Abstract
Objective
Hydroxychloroquine (HCQ) was not approved in Japan until 2015, and its therapeutic potential has not been explored in depth. We evaluated the additional therapeutic effect of HCQ in Japanese patients with systemic lupus erythematosus (SLE) on maintenance therapy.
Methods
Patients with SLE who visited our hospital from 2015 to 2016 and were taking prednisolone (PSL) at <20 mg/day were retrospectively evaluated. All patients were divided into three groups according to their maintenance treatment regimen: PSL + immunosuppressant, PSL alone, and no treatment. We compared the changes in the SLE disease activity index (SLEDAI), PSL dose, and cumulative flare rate between patients who were and were not treated with HCQ.
Results
Among the 165 patients evaluated, 35 (21.2%) were treated with HCQ. The mean period of observation did not differ markedly between patients who did and did not receive HCQ (p=0.3). The SLEDAI and PSL dose were significantly reduced in patients who received HCQ, regardless of their background treatment regimen. The cumulative flare rate was lower in patients who received HCQ than in those who did not in the PSL + immunosuppressant and no maintenance treatment groups (p=0.03 and 0.05, respectively).
Conclusion
The addition of HCQ reduced the disease activity and allowed PSL dose reduction, regardless of background treatment, in Japanese patients with SLE.
Keywords: systemic lupus erythematosus, hydroxychloroquine, SLEDAI
Introduction
Hydroxychloroquine (HCQ) is an antimalarial drug that is recommended for patients with systemic lupus erythematosus (SLE) because of its beneficial effect on decreasing the risk of flares (1), diabetes mellitus (2), thrombotic events (3,4), and dyslipidaemia (5). HCQ also reportedly reduces damage accrual (6) and improves the survival (7).
Many investigators have recently examined the association between the blood HCQ concentration and the clinical outcome (8-11). According to Mok et al., an increased concentration of HCQ is associated with a reduced number of flares in patients in clinical remission (8). Yeon et al. examined factors related to the blood HCQ concentration in SLE patients and concluded that taking an additional immunosuppressant other than a corticosteroid is associated with increased HCQ concentrations (9). Therefore, the therapeutic effect of HCQ may differ depending on the background treatment.
Given that HCQ was not approved in Japan until 2015, its therapeutic potential remains poorly understood in the Japanese population. In one study, a randomized trial showed that the mean cutaneous lupus erythematosus disease area and severity index (CLASI) were significantly improved in the HCQ group compared with the placebo group among Japanese patients (12). However, the additional effects of HCQ on reducing disease activity other than skin manifestation have not been well investigated.
In the present study, we evaluated the additional therapeutic effects of HCQ in Japanese patients with SLE on background maintenance therapy.
Materials and Methods
Patients
We performed a retrospective study in Japanese patients who met the American College of Rheumatology (ACR) classification criteria for SLE (13) and who visited St. Marianna University Hospital from 2015 through 2016. Patients who were taking prednisolone (PSL) at <20 mg/day were selected and divided into 3 groups according to their maintenance treatment regimen: PSL + immunosuppressant (IS), PSL alone, and no treatment. We compared the clinical characteristics between the patients who were and were not treated with HCQ up to February 2018. In this study, HCQ users were those who had taken HCQ for more than 3 months with a daily dose exceeding 200 mg/day. Patients who were not taking HCQ at baseline or newly started HCQ during the study observation period were excluded. Patients who discontinued HCQ because of adverse events were also excluded from the efficacy analysis. Among the 154 non-HCQ users, 24 newly started HCQ during the observation period. Among the 37 HCQ users, 2 discontinued HCQ because of a skin rash. A total of 130 patients in the non-HCQ group and 35 in the HCQ group were therefore included in this study.
This study was approved by the Ethics Committee of St. Marianna University School of Medicine. Because the study had a retrospective cohort design that did not involve any investigations/interventions other than those for clinical use, written informed consent was not required. This study was carried out as per routine clinical care, and HCQ was initiated at the attending physician's discretion.
