Abstract
Rationale: Although gastrostomy tubes have shown to be of limited benefit in patients with advanced dementia, they continue to be used to deliver nutritional support in critically ill patients. The epidemiology and short-term outcomes are unclear.
Objectives: To quantify national practice patterns and short-term outcomes of gastrostomy tube placement among the critically ill over the last two decades in the United States.
Methods: Using the U.S. Agency for Healthcare and Research Quality’s Healthcare Cost and Utilization Project’s National Inpatient Sample, we evaluated trends in annual population-standardized rates of gastrostomy tube placement among critically ill adults from 1994 to 2014; we also quantified trends in length of stay, in-hospital mortality, and discharge location. We conducted sensitivity analyses among mechanically ventilated patients, survivors, and decedents of critical illness, and in a critically ill population excluding patients with dementia.
Results: From 1994 to 2014, population-based rates of gastrostomy tube use in critically ill patients increased from 11.9 to 28.8 gastrostomies per 100,000 U.S. adults (peak in incidence in 2010), an increase of 142% (31,392–91,990 gastrostomy tubes in critically ill patients; P < 0.001). Patients receiving gastrostomy tubes during critical illness occupied a growing proportion of all gastrostomy tube placements, accounting for 19.6% of all gastrostomy tubes placed in 1994 and 50.8% in 2014. The rate of gastrostomies in critically ill patients remained roughly stable, from 2.5% of critically ill patients in 1994 to a peak of 3.7% in 2002 before declining again to 2.4% in 2014. Hospital length of stay and in-hospital mortality decreased among gastrostomy tube recipients (28.7 d to 20.5 d, P < 0.001; 25.9–11.3%, P < 0.001; respectively), whereas discharges to long-term facilities increased significantly (49.6–70.6%; P < 0.001). Sensitivity analyses among mechanically ventilated patients revealed similar increases in population-based estimates of gastrostomy tube placement.
Conclusions: The incidence of gastrostomy tube placement among critically ill patients more than doubled between 1994 and 2014, with most patients being discharged to long-term care facilities. Critically ill patients are now the primary utilizer of gastrostomy tubes placed in the United States. Additional research is needed to better characterize the long-term risk and benefits of gastrostomy tube use in critically ill patients.
Keywords: gastrostomy, critical illness, patient care planning
Improvements in the short-term mortality of critically ill patients have led to an increasing number of patients surviving to discharge with persistent, severe organ failure after critical illness. Patients faced with the prospect of prolonged life-supportive measures following critical illness often enter a state termed “chronic critical illness,” a condition frequently associated with rehospitalizations, high short-term mortality rates, and large financial burdens to patients, caretakers, and the healthcare system (1). For example, fewer than 10% of patients with chronic critical illness achieve functional independence at home, even 1 year after the inciting critical illness (2–4); 1-year mortality approaches 50% (1). However, the epidemiology of some interventions that are frequently used during or just before chronic critical illness, such as gastrostomy tube placement to deliver nutritional support, are understudied among the critically ill.
In contrast to critical illness, practice patterns and outcomes of gastrostomy tube placement during dementia have been well documented (5–7). In the 1990s, placement of gastrostomy tubes rose rapidly in elderly patients, doubling among patients with Alzheimer dementia from 1993 to 2003 (5–7). However, in the absence of a demonstrable benefit in the setting of dementia (and harms from local inflammation, infection, tube dislodgement, and concurrent use of restraints) the American Geriatrics Society issued guidelines recommending against the placement of gastrostomy tubes for provision of nutrition during advanced dementia in 1993, 2005, and 2014 (8–10). Gastrostomy tube placement in nursing home residents with advanced dementia subsequently declined (9), with “hand comfort-feeding” proposed as a potential humane alternative (11). Critically ill patients with significant preexisting frailty or severe new deficits may have prognoses similar to patients with advanced dementia (12), but the epidemiology, benefits, and harms of gastrostomy tube placement in the critically ill remain unclear.
To address these knowledge gaps, we sought to quantify national practice patterns in gastrostomy placement among the critically ill over the last two decades in the United States. We hypothesized that as the overall critically ill population grew (13) and gastrostomy tube placement in patients with dementia declined, that there would be a significant increase in both the population-based incidence of gastrostomy tube placement in the critically ill, and the proportion of all gastrostomy tubes represented by the critically ill, over the last 20 years.
Methods
Study Design
We used data from the National Inpatient Sample and the Nationwide Inpatient Sample, Healthcare Cost and Utilization Project, Agency for Healthcare Research and Quality, from 1994 to 2014 (14, 15). The National Inpatient Sample is a 20% stratified sample of all nonfederal U.S. acute care hospitalizations that contains deidentified administrative claims data from 5 to 8 million hospital discharges per year. It is the largest publicly available all-payer inpatient health care database in the United States, and represents more than 96% of the U.S. population (14).
We identified adults (≥18 yr of age) with a hospitalization that included presence of any one of a list of critical illness diagnosis and procedure codes from the International Classification of Diseases, Ninth Revision (ICD-9-CM) (see the online supplement); these patients are hereafter referred to as our critically ill population (16, 17). This algorithm has been used in prior literature to define critical illness as an alternative to simple admission to an ICU, (18–20), which can be influenced by local practice variation and ICU bed availability. We identified gastrostomies with previously validated (21) ICD-9-CM codes 43.1 (gastrostomy), 43.11 (percutaneous gastrostomy), 43.19 (other gastrostomy), and 44.32 (percutaneous gastrojejunostomy) (22–24). Patients that may have received gastrostomies for anatomic reasons unrelated to critical illness were excluded (i.e., face, head, neck, esophageal conditions) (see online supplement). We used the Healthcare Cost and Utilization Project procedure classification system to identify surgical patients (25). Patients receiving a Major Therapeutic ICD-9-CM procedure code (as opposed to Minor Diagnostic, Minor Therapeutic, and Major Diagnostic), excluding gastrostomy tube or tracheostomy (ICD-9-CM codes 31.1, 31.2, 31.21, 31.29), were considered “surgical” admissions; all others were considered “nonsurgical.” Comorbid conditions were identified in hospital discharge records using Elixhauser Comorbidity Software (26, 27); comorbidities were also indexed into readmission and mortality scores as a proxy for severity of comorbid illness (28).
