To the Editor:
A key strategy of tuberculosis (TB) control programs in high-resource countries is identification of latent tuberculosis infection (LTBI) and preventive therapy to avert progression to TB disease (1). Currently, only tuberculin skin tests (TSTs) and interferon-γ release assays (IGRAs) are used for LTBI screening (2). IGRAs are functional blood-based assays that detect interferon-γ produced by memory T cells after stimulation with mycobacterial antigens (2). Currently, two IGRAs are available: the T-SPOT.TB assay (Oxford Immunotec) and the more widely used QuantiFERON-TB Gold (QFT) assay (Cellestis/Qiagen) (3).
Globally, the number of hematopoietic stem cell transplant and solid organ transplant recipients is rising steadily. Transplant recipients require long-term immunosuppression and consequently have a much greater risk of developing TB disease than the general population (4). Furthermore, mortality associated with TB disease is higher (4–6). Calcineurin inhibitors, including cyclosporin and tacrolimus, are the most commonly used immunosuppressive agents after transplant (7). They reduce T cell activation, thereby inhibiting production of various cytokines, including interferon-γ and interleukin 2 (IL-2) (8). Both cytokines play crucial roles in human antimycobacterial immune responses (9, 10).
TB screening in patients receiving immunosuppressive medication is complex (4, 11–13). Considerable evidence shows that the sensitivity of TSTs is reduced in immunocompromised individuals (2, 14). Previous studies investigating IGRAs in the transplant setting have reported conflicting results, some suggesting that they are reliable and others concluding that their performance is impaired (15–18). The key limitation of all previous clinical studies is that no gold standard for LTBI exists (2). Therefore, the interpretation of negative IGRA results in immunosuppressed patients is difficult, because it is currently impossible to distinguish true absence of TB infection from a false-negative result caused by immunosuppression.
The aim of this study was to determine the impact of calcineurin inhibitors on the performance of QFT assays using an ex vivo model. In addition, we investigated their impact on recently identified biomarkers of TB infection: mycobacteria-specific IL-2, interferon-γ–inducible protein 10 (IP-10), and tumor necrosis factor-α (TNF-α) responses (9, 10).
Methods
Study population
Adults with a previous positive IGRA result or recent TB exposure were recruited at a TB clinic after written informed consent was obtained. Potential participants with known immunodeficiency or receiving immunosuppressive medication were excluded. The study was approved by the National Research Ethics Service Committee (13/SC/0043).
Interferon-γ release assays
From each participant, three sets of QFT assays comprising an antigen-stimulated, a positive (mitogen) control, and a negative control tube were obtained. No reagents were added to the first set (“standard assay”). In the second set, cyclosporin (Sandimmune; Novartis) was added to each tube to a final concentration of 200 ng/ml, a common target level in the hematopoietic stem cell transplant setting (19). In the third set, tacrolimus (Prograf; Astellas Pharma) was added to each tube to a final concentration of 10 ng/ml, a typical target level in the solid organ transplant setting (20). Drugs were added within 4 hours of phlebotomy, and samples were immediately transferred into a 37°C incubator. After 24 hours, supernatants were harvested as per the manufacturer’s instructions, followed by cryopreservation.
Cytokine measurements
Cytokine concentrations in supernatants were determined with ProcartaPlex xMAP assays (Affymetrix/eBioscience) measuring interferon-γ, IP-10, IL-2, and TNF-α according to the manufacturer’s instructions. Their broad dynamic range allows accurate measurement of the high interferon-γ concentrations that often occur in QFT assays, which exceed the upper limit of QFT enzyme-linked immunosorbent assays (13). Assays were read with a Luminex 100 Bioanalyzer with xPONENT software (Luminex Corporation).
Interpretation of QFT results
QFT results were interpreted according to the latest version of the manufacturer’s package insert (U.K. version). Briefly, a positive result was defined as a background-corrected interferon-γ response ≥ 0.35 IU/ml and simultaneously ≥ 25% of the nil control sample interferon-γ concentration. A negative result was defined as a response below this threshold in the presence of a valid positive control (i.e., background-corrected interferon-γ concentration ≥0.5 IU/ml). An indeterminate assay result was defined as a sample set in which the negative control failed (i.e., interferon-γ concentration >8.0 IU/ml) or in which the positive control failed (background-corrected interferon-γ concentration <0.5 IU/ml).
Statistical analyses
All cytokines were analyzed in picograms per milliliter, except interferon-γ, which was measured in picograms per milliliter and then converted to international units per milliliter (the units used in QFT assays) for analysis, as previously described (21). Statistical comparisons were done in Prism software (version 6.0; GraphPad Software) using Wilcoxon matched-pairs signed-rank tests.
