Abstract
Background:
Analysis of subtle vital sign changes could facilitate earlier treatment of acute inflammatory illnesses. We previously showed that high cross-correlation of heart rate and oxygen saturation (XCorr-HR-SpO2) occurs in some very low birthweight infants with sepsis, and hypothesized that this corresponds to apnea.
Methods:
In 629 VLBW infants, we analyzed XCorr-HR-SpO2 in relation to central apnea with bradycardia and desaturation (ABD), BD with or without central apnea (BD), and percent time in periodic breathing (PB) throughout the NICU stay (75 infant-years). We reviewed 100 days with extremely high XCorr-HR-SpO2 (>0.7) and control days for clinical associations. Next, we identified all cases of late-onset septicemia (LOS) and necrotizing enterocolitis (NEC) and analyzed change in XCorr-HR-SpO2 before diagnosis.
Results:
Mean XCorr-HR-SpO2 was ~0.10, and increasing XCorr-HR-SpO2 was associated with increasing ABD, BD, and PB (correlation coefficients >0.93). Days with maximum XCorr-HR-SpO2 >0.7 were more likely to have an adverse event than control days (49% versus 13%). In 93 cases of LOS or NEC, there was a 67% increase in XCorr-HR-SpO2 in the 24h period prior to diagnosis compared to the previous day (p<0.01).
Conclusion:
High XCorr-HR-SpO2 is associated with apnea and adverse events including LOS and NEC.
Background
In spite of preventative efforts, late-onset septicemia and necrotizing enterocolitis (LOS and NEC) remain major causes of mortality and morbidity among preterm infants(1). The associated systemic inflammatory response leads to changes in vital signs which may be subtle in the early phase of illness(2). Analyzing and displaying these abnormal vital sign patterns to clinicians is an important goal of early warning systems designed to facilitate more timely treatment which would be expected to lead to improved outcomes.
Heart rate characteristics analysis is the first example of an early warning system for sepsis in preterm infants and has been shown to reduce sepsis-associated mortality(3–6). Adding respiratory analysis to heart rate analysis might improve sepsis detection, since prostaglandins (7–9)and inflammatory cytokines(10,11) released during infection have a respiratory depressant effect and may increase apnea with associated bradycardia and oxygen desaturation. We developed a system for analyzing chest impedance waveform data from NICU bedside monitors to quantitate central apnea and periodic breathing (repetitive cycles of brief apneic pauses and breathing)(12,13). Using this system we showed that many preterm infants experience a significant increase in these immature breathing patterns hours before clinicians recognize signs of illness and initiate testing and treatment(14,15).
Apnea quantitation requires analysis of waveform data and considerable computer processing capacity that is not available at many centers, and we sought to develop simpler analytics for early warning systems. In a prior two-center study of over 1000 very low birthweight infants, we analyzed three vital signs collected every 2 seconds (heart rate, respiratory rate, and oxygen saturation) and found that the best metric for preclinical detection of LOS or NEC was cross-correlation of heart rate and oxygen saturation (XCorr-HR-SpO2)(16).
Cross-correlation is a mathematical calculation that represents similarity of two signals changing over time, allowing for a defined lag time. Positive XCorr indicates signals moving in the same direction, and negative XCorr signals moving in opposite directions. We hypothesized that a strongly positive XCorr would correspond to pauses in breathing with entrainment of both HR and SpO2 (deceleration-desaturation) and might serve as a surrogate measure for apnea and exaggerated periodic breathing which may occur in acute illnesses in preterm infants. To test this hypothesis, using the previously studied cohort of VLBW infants we first analyzed the association between XCorr-HR-SpO2 and events of central apnea, bradycardia-desaturation, and periodic breathing. We then sought clinical associations occurring at the time of very high XCorr-HR-SpO2. Finally, we assessed the maximum XCorr in the 24 hour period prior to diagnosis of LOS and NEC to begin to determine how XCorr might be incorporated into early warning systems for these critical illnesses.
Methods
Patient Population
This study was approved by the University of Virginia (UVA) Institutional Review Board with waiver of consent due to the non-interventional nature of the study and the use of deidentified patient data. We collected clinical and demographic data on all very low birthweight infants (VLBW, <1500 grams) admitted to the UVA NICU from January 2009-April 2014 for whom we had stored monitor waveform and vital sign data for analysis.
