Abstract
Objective
To elucidate whether cardiorespiratory fitness (CRF) is protective or contributory to coronary artery disease plaque burden.
Patients and Methods
Study participants were working middle-aged men from the Mayo Clinic Executive Health Program who underwent coronary artery calcium (CAC) assessment and exercise treadmill testing for risk stratification. Data from January 1, 1995, through December 31, 2008, were considered. The CAC assessment score was used for lifelong plaque burden analysis; functional aerobic capacity (FAC) from treadmill testing was analyzed as 4 ranked categories of CRF. Known risk factors for cardiovascular disease, including family history, were also considered.
Results
In 2946 male patients in this retrospective, cross-sectional, observational study, known cardiovascular risk factor profiles and risk calculations tended to uniformly improve with increasing CRF, defined by the FAC level. Only the above-average group, or the third of 4 levels, was found consistently lower than other levels of FAC for CAC scores. The above-average group also had statistical significance after controlling for age, body mass index, and family history of coronary artery disease in a U-shaped distribution rather than the expected linear dose-response relationship. Plaque burden was significantly increased in patients with the highest FAC level (P=.005) compared with the above-average group despite the observed maximal risk factor optimization in all known conventional cardiovascular risk factors.
Conclusion
For men, maximal CRF is associated with increased atherosclerosis, established with CAC scores. By comparison, average-to-moderate CRF appears to be cardioprotective regardless of either age or the influence of other contributing, recognized cardiac risk factors.
Abbreviations and Acronyms: BMI, body mass index; CAC, coronary artery calcium; CAD, coronary artery disease; CRF, cardiorespiratory fitness; CT, computed tomography; FAC, functional aerobic capacity; IQR, interquartile range; MET, metabolic equivalent
Cardiovascular disease prevention has been designed with exercise at its center. Nevertheless, a number of published studies have questioned whether more intense activity and maximal cardiorespiratory fitness (CRF) are actually a best practice and cardioprotective. Increased atherosclerosis has been noted in endurance athletes in a number of studies and reports. Greater amounts of coronary artery calcium (CAC) have been found in marathon runners than in controls matched for Framingham risk score.1 Long-term marathon runners who completed at least 25 marathons over 25 years were reported to have increased CAC and calcified plaque volume on coronary computed tomography (CT) angiography.2 More recently, an analysis of the Coronary Artery Risk Development in Young Adults study3 found that persons partaking in higher-than-recommended levels of physical activity by self-report had increased odds of subclinical coronary atherosclerosis by middle age.
Currently, the presumption is that higher exercise frequency, intensity, and duration and increased fitness capacity are cardioprotective. However, the dose-response association may not be a simple linear relationship. The aims and findings of recent studies scrutinize this relationship at the highest levels of CRF.4, 5, 6, 7 An important aspect is whether a serious departure from clinician understanding exists for patients at the highest level of CRF. Our study investigated whether a quantifiable indicator of maximal CRF contributes to disease burden, defined by the CAC score in a primary prevention population in which exercise is considered only for its health benefits and not for a competitive sport. We hypothesized that a high level of cardiovascular fitness, achievable only through long-term aerobic conditioning (maximal CRF and functional aerobic capacity [FAC] ≥130%), would be paradoxically associated with a higher level of CAC than average or lower levels of fitness. Poor fitness (poor CRF, deconditioned, FAC ≤69%), generally associated with increased risk, would be associated with the highest CAC level. We would expect that traditional Framingham risk factors would optimize in response to exercise. Knowledge gained from this study may inform and improve the accuracy of exercise recommendations for patients in the primary prevention of coronary artery disease (CAD).
Patients and Methods
For this retrospective, cross-sectional, observation study, we evaluated a homogeneous group of middle-aged men from the Mayo Clinic Executive Health Program who chose to come to our institution for a comprehensive preventive medical examination. From January 1, 1995, through December 31, 2008, CAC assessments were performed for patients without known CAD to identify those patients at highest risk and to target them for earlier medical intervention, including medication management and an exercise prescription to enhance CRF.
The patient cohort was further delimited to only those patients who underwent exercise treadmill testing within a few days or maximally within 1 year of this assessment and before an exercise prescription. Patients were excluded if they did not sign the required authorization for use of their health records for research. Because our aim was to study the cause of disease, we focused our assessments on men, given that (1) relatively few women were present in the Mayo Clinic Executive Health Program (16.5%; n=628) and (2) in general, the later development of plaque burden in women could potentially skew the analysis. Patients who had received a β-blocker treatment regimen were also excluded from the study because β-blockers may notably affect heart rate response to exercise and limit exercise capacity generally in the first 6 months of treatment onset. We were unable to control the onset and duration of β-blockade. Of the records that remained, coronary calcification reports presented percentile risk but no actual score, further reducing our study cohort.
The original cohort had 3828 patients. Patient exclusions were those who did not sign the required authorization (n=29); female patients (n=628); those treated with β-blockers (n=139); and those who had no score and only percentile risk (n=86). After these exclusions, the study population totaled 2946 patients.
Coronary artery calcium was used as a surrogate marker of atherosclerotic burden. Coronary artery calcium burden was quantified with CAC scoring of noncontrast medium cardiac CT images. Continuous 3-mm-thick images were obtained using either electron beam or multislice CT scanners; noncontrast medium scans were obtained using a C-150 or C-100 scanner (Imatron Inc). Calcium scoring was performed with automated scoring software using the method described previously.8, 9, 10
The main limiting symptom (eg, angina, chest pain, fatigue, and orthopedic) was selected as the test end point for the exercise treadmill test with use of the standard Bruce protocol on a motor-driven treadmill11 (Quinton Instrument Co or Marquette Electronics Inc). These limiting symptoms were selected as the test end point, although overwhelmingly present as fatigue in our cohort, as rated on a standard Borg perceived exertion scale.12 Active use of the treadmill handrails was not permitted.
