Abstract
Rationale: Cigarette smoke exposure is a risk factor for many lung diseases, and histologic studies suggest that tobacco-related vasoconstriction and vessel loss plays a role in the development of emphysema. However, it remains unclear how tobacco affects the pulmonary vasculature in general populations with a typical range of tobacco exposure, and whether these changes are detectable by radiographic methods.
Objectives: To determine whether tobacco exposure in a generally healthy population manifests as lower pulmonary blood vessel volumes and vascular pruning on imaging.
Methods: A total of 2,410 Framingham Heart Study participants with demographic data and smoking history underwent volumetric whole-lung computed tomography from 2008 to 2011. Automated algorithms calculated the total blood volume of all intrapulmonary vessels (TBV), smaller peripheral vessels (defined as cross-sectional area <5 mm2 [BV5]), and the relative fraction of small vessels (BV5/TBV). Tobacco exposure was assessed as smoking status, cumulative pack-years, and second-hand exposure. We constructed multivariable linear regression models to evaluate associations of cigarette exposure and pulmonary blood vessel volume measures, adjusting for demographic covariates, including age, sex, height, weight, education, occupation, and median neighborhood income.
Results: All metrics of tobacco exposure (including smoking status, pack-years, and second-hand exposure) were consistently associated with higher absolute pulmonary blood vessel volume, higher small vessel volume, and/or higher small vessel fraction. For example, ever-smokers had a 4.6 ml higher TBV (95% confidence interval [CI] = 2.9–6.3, P < 0.001), 2.1 ml higher BV5 (95% CI = 1.3–2.9, P < 0.001), and 0.28 percentage-point-higher BV5/TBV (95% CI = 0.03–0.52, P = 0.03) compared with never-smokers. These associations remained significant after adjustment for percent predicted forced expiratory volume in 1 second, cardiovascular comorbidities, and did not differ based on presence or absence of airflow obstruction.
Conclusions: Using computed tomographic imaging, we found that cigarette exposure was associated with higher pulmonary blood vessel volumes, especially in the smaller peripheral vessels. Although, histologically, tobacco-related vasculopathy is characterized by vessel narrowing and loss, our results suggest that radiographic vascular pruning may not be a surrogate of these pathologic changes.
Keywords: image analysis, epidemiology
Cigarette smoke is a major risk factor for many chronic lung diseases, including chronic obstructive pulmonary disease (COPD) (1), interstitial diseases (2, 3), and diffuse cystic lung disease (4). In addition, both animal models and small, human studies have shown pathologic effects of tobacco on pulmonary vessels, including endothelial dysfunction, smooth muscle proliferation, and intimal thickening (5–8), which lead to progressive vasoconstriction, vessel occlusion, and vascular “pruning” seen angiographically in smokers with COPD (9, 10). Tobacco-related vasculopathy may be central to the pathogenesis of emphysema in particular (11–13), and vascular abnormalities are frequently present in other lung diseases (14–16). However, the pulmonary vascular effects of cigarette smoke exposure have not been well studied in large populations of adults not selected on the basis of pre-existing lung disease, partly because detailed vascular assessment historically has required invasive angiography or tissue specimens (11). It remains unclear whether tobacco-related pulmonary vasculopathy occurs generally in all smokers, or only in the minority of smokers who go on to develop chronic lung disease.
Through computer-based image analysis of computed tomography (CT) scans, total volume of the pulmonary blood vessels and relative reductions of the smallest pulmonary vessels (a radiographic surrogate of vascular pruning) can be quantified noninvasively (17). No studies have used this technique to evaluate the effect of tobacco exposure on the pulmonary vasculature. In the MESA (Multi-Ethnic Study of Atherosclerosis) Study (18), a cohort not screened on the basis of smoking or lung disease, ever-smokers were found to have higher total pulmonary vascular volume on average compared with never-smokers, but, given that this finding was not a focus of the study, a formal statistical comparison with adjustment for potential confounders was not performed; in addition, MESA did not assess CT measures of vascular pruning. In COPDGene, heavy smokers and nonsmokers were observed to have similar total pulmonary blood vessel volumes in a univariate analysis, but smokers who had relatively less small vessel blood volume (i.e., more pruning) had lower forced expiratory volume in 1 second (FEV1) % predicted, oxygen saturation, and 6-minute walk distance (19). Although these studies suggest that lower total and peripheral pulmonary blood vessel volumes on CT may predict worse respiratory health, it remains unclear if CT-based assessment can detect the tobacco-related pulmonary vascular changes that are known to occur histologically.
To address this knowledge gap, we investigated associations of long-term cigarette smoke exposure with absolute and relative pulmonary blood vessel volumes in the Framingham Heart Study, a large, community-dwelling cohort without high burdens of smoking or lung disease. Based on histologic studies, we hypothesized that cigarette smoke exposure would be associated with lower total pulmonary blood vessel volumes and more severe vascular pruning on CT.
Methods
Study Sample
The study sample consists of participants of the Framingham Heart Study Offspring and Third Generation cohorts, described previously (20, 21). From 2008 to 2011, 2,764 participants underwent volumetric chest CT scans as part of the Multi-Detector Computed Tomography 2 substudy, with the primary goal of assessing coronary artery calcification (22–24). A total of 2,484 participants had image data satisfactory for measuring vascular morphology; those without demographic data or information on smoking status were excluded, leaving 2,410 participants in the analysis. Most participants attended Framingham Offspring Exam 8 (2005–2008) and Third Generation Exam 2 (2008–2011), except 9 participants (8 from Offspring) who attended Offspring Exam 7 (1998–2001) or Third Generation Exam 1 (2002–2005). This study was conducted in accordance with the World Medical Association Declaration of Helsinki. The Institutional Review Boards of the Beth Israel Deaconess Medical Center and Boston University Medical Center approved this study. All participants provided written consent.