Our primary outcome was annual population-standardized rates of gastrostomy placement among critically ill patients. Secondary analyses included annual rates of gastrostomy placement among all patients, trends in severity of illness, and trends in hospital mortality and discharge location. In the event that coding sensitivity for critical illness diagnoses may have changed over time, we conducted a sensitivity analysis where annual population-standardized rates of gastrostomy placement among mechanically ventilated (MV) patients (ICD-9-CM code 96.7x) were determined.
Sensitivity Analyses
We performed three sensitivity and subgroup analyses. First, In the event that coding sensitivity for critical illness diagnoses may have changed over time or that using ICD-9-CM codes to define critical illness may lead to misclassification, we conducted a sensitivity analysis where annual population-standardized rates of gastrostomy placement among MV patients (ICD-9-CM code 96.7x) were determined.
Second, to evaluate the impact of a decrease in the competing risk of hospital death on gastrostomy tube use with time, we conducted a sensitivity analysis to compare the proportion of survivors and decedents receiving gastrostomy tubes over time.
Finally, because trends in gastrostomy tubes placed during critical illness may be affected by secular trends in patients receiving gastrostomy tubes for dementia, we performed a sensitivity analysis whereby patients with dementia diagnoses were excluded. We identified patients with dementia using ICD-9-CM codes validated among critically ill patients (29); population-standardized rates of gastrostomy tube use were recalculated with this population excluded (see online supplement).
Statistical Analysis
We derived national estimates using survey-weighted methods and used U.S. Census Bureau yearly population estimates to determine population-standardized rates of gastrostomy tube placement per 100,000 U.S. adults. We tested for significant trends in gastrostomy tube placement among critically ill patients, MV patients, survivors and decedents of critical illness, and in a critically ill population excluding patients with dementia. We also tested for significant linear trends in patient and hospital characteristics over time among critically ill patients receiving gastrostomy tubes with Mantel-Haenszel chi-square tests (categorical variables) and linear regression (continuous variables).
All statistical testing was through SAS version 9.4 (SAS Institute), with two-tailed and performed with α = 0.05. This study was deemed exempt from review by the Beth Israel Deaconess Medical Center institutional review.
Results
Cohort Characteristics
Among 50,063,564 (10,425,023 unweighted) hospitalizations with critical illness from 1994 to 2014, a total of 1,465,956 (309,040 unweighted; 2.9% of critically ill patients) received a gastrostomy tube. During this period, 44.7% of critically ill patients receiving gastrostomy tubes were female, 54.9% were white, and the mean age of the group was 67.6 years (standard error, 0.14).
Temporal Trends in Gastrostomy Use
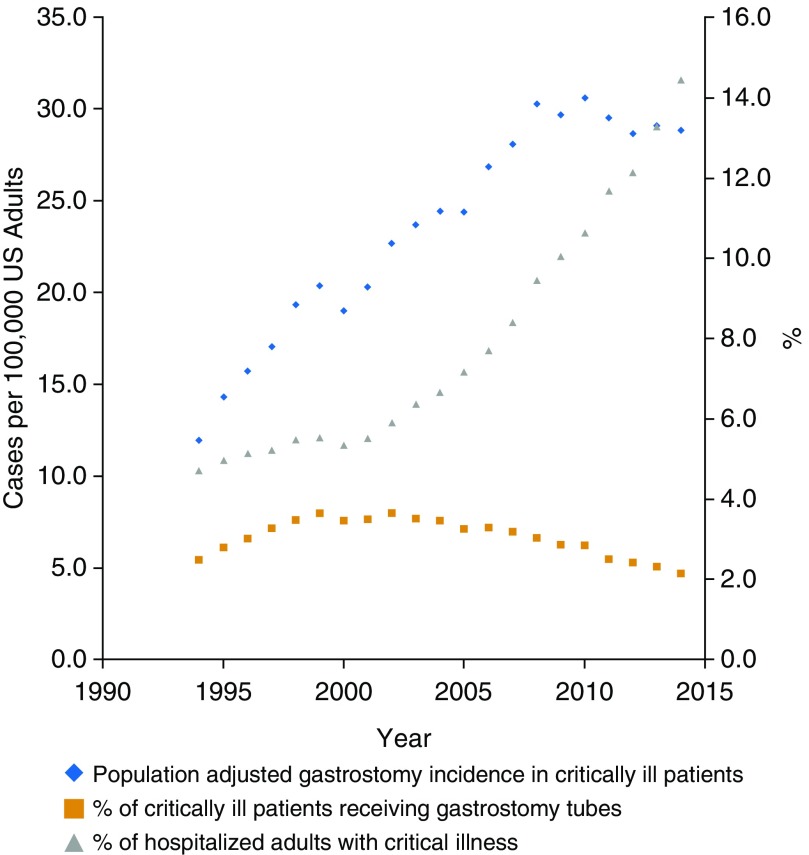
From 1994 to 2014, population-based rates of gastrostomy use in critically ill patients increased from 11.9 to 28.8 gastrostomies per 100,000 U.S. adults (with a peak of 30.6 per 100,000 adults in 2010), an overall increase of 142% (31,392 gastrostomy tubes in critically ill patients in 1994 to 91,990 in 2014; P < 0.001). This roughly parallels the rise in the critically ill population itself, which continuously rose by 180%, from 480.1 hospitalizations with critical illness in 1994 to 1,346.5 in 2014 per 100,000 U.S. adults (1,263,340 hospitalizations with critical illness in 1994 to 4,293,521 in 2014; P < 0.001). Among the population of critically ill patients, the rate of gastrostomy use remained roughly stable, from 2.5% of critically ill in 1994 to a peak of 3.7% in 2002 before declining again to 2.4% in 2014 (Table 1, Figure 1).
