Abstract
Hepatocellular carcinoma (HCC) is the fifth most common cancer, and its incidence is rapidly increasing in North America and Western Europe as well as South-East Asia. Patients with advanced stage HCC have very poor outcomes; therefore, the discovery of new innovative approaches is urgently needed. Cancer immunotherapy has become a game-changer and revolutionized cancer treatment. A comprehensive understanding of tumor-immune interactions led to the development of immune checkpoint inhibitors (ICIs) as new therapeutic tools, which have been used with great success. Targeting immune checkpoint molecules such as programmed cell death-1 (PD-1) and cytotoxic T lymphocyte-associated protein-4 (CTLA-4) reinvigorates anti-tumor immunity by restoring exhausted T cells. Despite their effectiveness in several types of cancer, of the many immune suppressive mechanisms limit the efficacy of ICI monotherapy. Radiation therapy (RT) is an essential local treatment modality for a broad range of malignancies, and it is currently gaining extensive attention as a promising combination partner with ICIs because of its ability to trigger immunogenic cell death. The efficacy of combination approaches using RT and ICIs has been well documented in numerous preclinical and clinical studies on various types of cancers but not HCC. The application of ICIs has now expanded to HCC, and RT is recognized as a promising modality in HCC. This review will highlight the current roles of PD-1 and CTLA-4 therapies and their combination with RT in the treatment of cancers, including HCC. In addition, this review will discuss the future perspectives of the combination of ICIs and RT in HCC treatment.
Keywords: Hepatocellular carcinoma, Radiation therapy, Immune checkpoint inhibitors, Abscopal effect
Core tip: In the era of cancer immunotherapy, the roles of radiation therapy (RT) are not limited to the direct killing of cancerous cells but have expanded to immune modulation. Numerous proof-of-concept preclinical studies have proven that combination approaches of RT and immune checkpoint inhibitors (ICIs) are highly promising for many types of cancers, and currently, many related clinical trials are ongoing. Historically, RT was considered ineffective for hepatocellular carcinoma (HCC), but the efficacy of RT in HCC has improved greatly with technical advances. Our goal of this review is to provide a rationale for the combined treatment with RT and ICIs in HCC.
INTRODUCTION
Hepatocellular carcinoma (HCC), which is the predominant type of liver cancer, is the fifth most common cancer[1,2]. The incidence of HCC is relatively high in South-East Asia, and that in North America and Western Europe is increasing[3]. Although hepatitis B and C infection are the main causes of HCC, alcohol consumption, liver cirrhosis, non-alcoholic fatty liver disease, aflatoxin exposure, and hereditary disorders such as hemochromatosis and alpha-1-antitrypsin deficiency can be also the etiologies of HCC[4-6]. The Barcelona Clinic Liver Cancer (BCLC) system recommends various treatment strategies, including curative local modalities, such as resection, liver transplantation, and ablation, and palliative chemoembolization and sorafenib according to the BCLC stage, performance status, and liver function[7]. However, patients with advanced stage HCC have very poor outcomes[8] and novel approaches to greatly improve clinical outcomes are urgently needed.
Cancer immunotherapy has become a game-changer in cancer treatment, and we have witnessed how it has led to a paradigm shift in cancer therapy. Although surgical removal or elimination of tumor cells by chemotherapy or radiotherapy is still a mainstay treatment, the reinvigoration of the antitumor immunity in the tumor microenvironment (TME) is gaining growing attention. Tumors escape from immune surveillance using various mechanisms[9]. Among them, a better understanding of the immune checkpoint mechanism has led to new therapeutic targets for cancer therapy, the so-called immune checkpoint inhibitors (ICIs). Programmed cell death-1 (PD-1), programmed death-ligand 1 (PD-L1), and cytotoxic T lymphocyte-associated protein-4 (CTLA-4) are the main targets of ICIs[10]. To date, beginning with the approval of ipilimumab [anti-CTLA-4 monoclonal antibodies (mAbs)] for malignant melanoma in 2011, a number of ICIs, including three anti-PD-1 antibodies (nivolumab, pembrolizumab, and cemiplimab) and three anti-PD-L1 antibodies (atezolizumab, durvalumab, and avelumab), have been approved by the Food and Drug Administration (FDA) for different types of cancers such as melanoma and non-small cell lung cancer[9]. In addition, the use of nivolumab and pembrolizumab has now extended to patients with HCC who have been previously treated with sorafenib.
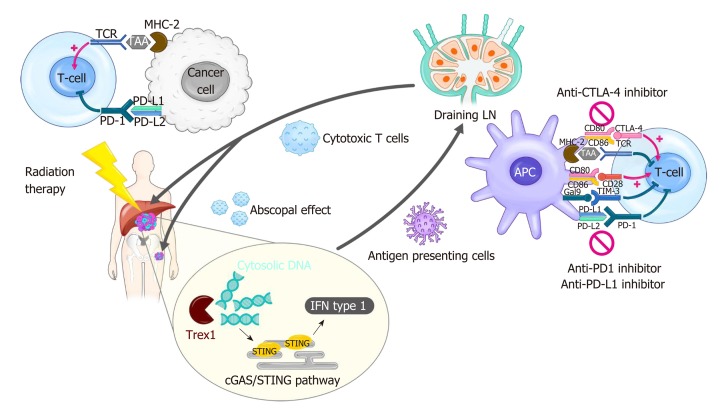
Radiation therapy (RT) has been used as an essential local treatment modality for a broad range of malignancies for over a century, and its immune-related effects have recently gained extensive attention in the era of immunotherapy[11,12]. Figure 1 illustrates the modulation of tumor immunity by RT and ICIs. RT has both pro-immunogenic and immunosuppressive effects on immune responses. RT triggers immunogenic cell death, which releases danger-associated molecular patterns (DAMPs) and primes immune cells, including dendritic cells (DCs), in the TME. RT also enhances immune cell infiltration by upregulating the expression of adhesion molecules on endothelial cells and the secretion of cytokines that can recruit cytotoxic T lymphocytes[13]. By contrast, RT directly kills radiosensitive CD8 effector T lymphocytes and preserves the less radiosensitive regulatory T lymphocytes (Tregs)[14]. Moreover, RT-induced production of transforming growth factor-beta (TGF-β) renders the TME more immunosuppressive[15]. RT-induced colony-stimulating factor-1 (CSF-1) also acts in immune suppression mechanisms such as the M2 polarization of tumor-associated macrophages and the recruitment of myeloid-derived suppressor cells (MDSCs)[16]. Furthermore, a substantial increase in the expression of PD-L1 and PD-1 in tumor cells and T lymphocytes, respectively, following RT weakens anti-tumor immunity, providing a rationale for combination treatment with ISIs.
Figure 1.
Modulation of tumor immunity by radiotherapy and immune checkpoint blockade. Radiation-induced cell death results in cytosolic DNA accumulation in the tumor, which in turn activates the production of type I interferon (IFN) genes via cGAS/STING pathway. Increased IFN activates antigen presenting cells such as dendritic cells (DCs), which can prime T cells within draining lymph node. IFN also mediates recruitment of effector CD8+ T cells capable of killing cancer cells into irradiated tumor sites. Radiation triggers the release of tumor antigens and danger-associated molecular patterns, which can also activate DCs. Radiation-induced secretion of cytokines and chemokines play both pro-immunogenic and immunosuppressive roles in the tumor microenvironment. The antitumor effect of radiation therapy (RT) is frequently hindered by activation of immune checkpoint pathways. Thus, the combination of RT and immune checkpoint inhibitors such as anti-programmed death 1 inhibitor shows a synergistic effect in many types of cancer. The immune checkpoint blockade also enhances RT-induced systemic effect, called abscopal effect, which refers to the regression of an unirradiated tumor. cGAS: Cyclic guanosine monophosphate-adenosine monophosphate synthase; CTLA-4: Cytotoxic T lymphocyte-associated protein 4; IFN: Interferon; LN: Lymph node; MHC: Major histocompatibility complex; PD-1: Programmed death 1; PD-L1: Programmed death-ligand 1; STING: Stimulator of interferon genes; TAA: Tumor-associated antigen; TCR: T-cell receptor; Trex1: Three prime repair exonuclease 1.
Numerous preclinical studies have provided convincing evidence that the combination of ICI and RT (iRT) can be more potent than either treatment alone[17]. The benefits of iRT have been reported in head and neck cancer, metastatic melanoma, metastatic pancreas cancer, and lung cancer[18,19], and clinical trials evaluating the outcomes of iRT are now ongoing[20]. The clinical use of immuno-therapy in the form of iRT has been extended to HCC[21], and several ongoing trials are investigating the benefits of immunotherapy for HCC[22]. In this review, we will discuss the basis of immunotherapy and iRT, and their application in HCC. Regarding immunotherapy, we will focus only on the CTLA-4 and PD-1/PD-L1 pathways in this review. Moreover, we will also discuss the future perspectives of immunotherapy and iRT for HCC.
IMMUNE CHECKPOINT INHIBITORS
The immunologic effect on the host has been an intriguing issue for the past several decades in cancer research. To date, a variety of cellular molecules relevant to the activation and inhibition of cancer immunity have been identified (Figure 1). Among these molecules, CTLA-4 and PD-1/PD-L1 have been proven to be effective targets for cancer immunotherapy, and their discovery opened a new landscape in cancer treatment[23,24].