ABD, BD, Periodic Breathing, and XCorr-HR-SpO2 Analysis
Bedside monitor data have been collected and stored since 2009 for all infants in the UVA NICU using a central network server (BedMaster, Excel Medical, Jupiter FL). Central apnea events were quantified all times when waveform chest impedance and electrocardiogram data and SpO2 were available and the infant was not on mechanical ventilation, using a published algorithm(12). Briefly, the chest impedance signal was processed with filters to remove motion and heart beat artifact, and central apnea was identified as at least 10 seconds of very low variance in filtered chest impedance associated with bradycardia <100 beats/minute and desaturation <80%. These were identified as ABD events. We also identified episodes of bradycardia-desaturation irrespective of central apnea (BD, using the same HR and SpO2 thresholds). Periodic breathing was quantified by a wavelet transform analysis as repetitive regular cycles of brief central apnea interspersed with regular breathing with cycle duration 10–30 seconds. The ABD and the PB algorithms underwent extensive clinical validation, as previously published(13).
Cross-correlation was analyzed from the every 2 second (0.5 Hz) HR and SPO2 data using the Matlab XCORR function with lag times from −30 to +30 seconds. Thirty percent of the lag times fell between −15 and −5 seconds, with deceleration preceding desaturation (data not shown). Cross-correlograms were calculated on sliding windows of length 10 minutes with 5 minute overlap and the maximum value over all lags found. The XCorr-HR-SPO2 was then recorded hourly as the average of these 12 maximum values in the previous 60 minutes. We report both the mean XCorr in specific time periods for the population of infants, and, where indicated, maximum XCorr over a specific time period.
Clinical events at the time of very high XCorr-HR-SpO2
In order to begin to determine the utility of high XCorr as an early warning system for illnesses such as LOS and NEC, we evaluated clinical associations with a very high XCorr following methodology we previously used for the heart rate characteristics index(17). We identified all days with at least one hourly XCorr-HR-SpO2 value ≥ 0.7 when there were no values this high the prior day. We then randomly selected 100 of these days and 100 control days matched for gestational and chronologic age with maximum XCorr < 0.7. Medical records were reviewed (blinded to XCorr) to identify clinical events occurring within one day. Infection-related events included late-onset septicemia (LOS, signs of sepsis and a positive blood culture at >72 hours of age treated with antibiotics for at least 5 days), clinical sepsis (negative cultures and at least 5 days antibiotics due to high clinical suspicion), other infection treated with antibiotics at least 5 days, and sepsis suspected but ruled out (negative cultures, < 5 days antibiotics). NEC was defined as clinical signs and a radiograph showing pneumatosis intestinalis, portal venous air, or pneumoperitoneum not classified as spontaneous intestinal perforation, and at least 5 days of treatment. Non-infectious events included surgery or procedure requiring anesthesia or sedation, very frequent apnea (≥ 20 algorithm-detected ABD events/day), or respiratory deterioration, defined as moving to a higher mode of respiratory support or at least 20% increase in ventilator settings due to respiratory acidosis or hypoxemia. Since frequent apnea or respiratory deterioration may be a sign of infection, and since each event was only assigned one clinical correlation, if antibiotics were initiated the event was included in an infection category.
XCorr-HR-SpO2 prior to septicemia and NEC
All episodes of LOS and NEC were identified in all VLBW infants during the study period. Time of septicemia was defined as the time of blood culture, and time of NEC was the time of the radiograph establishing the diagnosis. Ventilator support at the time of LOS or NEC diagnosis was recorded. XCorr-HR-SpO2 was analyzed in the 24 hour period prior to diagnosis of LOS or NEC, and in the period from 24–48h prior to diagnosis, whenever stored bedside monitor data were available.
Statistics
Demographic data are presented as mean unless otherwise noted. Associations between XCorr-HR-SpO2 and ABD, BD, and periodic breathing were analyzed with the correlation coefficient from standard linear regression with initial logarithmic transformation of ABD and BD rate. Increases in XCorr the day prior to LOS or NEC diagnosis compared to the previous day were analyzed by paired signed rank test. Statistics were performed in MATLAB (Mathworks, Natick, MA) with p<0.05 considered statistically significant.