Data collection
Clinical information was obtained at baseline and at the last visit. Data included the demographic and clinical features, PSL dose, and SLE disease activity index (SLEDAI) (14). Changes in the SLEDAI and PSL dose were compared between patients who did and did not receive HCQ. The incidence of newly started PSL or hospitalization due to lupus activity was also determined. The incidence of flare during the observation period was assessed using the SLE flare index (SFI) (15); we investigated only moderate/mild and severe flare in this study. To evaluate the clinical features associated with flare, we divided all patients into two groups depending on the experience of flare. We also investigated flare using the revised-SFI (SFI-R) (16).
Statistical analyses
Continuous values are shown as the mean ± standard deviation. Differences between the two groups were analysed using the Mann-Whitney U-test for nonparametric data and the chi-squared test for categorical data. The cumulative flare rate was calculated using the Kaplan-Meier method, and differences between the two groups were tested using a log-rank test. To identify parameters that were independently associated with flare, we performed a multivariate analysis with the initial characteristics that were significantly different at the baseline assessment or deemed likely to affect flare, such as selecting HCQ use, no drugs, IS use, age, sex, disease duration, white blood cell (WBC) count, and SLEDAI, as covariates.
Results
Baseline clinical characteristics and treatment regimen
Of a total of 165 patients evaluated, 35 (21.2%) received HCQ. The clinical features at baseline were compared between the patients who did and did not receive HCQ separated by maintenance treatment (Table 1). The length of the observation periods among the groups was not significantly different. Patients in the PSL + IS group who did not receive HCQ were older, received a lower dose of PSL, and comprised a lower percentage of mycophenolate mofetil users than those on HCQ (p=0.04, 0.03, and <0.01, respectively). In the PSL group, the mean age of patients who did not receive HCQ was higher than that of those who did (p=0.04). The patients who did not receive HCQ in the no maintenance treatment group had higher lupus anticoagulant positivity than those who did receive HCQ (p=0.03).
Table 1.
Baseline Clinical Characteristics.
| PSL+IS | PSL | No maintenance treatment | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Clinical feature | HCQ (+) (n=19) |
HCQ (−) (n=71) |
HCQ (+) (n=11) |
HCQ (−) (n=49) |
HCQ (+) (n=5) |
HCQ (−) (n=10) |
||||||
| Sex (female), n (%) | 17 (89.5) | 62 (87.3) | 10 (90.1) | 49 (100.0) | 5 (100.0) | 10 (100.0) | ||||||
| Age (years) | 38.4±13.9 | 45.5±14.3* | 36.3±12.7 | 48.3±14.1¶ | 36.8±5.3 | 48.2±18.4 | ||||||
| Disease duration (months) | 102.1±111.7 | 121.2±122.1 | 95.0±112.9 | 204.7±313.3 | 61.3±75.2 | 41.5±65.3 | ||||||
| Observation (months) | 13.1±6.1 | 17.0±4.6 | 11.9±5.9 | 14.6±4.1 | 10.5±7.0 | 14.0±3.9 | ||||||
