Abstract
Background:
With facet interventions under scrutiny, the authors’ objectives were to determine the effectiveness of different lumbar facet blocks and their ability to predict radiofrequency ablation outcomes.
Methods:
A total of 229 participants were randomized in a 2:2:1 ratio to receive intraarticular facet injections with bupivacaine and steroid, medial branch blocks, or saline. Those with a positive 1-month outcome (a 2-point or more reduction in average pain score) and score higher than 3 (positive satisfaction) on a 5-point satisfaction scale were followed up to 6 months. Participants in the intraarticular and medial branch block groups with a positive diagnostic block (50% or more relief) who experienced a negative outcome proceeded to the second phase and underwent radiofrequency ablation, while all saline group individuals underwent ablation. Coprimary outcome measures were average numerical rating scale pain scores 1 month after the facet or saline blocks and 3 months after ablation.
Results:
Mean reduction in average numerical rating scale pain score at 1 month was 0.7 ± 1.6 in the intraarticular group, 0.7 ± 1.8 in the medial branch block group, and 0.7 ± 1.5 in the placebo group; P = 0.993. The proportions of positive blocks were higher in the intraarticular (54%) and medial branch (55%) groups than in the placebo group (30%; P = 0.01). Radiofrequency ablation was performed on 135 patients (45, 48, and 42 patients from the intraarticular, medial branch, and saline groups, respectively). The average numerical rating scale pain score at 3 months was 3.0 ± 2.0 in the intraarticular, 3.2 ± 2.5 in the medial branch, and 3.5 ± 1.9 in the control group (P = 0.493). At 3 months, the proportions of positive responders in the intraarticular, medial branch block, and placebo groups were 51%, 56%, and 24% for the intraarticular, medial branch, and placebo groups, respectively (P = 0.005).
Conclusions:
This study establishes that facet blocks are not therapeutic. The higher responder rates in the treatment groups suggest a hypothesis that facet blocks might provide prognostic value before radiofrequency ablation.
FACET interventions are the second most commonly performed procedures for chronic pain, yet they remain mired in controversy.1,2 Whereas most reviews assert that radiofrequency ablation is effective for lumbar facet joint pain,3–6 others dispute this.2,7 For intraarticular injections, most reviews conclude the injections are ineffective,2–4,8,9 although some studies indicate they may provide some benefit compared to sham interventions10 and conservative therapy.11,12 Unlike negative randomized studies that utilized an intraarticular control group, a recent positive study10 compared intraarticular steroids to intramuscular steroids, as research has revealed that intraarticular nonsteroids may exert an analgesic effect.11 Intraarticular steroids may work in inflammatory conditions, as randomized13 and uncontrolled14–18 studies have shown effectiveness in individuals by positive single-photon emission computed tomography imaging.
For therapeutic medial branch blocks, the evidence supporting their benefit is based on several randomized trials performed by the same group.3,5,19 Theoretically, medial branch blocks may provide long-term benefit by the release or suppression of ectopic discharges from medial branches trapped beneath the mamilloaccessory ligament, which occurs in up to 20% of individuals at L5,20 as well as reversal of central sensitization.21
One should always consider that proper selection and technical factors can play an equal or greater role in the results of an interventional study than methodologic variables.22 For example, placebo-controlled studies that selected patients for radiofrequency ablation via intraarticular injections have yielded equivocal or negative results,23–25 while those that selected patients by medial branch blocks generally,26–29 but not always,30 reported positive findings, although methodologic differences preclude direct comparisons. The argument supporting a prognostic procedure that targets the nerves rather than the joints themselves to be ablated is intuitive and supported by a multicenter study demonstrating better radiofrequency ablation outcomes in patients who undergo medial branch blocks rather than intraarticular injections,31 a small study comparing the prognostic value of medial branch blocks and pericapsular facet injections before cryodenervation,32 and an experimental study demonstrating that 11% of individuals who receive medial branch blocks will continue to experience pain from facet joint capsular distension, which suggests aberrant innervation.33 Whereas it is commonly written that medial branch blocks and intraarticular injections are diagnostically equivalent,34–38 this assertion is based on two small studies, only one of which assessed postblock pain.39,40 To date, no randomized studies have examined the relative prognostic values of medial branch blocks and intraarticular injections before radiofrequency ablation.
In order to determine the prognostic and therapeutic value before radiofrequency ablation of intraarticular injections and medial branch blocks for suspected lumbar facet joint pain, we conducted a randomized study comparing both procedures to a sham injection. We hypothesized that intraarticular injections performed with local anesthetic and steroid may have therapeutic effect in a small segment of the population compared to placebo and medial branch blocks and that medial branch blocks would have better predictive value before denervation than intraarticular and placebo injections.
Materials and Methods
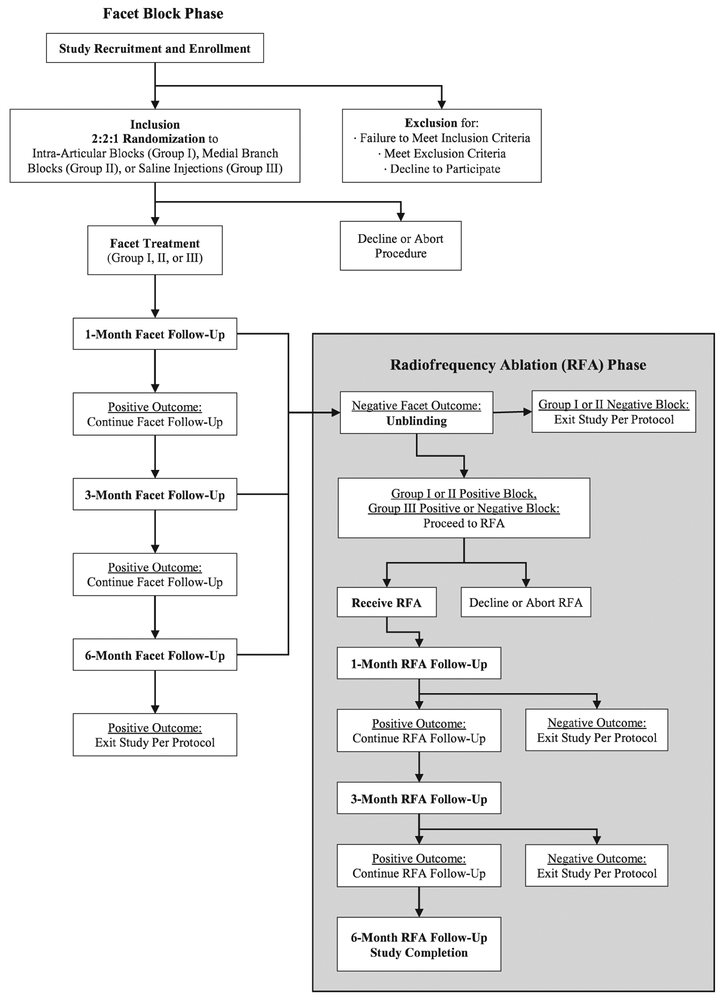
A diagram of the overall study design is shown in figure 1. Permission to conduct this multicenter randomized study was granted by the Internal Review Boards at Walter Reed National Military Medical Center (Bethesda, Maryland), San Diego Naval Medical Center (San Diego, California), District of Columbia Veterans Administration Medical Center (Washington, District of Columbia), Puget Sound Veterans Administration Hospital (Seattle, Washington), Landstuhl Regional Medical Center (Landstuhl, Germany), and The Johns Hopkins Medical Institutions (Baltimore, Maryland), the Board of Directors at Parkway Neuroscience and Spine Institute (Hagerstown, Maryland), as well as all participants who provided informed consent. These sites include U.S. military treatment facilities in the continental United States and Europe, Veterans Administration Hospitals, one academic pain treatment center situated in an urban setting, and one private practice pain treatment facility located in a suburban environment. The standardized protocol was performed at all participating institutions, with enrollment and follow-up taking place between March 2014 and August 2017. The trial was registered in December 2013 on clinicaltrials.gov, ClinicalTrials.gov identifier NCT02002429. To correct errors, slight modifications were made after completion of the trial to reflect the original protocol and conduct of the study and to include the addition of the coprimary outcome measure of average pain score 3 months after radiofrequency ablation, upon which the power analysis was based. The study protocol or the analysis plan were not altered.
Fig. 1.
Omnibus study flowchart.
Inclusion and Exclusion Criteria
Participants were recruited from pain treatment centers at participating institutions. Inclusion criteria were 18 yr of age or older, predominantly axial low back pain for 3 months or more, average back pain score more than 3 out of 10 over the past week on a numerical rating scale, failure to respond to more conservative therapy (e.g., physical therapy, integrative therapy, and pharmacotherapy) and paraspinal tenderness. Excluded from participation were patients with a known, specific etiology for low back pain (e.g., significant spinal stenosis or grade II or III spondylolisthesis), focal neurologic signs or symptoms, a positive response to previous spine interventions such as epidural steroids or sacroiliac joint blocks for the current pain episode, previous facet interventions, lumbar spine fusion, untreated coagulopathy, and concomitant medical (e.g., unstable angina) or psychiatric condition likely to undermine the diagnostic work-up or treatment response.
Randomization
A total of 229 participants were randomized in a 2:2:1 ratio by computer-generated randomization tables into the following three groups:
Group I received facet joint intraarticular injections with bupivacaine and corticosteroid;
Group II received medial branch blocks with bupivacaine and corticosteroid;
Group III received saline injections, with needle placement in the same location as group II.
Enrollment was done by an investigator physician and stratified by study site. Allocation was performed by research nurses in blocks of 30 at Walter Reed, Johns Hopkins, and Landstuhl, and in blocks of 10 at other sites, with treatment divulged via sequentially numbered sealed envelopes before injection. Larger allocation blocks were used to promote allocation concealment with investigators. Briefings were performed among clinical staff (e.g., nurse, medical assistant, radiology technician) before each procedure to facilitate blinding. The patient, research nurse, and evaluating physician (i.e., outcome adjudicator) were blinded to assignment.
Facet Injection Study Phase
Diagnostic/Prognostic Injections.
All prognostic injections (i.e., blocks used to select radiofrequency ablation candidates) were performed with superficial local anesthetic and fluoroscopic guidance by an attending physician board certified in pain medicine or by a trainee under their supervision. Except in rare circumstances, sedation was not administered. Levels to be injected were determined via the elicitation of tenderness under fluoroscopic imaging (approximately 4 kg of pressure, the amount of pressure required to blanch a thumbnail and similar to what is used to elicit tender points in fibromyalgia),41,42 referral patterns,43 and/or imaging. Individuals with unilateral pain underwent single one-sided blocks, while those with bilateral pain received bilateral procedures.
