Abstract
Cardiac beriberi, or heart failure due to thiamine deficiency, is considered rare in the developed world. The diagnosis is often only considered in limited populations such as those with chronic alcoholism. Alternatively, the disease can be mislabeled as “alcoholic cardiomyopathy” or “nonischemic cardiomyopathy.” The following 2 cases illustrate the need to expand our vigilance to other at-risk populations.
Case 1
A 68-year-old homeless man presented to the emergency department with 5 weeks of progressive dyspnea. He noticed increased swelling in his legs but denied cough, fevers, or calf pain. He denied any recent drug use. He had a remote history of alcoholism but had quit 10 years ago and had no recent medical care.
On admission, he was afebrile with a heart rate of 81 beats per minute (bpm), blood pressure (BP) of 156/105 mm Hg, respiratory rate of 22 breaths per minute, and oxygen saturation of 99% on 2L/min of oxygen. He weighed 201 lb (body mass index [BMI] 33kg/m2). Jugular venous pressure (JVP) was elevated to the angle of the mandible, and cardiac examination showed a regular rate with no murmurs. Trace bibasilar crackles were auscultated, and mild bilateral lower extremity edema was evident. No focal deficits were noted on neurologic examination. Electrocardiogram demonstrated normal sinus rhythm. His chest x-ray showed cardiomegaly with suggestion of Kerley B lines (Figure 1). Laboratory results were remarkable for an elevated B-type natriuretic peptide (BNP) level of 1017 pg/mL and troponin-I of 0.107 ng/mL. Complete blood count was significant for normocytic anemia with a hemoglobin level of 10.5 g/dL. His urine toxicology screen showed no illicit substances. Transthoracic echocardiography (TTE) revealed global left ventricle hypokinesis with a severely reduced ejection fraction of 25% with moderately reduced right ventricular systolic function (Figure 2 B, C). Results of a nuclear myocardial perfusion stress test were inconclusive. The patient subsequently underwent cardiac catheterization and was found to have nonobstructive coronary artery disease. The patient was diagnosed with new-onset nonischemic cardiomyopathy and was medically treated with furosemide 20 mg twice daily, carvedilol 12.5 mg twice daily, lisinopril 10 mg daily, and atorvastatin 40 mg daily.
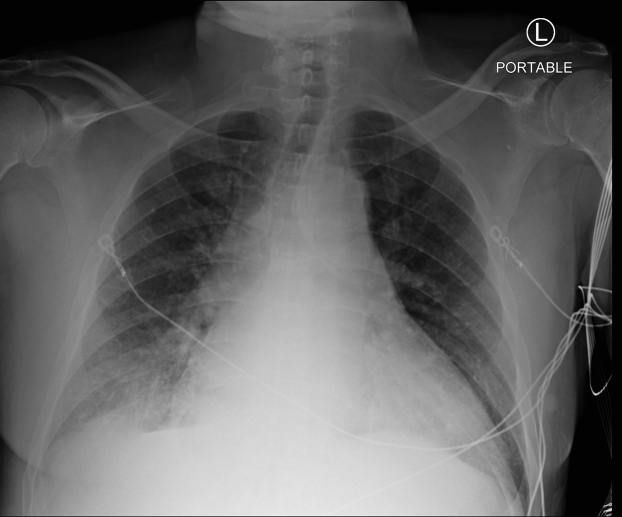
Figure 1.
Case 1: Initial chest x-ray with cardiomegaly, pulmonary venous congestion, and Kerley B lines suggestive of acute heart failure.
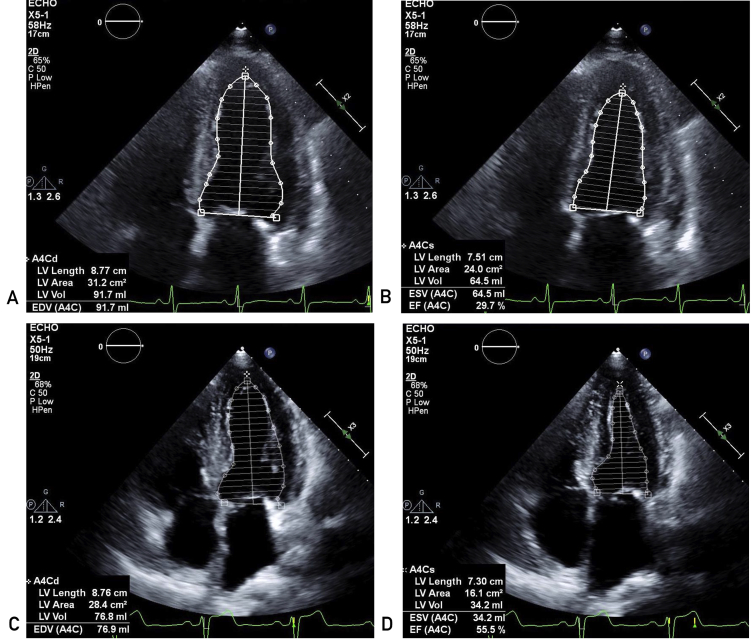
Figure 2.