Results
XCorr-HR-SpO2 and its association with ABD, BD, and periodic breathing
Of 755 VLBW infants admitted to the NICU in the 5 years of the study, 629 had stored bedside monitor waveform and vital sign data for analysis of XCorr-HR-SpO2 and apnea. Gestational age was 27.7 ± 3.0 weeks and birthweight 1019 ± 292 grams (mean, SD). Number of hours of XCorr analyzed was 659,824, which translates to 75.3 infant-years of data (on average, approximately 1000 hours or 42 days per infant).
Figure 1 shows average XCorr-HR-SpO2 for 10 weeks after birth for all VLBW infants while on mechanical ventilation (n=342), and for two birthweight categories while not on mechanical ventilation (<1000 grams birthweight, n=278, and 1000–1499 grams, n=314). Average XCorr is about 0.06–0.18 over the ten weeks after birth. There was no significant difference in average XCorr based on mechanical ventilation status, nor was there a significant difference in birth weight categories. For example, at age 4 weeks the 95% confidence intervals for the 3 groups are, respectively, 0.057–0.134, 0.081–0.144, and 0.075–0.176.
Figure 1: XCorr-HR-SpO2 in VLBW infants.
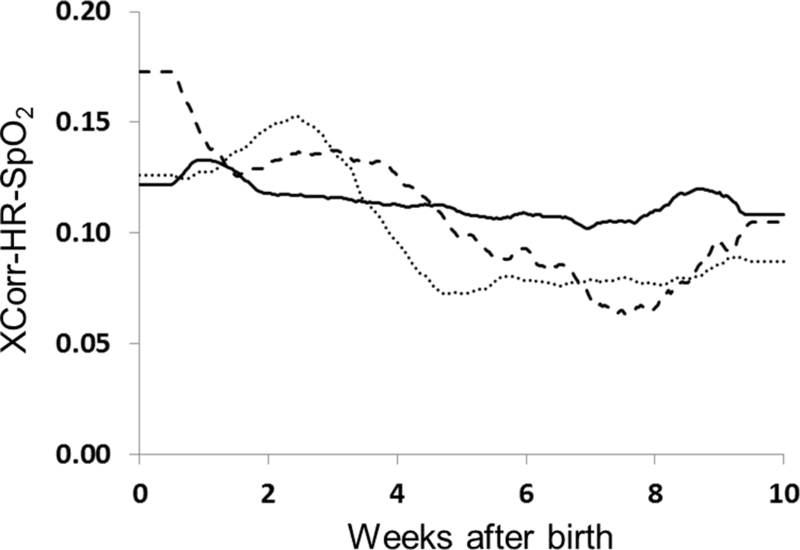
Cross-correlation of HR-SpO2 was analyzed continuously for 10 weeks after birth in VLBW infants on mechanical ventilation (n=342, dashed line) and infants not on mechanical ventilation (<1000 grams birthweight n=278 dotted line, 1000–1499 grams birthweight n=314, solid line). XCorr-HR-SpO2 was similar regardless of ventilator status, birth weight, and chronologic age.
Figure 2 shows the relationship of XCorr-HR-SpO2 and the number of ABD and BD events and percentage of time spent in PB for all times infants were not on mechanical ventilation. There was a very strong log-linear relationship between XCorr and ABD and BD (rho=.985 and .961 respectively) and a strong linear relationship with PB (rho=.933).
Figure 2: XCorr-HR-SpO2 association with apnea, bradycardia, desaturation, and periodic breathing.