Although exercise workload was estimated in metabolic equivalents (METs) (1 MET = 3.5 mL/kg per minute of oxygen consumption), fitness was quantified with FAC, a term interchangeable with aerobic or functional capacity. Functional aerobic capacity is calculated as achieved METs divided by predicted METs based on age and sex and is considered a reliable and stronger predictor of death than other established risk factors for CAD.13, 14, 15 This parameter had been taken from the CAC and FAC baseline assessments upon entry into the program, before any exercise recommendations, and with further validation provided by self-reported exercise frequency. Functional aerobic capacity data had been derived on the basis of indexed age- and sex-specific METs on exercise treadmill testing.
Operationally, we defined CRF as meeting the 4 ranked categories based on FAC determination of the exercise treadmill test. Category A (FAC ≤69%) refers to poor CRF, deconditioned with minimal or no exercise, and sedentary; B (FAC 70%-99%), CRF below average; C (FAC 100%-129%), CRF above average; and D (FAC ≥130%), maximal CRF, superconditioned with high-intensity exercise. Both CAC and log(CAC+1) were profiled as continuous outcome variables. The latter variable was thought to be most accurate and representative of the effect because it normalized the outcome data best, given that a number of zero scores had skewed the data.
We also had included age, body mass index (BMI; calculated as the weight in kilograms divided by the height in meters squared), and family history of CAD to control for these variables in our analyses because they may influence FAC. Furthermore, we provided descriptive epidemiological detail on traditional Framingham risk factors for our study cohort. The distribution of the Framingham risk factors had differed across levels of CRF, but the observed difference did not raise concern that would explain an increased risk in those with high CRF ranking.
Statistical Analyses
Data are presented as mean ± SD or median (interquartile range [IQR]) for continuous variables and as frequency count (percentage) for nominal variables. Baseline patient characteristics were compared across FAC categories using analysis of variance for continuous variables and the chi-square test or Fisher exact test for nominal variables. Because the distribution of CAC was highly skewed, this variable was compared across FAC groups using the Kruskal-Wallis test, with pairwise comparisons of each FAC group with the 100% to 129% group performed using the Wilcoxon rank-sum test. In addition, subgroup analyses were performed for 3 age groups (≤49, 50-59, and ≥60 years) and for family history of premature CAD. A multivariable analysis was performed using log(CAC+1) as the dependent variable; FAC group, age group, family history of premature CAD, and BMI were included as explanatory variables. In all cases, a 2-tailed P value of ≤.05 was considered statistically significant. The Mayo Clinic Institutional Review Board in Rochester, Minnesota, approved the study. All analyses were performed with SAS version 9.2 (SAS Institute Inc). The Strengthening the Reporting of Observational Studies in Epidemiology guidelines were used in reporting this study.
Results
In total, the study involved 2946 patients. Patient characteristics are presented in Table 1. Particularly of note is the favorable trend direction (eg, lower level of low-density lipoprotein cholesterol and higher level of high-density lipoprotein cholesterol) of all known risk factors for CAD with increasing fitness levels. Participants with the highest CRF ranking (FAC ≥130%) were older—a trend that likely influenced their Framingham risk score—and reported a family history of CAD. A subgroup analysis of patients 60 years and older revealed that the Framingham risk score was lower, and thus ideal, with increasing fitness levels (P<.001) (Table 2). No difference was observed among the various fitness groups for family history of either CAD (P=.59) or premature CAD (P=.15).