Case 1: Echocardiogram showing left ventricular cavity size in diastole and systole (Panel A and B, respectively) prior to thiamine treatment and after treatment (diastole and systole, Panel C and D, respectively). Patient was noted to have significant improvement in contractility with improvement of his ejection fraction from 25% to 55%.
Over the next 3 months, the patient presented to the emergency department multiple times with acute heart failure exacerbations that were suspected to be secondary to medication noncompliance. On the third ED visit, he was admitted for further workup, as he appeared confused. Neurologic testing revealed significant neurocognitive deficits as well as bilateral cranial nerve 6 palsies. He was presumed to have Wernicke's encephalopathy caused by his history of alcohol abuse, although he denied recent use. Oral thiamine at 100 mg per day was started. After 16 days, he became progressively hypotensive, requiring discontinuation of all his cardiac medications. A repeat TTE showed dramatic improvement in left ventricular systolic function with ejection fraction of 55% (Figure 2 D, E). Moreover, the patient's cognitive status significantly improved with his Montreal Cognitive Assessment (MOCA) scores increasing from 6/30 to 18/30. Review of his records showed that the patient had a random, nonfasting thiamine level of 12 nmol/L (normal: 8 to 30 nmol/L) a few days after his initial admission.
Case 2
A 63-year-old man with diabetes, hypertension, and remote history of polysubstance and alcohol abuse presented with dyspnea and altered mental status of unclear duration. The patient reported exertional dyspnea, leg swelling, and orthopnea. He had been severely limiting caloric intake to encourage weight loss. He often ate less than 1 meal a day and relied on convenience foods, as he had limited access to transportation to the grocery store. His home medications included aspirin 81 mg, atorvastatin 40 mg, lisinopril 10 mg, metformin 1000 mg twice daily, and repaglinide 2 mg with meals.
On examination, he was tachycardic, with a heart rate of 117 bpm; BP of 160/120 mm Hg; and tachypneic, saturating 96% on room air. He weighed 286 lb (BMI of 39 kg/m2), with a known baseline weight of 243 lb. He was somnolent, with periods of apnea consistent with Cheyne-Stokes breathing. His JVP was elevated; he had bilateral rales. Cardiac auscultation revealed a normal S1 and S2 with no extra heart sounds. His extremities were cold with significant edema. Laboratory evaluation revealed: sodium 123 mmol/L, BNP 1892 pg/mL, troponin 0.18 ng/mL, and negative urine toxicology. Electroencephalogram, blood cultures, ammonia, vitamin B12, folate, and thiamine levels were ordered to evaluate his confusion.
Electrocardiogram showed sinus tachycardia with left-axis deviation, and infrequent premature ventricular contractions. Chest x-ray showed a right-sided pleural effusion and cardiomegaly. TTE showed severe, globally reduced left ventricular ejection fraction of less than 20%, reduced right ventricular systolic function, and moderate mitral regurgitation.
The patient was diagnosed with decompensated severe heart failure. He was started on intravenous (IV) diuretics and nitroprusside drip. IV thiamine therapy was also initiated, given high clinical suspicion for Wernicke's encephalopathy. After 4 days of significant diuresis, patient underwent left-heart catheterization, which showed nonobstructive coronary artery disease. The patient's thiamine level later resulted as undetectable. His heart failure improved significantly after 14 days of treatment, which included IV thiamine. He was discharged on a regimen of furosemide 40 mg once a day, valsartan 160 mg twice a day, hydralazine 100 mg 3 times a day, carvedilol 12.5 mg twice a day, isosorbide mononitrate 120 mg once a day, spironolactone 25 mg once a day, atorvastatin, and oral thiamine.