Group I (Intraarticular Injections).
The image intensifier was angled obliquely and sometimes cephalad to optimize the view of the joint space at the targeted level. A 22-gauge needle was then inserted into the joint with oblique, anteroposterior, and lateral fluoroscopic imaging. To confirm placement, 0.2 ml of contrast dye was injected. In those instances (e.g., patient movement, extremely narrowed joint space, inability to discern the optimal angle) where an arthrogram could not be obtained after several attempts, an injection just outside of the joint was performed.44 Once needle placement was deemed acceptable, 0.5 ml of solution containing 0.25 ml of 0.5% bupivacaine mixed with 0.25 ml of 40 mg/ml depomethylprednisolone was injected. The dose of steroid used is higher than that used for clinical trials evaluating therapeutic medial branch blocks,19 but slightly lower than the equipotent dosages employed in most,9 but not all,11,14 studies evaluating intraarticular steroids. After the procedure, the number of intra- and extraarticular injections was recorded.
Group II (Medial Branch Blocks).
Medial branch blocks were performed in accordance with previously published standards and techniques.42 L5 dorsal rami blocks were performed by placing a 22-gauge needle in the groove between the sacral ala and articular process, while higher level lumbar medial branch blocks were done by inserting 22-gauge needles in an oblique trajectory at a point several millimeters below the junction of the upper transverse process and the superior articular process. After confirmation of needle placement in anteroposterior and lateral views, contrast was injected to ascertain appropriate spread and absence of intravascular uptake. When needle placement was deemed appropriate, 0.5 ml of solution containing 0.25 ml of 0.5% bupivacaine mixed with 0.25 ml of 40 mg/ml depomethylprednisolone was administered.
Group III (Placebo Injection).
This group received the same procedure as group II, except that 0.5 ml of saline was given once needle placement was optimized.
Postprocedure Instructions and Assessment of Blinding.
In the recovery area, participants were given a 6-h pain diary broken down into 30-min intervals containing spaces to annotate their 0 to 10 pain score as well as their activities at the time, to fax or e-mail back the following day. They were also instructed before discharge to engage in their normal activities and discount procedure-related pain. A positive block was defined as 50% or more pain relief sustained for at least 3h, to control for concomitant pain generators.3,42 To facilitate blinding, the same technique (e.g., trajectory and use of multiplanar fluoroscopy) was used for verum and sham injections, and no contact with any investigator was permitted. The same generic procedure note was entered into the chart for all injections, and images were withheld from the medical record until completion of the study. To assess blinding, patients were asked before discharge which treatment they thought they received. No concurrent interventions besides rescue medications were permitted during the course of the study.
Disposition and Progression to Radiofrequency Ablation.
The first follow-up visit was performed 1 month after the diagnostic or therapeutic injection (24- to 40-day window) by a disinterested investigator blinded to treatment. A positive categorical response (i.e., outcome) was predesignated as a 2-point or more decrease in average back pain, coupled with a score higher than 3 on a 5-point Likert scale measuring satisfaction with the results (0 = very unsatisfied, 3 = neither unsatisfied nor satisfied, 5 = very satisfied).
Intraarticular and Medial Branch Block Groups.
Those participants with a positive pain diary (i.e., positive block) and positive 1-month outcome proceeded to their 3- and 6-month follow-ups in a blinded fashion. If at any point between 1 month and 6 months their outcome became negative, they continued to the radiofrequency phase of the study with blinding maintained. Those with a negative diagnostic block exited the study at the time in which their follow-up categorical outcome was negative.
Placebo Group.
Individuals in the placebo group who experienced a negative diagnostic block (no pain relief from the saline injection) and negative categorical outcome at 1, 3, or 6 months proceeded to radiofrequency ablation in an unblinded fashion, while those with a positive pain diary were treated in a manner similar to the intraarticular and medial branch block groups (they proceeded to radiofrequency ablation at the point their categorical outcome became negative, in a blinded fashion; fig. 2).
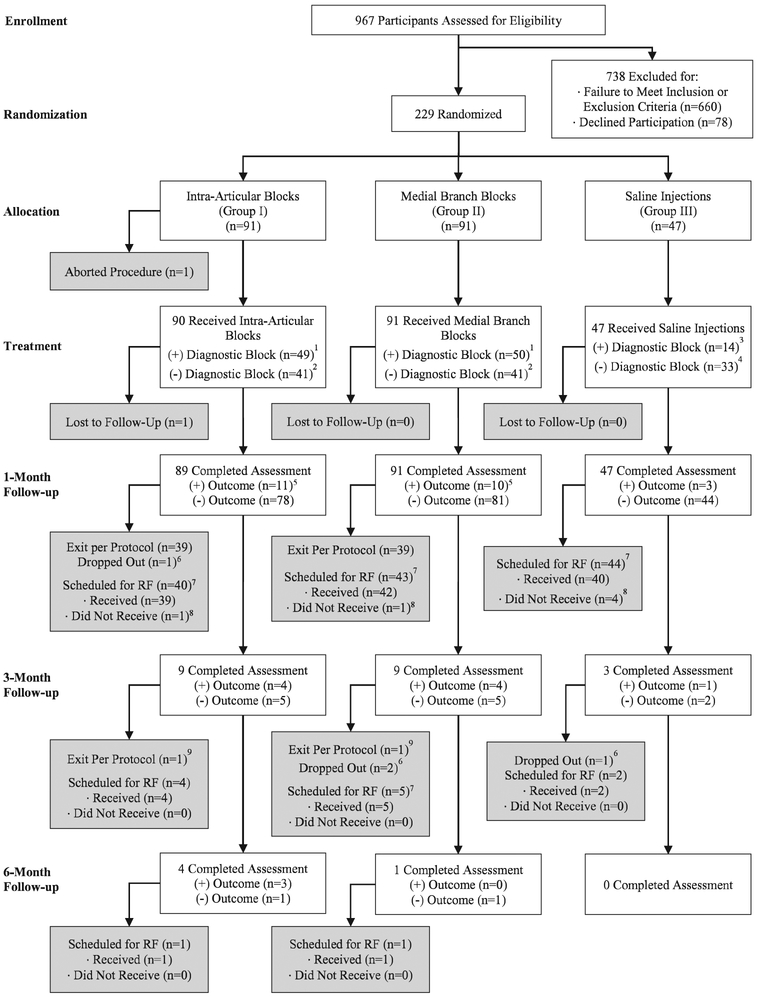
Fig. 2.
Flowchart demonstrating progression through diagnostic and therapeutic facet injection study phase. A positive outcome is defined as 2-point or more decrease in average back pain score coupled with a satisfaction score of 3 out of 5 or higher. (1) Participants with a positive diagnostic block continued the study until a negative follow-up visit, then proceeded with radiofrequency (RF) ablation in an unblinded manner.(2) Participants with a negative diagnostic block continued in the study until a negative follow-up visit, then exited the study. (3) Participants with a positive diagnostic saline injection were treated the same as those in groups I and II (continued in the study until they had a negative follow-up outcome, then proceeded to radiofrequency denervation in an unblinded manner). (4) Participants with a negative diagnostic saline block were unblinded at the point of their negative follow-up visit, then proceeded to radiofrequency denervation. (5) Includes individuals with a negative diagnostic block but positive follow-up outcome that did not exit the study. (6) Includes individuals who were lost to follow-up, chose to exit the study before a negative follow-up visit, or withdrew to receive nonpermitted treatments (e.g., nonradiofrequency denervation treatments or opioid therapy). (7) Scheduled for radiofrequency includes participants who had a negative outcome and a positive block and those who had a positive outcome and a positive block but developed pain before the next follow-up period. (8) Individuals who declined radiofrequency ablation or withdrew from the study before radiofrequency ablation were considered dropouts for the purposes of the study. (9) Includes all those with a negative diagnostic block with initial positive outcome at 1 month, then subsequent negative outcome.
Radiofrequency Ablation Study Phase
Radiofrequency Denervation Procedure.
Radiofrequency procedures were done whenever possible within 2 weeks of the first negative follow-up visit (or when their pain returned in those with a positive 1-month outcome) in participants in the intraarticular and medial branch block groups who experienced a positive block and all placebo group patients. For example, placebo group participants and individuals in the intraarticular and medial branch block groups with a positive diagnostic block, who experienced a positive 1-month outcome and whose pain returned at 2 months or 3-month follow-up, would undergo radiofrequency ablation at these time points.
Radiofrequency ablation procedures were done in accordance with our previously published standards and techniques, at the spinal levels targeted for the diagnostic injections.42 To alleviate procedure-related pain, superficial anesthesia was administered, along with light sedation as needed. With the image intensifier positioned in an ipsilateral oblique and sharp caudad-cephalad direction in order to maximize lesion size in an orientation parallel to the course of the target nerve, 18- or 20-gauge curved radiofrequency needles with 10-mm active tips (BMC RF Cannula, Baylis Medical, Canada) were inserted in coaxial views until bone was contacted between the superomedial border of the transverse and superior articular processes, and the inferior portion of the lateral neck of the superior articular process, with the convex surface apposed to bone. For L5 dorsal rami lesioning, the cannula was positioned in the crevice between the lateral aspect of the S1 articular process and the sacral ala. For each nerve, needles were adjusted to optimize sensory and motor stimulation. For each nerve lesion, electrodes were inserted and adjusted until correct placement was confirmed by electrostimulation at 50 Hz, with the goal being concordant sensation at 0.5 V or less. Before denervation, multifidus stimulation and the absence of leg contractions were verified with electrostimulation at 2 Hz. After optimal electrode placement was ascertained, 1 ml of 2% lidocaine was injected to reduce procedure-related pain and enhance lesion size. Ablation was then commenced at 90°C for 135 s with a radiofrequency generator (Electrothermal 20S Spine System, Smith and Nephew, USA; Baylis Medical Pain Management Generator 115V, Baylis Medical, Canada; or Radionics RF Lesion Generator System, Model RFG-3C, Radionics, Valleylab, USA). At the conclusion of lesioning, 10 mg depomethylprednisolone mixed with saline (total, 0.5 ml) was administered at each site to reduce the risk of neuritis.45
Follow-up
As noted in figure 1, intraarticular and medial branch block group participants remained blinded in the initial facet joint steroid injection study phase until they exited the study for a negative outcome. At this juncture, those with a positive diagnostic block proceeded to the radiofrequency ablation phase in a blinded fashion, while those with a negative diagnostic block exited the study. Placebo group participants with a positive diagnostic block were treated in the same fashion as those in the intraarticular and medial branch block groups, while those with a negative pain diary who were unblinded when their outcome became negative proceeded to the radiofrequency ablation phase. In all participants whose pain recurred in between follow-up visits, their negative data were recorded as part of their next follow-up, at which point disposition was decided (i.e., placebo group participants and those in the intraarticular and medial branch block groups with positive pain diaries proceeded to radiofrequency ablation). Participants in all groups who obtained a positive categorical outcome after their facet block past 3 months but before 6 months were not considered “dropouts” for the radiofrequency phase if they elected to undergo a repeat “therapeutic” injection, but they did exit the study (fig. 3).
