Abstract
BACKGROUND
Trimethylamine N-oxide (TMAO) has been shown to be involved in cardiovascular disease (CVD). However, its role in nonalcoholic steatohepatitis (NASH) is unknown.
AIM
To determine the effect of TMAO on the progression of NASH.
METHODS
A rat model was induced by 16-wk high-fat high-cholesterol (HFHC) diet feeding and TMAO was administrated by daily oral gavage for 8 wk.
RESULTS
Oral TMAO intervention attenuated HFHC diet-induced steatohepatitis in rats. Histological evaluation showed that TMAO treatment significantly alleviated lobular inflammation and hepatocyte ballooning in the livers of rats fed a HFHC diet. Serum levels of alanine aminotransferase and aspartate aminotransferase were also decreased by TMAO treatment. Moreover, hepatic endoplasmic reticulum (ER) stress and cell death were mitigated in HFHC diet-fed TMAO-treated rats. Hepatic and serum levels of cholesterol were both decreased by TMAO treatment in rats fed a HFHC diet. Furthermore, the expression levels of intestinal cholesterol transporters were detected. Interestingly, cholesterol influx-related Niemann-Pick C1-like 1 was downregulated and cholesterol efflux-related ABCG5/8 were upregulated by TMAO treatment in the small intestine. Gut microbiota analysis showed that TMAO could alter the gut microbial profile and restore the diversity of gut flora.
CONCLUSION
These data suggest that TMAO may modulate the gut microbiota, inhibit intestinal cholesterol absorption, and ameliorate hepatic ER stress and cell death under cholesterol overload, thereby attenuating HFHC diet-induced steatohepatitis in rats. Further studies are needed to evaluate the influence on CVD and define the safe does of TMAO treatment.
Keywords: Gut microbiota, Trimethylamine N-oxide, Nonalcoholic steatohepatitis, Endoplasmic reticulum stress, Cholesterol
Core tip: The function of trimethylamine N-oxide (TMAO) in nonalcoholic steatohepatitis (NASH) remains unexplored. We investigated the effect of oral TMAO administration on the progression of NASH in a rat model induced with a high-fat high-cholesterol (HFHC) diet. This study demonstrated for the first time that the gut microbial metabolite TMAO restores gut microbiota diversity, inhibits intestinal cholesterol absorption, and reduces hepatic cholesterol overload, thus attenuating cholesterol-induced endoplasmic reticulum stress and hepatocyte cell death. These functions facilitate the protection of TMAO against HFHC diet-induced steatohepatitis in rats.
INTRODUCTION
Nonalcoholic fatty liver disease (NAFLD) has emerged as a leading cause of chronic liver diseases worldwide, affecting about a quarter of the adult population[1,2]. Nonalcoholic steatohepatitis (NASH) is a serious subtype, which can progress to end-stage liver diseases such as cirrhosis and hepatocellular carcinoma without effective intervention. The pathogenesis of NASH is considered to be multifactorial, and the role of gut microbiota has attracted increasing attention over the past decade[3,4]. Evidence has accumulated suggesting that many effects of the gut microbiota are mediated by metabolites produced by the gut commensal bacteria utilizing dietary nutrients[5]. For example, short-chain fatty acids derived from dietary fibers are shown to regulate metabolic processes and the inflammatory response, and thus may exert a beneficial impact on NASH[4,6].
Trimethylamine N-oxide (TMAO) is a metabolite produced by the host in cooperation with the gut microbiota. Dietary choline and L-carnitine can serve as precursors and be degraded by gut commensal bacteria to produce trimethylamine which is absorbed and further metabolized into TMAO by hepatic flavin-containing monooxygenase 3[7]. Further, fish consumption also contributes to the presence and elevation of circulating TMAO as fish meat is enriched in TMAO. Recent studies have established a link between TMAO and cardiovascular disease (CVD). It has been shown that plasma levels of TMAO are positively associated with the risk of adverse cardiovascular events[8]. Moreover, several studies have found that TMAO can promote the initiation and progression of CVD[9-12]. On the other hand, beneficial roles of TMAO in diabetic peripheral neuropathy, glucose tolerance, and arterial hypertension have also been reported[13-15]. Despite the debate on the function of TMAO in CVD and diabetes, its role in NASH has not been determined.
In this study, we investigated the role of TMAO in a rat model of high-fat high-cholesterol (HFHC) diet-induced steatohepatitis. This in vivo study demonstrated that oral TMAO administration improved the histological alterations in HFHC diet-induced steatohepatitis. TMAO alleviated hepatic endoplasmic reticulum (ER) stress and cell death to attenuate liver injury in rats. The expression levels of intestinal cholesterol transporters were altered and hepatic cholesterol overload was reduced by TMAO intervention. TMAO treatment was sufficient to restore the depleted diversity of gut microbiota induced by the HFHC diet.
MATERIALS AND METHODS
Animal model and diets
Male Sprague-Dawley rats at six weeks of age were purchased from Shanghai Laboratory Animal Co. Ltd. (Shanghai, China). Rats were fed a standard chow diet or a HFHC diet[16,17] (fat 33 kcal%, carbohydrates 50 kcal%, protein 17 kcal%, and 2% cholesterol; TrophicDiet, Nantong, China) for 8 wk. Then, the rats fed with a chow diet or HFHC diet were randomly divided into two groups and treated with TMAO (120 mg/kg/day) (cat. 317594, Sigma-Aldrich, St. Louis, MO, United States) or vehicle (phosphate buffered saline, Corning, NYC, NY, United States) by gavage once daily for 8 wk. All rats were housed under a 12:12-h light/dark cycle at a controlled temperature. All animal experimental protocols were approved by the Institutional Animal Care and Use Committee of Xinhua Hospital Affiliated to Shanghai Jiao Tong University School of Medicine.
Histological analysis
At the end of the 16th wk, the rats were fasted overnight and were euthanized with pentobarbital before the tissues were harvested in the morning. Livers were fixed in 10% phosphate-buffered formalin acetate at 4 °C overnight and embedded in paraffin wax. Paraffin sections were cut and mounted on glass slides for hematoxylin and eosin (H&E) staining. Histological alterations were evaluated based on the steatosis, activity, and fibrosis (SAF) scoring system[18].
