Abstract
A 26-year-old woman with Takayasu's arteritis (TAK) experienced back and neck pain during tocilizumab (TCZ) treatment. The levels of C-reactive protein were normal, and ultrasonography revealed no significant changes. Diffusion-weighted whole-body imaging with background body signal suppression (DWIBS) showed signal enhancement in the walls of several arteries. Contrast computed tomography showed arterial inflammation in the same lesion. After increasing the dose of prednisolone and TCZ, all signal enhancements decreased and continued to decrease, as observed on days 76 and 132. Thus, DWIBS may be a novel imaging modality for assessing the disease activity of TAK, particularly during follow-up.
Keywords: Takayasu's arteritis, DWIBS, MRI
Introduction
Takayasu's arteritis (TAK) is a large-vessel arteritis that affects the aorta, its major branches, and the coronary and pulmonary arteries. Approximately 70% of TAK patients exhibit recurrence. Thus, to prevent life-threatening complications, monitoring the disease activity is important.
The disease activity in TAK is usually assessed by clinical symptoms, serological markers, and imaging. However, patients with TAK often show recurrence without the elevation of serological markers, such as C-reactive protein (CRP) (1) and the erythrocyte sedimentation rate (ESR). Since the approval of tocilizumab (TCZ) [an inhibitor of interleukin (IL)-6] for the treatment of TAK, assessing the disease activity using CRP levels has become less feasible. Therefore, imaging modalities, including ultrasonography, computed tomography (CT) angiography, magnetic resonance imaging (MRI), and 18F-fluorodeoxyglucose positron emission tomography (FDG-PET), are occasionally combined to assess the disease activity. However, a whole-body assessment of TAK activity is not possible using ultrasonography or MRI. Furthermore, CT angiography and FDG-PET pose other problems, including radiation exposure, contrast medium, and a high cost. Therefore, these modalities are not suitable for routine follow-up.
Diffusion-weighted whole-body imaging with background body signal suppression (DWIBS) is a relatively new imaging modality (2). DWIBS allows for the acquisition of volumetric diffusion-weighted images of the whole body without radiation or the injection of contrast agents. DWIBS is primarily used for cancer screening or staging, and a few reports have addressed the usefulness of DWIBS for patients with rheumatic disease, but the utility of DWIBS for TAK has never been reported.
Case Report
A 26-year-old Japanese woman presented to National Hospital Organization Osaka Minami Medical Center with a two-month history of a fever and neck pain in July 2016. CT angiography revealed stenosis in both the common carotid arteries and the left subclavian artery. She met the American College of Rheumatology 1990 criteria for the classification of TAK (3). Oral prednisolone (PSL) (50 mg) was administered for induction therapy. However, she repeatedly relapsed upon tapering of the PSL dose. Immunosuppressive treatments, including methotrexate and intravenous immunoglobulin therapy, failed to control the disease activity. The ethics committee of the National Hospital Organization Osaka Minami Medical Center approved the use of TCZ for this patient, and informed consent was obtained from the patient. TCZ treatment was initiated in December 2016. She experienced remission, and the PSL dosage was tapered. She was maintained on PSL (9 mg) and the intravenous infusion of TCZ (8 mg/kg/3 weeks).