Table 1.
Characteristics of critically ill patients receiving gastrostomy tubes in select years
| 1994 (n = 31,392) | 1999 (n = 56,802) | 2004 (n = 71,522) | 2009 (n = 91,004) | 2014 (n = 91,990) | P Value for Trend | |
|---|---|---|---|---|---|---|
| Gastrostomies/100,000 U.S. population | 11.9 | 20.4 | 24.4 | 29.7 | 28.8 | <0.001 |
| % of CI receiving gastrostomy (n of Cl patients) | 2.5 (1,263,340) | 3.6 (1,557,447) | 3.5 (2,061,987) | 2.9 (3,179,122) | 2.1 (4,293,521) | <0.001 |
| Demographic factors | ||||||
| Female, % | 45.8 | 46.5 | 44.6 | 43.9 | 43.3 | <0.001 |
| Age | <0.001 | |||||
| Mean (SE), yr | 68.3 (0.5) | 69.0 (0.4) | 67.4 (0.4) | 66.9 (0.3) | 66.2 (0.2) | |
| Age <65, % | 31.4 | 31.1 | 36.2 | 39.5 | 41.1 | |
| Age ≥65 and <85, % | 53.7 | 53.0 | 50.2 | 47.3 | 46.6 | |
| Age ≥85, % | 14.9 | 15.8 | 13.6 | 13.2 | 12.2 | |
| Race, % | <0.001 | |||||
| White | 61.8 | 55.1 | 47.7 | 54.5 | 60.1 | |
| Black | 14.6 | 15.2 | 16.1 | 16.0 | 19.3 | |
| Hispanic | 6.6 | 5.3 | 7.3 | 8.9 | 9.3 | |
| Other | 2.5 | 4.7 | 4.6 | 7.8 | 7.3 | |
| Missing | 14.5 | 19.8 | 24.3 | 12.8 | 4.0 | |
| Primary payer, % | <0.001 | |||||
| Medicare | 65.9 | 65.9 | 64.4 | 62.9 | 63.2 | |
| Medicaid | 9.2 | 10.2 | 10.9 | 13.1 | 14.6 | |
| Private insurance | 19.0 | 19.1 | 19.5 | 18.4 | 17.0 | |
| Self-pay | 3.1 | 2.1 | 2.7 | 2.9 | 2.6 | |
| Other, includes missing | 2.4 | 4.1 | 4.9 | 6.5 | 6.1 | |
| Median income for patient zip code, % | <0.001 | |||||
| Income quartile 1 | 29.9 | n/a | 31.3 | 30.0 | 32.9 | |
| Income quartile 2 | 20.4 | n/a | 25.3 | 24.0 | 25.8 | |
| Income quartile 3 | 15.4 | n/a | 20.8 | 23.4 | 21.5 | |
| Income quartile 4 | 30.2 | n/a | 20.2 | 19.4 | 17.4 | |
| Missing | 4.0 | n/a | 2.4 | 3.1 | 2.4 | |
| Hospital location and teaching status, % | <0.001 | |||||
| Rural | 7.5 | 5.6 | 5.0 | 5.2 | 4.0 | |
| Urban nonteaching | 43.4 | 42.7 | 41.1 | 39.9 | 24.2 | |
| Urban teaching | 49.0 | 51.6 | 53.9 | 52.5 | 71.7 | |
| Hospital size, % | <0.001 | |||||
| Small | 9.0 | 8.3 | 8.3 | 7.1 | 13.1 | |
| Medium | 29.5 | 27.3 | 23.1 | 21.9 | 28.0 | |
| Large | 61.4 | 64.3 | 68.6 | 68.6 | 58.9 | |
| Hospital region, % | <0.001 | |||||
| Northeast | 22.6 | 22.7 | 23.6 | 19.8 | 17.3 | |
| Midwest | 14.8 | 20.5 | 24.2 | 22.2 | 22.0 | |
| South | 45.9 | 41.0 | 36.7 | 37.1 | 42.4 | |
| West | 16.7 | 15.8 | 15.5 | 20.9 | 18.3 | |
| Clinical factors | ||||||
| Elixhauser comorbidities, mean (SE) | ||||||
| Number | 2.4 (0.04) | 2.6 (0.04) | 2.9 (0.05) | 3.2 (0.03) | 3.4 (0.02) | <0.001 |
| Mortality score | 12.5 (0.3) | 12.7 (0.2) | 14.3 (0.3) | 15 (0.2) | 16.7 (0.1) | <0.001 |
| Readmission score | 18.7 (0.4) | 19.7 (0.3) | 22.1 (0.4) | 23.6 (0.2) | 24.3 (0.1) | <0.001 |
| Length of stay, median (IQR) | 28.7 (32.5) | 24.3 (23.6) | 24.4 (22.4) | 22.0 (20.0) | 20.5 (17.6) | <0.001 |
| Type of critical illness, % | ||||||
| Severe sepsis | 29.9 | 34.0 | 42.2 | 48.9 | 49.2 | <0.001 |
| Shock | 7.6 | 8.8 | 13.9 | 21.7 | 27.4 | |
| Shock no trauma | 6.8 | 8.0 | 13.0 | 20.7 | 25.9 | <0.001 |
| Trauma shock | 0.4 | 0.3 | 0.7 | 0.6 | 0.9 | <0.001 |
| Postoperative shock | 0.4 | 0.5 | 0.3 | 0.6 | 0.9 | <0.001 |
| Acute respiratory failure | 55.9 | 63.0 | 65.2 | 67.5 | 69.6 | <0.001 |
| Hypotension | 10.0 | 9.1 | 8.3 | 10.0 | 11.1 | <0.001 |
| Arrest | 12.2 | 10.3 | 9.1 | 8.2 | 9.3 | <0.001 |
| Long MV | 50.9 | 55.0 | 57.0 | 57.6 | 55.6 | <0.001 |
| Surgical, % | 38.9 | 37.4 | 38.9 | 39.8 | 38.3 | 0.05 |
| Also received tracheostomy, % | 39.6 | 43.8 | 47.0 | 45.9 | 44.6 | <0.001 |
Definition of abbreviations: CI = critically ill; IQR = interquartile range; MV = mechanically ventilated; SE = standard error.