| WBC (×1,000/μL) | 6.7±2.6 | 5.8±2.4 | 6.2±2.4 | 5.8±2.4 | 3.7±0.9 | 4.3±1.9 | ||||||
| Hb (g/dL) | 11.2±2.1 | 12.1±1.5 | 11.4±1.6 | 12.3±1.7 | 12.6±0.7 | 11.3±2.1 | ||||||
| Plt (/μL) | 25.5±8.4 | 22.4±7.8 | 25.0±4.3 | 21.5±6.3 | 20.9±4.9 | 18.9±8.7 | ||||||
| SLEDAI | 3.8±3.8 | 3.4±4.4 | 2.8±2.6 | 2.2±3.9 | 3.0±2.2 | 4.5±5.3 | ||||||
| Proteinuria (g/gCr) | 0.4±0.8 | 0.6±1.3 | 0.1±0.1 | 0.4±1.7 | 0.4±0.5 | 0.3±0.6 | ||||||
| eGFR (mL/min) | 76.8±22.1 | 74.2±22.6 | 84.8±26.8 | 73.0±28.1 | 88.6±16.6 | 73.9±20.7 | ||||||
| CRP (mg/dL) | 0.3±0.7 | 0.2±0.5 | 0.3±0.4 | 0.3±0.6 | 0.1±0.1 | 0.8±1.8 | ||||||
| CH50 (U/mL) | 34.4±8.0 | 35.8±11.3 | 29.9±13.0 | 35.9±11.3 | 32.5±12.0 | 31.4±11.5 | ||||||
| LDL-C (mg/dL) | 93.2±17.8 | 104.0±33.9 | 97.2±16.5 | 116.0±30.4 | NA | NA | ||||||
| HbA1c, n (%) | 5.4±0.4 | 5.7±0.6 | 5.7±0.3 | 5.5±0.4 | NA | NA | ||||||
| Anti-Sm antibody positive, n (%) | 4 (21.1) | 15 (21.1) | 1 (9.1) | 4 (8.2) | 1 (20.0) | 4 (40.0) | ||||||
| Anti-dsDNA antibody (IU/mL) | 17.6±17.2 | 16.1±27.2 | 11.3±18.0 | 13.9±19.5 | 11.5±11.2 | 62.4±115.1 | ||||||
| Anti-cardiolipin antibody (IU/mL) | 15.0±16.2 | 13.9±13.7 | 10.0±4.3 | 8.5±2.7 | 8.3±0.6 | 9.7±3.0 | ||||||
| Lupus anticoagulant positive, n (%) | 5 (26.3) | 21 (29.6) | 4 (36.4) | 7 (14.3) | 1 (20.0) | 8 (80.0) § | ||||||
| Organ manifestation | ||||||||||||
| Skin, n (%) | 3 (15.8) | 11 (15.5) | 2 (18.2) | 12 (24.5) | 1 (20.0) | 0 (0) | ||||||
| Arthritis, n (%) | 3 (15.8) | 19 (26.8) | 2 (18.2) | 10 (20.4) | 1 (20.0) | 0 (0) | ||||||
| Cytopenia, n (%) | 8 (42.1) | 30 (43.7) | 6 (54.5) | 22 (44.9) | 4 (80.0) | 6 (60.0) | ||||||
| Serositis, n (%) | 0 (0) | 4 (5.6) | 1 (18.2) | 2 (4.1) | 0 (0) | 0 (0) | ||||||
| LN class III/IV, n (%) | 6 (31.6) | 14 (19.7) | 1 (9.1) | 3 (6.1) | 0 (0) | 0 (0) | ||||||
| NPSLE, n (%) | 1 (5.2) | 4 (5.6) | 1 (9.1) | 0 (0) | 0 (0) | 0 (0) | ||||||
| Others, n (%) | 2 (10.5) | 1 (1.4) | 0 (0) | 2 (4.1) | 1(20.0) | 0 (0) | ||||||
| Prednisolone (mg/day) | 8.0±4.2 | 5.9±3.6* | 6.8±3.8 | 6.6±4.5 | - | - | ||||||
| MMF use, n (%) | 10 (52.6) | 24 (33.8)* | - | - | - | - | ||||||
| TAC use, n (%) | 4 (21.1) | 26 (36.6) | - | - | - | - | ||||||
| CyA use, n (%) | 0 (0.0) | 8 (11.3) | ||||||||||
| MTX use, n (%) | 1 (5.3) | 3 (4.2) | - | - | - | - | ||||||
| AZP use, n (%) | 4 (21.1) | 16 (22.5) | - | - | - | - | - | |||||
* HCQ (−) vs. HCQ (+) in the PSL+IS group, p<0.05; ¶ HCQ (−) vs. HCQ (+) in the PSL group, p<0.05; § HCQ (−) vs. HCQ (+) in the no maintenance treatment group, p<0.05
PSL: prednisolone, IS: immunosuppressant, HCQ: hydroxychloroquine, NA: not available, WBC: white blood cells, Hb: hemoglobin, Plt: platelets, GFR: glomerular filtration rate, SLEDAI: Systemic Lupus Erythematosus Disease Activity Index, CRP: c-reactive protein, LDL-C: low-density lipoprotein cholesterol, dsDNA: double-stranded DNA, LN: lupus nephritis, NPSLE: neuropychiatric systemic lupus erythematosus, MMF: mycophenolate mofetil, TAC: tacrolimus, CyA: cyclosporine, MTX: methotrexate, AZA: azathioprine
Values indicate mean±standard deviation unless otherwise indicated.