Table 1.
| Characteristic | Overall (N=2946) |
Functional aerobic capacity (% predicted) |
P valuee | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| ≤69 (n=131) |
70-99 (n=1314) |
100-129 (n=1329) |
≥130 (n=172) |
||||||||
| No.d | Value | No.d | Value | No.d | Value | No.d | Value | No.d | Value | ||
| Age (y) | 2946 | 52.1±7.5 | 131 | 51.9±8.0 | 1314 | 51.1±7.3 | 1329 | 52.7±7.4 | 172 | 55.4±6.9 | <.001 |
| Race | 2518 | 113 | 1108 | 1146 | 151 | .39 | |||||
| White non-Hispanic | 2455 (97.5) | 108 (95.6) | 1078 (97.3) | 1120 (97.7) | 149 (98.7) | ||||||
| Other | 63 (2.5) | 5 (4.4) | 30 (2.7) | 26 (2.3) | 2 (1.3) | ||||||
| BMI (kg/m2) | 2797 | 29.0±4.4 | 124 | 36.2±6.2 | 1252 | 30.4±4.1 | 1255 | 27.4±3.3 | 166 | 26.0±2.6 | <.001 |
| Diabetes mellitus | 2946 | 164 (5.6) | 131 | 26 (19.8) | 1314 | 89 (6.8) | 1329 | 47 (3.5) | 172 | 2 (1.2) | <.001 |
| Renal disease | 2946 | 34 (1.2) | 131 | 5 (3.8) | 1314 | 16 (1.2) | 1329 | 13 (1.0) | 172 | 0 (0.0) | .03 |
| Hypertension | 2946 | 131 | 1314 | 1329 | 172 | <.001 | |||||
| None | 2374 (80.6) | 80 (61.1) | 1033 (78.6) | 1114 (83.8) | 147 (85.5) | ||||||
| Treated | 427 (14.5) | 40 (30.5) | 210 (16.0) | 156 (11.7) | 21 (12.2) | ||||||
| Untreated | 145 (4.9) | 11 (8.4) | 71 (5.4) | 59 (4.4) | 4 (2.3) | ||||||
| Smoking status | 2598 | 117 | 1124 | 1197 | 160 | <.001 | |||||
| Never | 1601 (61.6) | 60 (51.3) | 671 (59.7) | 769 (64.2) | 101 (63.1) | ||||||
| Former | 791 (30.5) | 43 (36.7) | 332 (29.5) | 362 (30.2) | 54 (33.8) | ||||||
| Current | 206 (7.9) | 14 (12.0) | 121 (10.8) | 66 (5.5) | 5 (3.1) | ||||||
| Family history of CAD | 2896 | 126 | 1295 | 1305 | 170 | ||||||
| Any CAD | 1206 (41.6) | 60 (47.6) | 525 (40.5) | 539 (41.3) | 82 (48.2) | .13 | |||||
| Premature CAD | 804 (27.8) | 37 (29.4) | 345 (26.6) | 359 (27.5) | 63 (37.1) | .04 | |||||
| Cholesterol level (mg/dL) | 2832 | 128 | 1258 | 1277 | 169 | ||||||
| HDL | 51.2±13.6 | 44.8±12.4 | 48.5±12.4 | 54.5±14.0 | 56.9±13.1 | <.001 | |||||
| Total | 205.1±38.2 | 203.9±39.4 | 208.1±39.9 | 203.0±36.7 | 198.5±33.5 | <.001 | |||||
| Fasting blood glucose level (mg/dL) | 2831 | 102.0±17.0 | 128 | 113.5±30.5 | 1258 | 103.4±17.0 | 1276 | 100.0±15.2 | 169 | 98.7±10.9 | <.001 |
| Blood pressure (mm Hg) | 2740 | 117 | 1230 | 1231 | 162 | ||||||
| Systolic | 124.2±14.7 | 130.7±15.9 | 125.4±14.5 | 122.4±14.8 | 123.5±13.1 | <.001 | |||||
| Diastolic | 79.3±9.2 | 83.0±9.7 | 80.5±9.0 | 77.9±9.1 | 78.2±8.3 | <.001 | |||||
| Resting HR (beats/min) | 2180 | 67.1±10.0 | 95 | 74.8±10.8 | 968 | 69.7±9.8 | 990 | 64.7±9.1 | 127 | 60.2±8.9 | <.001 |
| Framingham risk score | 2946 | 7.2±4.2 | 131 | 8.0±4.7 | 1314 | 7.0±4.3 | 1329 | 7.1±4.1 | 172 | 8.2±3.3 | <.001 |
| Exercise | 1621 | 52 | 657 | 795 | 117 | <.001 | |||||
| None | 93 (5.7) | 8 (15.4) | 52 (7.9) | 31 (3.9) | 2 (1.7) | ||||||
| Light | 1035 (63.9) | 38 (73.1) | 479 (72.9) | 469 (60.0) | 49 (41.9) | ||||||
| Moderate | 66 (30.4) | 6 (11.5) | 126 (19.2) | 295 (37.1) | 66 (56.4) | ||||||
| Treadmill test results | 2946 | 131 | 1314 | 1329 | 172 | ||||||
| MET | 11.2±2.0 | 7.2±1.0 | 10.2±1.2 | 12.2±1.2 | 14.8±1.6 | <.001 | |||||
| Peak HR (beats/min) | 168.6±15.4 | 154.8±18.3 | 168.1±15.9 | 170.5±13.9 | 169.0±14.4 | <.001 | |||||
BMI = body mass index; CAD = coronary artery disease; HDL = high-density lipoprotein; HR = heart rate; MET = metabolic equivalent.
SI conversion factor: To convert to mg/dL values to mmol/L, multiply by 0.0259.
Data are presented as mean ± SD or as No. (percentage).
Patients with information available for the characteristic.
Functional aerobic capacity groups were compared using analysis of variance for continuous variables and the χ2 test or Fisher exact test for nominal variables.
Table 2.