Figure 1.
Gastrostomy use rates in the critically ill in the United States, 1994–2014. (Left axis) Population-adjusted incidence of gastrostomy tubes among the critically ill (per 100,000 U.S. adults). (Right axis) Percent of hospitalized adults with critical illness (gray triangles), percent of adults with critical illness receiving a gastrostomy tube (orange squares).
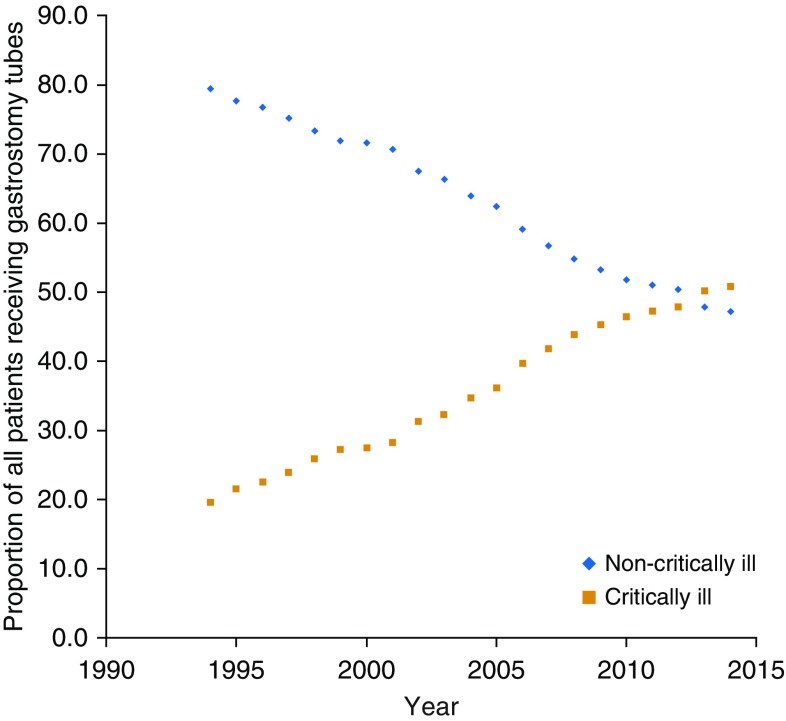
Patients receiving gastrostomy tubes during critical illness also occupied a growing proportion of the country’s total gastrostomy tube placements. In 1994, a total of 160,332 gastrostomy tubes were placed (61 per 100,000 U.S. adults); this peaked at 212,835 gastrostomy tubes in 2003 (73 per 100,000 U.S. adults), before decreasing back down to 181,250 in 2014 (57 per 100,000 U.S. adults). At the beginning of this 21-year period, most gastrostomy tube placements were in patients without critical illness (79.4%); the proportion of patients receiving gastrostomy use with critical illness gradually increased so that by 2014, patients with critical illness accounted for more than half of all gastrostomy tubes (from 19.6 to 50.8% of all gastrostomy tubes) (Figure 2).
Figure 2.
Proportion of gastrostomy tubes placed in critically ill and noncritically ill hospitalized patients, 1994–2014. Among all recipients of gastrostomy tube, the proportion of gastrostomy tubes placed in critically ill patients (orange squares) has steadily increased and ultimately superseded the proportion of gastrostomy tubes placed in noncritically ill (blue diamonds) over the last 20 years.
Comparing 2014 with 1994, critically ill patients receiving gastrostomy tubes on average were younger, (68.3 yr in 1994; 66.2 yr in 2014; P < 0.001), more likely to be treated at urban teaching hospitals (49.0–71.7% vs. rural and nonteaching hospitals; P < 0.001), on Medicaid (9.2–14.6%; P < 0.001), have a diagnosis of severe sepsis (29.9–49.2%; P < 0.001), receive a tracheostomy during the same hospitalization (39.6–44.6%; P < 0.001) and have a higher risk of readmission and in-hospital death (Elixhauser readmission and mortality index scores 18.7–24.3 [P < 0.001] and 12.5–16.7 [P < 0.001], respectively) (Table 1). There was not a significant change in the percent of critically ill gastrostomy tube recipients that were surgical patients (38.9–38.3%; P = 0.05) between 1994 and 2014.