CTLA-4 is an immune checkpoint receptor that is upregulated in activated T cells and constitutively expressed in Treg cells, and it negatively regulates the priming phase of the immune response. It outcompetes CD28 stimulatory protein for binding to CD80/CD86 (also called B7-1/2) located on the surface of antigen presenting cells (APCs), including DCs, and the interaction between CTLA-4/CD80 transmits inhibitory signals to T cells. CTLA-4 also facilitates immunosuppression by activating Tregs and upregulating indoleamine 2,3-dioxygenase (IDO) and IL-10 in DCs. Anti-CTLA-4 antibodies were designed to release T cells from the inhibitory signals and reactivate them, resulting in strong antitumor immunity[25]. Ipilimumab, the first humanized anti-CTLA4 mAb, produces remarkable responses in patients with metastatic melanoma[23]. Superior treatment outcomes following combination treatment with ipilimumab and nivolumab (PD-1 inhibitor) have been reported in advanced melanoma, although toxicities were higher with combination treatment than with monotherapy[26].
PD-1, firstly discovered in 1992, is another immune inhibitory receptor for the effector phase of the immune response[27]. It is primarily expressed by mature T cells in peripheral tissues and is also expressed in other immune cells including B Cells, natural killer (NK) cells, Tregs, MDSCs, and DCs. It has high binding affinity to PD-L1 (also called B7-H1), which is broadly expressed in hematopoietic cells such as APCs and MDSCs and non-hematopoietic cells such as parenchymal cells. The PD-1/PD-L1 interaction plays key roles in maintaining immune homeostasis in normal tissues. Tumor cells also express PD-L1, which allows them to escape immune surveillance in the TME. In the TME, antigen-specific T cells produce interferon-gamma (IFN-γ), which in turn induces PD-1 and PD-L1 expression on T cells and tumor cells, respectively. This ligand/receptor binding leads to T-cell exhaustion. Nivolumab was the first PD-1 inhibitor approved by the FDA; it was first approved for melanomas, followed by non-small-cell lung cancer and other cancers. Anti-PD-L1 antibodies such as atezolizumab, durvalumab, and avelumab were also developed to block the PD-1/PD-L1 axis and are now on the market.
ICI IN HCC
HCC has distinct characteristics compared to those of other cancers regarding the application of ICIs. Although a variety of etiologies link to the development of HCC, viral infection comprises the largest proportion, particularly in HBV and HCV epidemic areas[28]. The chronic inflammatory status stimulated by viral infection can effectively exhaust immune systems, thereby facilitating immune tolerance. Furthermore, the liver is an organ into which large amounts of antigens from the intestines drain via the portal vein, which also attenuates the immune surveillance system[29,30]. Indeed, pre-clinical and clinical studies have indicated that advanced HCC has a highly immunosuppressive TME, as indicated by intratumor CD8+ T cell exhaustion and inefficient T cell infiltration[31,32].
Hepatic immune tolerance is mainly mediated by specialized APCs such as resident DCs, liver sinusoidal endothelial cells (LSECs), Kupffer cells (KCs), and hepatic stellate cells (HSCs), as well as Treg cells and MDSCs[33]. They express inhibitory cytokines and immune checkpoint molecules including CTLA-4, PD-1, TIM-3, LAG-3 and BTLA[34]. Among them, PD-1 expression is high on effector CD8+ T cells within tumors of patients with HCC[35], which is associated with poor disease progression and postoperative recurrence[36]. Overexpression of PD-L1 is also seen on tumor cells as well as non-parenchymal liver cells such as KCs and LSECs, predicting tumor aggressiveness and postoperative recurrence in HCC[37]. In this background, ICIs were anticipated to be highly effective in HCC and therapeutic efficacy of CTLA-4 and PD-1/PD-L1 targeted therapies was clinically evaluated in HCC. Treatment-related hepatic toxicity is one of the most concerning issues when applying ICIs to patients with HCC because most patients with HCC have liver cirrhosis and are usually more vulnerable to systemic therapeutics than those with other malignancies.
The first study to evaluate the treatment outcomes of a CTLA-4 inhibitor, tremelimumab, was conducted in HCV-related HCC. This pilot clinical trial found a partial response rate of 17.6% and disease control rate of 76.4[38]. Interestingly, tremelimumab showed both anti-tumoral and anti-viral effects. Treatment-related hepatic toxicities were observed in approximately one-half of the patients, but they were all reversible. This success with tremelimumab encouraged the testing of other ICIs in HCC. In the CheckMate 040 trial, the feasibility of a PD-1 inhibitor, nivolumab, was evaluated in patients with HCC, finding an overall response rate of 15-20% with no severe toxicity[39]. Another phase 2 trial (KEYNOTE-224) investigating the efficacy of pembrolizumab in patients with advanced HCC who had been previously treated with sorafenib[40]. In this trial, the objective response rate was 17% and 12-mo progression-free survival and overall survival were 28% and 54%, respectively. While safety of combination treatment with PD-1 and CTLA-4 inhibitors could be proven in phase I/II, efficacy should be proven in phase III[41]. CheckMate-459 (NCT02576509) trial is an ongoing phase III trial to evaluate the role of nivolumab in frontline setting for advanced HCC[42]. Another ongoing phase III trial, Keynote-240 trial (NCT02702401), is to investigate the benefit of pembrolizumab in previously treated advanced HCC[43]. Most recently, Merck released update on Keynote-240 study. They announced that the patients who have been treated with pembrolizumab had superior OS to those treated with placebo, but the superiority failed to reach pre-specified statistical significance [HR=0.78 (95%CI: 0.611-0.998); P = 0.0238][44]. Although they failed to meet primary endpoints, favorable OS in pembrolizumab-treated group suggests that more evidence be needed to confirm the role of this drug. The final result from Keynote-240 and other ongoing trials are awaited. The combination treatment with durvalumab and tremelimumab showed better outcomes compared to monotherapy, but it showed higher toxicity rates. Based on the phase I/II trial outcomes, a large phase III trial, the HIMALAYA study, is ongoing to examine the feasibility of durvalumab and tremelimumab as first-line treatment for advanced HCC[45].
RADIOTHERAPY IN HCC
Traditionally, external beam RT (EBRT) has been limited role in the treatment of HCC[46]. Because of several obstacles facing the use of liver EBRT such as respiratory motion control, target delineation of hepatoma, and difficulty in image-guidance, few institutions had the technical ability to perform EBRT for patients with HCC[47]. Therefore, the role of EBRT was restricted in the palliation of metastatic HCC. In recent years, there has been growing interest in the use of EBRT in patients with HCC[46,48]. The recent technical advances ensure that high doses of radiation will be precisely delivered to the target in the liver while sparing the normal tissue[8]. As a result, EBRT has been utilized increasingly and practice guidelines of EBRT in HCC have been presented especially in Asian area[49,50].
The use of EBRT for primary HCC has various aims, including ablation of HCC; consolidation of other local treatments, mainly chemoembolization; bridging to liver transplantation; salvation from disease refractory to other treatment; and palliation[48,50-52]. Specifically, as higher doses of irradiation are widely attempted, stereotactic body RT (SBRT) or hypofractionated EBRT with ablative doses is increasingly used, with comparable local control compared to that with radiofrequency ablation[53-56]. Proton beam therapy (PBT) is another optimized EBRT tool for high-dose irradiation[57,58]. The physical properties of PBT with no exit dose minimize the integral doses in the normal liver parenchyma[59,60]. Therefore, PBT enables dose escalation without increasing the risk of radiation-induced liver toxicities, as evidenced by retrospective studies reporting that PBT showed excellent local control and low incidences of toxicities[57,58]. RT challenged the treatment guidelines of the BCLC system for HCC with macroscopic vascular invasion, which recommend sorafenib[8,61]. Recently, Yoon et al[62] showed in a phase II randomized controlled trial that the outcomes of chemoembolization followed by EBRT were superior to those of sorafenib in patients with HCC with macrovascular invasion, providing improved progression-free survival, objective response rate, time to progression, and overall survival. With the accumulation of evidence regarding the benefits of RT for primary HCC, other guidelines for the treatment of primary HCC have extended the range for the application of RT[50].
Aside from EBRT, selective internal RT (SIRT) using yttrium-90 (90Y) microspheres is also increasingly utilized in the treatment of primary HCC[63]. SIRT, also known as radioembolization, is a form of brachytherapy in which microspheres loaded with 90Y that emit high-energy beta radiation are administrated via a microcatheter positioned within the hepatic artery[64]. Because of the limited penetration depth of 2.5 mm of the beta-radiation, this approach spares much of normal liver tissue. The clinical application of SIRT is largely limited because of the lack of clinical evidence, but its roles in tumor control and immune activation are actively being investigated[65].
The role of palliative RT for HCC metastases has been expanded as well. Although the development of new systemic agents has improved the overall survival of patients with cancer, efficacious local modalities that palliate symptoms are frequently needed as the disease progresses[66]. In metastatic HCC, the need for palliative care has been raised, and technological advances have led to an increase in the use of palliative RT[67]. Bone metastasis from a primary HCC represents the type of soft tissue formation that requires high-dose irradiation for the long-term control of the tumor and symptoms[68] and in retrospective studies, the use of SBRT or hypofractionated RT with high doses was proven to be an effective way to control painful bone metastases from HCC[67,69,70]. This trend would be in accord with the scheme for iRT in which hypofractionated RT is preferred for immune boosting, as described below.