| Characteristic | Overall (n=502) |
Functional aerobic capacity (% predicted) |
P valuee | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| ≤69 (n=26) |
70-99 (n=178) |
100-129 (n=248) |
≥130 (n=50) |
||||||||
| No.d | Value | No.d | Value | No.d | Value | No.d | Value | No.d | Value | ||
| Age (y) | 502 | 63.3±3.3 | 26 | 62.2±3.2 | 178 | 63.2±3.4 | 248 | 63.5±3.3 | 50 | 63.6±3.3 | |
| Race | 444 | 21 | 154 | 223 | 46 | .11 | |||||
| White non-Hispanic | 438 (98.6) | 20 (95.2) | 150 (97.4) | 222 (99.6) | 46 (100.0) | ||||||
| Other | 6 (1.4) | 1 (4.8) | 4 (2.6) | 1 (0.4) | 0 (0.0) | ||||||
| BMI (kg/m2) | 476 | 29.0±4.4 | 24 | 35.4±7.5 | 169 | 30.7±4.3 | 235 | 27.7±2.9 | 48 | 26.2±2.9 | <.001 |
| Diabetes mellitus | 502 | 42 (8.4) | 26 | 6 (23.1) | 178 | 19 (10.7) | 248 | 17 (6.8) | 50 | 0 (0.0) | .002 |
| Renal disease | 502 | 12 (2.4) | 26 | 1 (3.8) | 178 | 4 (2.2) | 248 | 7 (2.8) | 50 | 0 (0.0) | .56 |
| Hypertension | 502 | 26 | 178 | 248 | 50 | .36 | |||||
| None | 338 (80.0) | 14 (53.8) | 116 (65.2) | 168 (67.7) | 40 (80.0) | ||||||
| Treated | 9 (18.0) | 10 (38.5) | 52 (29.2) | 65 (26.2) | 9 (18.0) | ||||||
| Untreated | 1 (2.0) | 2 (7.7) | 10 (5.6) | 15 (6.1) | 1 (2.0) | ||||||
| Smoking status | 459 | 25 | 152 | 235 | 47 | .80 | |||||
| Never | 222 (48.4) | 11 (44.0) | 68 (44.7) | 121 (51.5) | 22 (46.8) | ||||||
| Former | 209 (45.5) | 12 (48.0) | 72 (47.4) | 102 (43.4) | 23 (48.9) | ||||||
| Current | 28 (6.1) | 2 (8.0) | 12 (7.9) | 12 (5.1) | 2 (4.3) | ||||||
| Family history of CAD | 495 | 25 | 176 | 244 | 50 | ||||||
| Any CAD | 206 (41.6) | 11 (44.0) | 69 (39.2) | 101 (49.3) | 25 (50.0) | .59 | |||||
| Premature CAD | 116 (23.4) | 6 (24.0) | 41 (23.3) | 51 (20.9) | 18 (36.0) | .15 | |||||
| Cholesterol level (mg/dL) | 486 | 25 | 170 | 241 | 50 | ||||||
| HDL | 54.0±15.1 | 47.6±13.9 | 50.2±13.3 | 56.1±15.6 | 59.7±14.6 | <.001 | |||||
| Total | 195.0±37.3 | 195.2±38.9 | 197.5±37.3 | 194.4±38.7 | 189.0±28.5 | .55 | |||||
| Fasting blood glucose level (mg/dL) | 485 | 104.5±17.9 | 25 | 111.8±26.3 | 169 | 106.5±16.9 | 241 | 103.3±18.7 | 50 | 99.9±8.7 | |
| Blood pressure (mm Hg) | 462 | 20 | 167 | 228 | 47 | ||||||
| Systolic | 127.7±15.3 | 130.8±17.8 | 129.5±16.0 | 126.9±14.7 | 124.0±13.4 | .09 | |||||
| Diastolic | 78.5±8.8 | 82.7±12.0 | 79.1±8.4 | 77.8±8.9 | 78.1±7.2 | .08 | |||||
| Resting HR (beats/min) | 390 | 67.1±9.4 | 18 | 72.0±7.5 | 142 | 70.2±9.0 | 189 | 65.4±9.1 | 41 | 62.2±8.1 | <.001 |
| Framingham risk score | 502 | 11.5±1.4 | 26 | 12.1±1.5 | 178 | 11.8±1.4 | 248 | 11.3±1.3 | 50 | 11.1±1.1 | <.001 |
| Exercise | 322 | 11 | 95 | 177 | 39 | <.001 | |||||
| None | 9 (2.8) | 0 (0.0) | 5 (5.3) | 3 (1.7) | 1 (2.6) | ||||||
| Light | 179 (55.6) | 9 (81.8) | 67 (70.5) | 90 (50.8) | 13 (33.3) | ||||||
| Moderate | 134 (41.6) | 2 (18.2) | 23 (24.2) | 84 (47.5) | 25 (64.1) | ||||||
| Treadmill test results | 502 | 26 | 178 | 248 | 50 | ||||||
| MET | 10.2±1.9 | 6.3±0.9 | 8.8±1.0 | 10.9±0.8 | 13.5±0.9 | <.001 | |||||
| Peak HR (beats/min) | 157.3±15.4 | 148.7±17.0 | 152.7±15.8 | 160.8±13.9 | 160.6±14.0 | <.001 | |||||
BMI = body mass index; CAD = coronary artery disease; HDL = high-density lipoprotein; HR = heart rate; MET = metabolic equivalent.
SI conversion factor: To convert mg/dL values to mmol/L, multiply by 0.0259.
Data are presented as mean ± SD or as No. (percentage).
Patients with information available for the characteristic.
Functional aerobic capacity groups were compared using analysis of variance for continuous variables and the χ2 test or Fisher exact test for nominal variables.
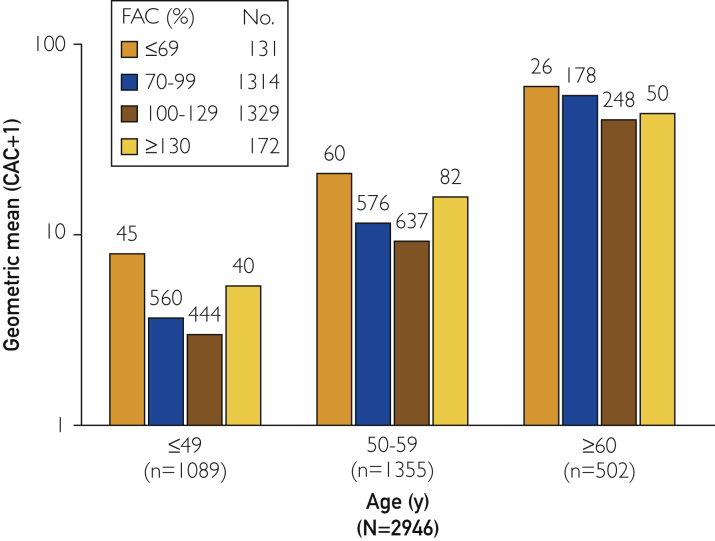
In an overall analysis, CAC differed significantly (Kruskal-Wallis, P<.001) across FAC groups; those with FAC of ≤69% and ≥130% had significantly higher CAC levels than did those with FAC of 100% to 129%. In age-specific analyses, significant differences across FAC groups were found for patients 49 years and younger and those aged 50 to 59 years, with a similar pattern of CAC across FAC categories (Table 3 and Figure 1).