Results
A total of 18 participants were recruited, of whom 13 had positive QFT results. For the analyses of antigen-stimulated cytokine responses, only data from these 13 participants were included, whereas for the analyses of positive control responses, data from all 18 were included.
Interferon-γ responses and categorical QFT results
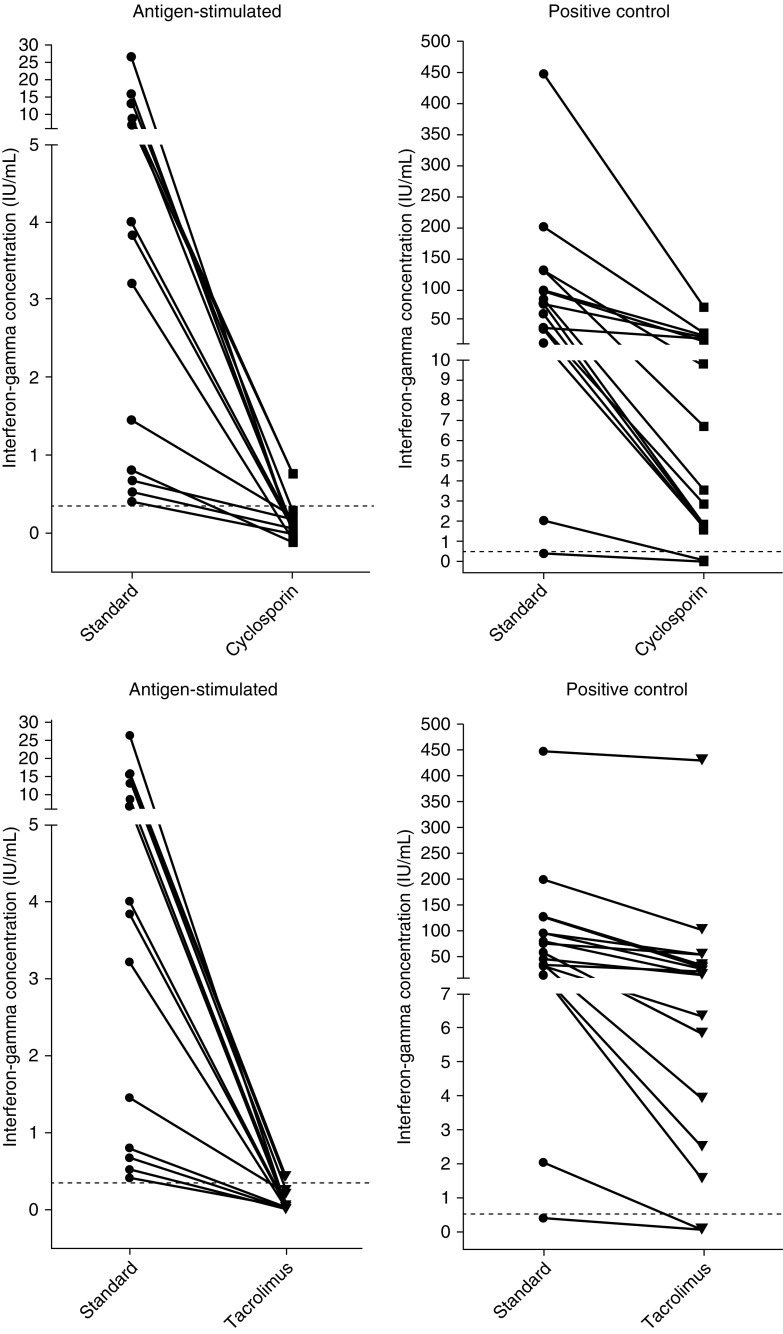
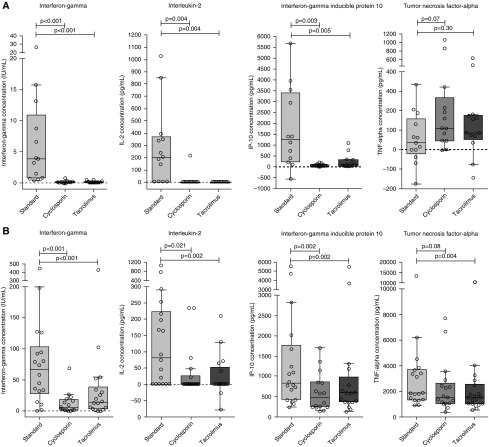
Both cyclosporin and tacrolimus caused considerable reductions in background-corrected interferon-γ concentrations in the antigen-stimulated samples in all participants (Figure 1). Compared with the standard assay (3.84 IU/ml; interquartile range [IQR], 0.74–10.9), the median interferon-γ concentrations were significantly lower in the cyclosporin- and tacrolimus-treated assay sets (0.0 IU/ml; IQR, −0.12 to 0.18; P < 0.001; and 0.02 IU/ml; IQR, −0.006 to 0.13; P < 0.001, respectively) (Figure 2A). In the cyclosporin- and tacrolimus-treated positive control samples, the median interferon-γ concentrations were also significantly lower (5.1 IU/ml; IQR, 1.6–18.9; and 14.3 IU/ml; IQR, 3.5–39.1, respectively) than in the standard assays (66.6 IU/ml; IQR, 28.0–103.3) but still considerably above the cutoff for classifying positive control samples as failed (Figure 2B).