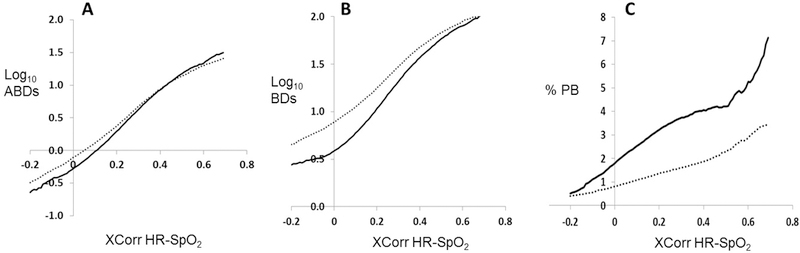
For 278 infants <1000 grams birthweight (dotted line) and 314 infants 1000–1499 grams (solid line), XCorr-HR-SpO2 is shown for all times infants were not on mechanical ventilation in relation to number of events of central apnea with associated bradycardia and desaturation (ABDs, panel A), BD events with or without central apnea (BDs, panel B), and percentage of time spent in periodic breathing (%PB, panel C). Note that, for panels A and B, the Y axis is plotted on a log base 10 scale. There was a very strong log-linear relationship between XCorr and ABD and BD (rho=.985 and .961 respectively) and a strong linear relationship with PB (rho=.933).
Clinical associations with extremely high XCorr-HR-SpO2
In 75 infant-years’ data from 629 infants, there were 266 days in 155 infants with at least one XCorr-HR-SpO2 value >0.7 and no value that high in the day prior. We randomly selected 100 of these days in 100 infants. An equal number of control days with XCorr-HR-SpO2 <0.7 matched for gestational and chronologic age were identified. Mean gestational age was 26 weeks and mean day of age of high XCorr or control was 28. Birthweight was similar in the two groups (mean 878 and 892 grams). A clinician performed blinded medical record review and found a clinical association within a day of 49% of the very high XCorr-HR-SpO2 time periods compared to 13% of control time periods (Table 1). About half of the associated events were suspected or proven infection or NEC.
Table 1:
Clinical associations at the time of extremely high XCorr HR-SpO2
| 100 high max Xcorr (≥0.7) |
100 matched controls |
|
|---|---|---|
| GA (weeks) | 26 | 26 |
| BW (mean, grams) | 878 | 892 |
| Age (mean, days) | 28 | 28 |
| EVENT* | n= | n= |
| Septicemia | 6 | 3 |
| Clinical sepsis or infection | 9 | 2 |
| Sepsis ruled out | 5 | 1 |
| NEC | 4 | 0 |
| Surgery or procedure | 10 | 2 |
| Frequent ABD (>20/day) | 4 | 0 |
| Respiratory deterioration | 11 | 5 |
| TOTAL EVENTS | 49 | 13 |
| No Event | 51 | 87 |
one event per period
XCorr-HR-SpO2 in 100 cases of septicemia and NEC
In the 5 years of the study there were 110 cases of LOS and 37 cases of NEC among 755 VLBW infants. Bedside monitor vital sign data were available for analysis of XCorr-HR-SpO2 the day prior to diagnosis in 73 LOS and 27 NEC cases (Table 2).
Table 2:
Clinical characteristics and XCorr-HR-SpO2 in LOS and NEC cases
| LOS no Vent (n=23) |
LOS Vent (n=50) |
NEC no Vent (n=19) |
NEC Vent (n=8) |
|
|---|---|---|---|---|
| GA (mean) | 27.2 | 24.7 | 28.3 | 25.5 |
| Birthweight (mean) | 1030 | 717 | 940 | 730 |
| % female | 43% | 42% | 28% | 25% |
| Age at diagnosis (mean days) | 32 | 26 | 30 | 30 |
| Gram-positive septicemia (#) | 17 | 40 | 2 | 1 |
| Gram-negative septicemia (#) | 6 | 10 | 2 | 1 |
| Max Xcorr HR-SpO2 within 24h prior to diagnosis (mean) | 0.48 | 0.45 | 0.49 | 0.54 |
Figure 3 shows six representative 24-hour cross-correlograms (at the top, a heat map of XCorr-HR-SpO2 plotted in relation to lag time, and at the bottom HR and SpO2). Four examples are shown in the 24 hour period leading up to the time of positive blood culture or time of X-ray establishing the diagnosis of LOS (A and D) or NEC (B and E). The HR and SpO2 tracings show many deceleration/desaturation events when the heat map shows high (yellow) XCorr and this was found both on and off mechanical ventilation (Fig. 3 compare A and B off ventilator with D and E on ventilator). Note that XCorr waxes and wanes over time. These examples show that the range of lag time on the y axis (time between the start of the change in HR and change in SpO2) tends to be more narrow for infants that are not on mechanical ventilation. Also shown are two examples matched for gestational and chronologic age of 24-hour time periods without LOS or NEC, off or on mechanical ventilation (Figure 3 panels C and F).