Changes in the SLEDAI, PSL dose, and hospitalization due to flare
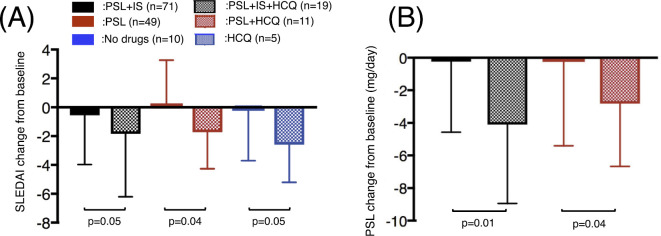
The SLEDAI and PSL dose were significantly lower in the HCQ group than in the non-HCQ group (Fig. 1). Furthermore, the degree by which they were reduced was similar, regardless of maintenance treatment (PSL + IS, PSL or no treatment). There were three patients who newly started PSL due to flare in the no treatment group. Hospitalization due to flare was seen in 3 patients (4.2%) in the PSL + IS group, 2 (4.1%) in the PSL group, and none in the no treatment group. There were no cases of hospitalization in the HCQ group.
Figure 1.
The changes in the SLEDAI and PSL dose between baseline and the final visit. The changes in the SLEDAI (A) and PSL (B) values are shown. PSL: prednisolone, IS: immunosuppressant, HCQ: hydroxychloroquine, SLEDAI: Systemic Lupus Erythematosus Disease Activity Index
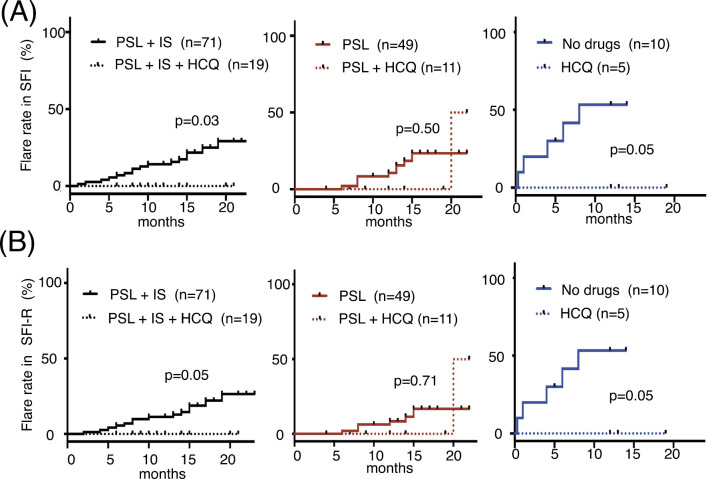
Cumulative flare rate
We also examined the flare rate (Fig. 2A). The cumulative flare rate was significantly lower in the patients who received HCQ in the PSL + IS and no maintenance treatment groups than in those who did not receive HCQ in those groups (p=0.03 and p=0.05, respectively). No significant difference in the flare rate was observed between patients who did and did not receive HCQ in the PSL group, as two patients on HCQ experienced flare. We next conducted the same analysis using the SFI-R (Fig. 2B). As there were no patients whose PSL was increased due solely to serological activity or cytopenia, the flare rate as assessed by the SFI-R was decreased compared to the SFI. There was one patient who experienced flare twice in the PSL + IS group during the observational period, while no cases were noted in the other groups.
Figure 2.
Cumulative flare rate. The cumulative flare rate was compared between patients who did and did not receive HCQ in the PSL+IS, PSL, and no maintenance treatment groups by the SFI (A) and R-SFI (B). SFI: systemic lupus erythematosus flare index, R-SFI: SFI-revised, PSL: prednisolone, IS: immunosuppressant, HCQ: hydroxychloroquine, SLEDAI: Systemic Lupus Erythematosus Disease Activity Index
Factors associated with flare
We next evaluated the clinical characteristics associated with flare. All of the patients were divided into two groups according to their history of flare (Table 2). The patients who had experienced flare had a significantly lower count of WBC, lower incidence of HCQ use, and higher incidence of no drugs than those with no such history (p<0.01, <0.01, and 0.02, respectively). A multivariate analysis revealed that HCQ use was negatively and no drug use was positively associated with flare [odds ratio (OR) 0.14, 95% confidence interval (CI) 0.007-0.71, p=0.01; and OR 4.21, 95% CI 1.09-16.36, p=0.04, respectively] (Table 3).