The patient did not follow up regularly because of transportation issues. During this time, he was only intermittently compliant with his cardiac medications, as they were making him “feel shaky.” Despite this, a repeat TTE showed improvement of his ejection fraction to 45% to 50%. BNP decreased to 286 pg/mL. His weight improved to 246 lb. The patient reported improvement in his dyspnea but noted episodes of dizziness, which he attributed to his cardiac medications. He was referred to cardiology for possible reduction of his cardiac medications.
Discussion
Thiamine, a water-soluble B vitamin, is a cofactor necessary for carbohydrate metabolism and production of energy. Thiamine is also essential in neurotransmitter synthesis.1 As thiamine has limited tissue storage and a half-life of 10 to 20 days, deficiency may develop within a few weeks but has been described in as little as 3 to 14 days.2, 3 The vitamin is primarily found in such foods as legumes, pork, brown rice, and cereals made from whole grains but is very low in white rice, milled cereals, milk products, fruits, and vegetables. In addition, although most grains are thiamine-enriched in the United States, processed meats and foods preserved in sulfites are low in the vitamin.4 Therefore, populations—such as the elderly or the homeless—who are at risk for food insecurity or rely heavily on convenience foods, may be at increased risk for thiamine deficiency. In addition, acute thiamine deficiency has been described in severe catabolic states such as sepsis, thyrotoxicosis, cancer, and end-stage renal disease.3, 5, 6, 7
Severe thiamine deficiency can cause cognitive impairment (Wernicke's encephalopathy), peripheral neuropathy (“dry beriberi”), or heart failure (cardiac, or “wet beriberi”). Cardiac beriberi occurs as a result of decreased cardiac function from impaired cellular metabolism. Thiamine deficiency impairs production of adenosine triphosphate (ATP), leading to accumulation of adenosine. This increase causes reduction in systemic vascular resistance via direct vasomotor depression, leading to a compensatory high-output state with increased blood volume.8 Eventually, myocardial weakness develops, leading to systolic dysfunction and a low-output state. Ultimately, patients develop hypotension and complete cardiovascular collapse unless thiamine is provided.9 An acute form of cardiac beriberi—Shoshin beriberi—can also present with more sudden cardiogenic shock and imminent death.
Classically, patients with cardiac beriberi have been described as having heart failure with significant lower-extremity edema but upper-body cachexia. However, patients with calorie-rich but nutritionally poor diets, or those with recent changes in their diets, may not appear emaciated. Echocardiography may reveal reduced ejection fraction that is indistinguishable from other dilated cardiomyopathies. Some studies suggest the use of cardiac magnetic resonance imaging to establish a diagnosis based on increased T2 signal intensity due to myocardial edema, but other studies find that myocardial edema may not always be present.10, 11
Diagnosing thiamine deficiency with blood tests has limitations. Plasma thiamine is often the initial test of choice, but levels are strongly influenced by recent caloric intake. The patient in Case 1 had thiamine levels in the low-normal range but was likely deficient, as the value was nonfasting. Moreover, as less than 1% of total body thiamine is found in whole blood, a low level may not always be a sensitive indicator of true deficiency. An alternative method for diagnosing thiamine deficiency is via a functional assay by measuring erythrocyte transketolase activity (ETKA), in which a low ETKA level is suggestive of thiamine deficiency.9 This test is affected by hemoglobin levels and is not widely available.