COMBINATION OF RT AND ICIs
Synergistic effects of RT and ICIs
Although ICIs have recently revolutionized cancer treatment, overall response rates remain around 20%, with much room for further improvement. RT is one of the most encouraging strategies that can be combined with ICIs to improve treatment outcomes because of its pro-immunogenic properties. On the other hand, the addition of ICIs could help overcome radiation resistance caused by radiation-induced immuno-suppressive effects. These synergistic effects of iRT have been proven in numerous preclinical studies and clinical settings[11,18,19,71,72]. Demaria et al[73] provided the first evidence of a synergistic effect when examining RT and CTLA-4 blockade using a murine 4T1 mammary carcinoma model; the survival of mice harboring a poorly immunogenic 4T1 tumor was not affected by CTLA-4 blockade alone but was significantly increased when combined with RT. Subsequently, the synergy between RT and PD-1 blockade was intensively investigated in preclinical settings[74-77]. For example, Zeng et al[74] showed that stereotactic radiation combined with anti-PD-1 antibodies markedly increased the survival of mice with intracranial gliomas with increased CD8+ T cells and decreased Treg cell infiltration. Another preclinical study using syngeneic mouse models revealed that PD-L1 blockade exerted a synergistical anti-tumor immunity with RT via MDSC reduction and cytotoxic T cell activation[78].
These lines of preclinical evidence provide a strong rationale for the use of combined iRT for cancer treatment, and numerous clinical evaluations are currently ongoing. A phase III trial (CA184-043) evaluating RT and ipilimumab in patients with metastatic castration-resistant prostate cancer that progressed after docetaxel chemotherapy has been conducted; post hoc subgroup analysis revealed a survival benefit with anti-CTLA-4 ipilimumab[79]. The efficacy of combining PD-1/PD-L1 blockade with RT has been tested more frequently in the clinical setting. A recent phase III PACIFIC trial for patients with unresectable stage III non-small lung cancer (NSCLC) receiving chemoradiation showed significant improvement of progression-free survival with anti-PD-L1 durvalumab vs placebo (median survival 16.8 mo vs 5.6 mo)[80]. According to a recent update of the PACIFIC trial, overall survival was also improved in the durvalumab group vs placebo group (2-year overall survival 66.3% vs 55.6%)[81]. The secondary analysis of the KEYNOTE-001 trial that studied patients with locally advanced or metastatic NSCLC treated with anti-PD-1 pembrolizumab revealed that patients who had previously received RT benefited from pembro-lizumab compared to patients without RT in terms of improved progression-free survival and overall survival with pembrolizumab[82].
RT is a type of local treatment but is also believed to induce systemic effects. An RT-induced systemic effect, called an “abscopal effect,” refers to the remission of a tumor outside the RT field. Because it was first reported in 1953[83], only anecdotal clinical evidence was reported for several decades[84]. However, in the era of cancer immunotherapy, abscopal effects are increasingly being reported when RT is given concomitantly with ICIs. Demaria et al[85] first showed evidence that an RT-induced abscopal effect is an immune-mediated response using a syngeneic mouse model bearing two tumors. Thereafter, numerous preclinical results using ICIs were reported[86-88]. Using PD-1 knockout mice, Park et al[88] showed that PD-1 blockade is a promising strategy to potentiate abscopal effects as well as anti-tumor effects caused by stereotactic ablative RT. In the clinical setting, Postow et al[89] reported the case of an abscopal effect in a patient with melanoma treated with anti-CTLA-4 ipilimumab and RT. Since then, several studies have reported that combined immunotherapy boosts the abscopal effects of RT, although the optimal dose/fraction size is still a matter of debate[84,90-92].
As described above, RT modulates immunity in the TME directly or indirectly, and a thorough understanding is important to design the optimal settings for iRT. RT exerts immunostimulatory effects by increasing CD8+ effector T cells[93] and type I IFN is a key modulator for their recruitment in response to RT[94]. The production of type I IFN required for anti-tumor immunity is mediated by stimulator of interferon genes (STING), and the upstream cyclic guanosine monophosphate-adenosine monophosphate synthase (cGAS) signaling pathways are initiated by sensing tumor-derived cytosolic DNA (Figure 1)[95-97]. On the other hand, RT exerts immune-inhibitory effects by upregulating PD-L1 expression or secreting cytokines such as TGF-β, which contributes to radio-resistance[98,99]. The balance between two opposite immune reactions depends on the radiation dose schemes, sequences between RT and ICIs, and treatment volume of radiation[100]. Therefore, these parameters need to be established in order to determine the optimal RT conditions that reach the maximum synergistic effect with ICIs.
Treatment sequences
When RT is given, ICIs can be administered before RT, after RT, or concurrently. Several preclinical studies have been performed to determine the optimal sequences of RT and ICIs. Dovedi et al[77] showed the concurrent administration of anti-PD-L1 mAb with RT of 2 Gy × 5 was more effective than their sequential application. On the other hand, another study showed that anti-CTLA-4 antibodies were most effective when given prior to RT[101], which reflects the function of CTLA-4 blockade in Treg depletion. Single large doses (> 20 Gy) of RT followed by anti-CTLA-4 also enhanced anti-tumor immunity in a Lewis lung carcinoma mouse model[102] and a mesothelioma mouse model[103], suggesting that dose and fraction size may also have an effect. A concurrent regimen for anti-PD-L1 and RT was also effective in a bladder cancer model[104]. Further retrospective or prospective studies are required to elucidate the optimal timing of RT that maximizes outcomes in patients co-treated with RT and ICIs.
Radiation dose and fractionation
It is widely accepted that SBRT or hypofractionated RT may be more immunogenic than conventional fractionated RT with 2 Gy per day. Lugade et al[105] showed that a single dose of 15 Gy boosted the immune reaction more effectively than a fractionated dose of 3 Gy × 5 in a B16 murine melanoma mouse model. Regarding the combination with ICIs, Dewan et al. reported that an 8 Gy × 3 regimen showed better local tumor control and a systemic abscopal effect than two other regimens, 20 Gy × 1 and 6 Gy × 5, plus anti-CTLA-4 mAb in a TSA mouse breast carcinoma model[86]. A recent study by Vanpouille-Box et al[106] provided an important mechanistic clue regarding the modulation of the immunogenic effect by different dose/fractionation schemes. They showed that 8 Gy × 3 but not a single 30 Gy dose when co-treated with anti-CTLA-4 antibodies induced abscopal responses with increased IFN-β production via the cGAS/STING pathway. A single radiation dose between 12 and 18 Gy upregulates the DNA exonuclease TREX, which in turn degrades cytoplasmic double-stranded DNA, resulting in turning off RT-induced immune stimulation. Thus, the use of a dose per fraction above 12 Gy for synergy with ICIs needs to be reconsidered.
Treatment volume
Draining lymph nodes (LNs) where APCs present antigens to T cells play a critical role in PD-1/PD-L1 therapy[75,107]. However, paradoxically, draining LNs are usually suspected to contain cancer cells as well as immune cells, which encourages radiation oncologists to encompass them in the RT field. In the setting of combination treatment with ICIs, the benefit of an RT target volume that includes draining LNs should be weighed against the risk of loss of tumor antigen-specific immune cells that help to reinvigorate anti-tumor immunity. Further studies to clarify the need for the modification of target delineation in the iRT setting may be warranted.
PRECLINICAL DATA AND ONGOING TRIALS FOR HCC
As described above, there are numerous studies and ongoing trials regarding the synergistic efficacy of ICIs and RT in various cancer types such as melanoma, head-and-neck cancer, non-small cell lung cancer, colorectal cancer, sarcoma, and renal cell carcinoma[11]. However, the studies on the combination effect in HCC are still underway, and just a few results have been reported to date. To the best of our knowledge, only two preclinical studies have been conducted to investigate the efficacy and the mechanism of ICIs and RT in murine HCC models[108,109]. Kim et al[108] showed that RT upregulated PD-L1 expression via IFN-γ/STAT3 signaling in the murine HCa-1 HCC cell line. The authors showed that the combination of anti-PD-L1 and RT of 10 Gy significantly suppressed Hca-1 tumor growth in syngeneic C3H mice and improved the survival of tumor-bearing mice compared to those with anti-PD-L1 alone or RT alone. The combination treatment increased the infiltration of effector CD8+ T cells and enhanced RT-induced apoptotic death within tumor tissue. These results suggest that iRT may hold promise as a potential strategy for HCC treatment. Another preclinical study supported the synergistic effect of the SBRT and anti-PD-1 combination in an orthotopic murine HCC model[109]. A Hep-55.1C tumor was injected into the liver right lobe, and 30 Gy in 3 fractions was delivered using a specialized irradiation system called the Small Animal Radiation Research Platform (SARRP). The combination of SBRT and anti-PD-1 antibodies markedly suppressed tumor growth and improved survival with increased infiltration of CD8+ cytotoxic T cells within the tumor.
There are only a limited number of studies on the combination effect of ICIs and RT in patients with HCC in the clinical setting. Kim et al[110] reported the clinical significance of the soluble PD-L1 level in blood samples from patients with HCC. The authors showed that EBRT significantly increased the soluble PD-L1 level and that a higher soluble PD-L1 level at 1 mo after EBRT was significantly associated with early lung metastasis and poor overall survival. Even though PD-1/PD-L1 therapy was not tested in this study, these findings support the rationale that iRT might be a promising therapeutic strategy in patients with HCC. In addition to the EBRT, the immune responses by the SIRT are under investigation. SIRT allows delivery of high-dose radiation up to 170 Gy penetrating the tissue with depth ranging from 2.5 to 11 mm[111]. Therefore, it was hypothesized that SIRT may have robust immunogenic effect by its similarity with high-dose EBRT with single fraction. However, there is very few literatures and only a case report showing the enhancement of the antitumoral immune response by the combination of nivolumab and SIRT with 90Y[112]. An analysis of tumor-infiltrating leukocytes isolated from patients with HCC after 90Y radioemboliztion revealed SIRT resulted in higher tumor infiltration of CD8+ T cells, and CD8+ and/or CD56+ NK cells and higher expression of tumor necrosis factor-α on both the CD8 and CD4 T cells and APCs in peripheral blood[65]. These implied that SIRT with 90Y enabled the activation of both local and systemic immune and potential of synergy by combination with ICI. There are needs for clinical data regarding the iRT and now several ongoing clinical trials are being conducted to investigate the efficacy of iRT using various ICIs and EBRT or SIRT for patients with HCC. The trials registered in www.ClinicalTrials.gov are listed in Table 1.
