Abstract
Introduction/Background
Adults with chest pain presenting to an emergency department are high-risk and high-volume. A methodology which gathers practicing physicians together to review evidence and share practice experience to formulate a written algorithm with key decision points and measures is discussed with implementation, based on change management principles, and results.
Methods
A methodology was followed to “establish the standard-of-care”. Literature and data were reviewed, a written consensus algorithm was designed with ability to track adherence and deviations. We performed a before and after analysis of a performance improvement intervention in adult patients with undifferentiated chest pain in our nine-campus hospital system in Florida between January 1st, 2014 and December 31st, 2018.
Results
A total of 200,691 patients were identified as adults with chest pain and the algorithm was used. A dramatic change in the disposition decision rate was noted. When the ‘Baseline-Year’ was compared with the ‘Performance-Year’, chest pain patients discharged from the ED increased by 99%, those going to the ‘Observation’ status decreased by 20%, and inpatient admissions decreased by 63% (p < 0.0001) All patients were tracked for 30-days for major adverse cardiac event (MACE) or return to the ED within the same system. If the s emergency physicians had not changed their practice/behavior and the Baseline-Year decision rate during the entire Performance-Year was unchanged, then 4563 more patients would have gone to Observation and 7986 patients to Inpatient. The opportunity costs avoided would be approximately $31million (US$.
Conclusions
For successful clinical transformation through change management, we learned: select strategic topics, get active physicians together, write a consensus algorithm with freedom to deviate, identify and remove barriers, communicate vision, pilot with feedback, implement, sustain by “hard wiring” into the electronic medical record and measure outputs.
Keywords: ED, SCAMPs, Chest pain, Heart score, Clinical transformation, Change management, Physician behavior and practice, Reduction in cost and resource utilization
Research in context
Evidence Before This Study
The research team reviewed the world literature in reference to chest pain risk stratification tools. The search terms included stratification of undifferentiated chest pain in the emergency department and run through PubMed. The body of evidence supported the use of HEART Score as an internationally accepted risk stratification tool.
Added Value of This Study
This study shows that clinician compliance to an evidence-based protocol can be sustained when coupled with a hospital supported outpatient care navigation platform. This conclusion is important as previous papers have suggested poor ability to sustain compliance to an outpatient chest pain algorithm.
Implication of All the Available Evidence
The results to this study point to the importance of a reliable process when a patient is transitioning to the outpatient care setting from an acute care setting. Emergency physicians may be able to implement more complex algorithms if there is timed accountable follow-up. Interestingly, an evidence-based algorithm is enough to foster compliance unless it is coupled with a population health mechanism. As healthcare goals are more focused on cost, quality and utilization, safe patient management outside the hospital becomes a priority. Future research on physician compliance with evidence-based algorithms other than chest pain, such as syncope and abdominal pain may benefit from the model highlighted in this paper.
Alt-text: Unlabelled Box
1. Introduction
Chest pain is one of the most common reasons for presentation to the emergency department (ED). Chest pain represents 5–10% of adult ED visits, however less than 1% of cases need acute intervention [1]. Clinical difficulty lies in identifying patients with acute coronary syndrome (ACS) needing prompt intervention from those that do not.
Mismanagement of ACS in the ED is a top medical-legal issue. The diagnosis of ACS is missed in approximately 2% leading to substantial consequences, including a short-term two-fold increase in mortality for patients with acute myocardial infarction (MI) who are mistakenly discharged from the ED. [2]. For patients at low-risk of ACS these concerns must be balanced against the cost and inconvenience of tests, procedures or admission, for individuals with low probability of improving the ability to discriminate patients with or without active cardiac ischemia or to improve patients' outcomes.
In 2014, AdventHealth (formerly Florida Hospital) became a founding partner of the Institute for Relevant Clinical Data Analytics (IRCDA), an organization who developed the Standardized Clinical Assessment and Management Plans (SCAMPs) methodology to improve patient outcomes while reducing practice variation and unnecessary resource utilization [3]. SCAMPs provide a better alternative to clinical practice guidelines [4] and are a preferred methodology to incorporate evidence-based medicine into practice and may fit better in the culture of medicine, obtaining clinician adoption and better influence the clinical decision making [5]. SCAMPs offer a pragmatic and well-accepted methodology to standardize practice, optimizing resource use while improving patient care [6], [7], [8], [9], [10].