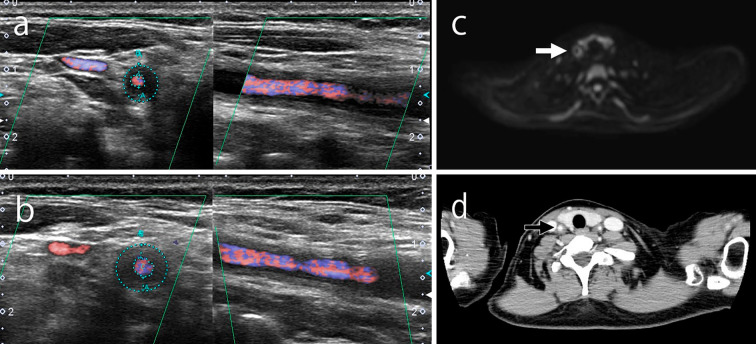
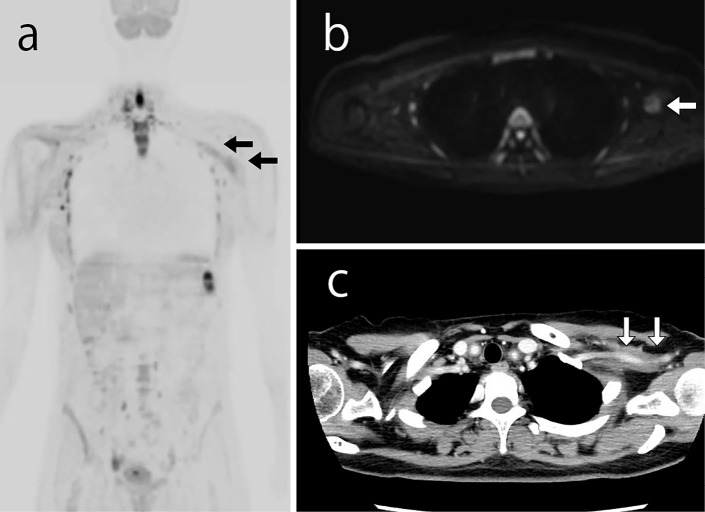
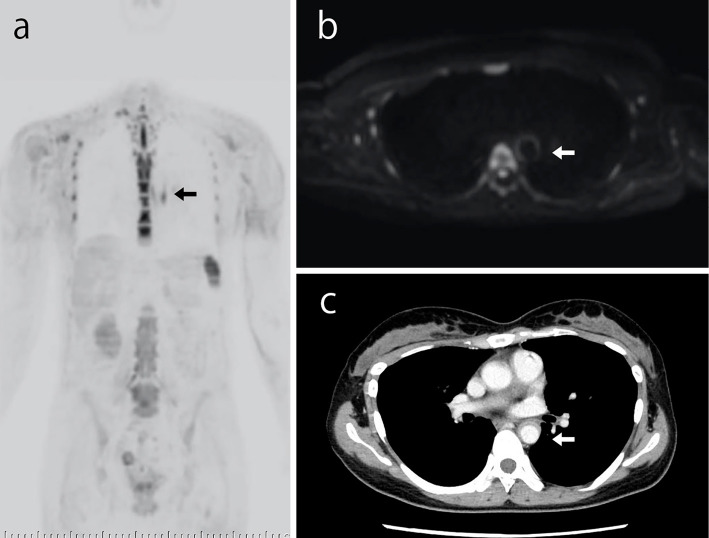
In February 2017, she was admitted to our hospital with back and neck pain without a fever. Her serum CRP level was 0.01 mg/dL, and her ESR was 7 mm/h. Ultrasonography showed no significant change from four weeks prior (Fig. 1a and b). DWIBS was performed to evaluate the inflammation in the artery walls. DWIBS revealed signal enhancement of the walls of the right common carotid artery (Fig. 1c), left subclavian artery (Fig. 2a and b), and thoracic aorta (Fig. 3a and b). DWIBS was the first imaging modality to indicate this thoracic aortic lesion. The lesion indicated by DWIBS was investigated on the same day using enhanced contrast CT. Both DWIBS and contrast CT showed artery inflammation in the same lesion (Fig. 1d, 2c, 3c).
Figure 1.
Ultrasonography showed a hypoechoic halo surrounding the right common carotid artery (a) on the day of admission. However, no significant changes were observed compared to four weeks prior (b). DWIBS (c) and contrast CT (d) showing inflammation on the same artery wall for which ultrasonography showed a halo on the day of admission. DWIBS: diffusion-weighted whole-body imaging with background body signal suppression
Figure 2.
Both DWIBS (a and b) and contrast CT (c) showing artery inflammation of the left subclavian artery. DWIBS: diffusion-weighted whole-body imaging with background body signal suppression
Figure 3.