Table 1.
On-going clinical trials for combination of immune checkpoint inhibitor and radiation
| NCT number | Institution | Phase | Disease | Intervention | Estimated enrollment | Primary endpoint |
| NCT03482102 | United States (MGH) | II | Locally advanced/ unresectable or metastatic disease HCC or biliary tract cancer | Experimental: Tremelimumab + Durvalumab + EBRT | 70 | Best overall response rate |
| NCT03203304 | United States (UCh) | I | HCC | SBRT Nivolumab → +/- ipilimumab + SBRT (8 Gy × 5) | 50 | Number of participants with adverse events |
| NCT03316872 | Canada (UHN) | II | HCC showing progression after sorafenib | Pembrolizumab + SBRT | 30 | Overall response rate |
| NCT03812562 | United States (NU) | I | HCC intended to be resected | 90Y SIRT → Nivolumab | 12 | Recurrence rate |
| NCT03033446 | Singapore (NCC) | II | HCC not suitable for resection or transplant | 90Y SIRT → Nivolumab | 40 | Response rate |
| NCT02837029 | United States (NU) | I/Ib | HCC stage IIIA - IVB | 90Y SIRT → Nivolumab | 35 | Maximum tolerated dose |
| NCT03099564 | United States (UNC) | I | HCC | 90Y SIRT + Pembrolizumab | 30 | Progression-free survival |
MGH: Massachusetts General Hospital; HCC: Hepatocellular carcinoma; EBRT: External beam radiotherapy; UCh: University of Chicago; SBRT: Stereotactic body radiotherapy; UHN: University Health Network; NU: Northwestern University; SIRT: Selective internal radiation therapy; NCC: National Cancer Center; UNC: University of North Carolina.
CONCLUSION
The advent of immunotherapy using ICIs had a great impact on melanoma treatment and is now expanding to the treatment of gastrointestinal malignancies, including HCC. Although the use of iRT in HCC treatment is anticipated, there remain many issues to be investigated. First, ICIs should not be considered the best partners to use with RT in HCC. Aside from ICIs, other immunotherapeutic strategies such as cytokine-induced killer cells or gene therapy using adenoviral vectors have already been assessed in patients with HCC[113,114]. The potential of IL-12 with RT was tested in a murine model, demonstrating that the combined therapy of RT and IL-12 showed better tumor regression compared to each treatment alone; however, this has not been evaluated in the clinical setting yet[115].
Another issue is that an optimized RT methodology for use in combination with ICIs has not been established. Although large amounts of preclinical and clinical data suggest optimal schemes for dose-fractionation, the timing of RT, and target delineation as described above, there is no general consensus that can be applied in the clinic. Further efforts are needed to understand the nature of RT-induced immune responses. In addition, RT-induced lymphopenia may suppress reinvigoration by ICIs, so a specialized RT protocol that can minimize lymphopenia needs to be newly designed for iRT[116]. The combination of ICIs and particle beam therapies is an emerging research field. Because of its dosimetric benefits, particle beam therapy can potentially prevent lymphopenia as well as save the normal liver. The immunologic effects of particle beam therapy are being investigated in other types of cancers such as NSCLC[117]. To date, the clinical trials to assess iRT have been focused on the treatment of primary HCC. However, iRT can potentially be effective in systemic disease control via abscopal effects[84]. As with other types of cancers in which the application of iRT for metastatic disease has been tested, iRT for the control of metastatic HCC can be considered a future new therapeutic strategy.
Finally, the appropriate selection of good candidates for iRT is required to improve the outcomes in patients with HCC. PD-L1 expression is a predictive biomarker for PD-1/PD-L1 blockade in patients with NSCLC, but it has not been tested in patients with HCC. Tumor mutation burden and infiltration of effector T lymphocytes are emerging biomarkers[118], but they also have not been evaluated in HCC. Next-generation sequencing-based profiling of tumor mutation burden, immune gene expression signatures, T-cell receptor repertoire, T-cell-inflamed gene expression, and the microbiome could help to predict the suitability of patients with HCC for iRT[118,119]. Such genetic information is useful for predicting not only the susceptibility to ICIs but also the individual radiation sensitivity[120-122]. Therefore, further efforts to identify the biomarkers to guide the selection of patients with HCC who are appropriate for iRT are needed.
Footnotes
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: South Korea
Peer-review report classification
Grade A (Excellent): A, A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
Conflict-of-interest statement: No potential conflicts of interest. No financial support.
Peer-review started: February 22, 2019
First decision: March 27, 2019
Article in press: April 19, 2019
P-Reviewer: Edeline J, Que JY S-Editor: Ma RY L-Editor: A E-Editor: Zhang YL
Contributor Information
Changhoon Choi, Department of Radiation Oncology, Samsung Medical Center, Seoul 06351, South Korea.
Gyu Sang Yoo, Department of Radiation Oncology, Samsung Medical Center, Seoul 06351, South Korea.
Won Kyung Cho, Department of Radiation Oncology, Samsung Medical Center, Seoul 06351, South Korea.
Hee Chul Park, Department of Radiation Oncology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul 06351, South Korea. hee.ro.park@samsung.com.
References
- 1.Lafaro KJ, Demirjian AN, Pawlik TM. Epidemiology of hepatocellular carcinoma. Surg Oncol Clin N Am. 2015;24:1–17. doi: 10.1016/j.soc.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 2.Ghouri YA, Mian I, Rowe JH. Review of hepatocellular carcinoma: Epidemiology, etiology, and carcinogenesis. J Carcinog. 2017;16:1. doi: 10.4103/jcar.JCar_9_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mittal S, El-Serag HB. Epidemiology of hepatocellular carcinoma: consider the population. J Clin Gastroenterol. 2013;47 Suppl:S2–S6. doi: 10.1097/MCG.0b013e3182872f29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fattovich G, Stroffolini T, Zagni I, Donato F. Hepatocellular carcinoma in cirrhosis: incidence and risk factors. Gastroenterology. 2004;127:S35–S50. doi: 10.1053/j.gastro.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 5.El-Serag HB. Hepatocellular carcinoma. N Engl J Med. 2011;365:1118–1127. doi: 10.1056/NEJMra1001683. [DOI] [PubMed] [Google Scholar]
- 6.Zhang DY, Friedman SL. Fibrosis-dependent mechanisms of hepatocarcinogenesis. Hepatology. 2012;56:769–775. doi: 10.1002/hep.25670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bruix J, Raoul JL, Sherman M, Mazzaferro V, Bolondi L, Craxi A, Galle PR, Santoro A, Beaugrand M, Sangiovanni A, Porta C, Gerken G, Marrero JA, Nadel A, Shan M, Moscovici M, Voliotis D, Llovet JM. Efficacy and safety of sorafenib in patients with advanced hepatocellular carcinoma: subanalyses of a phase III trial. J Hepatol. 2012;57:821–829. doi: 10.1016/j.jhep.2012.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yu JI, Park HC. Radiotherapy as valid modality for hepatocellular carcinoma with portal vein tumor thrombosis. World J Gastroenterol. 2016;22:6851–6863. doi: 10.3748/wjg.v22.i30.6851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Okusaka T, Ikeda M. Immunotherapy for hepatocellular carcinoma: current status and future perspectives. ESMO Open. 2018;3:e000455. doi: 10.1136/esmoopen-2018-000455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elsegood CL, Tirnitz-Parker JE, Olynyk JK, Yeoh GC. Immune checkpoint inhibition: prospects for prevention and therapy of hepatocellular carcinoma. Clin Transl Immunology. 2017;6:e161. doi: 10.1038/cti.2017.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang Y, Deng W, Li N, Neri S, Sharma A, Jiang W, Lin SH. Combining Immunotherapy and Radiotherapy for Cancer Treatment: Current Challenges and Future Directions. Front Pharmacol. 2018;9:185. doi: 10.3389/fphar.2018.00185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Van Limbergen EJ, De Ruysscher DK, Olivo Pimentel V, Marcus D, Berbee M, Hoeben A, Rekers N, Theys J, Yaromina A, Dubois LJ, Lambin P. Combining radiotherapy with immunotherapy: the past, the present and the future. Br J Radiol. 2017;90:20170157. doi: 10.1259/bjr.20170157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jiang W, Chan CK, Weissman IL, Kim BYS, Hahn SM. Immune Priming of the Tumor Microenvironment by Radiation. Trends Cancer. 2016;2:638–645. doi: 10.1016/j.trecan.2016.09.007. [DOI] [PubMed] [Google Scholar]
