Abstract
A 61-year-old man with essential thrombocythemia (ET) presented with acute myocardial infarction (AMI) and underwent primary percutaneous coronary intervention. After stent deployment from the left main (LM) to the left anterior descending artery, intravascular ultrasound revealed thrombi formation in the whole stent. Two days later, optical frequency domain imaging confirmed stent malapposition and thrombi remaining in only the LM. The stent malapposition and ET might have contributed to this phenomenon. He underwent an additional stent expansion and aggressive anti-thrombotic regimen. AMI complicated with ET carries increased risks of in-stent thrombi formation and requires careful revascularization and aggressive pharmacotherapy.
Keywords: essential thrombocythemia, acute myocardial infarction, in-stent thrombi formation
Introduction
Essential thrombocythemia (ET) is a chronic myeloproliferative neoplasm of unknown cause characterized by an increased number of platelets. The disease provokes thrombosis and hemorrhaging as typical complications. The complication of thrombosis in particular affects the prognosis of patients with ET. Treatment strategies for ET are stratified by age, the number of platelets and the history of thrombosis and hemorrhaging. In general, anagrelide and hydroxycarbamide are effective in cases at a high risk of thrombosis.
Some cases of acute coronary syndrome (ACS) related to ET have been reported. However, treatment strategies for ACS with ET have not been sufficiently established. We herein report our experience of a case of acute myocardial infarction (AMI) complicated with in-stent massive thrombi formation, probably due to stent malapposition and ET. In addition, based on the present experience, we discuss strategies of primary percutaneous coronary intervention (PCI) and drug therapies after primary PCI.
Case Report
A 61-year-old man presented with acute onset of chest pain at rest. He called an ambulance and suffered cardiopulmonary arrest in the ambulance. He was transferred to our emergency department after successful defibrillation. He had a medical history of ET and had been treated with anagrelide 2 mg and clopidogrel 75 mg per day. His coronary risk factors were hypertension and dyslipidemia.
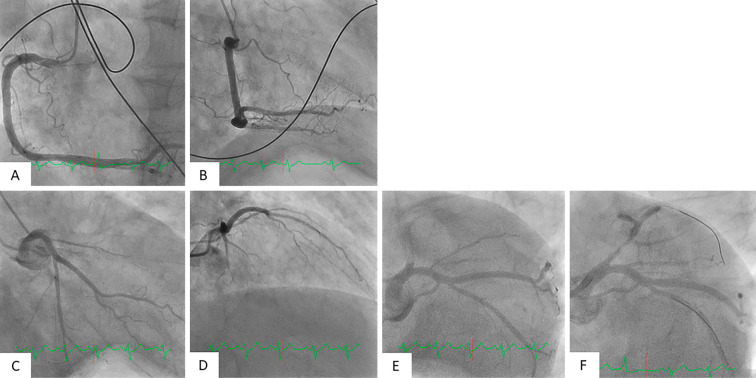
On an initial examination, his blood pressure was 162/98 mmHg, and his pulse rate was 105 bpm. A physical examination showed a regular heart rhythm with no murmurs, rough respiratory sounds in both lungs, and no edema in the lower extremities. Laboratory tests were almost normal except for leukocytosis and thrombocytosis (white blood cells: 14,470/μL, platelets: 86.3×104/μL). Electrocardiography showed ST-segment elevation in the V1 to V4 leads. Transthoracic echocardiography revealed severe hypokinesis in the anteroseptal wall. Emergency coronary angiography (CAG) showed total occlusion in only the proximal left anterior descending artery (LAD) and no significant stenosis in other coronary arteries (Fig. 1A-E).
Figure 1.
Findings of initial and post-reperfusion CAG. Initial and post-reperfusion CAG findings are shown. The right coronary artery (A, B) and left circumflex coronary artery had no severe stenosis. There was total occlusion from the LAD ostium (C, D, E). Initial CAG after recanalization showed thrombolysis in myocardial infarction (TIMI) grade II flow in the LAD (F).