Radiographic Pulmonary Vascular Assessment
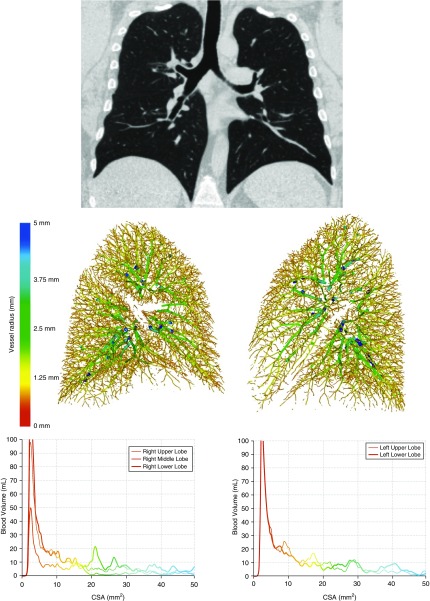
Inspiratory, noncontrast, whole-lung CT scans were performed in the supine position using a 64-detector-row scanner (Discovery; GE Healthcare), with uniform acquisition and reconstruction protocols. Scan parameters were 120 kVp, 300–350 mA (based on body weight), 350-ms rotation time, and 0.625-mm slice thickness. Image analysis was performed in the Applied Chest Imaging Laboratory at Brigham and Women’s Hospital using software based on the Chest Imaging Platform (www.chestimagingplatform.org), as previously described (19). An automated algorithm generated three-dimensional reconstructions of the pulmonary vasculature from which the volume of vessels of varying cross-sectional area were calculated (Figure 1), including the total blood volume of all intraparenchymal vessels (TBV) and of small vessels (defined as cross-sectional area < 5 mm2 [BV5]). The measures generated by this algorithm reflect the volume of the entire vessel, including the vascular wall and lumen; both arteries and veins are included. The small vessel fraction (BV5/TBV), representing the relative distribution of blood vessel volume in the smallest, most peripheral blood vessels detectable by CT, was calculated as a radiographic measure of pulmonary vascular pruning (19, 25).
Figure 1.
Top panel: coronal chest computed tomography of a healthy nonsmoker. Bottom panels: example volumetric vascular reconstruction and quantitative histogram demonstrating distribution of blood volume as a function of cross-sectional area (CSA). Image adapted from original to display vascular reconstructions of normal lungs. Adapted by permission from Reference 19.
Statistical Analysis
Multivariable linear regression models were used to examine associations of cigarette exposure with radiographic vascular measures (TBV, BV5, and BV5/TBV). Smoke exposure was assessed via multiple metrics, including smoking status (life-long never-smokers vs. ever-smokers, which included both current and former smokers), cumulative pack-years of exposure, and any second-hand smoke exposure in the home during adulthood. Due to expected collinearity, we first assessed the main effects of each of these exposure metrics individually and also ran models additionally adjusting for the other metrics. We tested for the linear component of trend for the associations of never-, former, and current smoking status with blood vessel volumes. For second-hand exposure, we performed a sensitivity analysis restricted to never-smokers. We evaluated departures from linearity for the association of total pack-years and radiographic vascular measures by plotting penalized splines with generalized additive models with a Gaussian distribution, and used the likelihood ratio test to compare the nested models. Models included potential confounders and predictors of outcome based on known or suspected associations with tobacco use, thoracic size, and/or abnormalities of pulmonary vessels; these included age at time of CT, sex, height, weight, personal educational attainment (26), occupation (laborer, sales/clerical, professional/technical, executive/supervisory, or other) (27), quartiles of census tract median neighborhood income (using home address at visit date and year 2000 census data) (27), and cohort identifier. We did not adjust for race/ethnicity because nearly all participants are of European ancestry (28).
In secondary analyses, we assessed whether associations between tobacco and radiographic pulmonary blood vessel volumes were explained by differences in lung function, given the known association of tobacco and pulmonary impairment, by adjusting for FEV1 % predicted and other lung volumes. In addition, we tested for differential associations of smoking with blood vessel volumes by sex and the presence of airflow limitation (defined as a fixed ratio of post-bronchodilator FEV1 to forced vital capacity [FVC] < 0.70 [29]) and moderate-to-severe airflow obstruction (defined by FEV1/FVC < 0.70 and FEV1 < 80% predicted) by adding interaction terms to our models. To determine whether radiographic assessment of pulmonary vessel volumes was affected by cardiac dysfunction, we performed additional adjustments for cardiovascular disease (defined as any cardiovascular disease-related event, including myocardial infarction, angina pectoris, congestive heart failure, cerebrovascular accident, or death due to cardiovascular disease or stroke), diabetes mellitus (defined as a fasting glucose > 126 mg/dl, any diabetic medication use, or any reported history of diabetes, excluding gestational diabetes), any antihypertensive medication use, or statin use (30). Finally, we constructed secondary models to adjust for a measure of physical inactivity (shown to predict incident heart and lung disease [31, 32]) as another possible confounder of the association of tobacco with pulmonary vascular measures. A physical activity index was calculated from a validated questionnaire of daily activities as described in prior Framingham Heart Study publications (33, 34). Regression coefficients were reported with 95% confidence intervals (CIs). For interaction terms, a threshold P value of less than 0.10 for the Wald test was used as statistically significant evidence of effect modification. All analyses were performed using SAS 9.4 (SAS Institute, Inc.), and penalized spline plots were produced using R version 3.5.1 (R Foundation for Statistical Computing).
Results
Study Participant Characteristics
Details of the study participants are provided in Table 1. The mean age (± standard deviation) of the cohort was 59.1 (±11.7) years, and 51.1% were female. Nearly one-half of the participants (48.5%) were never-smokers, whereas 44.2% were former smokers and 7.4% were current smokers at the time of the visit. Former and current smokers reported an average of 16.9 (±16.7) and 32.7 (±15.5) pack-years, respectively, whereas roughly one-quarter (25.7%) of study participants reported second-hand smoke exposure during adulthood. Mean FEV1 % predicted was normal (97.9 ± 15.2%); 480 participants (21.2%) had airflow limitation on spirometry, and of those, 167 (7.3% of the entire cohort) met criteria for moderate-to-severe obstruction. Mean total (TBV) and small blood vessel volumes (BV5) were 143.0 (±30.8) ml and 55.9 (±11.5) ml, respectively, and the small vessel fraction (BV5/TBV) was 39.3 (±4.1)%. Compared with the rest of the cohort, participants who met criteria for moderate-to-severe airflow obstruction had similar total blood vessel volumes, but lower small vessel volume and lower small vessel fraction (see Table E2 in the online supplement). Details regarding the distribution of cardiovascular disease and related comorbidities are shown in Table E3.