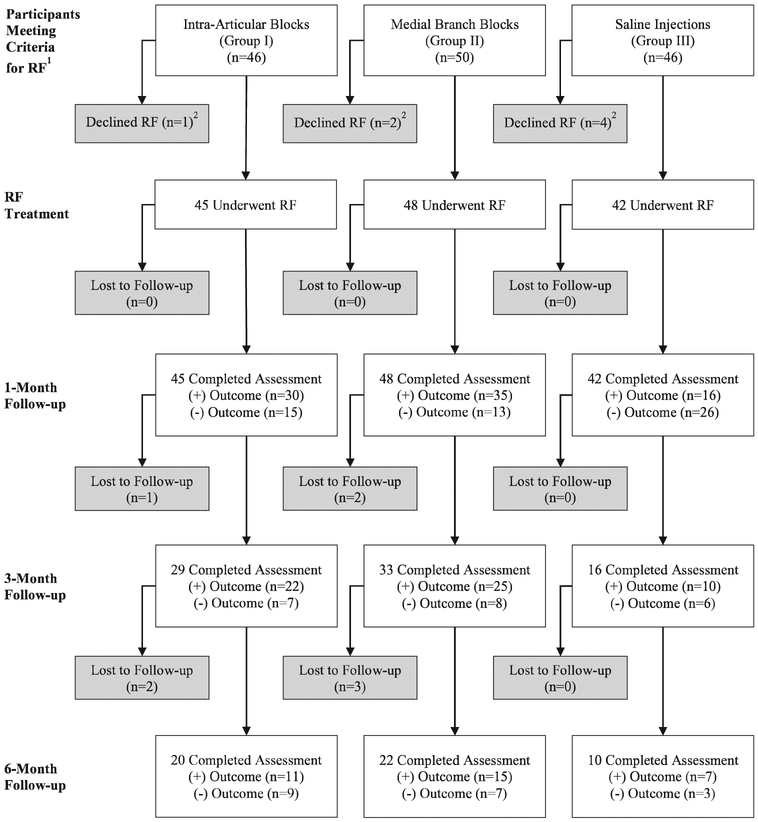
Fig. 3.
Flowchart demonstrating progression through radiofrequency (RF) ablation study phase. A positive outcome is defined as 2-point or more decrease in average back pain score coupled with a satisfaction score of 3 out of 5 or higher. (1) In individuals receiving intraarticular (Group I) and medial branch (group II) blocks, eligible patients were those obtaining a positive diagnostic block and not obtaining ongoing relief at 6 months from the block. In saline injection patients (group III), all patients not obtaining relief at 6-month follow-up during the facet phase met criteria for proceeding to radiofrequency ablation regardless of the results of their diagnostic block. (2) Individuals who declined radiofrequency ablation or withdrew from the study before radiofrequency ablation were considered dropouts.
Data Collection
Baseline data recorded included average and worst 0 to 10 numerical rating scale low back pain scores over the past week, an Oswestry disability index score (version 2.0, MODEMS, USA; scored from 0% to 100% with higher numbers indicating greater back pain disability),46 baseline demographic and clinical variables such as age, sex, etiology of pain, analgesic medication usage to include opioids, duration of pain, active duty status, tobacco use, coexisting psychiatric and pain conditions, as well as the levels injected and laterality. Immediately after the procedure, the number of intra- and extraarticular injections was annotated in the intraarticular group, with the distinction based on whether or not an arthrogram was visualized. The day after the procedure when the pain diary was returned, the percentage of pain relief was determined by a disinterested adjudicator based on pre- and postblock pain scores and activity levels, as well as a determination regarding whether the block was positive or negative. The first follow-up was 1-month postblock, at which time the same clinical variables were recorded in addition to a validated 5-point Likert scale (1 = very unsatisfied, 2 = unsatisfied, 3 = neutral, 4 = satisfied, 5 = very satisfied) grading satisfaction with back pain treatment outcomes47 and complications.
For the facet phase of the study, the primary outcome measure was average 0 to 10 numerical rating scale pain score (10 cm written pain scale showing all integers) 1 month after the facet injection. Secondary outcome measures included analgesic reduction (predefined as a more than 20% reduction in opioid use or cessation of a nonopioid analgesic),42 numerical rating scale pain score with activity, satisfaction and Oswestry disability index. A positive categorical outcome was predesignated to be a 2-point or more decrease in average pain score over the past week, which is deemed to constitute clinically meaningful improvement,48 coupled with a score higher than 3 out of 5 on the patient-reported satisfaction scale. In participants who experienced a positive 1-month outcome, subsequent follow-ups were performed at 3 months, and in those who continued to experience benefit after 3 months, at 6 months. As noted above, those in the intraarticular and medial branch block groups with a negative pain block exited the study if they had a negative outcome at any follow-up period, while those in the placebo group proceeded to radiofrequency ablation.
The second phase of the study was designed to evaluate the prognostic value of medial branch and intraarticular blocks before radiofrequency ablation compared to each other and a saline control block. For this part, the same baseline and follow-up information were recorded, and the coprimary outcome measure was designated as the average pain score at 3 months postprocedure. Guidelines for continuing or exiting this phase were similar to those in the facet block follow-up phase in that those who failed to achieve a positive categorical outcome exited the study per protocol, while those with a positive 1-month outcome continued to follow-up for 3-month and (if their benefit persisted) 6-month visits.
Statistical Analysis
A power analysis to determine sample size was performed before initiation of the study, based on the estimated difference between the two local anesthetic treatment groups for the coprimary outcome measure 0 to 10 numerical rating scale average pain score at 3 months postradiofrequency ablation. The assumptions included that 50% of participants in the intraarticular and medial branch block groups would undergo radiofrequency ablation, a baseline pain score of 6.2 ± 1.9 in each group, and postprocedure mean numerical rating scale pain scores of 4.4 in the intraarticular group and 3.4 in the medial branch blocks group, with a common SD of 1.45.31 Using an alpha level of 0.016 to correct for a type I error with three treatment groups, we determined that 31 patients per group who received radiofrequency ablation would have 80% power to detect a difference of 1.2 in pain scores and that 40 participants per group would have 90% power. To account for an anticipated 10% dropout rate, 90 participants in groups I and II were needed. Group III (saline) patients formed the control group; assuming that their 3-month postradiofrequency ablation average numerical rating scale pain score would be 5.4 ± 1.45, we determined that there was a 99% chance of detecting a significant difference between groups II and III in the coprimary outcome measure. Since the two primary end points were designed to address different study outcomes, each was assigned a 5% type I error rate.
All statistical analyses were performed in Stata 14 (StataCorp, USA). The coprimary outcomes of difference in numerical rating scale pain score at 1 month after facet injection and 3 months after radiofrequency ablation among the three treatment/control groups were evaluated with an ANOVA. For continuous outcomes, group means and standard deviations are reported, and ANOVA was used to compare the three treatment/control groups. For categorical outcomes, percentages are reported, with chi-square tests or Fisher exact tests used as indicated in the results, based on expected cell counts. For comparisons between two groups, a P value less than alpha of 0.05 was considered statistically significant, whereas for comparisons among three groups, a P value less than the Bonferroni-corrected alpha of 0.05/3 = 0.016 was deemed statistically significant.
The primary outcome variable, 0 to 10 numerical rating scale average pain score, was measured at 1 month after facet injection or 3 months after radiofrequency ablation. Data were analyzed by an intention-to-treat approach, with the last observation carried forward for the radiofrequency ablation data. Given the high proportion of patients exiting the study per protocol after facet injection, no observations were carried forward and only descriptive statistics were calculated for facet injection follow-up beyond 1 month.
In order to identify potential differences in covariates between positive and negative outcome groups for facet injections at 1 month and radiofrequency ablation at 3 months, Student’s t tests were used for parametric data and Mann–Whitney U tests were used for nonparametric data. Covariates in the analysis included treatment group, block outcome, age, sex, duration of pain, number of levels, unilateral versus bilateral procedure, opioids, military status, disability, inciting event, other pain conditions, comorbid psychiatric conditions, traumatic brain injury, smoking status, preprocedure pain scores, and Oswestry disability index. In order to determine factors associated with treatment outcome after facet injections at 1 month and radiofrequency ablation at 3 months, exploratory multivariable logistic-regression analyses were performed with the same covariates identified above in a backward stepwise approach. A two-sided P value less than 0.05 was considered to indicate statistical significance. A nonparametric bootstrap method using 500 resamples was used to internally validate the proposed logistic-regression models.
Results
Among the 229 patients enrolled in the study, 228 completed the assigned facet injection, and 208 completed the study per protocol. Table 1 shows baseline demographic and clinical data by group assignment. There were no statistically significant differences in any variables at baseline among the three groups.
Table 1.