After administration of oral aspirin 200 mg and intravenous heparin (total dose: 8,000 U), he underwent primary PCI. An intra-aortic balloon pump (IABP) was inserted via the right femoral artery, and a 7-Fr guiding catheter was advanced through the left radial artery to the left coronary artery ostium. A guidewire was successfully crossed into the diagonal branch distal to the occlusion, and initial recanalization was achieved by an aspiration thrombectomy catheter. Angiography showed thrombolysis in myocardial infarction (TIMI) grade II flow in the LAD (Fig. 1F). After advancing another guidewire to the LAD, intravascular ultrasound (IVUS) revealed diffuse plaque and considerable thrombi formation from the left main (LM) to the LAD. After pre-dilatation with a 2.5×13-mm balloon, a 4.0×24-mm drug-eluting stent (DES) was deployed from the LM to the LAD. Post-dilatation with a 3.5×12-mm balloon to the LAD and a 4.5×6.0-mm balloon to the LM was performed. Finally, another guidewire was re-crossed via the strut of the main branch stent to the left circumflex (LCX), and the stent strut was dilated from the LM to the LCX by a 3.0×4.0-mm balloon.
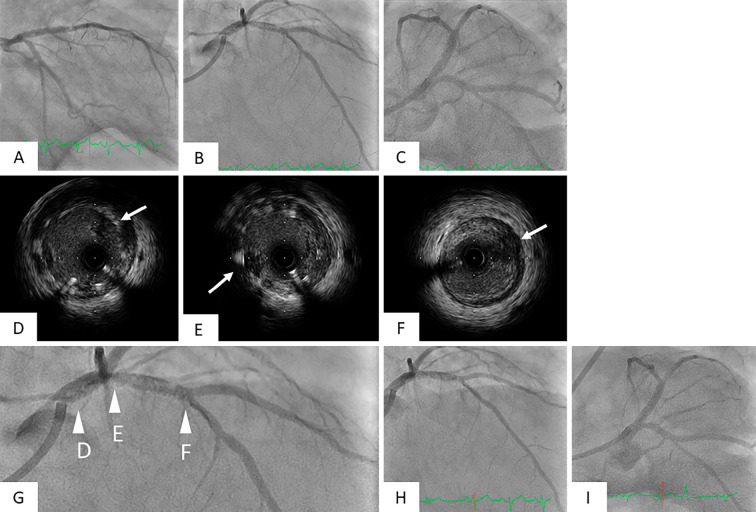
However, CAG revealed multiple dye defects in the stented lesion (Fig. 2A-C). Repeated IVUS revealed significant thrombi formation in the whole stent (Fig. 2D and E), although the activated clotting time (ACT) was sufficiently prolonged to 240 seconds. Argatroban and prasugrel 20 mg were immediately administered due to apprehension of heparin-induced thrombocytopenia (HIT) or clopidogrel resistance, and aspiration thrombectomy was repeatedly performed. Given the final IVUS findings of reduced thrombi in the stent and no obvious stent malapposition, we finished the primary PCI procedure (Fig. 2H and I).
Figure 2.
Findings of CAG and IVUS immediately after deploying a DES at primary PCI. CAG immediately after deploying a DES revealed a lot of defects in the stent (A, B, C). IVUS showed multiple thrombi in the whole stent and near the stent distal edge (D, E, F white arrow). Figure G is an enlarged version of Fig. B. The arrowheads of D, E, and F in Fig. G indicate the points of the IVUS images of Fig. D, E, and F. Final CAG revealed a reduction of thrombi in the stented lesion and thrombolysis in myocardial infarction (TIMI) grade III flow in the LAD (H, I).
After being transferred to the intensive-care unit, the patient continued to receive dual anti-platelet therapy (DAPT) with aspirin 100 mg and prasugrel 3.75 mg. In addition, ACT and activated partial thromboplastin time (APTT) were controlled with argatroban infusion (180 seconds < ACT <220 seconds or 1.5-3.0 times the reference value APTT) while IABP support was also provided. The peak creatine phosphokinase (CPK) level was 4,525 IU/L.