Table 3.
CAC Levels According to FAC Ranked Categoriesa
| Characteristic | FAC (% predicted) |
P valuec | |||||||
|---|---|---|---|---|---|---|---|---|---|
| ≤69 |
70-99 |
100-129 |
≥130 |
||||||
| No.b | Median (IQR) | No.b | Median (IQR) | No.b | Median (IQR) | No.b | Median (IQR) | ||
| Overall | 131 | 21 (1-125) | 1314 | 4 (0-60) | 1329 | 2 (0-59) | 172 | 18 (0-129) | <.001d,e,f,g |
| Age group (y) | |||||||||
| ≤49 | 45 | 3 (0-29) | 560 | 0 (0-7) | 444 | 0 (0-5) | 40 | 0 (0-36) | .002d,e |
| 50-59 | 60 | 21 (1-165) | 576 | 8.5 (0-83.5) | 637 | 4 (0-59) | 82 | 15.5 (0-128) | .002f,h |
| ≥60 | 26 | 83.5 (9-298) | 178 | 82.5 (12-306) | 248 | 59 (3.5-243.5) | 50 | 69 (1-281) | .58 |
| Family history of premature CAD | |||||||||
| No | 89 | 21 (0-88) | 950 | 3 (0-59) | 946 | 1.4 (0-54) | 107 | 13 (0-100) | .001d,e,g |
| Yes | 37 | 10 (0-158) | 345 | 6 (0-66) | 359 | 6 (0-77) | 63 | 34 (0-270) | .11 |
CAC = coronary artery calcium; CAD = coronary artery disease; FAC = functional aerobic capacity; IQR = interquartile range.
Number of patients with information available.
CAC was compared across FAC groups using the Kruskal-Wallis test. If the overall comparison across groups was statistically significant, supplemental pairwise group comparisons were performed using the Wilcoxon rank-sum test.
P<.05 for pairwise comparison of ≤69% vs 70%-99%.
P<.05 for pairwise comparison of ≤69% vs 100%-129%.
P<.05 for pairwise comparison of 70%-99% vs ≥130%.
P<.05 for pairwise comparison of 100%-129% vs ≥130%.
P<.05 for pairwise comparison of 70%-99% vs 100%-129%.
Figure 1.
CAC and FAC stratified by age. CAC = coronary artery calcium; FAC = functional aerobic capacity.
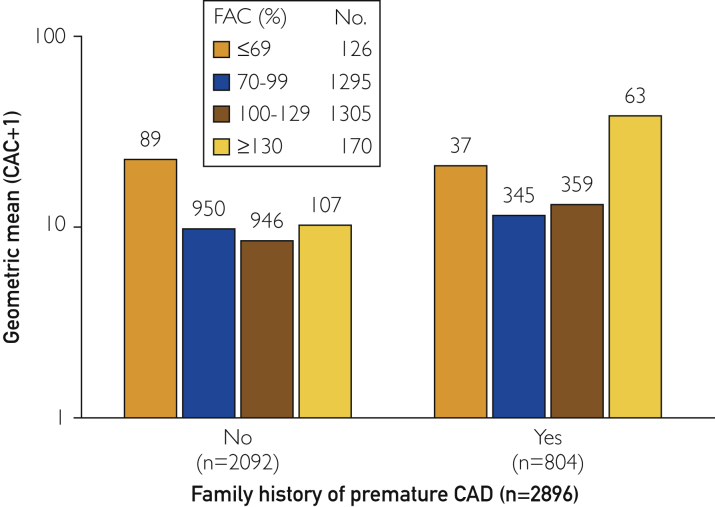
Family history of premature CAD differed significantly across FAC groups (P=.04.), and sensitivity analyses were performed to assess the differences among the groups after this covariate was taken into account. Among patients without a family history of premature CAD, the median (IQR) CAC level was 21 (0-88), 3 (0-59), 1.4 (0-54), and 13 (0-100) for those with FAC percentiles of ≤69%, 70% to 99%, 100% to 129%, and ≥130%, respectively. Among participants with a family history of premature CAD, the median (IQR) CAC level was 10 (0-158), 6 (0-66), 6 (0-77), and 34 (0-270), respectively. Among patients with and without a family history of premature CAD, those with an FAC percentile of ≥130% had a significantly higher CAC level than did those with an FAC percentile of 100% to 129% (Wilcoxon rank-sum, P=.03 and P=.04 with and without a family history of premature CAD, respectively) (Figure 2). A multivariable analysis was performed using log(CAC+1) as the dependent variable. After adjustment for age, BMI, and family history of premature CAD, CAC still differed significantly (P=.03) across FAC groups, with patients with FAC percentiles of 70% to 99% (P=.04) and 100% to 129% (P=.005) having lower CAC levels than did those with FAC ≥130%.
Figure 2.
CAC and FAC stratified by family history of premature coronary artery disease. CAC = coronary artery calcium; FAC = functional aerobic capacity.
