Abstract
We describe a 55-year-old woman with lung cancer complicated by bone metastases. Treatment with denosumab (120 mg monthly) was interrupted after 9 doses because of concern for potential osteonecrosis of the jaw during upcoming dental work. Fifteen months after receiving the last dose of denosumab, the patient presented with 7 atraumatic spinal compression fractures requiring kyphoplasty for symptom relief. No malignancy was found in pathology specimens. Evaluation for secondary causes of osteoporosis was negative. This phenomenon of rebound fractures after discontinuing the use of denosumab, an inhibitor of RANK ligand, has been well described in patients with osteoporosis, who receive much lower doses than do patients with cancer. However, this has not been previously reported in oncology patients, likely because most succumb to their disease before denosumab therapy is stopped.
Denosumab, a human monoclonal antibody that binds RANK ligand, is an important treatment for patients with bone metastases from various malignancies. An osteoclast inhibitor, denosumab is more effective than zoledronic acid in prolonging time to skeletal-related events (defined as pathologic fracture, radiation/surgery to bone, or spinal cord compression) in patients with solid tumors.1 Higher doses of denosumab are used in malignancy (120 mg monthly) than in osteoporosis (60 mg every 6 months). However, because the oncology dose is greater and possibly because of a more vulnerable patient population, rates of osteonecrosis of the jaw are higher, 1.7%, vs 0.1% in patients with osteoporosis.2 To reduce this risk, denosumab therapy is held before dental work. In the osteoporosis literature, discontinuation of denosumab therapy has been associated with rebound vertebral fractures,3, 4, 5 although this has not yet been described in the oncology-dose protocol recipients. We present a patient with bone metastases from lung cancer who suffered multiple vertebral compression fractures after holding denosumab therapy.
Case Report
A 55-year-old woman with stage IV lung adenocarcinoma receiving chemotherapy who had previously been treated with denosumab presented to endocrinology with 7 vertebral fractures that had occurred over 4 months. Originally diagnosed as having lung cancer in 2013, she underwent surgery and then 4 cycles of cisplatin/pemetrexed, with subsequent negative positron emission tomography. Repeated positron emission tomography/computed tomography (CT) (December 2014) showed multiple hypermetabolic lymph nodes and a destructive rib lesion. Treatment with erlotinib, 150 mg daily, and denosumab, 120 mg monthly, was started. After 8 doses (December 2014 through September 2015), denosumab therapy was held for dental procedures. She received another dose of denosumab in May 2016 but no more due to ongoing dental issues. Serial imaging through 2015 and 2016 showed a positive cancer response to chemotherapy until February 2017, when CT showed new lung nodules.
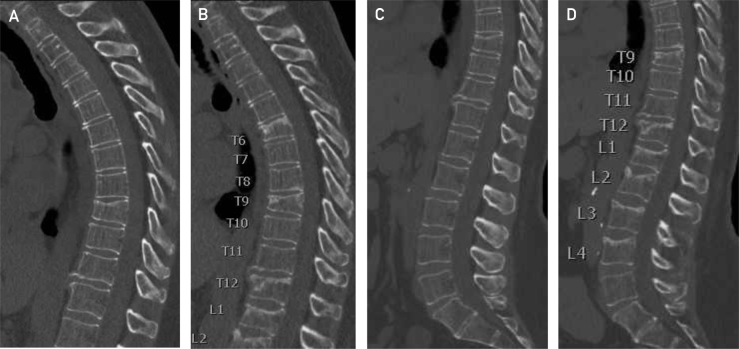
In August 2017, chest CT noted a new compression deformity of the thoracic T9 vertebra. In October 2017, the patient lifted a heavy object and had the acute onset of midthoracic spine pain. Imaging revealed new fractures of the T6, T12, and L1 vertebral bodies, with no underlying osseous metastases identified (Figure). In December 2017, radiography showed new lumbar compression fractures at L2, L3, and L4. Kyphoplasty was performed at T6, T9, T12, and L1 through L4 for persistent pain. Biopsies of these 7 vertebral bodies were negative for malignancy.
Figure.
Sagittal reconstruction of the thoracic and lumbar spines from computed tomographic scans of the thorax and abdomen/pelvis, initially and 4 months later. The images demonstrate the evolution of the fractures from August 2017 (A and C) through December 2017 (B and D) at T6, T9, T12, L1, L2, L3, and L4, with a combination of sclerosis, principally at the superior end plates, and loss of vertebral height. The images were obtained using the Revolution CT (GE Healthcare) (displayed at 3.75 mm thick, reconstructed from 0.625-mm helical acquisition and on a bone algorithm of width 2000 and level 500).
Previously, she had experienced neither fragility fractures nor height loss. Family history was positive for an aunt with osteoporosis. She has taken vitamin D, 2000 IU daily, for many years. Her only other risk factors include mild secondary hyperparathyroidism (parathyroid hormone level range, 50-112 pg/mL [to convert to ng/L, multiply by 1] on several occasions in 2017-2018), presumably related to chronic kidney disease (estimated glomerular filtration rate range, 30-40 mL/min) or inadequate intake/absorption of calcium. Investigation for secondary causes of osteoporosis was otherwise negative. 25-Hydroxyvitamin D concentration was 38 ng/mL (to convert to nmol/L, multiply by 2.496). Bone densitometry in January 2018 showed a left femur T score of –1.7 with a Z score of –1.0, and a left radius one-third T score of –0.6. Lumbar spine densitometry was uninterpretable due to kyphoplasty.