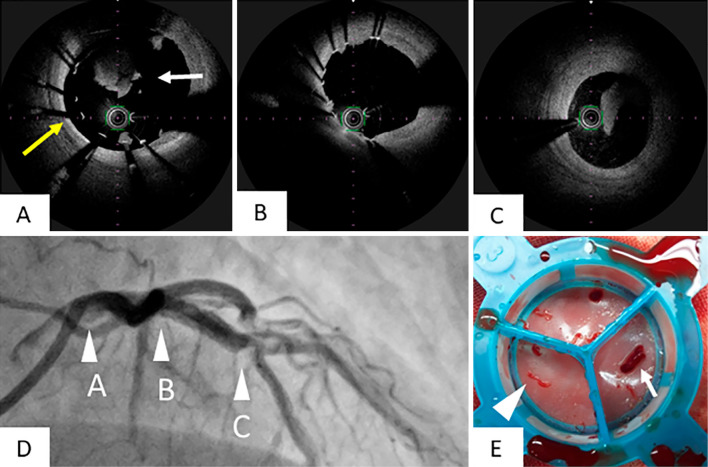
Two days after primary PCI (Day 3), CAG showed no apparent thrombi in the stent and moderate stenosis near the stent distal edge. Optical frequency domain imaging (OFDI) showed intimal dissection with thrombus near the stent distal edge and no thrombi in the LAD but revealed that in-stent thrombi and incomplete stent apposition still remained in the LM (Fig. 3A-C). Aspiration thrombectomy, intracoronary thrombolysis with urokinase (60,000 U) and additional stent expansion with a 5.0×6.0-mm balloon were performed. However, newer thrombi formation appeared in the guiding catheter during the procedure (Fig. 3E). Therefore, we decided to discontinue further PCI procedures for the moderate stenosis near the stent distal edge because of procedure-related risks of thrombi formation.
Figure 3.
Findings of CAG and OFDI 2 days later after primary PCI. We performed CAG and OFDI two days after primary PCI. In OFDI, we noted remnant thrombi (A, white arrow) and incomplete stent apposition in the LM (A, yellow arrow) and no thrombi and complete apposition in the LAD (B). Intimal dissection and thrombi were observed near the stent distal edge (C). The arrowheads of A, B, and C in Fig. D showed points of OFDI views of Fig. A, B, and C. The thrombus was retrieved using an aspiration catheter from the coronary artery (E, arrowhead) and guiding catheter (E, small arrow).
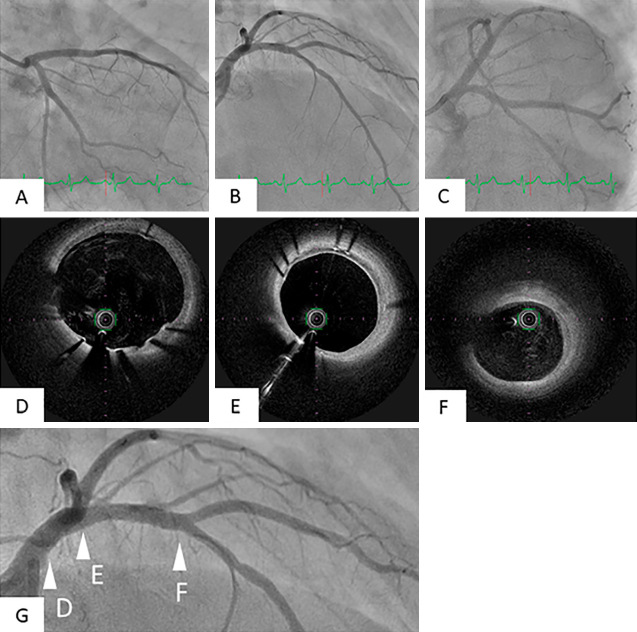
A further examination for excessive thrombophilia indicated no coagulopathy, including HIT. The dosage of anagrelide was increased from 2 to 3 mg, and apixaban 10 mg was added as an anti-coagulant drug. Platelet function testing showed that the effects of aspirin and prasugrel were in the therapeutic range. He had no additional thromboembolism or cardiovascular events during hospitalization and was discharged with a platelet count of 40.6×104/μL. The administration of apixaban was ended after three months because follow-up CAG revealed no restenosis, and the platelet count was able to be controlled within the normal range. CAG after nine months showed no in-stent restenosis and regression of the moderate stenosis in the mid-LAD (Fig. 4A-C). OFDI showed that the stent was completely covered with neointima over the full length, and the intimal dissection in the mid LAD was completely healed (Fig. 4D-F).