As there are no pathognomonic manifestations of cardiac beriberi, it can be easily overlooked. The diagnosis entails the presentation of an unexplained cardiomyopathy, a history of poor nutrition, a therapeutic response to thiamine, and exclusion of other etiologies of heart disease.12 The ideal treatment regimen has not been well studied and can vary depending on the severity of presentation. The patient in the first case improved after oral supplementation, but IV supplementation is commonly recommended with a loading dose of 100 mg to 500 mg parenteral thiamine followed by an oral regimen of 25 to 100 mg daily.13
The prevalence of thiamine deficiency in patients with heart failure in acute-care settings has been estimated as high as 30%.14 Small randomized control studies have shown improvement in cardiac function after adequate thiamine supplementation, regardless of the etiology of the cardiomyopathy.15, 16, 17 A randomized double-blind crossover trial found an approximate 10% improvement in ejection fraction after 300 mg per day of thiamine.15 Other studies report an approximate 5% improvement with supplementation.16, 18 Studies showing no benefit typically used low-dose oral supplementation or had limited follow-up.19, 20 A recent systematic review and meta-analysis concluded that patients with heart failure are at risk of thiamine deficiency and that supplementation was likely beneficial in improving left ventricular function as well as quality of life.21 Some studies have suggested association with diuretic use, but other studies have not been able to duplicate this finding.22, 23 Current guidelines from the American Heart Association/ American College of Cardiology and the European Society of Cardiology note thiamine deficiency as a cause of cardiomyopathy but do not emphasize any specific evaluation.24, 25 With the increased prevalence of food insecurity estimated at 11% to 18% of US households, nutritional deficiencies may be underappreciated and warrant further research.26
We present 2 patients with cardiac beriberi whose diagnoses could have been overlooked were it not for initiation of thiamine because of patients' concomitant confusion and an erroneous assumption of alcohol abuse. The cases underscore a need to expand our vigilance for thiamine deficiency to other populations that present with heart failure of unclear etiology. The patient in Case 1 presented with a moderate heart failure, had normalization of his ejection fraction, and was able to discontinue all cardiac medications after a short treatment course with oral thiamine. The patient in Case 2 had a sudden, severe presentation of heart failure. After treatment, his echocardiogram normalized, despite poor compliance with his cardiac medications.
Conclusions
Patients who present with an unexplained cardiomyopathy should be evaluated for thiamine deficiency. Persons most at risk include those with high chronic disease burden, alcoholism, and those with food insecurities such as with the homeless and the elderly. Larger studies are needed to evaluate and diagnose nutritional deficiencies, even in those with modern-day Western diets.
Footnotes
Potential Competing Interests: The authors report no competing interest.
References
- 1.Gibson G.E., Hirsch J.A., Fonzetti P., Jordan B.D., Cirio R.T., Elder J. Vitamin B1 (thiamine) and dementia. Ann NY Acad Sci. 2016;1367(1):21–30. doi: 10.1111/nyas.13031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.DiNicolantonio J.J., Liu J., O'Keefe J.H. Thiamine and cardiovascular disease: a literature review. Prog Cardiovasc Dis. 2018;61(1):27–32. doi: 10.1016/j.pcad.2018.01.009. [DOI] [PubMed] [Google Scholar]
- 3.Donnino M.W., Carney E., Cocchi M.N. Thiamine deficiency in critically ill patients. J Crit Care. 2010;25(4):576–581. doi: 10.1016/j.jcrc.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 4.Hoffman R. Thiamine deficiency in the Western diet and dementia risk. Br J Nutr. 2016;116(1):188–189. doi: 10.1017/S000711451600177X. [DOI] [PubMed] [Google Scholar]
- 5.Ohmori N., Tushima T., Sekine Y. Gestational thryotoxicosis with acute Wernicke encephalopathy: a case report. Endocrinol J. 1999;46(6):787–793. doi: 10.1507/endocrj.46.787. [DOI] [PubMed] [Google Scholar]
- 6.Isenberg-Grzeda E., Shen M.J., Alici Y., Wills J., Nelson C., Breitbart W. High rate of thiamine deficiency among inpatients with cancer referred for psychiatric consultation: results of a single site prevalence study. Psycho-oncology. 2017;26(9):1384–1389. doi: 10.1002/pon.4155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hung S.C., Hung S.H., Tarng D.C., Yang W.C., Chen T.W., Huang T.P. Thiamine deficiency and unexplained encephalopathy in hemodialysis and peritoneal dialysis patients. Am J Kidney Dis. 2001;38(5):941–947. doi: 10.1053/ajkd.2001.28578. [DOI] [PubMed] [Google Scholar]