Immunoblotting analysis
Immunoblotting analysis was performed as previously described[6]. Briefly, rat liver tissues were homogenized and lysed at 4 °C in a lysis buffer (50 mM Tris-HCl, pH 8.0, 1% (v/v) Nonidet P-40, 150 mM NaCl, 5 mM EDTA, 1 mM EGTA, 1 mM sodium orthovanadate, 10 mM sodium fluoride, 1 mM phenylmethylsulfonyl fluoride, 2 μg/mL aprotinin, 5 μg/mL leupeptin, and 1 μg/mL pepstatin), and the supernatant was used for immunoblotting. The protein concentrations in the cell lysates were measured using the BCA method. Protein samples were separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and then electrophoretically transferred to polyvinylidene difluoride membranes (BIO-RAD, Hercules, CA, United States). The membranes were blocked with 5% nonfat milk in Tris-buffered saline with 0.1% Tween 20 (TBST) and incubated with specific primary antibodies, followed by incubation with horseradish peroxidase-conjugated secondary antibodies (Santa Cruz Biotechnology, Santa Cruz, CA, United States). Immunoblots were visualized with a LumiGLO chemiluminescence detection kit (Cell Signaling Technology, Beverly, MA, United States). The intensity of bands was quantified using ImageJ (National Institutes of Health, Bethesda, MD, United States). Antibodies against phospho-JNK (cat. 9251), C/EBP homologous protein (CHOP) (cat. 2895), cleaved Caspase-3 (cat. 9664), and binding immunoglobulin protein (BiP) (cat. 3177) were from Cell Signaling Technology (Beverly, MA). Antibody against JNK (cat. 610628) was from BD Biosciences (San Jose, CA, United States). Antibody against β-actin (cat. sc-69879) was obtained from Santa Cruz Biotechnology (Santa Cruz, CA, United States).
RNA isolation and quantitative RT-PCR analysis
Liver tissues were homogenized in TRIzol Reagent (Life Technologies, Carlsbad, CA, United States). The quantity and quality of extracted RNA were assessed using NanoDrop 2000 (Thermo Fisher Scientific, Wilmington, DE, United States). The 260/280 and 260/230 ratios were used for RNA purity assessment[19]. The total RNA was then reverse transcribed to cDNA using SuperScript II reverse transcriptase (Life Technologies, Carlsbad, CA, United States) and Oligo dT. The resulting cDNA was subjected to real-time PCR with gene-specific primers in the presence of SYBR Green Master Mix (Yeasen, Shanghai, China) using the StepOnePlus Real-Time PCR System (Applied Biosystems, Waltham, MA, United States). The following primer sequences were used: CCCCAAACTCCCTCATAAGCA (forward) and TATCCCCCAAC AGCAAGGAAG (reverse) for rat Niemann-Pick C1-like 1 (NPC1L1); AAAAGG CTGCTGATTGCCC (forward) and GCAGGACAATCTGAGCAAAGAA (reverse) for rat ATP-binding cassette transporter A1 (ABCA1); TTGGCCCCTCACTTAATTGGA (forward) and GGACCATACCAAGCAGCACAAG (reverse) for rat ABCG5; ACTGCCATGGACCTGAACTCA (forward) and GCTGATGCCAATGACGATGA (reverse) for rat ABCG8; GGGCAGCCCAGAACATCAT (forward) and CCA GTGAGCTTCCCGTTCAG (reverse) for rat GAPDH. The amplification procedure was as follows: The initial step was 95 °C for 5 min, followed by 40 cycles of 95 °C for 30 s, 60 °C for 30 s, and 72 °C for 30 s. Data were analyzed using the ΔΔCT threshold cycle method. The mRNA levels of genes were normalized to those of GAPDH and are presented as relative levels to the control.
Gut microbiota analysis
Fecal samples were collected immediately upon defecation and stored at - 80 °C. Fecal DNA was extracted from fecal samples using the TIANamp Stool DNA Kit (Tiangen, Beijing, China) according to the manufacturer’s protocols. The quality and quantity of DNA were verified with NanoDrop (Thermo Fisher Scientific, Wilmington, DE, United States) and by agarose gel electrophoresis. Extracted DNA was diluted to a concentration of 1 ng/μL and stored at - 20 °C until further processing. The V4-V5 region of the bacterial 16S ribosomal RNA gene was amplified by PCR. Amplicons were extracted from 2% agarose gels and purified using the AxyPrep DNA Gel Extraction Kit (Axygen Biosciences, Union City, CA, United States) according to the manufacturer’s instructions and quantified using QuantiFluor™-ST (Promega, Wisconsin, USA). Purified amplicons were pooled at equimolar concentrations and sequenced on an Illumina MiSeq Platform (Illumina, San Diego, CA, United States) according to standard protocols. Raw sequencing data were in FASTQ format. Paired-end reads were then preprocessed using Trimmomatic software[20] to detect and cut off ambiguous bases. After trimming, paired-end reads were assembled using FLASH software[21]. Clean reads were subjected to primer sequence removal and clustering to generate operational taxonomic units using Vsearch software with a 97% similarity cutoff[22]. All representative reads were annotated and blasted against Silva database using RDP classifier (confidence threshold, 70%).
Statistical analysis
Data are expressed as the mean ± SE. Statistical significance was evaluated using the unpaired two-tailed Student's t-test or nonparametric tests and among more than two groups by analysis of two-way ANOVA. Differences were considered significant at a P value < 0.05.
RESULTS
TMAO attenuates histological alterations without affecting body weight in HFHC diet-induced steatohepatitis
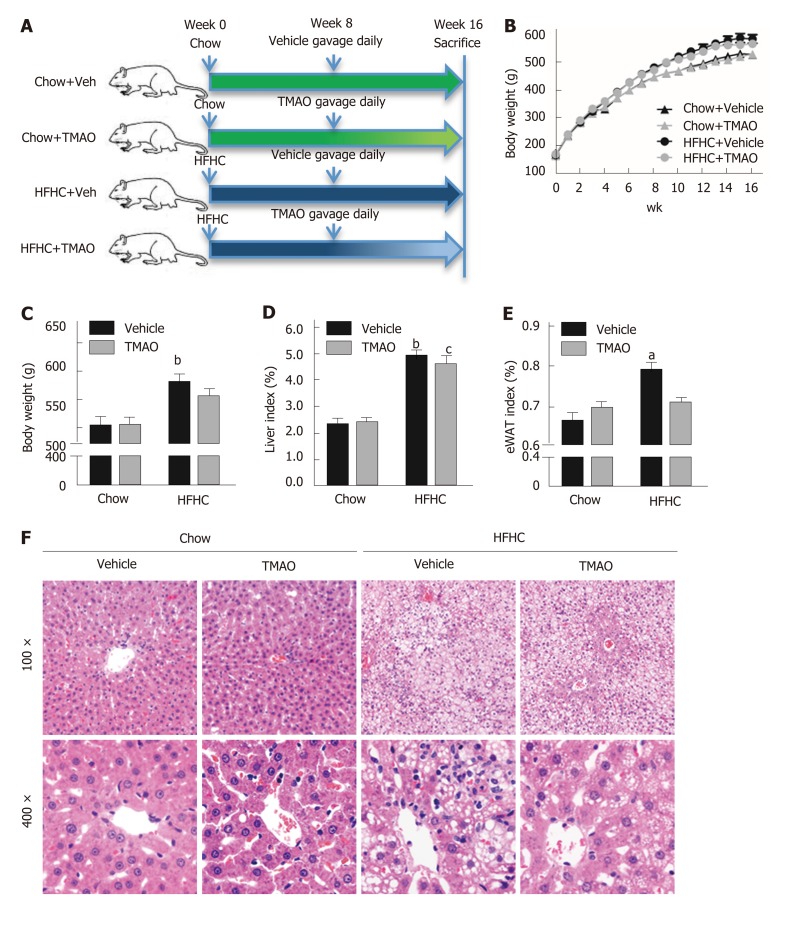
We first examined the effect of TMAO on metabolic parameters in rats fed a HFHC diet. Food intake was similar in the four groups (data not shown) and the body weight curves showed that TMAO treatment had little effect on the body weight (Figure 1B). The body weight at sacrifice was greatly increased in HFHC diet-fed rats. However, there was no significant difference between the TMAO-treated and vehicle-treated groups (Figure 1C). The liver index (liver weight/body weight × 100%) and epididymal white adipose tissue (eWAT) index (eWAT weight/body weight × 100%) were measured. Compared to the Chow + Vehicle group, the liver index and eWAT index were both increased in the HFHC + Vehicle group. Although the liver index was decreased by TMAO treatment in HFHC diet-fed rats, the eWAT index was not significantly changed in the TMAO-treated groups (Figure 1D and E). We further evaluated the effect of TMAO intervention on hepatic histological alterations in rats. H&E staining results showed that TMAO treatment had no effect in the chow diet-fed rats. Steatohepatitis was induced by HFHC diet feeding for 16 wk, as manifested by the presence of moderate to severe hepatic steatosis with lobular inflammation and hepatocyte ballooning, and TMAO treatment significantly improved the hepatic histological alterations in HFHC diet-fed rats. The HFHC diet-induced hepatic steatosis was mildly attenuated and the hepatic inflammation and hepatocyte ballooning were greatly alleviated by TMAO intervention (Figure 1F). These results suggest that TMAO treatment can significantly attenuate the histological alterations of steatohepatitis, while hardly affecting body weight gain and adiposity in rats fed a HFHC diet.