Table 2.
Baseline Clinical Characteristics Depending on Flare.
| Clinical feature | Flare (+) (n=29) |
Flare (−) (n=136) |
p | |||
|---|---|---|---|---|---|---|
| Sex (female), n (%) | 26 (89.7) | 127 (93.3) | 0.38 | |||
| Age (years) | 43.1±15.2 | 47.0±14.1 | 0.13 | |||
| Disease duration (months) | 115.5±104.8 | 160.1±220.2 | 0.32 | |||
| WBC (×103/μL) | 4.7±2.1 | 6.1±2.3 | <0.01 | |||
| Hb (g/dL) | 12.0±1.5 | 12.1±1.6 | 0.47 | |||
| Plt (104/μL) | 22.7±8.6 | 22.3±7.2 | 0.59 | |||
| SLEDAI | 2.3±3.4 | 2.7±3.6 | 0.55 | |||
| Proteinuria (g/gCr) | 0.2±0.6 | 0.3±1.0 | 0.44 | |||
| eGFR (mL/min) | 84.1±20.9 | 72.9±24.7 | 0.10 | |||
| CRP (mg/dL) | 0.4±1.3 | 0.3±0.6 | 0.53 | |||
| CH50 (U/mL) | 34.9±10.7 | 36.5±10.7 | 0.42 | |||
| Anti-Sm antibody positive, n (%) | 5 (17.2) | 11 (8.1) | 0.13 | |||
| Anti-dsDNA antibody (IU/mL) | 14.7±24.4 | 13.7±23.6 | 0.77 | |||
| Anti-cardiolipin antibody (IU/mL) | 13.3±11.4 | 12.2±12.3 | 0.46 | |||
| Lupus anticoagulant positive, n (%) | 8 (27.6) | 32 (23.5) | 0.64 | |||
| PSL use, n (%) | 24 (82.8) | 123 (90.4) | 0.22 | |||
| PSL (mg/day) | 5.1±4.0 | 6.0±4.3 | 0.27 | |||
| HCQ use, n (%) | 1 (3.4) | 30 (22.1) | <0.01 | |||
| IS use, n (%) | 1 (3.4) | 16 (11.7) | 0.18 | |||
| HCQ-monotherapy, n (%) | 0 (0) | 4 (2.9) | 0.35 | |||
| HCQ+PSL, n (%) | 0 (0) | 10 (7.3) | 0.13 | |||
| HCQ+PSL+IS, n (%) | 1 (3.4) | 16 (11.7) | 0.18 | |||
| Drug free, n (%) | 5 (17.2) | 5 (3.6) | 0.02 | |||
| PSL-monotherapy, n (%) | 7 (24.1) | 42 (30.9) | 0.67 | |||
| PSL+IS, n (%) | 16 (55.2) | 55 (40.4) | 0.14 |
Values indicate mean±standard deviation unless otherwise indicated.
WBC: white blood cells, Hb: hemoglobin, Plt: platelets, SLEDAI: Systemic Lupus Erythematosus Disease Activity Index, GFR: glomerular filtration rate, CRP: c-reactive protein, PSL: prednisolone, HCQ: hydroxychloroquine, IS: immunosuppressant
Table 3.
Univariate and Multivariate Analysis Associating with Flare.