AdventHealth (formerly Florida Hospital) with their nine geographic campuses under a single hospital license had 414,005 adult ED patient visits during the year 2014. The chief complaint with the highest volume, highest risk, and highest variability was undifferentiated chest pain or angina with 28,324 adult patients with a final primary or secondary diagnosis of R07.2, R07.89, R07.9, I120.0, I120.1, I120.8 or I120.9 (Table 1).
Table 1.
Chest pain/angina definitions by the ICD-10a.
| ICD-10 | |
|---|---|
| R07.2 | Precordial pain |
| R07.89 | Other chest pain |
| R07.9 | Chest pain, unspecified |
| I120.0 | Unstable angina |
| I120.1 | Angina pectoris with documented spasm |
| I120.8 | Other forms of angina pectoris |
| I120.9 | Angina pectoris, unspecified |
Abbreviations: ICD = international classification of diseases; ACT = AdventHealth Clinical Transformation
Patients receiving an emergent cardiac intervention were excluded from the study set as accorded by the algorithm.
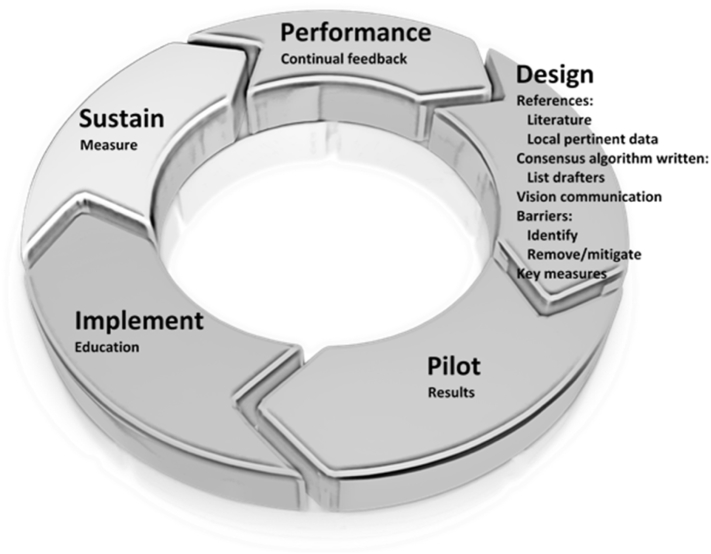
A Clinical Transformation department was established. Inspired by the SCAMPs methodology, adapted from change management principles,11 an AdventHealth Clinical Transformation (ACT) method was conceived and implemented (Fig. 1).
Fig. 1.
AdventHealth Clinical Transformation (ACT) cycle.
Design
•Demonstrate change needed now (evidence, data)
•Assemble guiding team, powerful coalition of active clinicians (written consensus algorithm)
•Develop motivating vision (best for patient)
•Communicate vision (urgency, honesty, clarity, passion)
•Identify and remove/mitigate barriers/obstacles (key measures)
Pilot
•Short run win (results, deviations, iterate)
Implement
•Maintain focus, build (education)
Sustain
•Institutionalize into culture (behaviors, attitudes, processes, ongoing measures)
Performance
•Continual feedback
2. Materials and Methods
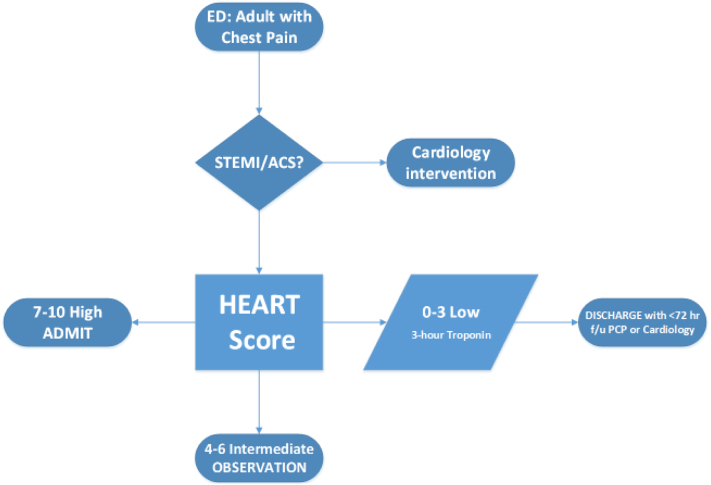
A guiding team of emergency medicine physicians, cardiologists, and nursing leadership was assembled representing an influential coalition. A vision to “establish the standard of care” for this patient population was developed and communicated. Following the ACT methodology, the pertinent systematic review of chest pain risk stratification was performed, current data was presented, a consensus algorithm (adapted from three SCAMPs pilots at Brigham and Women's Hospital; 10/14/2012 to 5/20/2014) was drafted (Fig. 2) with plans to capture the data from adherence to and deviations from the algorithm.