Figure 4.
Findings of follow-up CAG and OFDI nine months later. Follow-up CAG nine months later revealed no in-stent restenosis. In addition, the moderate stenosis in the mid LAD had improved (A, B, C). OFDI showed that the stent was completely covered with neointima over the full length with no thrombi from the LM to the LAD. (D, E, F). The arrowheads of D, E, and F in Fig. G showed points of OFDI views of Fig. D, E, and F.
Discussion
We experienced a case of AMI with ET that presented with massive in-stent thrombi formation in the culprit lesion. Our findings suggested that the primary causes of thrombi formation were the thrombophilia predisposition based on ET and the stent malapposition of the LM. We will now discuss potential drug treatments after PCI and strategies of primary PCI for cases of AMI with ET.
ET is a clonal disorder of multipotential stem cells and characterized by an absolute increase in the platelet count and an increase in vascular complications. Thrombosis is a major complication of ET and worsens the life expectancy of the patient (1). ACS is also a thrombotic complication of ET, and the incidence of MI in patients with ET was reported to be 9.4% (2). Even young patients with ET developed ACS in some cases (3,4), and ET might also be a risk factor of ACS. Treatment strategies for ACS with ET have not been fully established yet. Primary PCI (4-8), percutaneous transluminal coronary recanalization (9) and coronary artery bypass grafting (10) were previously reported as methods of revascularization. When AMI patients with ET present with massive thrombi in the culprit coronary lesions, aspiration thrombectomy or intracoronary thrombolysis without stent deployment might be optimal strategies for revascularization. In cases with underlying atherosclerotic plaque in the culprit lesions, additional balloon angioplasty or stent deployment should also be considered.
An underlying abnormal status of coagulation and fibrinolysis, such as HIT or anti-phospholipid antibody syndrome, might accelerate thrombi formation in the stented site. Some ACS patients with ET developed sub-acute thrombosis (SAT) after stent deployment. Possible reasons for SAT development were withdrawal of anti-platelet drugs because of bleeding complications in the puncture site (11) or the absence of cytoreductive drugs because of occult ET (12).
Adequate anti-platelet therapy for preventing thrombotic events and cytoreduction therapy for controlling platelet count play key roles in treating ET. Low-dose aspirin was recommended for preventing thrombotic events in ET cases (13,14). Although DAPT was also established after stent-based PCI for ACS or stable coronary artery disease, appropriate anti-platelet therapy after stent deployment for ACS patients with ET has not been established. Some reports describe the combination of aspirin and thienopyridines as the usual DAPT administered after PCI for ACS with ET, with these patients showing no major cardiac complications.
In the present patient, we considered the additional administration of other platelet P2Y12 inhibitors at loading doses, as clopidogrel resistance was suspected immediately after we confirmed significant thrombi formation in the whole stent by IVUS. It was reported that prasugrel and ticagrelor reduced the rate of MI or stent thrombosis (ST) compared with clopidogrel (15,16). The PRAGUE-18 trial reported that the primary endpoint (i.e., death, reinfarction, urgent vessel revascularization or serious bleeding) did not show any significant differences between ticagrelor and prasugrel (17). However, prasugrel and ticagrelor tended to increase the rate of major bleeding compared with clopidogrel. Based on these reports, if clopidogrel resistance is suspected, we suggest that prasugrel or ticagrelor at a loading dose be actively administered. Because it was reported that ticagrelor exhibited an antiplatelet effect earlier than prasugrel, ticagrelor at a loading dose might be suitable in cases of ST, such as in the present patient.