Baseline Demographic and Clinical Characteristics of Study Participants
| Characteristic | Intraarticular Facet Block (n = 91) |
Medial Branch Block (n = 91) |
Saline Injection (n = 47) |
Total (n = 229) |
|---|---|---|---|---|
| Age, years (mean ± SD) | 48±15 | 46±13 | 48±15 | 47±14 |
| Female (n, %) | 27 (30%) | 34 (37%) | 17 (36%) | 78 (34%) |
| Duration of pain, years (mean ± SD) | 8±7 | 6±6 | 5±5 | 7±7 |
| Levels (mean ± SD) | 2.2±0.6 | 2.0±0.5 | 2.1±0.4 | 2.1±0.5 |
| Bilateral (n, %) | 60 (66%) | 63 (69%) | 27 (57%) | 150 (66%) |
| Opioid use | ||||
| None | 76 (84%) | 79 (87%) | 39 (83%) | 194 (85%) |
| < 90 mg daily morphine equivalents | 15 (16%) | 11 (12%) | 8 (17%) | 34 (15%) |
| ≥90 mg daily morphine equivalents | 0 | 1 (1%) | 0 | 1 (0.4%) |
| Military status (n, %) | ||||
| Officer | 15 (16%) | 14 (15%) | 5 (11%) | 114, 50% |
| Enlisted | 29 (32%) | 34 (38%) | 18 (38%) | 81, 35% |
| Civilian | 47 (52%) | 43 (47%) | 24 (51%) | 34, 15% |
| Disability, worker’s compensation, or medical board* | 13 (14%) | 14 (15%) | 9 (19%) | 36 (16%) |
| Inciting event (n, %) | ||||
| Motor vehicle collision | 5 (5%) | 4 (4%) | 1 (2%) | 10 (4%) |
| Fall | 5 (5%) | 6 (7%) | 0 | 11 (5%) |
| Work related | 18 (20%) | 11 (12%) | 5 (11%) | 34 (15%) |
| Sports | 10 (11%) | 8 (9%) | 8 (17%) | 26 (11%) |
| Other | 7 (8%) | 9 (10%) | 3 (6%) | 19 (8%) |
| None | 46 (51 %) | 55 (60%) | 30 (64%) | 131 (43%) |
| Concomitant pain condition (n, %) | ||||
| Neck pain | 21 (23%) | 19 (21%) | 7 (15%) | 47 (21%) |
| Arthralgia | 30 (33%) | 35 (38%) | 15 (32%) | 80 (35%) |
| Headache | 8 (9%) | 5 (5%) | 2 (4%) | 15 (7%) |
| Neuropathic | 3 (3%) | 4 (4%) | 1 (2%) | 8 (4%) |
| Other | 11 (12%) | 15 (16%) | 4 (9%) | 30 (13%) |
| None | 35 (38%) | 33 (36%) | 24 (51%) | 137 (60%) |
| Coexisting psychiatric condition (n, %) | ||||
| Mood | 19 (21%) | 20 (22%) | 6 (13%) | 45 (20%) |
| Anxiety | 13 (14%) | 20 (22%) | 7 (15%) | 40 (18%) |
| Posttraumatic stress disorder† | 9 (10%) | 9 (10%) | 3 (6%) | 21 (9%) |
| Substance abuse† | 2 (2%) | 2 (2%) | 1 (2%) | 5 (2%) |
| Other | 3 (3%) | 3 (3%) | 1 (2%) | 7 (3%) |
| None | 61 (67%) | 55 (61%) | 35 (74%) | 78 (34%) |
| Deployment related (n, %) | 9 (10%) | 11 (12%) | 4 (9%) | 24 (11%) |
| Traumatic brain injury (n, %) | 4 (4%) | 6 (7%) | 1 (2%) | 11 (5%) |
| Smoking (n, %) | 14 (15%) | 17 (19%) | 7 (15%) | 38 (17%) |
| Average numerical rating scale back pain (mean ± SD) | 5.5±1.6 | 5.4±1.4 | 5.1±1.5 | 5.4±1.5 |
| Worst numerical rating scale back pain (mean ± SD) | 8.0±1.4 | 8.2 ±1.4 | 7.7±1.5 | 8.0±1.4 |
| Oswestry disability score (mean ± SD) | 37±15 | 35±13 | 34±14 | 35±14 |
Military equivalent of civilian disability.
Denotes controlled or inactive.
Facet Injection Study Phase
Postblock Pain Relief.
Treatment results following facet injection are shown in table 2. In patients who underwent facet injection, 50% had a positive block in the immediate postprocedural period; this difference was significantly different in the three treatment groups (54% vs. 55% vs. 30% for the intraarticular, medial branch block, and placebo groups, respectively; P = 0.946 for intraarticular vs. medial branch block group, P = 0.006 for intraarticular vs. placebo group, and P = 0.005 for medial branch block vs. placebo group; overall P = 0.010). There were no significant differences among the three groups in terms of numerical percent reduction in preblock pain score (P = 0.999 for intraarticular vs. medial branch block group, P = 0.188 for intraarticular vs. placebo group, and P = 0.195 for medial branch block vs. placebo group; overall P = 0.163).
Table 2.
Treatment Results and Disposition after Facet Block
| Intraarticular Facet Block |
Medial Branch Block |
Saline Injection |
P Value* | ||
|---|---|---|---|---|---|
| Facet block | Number of participants assessed | 90 | 91 | 47 | |
| Percent reduction in preblock pain score (mean ± SD)† | 47±31 | 47±32 | 37±31 | 0.163 | |
| Facet block positive‡ (number, %) | 49 (54%) | 50 (55%) | 14 (30%) | 0.010 | |
| Complications (number, %) | 6 (7%) | 8 (9%) | 2 (4%) | 0.617 | |
| 1-Month outcomes | Number of participants assessed | 89 | 91 | 47 | |
| Reduction in average NRS pain score from baseline (mean ± SD) | 0.7±1.6 | 0.7±1.8 | 0.7±1.5 | 0.993 | |
| Reduction in worst NRS pain score from baseline (mean ± SD) | 1.0±1.9 | 0.9±1.9 | 1.1±2.1 | 0.928 | |
| Reduction in ODI score (mean ± SD) | 3±9 | 3±11 | 2±11 | 0.668 | |
| Medication reduction§ (n/N, %) | 10/75 (13%) | 9/74 (12%) | 6/35 (17%) | 0.775 | |
| Satisfaction score∥ (mean ± SD) | 3.3±1.3 | 3.1±1.3 | 3.1±1.2 | 0.378 | |
| Positive outcome# (n, %) | 11 (12%) | 10 (11%) | 3 (6%) | 0.6177 | |
| 3-Month outcomes | Number of participants assessed | 9 | 9 | 3 | |
| Average NRS pain score (mean ± SD) | 3.2±2.2 | 2.5±2.0 | 2.3±1.2 | 0.724 | |
| Worst NRS pain score (mean ± SD) | 5.0±2.8 | 4.8±2.2 | 7.2±3.7 | 0.410 | |
| ODI score (mean ± SD) | 30±13 | 17±12 | 28±12 | 0.093 | |
| Medication reduction§ (n/N, %) | 1/7 (14%) | 1/5 (33%) | 1/3 (33%) | > 0.9997 | |
| Satisfaction score∥ (mean ± SD) | 4.2±0.9 | 4.2±1.2 | 4.2±1.0 | 0.993 | |
| Positive outcome# (n, %) | 4 (4%) | 4 (4%) | 1 (2%) | > 0.9997 | |
| 6-Month outcomes | Number of participants assessed | 4 | 1 | 0 | — |
| Average NRS pain score (mean ± SD) | 2.6±1.7 | 6 | – | 0.174 | |
| Worst NRS pain score (mean ± SD) | 5.3±3.9 | 8 | – | 0.577 | |
| ODI score (mean ± SD) | 22±9 | 40 | – | 0.148 | |
| Medication reduction§ (n/N, %) | 0/2 (0%) | 0/1 (0%) | – | – | |
| Satisfaction score∥ (mean ± SD) | 4.6±0.8 | 5 | – | 0.685 | |
| Positive outcome# (n, %) | 3 (3%) | 0 (0%) | 0 (0%) | 0.400** | |
| Eligible for radiofrequency (n, %)†† | 46 (51%) | 49 (54%) | 47 (100%) | 0.713 (Groups I and II only) | |
| Proceeded to radiofrequency at any time (n, %)†† | 45 (49%) | 48 (53%) | 42 (89%) | 0.712 (Groups I and II only) | |
For continuous variables, P values for three-way ANOVA are shown. For categorical variables, P values for chi-square tests are shown, except for those in which cell count was less than 5, in which case the P value for Fisher exact test is shown and denoted as below.
Percent pain relief from diagnostic injection calculated by independent observer, based on comments and recorded pain scores from pain diary.
Positive block defined as 50% or more pain relief from baseline lasting 3h or more.
Medication reduction defined as a more than 20% reduction in opioid dose from baseline or complete cessation of a nonopioid analgesic.
Satisfaction score on 1 to 5 scale.
Positive outcome defined as 50% or more pain relief from baseline at rest combined with satisfaction score of 3 out of 5 or higher.
Based on Fisher exact test.
Facet (group I) and medial branch (group II) block patients with (+) block who fail to experience a positive categorical outcome at follow-up designated for radiofrequency denervation per protocol; all saline (group III) patients who fail to experience a positive outcome designated tor radiofrequency denervation.
NRS = numerical rating scale; ODI = Oswestry Disability Index score.
1-Month Coprimary Outcome Measure and Follow-ups.
