (%) | 0.071 | NS | ||
| dHbA1c (%) | -0.328 | 0.0224 | ||
| BMIpre (kg/m2) | -0.128 | NS | ||
| BMIpost(kg/m2) | -0.057 | NS | ||
| dBMI (kg/m2) | 0.235 | NS | ||
| HOMA-IRpre | -0.152 | NS | ||
| HOMA-IRpost | -0.045 | NS | ||
| dHOMA-IR | 0.049 | NS | ||
| sdLDLpre (mg/dL) | 0.283 | 0.0539 | ||
| sdLDLpost (mg/dL) | 0.281 | 0.0587 | ||
| dsdLDL (mg/dL) | 0.108 | NS | ||
| %sdLDLpre | 0.391 | 0.0062 | ||
| %sdLDLpost | 0.298 | 0.0441 | ||
| d%sdLDL | -0.004 | NS |
dfactor=the factor value after a year (post) - factor value at the start of treatment (pre). Laboratory results were compared using a correlation analysis.
Finally, we explored the effect of contributing factors on the dmax-IMT by a multilinear regression analysis (Table 5A) and multivariate logistic regression analyses (Table 5B). A positive correlation was observed between the %sdLDL at the start of treatment and the dmax-IMT, but the dHbA1c did not have any statistical relationship. Because the median %sdLDL was 16%, we divided the patients into those with %sdLDL<16% and ≥16%. The outcomes of patients with %sdLDL<16% at the start of treatment and male gender were evaluated by a logistic regression analysis (Table 5B). In uni- and multivariate analyses, both of these factors significantly contributed to the dmax-IMT.
Table 5.
Uni- and Multivariate Analyses of Changes in the dmax-IMT.
| A. | |||||||
|---|---|---|---|---|---|---|---|
| Uni-variate | Multi-variate | ||||||
| β | p value | B | p value | ||||
| %sdLDLpre | 0.391 | 0.0066 | 0.334 | 0.0189 | |||
| dHbA1c (%) | -0.328 | 0.0229 | -0.254 | 0.071 | |||
| Uni-variate | Multi-variate | ||||||
|---|---|---|---|---|---|---|---|
| Odds ratio | 95% CI | p value | Odds ratio | 95% CI | p value | ||
| %sdLDL<16% | 0.081 | 0.009-0.698 | 0.02 | 0.074 | 0.008-0.698 | 0.02 | |
| Gender M | 6.818 | 1.542-30.157 | 0.01 | 7.528 | 1.485-38.165 | 0.01 | |
Multivariate analyses were performed with a multiple linear regression analysis (A) and logistic regression tests (B).
In patients with %sdLDL≥16%, the dmax-IMT did not differ markedly between sexes [women: (median) 0; (range) -0.1-1.5, men: 0.4; -0.2-1.9]; however, the dmax-IMT in men (0.1; -0.1-0.6) was higher than that in women (-0.1; -0.67-0.1) with a %sdLDL<16%. The dmax-IMT in women with a %sdLDL≥16% was higher than that in women with a %sdLDL<16%; however, the dmax-IMT in men remained unaltered (Figure d). Similarly, the dsdLDL [mean (SD): 15.1 (10.2)] in men with %sdLDL>16% was larger than in other groups [8.28 (7.39)] (p=0.0171).
Discussion
In patients achieving an SVR after DAA treatment, the LDL, HDL, sdLDL, %sdLDL, and max-IMT increased a year after starting treatment. However, the HOMA-IR, body weight, and TG level remained unaltered. An elevated max-IMT was associated with a %sdLDL≥16% at the start of treatment and male gender. After a year of such treatment, the atherosclerosis status should be evaluated in patients achieving an SVR.
The clearance of HCV by interferon-based therapy or treatment with any DAA is accompanied by an elevation in the serum LDL level (10-15,20), indicating a risk of coronary (11) and metabolic diseases (20); however, patients with SVR exhibit decreased extrahepatic mortality due to cardiovascular diseases after interferon-based therapy (8,9). Interferon-based and DAA treatments differ with respect to the period of treatment, adverse effects, and SVR rate. Because the duration of interferon-based treatment is long and because such therapy causes severe adverse effects, the serum total cholesterol decreases in the treatment period (11). However, after DAA treatment, the serum LDL decreases in the early treatment period (12); thus, the elevation in the LDL level is greater after DAA treatment than after interferon-based therapy. In addition, a high serum LDL level before treatment may be a significant prognostic indicator for the treatment outcome in patients with chronic HCV infection, particularly in those with genotype 1 and 2 infections (21). In contrast, no association between SVR and the pre-treatment lipid profile has been reported for DAA therapy (1-3). Nevertheless, it has been reported that HCV is associated with atherosclerosis (22-24). Effective HCV clearance may prevent atherosclerosis; however, the elevation in the LDL level must be evaluated in the future.