Table 1.
Characteristics of study participants (n = 2,410)
| Characteristics | Values |
|---|---|
| Age, yr, mean ± SD | 59.1 ± 11.7 |
| Female, n (%) | 1,232 (51.1) |
| Body mass index, kg/m2, mean ± SD | 28.4 ± 5.4 |
| Occupation category, n (%) | |
| Laborer | 192 (8.0) |
| Sales/clerical | 617 (25.6) |
| Professional/executive/supervisory/technical | 990 (41.1) |
| Other | 611 (25.4) |
| Educational attainment, n (%) | |
| High school or less | 513 (21.3) |
| Some college | 764 (31.7) |
| College/grad school | 1,133 (47.0) |
| Median census tract income, $, median (IQR) | 83,101 (36,754) |
| Offspring cohort, n (%) | 1,114 (46.2) |
| Physical activity index, point, mean ± SD | 36.0 ± 6.3 |
| Smoking status, n (%) | |
| Never | 1,168 (48.5) |
| Former | 1,064 (44.2) |
| Current | 178 (7.4) |
| Pack-years of smoking, mean ± SD | |
| Entire sample | 9.8 ± 15.7 |
| Former smokers | 16.9 ± 16.7 |
| Current smokers | 32.7 ± 15.5 |
| Second-hand smoke at home as adult, n (%) | 617 (25.7) |
| Airflow limitation (FEV1/FVC < 0.7), n (%) | 482 (21.2) |
| Moderate-to-severe obstruction (FEV1/FVC < 0.7 and FEV1 < 80% predicted), n (%) | 167 (7.3) |
| FEV1 % predicted, mean ± SD | 97.9 ± 15.2 |
| FVC % predicted, mean ± SD | 101.8 ± 13.3 |
| Radiographic pulmonary vascular measures | |
| TBV, ml, mean ± SD | 143.0 ± 30.8 |
| BV5, ml, mean ± SD | 55.9 ± 11.5 |
| BV5/TBV, %, mean ± SD | 39.3 ± 4.1 |
Definition of abbreviations: BV5 = blood volume of smaller peripheral vessels, defined as cross-sectional area <5 mm2; FEV1 = forced expiratory volume in 1 second; FVC = forced vital capacity; IQR = interquartile range; SD = standard deviation; TBV = total blood volume.
Associations with Radiographic Vascular Outcomes
Associations of preselected covariates with radiographic pulmonary vascular measures are presented in Table E1. Greater age, lower height, and greater weight were each significantly associated with lower TBV, BV5, and BV5/TBV. Males had higher TBV, but lower BV5/TBV compared with females.
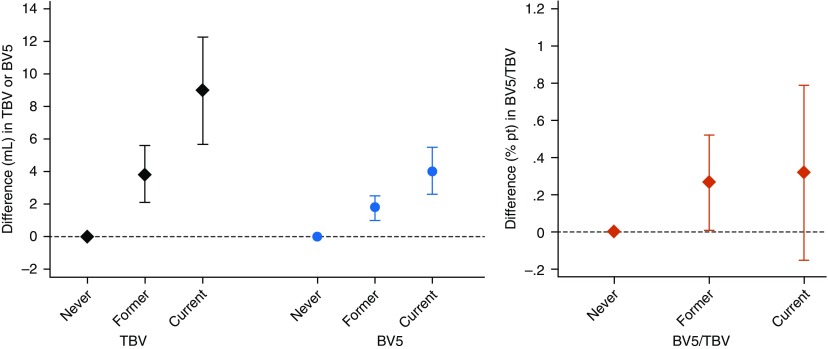
Associations of cigarette smoke exposure with pulmonary blood vessel volumes are reported in Table 2. Across various metrics of exposure, cigarette smoke was consistently associated with higher absolute pulmonary blood vessel volumes and/or higher small vessel fraction. Ever-smoking status was associated with higher TBV, BV5, and BV5/TBV compared with never-smokers, and the magnitude of effect of tobacco exposure on blood vessel volumes increased linearly when comparing never-, former, and current smokers (Ptrend < 0.001 for both TBV and BV5; Figure 2). Associations were slightly attenuated after adjustment for pack-years, but remained statistically significant. Second-hand exposure was associated with higher small vessel fraction, which remained significant after adjustment for pack-years and smoking status, and when restricting the analysis to never-smokers.
Table 2.