- 14.Kachikwu EL, Iwamoto KS, Liao YP, DeMarco JJ, Agazaryan N, Economou JS, McBride WH, Schaue D. Radiation enhances regulatory T cell representation. Int J Radiat Oncol Biol Phys. 2011;81:1128–1135. doi: 10.1016/j.ijrobp.2010.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barcellos-Hoff MH, Derynck R, Tsang ML, Weatherbee JA. Transforming growth factor-beta activation in irradiated murine mammary gland. J Clin Invest. 1994;93:892–899. doi: 10.1172/JCI117045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu J, Escamilla J, Mok S, David J, Priceman S, West B, Bollag G, McBride W, Wu L. CSF1R signaling blockade stanches tumor-infiltrating myeloid cells and improves the efficacy of radiotherapy in prostate cancer. Cancer Res. 2013;73:2782–2794. doi: 10.1158/0008-5472.CAN-12-3981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kalbasi A, June CH, Haas N, Vapiwala N. Radiation and immunotherapy: a synergistic combination. J Clin Invest. 2013;123:2756–2763. doi: 10.1172/JCI69219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang SJ, Haffty B. Radiotherapy as a New Player in Immuno-Oncology. Cancers (Basel) 2018;10 doi: 10.3390/cancers10120515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sharabi AB, Lim M, DeWeese TL, Drake CG. Radiation and checkpoint blockade immunotherapy: radiosensitisation and potential mechanisms of synergy. Lancet Oncol. 2015;16:e498–e509. doi: 10.1016/S1470-2045(15)00007-8. [DOI] [PubMed] [Google Scholar]
- 20.Kang J, Demaria S, Formenti S. Current clinical trials testing the combination of immunotherapy with radiotherapy. J Immunother Cancer. 2016;4:51. doi: 10.1186/s40425-016-0156-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mukaida N, Nakamoto Y. Emergence of immunotherapy as a novel way to treat hepatocellular carcinoma. World J Gastroenterol. 2018;24:1839–1858. doi: 10.3748/wjg.v24.i17.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kudo M. Immune Checkpoint Inhibition in Hepatocellular Carcinoma: Basics and Ongoing Clinical Trials. Oncology. 2017;92 Suppl 1:50–62. doi: 10.1159/000451016. [DOI] [PubMed] [Google Scholar]
- 23.Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, Akerley W, van den Eertwegh AJ, Lutzky J, Lorigan P, Vaubel JM, Linette GP, Hogg D, Ottensmeier CH, Lebbé C, Peschel C, Quirt I, Clark JI, Wolchok JD, Weber JS, Tian J, Yellin MJ, Nichol GM, Hoos A, Urba WJ. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, Pitot HC, Hamid O, Bhatia S, Martins R, Eaton K, Chen S, Salay TM, Alaparthy S, Grosso JF, Korman AJ, Parker SM, Agrawal S, Goldberg SM, Pardoll DM, Gupta A, Wigginton JM. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science. 1996;271:1734–1736. doi: 10.1126/science.271.5256.1734. [DOI] [PubMed] [Google Scholar]
- 26.Wolchok JD, Chiarion-Sileni V, Gonzalez R, Rutkowski P, Grob JJ, Cowey CL, Lao CD, Wagstaff J, Schadendorf D, Ferrucci PF, Smylie M, Dummer R, Hill A, Hogg D, Haanen J, Carlino MS, Bechter O, Maio M, Marquez-Rodas I, Guidoboni M, McArthur G, Lebbé C, Ascierto PA, Long GV, Cebon J, Sosman J, Postow MA, Callahan MK, Walker D, Rollin L, Bhore R, Hodi FS, Larkin J. Overall Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N Engl J Med. 2017;377:1345–1356. doi: 10.1056/NEJMoa1709684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ishida Y, Agata Y, Shibahara K, Honjo T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 1992;11:3887–3895. doi: 10.1002/j.1460-2075.1992.tb05481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shlomai A, de Jong YP, Rice CM. Virus associated malignancies: the role of viral hepatitis in hepatocellular carcinoma. Semin Cancer Biol. 2014;26:78–88. doi: 10.1016/j.semcancer.2014.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bogdanos DP, Gao B, Gershwin ME. Liver immunology. Compr Physiol. 2013;3:567–598. doi: 10.1002/cphy.c120011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Doherty DG. Immunity, tolerance and autoimmunity in the liver: A comprehensive review. J Autoimmun. 2016;66:60–75. doi: 10.1016/j.jaut.2015.08.020. [DOI] [PubMed] [Google Scholar]
- 31.Willimsky G, Schmidt K, Loddenkemper C, Gellermann J, Blankenstein T. Virus-induced hepatocellular carcinomas cause antigen-specific local tolerance. J Clin Invest. 2013;123:1032–1043. doi: 10.1172/JCI64742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Flecken T, Schmidt N, Hild S, Gostick E, Drognitz O, Zeiser R, Schemmer P, Bruns H, Eiermann T, Price DA, Blum HE, Neumann-Haefelin C, Thimme R. Immunodominance and functional alterations of tumor-associated antigen-specific CD8+ T-cell responses in hepatocellular carcinoma. Hepatology. 2014;59:1415–1426. doi: 10.1002/hep.26731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Crispe IN. Immune tolerance in liver disease. Hepatology. 2014;60:2109–2117. doi: 10.1002/hep.27254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Prieto J, Melero I, Sangro B. Immunological landscape and immunotherapy of hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2015;12:681–700. doi: 10.1038/nrgastro.2015.173. [DOI] [PubMed] [Google Scholar]
- 35.Wang BJ, Bao JJ, Wang JZ, Wang Y, Jiang M, Xing MY, Zhang WG, Qi JY, Roggendorf M, Lu MJ, Yang DL. Immunostaining of PD-1/PD-Ls in liver tissues of patients with hepatitis and hepatocellular carcinoma. World J Gastroenterol. 2011;17:3322–3329. doi: 10.3748/wjg.v17.i28.3322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shi F, Shi M, Zeng Z, Qi RZ, Liu ZW, Zhang JY, Yang YP, Tien P, Wang FS. PD-1 and PD-L1 upregulation promotes CD8(+) T-cell apoptosis and postoperative recurrence in hepatocellular carcinoma patients. Int J Cancer. 2011;128:887–896. doi: 10.1002/ijc.25397. [DOI] [PubMed] [Google Scholar]
- 37.Gao Q, Wang XY, Qiu SJ, Yamato I, Sho M, Nakajima Y, Zhou J, Li BZ, Shi YH, Xiao YS, Xu Y, Fan J. Overexpression of PD-L1 significantly associates with tumor aggressiveness and postoperative recurrence in human hepatocellular carcinoma. Clin Cancer Res. 2009;15:971–979. doi: 10.1158/1078-0432.CCR-08-1608. [DOI] [PubMed] [Google Scholar]
- 38.Sangro B, Gomez-Martin C, de la Mata M, Iñarrairaegui M, Garralda E, Barrera P, Riezu-Boj JI, Larrea E, Alfaro C, Sarobe P, Lasarte JJ, Pérez-Gracia JL, Melero I, Prieto J. A clinical trial of CTLA-4 blockade with tremelimumab in patients with hepatocellular carcinoma and chronic hepatitis C. J Hepatol. 2013;59:81–88. doi: 10.1016/j.jhep.2013.02.022. [DOI] [PubMed] [Google Scholar]
- 39.Sangro B, Yau T, Crocenzi TS, Kudo M, Hsu C, Kim TY, Choo SP, Trojan J, Meyer T, Kang YK, Yeo W, Chopra A, Anderson J, Dela Cruz C, Lang L, Neely J, Tang H, Dastani HB, Melero I, El-Khoueiry AB, Welling TH Rd. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017;389:2492–2502. doi: 10.1016/S0140-6736(17)31046-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhu AX, Finn RS, Edeline J, Cattan S, Ogasawara S, Palmer D, Verslype C, Zagonel V, Fartoux L, Vogel A, Sarker D, Verset G, Chan SL, Knox J, Daniele B, Webber AL, Ebbinghaus SW, Ma J, Siegel AB, Cheng AL, Kudo M KEYNOTE-224 investigators. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial. Lancet Oncol. 2018;19:940–952. doi: 10.1016/S1470-2045(18)30351-6. [DOI] [PubMed] [Google Scholar]
- 41.Kelley RK, Abou-Alfa GK, Bendell JC, Kim T-Y, Borad MJ, Yong W-P, Morse M, Kang Y-K, Rebelatto M, Makowsky M, Xiao F, Morris SR, Sangro B. Phase I/II study of durvalumab and tremelimumab in patients with unresectable hepatocellular carcinoma (HCC): Phase I safety and efficacy analyses. J Clin Oncol. 2017;35:4073–4073. [Google Scholar]
- 42.Sangro B, Park J-W, Cruz CMD, Anderson J, Lang L, Neely J, Shaw JW, Cheng A-L. A randomized, multicenter, phase 3 study of nivolumab vs sorafenib as first-line treatment in patients (pts) with advanced hepatocellular carcinoma (HCC): CheckMate-459. J Clin Oncol. 2016;34:TPS4147–TPS4147. [Google Scholar]