Fig. 2.
High level chest pain algorithm*.
*This high level algorithm is an abdriged version of the full interdisciplinary algorithm.
During the planning process, four critical components emerged as essential elements to algorithm formation:
-
I.
Identification: Adults with undifferentiated chest pain presenting to the ED.
-
II.
Indications: Inclusions: Chest discomfort/pain, chest pressure/fullness, pain radiating to left/both arms, jaw pain, pain in back/neck/stomach, shortness of breath, cold sweats, nausea/vomiting, lightheadedness. Exclusions: High concern for ACS on presentation [patients with STEMI (ST segment elevation myocardial infarction), definite NSTEMI, heart failure, arrhythmia, or non-cardiac etiology such as gastrointestinal, musculoskeletal or pulmonary.
-
III.
Stratification: The HEART (History, EKG, Age, Risk factors, Troponin) score was chosen as the best stratification tool. The HEART score [12], [13] had been prospectively validated for ED chest pain patients in The Netherlands via risk stratification with significantly higher concordance (c-statistic) than the Thrombolysis in Myocardial Infarction (TIMI) or the Global Registry of Acute Coronary Events (GRACE) scores. A secondary analysis of the Myeloperoxidase In the Diagnosis of Acute coronary syndromes Study (MIDAS) at 18 tertiary referral centers in the United States showed the HEART score identified a substantial number of low-risk patients for the early discharge, by incorporating a serial troponin measure, while maintaining high sensitivity for ACS [14].
-
IV.
Actions: Patients in the low-risk category (HEART score 0–3) would have a repeat troponin blood test in three hours and if normal would be discharged with cardiology or primary care physician follow-up within 72 h. Patients in the intermediate-risk category (HEART score 4–6) were recommended for observation status. Patients with a high-risk for ACS (HEART score 7–10) were recommended to be admitted for further evaluation and treatment.
After the consensus algorithm was defined, obstacles and barriers were identified (and mitigated):
-
•
Lack of ability of “low-risk” patients to get an appointment with primary care physician or cardiologist within 72 h of ED visit. (Establishment of care coordination center able to make outpatient appointments, before patient even left the ED)
-
•
Assurance of fairness: Patients with the established medical staff relationship return to their physician, un-assigned patients (payor agnostic) to primary care physician (PCP) or non-interventional cardiologist on call for the campus ED. (Adherence to medical staff by-laws and utilization of the campus call schedule)
-
•
Need for feedback to emergency physicians regarding the appointment compliance and 30-day outcomes of those transitioned to outpatient follow-up. (ACT team provided monthly report)
-
•
Concern regarding shifting the medical-legal risk from in the hospital to the doctors' offices. (Discussion and education)
-
•
Concern regarding shifting the financial burden of self-pay patients from the hospital to doctors' offices. (Awareness of hotline for scheduling follow-up for 45-days after the ED visit with hospital covering self-pay or narrow network patients)
The pilot was submitted for our Institutional Review Board (IRB) vetting and it was determined this study was classified by the IRB as a “performance improvement initiative and not human subject research”. A 400-patient pilot was planned at a 398-bed hospital for two months, June–July of 2015.
2.1. Lessons Learned From the Pilot
From the pilot study, we learned the following:
-
i)
Many physicians voiced opposition to “recipe medicine”, but no clinician disagreed with the consensus algorithm.
-
ii)
Communication with the physicians (emergency medicine, primary care, cardiologists) is never enough, and needs to be done in many venues.