- 8.Akbarian M., Yankopoulos N.A., Abelmann W.H. Hemodynamic studies in beriberi heart disease. Am J Med. 1966;41(2):197–212. doi: 10.1016/0002-9343(66)90016-7. [DOI] [PubMed] [Google Scholar]
- 9.DiNicolantonio J.J. Thiamine supplementation for the treatment of heart failure: a review of the literature. Congest Heart Fail. 2013;19(4):214–222. doi: 10.1111/chf.12037. [DOI] [PubMed] [Google Scholar]
- 10.Essa E., Velez M.R., Smith S., Giri S., Raman S.V., Gumina R.J. Cardiovascular magnetic resonance in wet beriberi. J Cardiovasc Magn Reson. 2011;13(1):41. doi: 10.1186/1532-429X-13-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee H.-S., Lee S.-A., Shin H.-S. A case of cardiac beriberi: a forgotten but memorable disease. Korean Circ J. 2013;43(8):569–572. doi: 10.4070/kcj.2013.43.8.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jones R.H., Jr. Beriberi heart disease. Circulation. 1959;19:275–283. doi: 10.1161/01.cir.19.2.275. [DOI] [PubMed] [Google Scholar]
- 13.Tanphaichitr V., Shils M.E., Olson J.A. Thiamin. In: Shils M.E., Olsen J.A., Ross A.C., editors. Modern Nutrition in Health and Disease. 9th ed. Williams & Wilkins; Baltimore, MD: 1999. pp. 381–389. [Google Scholar]
- 14.Hanninen S., Darling P., Sole M. The prevalence of thiamine deficiency in hospitalized patients with congestive heart failure. J Am Coll Cardiol. 2006;47(2):354–361. doi: 10.1016/j.jacc.2005.08.060. [DOI] [PubMed] [Google Scholar]
- 15.Schoenenberger A.W., Schoenenberger-Berzins R., de Maur C.A., Suter P.M., Vergopoulos A., Erne P. Thiamine supplementation in symptomatic chronic heart failure: a randomized, double-blind, placebo-controlled, cross-over pilot study. Clin Res Cardiol. 2012;101(3):159–164. doi: 10.1007/s00392-011-0376-2. [DOI] [PubMed] [Google Scholar]
- 16.Shimon I., Almog S., Vered Z. Improved left ventricular function after thiamine supplementation in patients with congestive heart failure receiving long-term furosemide therapy. Am J Med. 1995;98(5):485–490. doi: 10.1016/s0002-9343(99)80349-0. [DOI] [PubMed] [Google Scholar]
- 17.Seligmann H., Halkin H., Rauchfleisch S. Thiamine deficiency in patients with congestive heart failure receiving long-term furosemide therapy: a pilot study. Am J Med. 1991;91(2):151–155. doi: 10.1016/0002-9343(91)90007-k. [DOI] [PubMed] [Google Scholar]
- 18.Witte K., Nikitin N.P., Parker A.C. The effect of micronutrient supplementation on quality of life with left ventricular function in elderly patients with chronic heart failure. Eur Heart J. 2005;26(21):2238–2244. doi: 10.1093/eurheartj/ehi442. [DOI] [PubMed] [Google Scholar]
- 19.Smithline H.A. Thiamine for the treatment of acute decompensated heart failure. Am J Emerg Med. 2007;25(1):24–26. doi: 10.1016/j.ajem.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 20.Pfitzenmeyer P., Guilland J.C., d'Athis P., Petit-Marnier C., Gaudet M. Thiamine status of elderly patients with cardiac failure including the effects of supplementation. Int J Vitam Nutr Res. 1994;64(2):113–118. [PubMed] [Google Scholar]
- 21.Jain A., Mehta R., Al-Ani M., Hill J.A. Determining the role of thiamine deficiency in systolic heart failure: a meta-analysis and systematic review. J Card Fail. 2015;21(12):1000–1007. doi: 10.1016/j.cardfail.2015.10.005. [DOI] [PubMed] [Google Scholar]
- 22.Suter P.M., Haller J., Hany A., Vetter W. Diuretic use: a risk for subclinical thiamine deficiency in elderly patients. J Nutr Health Aging. 2000;4(2):69–71. [PubMed] [Google Scholar]
- 23.Teigen L.M., Twernbold D.D., Miller W.L. Prevalence of thiamine deficiency in a stable heart failure outpatient cohort on standard loop diuretic therapy. Clin Nutr. 2016;35(6):1323–1327. doi: 10.1016/j.clnu.2016.02.011. [DOI] [PubMed] [Google Scholar]
- 24.Yancy C.W., Jessup M., Bozkurt B. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62(16):e147–e239. doi: 10.1016/j.jacc.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 25.Ponikowski P., Voors A.A., Anker S.D. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37(27):2129–2200. doi: 10.1093/eurheartj/ehw128. [DOI] [PubMed] [Google Scholar]
- 26.Gundersen C., Ziliak J.P. Food insecurity and health outcomes. Health Affairs. 2015;34(11):1830–1839. doi: 10.1377/hlthaff.2015.0645. [DOI] [PubMed] [Google Scholar]