Figure 1.
Background-corrected interferon-γ concentrations in antigen-stimulated (left) and positive control (right) samples in individual participants in the standard assay set compared with sets with added cyclosporin (upper panel; n = 13) and tacrolimus (lower panel; n = 13). Dotted lines indicate the cutoff for a positive test result in antigen-stimulated samples (0.35 IU/ml) and the cutoff for a valid positive control response (0.5 IU/ml).
Figure 2.
Background-corrected interferon-γ, interleukin 2 (IL-2), interferon-γ–inducible protein 10 (IP-10), and tumor necrosis factor-α (TNF-α) concentrations in (A) antigen-stimulated (n = 13) and (B) positive control (n = 18) samples in standard assay sets and sets with added cyclosporin and tacrolimus. Box plot with Tukey whiskers; horizontal lines depict the medians; P values were calculated with Wilcoxon matched-pairs signed-rank tests. Negative values are due to background correction (see the Methods section).
Of the 13 participants with a positive QFT result in the standard assay, 10 converted to a negative result in the cyclosporin-treated set and 2 to an indeterminate result, and 1 (participant 4) continued to have a positive result despite a markedly reduced antigen-stimulated interferon-γ response (0.76 vs. 6.59 IU/ml in the standard assay). In the tacrolimus-treated set, 10 individuals converted to a negative and 2 to an indeterminate result, and 1 (participant 1) remained positive, again with markedly reduced response (0.43 vs. 13.1 IU/ml).
IL-2, IP-10, and TNF-α responses
Background-corrected IL-2 and IP-10 concentrations were significantly lower in the antigen-stimulated samples in the cyclosporin- and tacrolimus-treated assay sets than in the standard assay (Figure 2A). In contrast, there was no significant difference in background-corrected TNF-α concentrations. TNF-α responses in the positive control samples were also largely maintained, although statistically there was a significant reduction in concentrations in tacrolimus-treated samples (Figure 2B).
Discussion
This study provides robust evidence that calcineurin inhibitors have a significant adverse effect on the performance of IGRAs. Our results suggest that the majority of patients with LTBI who are receiving treatment with cyclosporin or tacrolimus would have false-negative IGRA results when screened for TB, such as in the context of contact screening after exposure to a case with pulmonary TB. Importantly, the ex vivo model used in this study cannot capture the long-term impact of calcineurin inhibitors on T cells, which may be even more pronounced.
The marked impact of calcineurin inhibitors on IGRAs is consistent with their known mechanism of action. A key property of this drug class is inhibition of T cell activation and suppression of proinflammatory cytokines, including interferon-γ and IL-2, in T cells (8, 22, 23), the main source of interferon-γ in functional assays determining antimycobacterial immune responses, including QFT assays (2). The observed reduction in IP-10 responses is also predicted, because IP-10 production is primarily induced by interferon-γ (24). It is unlikely that those observations are due to cytotoxicity, because previous data show that even at a 100-fold greater concentration than used in this study cyclosporin has no significant cytotoxic effects on T cells (25).
In contrast, TB antigen–induced TNF-α responses were not suppressed by cyclosporin or tacrolimus. This suggests that calcineurin inhibitors have only limited effect on macrophages, the principal source of TNF-α in immune responses directed against mycobacteria, consistent with published data (26). Furthermore, this observation suggests that in patients receiving calcineurin inhibitors, novel TB assays based on TNF-α responses, which are currently in development (9, 10), may prove more robust than IGRAs.