-
iii)
An electronic order needed to be created in the electronic medical record (EMR) for the call coordination center to reach into a physician's office schedule and make a follow-up appointment for a patient while still in the ED.
-
iv)
The hospital has a “45-day tail” and phone hotline for any patient who was discharged from the ED to receive any pertinent testing ordered by the PCP or cardiologist, and costs are covered by the hospital.
-
v)
Patients identified as “low-risk” were more appropriate for primary care follow-up to address modifiable risk factors vice cardiologists.
-
vi)
The “ACT effect” was felt, as the emergency medicine physicians would, on occasion, use the algorithm follow-up on patients they were caring for, whether they were ACT patients or not.
-
vii)
The “hard wiring” of the HEART score into the emergency physicians electronic work flow is critical for the maintenance and sustainment of the effort.
2.2. Full Implementation
Results and lessons learned from the pilot were presented and discussed with the entire healthcare system cardiologists and emergency medicine physicians. Unanimous approval was given to implement it system-wide, which was done campus by campus in the fall of 2015.
2.3. Maintenance and Spread
Every emergency medicine department and the core physicians' meetings were attended by the Clinical Transformation leaders, and physicians were educated on the ACT process, including a visit to all the cardiologists' offices.
2.4. Process Measures/Data Collection
Data were collected and reported to the ED leaders for evaluation, which included:
-
i)
The number of forms completed by the emergency physician (to assess adoption and need to education).
-
ii)
Follow-up on the number of patients in ACT algorithm.
-
iii)
The HEART score distribution.
-
iv)
Appointment recommended (no-show/decline vs. attend).
-
v)
Re-visit status of patients who received/recommended appointments when discharged from the ED.
The ACT algorithm was fully implemented on November 1, 2015. The HEART score was “hard wired in the EMR” on December 7, 2015. Full system-wide implementation was completed on January 1, 2016 with monthly feedback of appointments and the HEART score use by the individual physicians.
3. Statistical Analyses
We performed statistical tests and CI (confidence interval) using “Two Proportions” method. We also used matching and stratification to deal with any confounding effect. p-Values < 0.05 were considered statistically significant.
4. Results
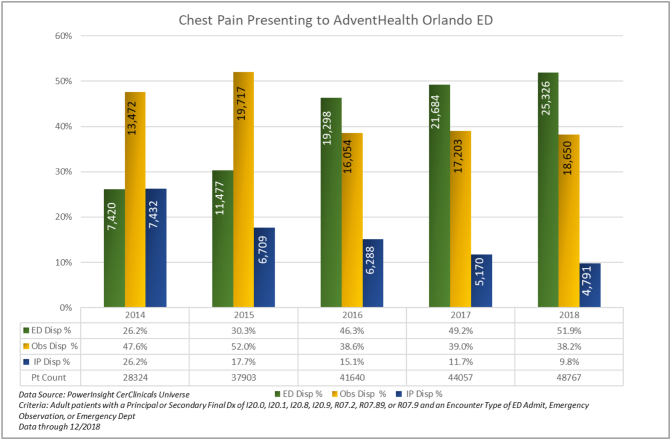
During the design/pilot, implement, sustain and the performance years, 200,691 patients were identified as adults with chest pain and the algorithm was used (Table 2). A dramatic change in the disposition decision rate was noted. When the ‘Baseline Year’ was compared with the ‘Performance Year’, chest pain patients discharged from the ED increased by 98% (26.2% to 51.9%), p < 0.0001), those going to the ‘Observation’ status decreased by 20% (47.6% to 38.2%; p < 0.0001) and ‘Inpatient’ admissions decreased by 62.5% (26.2% to 9.8%); p < 0.0001) (Fig. 3).
Table 2.
Performance of change management stages during the design/pilot years implementation.
| Adults ED | 2014 Y0 Baseline |
2015 Y1 Design/pilot |
2016 Y2 Implement |
2017 Y3 Sustain |
2018 Y4 Performance |
|---|---|---|---|---|---|
| Chest Pain | 28,324 | 37,903 | 41,640 | 44,057 | 48,767 |
| Total | 414,005 | 441,932 | 480,110 | 502,109 | 511,395 |
| 6.8% | 8.6% | 8.7% | 8.8% | 9.5% |
Abbreviations: ED – emergency department; Y = year.