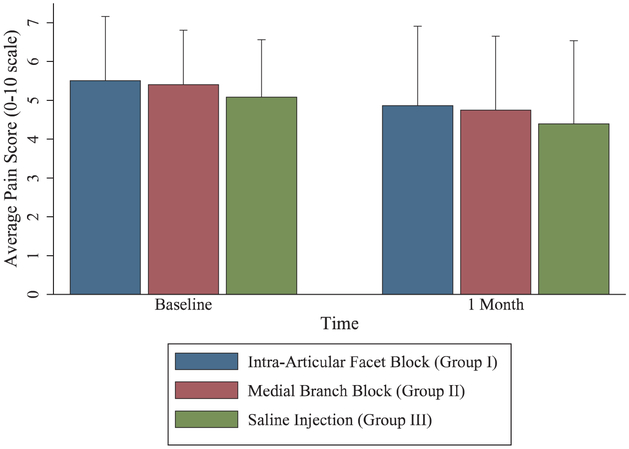
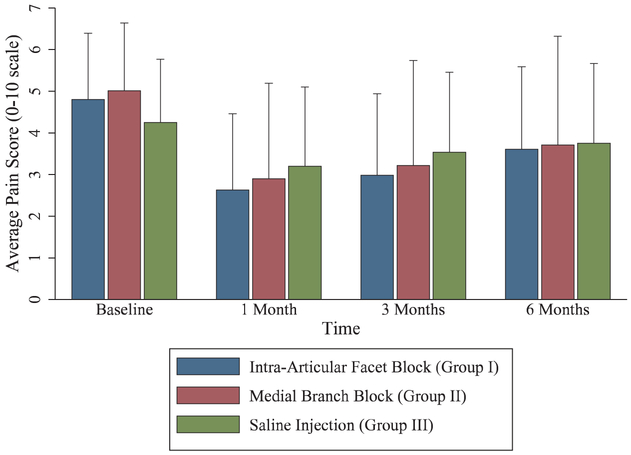
At 1 month after facet injection, only 24 patients had a positive categorical outcome. For the intraarticular and medial branch block groups, 12% and 11% of patients, respectively, had a positive categorical outcome, compared to 6% in the control group (P = 0.820 for intraarticular vs. medial branch block group, P = 0.379 for intraarticular vs. placebo, P = 0.542 for medial branch block vs. placebo group). The coprimary outcome of mean reduction in average numerical rating scale pain score at 1 month was 0.7 ± 1.6 (95% CI, 0.3 to 1.0) in the intraarticular group, 0.7 ± 1.8 (95% CI, 0.3 to 1.0) in the medial branch block group, and 0.7 ± 1.5 (95% CI, 0.2 to 1.1) in the placebo group (P = 0.999 for intraarticular vs. medial branch block, P = 0.997 intraarticular vs. placebo group, and P = 0.992 for medial branch block vs. placebo group). There were also no significant differences in the secondary outcome measures medication reduction, Oswestry disability index, and satisfaction scores. In the intraarticular group, 39 participants (44%) exited per protocol due to a negative diagnostic block and 40 (45%) qualified for radiofrequency ablation on the basis of a positive diagnostic block. Medial branch block group outcomes were similar, with 39 (43%) exiting per protocol and 43 (47%) proceeding to radiofrequency ablation. Due to the high rates of negative outcomes at 1 month, few participants continued in the facet phase follow-up beyond 1 month (fig. 4). Overall, during the entire facet portion of the study, 51% of the intraarticular group and 54% of the medial branch block group participants became eligible for radiofrequency ablation (P = 0.713), with 49% and 53%, respectively, proceeding to denervation (P = 0.712).
Fig. 4.
Pain relief after different lumbar facet blocks. Data presented as means with error bars representing 1 SD. For outcomes, n = 89 for intraarticular group, 91 for medial branch group, and 47 for placebo group.
Radiofrequency Ablation Study Phase
1-Month Follow-up.
Treatment results following radiofrequency ablation with a last-observation-carried-forward approach are shown in table 3. The mean reduction in average numerical rating scale pain score 1 month after ablation was 2.2 ± 2.1 (95% CI, 1.6 to 2.8) in the intraarticular group, 2.1 ± 2.0 (95% CI, 1.5 to 2.7) in the medial branch block group, and 1.0 ± 1.6 (95% CI, 0.5 to 1.5) in the control group (P = 0.986 for intraarticular vs. medial branch block group, P = 0.017 for intraarticular vs. placebo group, P = 0.023 for medial branch block vs. placebo group), with similar reductions in worst numerical rating scale pain score and medication use. There was a significant difference in patients experiencing a positive categorical outcome at 1 month among the three groups, with 67% of the intraarticular group, 73% of the medial branch block group, and 38% of the control group participants reporting 2-point or more average pain relief and a satisfaction score higher than 3 out of 5 (P = 0.511 for intraarticular vs. medial branch block group, P = 0.008 for intraarticular vs. placebo group, P = 0.001 for medial branch block vs. placebo group).
Table 3.
Treatment Results and Disposition after Radiofrequency Ablation
| Intraarticular Facet Block (n = 45)* |
Medial Branch Block (n = 48)* |
Saline Injection (n = 42)* |
P Value† | ||
|---|---|---|---|---|---|
| PreRFA | Average NRS pain score (mean ± SD) | 4.8±1.6 | 5.0±1.6 | 4.3±1.5 | 0.067 |
| Worst NRS pain score (mean ± SD) | 7.3±1.9 | 7.4±1.8 | 6.6±1.7 | 0.096 | |
| ODI score, (mean ± SD) | 35±15 | 32±12 | 31±13 | 0.406 | |
| RFA complications (n, %) | 3 (7%) | 2 (4%) | 8 (19%) | 0.052‡ | |
| 1-Month RFA outcomes | Number of participants assessed | 45 | 48 | 42 | |
| Average NRS pain score (mean ± SD) | 2.6±1.8 | 2.9±2.3 | 3.2±1.9 | 0.421 | |
| Reduction in average NRS pain score from baseline (mean ± SD) | 2.2±2.1 | 2.1±2.0 | 1.0±1.6 | 0.009 | |
| Worst NRS pain score (mean ± SD) | 4.5±2.4 | 4.7±2.8 | 5.4±2.4 | 0.236 | |
| Reduction in worst NRS pain score from baseline (mean ± SD) | 2.9±2.5 | 2.7±2.4 | 1.3±2.2 | 0.003 | |
| ODI score (mean ± SD) | 25±15 | 25±16 | 27±17 | 0.867 | |
| Reduction in ODI score (mean ± SD) | 10±12 | 7±13 | 4±13 | 0.098 | |
| Medication reduction§ (n/N, %) | 11/39 (28%) | 18/44 (42%) | 5/33 (15%) | 0.048 | |
| Satisfaction score∥ (mean ± SD) | 4.1±1.1 | 3.9±1.1 | 3.8±1.2 | 0.380 | |
| Positive outcome# (n, %) | 30 (67%) | 35 (73%) | 16 (38%) | 0.002 | |
| 3-Month RFA outcomes | Number of participants assessed | 29 | 34 | 16 | |
| Average NRS pain score** (mean ± SD) | 3.0±2.0 | 3.2±2.5 | 3.5±1.9 | 0.493 | |
| Reduction in average NRS pain score from baseline (mean ± SD) | 1.8±2.3 | 1.8±2.4 | 0.7±1.5 | 0.025 | |
| Worst NRS pain score** (mean ± SD) | 4.9±2.4 | 5.5±3.0 | 5.8±2.6 | 0.316 | |
| Reduction in worst NRS pain score from baseline (mean ± SD) | 2.4±2.8 | 1.9±2.8 | 0.8±2.4 | 0.024 | |
| ODI score** (mean ± SD) | 26±16 | 26±18 | 29±18 | 0.726 | |
| Reduction in ODI score (mean ± SD) | 9±13 | 7±13 | 3±12 | 0.081 | |
| Medication reduction§ (n/N, %) | 11/25 (44%) | 16/30 (55%) | 2/10 (20%) | 0.185 | |
| Satisfaction score∥ (mean ± SD) | 4.4±1.0 | 4.3±0.9 | 4.3±0.9 | 0.792 | |
| Positive outcome#,** (n, %) | 23 (51%) | 27 (56%) | 10 (24%) | 0.005 | |
| 6-Month RFA outcomes | Number of participants assessed | 20 | 22 | 10 | |
| Average NRS pain score** (mean ± SD) | 3.6±2.0 | 3.7±2.6 | 3.8±1.9 | 0.951 | |
| Reduction in average NRS pain score from baseline (mean ± SD) | 1.2±2.1 | 1.3±2.3 | 0.5±1.5 | 0.134 | |
| Worst NRS pain score** (mean ± SD) | 5.7±2.6 | 5.9±3.0 | 5.9±2.5 | 0.865 | |
| Reduction in worst NRS pain score from baseline (mean ± SD) | 1.7±2.7 | 1.5±2.6 | 0.7±2.2 | 0.180 | |
| ODI score** (mean ± SD) | 26±16 | 26±18 | 29±18 | 0.665 | |
| Reduction in ODI score (mean ± SD) | 6±14 | 6±13 | 1±12 | 0.170 | |
| Medication reduction§ (n/N, %) | 8/18 (44%) | 6/19 (32%) | 0/3 (0%) | 0.298 | |
| Satisfaction score∥ (mean ± SD) | 4.3±1.1 | 4.5±1.0 | 4.7±0.7 | 0.647 | |
| Positive outcome#,** (n, %) | 14 (31%) | 20 (42%) | 7 (17%) | 0.036 | |
Facet (group I) and medial branch (group II) block patients with (+) block who fail to experience a positive categorical outcome at follow-up designated for RF denervation per protocol; all saline (group III) patients who fail to experience a positive outcome designated for radiofrequency denervation.
For continuous variables, P values for three-way ANOVA are shown. For categorical variables, P values for chi-square tests are shown, except for those in which cell count was less than 5, in which case the P value for Fisher exact test is shown and denoted as below.
Based on Fisher exact test.
Medication reduction defined as a more than 20% reduction in opioid dose from baseline or complete cessation of a nonopioid analgesic, for patients who were assessed at that follow-up visit (i.e., medication reduction was not carried forward).
Satisfaction score on 1 to 5 scale, for patients who were assessed at that follow-up visit (i.e., satisfaction scores were not carried forward).
Positive outcome defined as 50% or more pain relief from baseline at rest combined with a satisfaction score of 3 out of 5 or higher.
For treatment failures at 1 and 3 months, last observed carried forward used for 3- and 6-month follow-up scores, respectively. NRS = numerical rating scale; ODI = Oswestry Disability Index score; RFA = radiofrequency ablation.
3-Month Radiofrequency Coprimary Outcome Measure and Follow-ups.
At the primary outcome time point of 3 months after radiofrequency ablation, there were no significant differences among the three groups in average or worst numerical rating scale score, and secondary outcome measures Oswestry disability index score, medication reduction, or satisfaction. The average numerical rating scale pain score at 3 months was 1.8 ± 2.3 (95% CI, 1.1 to 2.5) in the intraarticular group, 1.8 ± 2.4 in the medial branch block group (95% CI, 1.1 to 2.5), and 0.7 ± 1.5 (95% CI, 0.3 to 1.2) in the control group (P = 0.998 for intraarticular vs. medial branch block group, P = 0.044 for intraarticular vs. placebo group, P = 0.046 for medial branch block vs. placebo group). Among other secondary outcomes, only 10 (24%) control group patients experienced a continued positive categorical outcome 3 months after radiofrequency ablation, compared to 23 (51%) and 27 (56%) in the intraarticular and medial branch block groups, respectively (P = 0.619 for intraarticular vs. medial branch block group, P = 0.009 for intraarticular vs. placebo group, P = 0.002 for medial branch block vs. placebo group). At the final follow-up at 6 months after radiofrequency ablation, 14 (31%) of the intraarticular group, 20 (42%) of the medial branch block group, and 7 (17%) of control group participants had ongoing pain relief and satisfaction. There were no other significant differences among groups at 6 months (fig. 5).