Associations of cigarette smoke exposure with radiographic pulmonary vascular measures
| TBV |
BV5 |
BV5/TBV |
||||
|---|---|---|---|---|---|---|
| Difference (95% CI) (ml) | P Value | Difference (95% CI) (ml) | P Value | Difference (95% CI) (pp) | P Value | |
| Pack-years of smoking (per 10 pack-years) | 1.5 (0.9 to 2.0) | <0.001 | 0.6 (0.4 to 0.9) | <0.001 | 0.04 (−0.04 to 0.1) | 0.3 |
| Adjusted for smoking status | 0.6 (−0.1 to 1.3) | 0.1 | 0.2 (−0.1 to 0.5) | 0.2 | −0.02 (−0.1 to 0.08) | 0.7 |
| Ever vs. never-smoking status | 4.6 (2.9 to 6.3) | <0.001 | 2.1 (1.3 to 2.9) | <0.001 | 0.28 (0.03 to 0.52) | 0.03 |
| Adjusted for pack-years | 3.1 (1.0 to 5.2) | 0.004 | 1.5 (0.6 to 2.4) | 0.002 | 0.28 (−0.02 to 0.58) | 0.07 |
| Smoking status | ||||||
| Former vs. never | 3.8 (2.1 to 5.6) | <0.001 | 1.8 (1.0 to 2.5) | <0.001 | 0.27 (0.01 to 0.52) | 0.04 |
| Current vs. never | 9.0 (5.7 to 12.3) | <0.001 | 4.0 (2.6 to 5.5) | <0.001 | 0.32 (−0.15 to 0.79) | 0.2 |
| Smoking status, adjusted for pack-years | ||||||
| Former vs. never | 3.0 (0.9 to 5.1) | 0.005 | 1.4 (0.5 to 2.4) | 0.002 | 0.28 (−0.02 to 0.58) | 0.07 |
| Current vs. never | 7.2 (3.2 to 11.3) | <0.001 | 3.4 (1.6 to 5.2) | <0.001 | 0.40 (−0.18 to 0.98) | 0.2 |
| Second-hand smoke exposure | 0.3 (−1.7 to 2.2) | 0.8 | 0.5 (−0.3 to 1.4) | 0.2 | 0.31 (0.03 to 0.58) | 0.03 |
| Adjusted for pack-years and smoking status | −1.5 (−3.5 to 0.5) | 0.1 | −0.2 (−1.0 to 0.7) | 0.7 | 0.31 (0.02 to 0.59) | 0.03 |
| Among never-smokers only | −1.7 (−4.9 to 1.6) | 0.3 | 0.1 (−1.3 to 1.6) | 0.8 | 0.57 (0.11 to 1.02) | 0.01 |
Definition of abbreviations: BV5 = blood volume of smaller peripheral vessels, defined as cross-sectional area <5 mm2; CI = confidence interval; pp = percentage-point; TBV = total blood volume.
Results of linear regression models with adjustment for age, sex, height, weight, personal educational attainment, occupation category, quartiles of census tract median neighborhood income, and Framingham Heart Study cohort (Offspring vs. Third Generation).
Figure 2.
Associations of smoking status with radiographic pulmonary vascular measures. Black diamonds, total blood volume (TBV); blue circles, blood volume of smaller peripheral vessels (defined as cross-sectional area <5 mm2 [BV5]); red diamonds, small vessel fraction (BV5/TBV). Results of linear regression models adjusting for age, sex, height, weight, personal educational attainment, occupation category, quartiles of census tract median neighborhood income, and Framingham Heart Study cohort (Offspring vs. Third Generation)
Secondary Analyses
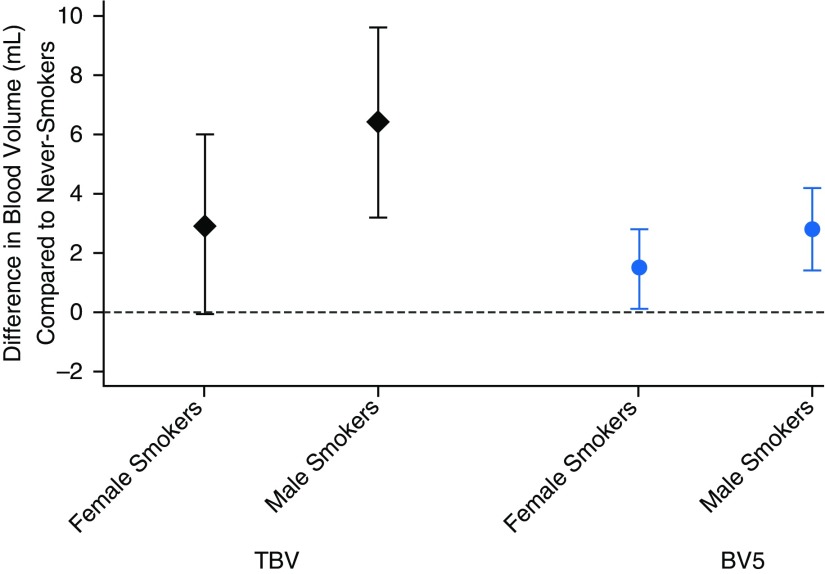
In secondary analyses, adjustment for FEV1 and FVC % predicted did not alter the pattern or statistical significance of the associations of cigarette smoke exposure with radiographic vascular volumes, nor was there evidence that these associations varied based on the presence/absence of airflow limitation or moderate-to-severe airflow obstruction (all Pinteraction > 0.1). The penalized spline for total pack-years and BV5/TBV (Figure E1) demonstrates a positive association of pack-years and BV5/TBV in the lower tobacco exposure range (0–30 pack-years), with a flattening or negative association in the higher tobacco exposure range, although the number of these observations was limited. The splines for pack-years and TBV and BV5 (Figure E2) also demonstrate evidence of nonlinearity (P values for all likelihood ratio tests < 0.05). We found differential effects of sex on the association between ever-smoking status and absolute pulmonary blood vessel volumes (Pinteraction = 0.04 for TBV and 0.08 for BV5), with a stronger association of smoking among males compared with females (Figure 3). For example, the main effect of ever-smoking status was a 4.6-ml-higher TBV (95% CI = 2.9–6.3, P < 0.001), but in males the difference was 6.4 ml (95% CI = 3.2–9.6, P < 0.001) compared with 2.9 ml in females (95% CI = −0.07 to 6.0, P = 0.058). A similar pattern was seen for BV5. We found no evidence of effect modification by sex in the association of second-hand smoke exposure with pulmonary vascular measures. Adjusting for cardiovascular disease and related comorbidities and physical activity index did not substantially alter associations of tobacco exposure with pulmonary vascular measures.
Figure 3.
Difference in pulmonary blood volumes for ever-smokers versus never-smokers, by sex. Black diamonds, total blood volume (TBV); blue circles, blood volume of smaller peripheral vessels (defined as cross-sectional area <5 mm2 [BV5]). Results of interaction analyses from fully adjusted linear regression models, including adjustment for age, sex, height, weight, personal educational attainment, occupation category, quartiles of census tract median neighborhood income, and Framingham Heart Study cohort (Offspring vs. Third Generation).
Discussion
In this large cohort of community-dwelling and generally healthy adults, measures of tobacco exposure were consistently associated with higher absolute pulmonary blood vessel volume, higher small vessel volume, and/or higher small vessel volume ratio in linear models. These findings were not explained by differences in lung function. This suggests that tobacco exposure itself may not, on average, appear radiographically as pruning. In fact, tobacco exposure may actually result in higher total and small vessel pulmonary vascular volumes.