Figure 3: Examples of high XCorr-HR-SpO2 in LOS and NEC compared to controls, on and off mechanical ventilation.
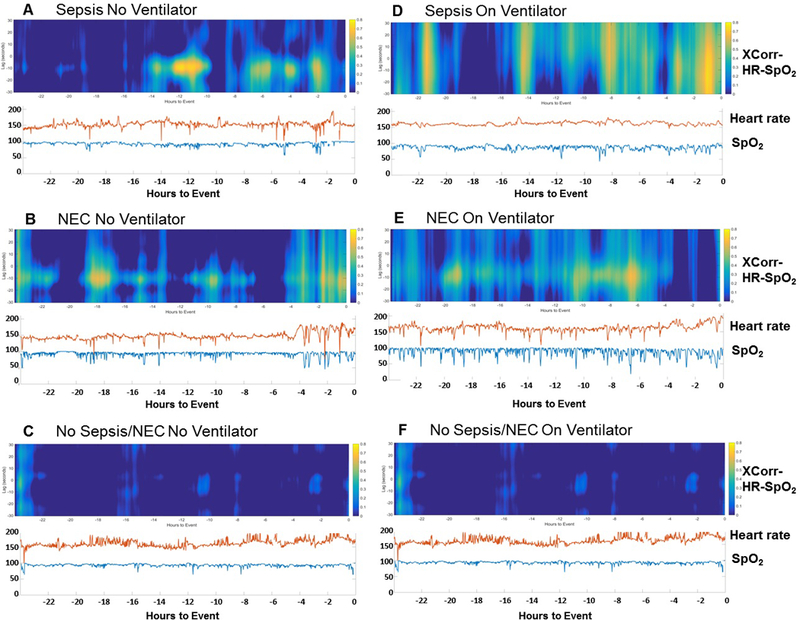
Continuous time cross-correlograms of cases of high maximum XCorr-HR-SpO2 and corresponding HR and SpO2 tracings in the 24 hours prior to diagnosis of LOS (A,D) or NEC (B,E), and age-matched control time periods without LOS or NEC (C, F). Representative examples are shown for infants off (A-C) or on (D-F) mechanical ventilation. The heat map depicts high XCorr in yellow, mid-range in green and light blue, and low in dark blue. The x axis shows hours prior to diagnosis (time zero at far right). The y axis on the heat map shows the seconds of lag time, with negative values indicating HR change preceding SpO2 change. The y axis on the vital sign graph indicates the corresponding heart rate (shown in red) and SpO2 (shown in blue).
Table 2 shows demographics and the mean of the maximum XCorr-HR-SpO2 in the 24 hour period prior to diagnosis of LOS or NEC. Infants on mechanical ventilation at the time of diagnosis were of lower gestational age, birthweight, and chronologic age at the time of diagnosis compared to those not on mechanical ventilation. Mean maximum XCorr-HR-SpO2 within 24 hours prior to diagnosis was similar for the 4 groups and significantly higher than mean maximum XCorr-HR-SpO2 for all 24 hour periods for all infants (max XCorr 0.48 versus 0.33, p<0.01). Twenty-one (21%) of the LOS/NEC cases had extremely high maximum XCorr >0.7 within a day of diagnosis, 13 of these spikes occurring in the day prior and 8 occurring the day after. Among 93 cases of LOS and NEC with monitor data available in the 48 hour period leading up to the event, the 24 hour period prior to diagnosis had 67% higher maximum XCorr compared to the maximum XCorr in the previous day and was statistically different using a paired signed rank test (p<0.01).