| Parameters | Univariate | Multivariate | ||||||
|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | |||||
| HCQ use | 0.12 (0.007-0.61) | <0.01 | 0.14 (0.007-0.71) | 0.01 | ||||
| No drugs | 5.29 (1.37-20.41) | 0.02 | 4.21 (1.09-16.36) | 0.04 | ||||
| IS use | 0.25 (0.01-1.35) | 0.12 | ||||||
| Age (year) | 1.02 (0.99-1.05) | 0.17 | ||||||
| Female | 1.25 (0.27-4.37) | 0.74 | ||||||
| Disease duration (months) | 1.00 (0.99-1.00) | 0.17 | ||||||
| WBC (×1,000/μL) | 1.00 (1.00-1.01) | 0.99 | - | - | ||||
| SLEDAI | 1.03 (0.91-1.18) | 0.62 | - | - | ||||
HCQ: hydroxychloroquine, IS: immunosuppressant, WBC: white blood cells, SLEDAI: Systemic Lupus Erythematosus Disease Activity Index
Discussion
We found that patients who were treated with HCQ had a significantly lower SLEDAI and PSL dose, regardless of their background treatment, than those who were not treated with HCQ. We also showed that HCQ reduced the incidence of flare in the PSL + IS and no maintenance treatment groups. This report may have advantages over previous studies describing HCQ treatment for SLE in its investigation of the Japanese population and its evaluate of the efficacy of HCQ depending on the background treatment.
HCQ is recommended for patients with SLE. Although many reports have focused on the effect of HCQ in preventing disease flare (1) and reducing the damage accumulation (6,7), few investigations have examined its therapeutic effect in reducing the disease activity. Antimalarial agents exert their effects via multiple molecular pathways (17). One mechanism of action is Toll-like receptor antagonism. Antimalarial agents reportedly antagonize Toll-like receptor-mediated immune responses and interferon-α (IFN-α) synthesis (17). Since IFN-α has a crucial role in the pathogenesis of SLE (18), the inhibition of IFN-α has been proposed as a potential therapeutic strategy. A recent study showed that the anti-IFN-α receptor monoclonal antibody anifrolumab successfully reduced the disease activity in SLE patients (19). Therefore, HCQ may reduce the disease activity by inhibiting IFN-α production; our results support this potential mechanism of action. The reduction in the SLEDAI in patients treated with HCQ in our study suggests that HCQ has therapeutic potential for reducing the disease activity. HCQ may exert disease-modifying actions, and a further analysis is required to confirm this hypothesis.
Our data showed that an additional therapeutic effect of HCQ was almost equally observed regardless of the background treatment. Yeon et al. recently reported that adding one more IS increased the blood concentration of HCQ, which might lead to a favourable outcome (9). Previously conducted randomized trials comparing the effect of standard and adjusted HCQ dosing schedules on SLE flares concluded that adjusting the HCQ dose did not reduce the incidence of SLE flares (10). A higher concentration of HCQ might therefore be unnecessary to increase its efficacy. While we cannot draw any conclusions on the relationship between efficacy and HCQ concentration because we did not examine the HCQ concentration in our study, we can conclude that its clinical efficacy was not affected by the type of background treatment in a small number of patients with a short observation period.
This study is limited by its single-centre nature, relatively short observation period, and small sample size. Statistical significance might not have been reached because of the small sample size. Since this study was conducted retrospectively, some important information was missing, such as including the attending physician's impression when evaluating flare in the patients. Furthermore, the addition of HCQ might have influenced the physicians' decision to reduce the dose of PSL. Since patients in the no treatment group had been treated with no drugs prior to this study period, their disease condition might have differed from that of patients in other groups, so comparisons among these groups might not be appropriate. A multi-centre prospective study is required to confirm our findings.
In conclusion, the addition of HCQ may be beneficial, regardless of the background treatment, in Japanese patients with SLE.
The authors state that they have no Conflict of Interest (COI).