Figure 1.
Trimethylamine N-oxide attenuates histological alterations with little effect on body weight in high-fat high-cholesterol diet-induced steatohepatitis. Six-week-old male rats were fed a high-fat high-cholesterol diet for 8 wk and then gavaged with trimethylamine N-oxide (120 mg/kg/day) once daily for 8 wk. Rats were sacrificed at the end of the 16th wk. A: Schematic illustration showing the design of the in vivo experiment; B: The body weight curves; C: The body weight at the 16th wk before sacrifice; D: The liver index; E: Epididymal white adipose tissue index; F: Representative hematoxylin and eosin staining of liver tissue. The data are presented as the mean ± SE (n = 10-14). aP < 0.05, vs rats fed a chow diet; bP < 0.01, vs rats fed a chow diet; cP < 0.05, vs rats fed a high-fat high-cholesterol diet; dP < 0.01, vs rats fed a high-fat high-cholesterol diet. eWAT: Epididymal white adipose tissue; TMAO: Trimethylamine N-oxide; HFHC: High-fat high-cholesterol.
TMAO leads to reduced activity and serological improvement in HFHC diet-induced steatohepatitis
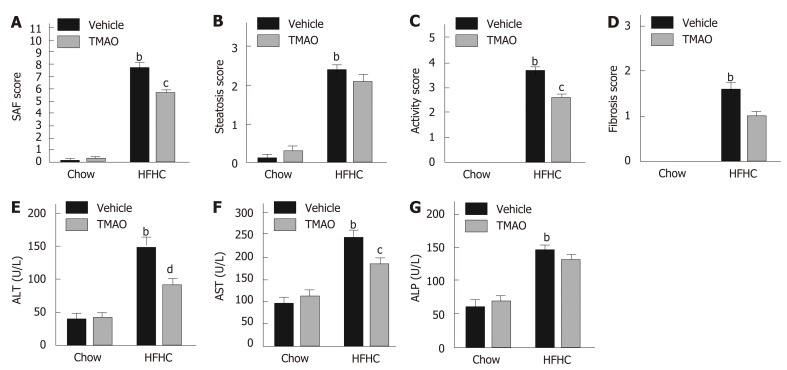
To further elucidate the effect of TMAO on HFHC diet-induced steatohepatitis, we semiquantified the histological alterations utilizing the SAF scoring system[18]. As shown in Figure 2A, the SAF score was significantly decreased by TMAO intervention in HFHC diet-fed rats. The activity score, which comprises lobular inflammation and hepatocyte ballooning, was mostly improved by TMAO treatment (Figure 2C), while the improvements in steatosis and fibrosis were not significant (Figure 2B and D). Moreover, serum levels of liver enzymes were measured. In HFHC diet-fed rats, serum levels of alanine transaminase and aspartate transaminase were greatly decreased by TMAO treatment while alkaline phosphatase was not significantly changed (Figure 2B-D), suggesting that TMAO attenuates hepatic inflammation and liver injury caused by HFHC diet feeding.
Figure 2.
Trimethylamine N-oxide reduces the activity of steatohepatitis and improves the serological alterations in rats fed a high-fat high-cholesterol diet. A: The steatosis, activity, and fibrosis scores; B: The scores of steatosis; C: The scores of activity; D: The scores of fibrosis; E: Serum levels of alanine transaminase; F: Serum levels of aspartate transaminase; G: Serum levels of alkaline phosphatase. The data are presented as the mean ± SE (n = 8-10). aP < 0.05, vs rats fed a chow diet; bP < 0.01, vs rats fed a chow diet; cP < 0.05, vs rats fed a high-fat high-cholesterol diet; dP < 0.01, vs rats fed a high-fat high-cholesterol diet. SAF: Steatosis, activity, and fibrosis; ALT: Alanine transaminase; AST: Aspartate transaminase; ALP: Alkaline phosphatase; TMAO: Trimethylamine N-oxide; HFHC: High-fat high-cholesterol.
TMAO improves liver injury by alleviating hepatic ER stress and cell death
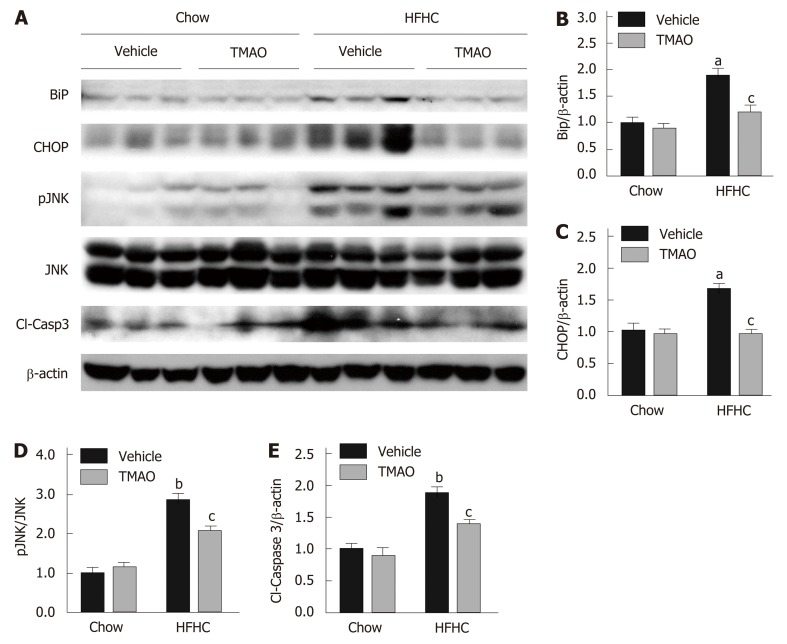
Cell death under hyperactive ER stress is a major cause of liver injury in NASH[23]. Therefore, we explored the effect of TMAO on hepatic ER stress and cell death in rats fed a HFHC diet. The hepatic protein levels of BiP and CHOP were greatly induced in the HFHC + Vehicle group, and were significantly inhibited by TMAO treatment, suggesting that TMAO can mitigate hepatic ER stress in HFHC diet-fed rats (Figure 3A-C). Furthermore, HFHC diet-induced increase in hepatic phosphorylated c-Jun N-terminal kinase (JNK) and cleaved caspase-3 was also decreased by TMAO treatment (Figure 3A, D and E), indicating that cell death is alleviated by TMAO intervention. Taken together, these data show that TMAO can alleviate HFHC diet-induced liver injury by inhibiting hepatic ER stress and cell death.