Both DWIBS (a and b) and contrast CT (c) showing artery inflammation of the thoracic aorta. DWIBS: diffusion-weighted whole-body imaging with background body signal suppression
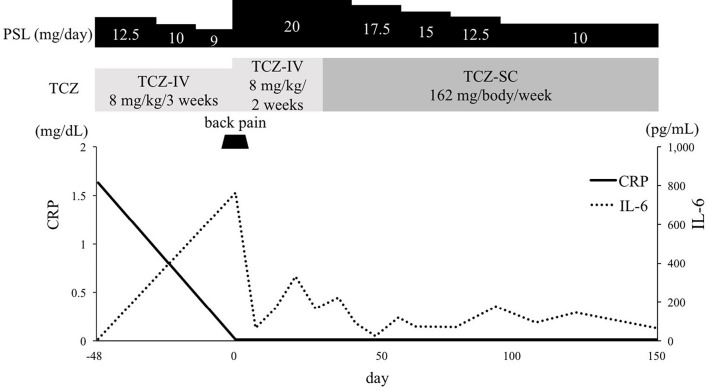
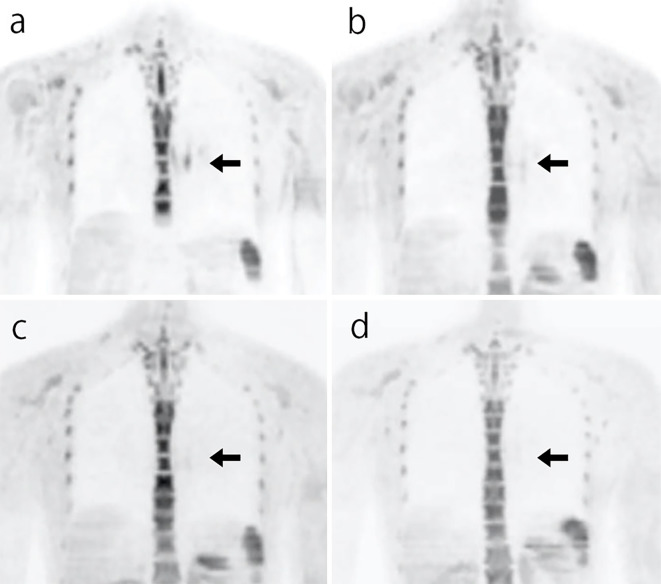
Based on the imaging data, we concluded that the patient was experiencing TAK recurrence. PSL was increased to 20 mg/day, and the intravenous infusion of TCZ was increased to 8 mg/kg/2 weeks. She improved clinically after several days (Fig. 4). Although the level of serum IL -6 was high on the day of admission, it decreased remarkably by day 7 and remained at a low level thereafter. Since she developed an allergic response to the contrast agent, we were unable to perform contrast CT as part of the follow-up. However, DWIBS was performed to follow the course of the lesion in the artery wall. On day 27, the signals from the wall of the right common carotid artery, left subclavian artery, and descending aorta had all decreased. These signals decreased further on days 76 and 132 (Fig. 5). We used DWIBS to monitor her disease activity. The PSL dose was decreased to 10 mg/day on day 66.
Figure 4.
Clinical course of the patient. CRP: C-reactive protein, IL-6: interleukin-6, PSL: prednisolone, TCZ: tocilizumab, TCZ-IV: intravenous infusion of tocilizumab, TCZ-SC: subcutaneous injection of tocilizumab
Figure 5.
(a) DWIBS showing signal enhancement in the wall of the thoracic aorta on day 0. The signal enhancement gradually decreased on (b) day 27, (c) day 76, and (d) day 132. DWIBS: diffusion-weighted whole-body imaging with background body signal suppression
Discussion
TAK is a large-vessel arteritis of unknown etiology. The disease is most common among women 15-40 years of age. TAK can also occasionally cause life-threatening vascular complications, including aortic regurgitation, aortic aneurysm, pulmonary hypertension, renal insufficiency, and refractory severe systemic hypertension. A prompt diagnosis and early initiation of proper treatment is therefore necessary for achieving a favorable outcome. Since TAK often recurs, monitoring the disease activity is important for preventing these vascular complications.
The initial diagnosis of TAK is made using serological markers, including CRP, ESR, and IL-6, combined with imaging modalities, including ultrasonography, CT angiography, MRI, and FDG-PET. According to the recommendations for the use of imaging in large-vessel vasculitis outlined by the European League Against Rheumatism (EULAR), MRI should be used as the first imaging test for diagnosing TAK (4). FDG-PET, CT, and ultrasound may be used as alternative imaging modalities in patients with suspected TAK. The sensitivity and specificity of these modalities for the diagnosis of TAK are listed in Table (5-7).
Table.