Fig. 5.
Pain relief after lumbar facet radiofrequency ablation stratified by treatment group. Data presented as means with error bars representing 1 SD. For outcomes, n = 45 for intraarticular group, 48 for medial branch group, and 42 for placebo group.
Cumulative Pain Relief
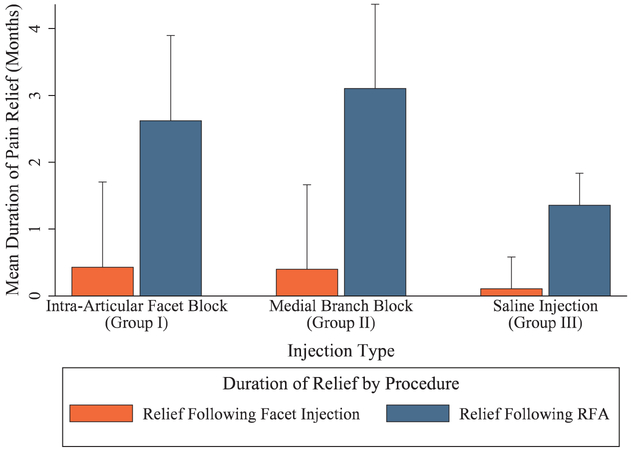
Figure 6 shows the cumulative pain relief (i.e., additive pain relief from facet block and radiofrequency ablation) broken down by treatment group. For patients with a positive block after an intraarticular facet injection, the mean total duration of pain relief was 2.8 ± 2.7 months, which broke down as 0.4 ± 1.3 months after the facet block and 2.6 ± 2.5 months after radiofrequency ablation. Patients with a positive medial branch block had a mean total duration of pain relief of 3.4 ± 2.8 months, or 0.4 ± 1.3 months after the medial branch block and 3.1 ± 2.6 months after radiofrequency ablation. The mean duration of pain relief was not significantly different between the two facet block groups (P = 0.540). The mean total duration of pain relief for all patients receiving placebo injections was 1.5 ± 2.3 months, or 0.1 ± 0.5 month after the injection and 1.4 ± 2.3 months after radiofrequency ablation, which was significantly different from intraarticular facet (P = 0.016) and medial branch block (P = 0.001) patients.
Fig. 6.
Cumulative pain relief broken down by study group. Relief after facet injection calculated based on the total number of months of pain relief from the diagnostic block, divided by the people who had a positive diagnostic block for groups I and II, or all group III participants. Relief after radiofrequency ablation (RFA) calculated based on the total number of months of pain relief from the radiofrequency ablation, divided by the number of participants undergoing RFA within each respective group. Data presented as means with SD error bars representing 1 SD. For facet injection outcomes, n = 49 for intraarticular group, 50 for medial branch group, and 47 for placebo group. For radiofrequency ablation outcomes, n = 45 for intraarticular group, 48 for medial branch group, and 42 for placebo group.
Factors Associated with Treatment Outcomes
The association between individual demographic factors and treatment outcome 1 month after facet block and 3 months after radiofrequency ablation is shown in table 4. There were no significant differences between those experiencing positive or negative outcomes after either procedure, with the exception of a lower preprocedural Oswestry disability index score in patients with a positive categorical outcome (P = 0.01).
Table 4.
Factors Associated with Treatment Outcome after Facet Block and Radiofrequency Denervation
| Characteristic | Negative 1-Month Outcome after Facet Block (N = 203) |
Positive 1-Month Outcome after Facet Block (N = 24) |
P Value* | Negative 3-Month Outcome after Radiofrequency Denervation (N = 74) |
Positive 3-Month Outcome after Radiofrequency Denervation (N = 60) |
P Value |
|---|---|---|---|---|---|---|
| Age (mean ± SD) | 48±14 | 46±11 | 0.640 | 48±14 | 46±14 | 0.441 |
| Female (n, %) | 71 (35%) | 7 (29%) | 0.571 | 25 (33%) | 23 (38%) | 0.546 |
| Duration of pain in years (mean ± SD) | 7±7 | 5±5 | 0.473† | 7±7 | 6±5 | 0.852† |
| Levels (mean ± SD) | 2.1±0.5 | 2.0±0.6 | 0.723† | 2.1±0.5 | 2.1±0.4 | 0.816† |
| Bilateral (n, %) | 133 (66%) | 16 (64%) | 0.911 | 51 (68%) | 36 (60%) | 0.335 |
| Opioid use (n, %) | 33 (16%) | 2 (8%) | 0.586† | 16 (21%) | 6 (10%) | 0.178 |
| Military status (n, %) | 0.434 | 0.388 | ||||
| Officer | 31 (15%) | 2 (8%) | 9 (12%) | 12 (20%) | ||
| Enlisted | 69 (34%) | 11 (48%) | 27 (36%) | 22 (37%) | ||
| Civilian | 103(51%) | 11 (44%) | 39 (52%) | 26 (43%) | ||
| Disability, worker’s compensation, or medical board‡ | 34 (17%) | 2 (8%) | 0.385† | 18 (24%) | 7 (12%) | 0.067 |
| Inciting event (n, %) | ||||||
| Motor vehicle collision | 9 (4%) | 1 (4%) | > 0.999† | 2 (3%) | 3 (5%) | 0.655† |
| Fall | 9 (4%) | 2 (8%) | 0.328† | 2 (3%) | 3 (5%) | 0.655† |
| Work related | 31 (15%) | 3 (13%) | > 0.999† | 8 (11%) | 9 (15%) | 0.451 |
| Sports | 23 (11%) | 3 (13%) | 0.744† | 10 (13%) | 10 (16%) | 0.588 |
| Other | 17 (8%) | 2 (8%) | > 0.999† | 10 (13%) | 4 (7%) | 0.262† |
| None | 116 (57%) | 13 (54%) | 0.781 | 43 (57%) | 32 (53%) | 0.642 |
| Concomitant pain condition (n, %) | ||||||
| Neck pain | 39 (19%) | 6 (25%) | 0.501 | 12 (16%) | 11 (18%) | 0.720 |
| Arthralgia | 75 (37%) | 5 (21%) | 0.118 | 30 (40%) | 16 (27%) | 0.104 |
| Headache | 13 (6%) | 1 (4%) | > 0.999† | 5 (7%) | 3 (5%) | 0.732† |
| Neuropathic | 6 (3%) | 2 (8%) | 0.202† | 1 (1%) | 3 (5%) | 0.323† |
| Other | 29 (14%) | 0 | 0.051† | 6 (8%) | 6 (10%) | 0.685 |
| None | 79 (39%) | 13 (54%) | 0.150 | 32 (43%) | 29 (48%) | 0.511 |
| Coexisting psychiatric condition (n, %) | ||||||
| Mood | 40 (20%) | 5 (21%) | 0.896 | 19 (25%) | 11 (18%) | 0.331 |
| Anxiety | 36 (18%) | 4 (17%) | > 0.999† | 17 (23%) | 9 (15%) | 0.262 |
| PTSD§ | 18 (9%) | 3 (13%) | 0.472† | 7 (9%) | 4 (7%) | 0.754† |
| Substance abuse§ | 4 (2%) | 1 (4%) | 0.431† | 2 (3%) | 3 (5%) | 0.655† |
| Other | 7 (3%) | 0 | > 0.999† | 2 (3%) | 2 (3%) | > 0.999† |
| None | 133 (66%) | 16 (67%) | 0.911 | 47 (63%) | 41 (68%) | 0.492 |
| Deployment related (n, %) | 22 (11%) | 2 (8%) | 0.701 | 6 (8%) | 8 (13%) | 0.325 |
| Traumatic brain injury (n, %) | 10 (5%) | 1 (4%) | > 0.999† | 3 (4%) | 4 (7%) | 0.487 |
| Smoking (n, %) | 32 (16%) | 5 (21%) | 0.525 | 10 (13%) | 9 (15%) | 0.782 |
| Average NRS back pain (mean ± SD) | 5.4±1.5 | 4.9±1.6 | 0.090† | 5.2±1.5 | 5.4±1.6 | 0.439† |
| Worst NRS back pain (mean ± SD) | 8.1±1.4 | 7.7± 1.5± | 0.210† | 8.1±1.4 | 8.0±1.6 | 0.979† |
| Oswestry disability score (mean ± SD) | 33±13 | 30±13 | 0.014† | 35±14 | 30±12 | 0.138† |
| Percent relief from diagnostic | (n = 158) | (n = 21) | 0.016 | (n = 42) | (n = 50) | 0.864 |
| block (mean ± SD)∥ | 44±32% | 60±32% | 74±15% | 73±17% | ||
| Percent relief from diagnostic | (n = 202) | (n = 24) | 0.037 | (n = 74) | (n = 60) | 0.004 |
| block (mean ± SD)# | 46±32% | 61±32% | 56±31% | 70±21% |
For continuous variables, P values for parametric tests are shown, except for nonparametric data, for which P values for the nonparametric tests are shown, as denoted below.
Based on nonparametric test.
Service members with a medical condition that renders them unfit for duty enter an integrated disability evaluation system to determine their eligibility to remain on active duty (i.e., equivalent to a civilian disability hearing).
Denotes controlled or inactive.
Excludes group III patients.
Includes group III patients.
NRS = numerical rating scale; PTSD = posttraumatic stress disorder.
After an exploratory multivariate logistic-regression analysis, treatment group, block outcome, age, sex, duration of pain, number of levels, unilateral vs. bilateral procedure, opioids, active duty and deployment status, disability, inciting event, other pain conditions, comorbid psychiatric conditions, traumatic brain injury, smoking status, preprocedure pain scores, and Oswestry disability index were not significantly associated with outcome at 1 month after facet injection. The strongest predictor of a positive categorical outcome at 3 months after radiofrequency ablation was the presence of a positive diagnostic block. Patients with a positive block had a 6.87 (95% CI, 2.32 to 20.33; P < 0.001) times increased odds of a positive categorical outcome compared to those who had a negative block. In the same regression analysis, the odds of a positive outcome were decreased by 0.67 (95% CI, 0.51 to 0.91; P = 0.009) per 10-point increase in preprocedural Oswestry disability index. There was a 0.32 (95% CI, 0.11 to 0.92; P = 0.034) times decreased odds of a positive radiofrequency ablation outcome for patients on preprocedural opioids compared to those not on opioids. Nonparametric bootstrap estimates in the logistic-regression model were similar for the three covariates noted: block outcome (OR, 6.87; 95% CI, 2.04 to 23.13; P = 0.002), preprocedural Oswestry disability index (OR, 0.68; 95% CI, 0.50 to 0.92; P = 0.012), and preprocedural opioid use (OR, 0.32; 95% CI, 0.10 to 1.07; P = 0.064). No other covariates assessed were significantly associated with radiofrequency ablation outcomes at 3 months.