Figure 3.
Trimethylamine N-oxide alleviates liver injury by mitigating hepatic endoplasmic reticulum stress and cell death in rats fed a high-fat high-cholesterol diet. A: Representative immunoblots from three rats in each group; B-E: Densitometric quantification from three rats in each group. Relative protein levels normalized to β-actin except for phosphorylated c-Jun N-terminal kinase (JNK), which was normalized to endogenous JNK. The data are presented as the mean ± SE (n = 3). aP < 0.05, vs rats fed a chow diet; bP < 0.01, vs rats fed a chow diet; cP < 0.05, vs rats fed a high-fat high-cholesterol diet; dP < 0.01, vs rats fed a high-fat high-cholesterol diet. JNK: c-Jun N-terminal kinase; TMAO: Trimethylamine N-oxide; HFHC: High-fat high-cholesterol.
TMAO reduces hepatic cholesterol overload and improves the serum lipid profile in HFHC-fed rats
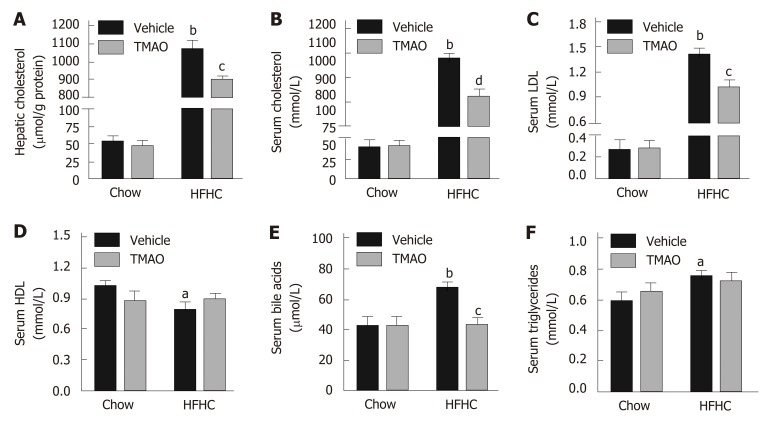
The pathogenic role of cholesterol in liver injury and inflammation has been demonstrated in the context of NASH[24]. Therefore, hepatic and serum levels of cholesterol were detected in rats. As shown in Figure 4A, the hepatic level of cholesterol was strikingly induced by the HFHC diet and significantly decreased by TMAO intervention. Similarly, serum levels of cholesterol and low-density lipoprotein were greatly decreased by TMAO treatment in rats fed a HFHC diet (Figure 4B and C), while serum level of high-density lipoprotein was not significantly changed in the TMAO-treated groups (Figure 4D). Notably, serum levels of bile acids were also greatly deceased by TMAO treatment in HFHC diet-fed rats (Figure 4E), whereas serum level of triglycerides was not significantly altered by TMAO (Figure 4F). These results imply a decreased overall cholesterol pool in HFHC diet-fed TMAO-treated rats.
Figure 4.
Trimethylamine N-oxide reduces hepatic cholesterol overload and improves serum lipid profile in rats fed a high-fat high-cholesterol diet. A: Hepatic levels of cholesterol; B: Serum levels of cholesterol; C: Serum levels of low-density lipoprotein; D: Serum levels of high-density lipoprotein; E: Serum levels of bile acids; F: Serum levels of triglycerides. The data are presented as the mean ± SE (n = 8-10). aP < 0.05, vs rats fed a chow diet; bP < 0.01, vs rats fed a chow diet; cP < 0.05, vs rats fed a high-fat high-cholesterol diet; dP < 0.01, vs rats fed a high-fat high-cholesterol diet. LDL: Low-density lipoprotein; HDL: High-density lipoprotein; TMAO: Trimethylamine N-oxide; HFHC: High-fat high-cholesterol.
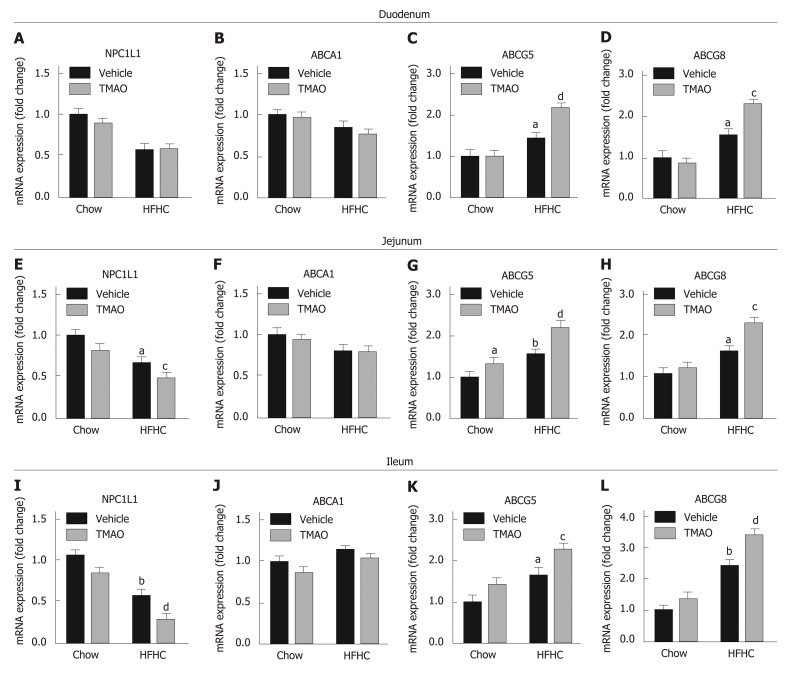
TMAO alters the expression of intestinal cholesterol transporters to inhibit cholesterol absorption
We next determined the effect of TMAO on intestinal cholesterol absorption. The expression levels of cholesterol transporters in the small intestine were detected. The expression level of NPC1L1, which is a key protein mediating the transportation of cholesterol from the gut lumen into enterocytes, was downregulated in the small intestine of HFHC diet-fed rats (Figure 5A, E, and I) and further decreased by TMAO treatment in the jejunum (Figure 5E) and ileum (Figure 5I). Furthermore, the expression levels of ATP-binding cassette subfamily G member 5 (ABCG5) and ABCG8, which facilitate the excretion of intracellular cholesterol into the gut lumen, were upregulated in the small intestine of HFHC diet-fed rats and further increased by TMAO treatment (Figure 5C, D, G, H, K, and L). The expression level of ABCA1, which mediates the efflux of intracellular cholesterol into the bloodstream, was not significantly altered by the HFHC diet and TMAO treatment (Figure 5B, F and J). Taken together, these data demonstrate that TMAO treatment alters the expression of cholesterol transporters in the small intestine to inhibit intestinal cholesterol absorption, reducing hepatic cholesterol overload in rats fed a HFHC diet.