HR-SpO2 patterns associated with high XCorr-HR-SpO2
Figure 4 shows three general patterns we have identified after reviewing multiple episodes of very high XCorr-HR-SpO2. The majority of cases have either large deceleration-desaturations (Fig. 4A) or frequent small dips in HR-SpO2 (Fig. 4B). A smaller number of cases have low variability of both signals (Fig. 4C). A representative example of HR and SpO2 with small declines in each signal but much lower XCorr is also shown (Fig. 4D).
Figure 4: Patterns of high XCorr-HR-SpO2.
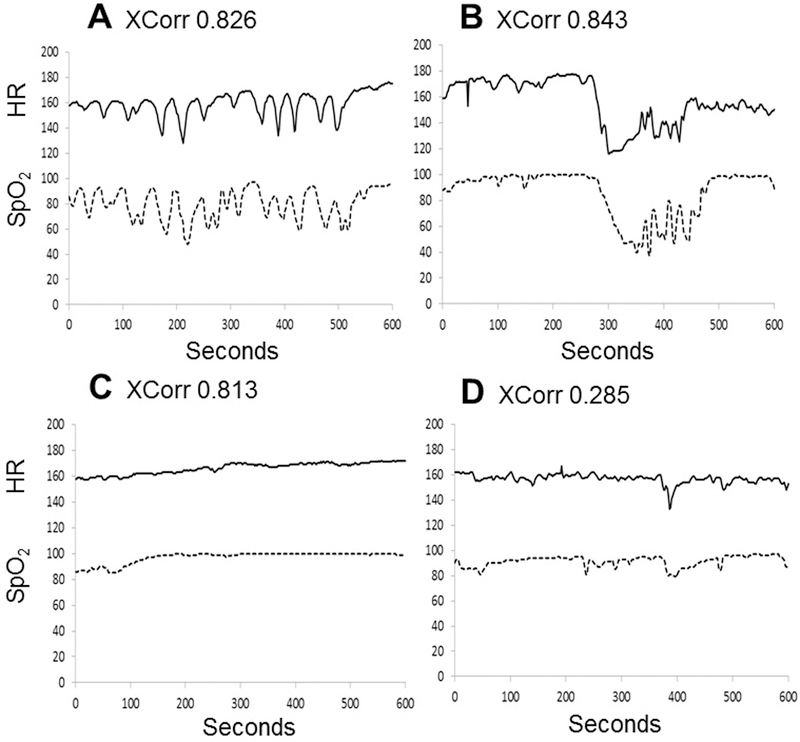
Examples are shown of three typical 600-second HR-SpO2 patterns corresponding to a very high XCorr-HR-SpO2, and one segment corresponding to a lower XCorr-HR-SpO2. A) Periodic deceleration-desaturations (XCorr 0.826) B) Prolonged deceleration-desaturation event (XCorr 0.843) C) Low variability of both HR and SpO2 (XCorr 0.813). D) Small decelerations and desaturations with lower XCorr-HR-SpO2 (XCorr 0.285)
Discussion
We previously reported, in VLBW preterm infants at two NICUs, that an increase in cross-correlation of heart rate and oxygen saturation (XCorr-HR-SpO2) often occurred in the early phases of LOS and NEC. In that study we speculated that this represented an increase in breathing pauses, and in the current single-center analysis we confirm an association between increased XCorr-HR-SpO2 and central apnea with bradycardia and desaturation (ABD) events, BD events with or without central apnea, and periodic breathing with associated deceleration/desaturation. We found that about half of cases of very high XCorr-HR-SpO2 were associated with a pathologic condition, and about half of those events were suspected or proven infection or NEC. Among 93 cases of LOS or NEC, there was a 67% increase in XCorr in the 24 hour period before diagnosis compared to one day prior.
Cross-correlation is a measure of similarity of two signals over time, allowing for a defined lag, and as such, measurement of cross-correlation of time series vital sign data can reveal entrainment of physiological processes. Inflammatory illnesses such as LOS and NEC are commonly associated with an increase in immature breathing patterns in preterm infants, due in part to release of prostaglandins which have a respiratory depressant effect(8,9), and apneic pauses often entrain a decline in HR and SpO2(18). Detection of increased central apnea may be a useful addition to early warning systems for sepsis, but apnea quantitation is complicated because it requires analysis of chest impedance waveform data. We previously developed an automated algorithm that analyzes the chest impedance, electrocardiogram, and pulse oximeter signals from standard NICU bedside monitors and quantifies central apnea events with associated HR deceleration and oxygen desaturation(12). Chest impedance and electrocardiogram waveform data are collected at very high frequency (up to 1000 Hz total) and their analysis requires significant signal processing, in contrast to HR and SpO2 which are typically recorded at 0.5–1 Hz and can yield important information with relatively simple analytics. XCorr may therefore be useful in resource-constrained settings since it can be measured from pulse oximetry data alone.