Outcomes
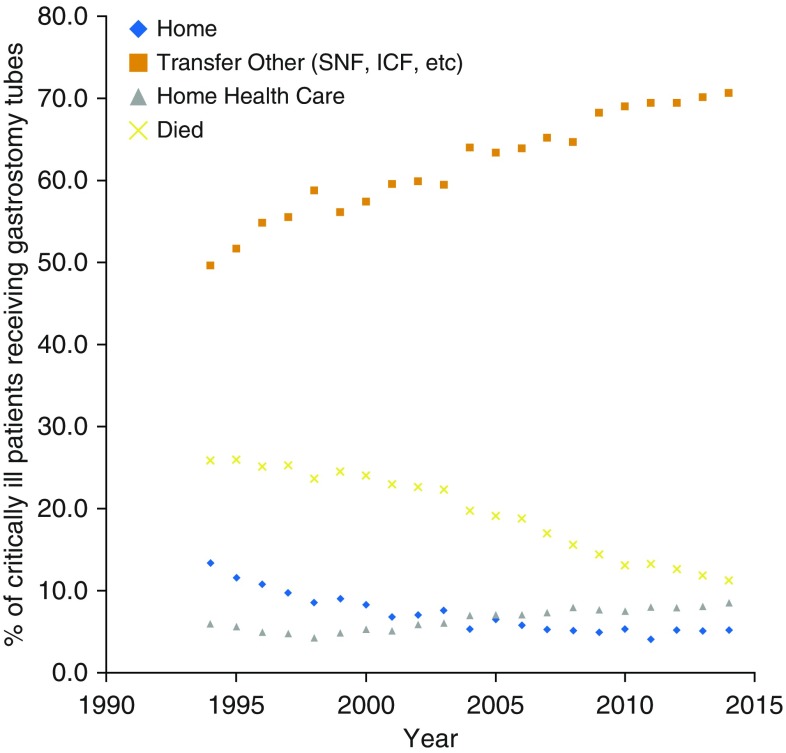
Hospital length of stay in critically ill recipients of gastrostomy tubes decreased from a median of 28.7 days (interquartile range, 32.5) in 1994 to 20.5 days (interquartile range, 17.6) in 2014 (P < 0.001). In-hospital mortality of gastrostomy tube recipients also decreased from 25.9% in 1994 to 11.3% in 2014 (P < 0.001). The decrease in hospital length of stay and in-hospital mortality was concurrent with a decrease in the number of discharges home (13.3% in 1994 to 5.2% in 2014; P < 0.001) and an increase in the number of discharges to long-term facilities including skilled nursing facilities and long-term care hospitals (49.6% in 1994 to 70.6% in 2014; P < 0.001) (Figure 3).
Figure 3.
Outcomes of critically ill patients receiving gastrostomies, 1994–2014. Over time, fewer patients died in the hospital (yellow x) or were discharged home (blue diamonds), but more patients were transferred to long-term facilities (orange squares). ICF = Intermediate Care Facility; SNF = Skilled Nursing Facility.
Sensitivity Analysis
In the event that changing coding patterns for critical illness may unduly affect our estimates of trends in gastrostomy tube incidence among critically ill patients, we investigated the incidence of gastrostomy tube placement among MV patients. From 1994 to 2014, population-based estimates of gastrostomy tube placement among MV patients similarly increased from 8.2 to 20.1 gastrostomies per 100,000 U.S. adults (with a peak of 21.5 per 100,000 adults in 2010), an overall increase of 145% (21,634 gastrostomy tubes in 1994 to 64,020 in 2014 among MV; P < 0.001). During this time, the MV population grew by 45%, from 214.8 hospitalization in 1994 to 311.5 hospitalization in 2014 per 100,000 U.S. adults (563,287 hospitalizations with MV in 1994 to 993,174 in 2014; P < 0.001). The rate of gastrostomy use in MV patients increased from 3.8% of MV in 1994 to 7.2% in 2008 before declining to 6.4% in 2014 (see Figure E1 in the online supplement). The proportion of gastrostomy tubes accounted for by MV patients increased with time, from 13.5% in 1994 to 35.3% in 2014, with a corresponding decline in the proportion of gastrostomy tubes accounted for by patients not receiving MV (see Figure E2).
In the event that changing rates of hospital deaths may have an impact on trends in gastrostomy tube use among critically ill patients, we compared the incidence gastrostomy tube placement among survivors and decedents of critical illness over time. From 1994 to 2014 population-based estimates of gastrostomy tube our results show very similar trends among the two groups, with survivors and decedents receiving gastrostomy tubes at approximately the same rate every year (2.6% and 2.2%, respectively, in 1994; a peak of 3.7% and 3.4% in 2002, and 2.2% and 2.0% in 2014) (see Figure E3).
Because trends in gastrostomy tube placement among critically ill patients may be affected by trends in gastrostomy tubes placed in patients with dementia, we assessed trends in gastrostomy tube placement excluding patients with dementia. Results were not substantively changed after excluding patients with dementia (see Figures E4 and E5).
Discussion
We performed an analysis of the trends in gastrostomy tube placement in the critically ill in the United States from 1994 to 2014. Overall, the incidence of gastrostomy tube placement among critically ill patients rose sharply during this period, in part caused by a large rise over that time period of critically ill patients in the U.S. population. Our sensitivity analysis of MV patients reveals that the observed increase in incidence of gastrostomy tube use is not wholly caused by changes in coding patterns of the critically ill. Among MV patients (which increased only moderately between 1994 and 2014), there remained a significant rise in both the percent of MV patients who received gastrostomy tubes (peaking in 2008) and the overall incidence of gastrostomy tubes among MV patients nationwide (peaking in 2010). As a result, the proportion of gastrostomy tubes placed in critically ill patients has steadily increased and ultimately superseded the proportion of gastrostomy tubes placed in noncritically ill over the last 20 years.