Figure 5.
Trimethylamine N-oxide modulates the expression of intestinal cholesterol transporters to inhibit cholesterol absorption in rats fed a high-fat high-cholesterol diet. A-D: Expression levels of cholesterol transporters in the duodenum; E-H: Expression levels of cholesterol transporters in the jejunum; I-L: Expression levels of cholesterol transporters in the ileum. The data are presented as the mean ± SE (n = 7-8). aP < 0.05, vs rats fed a chow diet; bP < 0.01, vs rats fed a chow diet; cP < 0.05, vs rats fed a high-fat high-cholesterol diet; dP < 0.01, vs rats fed a high-fat high-cholesterol diet. TMAO: Trimethylamine N-oxide; HFHC: High-fat high-cholesterol; NPC1L1: Niemann-Pick C1-Like 1; ABCA1: ATP-binding cassette transporter A1; ABCG: ATP-binding cassette subfamily G member.
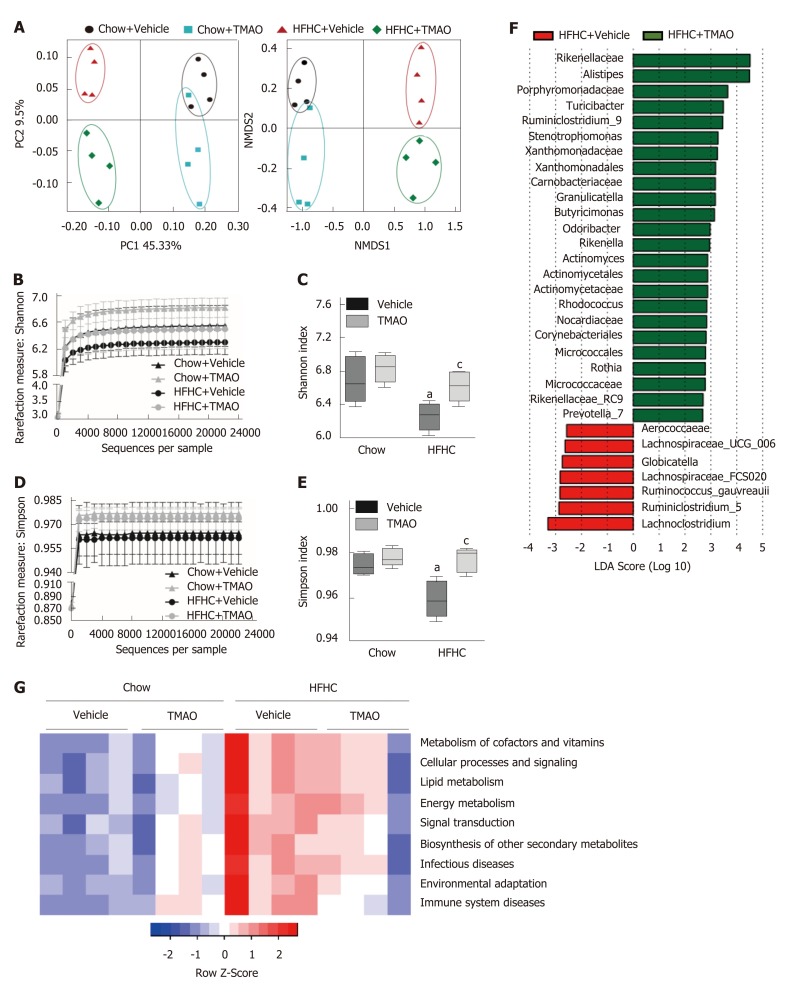
TMAO restores the diversity of the gut microbiota in rats fed a HFHC diet
Gut microbiota dysbiosis is frequently observed in patients with NAFLD and is considered to contribute to the pathogenesis of NASH[25,26]. We collected fecal samples at the end of the study (i.e., week 16) and performed 16S rRNA sequencing to examine the effect of TMAO on the gut microbial profile. As shown in Figure 6A, principal coordinate analysis and nonmetric multidimensional scaling analysis revealed that oral TMAO administration caused a major shift in the overall structure of the gut microbiota in both chow diet-fed and HFHC diet-fed rats. The depleted alpha diversity of the gut flora is a hallmark in multiple metabolic diseases, including NAFLD[27,28]. Therefore, we determined the effect of TMAO on the alpha diversity of the gut microbiota based on the Shannon and Simpson measurements. The rarefaction curve (Figure 6B) and index bar (Figure 6C) of the Shannon measurement both showed a restoration of alpha diversity with TMAO intervention in HFHC-fed rats. Similar results were observed when applying the Simpson measurement (Figure 6D and E). Next, we performed linear discriminant analysis coupled with effect size measurements to discriminate the altered gut bacteria by TMAO treatment. Compared to the HFHC + Vehicle group, an increased abundance of several families such as Rikenellaceae and Porphyromonadaceae, and genera such as Alistipes and Turicibacter, and a decreased abundance of several families such as Aerococcaeae and Lachnospiraceae were observed in the HFHC + TMAO group (Figure 6F). Moreover, we applied the PICRUSt (Phylogenetic Investigation of Communities by Reconstruction of Unobserved States) method to predict functional alterations of the gut microbiota by the HFHC diet and TMAO treatment. As shown in Figure 6G, TMAO partially reversed the HFHC diet-induced alterations of pathways related to nutrient and energy metabolism, suggesting the improved microbial profile by TMAO may facilitate the restoration of energy homeostasis under nutrients overload conditions. Taken together, these findings suggest that TMAO can regulate the structure of the gut microbiota and restore the depleted diversity under HFHC diet feeding conditions.
Figure 6.