In addition to an increase in the LDL level after a year of starting treatment, the sdLDL and %sdLDL were also elevated in the present study. The association between HCV clearance and sdLDL has not been evaluated previously. Patients with non-alcoholic fatty liver disease had increased levels of sdLDL and %sdLDL, and ex vivo studies showed that these patients exhibited increased sensitivity of hepatic TG levels and cholesterol synthesis to insulin (25). Central obesity with hypertriglyceridemia is associated with high hepatic lipase activity that leads to the formation of proatherogenic sdLDL (26). In addition, HCV inhibits hepatic TG lipase production in hepatocytes, thereby elevating the TG-rich LDL level (27). sdLDL concentrations predict the risk for coronary heart disease (28) and exacerbation of the IMT (29). The change in the IMT in diabetes patients has been predicted by the comparison of the sdLDL level at baseline to that after two years (26). Consistent with previous reports, HCV-induced sdLDL production by hepatic TG lipase before DAA treatment and increased %sdLDL at the start of treatment were associated with an increased IMT after a year. However, while it has been reported that changes in the sdLDL are related to obesity, TG, and insulin resistance (25,26,29), the body weight, TG, and insulin resistance remained unaltered after a year of treatment in the present study despite an elevated sdLDL level. The association of the elevated sdLDL level and HCV clearance must be evaluated, and the IMT in patients with %sdLDL≥16% at the start of DAA treatment must be evaluated a year after starting DAA treatment.
Men are more prone to an exacerbated dmax-IMT than women. It has been reported that carotid plaque formation is associated with men, but sex does not affect the association between the HCV core protein and carotid atherosclerosis (30). Men with sdLDL levels in the high quartile were found to have diabetes, hypertension, and metabolic syndrome (28). In patients treated with interferon-based therapy, a rebound increase in the LDL and total cholesterol levels was not associated with sex (11); however, the IMT in men treated with DAAs must be evaluated after one year of DAA treatment. The max-IMT in men at the start of treatment was higher than that in women (Figure c). According to the clinical features shown in Table 1, the ALT value in men was higher than that in women only at the start of treatment (mean value in men, 63.35 and in women, 35.75; p=0.025). There was no marked difference between men and women regarding the demographic and clinical features except for the max-IMT and the serum ALT level at the start of treatment. Sex-related differences in the IMT after DAA treatment should also be examined in the future.
This study had a relatively short observation period and included a small patient sample size. Thus, the long-term changes in sdLDL and IMT must be observed in another study. Because the sdLDL and IMT values were exacerbated after a year of DAA treatment, atherosclerosis, including cardio- and cerebrovascular diseases, must also be evaluated in patients achieving an SVR at least one year after starting DAA treatment.
The authors state that they have no Conflict of Interest (COI).
References
- 1.Kumada H, Suzuki Y, Ikeda K, et al. Daclatasvir plus asunaprevir for chronic HCV genotype 1b infection. Hepatology 59: 2083-2091, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mizokami M, Yokosuka O, Takehara T, et al. Ledipasvir and sofosbuvir fixed-dose combination with and without ribavirin for 12 weeks in treatment-naive and previously treated Japanese patients with genotype 1 hepatitis C: an open-label, randomised, phase 3 trial. Lancet. Infect. Dis. 15: 645-653, 2015. [DOI] [PubMed] [Google Scholar]
- 3.Omata M, Nishiguchi S, Ueno Y, et al. Sofosbuvir plus ribavirin in Japanese patients with chronic genotype 2 HCV infection: an open-label, phase 3 trial. J Viral Hepat 21: 762-768, 2014. [DOI] [PubMed] [Google Scholar]
- 4.Waziry R, Hajarizadeh B, Grebely J, et al. Hepatocellular carcinoma risk following direct-acting antiviral HCV therapy: a systematic review, meta-analyses, and meta-regression. J Hepatol 67: 1204-1212, 2017. [DOI] [PubMed] [Google Scholar]
- 5.Flemming JA, Kim WR, Brosgart CL, Terrault NA. Reduction in liver transplant wait-listing in the era of direct-acting antiviral therapy. Hepatology 65: 804-812, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nahon P, Bourcier V, Layese R, et al. Eradication of hepatitis c virus infection in patients with cirrhosis reduces risk of liver and non-liver complications. Gastroenterology 152: 142-156.e2, 2017. [DOI] [PubMed] [Google Scholar]
- 7.Arase Y, Suzuki F, Suzuki Y, et al. Sustained virological response reduces incidence of onset of type 2 diabetes in chronic hepatitis C. Hepatology 49: 739-744, 2009. [DOI] [PubMed] [Google Scholar]
- 8.Hsu Y-C, Ho HJ, Huang Y-T, et al. Association between antiviral treatment and extrahepatic outcomes in patients with hepatitis C virus infection. Gut 64: 495-503, 2015. [DOI] [PubMed] [Google Scholar]