- 43.Finn RS, Chan SL, Zhu AX, Knox JJ, Cheng A-L, Siegel AB, Bautista O, Watson P, Kudo M. KEYNOTE-240: Randomized phase III study of pembrolizumab versus best supportive care for second-line advanced hepatocellular carcinoma. J Clin Oncol. 2017;35:TPS503–TPS503. [Google Scholar]
- 44.Available from: https://investors. merck.com/news/press-release-details/2019/Merck-Provides-Update-on-KEYNOTE-240-a-Phase-3-Study-of-KEYTRUDA-pembrolizumab-in-Previously-Treated-Patients-with-Advanced-Hepatocellular-Carcinoma/default.aspx. [Google Scholar]
- 45.Abou-Alfa GK, Chan SL, Furuse J, Galle PR, Kelley RK, Qin S, Armstrong J, Darilay A, Vlahovic G, Negro A, Sangro B. A randomized, multicenter phase 3 study of durvalumab (D) and tremelimumab (T) as first-line treatment in patients with unresectable hepatocellular carcinoma (HCC): HIMALAYA study. J Clin Oncol. 2018;36:TPS4144–TPS4144. [Google Scholar]
- 46.Kalogeridi MA, Zygogianni A, Kyrgias G, Kouvaris J, Chatziioannou S, Kelekis N, Kouloulias V. Role of radiotherapy in the management of hepatocellular carcinoma: A systematic review. World J Hepatol. 2015;7:101–112. doi: 10.4254/wjh.v7.i1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jung J, Kim H, Yoon SM, Cho B, Kim YJ, Kwak J, Kim JH. Targeting Accuracy of Image-Guided Stereotactic Body Radiation Therapy for Hepatocellular Carcinoma in Real-Life Clinical Practice: In Vivo Assessment Using Hepatic Parenchymal Changes on Gd-EOB-DTPA-Enhanced Magnetic Resonance Images. Int J Radiat Oncol Biol Phys. 2018;102:867–874. doi: 10.1016/j.ijrobp.2018.05.018. [DOI] [PubMed] [Google Scholar]
- 48.Klein J, Dawson LA. Hepatocellular carcinoma radiation therapy: review of evidence and future opportunities. Int J Radiat Oncol Biol Phys. 2013;87:22–32. doi: 10.1016/j.ijrobp.2012.08.043. [DOI] [PubMed] [Google Scholar]
- 49.Korean Liver Cancer Study Group; National Cancer Center; Korea (NCC) 2014 KLCSG-NCC Korea Practice Guideline for the Management of Hepatocellular Carcinoma. Gut Liver. 2015;9:267–317. doi: 10.5009/gnl14460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Park HC, Yu JI, Cheng JC, Zeng ZC, Hong JH, Wang ML, Kim MS, Chi KH, Liang PC, Lee RC, Lau WY, Han KH, Chow PK, Seong J. Consensus for Radiotherapy in Hepatocellular Carcinoma from The 5th Asia-Pacific Primary Liver Cancer Expert Meeting (APPLE 2014): Current Practice and Future Clinical Trials. Liver Cancer. 2016;5:162–174. doi: 10.1159/000367766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cha H, Park HC, Yu JI, Kim TH, Nam TK, Yoon SM, Yoon WS, Kim JW, Kim MS, Jang HS, Choi Y, Kim JH, Kay CS, Jung I, Seong J. Clinical Practice Patterns of Radiotherapy in Patients with Hepatocellular Carcinoma: A Korean Radiation Oncology Group Study (KROG 14-07) Cancer Res Treat. 2017;49:61–69. doi: 10.4143/crt.2016.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yu JI, Park JW, Park HC, Yoon SM, Lim DH, Lee JH, Lee HC, Kim SW, Kim JH. Clinical impact of combined transarterial chemoembolization and radiotherapy for advanced hepatocellular carcinoma with portal vein tumor thrombosis: An external validation study. Radiother Oncol. 2016;118:408–415. doi: 10.1016/j.radonc.2015.11.019. [DOI] [PubMed] [Google Scholar]
- 53.Lee KH, Yu JI, Park HC, Park SY, Shin JS, Shin EH, Cho S, Jung SH, Han YY, Lim DH. Is higher dose always the right answer in stereotactic body radiation therapy for small hepatocellular carcinoma? Radiat Oncol J. 2018;36:129–138. doi: 10.3857/roj.2017.00598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bae SH, Park HC, Lim DH, Lee JA, Gwak GY, Choi MS, Lee JH, Koh KC, Paik SW, Yoo BC. Salvage treatment with hypofractionated radiotherapy in patients with recurrent small hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2012;82:e603–e607. doi: 10.1016/j.ijrobp.2011.09.053. [DOI] [PubMed] [Google Scholar]
- 55.Rajyaguru DJ, Borgert AJ, Smith AL, Thomes RM, Conway PD, Halfdanarson TR, Truty MJ, Kurup AN, Go RS. Radiofrequency Ablation Versus Stereotactic Body Radiotherapy for Localized Hepatocellular Carcinoma in Nonsurgically Managed Patients: Analysis of the National Cancer Database. J Clin Oncol. 2018;36:600–608. doi: 10.1200/JCO.2017.75.3228. [DOI] [PubMed] [Google Scholar]
- 56.Wahl DR, Stenmark MH, Tao Y, Pollom EL, Caoili EM, Lawrence TS, Schipper MJ, Feng M. Outcomes After Stereotactic Body Radiotherapy or Radiofrequency Ablation for Hepatocellular Carcinoma. J Clin Oncol. 2016;34:452–459. doi: 10.1200/JCO.2015.61.4925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yoo GS, Yu JI, Park HC. Proton therapy for hepatocellular carcinoma: Current knowledges and future perspectives. World J Gastroenterol. 2018;24:3090–3100. doi: 10.3748/wjg.v24.i28.3090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yu JI, Yoo GS, Cho S, Jung SH, Han Y, Park S, Lee B, Kang W, Sinn DH, Paik YH, Gwak GY, Choi MS, Lee JH, Koh KC, Paik SW, Park HC. Initial clinical outcomes of proton beam radiotherapy for hepatocellular carcinoma. Radiat Oncol J. 2018;36:25–34. doi: 10.3857/roj.2017.00409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang X, Krishnan S, Zhang X, Dong L, Briere T, Crane CH, Martel M, Gillin M, Mohan R, Beddar S. Proton radiotherapy for liver tumors: dosimetric advantages over photon plans. Med Dosim. 2008;33:259–267. doi: 10.1016/j.meddos.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 60.Gandhi SJ, Liang X, Ding X, Zhu TC, Ben-Josef E, Plastaras JP, Metz JM, Both S, Apisarnthanarax S. Clinical decision tool for optimal delivery of liver stereotactic body radiation therapy: Photons versus protons. Pract Radiat Oncol. 2015;5:209–218. doi: 10.1016/j.prro.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 61.Yu JI, Park HC, Lim DH, Park W, Yoo BC, Paik SW, Koh KC, Lee JH. Prognostic index for portal vein tumor thrombosis in patients with hepatocellular carcinoma treated with radiation therapy. J Korean Med Sci. 2011;26:1014–1022. doi: 10.3346/jkms.2011.26.8.1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yoon SM, Ryoo BY, Lee SJ, Kim JH, Shin JH, An JH, Lee HC, Lim YS. Efficacy and Safety of Transarterial Chemoembolization Plus External Beam Radiotherapy vs Sorafenib in Hepatocellular Carcinoma With Macroscopic Vascular Invasion: A Randomized Clinical Trial. JAMA Oncol. 2018;4:661–669. doi: 10.1001/jamaoncol.2017.5847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang EA, Broadwell SR, Bellavia RJ, Stein JP. Selective internal radiation therapy with SIR-Spheres in hepatocellular carcinoma and cholangiocarcinoma. J Gastrointest Oncol. 2017;8:266–278. doi: 10.21037/jgo.2016.11.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Salem R, Thurston KG. Radioembolization with 90Yttrium microspheres: a state-of-the-art brachytherapy treatment for primary and secondary liver malignancies. Part 1: Technical and methodologic considerations. J Vasc Interv Radiol. 2006;17:1251–1278. doi: 10.1097/01.RVI.0000233785.75257.9A. [DOI] [PubMed] [Google Scholar]
- 65.Chew V, Lee YH, Pan L, Nasir NJM, Lim CJ, Chua C, Lai L, Hazirah SN, Lim TKH, Goh BKP, Chung A, Lo RHG, Ng D, Filarca RLF, Albani S, Chow PKH. Immune activation underlies a sustained clinical response to Yttrium-90 radioembolisation in hepatocellular carcinoma. Gut. 2019;68:335–346. doi: 10.1136/gutjnl-2017-315485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cho HS, Oh JH, Han I, Kim HS. Survival of patients with skeletal metastases from hepatocellular carcinoma after surgical management. J Bone Joint Surg Br. 2009;91:1505–1512. doi: 10.1302/0301-620X.91B11.21864. [DOI] [PubMed] [Google Scholar]
- 67.Yoo GS, Park HC, Yu JI, Lim DH, Cho WK, Lee E, Jung SH, Han Y, Kim ES, Lee SH, Eoh W, Park SJ, Chung SS, Lee CS, Lee JH. Stereotactic ablative body radiotherapy for spinal metastasis from hepatocellular carcinoma: its oncologic outcomes and risk of vertebral compression fracture. Oncotarget. 2017;8:72860–72871. doi: 10.18632/oncotarget.20529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kim S, Chun M, Wang H, Cho S, Oh YT, Kang SH, Yang J. Bone metastasis from primary hepatocellular carcinoma: characteristics of soft tissue formation. Cancer Res Treat. 2007;39:104–108. doi: 10.4143/crt.2007.39.3.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Seong J, Koom WS, Park HC. Radiotherapy for painful bone metastases from hepatocellular carcinoma. Liver Int. 2005;25:261–265. doi: 10.1111/j.1478-3231.2005.01094.x. [DOI] [PubMed] [Google Scholar]