Diversity of gut microbiota is restored by trimethylamine N-oxide treatment in rats fed a high-fat high-cholesterol diet. A: The beta diversity of the gut microbiota (n = 4); B: The rarefaction curve of the Shannon measurement (n = 4); C: Index bar of the Shannon measurement (n = 4); D: The rarefaction curve of the Simpson measurement (n = 4); E: Index bar of the Simpson measurement (n = 4); F: Linear discriminant analysis coupled with effect size measurements analysis (n = 4); G: Prediction of the functional genes in the sampled bacterial community using phylogenetic investigation of communities by reconstruction of unobserved states (n = 4). aP < 0.05, vs rats fed a chow diet; bP < 0.01, vs rats fed a chow diet; cP < 0.05, vs rats fed a high-fat high-cholesterol diet; dP < 0.01, vs rats fed a high-fat high-cholesterol diet. TMAO: Trimethylamine N-oxide; HFHC: High-fat high-cholesterol.
DISCUSSION
Although TMAO has been shown to be involved in the pathogenesis of CVD, its role in NASH remains undetermined. We demonstrated, for the first time, that oral TMAO administration restored the diversity of gut flora, inhibited intestinal cholesterol absorption, reduced hepatic cholesterol overload, and attenuated ER stress and cell death induced liver injury, which contributed to the histological and serological improvements of NASH in HFHC diet-fed rats.
One of the most important findings of this study is the identification of an important role of TMAO in cholesterol metabolism and metabolic stress under cholesterol overload. ER is a major storage organelle of intracellular cholesterol. Disruption of cholesterol homeostasis and excess cholesterol challenge will disrupt the physiological function of ER to correctly fold and modify proteins, resulting in ER stress. Under this circumstance, the unfolded protein response is activated to protect against ER stress, and if the adaptive reactions fail to compensate, apoptosis will be induced[29]. Our data show that hepatic ER stress and cell death were decreased by TMAO intervention. This may be due to the reduction of hepatic cholesterol by TMAO treatment. TMAO may also serve as a chemical chaperone to facilitate protein folding and stabilize protein structure and directly alleviate ER stress. Several studies have proposed that the alleviation of ER stress by TMAO may mediate the beneficial roles of TMAO[13,14]. To further verify the direct effect of TMAO on ER stress in hepatocytes, in vitro assays will be needed in future studies.
Our study also showed that oral TMAO administration altered the expression levels of cholesterol transporters in the small intestine, especially in the jejunum and ileum. The downregulated NPC1L1 and upregulated ABCG5/8 in the TMAO-treated group could lead to decreased intestinal cholesterol absorption from the diet. It is interesting that the effect of TMAO on the expression of intestinal cholesterol transporters was magnified under HFHC-diet feeding conditions. A possible explanation is that activated adaptive regulation of intestinal cholesterol transporters under high cholesterol influx conditions synergizes with TMAO functions. Moreover, it has also been demonstrated that dietary TMAO supplementation can cause a 26% reduction in intestinal cholesterol absorption under normal chow diet feeding conditions[10], which partially supports our findings in the context of HFHC diet-induced steatohepatitis.
Another finding of our study is that TMAO restored the diversity of gut commensal bacteria in rats fed a HFHC diet. Gut dysbiosis is a hallmark of NAFLD and NASH, in which reduced diversity of gut bacteria is an important characteristic[25,30]. Gut dysbiosis leads to gut bacteria translocation due to the impaired intestinal barrier and increased production of harmful microbial metabolites such as lipopolysaccharide and endogenous ethanol, which contribute to the pathogenesis and progression of NAFLD and NASH[31]. The restoration of the gut microbiota diversity by TMAO may mediate its beneficial role in HFHC diet-induced steatohepatitis. It has been demonstrated that bile acids have a great impact on the composition of the gut microbiota[32,33]. Bile acids can directly impair membrane integrity, cause oxidative stress, damage DNA structure, and indirectly sabotage microbial growth via the intestinal farnesoid X receptor, leading to an overall negative effect on the gut bacteria[34,35]. On the other hand, TMAO has been shown to inhibit bile acid synthesis by downregulating Cyp7A1 and Cyp27A1, which results in a reduced total bile acid pool size[10]. In agreement with this finding, a decreased serum level of bile acids in TMAO-treated rats was also observed in our study. Taken together, these findings suggest that TMAO may restore gut microbial diversity by affecting bile acid metabolism. Nevertheless, further studies are needed to determine the direct and indirect effects of TMAO on the gut microbial profile.
There are some limitations to the present study. First, the potential harmful effect of TMAO on the cardiovascular system was not excluded in our study. However, it has been shown that TMAO treatment at lower doses may not exert negative effects on the circulatory system[15]. The minimal effective dose of TMAO in the NASH intervention will be defined in our further study. Second, the contribution of improved gut microbial profile to the attenuation of HFHC diet-induced steatohepatitis by TMAO treatment was not fully illuminated. More investigations concerning the effect of TMAO on gut microbiota and metabolic consequences are needed. Third, the direct targets and exact mechanism of the regulation of intestinal cholesterol absorption by TMAO need to be further explored.
In conclusion, the current study demonstrates that the gut microbial metabolite TMAO restores the diversity of gut flora, inhibits intestinal cholesterol absorption, and reduces hepatic cholesterol overload, leading to the attenuation of cholesterol-induced hepatic ER stress and cell death. These functions facilitate the protection of TMAO against HFHC diet-induced steatohepatitis in rats. Our data highlight the important role of TMAO in cholesterol metabolism and its beneficial effect on metabolic stress under cholesterol overload. The safety of TMAO treatment for CVD should be further evaluated and the effective dose of TMAO in the NASH intervention should be further defined.
ARTICLE HIGHLIGHTS
Research background
The gut microbial metabolites have been shown to be mediators in the gut-liver axis and play important role in non-alcoholic fatty liver disease. Trimethylamine N-oxide (TMAO) is a gut microbial metabolite derived from dietary choline and L-carnitine and is implied to be involved in the pathogenesis of cardiovascular disease.
Research motivation
Although the gut microbiota has long been found to play important roles in maintaining health, its function and regulation pathways are largely unknown. The gut microbial metabolites are considered to mediate the interaction between the gut commensal bacteria and the host. Exploring the function of gut microbial metabolites may help develop novel therapeutic approaches.
Research objectives
The main objective of the present study was to explore the function of TMAO in the progression of NASH and identify the targets and mechanisms underlying the effect of TMAO.
Research methods
A rat model of NASH was induced by high-fat high-cholesterol (HFHC) diet feeding for 16 wk. TMAO was orally administrated daily for 8 wk. Histological analysis was performed to evaluate the effect of TMAO on steatohepatitis. Hepatic and serum lipid profiles were measured. Endoplasmic reticulum (ER) stress-related pathways were detected by Western blot and expression levels of intestinal cholesterol transporters were detected by qRT-PCR. 16s rDNA sequencing was preformed to examine the effect of TMAO on gut microbial profile.
Research results
Oral TMAO administration significantly improved the histological and serological alterations in HFHC diet-induced steatohepatitis. Hepatic ER stress and cell death were ameliorated by TMAO treatment. Both hepatic and serum levels of cholesterol were decreased by TMAO intervention. The expression levels of intestinal cholesterol transporters were altered by TMAO treatment. And the diversity of gut microbial profile was restored by TMAO treatment.