In our patient, an anticoagulant drug was administered in addition to DAPT after the second PCI in order to prevent stent thrombosis. We considered this anticoagulant drug to be effective because it exerted its antiplatelet action by suppressing thrombin generation. In ET cases complicated with recurrent thrombotic events (brachial artery occlusion) after PCI, warfarin in addition to DAPT and cytoreduction therapy was administered in order to prevent repeat thrombosis (18). The European Society of Cardiology stated that triple therapy with aspirin, clopidogrel and an anticoagulant drug should be considered in patients with a high ischemic risk due to ACS or other anatomical/procedure characteristics that outweigh the bleeding risk (19). Warfarin, in combination with aspirin or given alone, was superior to aspirin alone in reducing death or nonfatal reinfarction after MI (20). Furthermore, rivaroxaban (2.5 mg twice daily) plus aspirin had better cardiovascular outcomes than aspirin alone among patients with stable atherosclerotic vascular disease (21). However, these reports showed that the additional administration of warfarin or rivaroxaban increased the risk of major bleeding than aspirin alone. At present, two types of anticoagulants-vitamin K antagonists (VKAs) and direct oral anticoagulants (DOACs)-are available, and DOACs have been shown to be superior to VKAs in preventing bleeding complications (19). In cases with ST, we suggest that an anticoagulant in addition to DAPT is effective in terms of preventing reinfarction after MI, but patients must be closely monitored for complications of major bleeding. The present patient suffered neither ischemic events nor major bleeding complications during the triple therapy regimen.
Generally, thrombolytic therapy with or without PCI is not recommended for cases of ACS other than ST-elevated myocardial infarction, even if thrombus is involved (22,23). In the case of platelet-based thrombus, thrombolytic therapy promotes the activity of platelets. For cases that show massive thrombosis in coronary arteries, the use of platelet membrane glycoprotein (GP) IIb/IIIa inhibitors is recommended in other countries. However, because the effectiveness was not proven in the trial of GP IIb/IIIa inhibitors, no GP IIb/IIIa inhibitors are currently available in Japan (24). Systemic thrombolytic therapy has been reported to be effective for early coronary stent thrombosis (25). We therefore decided to administer a small amount of urokinase on the premise of performing PCI. However, it was difficult to determine whether or not thrombolytic therapy produced good outcomes in the present case. Because no GP IIb/IIIa inhibitors are currently available in Japan, it may be necessary to consider systemic thrombolytic therapy, such as urokinase or tissue plasminogen activator (t-PA), depending on the patient. Therefore, further evidence concerning thrombolytic therapy in patients with ST is desired.
It is also important to control the platelet count in patients who present with thromboembolism related to ET. Reducing the number of platelets is a particularly important treatment target in high-risk ET cases in order to prevent thrombus or hemorrhagic events, which may lead to serious outcomes. At present, anagrelide is approved for the treatment of ET or chronic myelogenous leukemia (26). It was reported that the main mechanism of action of thrombocytopenia is the suppression of platelet production by selectively acting on megakaryocytes, a precursor of platelets. The target platelet count using cytoreduction therapy is uncertain for thromboembolism related to ET after PCI. According to the British Society for Haematology guideline, the treatment target of platelet count should be <40×104/μL in high-risk patients (>60 years of age, ET-related thrombotic or hemorrhagic event, or a platelet count of >150×104/μL) (27). In our case, the platelet count was successfully controlled by increasing the anagrelide dosage.
Incomplete stent apposition to the culprit vessel wall may be a major cause of thrombus formation. In our case, stent malapposition that was not detected by the initial IVUS survey might have contributed to the massive thrombi formation. However, OFDI revealed that the stent had been successfully implanted in the LAD with complete strut apposition, and stent apposition was incomplete only in the LM. We believed that the stent deployed in primary PCI was not crimped to the vessel wall of the LM due to the large amount of thrombi. Furthermore, we were able to detect stent malapposition by OFDI two days after primary PCI because the thrombus attached to the LM disappeared following the administration of DAPT and an anticoagulant drug. Given these findings, we suggested that LM stent malapposition was a major factor influencing thrombi formation throughout the stented lesion in the first PCI procedure.
Primary stenting is an established revascularization strategy of PCI for ACS. However, we must consider the PCI strategy carefully, as stent deployment in the acute phase in ET cases carries a risk of SAT. If it seems that TIMI III can be obtained by aspiration thrombectomy or balloon expansion, it may be preferable to perform drug therapies or IABP without stent deployment. However, stent deployment is often necessary in clinical practice due to dissection by balloon expansion and massive thrombi. We therefore suggest that the minimum lumen area inside the stent must first be obtained by balloon expansion. In addition, it may be necessary to confirm complete stent apposition by OFDI or optical coherence tomography (OCT), which can assess the vascular wall more clearly than conventional IVUS.