In conclusion, considering our results together with previous data showing that the performance of TSTs is also impaired in immunosuppressed patients, both currently used LTBI screening tests should be regarded as unreliable in patients receiving calcineurin inhibitors. Although a positive IGRA result remains useful in this patient population, a negative result provides no meaningful information regarding TB infection status.
Supplementary Material
Footnotes
Supported by a clinical lectureship provided by the National Institute for Health Research (M.T.).
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1.World Health Organization (WHO) Global tuberculosis report 2017. Geneva: WHO; 2017.
- 2.Tebruegge M, Ritz N, Curtis N, Shingadia D. Diagnostic tests for childhood tuberculosis: past imperfect, present tense and future perfect? Pediatr Infect Dis J. 2015;34:1014–1019. doi: 10.1097/INF.0000000000000796. [DOI] [PubMed] [Google Scholar]
- 3.Tebruegge M, Ritz N, Koetz K, Noguera-Julian A, Seddon JA, Welch SB, et al. Availability and use of molecular microbiological and immunological tests for the diagnosis of tuberculosis in Europe. PLoS One. 2014;9:e99129. doi: 10.1371/journal.pone.0099129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bumbacea D, Arend SM, Eyuboglu F, Fishman JA, Goletti D, Ison MG, et al. The risk of tuberculosis in transplant candidates and recipients: a TBNET consensus statement. Eur Respir J. 2012;40:990–1013. doi: 10.1183/09031936.00000712. [DOI] [PubMed] [Google Scholar]
- 5.Benito N, García-Vázquez E, Horcajada JP, González J, Oppenheimer F, Cofán F, et al. Clinical features and outcomes of tuberculosis in transplant recipients as compared with the general population: a retrospective matched cohort study. Clin Microbiol Infect. 2015;21:651–658. doi: 10.1016/j.cmi.2015.03.010. [DOI] [PubMed] [Google Scholar]
- 6.Russo RL, Dulley FL, Suganuma L, França IL, Yasuda MA, Costa SF. Tuberculosis in hematopoietic stem cell transplant patients: case report and review of the literature. Int J Infect Dis. 2010;14:e187–e191. doi: 10.1016/j.ijid.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 7.Ruutu T, Gratwohl A, de Witte T, Afanasyev B, Apperley J, Bacigalupo A, et al. Prophylaxis and treatment of GVHD: EBMT-ELN working group recommendations for a standardized practice Bone Marrow Transplant 201449168–173.[Published erratum appears in Bone Marrow Transplant 2014;49:319.] [DOI] [PubMed] [Google Scholar]
- 8.Rao A, Luo C, Hogan PG. Transcription factors of the NFAT family: regulation and function. Annu Rev Immunol. 1997;15:707–747. doi: 10.1146/annurev.immunol.15.1.707. [DOI] [PubMed] [Google Scholar]
- 9.Clifford V, Tebruegge M, Zufferey C, Germano S, Forbes B, Cosentino L, et al. Mycobacteria-specific cytokine responses as correlates of treatment response in active and latent tuberculosis. J Infect. 2017;75:132–145. doi: 10.1016/j.jinf.2017.04.011. [DOI] [PubMed] [Google Scholar]
- 10.Tebruegge M, Dutta B, Donath S, Ritz N, Forbes B, Camacho-Badilla K, et al. Mycobacteria-specific cytokine responses detect tuberculosis infection and distinguish latent from active tuberculosis. Am J Respir Crit Care Med. 2015;192:485–499. doi: 10.1164/rccm.201501-0059OC. [DOI] [PubMed] [Google Scholar]
- 11.Clifford V, Tebruegge M, Curtis N. Limitations of current tuberculosis screening tests in immunosuppressed patients. BMJ. 2015;350:h2226. doi: 10.1136/bmj.h2226. [DOI] [PubMed] [Google Scholar]
- 12.Clifford V, Zufferey C, Germano S, Ryan N, Leslie D, Street A, et al. The impact of anti-tuberculous antibiotics and corticosteroids on cytokine production in QuantiFERON-TB Gold In Tube assays. Tuberculosis (Edinb) 2015;95:343–349. doi: 10.1016/j.tube.2015.02.039. [DOI] [PubMed] [Google Scholar]
- 13.Edwards A, Gao Y, Allan RN, Ball D, de Graaf H, Coelho T, et al. Corticosteroids and infliximab impair the performance of interferon-γ release assays used for diagnosis of latent tuberculosis. Thorax. 2017;72:946–949. doi: 10.1136/thoraxjnl-2016-209397. [DOI] [PubMed] [Google Scholar]