In small studies of animals and human specimens, tobacco exposure has been found to cause pathologic changes in the pulmonary vasculature, including vasoconstriction, vessel occlusion and destruction, and vascular pruning (5–10). Although the pulmonary vessels are difficult to assess noninvasively, CT imaging may provide a new opportunity to study tobacco-related vascular effects, and compares favorably with existing diagnostic modalities. CT-based measures of vascular pruning are associated with lower lung perfusion on scintigraphy and with higher pulmonary artery pressures on right heart catheterization, and demonstrate within-subject reproducibility (17, 19, 35). Compared with magnetic resonance imaging, CT imaging also provides similarly detailed anatomic and quantitative information regarding the structure of the pulmonary veins (36).
However, using chest imaging technology to characterize pulmonary vascular morphology, we found a strikingly different pattern of association with tobacco exposure: exposure to cigarette smoke (whether measured as pack-years of exposure, smoking status, or second-hand exposure) was associated with higher absolute pulmonary blood volume, small vessel volume, and/or higher small vessel fraction. Interestingly, in the MESA Study, the only other large cohort of generally healthy adults to examine associations of tobacco with radiographic pulmonary vascular measures, ever-smokers also had a higher total pulmonary vascular volume compared with never-smokers (18). However, this was an unadjusted univariate comparison, and, in addition, MESA did not assess the relative small vessel blood volume (BV5/TBV), which is felt to be a clinically meaningful radiographic surrogate of pulmonary vascular disease. The COPDGene Study compared whole-lung pulmonary vascular morphology in a small cohort of never-smokers (n = 82) and smokers with normal lung function (n = 104), and, in univariate comparisons, found no differences in BV5, TBV, or BV5/TBV between these groups (19). In addition, COPDGene demonstrated that participants with moderate-to-severe airflow obstruction had lower BV5 and BV5/TBV, but not TBV, compared with those without (although COPDGene did not analyze how tobacco exposure affected these results). In our healthier Framingham cohort, we also similarly found lower BV5 and BV5/TBV in participants with moderate-to-severe obstruction. These consistent radiographic results likely indicate the pulmonary vasculopathy and vascular pruning that is known to occur in the setting of chronic obstructive lung disease. Our analysis additionally demonstrated that the presence of obstructive lung disease did not affect the association of tobacco exposure with higher pulmonary vascular volumes. In both MESA and COPDGene, among smokers, differences in pulmonary vascular morphology (lower total vascular volumes in MESA Lung and lower BV5/TBV in COPDGene) were associated with worse respiratory health (18, 19). Taken together, our study and these findings suggest that, whereas radiographic pruning and lower blood vessel volumes may be an indicator of pulmonary vascular pathology and respiratory limitation among smokers and subpopulations with COPD, higher tobacco exposures do not appear to be associated with more severe radiographic pruning in a generally healthy population.
There are several possible explanations for our findings of higher total and peripheral pulmonary blood vessel volumes in those exposed to tobacco. Physiologically, although the effect of tobacco on vasoactive mediators overall favors vasoconstriction, certain elements of the nitric oxide pathway in mice are upregulated after tobacco exposure (6), and carbon monoxide (a byproduct of tobacco combustion) is known to induce vasodilation via nitric oxide–mediated mechanisms (37, 38). However, a mechanism involving these acute effects would only explain higher blood vessel volumes in active (but not former) smokers, which was not observed. Technically, this image analysis technique cannot differentiate the volume of the vessel wall from the volume of blood (i.e., the volume of the vessel lumen), and thus our results may reflect an increase in vessel wall thickness (due to smooth muscle hyperplasia and intimal thickening) rather than, or in addition to, an expansion of blood volume within the lungs. Another technical limitation may be that certain radiographic abnormalities, such as interstitial fibrosis or inflammation, within the lung compartments immediately adjacent to the vessels may be misclassified by the algorithm as being part of the outer aspect of the vessel, a possibility that has been raised by other investigators (16). Subvisual perivascular fibrosis may play a role in explaining our results, although the overall prevalence of definite interstitial change detectable by CT in the Framingham Heart Study has previously been found to be quite low (39). Perivascular inflammation due to tobacco exposure, on the other hand, would likely be an acute process that would predominantly affect active (but not former) smokers.
An additional explanation is that the pulmonary microvessels with cross-sectional area less than 1 mm2 that are below the resolution limit for this CT algorithm (40) (which include the smallest pulmonary arterioles, venules, and the capillary bed) may be the vessels that are most affected by the expected tobacco-mediated vasoconstriction. Vascular congestion upstream of these microvessels might lead to observed increases in BV5 and TBV, although this mechanism would only apply to the larger upstream arterial vessels. Cardiac effects may also explain our findings: tobacco exposure has been associated with reduced diastolic compliance (41), and with higher pulmonary venous pressures and vessel engorgement (42). In secondary analyses, we found that additional adjustments for cardiovascular disease, diabetes, antihypertensive medication use, and statin use did not alter the association of tobacco with pulmonary vascular volumes. Finally, smoking-related pulmonary effects, such as airflow limitation leading to air trapping and parenchymal hyperinflation, might affect the appearance of the pulmonary vasculature. However, our findings were robust to adjustment for lung volumes, and did not differ in those with and without airflow obstruction. If anything, higher intrathoracic pressures due to hyperinflation may compress cardiac and vascular structures, especially in smaller peripheral vessels (43–45). It is also possible that ever-smokers (with or without overt lung disease) may have stronger inspiratory effort and better end-inspiratory breath hold during the CT, leading to more recruitment and thus a larger appearance of the pulmonary vessels on imaging, an effect that cannot be accounted for in this analysis.