Although fewer than half of patients receiving gastrostomy tubes also received a tracheostomy, indicating that these two procedures were frequently performed independently rather than as a package, we found some similarities in trends. In critically ill recipients of gastrostomy tubes, incidence peaked in 2010, and we observed shifts in the demographics toward younger, male, and Medicaid patients; we also found shorter hospital length of stay, more frequent discharges to long-term care facilities, and fewer discharges from hospital to home in 2014 as compared with 1994. Prior literature has shown that incidence of tracheostomy placement peaked in 2008, and shifted toward the same demographics and hospital outcomes over time (30). Decreases in hospital length of stay and in hospital mortality with increases in discharge to long-term acute care hospitals also echo trends seen in many other epidemiologic studies of critical illness. Other authors have suggested this trend reflects a shift in mortality from the hospital to long-term acute care hospitals, rather than a true improvement in outcomes (30, 31). Our findings highlight the importance of future studies tracking patient outcomes beyond the initial hospitalization, because much of these patients’ morbidity and mortality has more recently shifted away from the acute care setting.
Several factors likely contributed to the observed rise in the incidence of gastrostomy among the critically ill over these 20 years. Although gastrostomy tubes have traditionally been placed surgically, the introduction of the percutaneous technique in the 1980s (32), which allowed for less-invasive placement under sedation, without general anesthesia, caused a substantial increase in the use of gastrostomy tubes for general indications. In the United States between 1988 and 1995, the number of gastrostomies placed in adults older than the age of 65 doubled from 61,000 to 121,000 (6, 7, 24), with gastrostomy placement further increasing by 38% among adults older than the age of 65 from 1993 to 2003 (5). The increased availability and use of percutaneous placement, combined with a growing number of critical care beds (33), likely contributed to the precipitous rise in gastrostomies among the critically ill up until the late 2000s. The role of other potential drivers, such as a desire for shorter hospital length of stay or for ease of placement in longer-term facilities, deserves exploration. For context, the absolute number of gastrostomy tubes we found placed in the critically ill in 2014 (a national estimate of almost 92,000 patients) exceeds the absolute number placed among U.S. hospitalized nursing home residents with advanced dementia, even at the peak in practice, by at least fivefold (on the order of ∼15,000 gastrostomy tubes in the early 2000s) (34).
Reasons for the subsequent decrease in gastrostomy incidence among both the critically ill and MV subgroups, even in the face of continued increases in the critically ill and MV populations, are less clear. The roughly contemporaneous downward trend in tracheostomy placements raises several possible hypotheses. Tracheostomies and gastrostomies may have simply decreased in parallel, because the increased use of advance directives (35) and greater awareness of long-term outcomes of chronic critical illness (1, 36, 37) may have led more families and patients to pursue more comfort-based care. It is also possible that to some degree the decrease in gastrostomy use is actually secondary to the decreased need for tracheostomies, because improved practices in sedative use, ventilator weaning (38), and a trend away from early tracheostomies (30) have led to fewer patients to be eligible for a “trach-and-peg” bundle of care. Additionally, it is possible that decreased enthusiasm for gastrostomy tubes among patients with dementia has diffused into the critically ill population, such that gastrostomy tube placement is deferred longer (e.g., until after ventilator weaning trajectory is established in tracheostomy patients, or more broadly, until after additional speech and swallow therapy is attempted).
Our sensitivity analysis among survivors and decedents of critical illness suggests that death does not represent a strong competing risk to gastrostomy placement; decedents and survivors had similar rates and trends in gastrostomy use. The similar rates of tube placement among survivors and decedents also suggests that the ability to predict whether a patient will be a survivor or decedent after gastrostomy has not improved with time, and that future research is needed to determine which patients are likely to benefit from a procedure intended to provide long-term nutrition.
Our study has several limitations. We relied on the use of ICD-9-CM and DRG coding to define both our patient cohorts (the critically ill and the MV) and our key outcome of interest, gastrostomy tube placement. This makes the estimates of our baseline population, critically ill patients, vulnerable to changes in coding practices. However, coding practices for MV were unlikely to be as vulnerable, because MV was associated with high levels of reimbursement throughout the study periods, and our key finding of a sharp increase in gastrostomy tube incidence was confirmed in this population. We also conducted a preliminary validation of gastrostomy tubes, and confirmed high positive and negative predictive value of our codes. Similarly, the algorithm we used to identify critical illness by ICD-9-CM codes has previously been published (16) but not been validated, and there is risk of misclassification of our exposure. The test characteristics of our criteria to identify critically ill patients (including sensitivity, specificity, and negative and positive predictive values) and its performance over time between are not known. The National Inpatient Sample does not identify admissions to an intensive care unit and a gold standard definition of what constitutes “critical illness” does not otherwise exist. This is a known obstacle of the current state of health services research of the ICU population (39). However, our criteria identify severe diagnoses that are likely to be among top-billed diagnoses at discharge, and identifying acutely critically ill patients by objective diagnoses may be a more consistent method of identifying our population than simply ICU admission, because thresholds for ICU admission vary greatly from hospital to hospital.
In summary, we found a significant growth in the population of critically ill patients receiving gastrostomy tubes in the United States over a 20-year period, most of whom are now being discharged to long-term facilities. Although the rate of critically ill patients receiving gastrostomy tubes has more or less remained stable, indicating no major differences in practice patterns, the sharp increase in gastrostomy utilization in the critically ill echoes the sharp growth previously seen in patients with advanced dementia, ultimately far surpassing the number of gastrostomy tubes ever placed annually in patients with advanced dementia. Whether this increase is driven by patient values, clinical uncertainty, or by a desire for shorter hospital length of stay of ease of placement in longer-term facilities, remains uncertain. After gastrostomy tubes were shown to offer limited benefit but greater harms to patients with advanced dementia (perhaps in part because of the irreversibility of their underlying need for gastrostomy feeds), the bulk of gastrostomy patients has since been supplanted by a new population, with as-yet unidentified outcomes. Given that many patients within this critically ill population may go on to become chronically critically ill with high resource utilization and similarly poor prognoses as patients with advanced dementia (because of preexisting poor health states in combination with new significant insults), our findings highlight the importance of recapitulating the analysis of benefits of gastrostomy use in the population that is now the primary utilizer of gastrostomy tubes. Future studies delineating the benefits, morbidity, long-term mortality, and associated predictors within the subgroups of critically ill population are critical so that we may refine our practices accordingly.