References
- 1.The Canadian Hydroxychloroquine Study Group.. A randomized study of the effect of withdrawing hydroxychloroquine sulfate in systemic lupus erythematosus. N Engl J Med 324: 150-154, 1991. [DOI] [PubMed] [Google Scholar]
- 2.Penn SK, Kao AH, Schott LL, et al. Hydroxychloroquine and glycemia in women with rheumatoid arthritis and systemic lupus erythematosus. J Rheumatol 37: 1136-1142, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaiser R, Cleveland CM, Criswell LA. Risk and protective factors for thrombosis in systemic lupus erythematosus: results from a large, multi-ethnic cohort. Ann Rheum Dis 68: 238-241, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tektonidou MG, Laskari K, Panagiotakos DB, Moutsopoulos HM. Risk factors for thrombosis and primary thrombosis prevention in patients with systemic lupus erythematosus with or without antiphospholipid antibodies. Arthritis Rheum 61: 29-36, 2009. [DOI] [PubMed] [Google Scholar]
- 5.Cairoli E, Rebella M, Danese N, Garra V, Borba EF. Hydroxychloroquine reduces low-density lipoprotein cholesterol levels in systemic lupus erythematosus: a longitudinal evaluation of the lipid-lowering effect. Lupus 21: 1178-1182, 2012. [DOI] [PubMed] [Google Scholar]
- 6.Alarcon GS, McGwin G, Bertoli AM, et al. Effect of hydroxychloroquine on the survival of patients with systemic lupus erythematosus: data from LUMINA, a multiethnic US cohort (LUMINA L). Ann Rheum Dis 66: 1168-1172, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Petri M, Purvey S, Fang H, Magder LS. Predictors of organ damage in systemic lupus erythematosus: the Hopkins Lupus Cohort. Arthritis Rheum 64: 4021-4028, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mok CC, Penn HJ, Chan KL, Tse SM, Langman LJ, Jannetto PJ. Hydroxychloroquine serum concentrations and flares of systemic lupus erythematosus: a longitudinal cohort analysis. Arthritis Care Res (Hoboken) 68: 1295-1302, 2016. [DOI] [PubMed] [Google Scholar]
- 9.Yeon Lee J, Lee J, Ki Kwok S, Hyeon Ju J, Su Park K, Park SH. Factors related to blood hydroxychloroquine concentration in patients with systemic lupus erythematosus. Arthritis Care Res (Hoboken) 69: 536-554, 2017. [DOI] [PubMed] [Google Scholar]
- 10.Costedoat-Chalumeau N, Galicier L, Aumaître O, et al. Hydroxychloroquine in systemic lupus erythematosus: results of a French multicentre controlled trial (PLUS Study). Ann Rheum Dis 72: 1786-1792, 2013. [DOI] [PubMed] [Google Scholar]
- 11.Lee SG, Park EK, Park JH, Kweon SM, Kim YK, Kim GT. Compliance and persistence with hydroxychloroquine in South Korean patients with systemic lupus erythematosus. Lupus 27: 753-761, 2018. [DOI] [PubMed] [Google Scholar]
- 12.Yokogawa N, Eto H, Tanikawa A, et al. Effects of hydroxychloroquine in patients with cutaneous lupus erythematosus: a multicenter, double-blind, randomized, parallel-group trial. Arthritis Rheumatol 69: 791-799, 2017. [DOI] [PubMed] [Google Scholar]
- 13.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 40: 1725, 1997. [DOI] [PubMed] [Google Scholar]
- 14.Bombardier C, Gladman DD, Urowitz MB, Caron D, Chang CH. Derivation of the SLEDAI. A disease activity index for lupus patients. The Committee on Prognosis Studies in SLE. Arthritis Rheum 35: 630-640, 1992. [DOI] [PubMed] [Google Scholar]
- 15.Petri M, Buyon J, Kim M. Classification and definition of major flares in SLE clinical trials. Lupus 8: 685-691, 1999. [DOI] [PubMed] [Google Scholar]
- 16.Thanou A, Chakravarty E, James JA, Merrill JT. How should lupus flares be measured? Deconstruction of the safety of estrogen in lupus erythematosus national assessment-systemic lupus erythematosus disease activity index flare index. Rheumatology (Oxford) 53: 2175-2181, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wallace DJ, Gudsoorkar VS, Weisman MH, Venuturupalli SR. New insights into mechanisms of therapeutic effects of antimalarial agents in SLE. Nat Rev Rheumatol 8: 522-533, 2012. [DOI] [PubMed] [Google Scholar]
- 18.Vallin H, Blomberg S, Alm GV, Cederblad B, Rönnblom L. Patients with systemic lupus erythematosus (SLE) have a circulating inducer of interferon-alpha (IFN-alpha) production acting on leucocytes resembling immature dendritic cells. Clin Exp Immunol 115: 196-202, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Furie R, Khamashta M, Merrill JT, et al. Anifrolumab, an anti-interferon-α receptor monoclonal antibody, in moderate-to-severe systemic lupus erythematosus. Arthritis Rheumatol 69: 376-386, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]