Sensitivity and Specificity of the Imaging Modalities Used in the Diagnosis of Takayasu’s Arteritis.
| Modality | Sensitivity (%) | Specificity (%) | ||
|---|---|---|---|---|
| CTA (5) | 95 | 100 | ||
| FDG-PET (6) | 92 | 100 | ||
| MRA (7) | 100 | 100 |
CTA: computed tomography angiography, FDG-PET: 18F-fluorodeoxyglucose positron emission tomography, MRA: magnetic resonance angiography
Although serological markers are commonly used to monitor the disease activity, these markers cannot be used alone to sufficiently evaluate the disease activity (8). In addition, TAK can relapse in the absence of elevated CRP and ESR. Kerr et al. reported that 44% of patients considered to be in remission display histologically active disease (9). Furthermore, while the serum IL-6 levels have been associated with the disease activity (10), the measurement of serum IL-6 is not yet standardized. Thus, imaging modalities are used in combination with serological markers to assess the disease activity. However, according to the EULAR recommendations, imaging is not routinely recommended for patients in clinical and biochemical remission (4), and these imaging modalities are associated with several problems.
Ultrasonography is a non-invasive, inexpensive, non-radiation modality. Furthermore, ultrasound data can be used to assess both the luminal status and vessel anatomy and can also identify early vessel wall changes before angiographically detectable lumen changes occur (11). In addition, ultrasound has a high resolution (approximately 0.1 mm) that is 10-fold greater than that of MRI (12); however, the results are operator-dependent, and ultrasound does not provide images of arterial segments (e.g., the thoracic aorta).
CT angiography enables the assessment of the vessel walls and lumen and can provide information regarding organ ischemia (13). However, this method is costly and exposes the patient to higher levels of radiation than other imaging modalities. CT also carries a risk of developing an iodine contrast allergy and contrast-induced nephropathy, making it impractical for frequent monitoring.
MRI can detect anatomical and pathophysiological changes, such as vascular lesions, inflammation sites, and wall thickening. T2-weighted short-tau inversion recovery (STIR) imaging is suitable for identifying inflammation in the artery wall, indicating edema. Budtz-Lilly et al. reported signal enhancement of the aortic wall in STIR sequencing (14); however, MRI can only evaluate limited regions at one time and is thus not suitable for a whole-body evaluation.
FDG-PET can assess the metabolic activity of the vascular wall, highlighting the regions of inflammation. FDG-PET is reported to have high sensitivity and specificity for the assessment of active TAK and correlates well with the disease activity (15). However, the FDG uptake is not specific to vessel wall inflammation. Thus, distinguishing between the FDG uptake due to active TAK and that due to subclinical atherosclerosis is difficult (16). Furthermore, FDG-PET is very expensive, not widely available, and exposes the patient to radiation.
DWIBS is a new imaging modality reported by Takahara et al. in 2004 (2). DWIBS is acquired using multiple signal averaging, prepulse fat suppression, and heavy diffusion weighting during free breathing. Diffusion-weighted imaging is based on the random movement of water at the molecular level. This imaging method provides a strong contrast between cancer and normal surrounding tissues and is therefore useful for cancer detection and staging. In addition, DWIBS detects a decrease in diffusion motion associated with interstitial edema and necrosis as signal enhancement (2). Since the diffusion of water molecules is suppressed in tumors and in acute inflammation, these conditions can be detected with DWIBS. Further advantages of DWIBS include its non-invasive nature, avoidance of exposure to ionizing radiation, and inexpensiveness compared to CT angiography in Japan. These characteristics make DWIBS an excellent tool for frequent monitoring. DWIBS also has a high sensitivity, equal to that of FDG-PET for diagnosing cancer (17). Further studies are needed to examine the use of DWIBS in other TAK patients.
In the present case, signal enhancement detected via DWIBS and vessel wall changes identified with contrast CT were observed in the same lesion. This signal enhancement likely reflects artery inflammation. We were unable to perform contrast CT imaging after treatment because the patient developed a contrast agent allergy. However, the level of IL-6 substantially decreased after treatment and remained relatively low, suggesting that the disease activity was adequately controlled. All signal enhancements detected with DWIBS decreased and continued to decline throughout the follow-up. Thus, we were able to successfully monitor the disease activity with DWIBS.
In summary, this report describes the first successful use of DWIBS for the evaluation of the disease activity in TAK. DWIBS may therefore be a novel imaging modality for assessing the disease activity of TAK, particularly during follow-up.
The authors state that they have no Conflict of Interest (COI).