Supplementary Material
Footnotes
Supported by National Institute on Aging 1F32AG058352 (A.C.L.), Agency for Healthcare Research and Quality 5K08HS024288 (J.P.S.), Doris Duke Charitable Foundation (J.P.S.), National Heart Lung Blood Institute 1R01HL136660 and 1R01HL139751 (A.J.W.), and Boston University School of Medicine Department of Medicine Career Investment Award (A.J.W.).
Author Contributions: Literature search, A.C.L. Study design, A.C.L., J.P.S., and A.J.W. Data analysis, A.C.L. Data interpretation, all authors. Writing, reviewing, and final approval of the manuscript, all authors.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Kahn JM, Le T, Angus DC, Cox CE, Hough CL, White DB, et al. ProVent Study Group Investigators. The epidemiology of chronic critical illness in the United States*. Crit Care Med. 2015;43:282–287. doi: 10.1097/CCM.0000000000000710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cox CE, Carson SS, Lindquist JH, Olsen MK, Govert JA, Chelluri L Quality of Life After Mechanical Ventilation in the Aged (QOL-MV) Investigators. Differences in one-year health outcomes and resource utilization by definition of prolonged mechanical ventilation: a prospective cohort study. Crit Care. 2007;11:R9. doi: 10.1186/cc5667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scheinhorn DJ, Hassenpflug MS, Votto JJ, Chao DC, Epstein SK, Doig GS, et al. Ventilation Outcomes Study Group. Post-ICU mechanical ventilation at 23 long-term care hospitals: a multicenter outcomes study. Chest. 2007;131:85–93. doi: 10.1378/chest.06-1081. [DOI] [PubMed] [Google Scholar]
- 4.Stoller JK, Xu M, Mascha E, Rice R. Long-term outcomes for patients discharged from a long-term hospital-based weaning unit. Chest. 2003;124:1892–1899. doi: 10.1378/chest.124.5.1892. [DOI] [PubMed] [Google Scholar]
- 5.Mendiratta P, Tilford JM, Prodhan P, Curseen K, Azhar G, Wei JY. Trends in percutaneous endoscopic gastrostomy placement in the elderly from 1993 to 2003. Am J Alzheimers Dis Other Demen. 2012;27:609–613. doi: 10.1177/1533317512460563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Graves EJ. Detailed diagnoses and procedures, National Hospital Discharge Survey, 1988. Vital Health Stat 13. 1991;107:1–239. [PubMed] [Google Scholar]
- 7.Graves EJGB, Gillum BS. Detailed diagnoses and procedures, National Hospital Discharge Survey, 1995. Vital Health Stat 13. 1997;130:1–146. [PubMed] [Google Scholar]
- 8.Sampson EL, Candy B, Jones L. Enteral tube feeding for older people with advanced dementia. Cochrane Database Syst Rev. 2009;2:CD007209. doi: 10.1002/14651858.CD007209.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mitchell SL, Mor V, Gozalo PL, Servadio JL, Teno JM. Tube feeding in US nursing home residents with advanced dementia, 2000-2014. JAMA. 2016;316:769–770. doi: 10.1001/jama.2016.9374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li I. Feeding tubes in patients with severe dementia. Am Fam Physician. 2002;65:1605–1610. [PubMed] [Google Scholar]
- 11.Palecek EJ, Teno JM, Casarett DJ, Hanson LC, Rhodes RL, Mitchell SL. Comfort feeding only: a proposal to bring clarity to decision-making regarding difficulty with eating for persons with advanced dementia. J Am Geriatr Soc. 2010;58:580–584. doi: 10.1111/j.1532-5415.2010.02740.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brummel NE, Bell SP, Girard TD, Pandharipande PP, Jackson JC, Morandi A, et al. Frailty and subsequent disability and mortality among patients with critical illness. Am J Respir Crit Care Med. 2017;196:64–72. doi: 10.1164/rccm.201605-0939OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Halpern NA, Pastores SM. Critical care medicine in the United States 2000-2005: an analysis of bed numbers, occupancy rates, payer mix, and costs. Crit Care Med. 2010;38:65–71. doi: 10.1097/CCM.0b013e3181b090d0. [DOI] [PubMed] [Google Scholar]
- 14.HCUP National Inpatient Sample (NIS) Healthcare Cost and Utilization Project (HCUP). 2012–2014. Rockville, MD: Agency for Healthcare Research and Quality; 2018 [accessed 2017 Jul 1]. Available from: www.hcup-us.ahrq.gov/nisoverview.jsp.
- 15.Nationwide Inpatient Sample HCUP. (NIS). Healthcare Cost and Utilization Project (HCUP). 1994–2011. Rockville, MD: Agency for Healthcare Research and Quality; 2018 [accessed 2017 Jul 1]. Available from: www.hcup-us.ahrq.gov/nisoverview.jsp.