Discussion
The present study suggests that higher levels of CRF may not confer additional cardioprotection beyond a certain threshold, which has been the conventional understanding. Instead, they may contribute to disease burden.
These data are in line with other studies2 that either suggested or found increased atherosclerosis in high-performance athletes (with adjustment for age and sex).
Strenuous exercise is associated with other cardiovascular complications. Only moderate exercise intensity and total physical activity have been associated with a protective effect on heart failure risk.16 Among apparently healthy men in the Physician’s Health Study, the frequency of vigorous exercise was associated with an increased risk of atrial fibrillation in young men and joggers.17 Over time, the long-term practice of robust training involving endurance exercise may stimulate unfavorable heart remodeling that causes a framework for cardiac arrhythmia.18 Animal studies corroborate this finding and indicate that cardiac fibrosis developed with long-term intensive exercise training, along with documented changes in ventricular function and increased arrhythmia.18, 19
Strenuous exercise has also been associated with an increase in all-cause mortality rate with a U-shaped distribution. The lowest hazard ratio for death has been found in persons who do light jogging, followed by moderate joggers and then strenuous joggers.6 The mortality rate of strenuous joggers was not statistically different from that of their most sedentary counterparts.
In this study, increasing fitness uniformly optimizes known cardiovascular risk factors. Paradoxically, it appears to have considerable adverse effect on plaque burden for those in the highest fitness and exercise parameters. Conventional risk factors may lose some of their protective and predictive power for patients with high CRF ranking. This outcome suggests an optimal level of exercise, beyond which other deleterious factors may have a role.
This study has multiple strengths. First, a large number of patients were identified in a primary prevention population typically associated with higher socioeconomic status, often called the working well. They have access and presumably lifelong exposure to a healthy lifestyle and health care, which may control other yet-unknown contributing and confounding risk factors, such as nutrition. Although a CAC score represents a single data point, it also represents the cumulative burden of disease over a lifetime, rather than relying on indirect risk factors that may waver over time and contribute only partially and minimally to disease burden. Direct relationships have been established between these scores and histologic and angiographic measures of CAD on a vessel-by-vessel and even segmental basis.20, 21
Second, we considered all conventional risk factors currently included in CAD risk calculations. Of interest, although maximal exercise (ie, maximal CRF) inferred improved risk factor profiles, it did not result in reduced atherosclerotic cardiovascular burden. This phenomenon suggests a more narrow range for CRF than previously thought, although different exercise regimens need to be considered with regard to their effect.
The use of an actually quantified FAC ensured that only participants capable of attaining the highest exercise endurance, and thus the highest CRF ranking, were accurately identified to avoid misclassification bias. One might argue that those already at risk given foreknowledge of a family history of CAD would be exercising to a greater degree to offset their risk and thereby potentially accounting for this phenomenon. However, this conclusion was refuted given the findings from our separate analyses for both groups, as was family history of CAD, biasing our findings.
We found many CAC scores of zero. This observation is not surprising given that participants in our study were relatively young and were interested in early subclinical disease identification. Outcome data were skewed to the left, accordingly, and affected our choice for study analysis. The CAC levels presented in Table 3 are low, even when looking at the upper end of the IQR, and most of the medians are 10 Agatston units or lower. Nevertheless, past published studies have noted increased mortality rates with increasing CAC scores, with a CAC score of zero having a statistically significant mortality advantage over CAC scores of 1 to 100.22
Although the present study focused on etiological understanding of the disease process, it is limited in its generalization to other patient populations, such as women, other ethnic groups, or patients of lower socioeconomic status. This study cannot assess varying training regimens from FAC and self-reported exercise frequency. Those patients with FAC ≥130% may also have had characteristics besides CRF that affected their CAC scores. For example, fitness, in part, may be genetically determined.23 In older men, the association between CRF and CAC seems to disappear. This finding may be explained by the additional time, provided by work retirement, that is devoted to fitness, nullifying an earlier effect or perhaps a phenomenon whereby the magnitude of difference within the CRF categories and capacity decreases with age.
Conclusion
Men from a primary prevention population who were capable of achieving the highest CRF ranking (FAC ≥130%) had higher levels of atherosclerotic disease burden in a U-shaped relationship. Average-to-moderate CRF was associated with the least CAC burden and thus may be the most cardioprotective. Increased microtrauma may be responsible for the amplification of the inflammatory cascade, a critical component of CAD development.24 This deleterious effect had not been offset by the conventional CAD risk factor optimization we observed. Further studies should examine the effects of sex and ethnicity and the interactions of specific exercise variables on disease progression.
A pathophysiological understanding is needed to assess the findings of increased CAC and presumed atherosclerotic burden of persons with maximal CRF. Although our study suggests that only average or moderate CRF should be attained, we focused on a single variable in the natural history of CAD. Other factors that may be associated with vigorous exercise may be cardioprotective and may offset the observed risk of plaque burden at the highest fitness parameters, such as optimized capillary oxygen delivery and solute transfer, coronary artery collateralization, and epicardial dilation with essentially a protective ischemic reserve.25, 26, 27, 28 Sex-based differences may exist, as recently published in a study of women in which no association was observed between CRF and CAC after risk factor adjustment.29
Additional research is needed to reconcile our findings with those indicative that increased exercise intensity is associated with better outcomes vs studies that claim a more complicated dose-response relationship, whereby risk alternatively is increased at the highest CRF parameters.6, 7, 30, 31 The need is urgent to identify components of exercise (ie, type, frequency, duration, and intensity), ethnicity and sex effects, and genetic variables that inform fitness to maximally protect against CAD. Such data would considerably inform and affect the advice clinicians deliver to patients as their current practice standard.