Our study is among the first to examine the association of tobacco exposure, including second-hand exposure, on radiographic pulmonary vascular structure in a large population of generally healthy adults in multivariate analysis, and is one of the largest to examine this quantitative radiographic pulmonary vascular assessment. Strengths of our study include the large cohort size, detailed measurement of potential confounders, and more comprehensive metrics of tobacco exposure than other reports in the literature. We also examined both absolute and relative pulmonary blood vessel volumes to assess for differences in the distribution of the pulmonary vasculature. One particular strength is that all CTs were performed on the same model scanner, at one institution, and using a uniform protocol, thereby reducing bias introduced by differences in image acquisition and manufacturer-related reconstruction algorithms—a significant concern for radiographic biomarkers (46).
There are several limitations. The cross-sectional study design limits conclusions regarding the causality of the associations. Although our models adjusted for many potential confounders, residual confounding cannot be excluded. Although the inspiratory, whole-lung CT scans enable the interpretation of lung and pulmonary vascular images, a potential limitation of the imaging protocol is that the CTs were gated to optimize assessment of coronary artery calcification. Due to the original recruitment strategy targeting residents of the town of Framingham in Massachusetts, our sample is entirely of European ancestry, and our findings may not be generalizable to other groups. In addition, our radiographic assessment of the pulmonary vascular structure does not enable us to directly assess the pulmonary microvasculature, nor distinguish arterial from venous vessels, although a prior study found that separately evaluating arterial and venous BV5/TBV yielded similar associations with measures of right ventricular dysfunction on cardiac magnetic resonance imaging (47). Although our primary findings were highly robust to adjustment for known cardiovascular disease and the presence of related comorbidities, we were not able to directly adjust for elevated left atrial pressure or left ventricular dysfunction in our analysis. However, in MESA, a similarly healthy cohort with similar prevalence of smoking, ever-smokers had similar left atrial volume, left ventricular volume, and left ventricular ejection fraction compared with never-smokers (18), arguing that left-sided cardiac effects likely do not entirely explain our findings. Future work may address the clinical implications of our findings, including the impact of longitudinal pulmonary vascular changes on objective and subjective pulmonary and cardiac outcomes.
Conclusions
In this study, current and former smokers had higher average total and peripheral vessel pulmonary vascular volumes compared with nonsmokers. This suggests that, although lower BV5/TBV may be a radiographic surrogate of pulmonary vasculopathy in populations with significant COPD, it may not directly represent tobacco-related vasculopathy in generally healthy individuals without high burdens of tobacco exposure and/or lung disease, and should be interpreted with caution in these settings. The mechanisms and potential health consequences of the observed association between tobacco smoke exposure and higher average pulmonary blood vessel volumes remain to be determined.
Supplementary Material
Acknowledgments
Acknowledgment
The authors thank the participants of the Framingham Offspring and Third Generation cohorts.
Footnotes
Supported by National Institutes of Health (NIH) grants R01 HL122464 and R01 HL116473 (G.R.W.), and 1R01 HL116931 and R01 HL116473 (R.S.J.E.), National Institute for Environmental Health Sciences grant K23 ES026204, the American Thoracic Society Foundation, and the American Lung Association (M.B.R.), and in part by National Heart, Lung and Blood Institute Framingham Heart Study contracts N01-HC-25195 and HHSN268201500001I.
Author Contributions: A.J.S. contributed to the conception of the research project, conducted the data analysis and interpretation, and contributed to the writing of the manuscript; M.B.R. provided supervision for the project, including data analysis, interpretation, and writing of the manuscript; G.R.W., M.A.M., and M.B.R. planned the overall study design of the project; G.R.W. and G.T.O’C. developed the computed tomography substudy within the Framingham Heart Study, which generated the radiographic vascular measures, provided expertise with pulmonary data from the Framingham Heart Study, and informed the statistical analysis; G.R.W. and R.S.J.E. provided technical expertise with radiographic vascular outcomes; W.L. provided expertise with demographic data in the Framingham Heart Study and contributed to the statistical analysis; C.Z. contributed to the data analysis and data presentation; all authors contributed to the interpretation of the data and critical revision of the manuscript, and approved the final manuscript.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Laniado-Laborín R. Smoking and chronic obstructive pulmonary disease (COPD). Parallel epidemics of the 21 century. Int J Environ Res Public Health. 2009;6:209–224. doi: 10.3390/ijerph6010209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vassallo R. Diffuse lung diseases in cigarette smokers. Semin Respir Crit Care Med. 2012;33:533–542. doi: 10.1055/s-0032-1325162. [DOI] [PubMed] [Google Scholar]