- 70.Lee E, Kim TG, Park HC, Yu JI, Lim DH, Nam H, Lee H, Lee JH. Clinical outcomes of stereotactic body radiotherapy for spinal metastases from hepatocellular carcinoma. Radiat Oncol J. 2015;33:217–225. doi: 10.3857/roj.2015.33.3.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Herrera FG, Bourhis J, Coukos G. Radiotherapy combination opportunities leveraging immunity for the next oncology practice. CA Cancer J Clin. 2017;67:65–85. doi: 10.3322/caac.21358. [DOI] [PubMed] [Google Scholar]
- 72.Weichselbaum RR, Liang H, Deng L, Fu YX. Radiotherapy and immunotherapy: a beneficial liaison? Nat Rev Clin Oncol. 2017;14:365–379. doi: 10.1038/nrclinonc.2016.211. [DOI] [PubMed] [Google Scholar]
- 73.Demaria S, Kawashima N, Yang AM, Devitt ML, Babb JS, Allison JP, Formenti SC. Immune-mediated inhibition of metastases after treatment with local radiation and CTLA-4 blockade in a mouse model of breast cancer. Clin Cancer Res. 2005;11:728–734. [PubMed] [Google Scholar]
- 74.Zeng J, See AP, Phallen J, Jackson CM, Belcaid Z, Ruzevick J, Durham N, Meyer C, Harris TJ, Albesiano E, Pradilla G, Ford E, Wong J, Hammers HJ, Mathios D, Tyler B, Brem H, Tran PT, Pardoll D, Drake CG, Lim M. Anti-PD-1 blockade and stereotactic radiation produce long-term survival in mice with intracranial gliomas. Int J Radiat Oncol Biol Phys. 2013;86:343–349. doi: 10.1016/j.ijrobp.2012.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sharabi AB, Nirschl CJ, Kochel CM, Nirschl TR, Francica BJ, Velarde E, Deweese TL, Drake CG. Stereotactic Radiation Therapy Augments Antigen-Specific PD-1-Mediated Antitumor Immune Responses via Cross-Presentation of Tumor Antigen. Cancer Immunol Res. 2015;3:345–355. doi: 10.1158/2326-6066.CIR-14-0196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dovedi SJ, Cheadle EJ, Popple AL, Poon E, Morrow M, Stewart R, Yusko EC, Sanders CM, Vignali M, Emerson RO, Robins HS, Wilkinson RW, Honeychurch J, Illidge TM. Fractionated Radiation Therapy Stimulates Antitumor Immunity Mediated by Both Resident and Infiltrating Polyclonal T-cell Populations when Combined with PD-1 Blockade. Clin Cancer Res. 2017;23:5514–5526. doi: 10.1158/1078-0432.CCR-16-1673. [DOI] [PubMed] [Google Scholar]
- 77.Dovedi SJ, Adlard AL, Lipowska-Bhalla G, McKenna C, Jones S, Cheadle EJ, Stratford IJ, Poon E, Morrow M, Stewart R, Jones H, Wilkinson RW, Honeychurch J, Illidge TM. Acquired resistance to fractionated radiotherapy can be overcome by concurrent PD-L1 blockade. Cancer Res. 2014;74:5458–5468. doi: 10.1158/0008-5472.CAN-14-1258. [DOI] [PubMed] [Google Scholar]
- 78.Deng L, Liang H, Burnette B, Beckett M, Darga T, Weichselbaum RR, Fu YX. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J Clin Invest. 2014;124:687–695. doi: 10.1172/JCI67313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kwon ED, Drake CG, Scher HI, Fizazi K, Bossi A, van den Eertwegh AJ, Krainer M, Houede N, Santos R, Mahammedi H, Ng S, Maio M, Franke FA, Sundar S, Agarwal N, Bergman AM, Ciuleanu TE, Korbenfeld E, Sengeløv L, Hansen S, Logothetis C, Beer TM, McHenry MB, Gagnier P, Liu D, Gerritsen WR CA184-043 Investigators. Ipilimumab versus placebo after radiotherapy in patients with metastatic castration-resistant prostate cancer that had progressed after docetaxel chemotherapy (CA184-043): a multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol. 2014;15:700–712. doi: 10.1016/S1470-2045(14)70189-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, Yokoi T, Chiappori A, Lee KH, de Wit M, Cho BC, Bourhaba M, Quantin X, Tokito T, Mekhail T, Planchard D, Kim YC, Karapetis CS, Hiret S, Ostoros G, Kubota K, Gray JE, Paz-Ares L, de Castro Carpeño J, Wadsworth C, Melillo G, Jiang H, Huang Y, Dennis PA, Özgüroğlu M PACIFIC Investigators. Durvalumab after Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. N Engl J Med. 2017;377:1919–1929. doi: 10.1056/NEJMoa1709937. [DOI] [PubMed] [Google Scholar]
- 81.Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, Kurata T, Chiappori A, Lee KH, de Wit M, Cho BC, Bourhaba M, Quantin X, Tokito T, Mekhail T, Planchard D, Kim YC, Karapetis CS, Hiret S, Ostoros G, Kubota K, Gray JE, Paz-Ares L, de Castro Carpeño J, Faivre-Finn C, Reck M, Vansteenkiste J, Spigel DR, Wadsworth C, Melillo G, Taboada M, Dennis PA, Özgüroğlu M PACIFIC Investigators. Overall Survival with Durvalumab after Chemoradiotherapy in Stage III NSCLC. N Engl J Med. 2018;379:2342–2350. doi: 10.1056/NEJMoa1809697. [DOI] [PubMed] [Google Scholar]
- 82.Shaverdian N, Lisberg AE, Bornazyan K, Veruttipong D, Goldman JW, Formenti SC, Garon EB, Lee P. Previous radiotherapy and the clinical activity and toxicity of pembrolizumab in the treatment of non-small-cell lung cancer: a secondary analysis of the KEYNOTE-001 phase 1 trial. Lancet Oncol. 2017;18:895–903. doi: 10.1016/S1470-2045(17)30380-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.MOLE RH. Whole body irradiation; radiobiology or medicine? Br J Radiol. 1953;26:234–241. doi: 10.1259/0007-1285-26-305-234. [DOI] [PubMed] [Google Scholar]
- 84.Abuodeh Y, Venkat P, Kim S. Systematic review of case reports on the abscopal effect. Curr Probl Cancer. 2016;40:25–37. doi: 10.1016/j.currproblcancer.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 85.Demaria S, Ng B, Devitt ML, Babb JS, Kawashima N, Liebes L, Formenti SC. Ionizing radiation inhibition of distant untreated tumors (abscopal effect) is immune mediated. Int J Radiat Oncol Biol Phys. 2004;58:862–870. doi: 10.1016/j.ijrobp.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 86.Dewan MZ, Galloway AE, Kawashima N, Dewyngaert JK, Babb JS, Formenti SC, Demaria S. Fractionated but not single-dose radiotherapy induces an immune-mediated abscopal effect when combined with anti-CTLA-4 antibody. Clin Cancer Res. 2009;15:5379–5388. doi: 10.1158/1078-0432.CCR-09-0265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Liu Y, Dong Y, Kong L, Shi F, Zhu H, Yu J. Abscopal effect of radiotherapy combined with immune checkpoint inhibitors. J Hematol Oncol. 2018;11:104. doi: 10.1186/s13045-018-0647-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Park SS, Dong H, Liu X, Harrington SM, Krco CJ, Grams MP, Mansfield AS, Furutani KM, Olivier KR, Kwon ED. PD-1 Restrains Radiotherapy-Induced Abscopal Effect. Cancer Immunol Res. 2015;3:610–619. doi: 10.1158/2326-6066.CIR-14-0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Postow MA, Callahan MK, Barker CA, Yamada Y, Yuan J, Kitano S, Mu Z, Rasalan T, Adamow M, Ritter E, Sedrak C, Jungbluth AA, Chua R, Yang AS, Roman RA, Rosner S, Benson B, Allison JP, Lesokhin AM, Gnjatic S, Wolchok JD. Immunologic correlates of the abscopal effect in a patient with melanoma. N Engl J Med. 2012;366:925–931. doi: 10.1056/NEJMoa1112824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rodriguez-Ruiz ME, Rodriguez I, Barbes B, Mayorga L, Sanchez-Paulete AR, Ponz-Sarvise M, Pérez-Gracia JL, Melero I. Brachytherapy attains abscopal effects when combined with immunostimulatory monoclonal antibodies. Brachytherapy. 2017;16:1246–1251. doi: 10.1016/j.brachy.2017.06.012. [DOI] [PubMed] [Google Scholar]