References
- 1.Ishihara T, Haraguchi G, Tezuka D, Kamiishi T, Inagaki H, Isobe M. Diagnosis and assessment of Takayasu arteritis by multiple biomarkers. Circ J 77: 477-483, 2013. [DOI] [PubMed] [Google Scholar]
- 2.Takahara T, Imai Y, Yamashita T, Yasuda S, Nasu S, Van Cauteren M. Diffusion weighted whole body imaging with background body signal suppression (DWIBS): technical improvement using free breathing, STIR and high resolution 3D display. Radiat Med 22: 275-282, 2004. [PubMed] [Google Scholar]
- 3.Arend WP, Michel BA, Bloch DA, et al. The American College of Rheumatology 1990 criteria for the classification of Takayasu arteritis. Arthritis Rheum 33: 1129-1134, 1990. [DOI] [PubMed] [Google Scholar]
- 4.Dejaco C, Ramiro S, Duftner C, et al. EULAR recommendations for the use of imaging in large vessel vasculitis in clinical practice. Ann Rheum Dis 77: 636-643, 2018. [DOI] [PubMed] [Google Scholar]
- 5.Yamada I, Nakagawa T, Himeno Y, et al. Takayasu arteritis: evaluation of the thoracic aorta with CT angiography. Radiology 209: 103-109, 1998. [DOI] [PubMed] [Google Scholar]
- 6.Webb M, Chambers A, Al-Nahhas A, et al. The role of 18F-FDG PET in characterising disease activity in Takayasu arteritis. Eur J Nucl Med Mol Imaging 31: 627-634, 2004. [DOI] [PubMed] [Google Scholar]
- 7.Yamada I, Nakagawa T, Himeno Y, Kobayashi Y, Numano F, Shibuya H. Takayasu arteritis: diagnosis with breath-hold contrast-enhanced three-dimensional MR angiography. J Magn Reson Imaging 11: 481-487, 2000. [DOI] [PubMed] [Google Scholar]
- 8.Keser G, Direskeneli H, Aksu K. Management of Takayasu arteritis: a systematic review. Rheumatology (Oxford) 53: 793-801, 2014. [DOI] [PubMed] [Google Scholar]
- 9.Kerr GS, Hallahan CW, Giordano J, et al. Takayasu arteritis. Ann Intern Med 1;120: 919-929, 1994. [DOI] [PubMed] [Google Scholar]
- 10.Park MC, Lee SW, Park YB, Lee SK. Serum cytokine profiles and their correlations with disease activity in takayasu's arteritis. Rheumatology 45: 545-548, 2006. [DOI] [PubMed] [Google Scholar]
- 11.Romera-Villegas A, Vila-Coll R, Poca-Dias V, Cairols-Castellote MA. The role of color duplex sonography in the diagnosis of giant cell arteritis. J Ultrasound Med 23: 1493-1498, 2004. [DOI] [PubMed] [Google Scholar]
- 12.Schmidt WA, Blockmans D. Use of ultrasonography and positron emission tomography in the diagnosis and assessment of large-vessel vasculitis. Curr Opin Rheumatol 17: 9-15, 2005. [DOI] [PubMed] [Google Scholar]
- 13.Bryl M, Guziński M, Rabczyński M, et al. Imaging difficulties in Takayasu arteritis - case report and review of the literature. Pol J Radiol 77: 67-71, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Budtz-Lilly JW, Paaske W, Thrysoe SA, Andersen G. Takayasu's arteritis and the utility of magnetic resonance imaging. J Vasc Surg 56: 832, 2012. [DOI] [PubMed] [Google Scholar]
- 15.Pipitone N, Versari A, Salvarani C. Role of imaging studies in the diagnosis and follow-up of large-vessel vasculitis: an update. Rheumatology (Oxford) 47: 403-408, 2008. [DOI] [PubMed] [Google Scholar]
- 16.Yun M, Yeh D, Araujo LI, Jang S, Newberg A, Alavi A. F-18 FDG uptake in the large arteries: a new observation. Clin Nucl Med 26: 314-319, 2001. [DOI] [PubMed] [Google Scholar]
- 17.Ochiai R, Kobayashi H, Yoshida T, Kitagawa M, Ono K, Omagari J. Comparison of body diffusion weighted imaging using diffusion weighted whole body imaging with background body signal suppression (DWIBS) and 18FDG-PET for the detection of tumors. NICHIDOKU-IHO 50: 86-98, 2005(in Japanese, Abstract in English). [Google Scholar]