Research conclusions
Under HFHC diet feeding conditions, TMAO inhibits intestinal cholesterol absorption, attenuates hepatic cholesterol overload, and alleviates ER stress mediated liver injury, which leads to the protective role of TMAO in HFHC diet-induced steatohepatitis in rats.
Research perspectives
The minimal effective dose of TMAO treatment needs to be defined to avoid the potential harmful effect of TMAO on the cardiovascular system in future studies.
Footnotes
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
Institutional review board statement: This study was approved by the institutional review board of Xinhua Hospital Affiliated to Shanghai Jiao Tong University School of Medicine.
Institutional animal care and use committee statement: The study was approved by the Institutional Animal Care and Use Committee of Xinhua Hospital Affiliated to Shanghai Jiao Tong University School of Medicine.
Conflict-of-interest statement: The authors declare that they have no conflict of interest.
Data sharing statement: No additional data are available.
ARRIVE guidelines statement: The ARRIVE Guidelines have been adopted.
Peer-review started: March 28, 2019
First decision: April 11, 2019
Article in press: May 8, 2019
P-Reviewer: Beltowski J, Hosomi R, Wada J S-Editor: Yan JP L-Editor: Wang TQ E-Editor: Zhang YL
Contributor Information
Ze-Hua Zhao, Center for Fatty Liver, Department of Gastroenterology, Xinhua Hospital Affiliated to Shanghai Jiao Tong University School of Medicine, Shanghai 200092, China.
Feng-Zhi Xin, Center for Fatty Liver, Department of Gastroenterology, Xinhua Hospital Affiliated to Shanghai Jiao Tong University School of Medicine, Shanghai 200092, China.
Da Zhou, Center for Fatty Liver, Department of Gastroenterology, Xinhua Hospital Affiliated to Shanghai Jiao Tong University School of Medicine, Shanghai 200092, China.
Ya-Qian Xue, CAS Key Laboratory of Nutrition, Metabolism and Food Safety, Shanghai Institute of Nutrition and Health, Shanghai Institutes for Biological Sciences, University of Chinese Academy of Sciences, Chinese Academy of Sciences, Shanghai 200031, China.
Xiao-Lin Liu, Center for Fatty Liver, Department of Gastroenterology, Xinhua Hospital Affiliated to Shanghai Jiao Tong University School of Medicine, Shanghai 200092, China.
Rui-Xu Yang, Center for Fatty Liver, Department of Gastroenterology, Xinhua Hospital Affiliated to Shanghai Jiao Tong University School of Medicine, Shanghai 200092, China.
Qin Pan, Center for Fatty Liver, Department of Gastroenterology, Xinhua Hospital Affiliated to Shanghai Jiao Tong University School of Medicine, Shanghai 200092, China.
Jian-Gao Fan, Center for Fatty Liver, Department of Gastroenterology, Xinhua Hospital Affiliated to Shanghai Jiao Tong University School of Medicine, Shanghai 200092, China; Shanghai Key Lab of Pediatric Gastroenterology and Nutrition, Shanghai 200092, China. fanjiangao@xinhuamed.com.cn.
References
- 1.Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73–84. doi: 10.1002/hep.28431. [DOI] [PubMed] [Google Scholar]
- 2.Fan JG, Kim SU, Wong VW. New trends on obesity and NAFLD in Asia. J Hepatol. 2017;67:862–873. doi: 10.1016/j.jhep.2017.06.003. [DOI] [PubMed] [Google Scholar]
- 3.Buzzetti E, Pinzani M, Tsochatzis EA. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD) Metabolism. 2016;65:1038–1048. doi: 10.1016/j.metabol.2015.12.012. [DOI] [PubMed] [Google Scholar]
- 4.Leung C, Rivera L, Furness JB, Angus PW. The role of the gut microbiota in NAFLD. Nat Rev Gastroenterol Hepatol. 2016;13:412–425. doi: 10.1038/nrgastro.2016.85. [DOI] [PubMed] [Google Scholar]
- 5.Chu H, Duan Y, Yang L, Schnabl B. Small metabolites, possible big changes: A microbiota-centered view of non-alcoholic fatty liver disease. Gut. 2019;68:359–370. doi: 10.1136/gutjnl-2018-316307. [DOI] [PubMed] [Google Scholar]
- 6.Zhou D, Chen YW, Zhao ZH, Yang RX, Xin FZ, Liu XL, Pan Q, Zhou H, Fan JG. Sodium butyrate reduces high-fat diet-induced non-alcoholic steatohepatitis through upregulation of hepatic GLP-1R expression. Exp Mol Med. 2018;50:157. doi: 10.1038/s12276-018-0183-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cho CE, Caudill MA. Trimethylamine-N-Oxide: Friend, Foe, or Simply Caught in the Cross-Fire? Trends Endocrinol Metab. 2017;28:121–130. doi: 10.1016/j.tem.2016.10.005. [DOI] [PubMed] [Google Scholar]
- 8.Tang WH, Wang Z, Levison BS, Koeth RA, Britt EB, Fu X, Wu Y, Hazen SL. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N Engl J Med. 2013;368:1575–1584. doi: 10.1056/NEJMoa1109400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang Z, Klipfell E, Bennett BJ, Koeth R, Levison BS, Dugar B, Feldstein AE, Britt EB, Fu X, Chung YM, Wu Y, Schauer P, Smith JD, Allayee H, Tang WH, DiDonato JA, Lusis AJ, Hazen SL. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472:57–63. doi: 10.1038/nature09922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koeth RA, Wang Z, Levison BS, Buffa JA, Org E, Sheehy BT, Britt EB, Fu X, Wu Y, Li L, Smith JD, DiDonato JA, Chen J, Li H, Wu GD, Lewis JD, Warrier M, Brown JM, Krauss RM, Tang WH, Bushman FD, Lusis AJ, Hazen SL. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med. 2013;19:576–585. doi: 10.1038/nm.3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang Z, Roberts AB, Buffa JA, Levison BS, Zhu W, Org E, Gu X, Huang Y, Zamanian-Daryoush M, Culley MK, DiDonato AJ, Fu X, Hazen JE, Krajcik D, DiDonato JA, Lusis AJ, Hazen SL. Non-lethal Inhibition of Gut Microbial Trimethylamine Production for the Treatment of Atherosclerosis. Cell. 2015;163:1585–1595. doi: 10.1016/j.cell.2015.11.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhu W, Gregory JC, Org E, Buffa JA, Gupta N, Wang Z, Li L, Fu X, Wu Y, Mehrabian M, Sartor RB, McIntyre TM, Silverstein RL, Tang WHW, DiDonato JA, Brown JM, Lusis AJ, Hazen SL. Gut Microbial Metabolite TMAO Enhances Platelet Hyperreactivity and Thrombosis Risk. Cell. 2016;165:111–124. doi: 10.1016/j.cell.2016.