- 3.Lederer DJ, Enright PL, Kawut SM, Hoffman EA, Hunninghake G, van Beek EJ, et al. Cigarette smoking is associated with subclinical parenchymal lung disease: the Multi-Ethnic Study of Atherosclerosis (MESA)-lung study. Am J Respir Crit Care Med. 2009;180:407–414. doi: 10.1164/rccm.200812-1966OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gupta N, Vassallo R, Wikenheiser-Brokamp KA, McCormack FX. Diffuse cystic lung disease: part I. Am J Respir Crit Care Med. 2015;191:1354–1366. doi: 10.1164/rccm.201411-2094CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferrer E, Peinado VI, Díez M, Carrasco JL, Musri MM, Martínez A, et al. Effects of cigarette smoke on endothelial function of pulmonary arteries in the guinea pig. Respir Res. 2009;10:76. doi: 10.1186/1465-9921-10-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seimetz M, Parajuli N, Pichl A, Veit F, Kwapiszewska G, Weisel FC, et al. Inducible NOS inhibition reverses tobacco-smoke-induced emphysema and pulmonary hypertension in mice. Cell. 2011;147:293–305. doi: 10.1016/j.cell.2011.08.035. [DOI] [PubMed] [Google Scholar]
- 7.Wright JL, Churg A. Effect of long-term cigarette smoke exposure on pulmonary vascular structure and function in the guinea pig. Exp Lung Res. 1991;17:997–1009. doi: 10.3109/01902149109064331. [DOI] [PubMed] [Google Scholar]
- 8.Santos S, Peinado VI, Ramírez J, Melgosa T, Roca J, Rodriguez-Roisin R, et al. Characterization of pulmonary vascular remodelling in smokers and patients with mild COPD. Eur Respir J. 2002;19:632–638. doi: 10.1183/09031936.02.00245902. [DOI] [PubMed] [Google Scholar]
- 9.Jacobson G, Turner AF, Balchum OJ, Jung R. Vascular changes in pulmonary emphysema. The radiologic evaluation by selective and peripheral pulmonary wedge angiography. Am J Roentgenol Radium Ther Nucl Med. 1967;100:374–396. [PubMed] [Google Scholar]
- 10.Scarrow GD. The pulmonary angiogram in chronic bronchitis and emphysema. Clin Radiol. 1966;17:54–67. doi: 10.1016/s0009-9260(66)80123-x. [DOI] [PubMed] [Google Scholar]
- 11.Voelkel NF, Gomez-Arroyo J, Mizuno S. COPD/emphysema: The vascular story. Pulm Circ. 2011;1:320–326. doi: 10.4103/2045-8932.87295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Voelkel NF. Cigarette smoke is an endothelial cell toxin. Am J Respir Crit Care Med. 2018;197:274. doi: 10.1164/rccm.201706-1123LE. [DOI] [PubMed] [Google Scholar]
- 13.Lu Q, Gottlieb E, Rounds S. Effects of cigarette smoke on pulmonary endothelial cells. Am J Physiol Lung Cell Mol Physiol. 2018;314:L743–L756. doi: 10.1152/ajplung.00373.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sverzellati N, Silva M. the matter of the lung: quantification of vascular substance in asthma. Am J Respir Crit Care Med. 2018;198:1–2. doi: 10.1164/rccm.201804-0804ED. [DOI] [PubMed] [Google Scholar]
- 15.Kovacs G, Agusti A, Barberá JA, Celli B, Criner G, Humbert M, et al. Pulmonary vascular involvement in chronic obstructive pulmonary disease—is there a pulmonary vascular phenotype? Am J Respir Crit Care Med. 2018;198:1000–1011. doi: 10.1164/rccm.201801-0095PP. [DOI] [PubMed] [Google Scholar]
- 16.Jacob J, Bartholmai BJ, Rajagopalan S, Kokosi M, Nair A, Karwoski R, et al. Mortality prediction in idiopathic pulmonary fibrosis: evaluation of computer-based CT analysis with conventional severity measures. Eur Respir J. 2017;49:1601011. doi: 10.1183/13993003.01011-2016. [DOI] [PubMed] [Google Scholar]
- 17.Matsuoka S, Washko GR, Yamashiro T, Estepar RS, Diaz A, Silverman EK, et al. National Emphysema Treatment Trial Research Group. Pulmonary hypertension and computed tomography measurement of small pulmonary vessels in severe emphysema. Am J Respir Crit Care Med. 2010;181:218–225. doi: 10.1164/rccm.200908-1189OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aaron CP, Hoffman EA, Lima JAC, Kawut SM, Bertoni AG, Vogel-Claussen J, et al. Pulmonary vascular volume, impaired left ventricular filling and dyspnea: the MESA Lung Study. PLoS One. 2017;12:e0176180. doi: 10.1371/journal.pone.0176180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Estépar RSJ, Kinney GL, Black-Shinn JL, Bowler RP, Kindlmann GL, Ross JC, et al. COPDGene Study. Computed tomographic measures of pulmonary vascular morphology in smokers and their clinical implications. Am J Respir Crit Care Med. 2013;188:231–239. doi: 10.1164/rccm.201301-0162OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kannel WB, Feinleib M, McNamara PM, Garrison RJ, Castelli WP. An investigation of coronary heart disease in families: the Framingham Offspring Study. Am J Epidemiol. 1979;110:281–290. doi: 10.1093/oxfordjournals.aje.a112813. [DOI] [PubMed] [Google Scholar]
- 21.Splansky GL, Corey D, Yang Q, Atwood LD, Cupples LA, Benjamin EJ, et al. The Third Generation Cohort of the National Heart, Lung, and Blood Institute’s Framingham Heart Study: design, recruitment, and initial examination. Am J Epidemiol. 2007;165:1328–1335. doi: 10.1093/aje/kwm021. [DOI] [PubMed] [Google Scholar]
- 22.Araki T, Nishino M, Zazueta OE, Gao W, Dupuis J, Okajima Y, et al. Paraseptal emphysema: prevalence and distribution on CT and association with interstitial lung abnormalities. Eur J Radiol. 2015;84:1413–1418. doi: 10.1016/j.ejrad.2015.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kliment CR, Araki T, Doyle TJ, Gao W, Dupuis J, Latourelle JC, et al. A comparison of visual and quantitative methods to identify interstitial lung abnormalities. BMC Pulm Med. 2015;15:134. doi: 10.1186/s12890-015-0124-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dorans KS, Wilker EH, Li W, Rice MB, Ljungman PL, Schwartz J, et al. Residential proximity to major roads, exposure to fine particulate matter, and coronary artery calcium: the Framingham Heart Study. Arterioscler Thromb Vasc Biol. 2016;36:1679–1685. doi: 10.1161/ATVBAHA.116.307141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ash SY, Rahaghi FN, Come CE, Ross JC, Colon AG, Cardet-Guisasola JC, et al. SARP Investigators. Pruning of the pulmonary vasculature in asthma: the Severe Asthma Research Program (SARP) cohort. Am J Respir Crit Care Med. 2018;198:39–50. doi: 10.1164/rccm.201712-2426OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rice MB, Li W, Dorans KS, Wilker EH, Ljungman P, Gold DR, et al. Exposure to traffic emissions and fine particulate matter and computed tomography measures of the lung and airways. Epidemiology. 