Acknowledgments
We thank Laurie M. Barr and Melissa S. Gregg for their assistance in data acquisition; Stephen Cha, MS, for assistance with statistical analysis; and Thomas A. Foley, MD, Thomas G. Allison, PhD, and Dawn P. Bergen for administrative, technical, or material support. Editing, proofreading, and reference verification were performed by Scientific Publications, Mayo Clinic.
Footnotes
For editorial comment, see page 103
Grant Support: The work was supported by the Division of Preventive, Occupational, and Aerospace Medicine, Department of Internal Medicine, Mayo Clinic. The funder had no role in the design, data, writing, and decision to submit the article for publication. In addition, this work was supported by Mayo Clinic STAR (Scheduled Time Away for Research) and WRAP (WRite up And Publish) grants (Dr Kermott).
Potential Competing Interests: Dr Kopecky has a board membership of the American Society of Men’s Health, serves as a consultant to Prime Therapeutics and Roche; has received grants from True Health, Amgen, and Regeneron; and is a board member of Mayo Clinic Support Services, Texas, a Board of Directors member of Mayo Clinic CV P&T Task Force, and a Data Safety Monitor Board member of Applied Clinical Intelligence. Dr Behrenbeck is Emeritus Member; he has received consultancy fees from the Eka Medical Group (outside the submitted work). The other authors report no competing interests.
References
- 1.Möhlenkamp S., Lehmann N., Breuckmann F., et al. Marathon Study Investigators; Heinz Nixdorf Recall Study Investigators. Running: the risk of coronary events: prevalence and prognostic relevance of coronary atherosclerosis in marathon runners. Eur Heart J. 2008;29(15):1903–1910. doi: 10.1093/eurheartj/ehn163. [DOI] [PubMed] [Google Scholar]
- 2.Schwartz J.G., Merkel-Kraus S., Duval S., et al. Does long-term endurance running enhance or inhibit coronary artery plaque formation? A prospective multidetector CTA study or men completing marathons for at least 25 consecutive years. J Am Coll Cardiol. 2010;55(10A) [abstract] [Google Scholar]
- 3.Laddu D.R., Rana J.S., Murillo R., et al. 25-year physical activity trajectories and development of subclinical coronary artery disease as measured by coronary artery calcium: the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Mayo Clin Proc. 2017;92(11):1660–1670. doi: 10.1016/j.mayocp.2017.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Armstrong M.E., Green J., Reeves G.K., Beral V., Cairns B.J., Million Women Study Collaborators Frequent physical activity may not reduce vascular disease risk as much as moderate activity: large prospective study of women in the United Kingdom. Circulation. 2015;131(8):721–729. doi: 10.1161/CIRCULATIONAHA.114.010296. [DOI] [PubMed] [Google Scholar]
- 5.Mons U., Hahmann H., Brenner H. A reverse J-shaped association of leisure time physical activity with prognosis in patients with stable coronary heart disease: evidence from a large cohort with repeated measurements. Heart. 2014;100(13):1043–1049. doi: 10.1136/heartjnl-2013-305242. [DOI] [PubMed] [Google Scholar]
- 6.Schnohr P., O’Keefe J.H., Marott J.L., Lange P., Jensen G.B. Dose of jogging and long-term mortality: the Copenhagen City Heart Study. J Am Coll Cardiol. 2015;65(5):411–419. doi: 10.1016/j.jacc.2014.11.023. [DOI] [PubMed] [Google Scholar]
- 7.Williams P.T., Thompson P.D. Increased cardiovascular disease mortality associated with excessive exercise in heart attack survivors. Mayo Clin Proc. 2014;89(9):1187–1194. doi: 10.1016/j.mayocp.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 8.Rumberger J.A., Sheedy P.F., Breen J.F., Schwartz R.S. Electron beam computed tomographic coronary calcium score cutpoints and severity of associated angiographic lumen stenosis. J Am Coll Cardiol. 1997;29(7):1542–1548. doi: 10.1016/s0735-1097(97)00093-4. [DOI] [PubMed] [Google Scholar]
- 9.Agatston A.S., Janowitz W.R., Hildner F.J., Zusmer N.R., Viamonte M., Jr., Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15(4):827–832. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 10.Rumberger J.A., Brundage B.H., Rader D.J., Kondos G. Electron beam computed tomographic coronary calcium scanning: a review and guidelines for use in asymptomatic persons. Mayo Clin Proc. 1999;74(3):243–252. doi: 10.4065/74.3.243. [published correction appears in Mayo Clin Proc. 1999;74(5):538] [DOI] [PubMed] [Google Scholar]
- 11.Bruce R.A. Exercise testing of patients with coronary heart disease: principles and normal standards for evaluation. Ann Clin Res. 1971;3(6):323–332. [PubMed] [Google Scholar]
- 12.Borg G. Perceived exertion as an indicator of somatic stress. Scand J Rehabil Med. 1970;2(2):92–98. [PubMed] [Google Scholar]
- 13.Morris C.K., Myers J., Froelicher V.F., Kawaguchi T., Ueshima K., Hideg A. Nomogram based on metabolic equivalents and age for assessing aerobic exercise capacity in men. J Am Coll Cardiol. 1993;22(1):175–182. doi: 10.1016/0735-1097(93)90832-l. [DOI] [PubMed] [Google Scholar]