Among all intraarticular group injections, 71% were deemed to be intraarticular (i.e., 29% failure rate), and 36% of intraarticular group participants had arthrograms visible for all joints targeted (i.e., all intraarticular). When stratified by injection location, there were no differences in the proportion of positive blocks (52% vs. 60%; P = 0.492), 1-month facet outcomes (18% vs. 8%; P = 0.170) or 3-month radiofrequency ablation outcomes (54% vs. 45%; P = 0.588) between those individuals in whom all injections were intraarticular and those in whom some were periarticular, respectively. Other covariates examined were also not significantly associated with radiofrequency treatment outcome at 3 months.
Effectiveness of Blinding
Among the 223 patients who were asked to guess their facet treatment allocation, 94 (42%) did not know at all. In the 129 patients that did guess, 35 (27% of guessers, 16% overall) correctly guessed their treatment allocation, which is approximately equal to the one-third probability of a correct guess by random chance.
Complications
There were no serious adverse events after any of the facet injections (table 5). There were no differences in incidence of complications after facet injection (P = 0.600). Facet injection complications were minor, occurring in 7% of patients, and included rash, localized skin infection, vasovagal episode, nausea, numbness, and worsening pain. A total of 13 patients (10%) developed adverse events after radiofrequency ablation, 7 of whom experienced minor events. Of the four serious adverse events reported, three were judged to be unrelated to the procedure, and one was a case of suspected medial branch neuritis resulting in an emergency department visit for worsening axial pain.
Table 5.
Adverse Events by Treatment
| Adverse Event | Intraarticular Facet Block (n = 91) |
Medial Branch Block (n = 92) |
Saline Block (n = 47) |
Radiofrequency Denervation (n = 136) |
|---|---|---|---|---|
| Serious adverse event* | 0 | 0 | 0 | 4 (3%)† |
| Minor adverse event‡ | 5 (6%) | 8 (9%) | 2 (4%) | 7 (5%) |
| Worsening pain | ||||
| Neurologic | 1 (1%) | 2 (2%) | – | 1 (1%) |
| Nonneurologic | 1 (1%) | 1 (1%) | – | 1 (1%) |
| Neuritis | – | – | – | 3 (2%) |
Serious adverse events were as defined by the Food and Drug Administration (resulting in death, life-threatening adverse drug experience, inpatient or prolonged hospitalization, disability or permanent damage, congenital anomaly or birth defect, or required intervention to prevent permanent impairment or damage).
Includes three events that were judged to be unrelated to procedure (emergency department visit with unspecified chest pain two months after procedure, admission for suicidal ideation unrelated to low back pain, and suicide unrelated to low back pain). One patient went to the emergency department for suspected medial branch neuritis that resolved within four days.
Included rash, localized skin infection, vasovagal episode, nausea, numbness, and worsening pain.
Discussion
The main findings in this placebo-controlled study are that facet injections are not therapeutic, and that while prognostic medial branch block and intraarticular injections may be associated with superior benefit in some outcomes before radiofrequency ablation compared to saline, with statistical correction there were no significant differences between groups for the coprimary outcome measure.
Facet Joint Study Phase Outcomes: Comparison to Other Studies
Our results are consistent with clinical trials and systematic reviews that show negative evidence for the therapeutic value of facet blocks.2–4,8,9 Although studies by Manchikanti et al.19,49 showed some benefit for lumbar medial branch blocks done with or without steroid, these studies lacked a true control group. Deriving long-term benefit from medial branch block would be analogous to the long-term treatment of knee arthritis from a local anesthetic block performed at the nerve(s) providing sensory innervation, which is inconsistent with the rationale behind the use of local anesthetic blocks as a prognostic procedure before radiofrequency ablation,50 and supported by only a single, underpowered study.19,49 They are also inconsistent with the results of most randomized trials, which failed to report that a significant proportion of patients experienced prolonged benefit from screening medial branch blocks.25,26,30,51 Although Nath et al.28 did report that a substantial percentage of people experienced “prolonged” relief outlasting the expected duration of analgesia during comparative local anesthetic blocks (wherein one should obtain longer pain relief from bupivacaine than lidocaine), the duration of benefit was not noted. Possible causes of prolonged relief from medial branch blocks and intraarticular steroids include entrapment of the medial branch beneath the mamilloaccessory ligament, which can occur in up to 20% of people at L5,20 and active inflammation of the joint.18
Radiofrequency Ablation Study Phase Outcomes: Comparison to Other Studies
The second part of our study examined whether medial branch block or intraarticular was better as a prognostic procedure before radiofrequency ablation. As noted above, the frequent contention that medial branch blocks and intraarticular blocks are diagnostically comparable are based on old studies that contained myriad technical limitations (e.g., not prescreening participants with diagnostic blocks, measurement of diagnostic effect at inappropriate intervals, use of excessive volumes, not using contrast to ensure proper positioning).39,40 We hypothesized that medial branch blocks would be superior for reasons that could include the following: less procedure-related pain (i.e., lower false-negative rate); the high failure rate of intraarticular injections, which were confirmed in our study; aberrant, nonmedial branch innervation in some people; greater face validity; and the extrapolation of clinical trials examining the prognostic value of other nerve blocks before ablation (e.g., celiac plexus neurolysis).52 Our studies are in contrast to a case-control study (n = 510) by Cohen et al.,31 in which the prognostic value of medial branch blocks and intraarticular injections performed by the same practitioners were compared to each other before radiofrequency ablation, with medial branch blocks found to be superior. They are also in contradistinction to a small (n = 32) randomized study by Birkenmaier et al.,32 which, similar to the study by Cohen et al.,31 found that medial branch blocks were associated with a better outcome than pericapsular facet blocks before cryodenervation. Notably, extraarticular injections are not advocated by any organization as a diagnostic or prognostic tool before radiofrequency ablation,36–38,53 although one might expect anesthetization of the medial branches in some instances. Comparisons of outcomes between studies that screened participants with medial branch blocks and intraarticular injections also suggest a slight benefit for the former, although other differences in methodology render a metaanalysis fraught with difficulty. Possible explanations for the lack of difference observed include methodologic flaws in the studies by Cohen et al.31 and Birkenmaier et al.,32 overly optimistic statistical assumptions that resulted in the study being underpowered, the robust placebo response we observed, and that no meaningful differences between the predictive value of the two blocks exists.
Technical Success Rate for Intraarticular Injections
We found a substantial failure rate (29%) of intraarticular injections, which, according to physicians doing the injections, was greatest at L5–S1. No difference was found between either short- or long-term relief when the results were stratified based on whether or not all injections were intraarticular. Our high failure rate is consistent with that of Lynch and Taylor,44 who found that 38% of attempted intraarticular injections were extraarticular and that only 54% of patients who underwent two-level attempted intraarticular injections had demonstrable intraarticular spread in both joints. However, they differ from this prospective study in that those authors found that a higher percentage of people who had two intraarticular injections experienced complete or partial relief at 2-week follow-up than those who had no or only 1 intraarticular injection (93% vs. 61%). This small (n = 50) study did not randomize patients, had a short follow-up period, and their outcomes were neither standardized nor included any measure besides whether pain relief was “total” or “partial.” As alluded to above, extraarticular injections may “inadvertently” block the medial branches, rendering them diagnostic.
Factors Associated with Radiofrequency Ablation Treatment Outcomes
The strongest factor associated with a positive radiofrequency ablation outcome was a positive outcome from the diagnostic block. Although the Spine Intervention Society Guidelines53 assert that only complete pain relief constitutes a positive block, prospective studies have found no difference in radiofrequency ablation outcomes when comparing people who obtain near-complete pain relief to those who obtain partial relief.54 Anatomically, facet joint arthropathy is rare in the absence of degenerative disc disease, which generally precedes it by many years.3 When participants who received placebo blocks were excluded from analysis, there was no difference in the percent pain relief from the diagnostic block stratified by treatment response, which is consistent with a strong placebo effect in some individuals.
The observation that the proportion of responders and some other secondary outcome measures after radiofrequency ablation were superior with medial branch block and intraarticular injections than in the placebo group provides some evidence for the validity of facet injections as diagnostic tools. However, there are other factors that could have contributed to the greater benefit found in the treatment groups. Single facet blocks carry a false-positive rate between 20% and 40%.3,36,38 Since only responders in the intraarticular and medial branch block groups proceeded to radiofrequency ablation, placebo nonresponders were essentially screened out, which was not the case for those allocated to the sham group, all of whom proceeded to denervation. Moreover, expectations may have been higher in the two-thirds of patients who received true facet blocks than in those who received saline injections, as well-informed patients who failed to derive relief from the sham injections may have known that only a minority of them would have true faceto-genic pain. Expectations play a key role in the placebo effect, which is particularly robust for interventional procedures.55
Drop-off in Success Rate
There was a modest drop-off in our success rate between the 1-month and 6-month follow-ups, which is consistent with nearly all pain treatments, including spine surgery.56 Although this decrease in outcomes is less than reported in some studies,29 it is more than what one might expect based on the distance of the medial branch to the facet joint and the typical rate of nerve regrowth, which is 1 to 2 mm/day. In other randomized trials, authors did not report interim outcomes between treatment and 6 months27,28 or also a reported slight reduction in successful outcomes (66.7% at 2 months vs. 46.7% at 6 months).26 Having our reporting periods in discrete intervals could also underestimate the duration of benefit. For example, a person who obtained 5 ½ months of relief would be classified as having only 3 months of relief.