Fig. 3.
Patient count and Disposition Decision Rate Breakdown for adults with chest pain/angina presenting to the emergency department from January 1, 2014 to Dec 31, 2018.
All ACT patients were tracked for 30-days for major adverse cardiac event (MACE) or return to the ED within the same healthcare system. Of the 40,791 patients identified as “low-risk” during the implement and sustain years (2016–2017), three cases returned within 30-days with MACE. A retrospective blinded review by a senior emergency medicine physician re-scored all three cases as “intermediate-risk” on the initial presentation and observation status would have been more appropriate. Refresher training was provided to all the emergency medicine physicians. Limitations to the study population include patients with 30-day MACE presenting to a hospital outside of our system or on the Social Security Death Index were not available. Limitations to the study also included the subjective nature inherent in one parameter of the HEART score.
Our findings were consistent with a contemporaneous multi-center study [15], which determined that “in adult patients with chest pain admitted with two negative findings for serial biomarkers, non-concerning vital signs, and non-ischemic electrocardiogram (EKG) findings, short-term clinically relevant adverse cardiac events were rare and commonly iatrogenic, suggesting routine inpatient admission may not be beneficial strategy for this group”.
5. Discussion
Many strategic initiatives are attempted in healthcare, often with initial success [11], [16] A more sustaining approach to changing the behavior and practice of physicians is by engaging physicians to design the care algorithm, track the data, embed decision making tools in the EMR, and encourage deviation for individual patient variation. ACT is an innovative method of examining relevant clinical data to improve patient outcomes while reducing practice variation and unnecessary resource utilization.
Our experience utilizing the ACT algorithm for patients with chest pain in the ED suggests that the percentage of patients identified as “low-risk” and safely transitioned to outpatient appointment for the continued evaluation and treatment nearly doubled while patients going to an observation decreased. Those found requiring inpatient admission were more than cut in half. By “hard-wiring”, the HEART score in the EMR and vigilantly monitoring the process measures (appointments and the HEART score utilization) by individual physician, the clinical change needed to improve the care of adults with chest pain presenting to an ED was maintained and sustained.
The change management approach enabled Emergency Physicians to develop an algorithm based on evidence and practice experience. The process and the results when communicated back to the physicians facilitated them to change their behaviors and practices to safely identify the appropriate venue to take care of adults with chest pain presenting to the emergency department. Equally important to the study was the continued maintenance (sustain) which had been a failing point in other studies to date.
In healthcare, the stroke of the pen (click of the mouse) is the determinant with the most powerful effect on cost of care. The difference in direct costs for a patient with ED discharge versus inpatient admission is US $3000 more for the admitted patient, with a final primary or secondary diagnosis of R07.2, R07.89, R07.9, I120.0, I120.1, I120.8 or I120.9. For Observation status, US $1500 more in direct costs is noted, compared to ED discharge [17].
An exercise of hypothetical analysis helps quantify the impact of the decision process (Table 3). If the Emergency Physicians had not changed their practice or behavior and the Baseline Year decision rate during the entire Performance year was unchanged, then 4563 more patients would have gone to Observation status and 7986 patients to Inpatient admissions. The one-year opportunity costs avoided would be approximately US $7 million (M) and $24M, respectively, for a total of $31M.
Table 3.
Decision rate comparison, baseline to performance.
| Y0 Baseline | Y4 Performance | Y4 Performance |
Y4 if Y0 Decision rate | Y4 if Y0 Decision rate | |
|---|---|---|---|---|---|
| ED to home | 26.2% | 51.9% | 25,326 | 26.2% | 12,777 |
| Observation | 47.6% | 38.2% | 18,650 | 47.6% | 23,213 |
| Inpatient admit | 26.2% | 9.8% | 4791 | 26.2% | 12,777 |
Reduced Observation annualized patients by 4563 ≥ $7M US$.
Reduced Inpatient Admits annualized patients by 7986 ≥ $24M US$.
Opportunity to save $31M US direct costs.
380 fewer Observation stays per month.
666 fewer Admissions per month.