- 14.Richeldi L, Losi M, D’Amico R, Luppi M, Ferrari A, Mussini C, et al. Performance of tests for latent tuberculosis in different groups of immunocompromised patients. Chest. 2009;136:198–204. doi: 10.1378/chest.08-2575. [DOI] [PubMed] [Google Scholar]
- 15.Hadaya K, Bridevaux PO, Roux-Lombard P, Delort A, Saudan P, Martin PY, et al. Contribution of interferon-γ release assays (IGRAs) to the diagnosis of latent tuberculosis infection after renal transplantation. Transplantation. 2013;95:1485–1490. doi: 10.1097/TP.0b013e3182907073. [DOI] [PubMed] [Google Scholar]
- 16.Redelman-Sidi G, Sepkowitz KA. IFN-γ release assays in the diagnosis of latent tuberculosis infection among immunocompromised adults. Am J Respir Crit Care Med. 2013;188:422–431. doi: 10.1164/rccm.201209-1621CI. [DOI] [PubMed] [Google Scholar]
- 17.Sester M, van Leth F, Bruchfeld J, Bumbacea D, Cirillo DM, Dilektasli AG, et al. TBNET. Risk assessment of tuberculosis in immunocompromised patients: a TBNET study. Am J Respir Crit Care Med. 2014;190:1168–1176. doi: 10.1164/rccm.201405-0967OC. [DOI] [PubMed] [Google Scholar]
- 18.Scholman T, Straub M, Sotgiu G, Elsäßer J, Leyking S, Singh M, et al. Superior sensitivity of ex vivo IFN-γ release assays as compared to skin testing in immunocompromised patients. Am J Transplant. 2015;15:2616–2624. doi: 10.1111/ajt.13330. [DOI] [PubMed] [Google Scholar]
- 19.Peters C, Minkov M, Gadner H, Klingebiel T, Vossen J, Locatelli F, et al. European Group for Blood and Marrow Transplantation (EBMT) Working Party Paediatric Diseases; International BFM Study Group–Subcommittee Bone Marrow Transplantation (IBFM-SG) Statement of current majority practices in graft-versus-host disease prophylaxis and treatment in children. Bone Marrow Transplant. 2000;26:405–411. doi: 10.1038/sj.bmt.1702524. [DOI] [PubMed] [Google Scholar]
- 20.Israni AK, Riad SM, Leduc R, Oetting WS, Guan W, Schladt D, et al. DeKAF Genomics Investigators. Tacrolimus trough levels after month 3 as a predictor of acute rejection following kidney transplantation: a lesson learned from DeKAF Genomics. Transpl Int. 2013;26:982–989. doi: 10.1111/tri.12155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Desem N, Jones SL. Development of a human γ interferon enzyme immunoassay and comparison with tuberculin skin testing for detection of Mycobacterium tuberculosis infection. Clin Diagn Lab Immunol. 1998;5:531–536. doi: 10.1128/cdli.5.4.531-536.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gelfand EW, Cheung RK, Mills GB. The cyclosporins inhibit lymphocyte activation at more than one site. J Immunol. 1987;138:1115–1120. [PubMed] [Google Scholar]
- 23.Kino T, Hatanaka H, Miyata S, Inamura N, Nishiyama M, Yajima T, et al. FK-506, a novel immunosuppressant isolated from a Streptomyces. II. Immunosuppressive effect of FK-506 in vitro. J Antibiot (Tokyo) 1987;40:1256–1265. doi: 10.7164/antibiotics.40.1256. [DOI] [PubMed] [Google Scholar]
- 24.Neville LF, Mathiak G, Bagasra O. The immunobiology of interferon-γ inducible protein 10 kD (IP-10): a novel, pleiotropic member of the C-X-C chemokine superfamily. Cytokine Growth Factor Rev. 1997;8:207–219. doi: 10.1016/s1359-6101(97)00015-4. [DOI] [PubMed] [Google Scholar]
- 25.Gertsch J, Güttinger M, Sticher O, Heilmann J. Relative quantification of mRNA levels in Jurkat T cells with RT-real time-PCR (RT-rt-PCR): new possibilities for the screening of anti-inflammatory and cytotoxic compounds. Pharm Res. 2002;19:1236–1243. doi: 10.1023/a:1019818814336. [DOI] [PubMed] [Google Scholar]
- 26.van den Bosch TP, Kannegieter NM, Hesselink DA, Baan CC, Rowshani AT. Targeting the monocyte-macrophage lineage in solid organ transplantation. Front Immunol. 2017;8:153. doi: 10.3389/fimmu.2017.00153. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.