There are a number of features other than simplicity that make XCorr attractive for predictive monitoring algorithms. First, it does not rely on thresholds or absolute values of vital signs. Our central apnea “ABD” algorithm uses 100 beats per minute as a bradycardia threshold and 80% SpO2 as a desaturation threshold, but XCorr can detect a downward co-trending of HR-SpO2 that does not cross these thresholds and does not activate bedside monitor alarms. Also, since XCorr is insensitive to absolute values, it may be more useful across centers that have different clinical practices with regard to SpO2 targeting. Another important consideration is mechanical ventilation status. In our prior two-center study of 1065 VLBW infants we found that XCorr performed better for spontaneously breathing infants for LOS or NEC prediction, but many infants on mechanical ventilation had a significant rise in XCorr prior to diagnosis(16). In the current analysis of cases of LOS or NEC, the maximum XCorr in the day prior to diagnosis was similarly high irrespective of ventilator status (Table 2). We speculate that an increase in deceleration-desaturation spells in mechanically ventilated infants with LOS or NEC might represent autonomic nervous system activation. We previously showed in a mouse model that pathogens induce vagus nerve-mediated repetitive transient HR decelerations(19), and parasympathetic activation might also contribute to acute changes in respiratory physiology leading to oxygen desaturation.
We measured XCorr-HR-SpO2 allowing for up to 30 second lag time, and found that in most cases of high XCorr-HR-SpO2 deceleration preceded desaturation by about 10–20 seconds. This is in line with the typical sequence of events that we have noted in our prior work, with an apneic pause followed after some seconds by a decline in HR and then a decline in SpO2(20,21). Infants on mechanical ventilation tend to have a broader range of lag time (compare Figure 3 panels C and D on ventilator and panels A and B not on ventilator), indicating a more variable timing of the decline in HR and SpO2 in ventilator-associated “BD” events. Whether analysis of the lag time can distinguish pathologic apnea associated with an acute illness from physiologic apnea of prematurity deserves further study.
The addition of SpO2 analysis may improve on heart rate analysis alone as an early warning system for sepsis, but several limitations deserve consideration. As with the heart rate characteristics measurement which captures decreased variability and decelerations, a high or rising XCorr-HR-SpO2 is not specific for sepsis but may occur with respiratory deterioration, other clinical conditions, or in absence of any apparent event. Also, the very high XCorr threshold of 0.7 used in our current analysis was selected to emphasize positive predictive value and further refinements could be made to increase sensitivity.Selecting a cut-off value for an early warning system alarm requires sacrificing sensitivity in favor of positive predictive accuracy so as not to create so many false alarms that caregivers ignore them or are distracted from other patient care responsibilities. Another approach to predictive monitoring, like the one used for the heart rate characteristics monitor, is to present a vital sign-based continuous risk indicator without a threshold alarm. A rising risk score above an infant’s prior baseline could then be considered in the context of other clinical and laboratory data to determine whether a change in clinical care might benefit the infant.
Conclusion
XCorr-HR-SpO2 is a measure of cardiorespiratory interaction that may identify some infants in the early phase of potentially catastrophic illnesses. More work is needed to determine how to incorporate this with other biomarkers and clinical data to create a useful decision support tool.