- 14.Myers J., Prakash M., Froelicher V., Do D., Partington S., Atwood J.E. Exercise capacity and mortality among men referred for exercise testing. N Engl J Med. 2002;346(11):793–801. doi: 10.1056/NEJMoa011858. [DOI] [PubMed] [Google Scholar]
- 15.Prakash M., Myers J., Froelicher V.F., et al. Clinical and exercise test predictors of all-cause mortality: results from >6,000 consecutive referred male patients. Chest. 2001;120(3):1003–1013. doi: 10.1378/chest.120.3.1003. [DOI] [PubMed] [Google Scholar]
- 16.Rahman I., Bellavia A., Wolk A., Orsini N. Physical activity and heart failure risk in a prospective study of men. JACC Heart Fail. 2015;3(9):681–687. doi: 10.1016/j.jchf.2015.05.006. [DOI] [PubMed] [Google Scholar]
- 17.Aizer A., Gaziano J.M., Cook N.R., Manson J.E., Buring J.E., Albert C.M. Relation of vigorous exercise to risk of atrial fibrillation. Am J Cardiol. 2009;103(11):1572–1577. doi: 10.1016/j.amjcard.2009.01.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Benito B., Gay-Jordi G., Serrano-Mollar A., et al. Cardiac arrhythmogenic remodeling in a rat model of long-term intensive exercise training. Circulation. 2011;123(1):13–22. doi: 10.1161/CIRCULATIONAHA.110.938282. [DOI] [PubMed] [Google Scholar]
- 19.Lee C.D., Jacobs D.R., Jr., Hankinson A., Iribarren C., Sidney S. Cardiorespiratory fitness and coronary artery calcification in young adults: the CARDIA Study. Atherosclerosis. 2009;203(1):263–268. doi: 10.1016/j.atherosclerosis.2008.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rumberger J.A., Simons D.B., Fitzpatrick L.A., Sheedy P.F., Schwartz R.S. Coronary artery calcium area by electron-beam computed tomography and coronary atherosclerotic plaque area: a histopathologic correlative study. Circulation. 1995;92(8):2157–2162. doi: 10.1161/01.cir.92.8.2157. [DOI] [PubMed] [Google Scholar]
- 21.Budoff M.J., Georgiou D., Brody A., et al. Ultrafast computed tomography as a diagnostic modality in the detection of coronary artery disease: a multicenter study. Circulation. 1996;93(5):898–904. doi: 10.1161/01.cir.93.5.898. [DOI] [PubMed] [Google Scholar]
- 22.Nasir K., Rubin J., Blaha M.J., et al. Interplay of coronary artery calcification and traditional risk factors for the prediction of all-cause mortality in asymptomatic individuals. Circ Cardiovasc Imaging. 2012;5(4):467–473. doi: 10.1161/CIRCIMAGING.111.964528. [DOI] [PubMed] [Google Scholar]
- 23.Bouchard C. Genomic predictors of trainability. Exp Physiol. 2012;97(3):347–352. doi: 10.1113/expphysiol.2011.058735. [DOI] [PubMed] [Google Scholar]
- 24.Teague H., Mehta N.N. The link between inflammatory disorders and coronary heart disease: a look at recent studies and novel drugs in development. Curr Atheroscler Rep. 2016;18(1):3. doi: 10.1007/s11883-015-0557-y. [DOI] [PubMed] [Google Scholar]
- 25.Behrenbeck T.R., McCollough C.H., Miller W.L., et al. Early changes in myocardial microcirculation in asymptomatic hypercholesterolemic subjects: as detected by perfusion CT. Ann Biomed Eng. 2014;42(3):515–525. doi: 10.1007/s10439-013-0934-z. [published correction appears in Ann Biomed Eng. 2014;42(6):1354] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Möbius-Winkler S., Uhlemann M., Adams V., et al. Coronary collateral growth induced by physical exercise: results of the Impact of Intensive Exercise Training on Coronary Collateral Circulation in Patients With Stable Coronary Artery Disease (EXCITE) Trial. Circulation. 2016;133(15):1438–1448. doi: 10.1161/CIRCULATIONAHA.115.016442. discussion 1448. [DOI] [PubMed] [Google Scholar]
- 27.Zbinden R., Zbinden S., Windecker S., Meier B., Seiler C. Direct demonstration of coronary collateral growth by physical endurance exercise in a healthy marathon runner. Heart. 2004;90(11):1350–1351. doi: 10.1136/hrt.2003.023267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hambrecht R., Wolf A., Gielen S., et al. Effect of exercise on coronary endothelial function in patients with coronary artery disease. N Engl J Med. 2000;342(7):454–460. doi: 10.1056/NEJM200002173420702. [DOI] [PubMed] [Google Scholar]
- 29.DeFina L., Radford N., Leonard D., Gibbons L., Khera A. Cardiorespiratory fitness and coronary artery calcification in women. Atherosclerosis. 2014;233(2):648–653. doi: 10.1016/j.atherosclerosis.2014.01.016. [DOI] [PubMed] [Google Scholar]
- 30.Kodama S., Saito K., Tanaka S., et al. Cardiorespiratory fitness as a quantitative predictor of all-cause mortality and cardiovascular events in healthy men and women: a meta-analysis. JAMA. 2009;301(19):2024–2035. doi: 10.1001/jama.2009.681. [DOI] [PubMed] [Google Scholar]
- 31.Swain D.P., Franklin B.A. Comparison of cardioprotective benefits of vigorous versus moderate intensity aerobic exercise. Am J Cardiol. 2006;97(1):141–147. doi: 10.1016/j.amjcard.2005.07.130. [DOI] [PubMed] [Google Scholar]