2018;29:333–341. doi: 10.1097/EDE.0000000000000809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li W, Dorans KS, Wilker EH, Rice MB, Ljungman PL, Schwartz JD, et al. Short-term exposure to ambient air pollution and biomarkers of systemic inflammation: the Framingham Heart Study. Arterioscler Thromb Vasc Biol. 2017;37:1793–1800. doi: 10.1161/ATVBAHA.117.309799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tsao CW, Vasan RS. Cohort profile: the Framingham Heart Study (FHS): overview of milestones in cardiovascular epidemiology. Int J Epidemiol. 2015;44:1800–1813. doi: 10.1093/ije/dyv337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vogelmeier CF, Criner GJ, Martinez FJ, Anzueto A, Barnes PJ, Bourbeau J, et al. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease 2017 Report: GOLD executive summary. Am J Respir Crit Care Med. 2017;195:557–582. doi: 10.1164/rccm.201701-0218PP. [DOI] [PubMed] [Google Scholar]
- 30.Li W, Wilker EH, Dorans KS, Rice MB, Schwartz J, Coull BA, et al. Short-term exposure to air pollution and biomarkers of oxidative stress: the Framingham Heart Study. J Am Heart Assoc. 2016;5:e002742. doi: 10.1161/JAHA.115.002742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kraigher-Krainer E, Lyass A, Massaro JM, Lee DS, Ho JE, Levy D, et al. Association of physical activity and heart failure with preserved vs. reduced ejection fraction in the elderly: the Framingham Heart Study. Eur J Heart Fail. 2013;15:742–746. doi: 10.1093/eurjhf/hft025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Garcia-Aymerich J, Lange P, Benet M, Schnohr P, Antó JM. Regular physical activity modifies smoking-related lung function decline and reduces risk of chronic obstructive pulmonary disease: a population-based cohort study. Am J Respir Crit Care Med. 2007;175:458–463. doi: 10.1164/rccm.200607-896OC. [DOI] [PubMed] [Google Scholar]
- 33.Kiely DK, Wolf PA, Cupples LA, Beiser AS, Kannel WB. Physical activity and stroke risk: the Framingham Study. Am J Epidemiol. 1994;140:608–620. doi: 10.1093/oxfordjournals.aje.a117298. [DOI] [PubMed] [Google Scholar]
- 34.Kannel WB, Sorlie P. Some health benefits of physical activity: the Framingham Study. Arch Intern Med. 1979;139:857–861. [PubMed] [Google Scholar]
- 35.Matsuoka S, Yamashiro T, Matsushita S, Fujikawa A, Yagihashi K, Kurihara Y, et al. Relationship between quantitative CT of pulmonary small vessels and pulmonary perfusion. AJR Am J Roentgenol. 2014;202:719–724. doi: 10.2214/AJR.13.11027. [DOI] [PubMed] [Google Scholar]
- 36.Hamdan A, Charalampos K, Roettgen R, Wellnhofer E, Gebker R, Paetsch I, et al. Magnetic resonance imaging versus computed tomography for characterization of pulmonary vein morphology before radiofrequency catheter ablation of atrial fibrillation. Am J Cardiol. 2009;104:1540–1546. doi: 10.1016/j.amjcard.2009.07.029. [DOI] [PubMed] [Google Scholar]
- 37.Thorup C, Jones CL, Gross SS, Moore LC, Goligorsky MS. Carbon monoxide induces vasodilation and nitric oxide release but suppresses endothelial NOS. Am J Physiol. 1999;277:F882–F889. doi: 10.1152/ajprenal.1999.277.6.F882. [DOI] [PubMed] [Google Scholar]
- 38.Papathanasiou G, Mamali A, Papafloratos S, Zerva E. Effects of smoking on cardiovascular function: the role of nicotine and carbon monoxide. Health Sci J. 2014;8:274–290. [Google Scholar]
- 39.Putman RK, Hatabu H, Araki T, Gudmundsson G, Gao W, Nishino M, et al. Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) Investigators; COPDGene Investigators. Association between interstitial lung abnormalities and all-cause mortality. JAMA. 2016;315:672–681. doi: 10.1001/jama.2016.0518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rahaghi FN, Come CE, Ross J, Harmouche R, Diaz AA, Estepar RS, et al. Morphologic response of the pulmonary vasculature to endoscopic lung volume reduction. Chronic Obstr Pulm Dis. 2015;2:214–222. doi: 10.15326/jcopdf.2.3.2014.0164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Leigh JA, Kaplan RC, Swett K, Balfour P, Kansal MM, Talavera GA, et al. Smoking intensity and duration is associated with cardiac structure and function: the ECHOcardiographic Study of Hispanics/Latinos. Open Heart. 2017;4:e000614. doi: 10.1136/openhrt-2017-000614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Perez VA, Haddad F, Zamanian RT. Diagnosis and management of pulmonary hypertension associated with left ventricular diastolic dysfunction. Pulm Circ. 2012;2:163–169. doi: 10.4103/2045-8932.97598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jörgensen K, Müller MF, Nel J, Upton RN, Houltz E, Ricksten SE. Reduced intrathoracic blood volume and left and right ventricular dimensions in patients with severe emphysema: an MRI study. Chest. 2007;131:1050–1057. doi: 10.1378/chest.06-2245. [DOI] [PubMed] [Google Scholar]
- 44.Jörgensen K, Houltz E, Westfelt U, Nilsson F, Scherstén H, Ricksten SE. Effects of lung volume reduction surgery on left ventricular diastolic filling and dimensions in patients with severe emphysema. Chest. 2003;124:1863–1870. doi: 10.1378/chest.124.5.1863. [DOI] [PubMed] [Google Scholar]
- 45.Mineo TC, Pompeo E, Rogliani P, Dauri M, Turani F, Bollero P, et al. Effect of lung volume reduction surgery for severe emphysema on right ventricular function. Am J Respir Crit Care Med. 2002;165:489–494. doi: 10.1164/ajrccm.165.4.2108129. [DOI] [PubMed] [Google Scholar]
- 46.Bodduluri S, Reinhardt JM, Hoffman EA, Newell JD, Jr, Bhatt SP. Recent advances in computed tomography imaging in chronic obstructive pulmonary disease. Ann Am Thorac Soc. 2018;15:281–289. doi: 10.1513/AnnalsATS.201705-377FR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rahaghi FN, Wells JM, Come CE, De La Bruere IA, Bhatt SP, Ross JC, et al. and the COPDGene Investigators. Arterial and venous pulmonary vascular morphology and their relationship to findings in cardiac magnetic resonance imaging in smokers. J Comput Assist Tomogr. 2016;40:948–952. doi: 10.1097/RCT.0000000000000465. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.