According to previous reports regarding unstable angina, DAPT and cytoreductive drugs were administered before performing PCI, and PCI was then performed without complications such as distal embolization and SAT (18,28). Under stable conditions, sufficient drug therapy should precede PCI, whereas under unstable conditions, stent-less PCI might be preferable as a revascularization strategy among ACS patients with ET. When deploying a stent in cases of ACS with high platelet counts or a large amount of thrombi in the culprit lesion due to ET, we should carefully perform PCI in order to ensure complete stent apposition and consider an aggressive anti-thrombotic therapy, such as triple therapy including an anticoagulant drug.
Conclusion
We encountered a case of AMI with ET that presented with massive in-stent thrombi formation in the culprit lesion. Appropriate stent apposition and aggressive medication combined with anti-thrombotic regimen and cytoreductive drugs contributed to good clinical outcomes in this patient.
The authors state that they have no Conflict of Interest (COI).
References
- 1. Dan K, Yamada T, Kimura Y, et al. Clinical features of polycythemia vera and essential thrombocythemia in Japan: retrospective analysis of a nationwide survey by the Japanese Elderly Leukemia and Lymphoma Study Group. Int J Hematol 83: 443-449, 2006. [DOI] [PubMed] [Google Scholar]
- 2. Rossi C, Randi ML, Zerbinati P, Rinaldi V, Girolami A. Acute coronary disease in essential thrombocythemia and polycythemia vera. J Intern Med 244: 49-53, 1998. [DOI] [PubMed] [Google Scholar]
- 3. Pande S, Joshi R, Pande R. Essential thrombocythemia in a young man treated for myocardial infarction. BMJ Case Reports 3: 2010 (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bhat T, Ahmed M, Baydoun H, Ghandour Z, Bhat A, McCord D. Acute ST-segment elevation myocardial infarction in a young patient with essential thrombocythemia: a case with long-term follow-up report. J Blood Med 5: 123-127, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chang H, Shim CY, Cheong JW, et al. Coronary artery intervention after cytostatics treatment in unstable angina patient with essential thrombocythemia. A case report and literature review. Korean J Intern Med 21: 146-149, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Doesch C, Krämer B, Geisler T, et al. Challenges in the treatment of patients with essential thrombocythemia and acute coronary syndrome. J Thromb Thrombolysis 25: 193-197, 2008. [DOI] [PubMed] [Google Scholar]
- 7. Tortorella G, Calzolari M, Tieghi A, Muià N, Piccin A, Gugliotta L. Acute coronary syndrome (ACS) in patients with essential thrombocytemia (ET). What is the best treatment? Int J Cradiol 203: 225-227, 2016. [DOI] [PubMed] [Google Scholar]
- 8. Watanabe T, Fujinaga H, Ikeda Y. Acute myocardial infarction in a patient with essential thrombocythemia who underwent successful stenting-a case report. Angiology 56: 771-774, 2005. [DOI] [PubMed] [Google Scholar]
- 9. Mizuta E, Takeda S, Sasaki N, et al. Acute myocardial infarction in a patient with essential thrombocythemia: successful treatment with percutaneous transluminal coronary recanalization. Circ J 69: 1000-1002, 2005. [DOI] [PubMed] [Google Scholar]
- 10. Daya SK, Gowda RM, Landis WA, Khan IA. Essential thrombocythemia-related acute ST-segment elevation myocardial infarction. A case report and literature review. Angiology 55: 319-323, 2004. [DOI] [PubMed] [Google Scholar]
- 11. Turgut T, Harjai KJ, Edupuganti R, et al. Acute coronary occlusion and in-stent thrombosis in a patient with essential thrombocythemia. Cathet Cardiovasc Diagn 45: 428-433, 1998. [DOI] [PubMed] [Google Scholar]