- 9.Negro F, Forton D, Craxì A, Sulkowski MS, Feld JJ, Manns MP. Extrahepatic morbidity and mortality of chronic hepatitis C. Gastroenterology 149: 1345-1360, 2015. [DOI] [PubMed] [Google Scholar]
- 10.Ramcharran D, Wahed AS, Conjeevaram HS, et al. Associations between serum lipids and hepatitis C antiviral treatment efficacy. Hepatology 52: 854-863, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Corey KE, Kane E, Munroe C, Barlow LL, Zheng H, Chung RT. Hepatitis C virus infection and its clearance alter circulating lipids: implications for long-term follow-up. Hepatology 50: 1030-1037, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hashimoto S, Yatsuhashi H, Abiru S, et al. Rapid increase in serum low-density lipoprotein cholesterol concentration during hepatitis C interferon-free treatment. PLoS One 11: e0163644, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Endo D, Satoh K, Shimada N, Hokari A, Aizawa Y. Impact of interferon-free antivirus therapy on lipid profiles in patients with chronic hepatitis C genotype 1b. World J Gastroenterol 23: 2355-2364, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Inoue T, Goto T, Iio E, et al. Changes in serum lipid profiles caused by three regimens of interferon-free direct-acting antivirals for patients infected with hepatitis C virus. Hepatol Res 48: E203-E212, 2018 (Epub ahead of print). [DOI] [PubMed] [Google Scholar]
- 15.Morales AL, Junga Z, Singla MB, Sjogren M, Torres D. Hepatitis C eradication with sofosbuvir leads to significant metabolic changes. World J. Hepatol 8: 1557-1563, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grundy SM, Cleeman JI, Merz CNB, et al. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation 110: 227-239, 2004. [DOI] [PubMed] [Google Scholar]
- 17.Pignoli P, Tremoli E, Poli A, Oreste P, Paoletti R. Intimal plus medial thickness of the arterial wall: a direct measurement with ultrasound imaging. Circulation 74: 1399-1406, 1986. [DOI] [PubMed] [Google Scholar]
- 18.Hirano T, Ito Y, Koba S, et al. Clinical significance of small dense low-density lipoprotein cholesterol levels determined by the simple precipitation method. Arterioscler Thromb Vasc Biol 24: 558-563, 2004. [DOI] [PubMed] [Google Scholar]
- 19.Vallet-Pichard A, Mallet V, Nalpas B, et al. FIB-4: an inexpensive and accurate marker of fibrosis in HCV infection. Comparison with liver biopsy and FibroTest. Hepatology 46: 32-36, 2007. [DOI] [PubMed] [Google Scholar]
- 20.Chang ML, Tsou YK, Hu TH, et al. Distinct patterns of the lipid alterations between genotype 1 and 2 chronic hepatitis C patients after viral clearance. PLoS One 9: e104783, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gopal K, Johnson TC, Gopal S, et al. Correlation between beta-lipoprotein levels and outcome of hepatitis C treatment. Hepatology 44: 335-340, 2006. [DOI] [PubMed] [Google Scholar]
- 22.Ishizaka N, Ishizaka Y, Takahashi E, et al. Association between hepatitis C virus seropositivity, carotid-artery plaque, and intima-media thickening. Lancet (London, England) 359: 133-135, 2002. [DOI] [PubMed] [Google Scholar]
- 23.The V, Fukui M, Kitagawa Y, Nakamura N, Yoshikawa T. Hepatitis C virus and atherosclerosis in patients with type 2 diabetes. JAMA 289: 1245-1246, 2014. [DOI] [PubMed] [Google Scholar]
- 24.Targher G, Bertolini L, Padovani R, Rodella S, Arcaro G, Day C. Differences and similarities in early atherosclerosis between patients with non-alcoholic steatohepatitis and chronic hepatitis B and C. J Hepatol 46: 1126-1132, 2007. [DOI] [PubMed] [Google Scholar]
- 25.Siddiqui MS, Fuchs M, Idowu MO, et al. Severity of nonalcoholic fatty liver disease and progression to cirrhosis are associated with atherogenic lipoprotein profile. Clin Gastroenterol Hepatol 13: 1000-1008.e3, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brunzell JD, Zambon A, Deeb SS. The effect of hepatic lipase on coronary artery disease in humans is influenced by the underlying lipoprotein phenotype. Biochim Biophys Acta 1821: 365-372, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shinohara Y, Imajo K, Yoneda M, et al. Hepatic triglyceride lipase plays an essential role in changing the lipid metabolism in genotype 1b hepatitis C virus replicon cells and hepatitis C patients. Hepatol Res 43: 1190-1198, 2013. [DOI] [PubMed] [Google Scholar]
- 28.Hoogeveen RC, Gaubatz JW, Sun W, et al. Small dense low-density lipoprotein-cholesterol concentrations predict risk for coronary heart disease: The Atherosclerosis Risk in Communities (ARIC) study. Arterioscler Thromb Vasc Biol 34: 1069-1077, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gerber PA, Thalhammer C, Schmied C, et al. Small, dense LDL particles predict changes in intima media thickness and insulin resistance in men with type 2 diabetes and prediabetes - a prospective cohort study. PLoS One 8: 1-8, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ishizaka Y, Ishizaka N, Takahashi E, et al. Association between hepatitis C virus core protein and carotid atherosclerosis. Circ J 67: 26-30, 2003. [DOI] [PubMed] [Google Scholar]