Reduction in bed use in the healthcare system with chronic overcapacity is also important. If one assumes 1.5 days hospital length-of-stay (LOS) for observation and 4-days LOS for inpatient, then a 19-bed observation unit and 88-bed inpatient unit would have been utilized. The “capital redirection” for optimized utilization could be hypothesized at US $100 M.
More importantly than the cost and utilization exercise are the intersecting premises of transformation and safety. The human story of methodically identifying an additional one in four adult patients with chest pain who can safely be further evaluated in the outpatient setting meets the quadruple aim of the improved outcomes, lower cost, higher satisfaction, and improved experience for the healthcare team [18].
6. Conclusions
As healthcare continually strives to be fiscally responsible while maintaining patient safety, it is becoming increasingly important to identify patients that can be cared for in low -cost environments outside the hospital. This paper suggests that the safe disposition of undifferentiated chest pain presenting to the emergency room can be accomplished using the HEART Score coupled with a supportive outpatient infrastructure. We have successfully shown the ability to change emergency physician behavior and the ability to sustain the outpatient disposition rate in contrast to recent studies [13], [19], [20]. Our primary addition to the HEART score stratification was a hospital supported care navigation pathway that guaranteed all patients a 72 h follow -up with a PCP. The inference here is that emergency physicians will support and sustain outpatient discharge of low-risk patients as long as the outpatient hand-off is structured, timely, and set at the time of ED discharge. This has important implications for other outpatient diagnostic pathways that have a readily identifiable low-risk population.
Lessons learned for successful clinical transformation through change management
-
•
Select strategic topics
-
•
Get active physicians together
-
•
Write a consensus algorithm with freedom to deviate
-
•
Identify barriers and remove obstacles
-
•
Communicate vision
-
•
Mandatory pilot and transparent feedback
-
•
Phased system implementation
-
•
Sustain by “hard wiring” EMR, and outputs
Alt-text: Unlabelled Box
Authors' Contributions
Dr. Jeffrey Kulhman: Conceived the study design and its implementation, performed literature search, data review and analyses, data interpretation, and manuscript writing.
Dr. David Moorhead: Discussed the study design and oversaw the project operation, and participated in data review, interpretation, and manuscript writing.
Ms. Joyce Kerpchar: Discussed the study design, participated in the project implementation and data review, interpretation and analyses.
Mr. Daniel J. Peach: Help conceived the study design and its implementation, performed literature search, data review, analyses, and interpretation, created figures, and writing.
Dr. Sarfraz Ahmad: Performed peer-reviewed literature search, scientific discussion, data interpretation, created tables, writing and preparation of the manuscript for publication.
Dr. Paul B. O'Brian: Help conceived the study design and its implementation in emergency medicine, performed literature search, data review, analyses, interpretation, and writing.
Conflict of Interest Statements
All the authors declare that there are no conflicts of interest associated with this research manuscript.
Role of Funding Source
None to disclose.
Ethics Committee Approval
This study was deemed exempt by our AdventHealth Institutional Review Board (IRB), and no patient consent was required.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.eclinm.2019.04.010.
Contributor Information
Jeffrey Kuhlman, Email: jeffrey.kuhlman@AdventHealth.com.
Sarfraz Ahmad, Email: sarfraz.ahmad@AdventHealth.com.
Appendix A. Supplementary data
The following is the supplementary data related to this article.
Full interdisciplinary algorithm chest pain.