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lupachyk S, Watcho P, Stavniichuk R, Shevalye H, Obrosova IG. Endoplasmic reticulum stress plays a key role in the pathogenesis of diabetic peripheral neuropathy. Diabetes. 2013;62:944–952. doi: 10.2337/db12-0716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dumas ME, Rothwell AR, Hoyles L, Aranias T, Chilloux J, Calderari S, Noll EM, Péan N, Boulangé CL, Blancher C, Barton RH, Gu Q, Fearnside JF, Deshayes C, Hue C, Scott J, Nicholson JK, Gauguier D. Microbial-Host Co-metabolites Are Prodromal Markers Predicting Phenotypic Heterogeneity in Behavior, Obesity, and Impaired Glucose Tolerance. Cell Rep. 2017;20:136–148. doi: 10.1016/j.celrep.2017.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huc T, Drapala A, Gawrys M, Konop M, Bielinska K, Zaorska E, Samborowska E, Wyczalkowska-Tomasik A, Pączek L, Dadlez M, Ufnal M. Chronic, low-dose TMAO treatment reduces diastolic dysfunction and heart fibrosis in hypertensive rats. Am J Physiol Heart Circ Physiol. 2018;315:H1805–H1820. doi: 10.1152/ajpheart.00536.2018. [DOI] [PubMed] [Google Scholar]
- 16.Fan JG, Zhong L, Xu ZJ, Tia LY, Ding XD, Li MS, Wang GL. Effects of low-calorie diet on steatohepatitis in rats with obesity and hyperlipidemia. World J Gastroenterol. 2003;9:2045–2049. doi: 10.3748/wjg.v9.i9.2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fan JG, Zhong L, Tia LY, Xu ZJ, Li MS, Wang GL. Effects of ursodeoxycholic acid and/or low-calorie diet on steatohepatitis in rats with obesity and hyperlipidemia. World J Gastroenterol. 2005;11:2346–2350. doi: 10.3748/wjg.v11.i15.2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bedossa P, Poitou C, Veyrie N, Bouillot JL, Basdevant A, Paradis V, Tordjman J, Clement K. Histopathological algorithm and scoring system for evaluation of liver lesions in morbidly obese patients. Hepatology. 2012;56:1751–1759. doi: 10.1002/hep.25889. [DOI] [PubMed] [Google Scholar]
- 19.Desjardins P, Conklin D. NanoDrop microvolume quantitation of nucleic acids. J Vis Exp. 2010;(45):pii: 2565. doi: 10.3791/2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bolger AM, Lohse M, Usadel B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30:2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reyon D, Tsai SQ, Khayter C, Foden JA, Sander JD, Joung JK. FLASH assembly of TALENs for high-throughput genome editing. Nat Biotechnol. 2012;30:460–465. doi: 10.1038/nbt.2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rognes T, Flouri T, Nichols B, Quince C, Mahé F. VSEARCH: A versatile open source tool for metagenomics. PeerJ. 2016;4:e2584. doi: 10.7717/peerj.2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schwabe RF, Luedde T. Apoptosis and necroptosis in the liver: A matter of life and death. Nat Rev Gastroenterol Hepatol. 2018;15:738–752. doi: 10.1038/s41575-018-0065-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marra F, Svegliati-Baroni G. Lipotoxicity and the gut-liver axis in NASH pathogenesis. J Hepatol. 2018;68:280–295. doi: 10.1016/j.jhep.2017.11.014. [DOI] [PubMed] [Google Scholar]
- 25.Shen F, Zheng RD, Sun XQ, Ding WJ, Wang XY, Fan JG. Gut microbiota dysbiosis in patients with non-alcoholic fatty liver disease. Hepatobiliary Pancreat Dis Int. 2017;16:375–381. doi: 10.1016/S1499-3872(17)60019-5. [DOI] [PubMed] [Google Scholar]
- 26.Saltzman ET, Palacios T, Thomsen M, Vitetta L. Intestinal Microbiome Shifts, Dysbiosis, Inflammation, and Non-alcoholic Fatty Liver Disease. Front Microbiol. 2018;9:61. doi: 10.3389/fmicb.2018.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fangmann D, Theismann EM, Türk K, Schulte DM, Relling I, Hartmann K, Keppler JK, Knipp JR, Rehman A, Heinsen FA, Franke A, Lenk L, Freitag-Wolf S, Appel E, Gorb S, Brenner C, Seegert D, Waetzig GH, Rosenstiel P, Schreiber S, Schwarz K, Laudes M. Targeted Microbiome Intervention by Microencapsulated Delayed-Release Niacin Beneficially Affects Insulin Sensitivity in Humans. Diabetes Care. 2018;41:398–405. doi: 10.2337/dc17-1967. [DOI] [PubMed] [Google Scholar]
- 28.Del Chierico F, Nobili V, Vernocchi P, Russo A, Stefanis C, Gnani D, Furlanello C, Zandonà A, Paci P, Capuani G, Dallapiccola B, Miccheli A, Alisi A, Putignani L. Gut microbiota profiling of pediatric nonalcoholic fatty liver disease and obese patients unveiled by an integrated meta-omics-based approach. Hepatology. 2017;65:451–464. doi: 10.1002/hep.28572. [DOI] [PubMed] [Google Scholar]
- 29.Sozen E, Ozer NK. Impact of high cholesterol and endoplasmic reticulum stress on metabolic diseases: An updated mini-review. Redox Biol. 2017;12:456–461. doi: 10.1016/j.redox.2017.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schneider KM, Mohs A, Kilic K, Candels LS, Elfers C, Bennek E, Schneider LB, Heymann F, Gassler N, Penders J, Trautwein C. Intestinal Microbiota Protects against MCD Diet-Induced Steatohepatitis. Int J Mol Sci. 2019;20:pii: E308. doi: 10.3390/ijms20020308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhao ZH, Lai JK, Qiao L, Fan JG. Role of gut microbial metabolites in nonalcoholic fatty liver disease. J Dig Dis. 2019;20:181–188. doi: 10.1111/1751-2980.12709. [DOI] [PubMed] [Google Scholar]
- 32.Islam KB, Fukiya S, Hagio M, Fujii N, Ishizuka S, Ooka T, Ogura Y, Hayashi T, Yokota A. Bile acid is a host factor that regulates the composition of the cecal microbiota in rats. Gastroenterology. 2011;141:1773–1781. doi: 10.1053/j.gastro.2011.07.046. [DOI] [PubMed] [Google Scholar]
- 33.Ridlon JM, Alves JM, Hylemon PB, Bajaj JS. Cirrhosis, bile acids and gut microbiota: Unraveling a complex relationship. Gut Microbes. 2013;4:382–387. doi: 10.4161/gmic.25723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Begley M, Gahan CG, Hill C. The interaction between bacteria and bile. FEMS Microbiol Rev. 2005;29:625–651. doi: 10.1016/j.femsre.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 35.Staley C, Weingarden AR, Khoruts A, Sadowsky MJ. Interaction of gut microbiota with bile acid metabolism and its influence on disease states. Appl Microbiol Biotechnol. 2017;101:47–64. doi: 10.1007/s00253-016-8006-6. [DOI] [PMC free article] [PubMed] [Google Scholar]