Acknowledgments
Funding: National Institutes of Health HD072071 and HD064488
References
- 1.Stoll BJ, Hansen NI, Bell EF, et al. Trends in Care Practices, Morbidity, and Mortality of Extremely Preterm Neonates, 1993–2012. JAMA 2015;314:1039–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sullivan B a., Fairchild KD. Predictive monitoring for sepsis and necrotizing enterocolitis to prevent shock. Semin Fetal Neonatal Med 2015;20:255–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fairchild KD, Schelonka RL, Kaufman D a, et al. Septicemia mortality reduction in neonates in a heart rate characteristics monitoring trial. Pediatr Res 2013;74:570–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moorman JR, Carlo WA, Kattwinkel J, et al. Mortality reduction by heart rate characteristic monitoring in very low birth weight neonates: A randomized trial. J Pediatr 2011;159:900–6.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moorman JR, Lake DE, Griffin MP. Heart rate characteristics monitoring for neonatal sepsis. IEEE Trans. Biomed. Eng. 2006;53:126–32. [DOI] [PubMed] [Google Scholar]
- 6.Griffin MP, Lake DE, Bissonette EA, Harrell FE, O’Shea TM, Moorman JR. Heart rate characteristics: novel physiomarkers to predict neonatal infection and death. Pediatrics 2005;116:1070–4. [DOI] [PubMed] [Google Scholar]
- 7.Hofstetter AO, Saha S, Siljehav V, Jakobsson P-J, Herlenius E. The induced prostaglandin E2 pathway is a key regulator of the respiratory response to infection and hypoxia in neonates. Proc Natl Acad Sci U S A 2007;104:9894–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Herlenius E An inflammatory pathway to apnea and autonomic dysregulation. Respir. Physiol. Neurobiol. 2011;178:449–57. [DOI] [PubMed] [Google Scholar]
- 9.Siljehav V, Hofstetter AM, Leifsdottir K, Herlenius E. Prostaglandin E2 Mediates Cardiorespiratory Disturbances during Infection in Neonates. J Pediatr 2015;167:1207–1213.e3. [DOI] [PubMed] [Google Scholar]
- 10.Balan KV, Kc P, Hoxha Z, Mayer CA, Wilson CG, Martin RJ. Vagal afferents modulate cytokine-mediated respiratory control at the neonatal medulla oblongata. Respir Physiol Neurobiol 2011;178:458–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gauda EB, Shirahata M, Mason A, Pichard LE, Kostuk EW, Chavez-Valdez R. Inflammation in the carotid body during development and its contribution to apnea of prematurity. Respir Physiol Neurobiol 2013;185:120–31. [DOI] [PubMed] [Google Scholar]
- 12.Lee H, Rusin CG, Lake DE, et al. A new algorithm for detecting central apnea in neonates. Physiol Meas 2011;33:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mohr MA, Fairchild KD, Patel M, et al. Quantification of periodic breathing in premature infants. Physiol Meas 2015;36:1415–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fairchild K, Mohr M, Paget-Brown A, et al. Clinical associations of immature breathing in preterm infants: part 1-central apnea. Pediatr Res 2016;80:21–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patel M, Mohr M, Lake D, et al. Clinical Associations with Immature Breathing in Preterm Infants. Part 2: Periodic Breathing. Pediatr Res 2016;80:28–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fairchild KD, Lake DE, Kattwinkel J, et al. Vital signs and their cross-correlation in sepsis and NEC: a study of 1,065 very-low-birth-weight infants in two NICUs. Pediatr Res 2016;81:315–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sullivan BA, Grice SM, Lake DE, Moorman JR, Fairchild KD. Infection and other clinical correlates of abnormal heart rate characteristics in preterm infants. J Pediatr 2014;164:775–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Finer NN, Higgins R, Kattwinkel J, Martin RJ. Summary proceedings from the apnea-of-prematurity group. Pediatrics 2006;117:S47–51. [DOI] [PubMed] [Google Scholar]
- 19.Fairchild KD, Srinivasan V, Moorman JR, Gaykema RPA, Goehler LE. Pathogen-induced heart rate changes associated with cholinergic nervous system activation. Am J Physiol Regul Integr Comp Physiol 2011;300:R330–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vergales B, Paget-Brown AAO, Lee H, et al. Accurate Automated Apnea Analysis in Preterm Infants. Am J Perinatol 2014;31:157–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mohr MA, Vergales BD, Lee H, et al. Very long apnea events in preterm infants. J Appl Physiol 2014;118:558–68. [DOI] [PMC free article] [PubMed] [Google Scholar]