- 91.Ngwa W, Irabor OC, Schoenfeld JD, Hesser J, Demaria S, Formenti SC. Using immunotherapy to boost the abscopal effect. Nat Rev Cancer. 2018;18:313–322. doi: 10.1038/nrc.2018.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chandra RA, Wilhite TJ, Balboni TA, Alexander BM, Spektor A, Ott PA, Ng AK, Hodi FS, Schoenfeld JD. A systematic evaluation of abscopal responses following radiotherapy in patients with metastatic melanoma treated with ipilimumab. Oncoimmunology. 2015;4:e1046028. doi: 10.1080/2162402X.2015.1046028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lee Y, Auh SL, Wang Y, Burnette B, Wang Y, Meng Y, Beckett M, Sharma R, Chin R, Tu T, Weichselbaum RR, Fu YX. Therapeutic effects of ablative radiation on local tumor require CD8+ T cells: changing strategies for cancer treatment. Blood. 2009;114:589–595. doi: 10.1182/blood-2009-02-206870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lim JY, Gerber SA, Murphy SP, Lord EM. Type I interferons induced by radiation therapy mediate recruitment and effector function of CD8(+) T cells. Cancer Immunol Immunother. 2014;63:259–271. doi: 10.1007/s00262-013-1506-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Burnette BC, Liang H, Lee Y, Chlewicki L, Khodarev NN, Weichselbaum RR, Fu YX, Auh SL. The efficacy of radiotherapy relies upon induction of type i interferon-dependent innate and adaptive immunity. Cancer Res. 2011;71:2488–2496. doi: 10.1158/0008-5472.CAN-10-2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Vanpouille-Box C, Formenti SC, Demaria S. TREX1 dictates the immune fate of irradiated cancer cells. Oncoimmunology. 2017;6:e1339857. doi: 10.1080/2162402X.2017.1339857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Deng L, Liang H, Xu M, Yang X, Burnette B, Arina A, Li XD, Mauceri H, Beckett M, Darga T, Huang X, Gajewski TF, Chen ZJ, Fu YX, Weichselbaum RR. STING-Dependent Cytosolic DNA Sensing Promotes Radiation-Induced Type I Interferon-Dependent Antitumor Immunity in Immunogenic Tumors. Immunity. 2014;41:843–852. doi: 10.1016/j.immuni.2014.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bouquet F, Pal A, Pilones KA, Demaria S, Hann B, Akhurst RJ, Babb JS, Lonning SM, DeWyngaert JK, Formenti SC, Barcellos-Hoff MH. TGFβ1 inhibition increases the radiosensitivity of breast cancer cells in vitro and promotes tumor control by radiation in vivo. Clin Cancer Res. 2011;17:6754–6765. doi: 10.1158/1078-0432.CCR-11-0544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Vanpouille-Box C, Diamond JM, Pilones KA, Zavadil J, Babb JS, Formenti SC, Barcellos-Hoff MH, Demaria S. TGFβ Is a Master Regulator of Radiation Therapy-Induced Antitumor Immunity. Cancer Res. 2015;75:2232–2242. doi: 10.1158/0008-5472.CAN-14-3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Formenti SC, Demaria S. Combining radiotherapy and cancer immunotherapy: a paradigm shift. J Natl Cancer Inst. 2013;105:256–265. doi: 10.1093/jnci/djs629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Young KH, Baird JR, Savage T, Cottam B, Friedman D, Bambina S, Messenheimer DJ, Fox B, Newell P, Bahjat KS, Gough MJ, Crittenden MR. Optimizing Timing of Immunotherapy Improves Control of Tumors by Hypofractionated Radiation Therapy. PLoS One. 2016;11:e0157164. doi: 10.1371/journal.pone.0157164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yoshimoto Y, Suzuki Y, Mimura K, Ando K, Oike T, Sato H, Okonogi N, Maruyama T, Izawa S, Noda SE, Fujii H, Kono K, Nakano T. Radiotherapy-induced anti-tumor immunity contributes to the therapeutic efficacy of irradiation and can be augmented by CTLA-4 blockade in a mouse model. PLoS One. 2014;9:e92572. doi: 10.1371/journal.pone.0092572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wu L, Wu MO, De la Maza L, Yun Z, Yu J, Zhao Y, Cho J, de Perrot M. Targeting the inhibitory receptor CTLA-4 on T cells increased abscopal effects in murine mesothelioma model. Oncotarget. 2015;6:12468–12480. doi: 10.18632/oncotarget.3487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wu CT, Chen WC, Chang YH, Lin WY, Chen MF. The role of PD-L1 in the radiation response and clinical outcome for bladder cancer. Sci Rep. 2016;6:19740. doi: 10.1038/srep19740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lugade AA, Moran JP, Gerber SA, Rose RC, Frelinger JG, Lord EM. Local radiation therapy of B16 melanoma tumors increases the generation of tumor antigen-specific effector cells that traffic to the tumor. J Immunol. 2005;174:7516–7523. doi: 10.4049/jimmunol.174.12.7516. [DOI] [PubMed] [Google Scholar]
- 106.Vanpouille-Box C, Alard A, Aryankalayil MJ, Sarfraz Y, Diamond JM, Schneider RJ, Inghirami G, Coleman CN, Formenti SC, Demaria S. DNA exonuclease Trex1 regulates radiotherapy-induced tumour immunogenicity. Nat Commun. 2017;8:15618. doi: 10.1038/ncomms15618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Fransen MF, Schoonderwoerd M, Knopf P, Camps MG, Hawinkels LJ, Kneilling M, van Hall T, Ossendorp F. Tumor-draining lymph nodes are pivotal in PD-1/PD-L1 checkpoint therapy. JCI Insight. 2018:3. doi: 10.1172/jci.insight.124507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kim KJ, Kim JH, Lee SJ, Lee EJ, Shin EC, Seong J. Radiation improves antitumor effect of immune checkpoint inhibitor in murine hepatocellular carcinoma model. Oncotarget. 2017;8:41242–41255. doi: 10.18632/oncotarget.17168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Friedman D, Baird JR, Young KH, Cottam B, Crittenden MR, Friedman S, Gough MJ, Newell P. Programmed cell death-1 blockade enhances response to stereotactic radiation in an orthotopic murine model of hepatocellular carcinoma. Hepatol Res. 2017;47:702–714. doi: 10.1111/hepr.12789. [DOI] [PubMed] [Google Scholar]
- 110.Kim HJ, Park S, Kim KJ, Seong J. Clinical significance of soluble programmed cell death ligand-1 (sPD-L1) in hepatocellular carcinoma patients treated with radiotherapy. Radiother Oncol. 2018;129:130–135. doi: 10.1016/j.radonc.2017.11.027. [DOI] [PubMed] [Google Scholar]
- 111.Kallini JR, Gabr A, Salem R, Lewandowski RJ. Transarterial Radioembolization with Yttrium-90 for the Treatment of Hepatocellular Carcinoma. Adv Ther. 2016;33:699–714. doi: 10.1007/s12325-016-0324-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wehrenberg-Klee E, Goyal L, Dugan M, Zhu AX, Ganguli S. Y-90 Radioembolization Combined with a PD-1 Inhibitor for Advanced Hepatocellular Carcinoma. Cardiovasc Intervent Radiol. 2018;41:1799–1802. doi: 10.1007/s00270-018-1993-1. [DOI] [PubMed] [Google Scholar]
- 113.Lee JH, Lee JH, Lim YS, Yeon JE, Song TJ, Yu SJ, Gwak GY, Kim KM, Kim YJ, Lee JW, Yoon JH. Adjuvant immunotherapy with autologous cytokine-induced killer cells for hepatocellular carcinoma. Gastroenterology. 2015;148:1383–1391.e6. doi: 10.1053/j.gastro.2015.02.055. [DOI] [PubMed] [Google Scholar]
- 114.Wang YG, Huang PP, Zhang R, Ma BY, Zhou XM, Sun YF. Targeting adeno-associated virus and adenoviral gene therapy for hepatocellular carcinoma. World J Gastroenterol. 2016;22:326–337. doi: 10.3748/wjg.v22.i1.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wu CJ, Tsai YT, Lee IJ, Wu PY, Lu LS, Tsao WS, Huang YJ, Chang CC, Ka SM, Tao MH. Combination of radiation and interleukin 12 eradicates large orthotopic hepatocellular carcinoma through immunomodulation of tumor microenvironment. Oncoimmunology. 2018;7:e1477459. doi: 10.1080/2162402X.2018.1477459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Liu J, Zhao Q, Deng W, Lu J, Xu X, Wang R, Li X, Yue J. Radiation-related lymphopenia is associated with spleen irradiation dose during radiotherapy in patients with hepatocellular carcinoma. Radiat Oncol. 2017;12:90. doi: 10.1186/s13014-017-0824-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lee HJ, Jr, Zeng J, Rengan R. Proton beam therapy and immunotherapy: an emerging partnership for immune activation in non-small cell lung cancer. Transl Lung Cancer Res. 2018;7:180–188. doi: 10.21037/tlcr.2018.03.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Chan TA, Yarchoan M, Jaffee E, Swanton C, Quezada SA, Stenzinger A, Peters S. Development of tumor mutation burden as an immunotherapy biomarker: utility for the oncology clinic. Ann Oncol. 2019;30:44–56. doi: 10.1093/annonc/mdy495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Bernicker E. Next-Generation Sequencing and Immunotherapy Biomarkers: A Medical Oncology Perspective. Arch Pathol Lab Med. 2016;140:245–248. doi: 10.5858/arpa.2015-0287-SA. [DOI] [PubMed] [Google Scholar]
- 120.Jang BS, Kim IA. A radiosensitivity gene signature and PD-L1 predict the clinical outcomes of patients with lower grade glioma in TCGA. Radiother Oncol. 2018;128:245–253. doi: 10.1016/j.radonc.2018.05.003. [DOI] [PubMed] [Google Scholar]
- 121.Lee JH, Lee JH, Lim YS, Yeon JE, Song TJ, Yu SJ, Gwak GY, Kim KM, Kim YJ, Lee JW, Yoon JH. Sustained efficacy of adjuvant immunotherapy with cytokine-induced killer cells for hepatocellular carcinoma: an extended 5-year follow-up. Cancer Immunol Immunother. 2019;68:23–32. doi: 10.1007/s00262-018-2247-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Cui Y, Li B, Pollom EL, Horst KC, Li R. Integrating Radiosensitivity and Immune Gene Signatures for Predicting Benefit of Radiotherapy in Breast Cancer. Clin Cancer Res. 2018;24:4754–4762. doi: 10.1158/1078-0432.CCR-18-0825. [DOI] [PMC free article] [PubMed] [Google Scholar]