Discussion
Based on her bone density and absence of major secondary risk factors, this patient was not at elevated risk for vertebral compression fractures. Although she had rib metastasis, there was no evidence of spine metastases, and her cancer was responding to therapy. After diagnosis of fractures, a thorough evaluation did not demonstrate a secondary etiology to explain the 7 vertebral fractures over a 4-month period. Her use and subsequent discontinuation of denosumab, however, puts her at high risk for rebound vertebral fractures that may be underappreciated by prescribing oncology providers.3, 4, 5
Recent osteoporosis literature has noted rebound vertebral fractures in patients in whom denosumab therapy was discontinued. Because denosumab is not incorporated into bone matrix the way that bisphosphonates are, bone turnover is no longer suppressed once denosumab use is stopped, and studies suggest accelerated bone resorption afterward. This patient did not experience vertebral fractures until 15 months after her last dose, consistent with previous reports of multiple vertebral fractures occurring 9 to 16 months after the last injection. Several studies also note markers of bone turnover rebound to values above average 1 to 2 years after stopping denosumab therapy.3 Patients exposed to denosumab in one study demonstrated relatively elevated concentrations of P1NP and CTX (markers of bone formation and resorption, respectively), suggesting acceleration in bone turnover (compared with drug-naive persons).6 Levels of micro-RNA known to be important in inhibition of osteoclastogenesis were also significantly lower than in denosumab-naive patients.6
There is also some evidence that the rise in bone turnover markers and the decline in bone density after discontinuation of denosumab use increases with the duration of denosumab treatment. It is unclear whether this is due to duration of treatment or accumulated dosage. If related to dosage, patients receiving the oncology doses of denosumab (12× the standard osteoporosis dose) may also be at higher risk for rebound fractures. This risk of rebound fracture is high enough that a systematic review recommends not to stop denosumab therapy in high-risk patients, or, alternatively, to switch to another therapy, such as a bisphosphonate. The risks and benefits of holding denosumab therapy in oncology patients for dental procedures due to rare osteonecrosis of the jaw must be balanced with the potential for rebound fractures. It is unclear whether it is safe to hold denosumab therapy in oncology patients for short durations (<6 months, which is the normal interval between doses in osteoporotic patients).
Conclusion
Although rebound fractures after denosumab therapy discontinuation have been reported in the osteoporosis literature, this is the first case report of multiple vertebral fractures occurring in a patient after discontinuation of oncology-dose protocol denosumab therapy. Presumably, many oncology patients treated with denosumab succumb to their cancer without denosumab interruption, hence the lack of reports of this phenomenon. Fractures in the present patient occurred 15 months after the last dose, timing that is consistent with previous reports. Given this, risks and benefits must be weighed before holding denosumab therapy to avoid osteonecrosis of the jaw. Emphasis needs to be placed on recommendations for exemplary oral hygiene for such patients and for completion of invasive dental procedures before initiation of denosumab therapy, while acknowledging the difficulty of such recommendations in a population with an urgent need for therapy. An alternative strategy is to prioritize the use of zolendronic acid every 12 weeks (instead of monthly) because rebound fractures have not been described after bisphosphonate discontinuation.7
Footnotes
Potential Competing Interests: Dr Patel receives scientific advisory income from AstraZeneca, Bristol-Myers Squibb, Illumina, Novartis, Nektar, and Tempus. Dr Patel's university receives research funding from Bristol-Myers Squibb, Eli Lilly, Incyte, AstraZeneca/MedImmune, Merck, Pfizer, Roche/Genentech, Xcovery, Fate Therapeutics, Genocea, and Iovance.
References
- 1.Henry D., Vadhan-raj S., Hirsh V., et al. Delaying skeletal-related events in a randomized phase 3 study of denosumab versus zoledronic acid in patients with advanced cancer: an analysis of data from patients with solid tumors. Support Care Cancer. 2014;22(3):679–687. doi: 10.1007/s00520-013-2022-1. [DOI] [PubMed] [Google Scholar]
- 2.Dodson T.B. The frequency of medication-related osteonecrosis of the jaw and its associated risk factors. Oral Maxillofac Surg Clin North Am. 2015;27(4):509–516. doi: 10.1016/j.coms.2015.06.003. [DOI] [PubMed] [Google Scholar]
- 3.Tsourdi E., Langdahl B., Cohen-Solal M., et al. Discontinuation of denosumab therapy for osteoporosis: a systematic review and position statement by ECTS. Bone. 2017;105:11–17. doi: 10.1016/j.bone.2017.08.003. [DOI] [PubMed] [Google Scholar]
- 4.Cummings S.R., Ferrari S., Eastell R., et al. Vertebral fractures after discontinuation of denosumab: a post hoc analysis of the randomized placebo-controlled FREEDOM trial and its extension. J Bone Miner Res. 2018;33(2):190–198. doi: 10.1002/jbmr.3337. [DOI] [PubMed] [Google Scholar]
- 5.Lamy O., Gonzalez-Rodriguez E., Stoll D., Hans D., Aubry-Rozier B. Severe rebound-associated vertebral fractures after denosumab discontinuation: 9 clinical cases report. J Clin Endocrinol Metab. 2017;102(2):354–358. doi: 10.1210/jc.2016-3170. [DOI] [PubMed] [Google Scholar]
- 6.Anastasilakis A.D., Yavropoulou M.P., Makras P., et al. Increased osteoclastogenesis in patients with vertebral fractures following discontinuation of denosumab treatment. Eur J Endocrinol. 2017;176(6):677–683. doi: 10.1530/EJE-16-1027. [DOI] [PubMed] [Google Scholar]
- 7.Himelstein A.L., Foster J.C., Khatcheressian J.L., et al. Effect of longer-interval vs standard dosing of zoledronic acid on skeletal events in patients with bone metastases: a randomized clinical trial. JAMA. 2017;317(1):48–58. doi: 10.1001/jama.2016.19425. [DOI] [PMC free article] [PubMed] [Google Scholar]