References
- 1.Mozaffarian D., Benjamin E.J., Go A.S. Heart disease and stroke statistics-2015 update: a report from the American Heart Association. Circulation. 2015;131:e29–322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 2.Mehta R.H., Eagle K.A. Missed diagnoses of acute coronary syndromes in the emergency room - continuing challenges. N Engl J Med. 2000;342:1207–1210. doi: 10.1056/NEJM200004203421610. [DOI] [PubMed] [Google Scholar]
- 3.Rathod R.H. SCAMPs: a new tool for an old problem. J Hosp Med. 2015;10:633–636. doi: 10.1002/jhm.2419. [DOI] [PubMed] [Google Scholar]
- 4.Farias M., Jenkins K., Lock J. Standardized clinical assessment and management plans (SCAMPs) provide a better alternative to clinical practice guidelines. Health Aff. 2013;32:911–920. doi: 10.1377/hlthaff.2012.0667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Farias M., Ziniel S., Rathod R.H. Provider attitudes toward standardized clinical assessment and management plans (SCAMPs) Congenit Heart Dis. 2011;6:558–565. doi: 10.1111/j.1747-0803.2011.00579.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Farias M., Friedman K.G., Lock J.E., Newburger J.W., Rathod R.H. Differentiating standardized clinical assessment and management plans from clinical practice guidelines. Acad Med. 2015;90:1002. doi: 10.1097/ACM.0000000000000783. [DOI] [PubMed] [Google Scholar]
- 7.Angoff G.H., Kane D.A., Giddins N., Paris Y.M., Moran A.M., Tantengco V. Regional implementation of a pediatric cardiology chest pain guideline using SCAMPs methodology. Pediatrics. 2013;132(4):e1010–e1017. doi: 10.1542/peds.2013-0086. [DOI] [PubMed] [Google Scholar]
- 8.Friedman K.G., Kane D.A., Rathod R.H., Renaud A., Farias M., Geggel R. Management of pediatric chest pain using a standardized assessment and management plan. Pediatrics. 2011;128(2):239–245. doi: 10.1542/peds.2011-0141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moodie D.S. Outcomes research-standardized clinical assessment and management plans. Congenit Heart Dis. 2010;5(4):337. doi: 10.1111/j.1747-0803.2010.00437.x. [DOI] [PubMed] [Google Scholar]
- 10.Sox H., Stewart W. Algorithms, clinical practice guidelines, and standardized clinical assessment and management plans: evidence-based patient management standards in evolution. Acad Med. 2015;90(2):129–132. doi: 10.1097/ACM.0000000000000509. [DOI] [PubMed] [Google Scholar]
- 11.Kotter J.P. Leading change: why transformation efforts fail. Harv Bus Rev. 2007:11. January 1. [Google Scholar]
- 12.Backus B.E., Six A.J., Kelder J.C. A prospective validation of the HEART score for chest pain patients at the emergency department. Int J Cardiol. 2013;168:2153–2158. doi: 10.1016/j.ijcard.2013.01.255. [DOI] [PubMed] [Google Scholar]
- 13.Poldervaart J.M., Reitsma J.B., Backus B.E. Effect of using the HEART score in patients with chest pain in the emergency department: a stepped-wedge, cluster randomized trial. Ann Intern Med. 2017;166:689–697. doi: 10.7326/M16-1600. [DOI] [PubMed] [Google Scholar]
- 14.Mahler S.A., Miller C.D., Hollander J.E. Identifying patients for early discharge: performance of decision rules among patients with acute chest pain. Int J Cardiol. 2013;168:795–802. doi: 10.1016/j.ijcard.2012.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weinstock M.B., Weingart S., Orth F. Risk for clinically relevant adverse cardiac events in patients with chest pain at hospital admission. JAMA Intern Med. 2015;175:1207–1212. doi: 10.1001/jamainternmed.2015.1674. [DOI] [PubMed] [Google Scholar]
- 16.Creasey T. Harnessing the power of change-enabling systems. Prosci®. https://www.prosci.com/resources/articles/harnessing-the-power-of-change-enabling-systems
- 17.Budryk Z. Fierce Healthcare; 2014. Two midnight rule means complications for hospitals, patients. July 15. [Google Scholar]
- 18.Bodenheimer T., Sinsky C. From triple to quadruple aim: care of the patient requires care of the provider. Ann Fam Med. 2014;12:573–576. doi: 10.1370/afm.1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Than M.P., Pickering J.W., Dryden J.M. Improving care processes for patients with suspected acute coronary syndrome (ICare-ACS): a study of cross-system implementation of a national clinical pathway. Circulation. 2018;137:354–363. doi: 10.1161/CIRCULATIONAHA.117.031984. [DOI] [PubMed] [Google Scholar]
- 20.Kwong R.Y., Schussheim A.E., Rekhraj S., Aletras A.H., Geller N., Davis J. Detecting acute coronary syndrome in the emergency department with cardiac magnetic resonance imaging. Circulation. 2003;107(4):531–537. doi: 10.1161/01.cir.0000047527.11221.29. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Full interdisciplinary algorithm chest pain.