- 12. Isilak Z, Tezcan M, Atalay M, Kardesoglu E. Acute myocardial infarction and sub-acute stent thrombosis associated with occult essential thrombocythemia. Chin Med J (Engl) 127: 3512-3513, 2014. [PubMed] [Google Scholar]
- 13. Tefferi A, Barbui T. Essential thrombocythemia and polycythemia vera: focus on clinical practice. Mayo Clin Proc 90: 1283-1293, 2015. [DOI] [PubMed] [Google Scholar]
- 14. Rumi E, Cazzola M. How I treat essential thrombocythemia. Blood 128: 2403-2414, 2016. [DOI] [PubMed] [Google Scholar]
- 15. Wiviott SD, Braunwald E, McCabe CH, et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 357: 2001-2015, 2007. [DOI] [PubMed] [Google Scholar]
- 16. Wallentin L, Becker RC, Budaj A, et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 361: 1045-1057, 2009. [DOI] [PubMed] [Google Scholar]
- 17. Motovska Z, Hlinomaz O, Miklik R, et al. Prasugrel versus ticagrelor in patients with acute myocardial infarction treated with primary percutaneous coronary intervention multicenter randomized PRAGUE-18 study. Circulation 134: 1603-1612, 2016. [DOI] [PubMed] [Google Scholar]
- 18. Kumagai N, Mitsutake R, Miura S, et al. Acute coronary syndrome associated with essential thrombocythemia. J Cardiol 54: 485-489, 2009. [DOI] [PubMed] [Google Scholar]
- 19. Valgimigli M, Bueno H, Byrne RA, et al. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: The Task Force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J 39: 213-260, 2018. [DOI] [PubMed] [Google Scholar]
- 20. Hurlen M, Abdelnoor M, Smith P, Erikssen J, Arnesen H. Warfarin, aspirin, or both after myocardial infarction. N Engl J Med 347: 969-974, 2002. [DOI] [PubMed] [Google Scholar]
- 21. Eikelboom JW, Connolly SJ, Bosch J, et al. Rivaroxaban with or without aspirin in stale cardiovascular disease. N Engl J Med 377: 1319-1330, 2017. [DOI] [PubMed] [Google Scholar]
- 22. Bar FW, Verheugt FW, Col J, et al. Thrombolysis in patients with unstable angina improves the angiographic but not the clinical outcome. Results of UNASEM, a multicenter, randomized, placebo-controlled, clinical trial with anistreplase. Circulation 86: 131-137, 1992. [DOI] [PubMed] [Google Scholar]
- 23. The Thrombolysis in Myocardial Ischemia (TIMI) III B investigators Effects of tissue plasminogen activator and a comparison of early invasive and conservative strategies in unstable angina and non-Q wave myocardial infarction. Results of TIMI IIIB trial. Circulation 89: 1545-1556, 1994. [DOI] [PubMed] [Google Scholar]
- 24. Nakagawa Y, Nobuyoshi M, Yamaguchi T, et al. Efficacy of abciximab for patients undergoing balloon angioplasty data from Japanese Evaluation of c7E3 Fab for Elective and Primary PCI Organization in Randomized Trial (JEPPORT). Circ J 73: 145-151, 2009. [DOI] [PubMed] [Google Scholar]
- 25. Koduganti SC, Shankar SJ. Systemic thrombolytic therapy for earlt coronary stent thrombosis. Indian Heart J 58: 245-248, 2006. [PubMed] [Google Scholar]
- 26. Silverstein MN, Petitt RM, Solberg LA Jr, Fleming JS, Knight RC, Schacter LP. Anagrelide: a new drug for treating thrombocytosis. N Eng J Med 318: 1292, 1988. [DOI] [PubMed] [Google Scholar]
- 27. Harrison CN, Bareford D, Butt N, et al. Guideline for investigation and management of adults and children presenting with a thrombocytosis. Br J Haematol 149: 352-375, 2010. [DOI] [PubMed] [Google Scholar]
- 28. Fujimura M, Akaike M, Kato M, et al. Aggressive antiplatelet therapy before coronary stent implantation in acute coronary syndrome with essential thrombocythemia-a case report. Angiology 54: 485-490, 2003. [DOI] [PubMed] [Google Scholar]