- 16.Ehlenbach WJHC, Hough CL, Crane PK, Haneuse SJ, Carson SS, Curtis JR, et al. Association between acute care and critical illness hospitalization and cognitive function in older adults. JAMA. 2010;303:763–770. doi: 10.1001/jama.2010.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348:1546–1554. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- 18.Seymour CW, Kahn JM, Cooke CR, Watkins TR, Heckbert SR, Rea TD. Prediction of critical illness during out-of-hospital emergency care. JAMA. 2010;304:747–754. doi: 10.1001/jama.2010.1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seymour CW, Iwashyna TJ, Ehlenbach WJ, Wunsch H, Cooke CR. Hospital-level variation in the use of intensive care. Health Serv Res. 2012;47:2060–2080. doi: 10.1111/j.1475-6773.2012.01402.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cooke CR. Risk of death influences regional variation in intensive care unit admission rates among the elderly in the United States. PLoS One. 2016;11:e0166933. doi: 10.1371/journal.pone.0166933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Law AC, Walkey AJ. Trends in gastrostomy and tracheostomy utilization during critical illness, 2008–2015: a single, tertiary care center study. Presented at the American Thoracic Society Conference. 2017, Washington, DC, May 23, 2017.
- 22.Teno JM, Gozalo PL, Mitchell SL, Kuo S, Rhodes RL, Bynum JP, et al. Does feeding tube insertion and its timing improve survival? J Am Geriatr Soc. 2012;60:1918–1921. doi: 10.1111/j.1532-5415.2012.04148.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chang WK, Lin KT, Tsai CL, Chung CH, Chien WC, Lin CS. Trends regarding percutaneous endoscopic gastrostomy: a nationwide population-based study from 1997 to 2010. Medicine (Baltimore) 2016;95:e3910. doi: 10.1097/MD.0000000000003910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grant MDRM, Rudberg MA, Brody JA. Gastrostomy placement and mortality among hospitalized Medicare beneficiaries. JAMA. 1998;279:1973–1976. doi: 10.1001/jama.279.24.1973. [DOI] [PubMed] [Google Scholar]
- 25.HCUP Procedure Classes. Healthcare Cost and Utilization Project (HCUP). Rockville, MD: Agency for Healthcare Research and Quality; 2009 [accessed 2017 Jan 15]. Available from: http://www.hcup-us.ahrq.gov/toolssoftware/procedure/procedure.jsp.
- 26.HCUP Comorbidity Software. Healthcare Cost and Utilization Project (HCUP). Rockville, MD: Agency for Healthcare Research and Quality; 2008 [accessed 2017 Jan 15]. Available from: www.hcup-us.ahrq.gov/toolssoftware/comorbidity/comorbidity.jsp.
- 27.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 28.Moore BJ, White S, Washington R, Coenen N, Elixhauser A. Identifying increased risk of readmission and in-hospital mortality using hospital administrative data: the AHRQ Elixhauser Comorbidity Index. Med Care. 2017;55:698–705. doi: 10.1097/MLR.0000000000000735. [DOI] [PubMed] [Google Scholar]
- 29.Amra S, O’Horo JC, Singh TD, et al. Derivation and validation of the automated search algorithms to identify cognitive impairment and dementia in electronic health records. J Crit Care. 2017;37:202–205. doi: 10.1016/j.jcrc.2016.09.026. [DOI] [PubMed] [Google Scholar]
- 30.Mehta AB, Syeda SN, Bajpayee L, Cooke CR, Walkey AJ, Wiener RS. Trends in tracheostomy for mechanically ventilated patients in the United States, 1993-2012. Am J Respir Crit Care Med. 2015;192:446–454. doi: 10.1164/rccm.201502-0239OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rhee C, Dantes R, Epstein L, Murphy DJ, Seymour CW, Iwashyna TJ, et al. CDC Prevention Epicenter Program. Incidence and trends of sepsis in US hospitals using clinical vs claims data, 2009-2014. JAMA. 2017;318:1241–1249. doi: 10.1001/jama.2017.13836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gauderer MW, Ponsky JL, Izant RJ., Jr Gastrostomy without laparotomy: a percutaneous endoscopic technique. J Pediatr Surg. 1980;15:872–875. doi: 10.1016/s0022-3468(80)80296-x. [DOI] [PubMed] [Google Scholar]
- 33.Wallace DJ, Seymour CW, Kahn JM. Hospital-level changes in adult ICU bed supply in the United States. Crit Care Med. 2017;45:e67–e76. doi: 10.1097/CCM.0000000000002051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Teno JM, Mitchell SL, Gozalo PL, Dosa D, Hsu A, Intrator O, et al. Hospital characteristics associated with feeding tube placement in nursing home residents with advanced cognitive impairment. JAMA. 2010;303:544–550. doi: 10.1001/jama.2010.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Silveira MJ, Wiitala W, Piette J. Advance directive completion by elderly Americans: a decade of change. J Am Geriatr Soc. 2014;62:706–710. doi: 10.1111/jgs.12736. [DOI] [PubMed] [Google Scholar]
- 36.Nelson JE, Cox CE, Hope AA, Carson SS. Chronic critical illness. Am J Respir Crit Care Med. 2010;182:446–454. doi: 10.1164/rccm.201002-0210CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Iwashyna TJ, Cooke CR, Wunsch H, Kahn JM. Population burden of long-term survivorship after severe sepsis in older Americans. J Am Geriatr Soc. 2012;60:1070–1077. doi: 10.1111/j.1532-5415.2012.03989.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Girard TD, Kress JP, Fuchs BD, Thomason JW, Schweickert WD, Pun BT, et al. Efficacy and safety of a paired sedation and ventilator weaning protocol for mechanically ventilated patients in intensive care (Awakening and Breathing Controlled trial): a randomised controlled trial. Lancet. 2008;371:126–134. doi: 10.1016/S0140-6736(08)60105-1. [DOI] [PubMed] [Google Scholar]
- 39.Wunsch H, Harrison DA, Rowan K. Health services research in critical care using administrative data. J Crit Care. 2005;20:264–269. doi: 10.1016/j.jcrc.2005.08.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.