Interpretation and Recommendations
How should our findings be interpreted? First, the relatively modest difference in pain relief and radiofrequency ablation response rates between verum and placebo injections suggests that unlike in clinical practice where the double-block paradigm is not cost-effective in the United States,42 for efficacy studies two blocks may be necessary. The placebo effect is particularly strong for subjective outcomes such as pain and is higher for procedures than for medications.55 Hence, the small effect size we observed, whereby the placebo effect is greater in magnitude than the treatment effect, is consistent with nearly all controlled trials evaluating pain treatments. The well-publicized study by Juch et al.51 was not designed as an efficacy study, but it has been used as evidence against the efficacy of radiofrequency ablation.57 Differences between their study and ours include their more liberal selection criteria, their higher positive rate for diagnostic injections, and that they placed small electrodes perpendicular rather than parallel to the nerves and heated for a shorter time, which will reduce lesion volume. Second, consistent with other reviews,2–4,8,9 our findings indicate medial branch block and intraarticular injections are unlikely to provide long-term benefit to most people and should not be marketed as treatments. Since medial branch blocks are easier to perform and associated with a lower technical failure rate,3,44 they should preferentially be employed. Scenarios in which intraarticular injections can be considered include young people with an acute inflammatory process and those in whom joint access may be technically easier, and individuals (e.g., athletes, those with weakened posterior spinal musculature) in whom denervation of the multifidus muscle, a dynamic stabilizer of the spine whose innervation derives from the medial branches, might carry negative consequences.
Limitations
There are several limitations to our study. First, our study was designed primarily as a comparative-effectiveness study and therefore utilized liberal selection criteria to enhance generalization, unlike studies designed to show efficacy, which ideally employ rigorous criteria. This may have resulted in smaller effect sizes and reduced any differences between treatment groups that might be realized under more stringent selection criteria (i.e., efficacy studies that employ real-world criteria). Second, our study, which used data from a retrospective study to calculate sample size, may have been underpowered to detect differences in success rates between medial branch blocks and intraarticular injections. Third, our study design precluded blinding of the physicians who performed the block and those in the control group who failed to derive benefit from their sham injections and were scheduled for radiofrequency ablation per protocol. This may have introduced bias and amplified differences in the placebo effect. Last, for ethical reasons we could not mandate that patients who failed treatment, or were unsatisfied with their results, be forced to remain in a study that precluded cointerventions. Although we tried to compensate for this by using the statistically conservative “last-observation-carried-forward” approach for radiofrequency ablation treatment failures, it is possible that some people who failed to experience benefit at 1 month (e.g., prolonged neuritis) might have derived long-term benefit, thereby underestimating effectiveness.
Conclusions
In summary, facet injections appear to have little long-term utility, and multicenter randomized trials are needed to ascertain the best way to diagnose facet joint pain, confirm the efficacy of radiofrequency ablation, and determine which patients benefit most.
What We Already Know about This Topic
Facet blocks, including intraarticular and medial branch blocks, are frequently used before radiofrequency ablation, but their validity as a predictive tool is unproven
Recently, the evidence supporting radiofrequency ablation has come under great scrutiny
What This Article Tells Us That Is New
This randomized study establishes the lack of long-term efficacy for intraarticular and medial branch facet blocks but suggests the possibility that when used as prognostic tools, these injections may possibly provide superior outcomes before radiofrequency ablation on some measures compared to control blocks
Acknowledgments
Research Support
This research was funded by the Center for Rehabilitation Sciences Research, Bethesda, Maryland.
This trial was registered in 2013 on clinicaltrials.gov: NCT02002429
Footnotes
Competing Interests
Dr. Cohen has served as a consultant to Halyard, Boston Scientific, and Abbott within the past 3 yr. The opinions or assertions contained herein are the private views of the authors and are not to be construed as official or as reflecting the views of the Department of the Army or the U.S. Department of Defense.
Reproducible Science
Full protocol available at: scohen40@jhmi.edu. Raw data available at: scohen40@jhmi.edu.
Contributor Information
Steven P. Cohen, Department of Anesthesiology and Critical Care Medicine, Department of Neurology and Physical Medicine and Rehabilitation, The Johns Hopkins School of Medicine, Baltimore, Maryland; Deptartment of Anesthesiology, Department of Physical Medicine and Rehabilitation, Uniformed Services University of the Health Sciences, Bethesda, Maryland
Tina L. Doshi, Department of Anesthesiology and Critical Care Medicine, The Johns Hopkins School of Medicine, Baltimore, Maryland
Octav C. Constantinescu, Department of Surgery, Landstuhl, Regional Medical Center, Landstuhl, Germany
Zirong Zhao, Department of Neurology, District of Columbia Veterans Affairs Hospital, Washington, District of Columbia
Connie Kurihara, Anesthesia Service, Walter Reed National Military Medical Center, Bethesda, Maryland
Thomas M. Larkin, Parkway Neuroscience and Spine Institute, Hagerstown, Maryland
Scott R. Griffith, Deptartment of Anesthesiology, Uniformed Services University of the Health Sciences, Bethesda, Maryland; Department of Surgery, Walter Reed National Military Medical Center, Bethesda, Maryland
Michael B. Jacobs, Department of Physical Medicine and Rehabilitation, Uniformed Services University of the Health Sciences, Bethesda, Maryland; Department of Surgery and Department of Orthopedic Surgery, Walter Reed National Military Medical Center, Bethesda, Maryland
William J. Kroski, Department of Physical Medicine and Rehabilitation, Uniformed Services University of the Health Sciences, Bethesda, Maryland; Department of Surgery, Walter Reed National Military Medical Center, Bethesda, Maryland
Timothy C. Dawson, Puget Sound Veteran’s Hospital, Seattle, Washington; Department of Anesthesiology, University of Washington, Seattle, Washington
Ian M. Fowler, Pain Medicine Center, Deptartment of Anesthesiology, Naval Medical Center-San Diego, San Diego, California
Ronald L. White, Department of Pain Medicine, David Grant U.S. Air Force Medical Center, Travis Air Force Base, California; Departments of Anesthesiology and Neurology, Penn State Hershey Medical Center, Hershey, Pennsylavania
Aubrey J. Verdun, Department of Anesthesiology and Critical Care Medicine, The Johns Hopkins School of Medicine, Baltimore, Maryland; Pain Treatment Center, Walter Reed National Military Medical Center, Bethesda, Maryland
David E. Jamison, Department of Anesthesiology and Critical Care Medicine, The Johns Hopkins School of Medicine, Baltimore, Maryland
Mirinda Anderson White, Pain Treatment Center, Walter Reed National Military Medical Center, Bethesda, Maryland
Stephanie E. Shank, Parkway Neuroscience and Spine Institute, Hagerstown, Maryland
Paul F. Pasquina, Deptartment of Physical Medicine and Rehabilitation, Uniformed Services University of the Health Sciences, Bethesda, Maryland; Physical Medicine and Rehabilitation Service, Walter Reed National Military Medical Center, Bethesda, Maryland
References
- 1.Manchikanti L, Hirsch JA, Pampati V, Boswell MV: Utilization of facet joint and sacroiliac joint interventions in medicare population from 2000 to 2014: Explosive growth continues! Curr Pain Headache Rep 2016; 20:58. [DOI] [PubMed] [Google Scholar]
- 2.Chou R: Low back pain (chronic). BMJ Clin Evid 2010; 2010: pii:1116. [PMC free article] [PubMed] [Google Scholar]
- 3.Cohen SP, Huang JH, Brummett C: Facet joint pain–advances in patient selection and treatment. Nat Rev Rheumatol 2013; 9:101–16 [DOI] [PubMed] [Google Scholar]
- 4.van Kleef M, Vanelderen P, Cohen SP, Lataster A, Van Zundert J, Mekhail N: 12. Pain originating from the lumbar facet joints. Pain Pract 2010; 10:459–69 [DOI] [PubMed] [Google Scholar]
- 5.Manchikanti L, Kaye AD, Boswell MV, Bakshi S, Gharibo CG, Grami V, Grider JS, Gupta S, Jha SS, Mann DP, Nampiaparampil DE, Sharma ML, Shroyer LN, Singh V, Soin A, Vallejo R, Wargo BW, Hirsch JA: A systematic review and best evidence synthesis of the effectiveness of therapeutic facet joint interventions in managing chronic spinal pain. Pain Physician 2015; 18:E535–82 [PubMed] [Google Scholar]
- 6.Leggett LE, Soril LJ, Lorenzetti DL, Noseworthy T, Steadman R, Tiwana S, Clement F: Radiofrequency ablation for chronic low back pain: A systematic review of randomized controlled trials. Pain Res Manag 2014; 19:e146–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maas ET, Ostelo RW, Niemisto L, Jousimaa J, Hurri H, Malmivaara A, van Tulder MW: Radiofrequency denervation for chronic low back pain. Cochrane Database Syst Rev 2015; 10:CD008572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chou R, Hashimoto R, Friedly J, Fu R, Dana T, Sullivan S, Bougatsos C, Jarvik J: Pain management injection therapies for low back pain. Rockville (MD): Agency for Healthcare Research and Quality (US); 2015 [PubMed] [Google Scholar]
- 9.Vekaria R, Bhatt R, Ellard DR, Henschke N, Underwood M, Sandhu H: Intra-articular facet joint injections for low back pain: A systematic review. Eur Spine J 2016; 25:1266–81 [DOI] [PubMed] [Google Scholar]
- 10.Ribeiro LH, Furtado RN, Konai MS, Andreo AB, Rosenfeld A, Natour J: Effect of facet joint injection versus systemic steroids in low back pain: A randomized controlled trial. Spine (Phila PA 1976) 2013; 38: 1995–2002 [DOI] [PubMed] [Google Scholar]
- 11.Celik B, Er U, Simsek S, Altug T, Bavbek M: Effectiveness of lumbar zygapophysial joint blockage for low back pain. Turk Neurosurg 2011; 21:467–70 [PubMed] [Google Scholar]
- 12.Kawu AA, Olawepo A, Salami AO: Facet joints infiltration: A viable alternative treatment to physiotherapy in patients with low back pain due to facet joint arthropathy. Niger J Clin Pract 2011; 14:219–22 [DOI] [PubMed] [Google Scholar]
- 13.Egsmose C, Lund B, Bach Andersen R: Hip joint distension in osteoarthrosis. A triple-blind controlled study comparing the effect of intra-articular indoprofen with placebo. Scand J Rheumatol 1984; 13:238–42 [DOI] [PubMed] [Google Scholar]
- 14.Ackerman WE 3rd, Ahmad M: Pain relief with intraarticular or medial branch nerve blocks in patients with positive lumbar facet joint SPECT imaging: A 12-week outcome study. South Med J 2008; 101:931–4 [DOI] [PubMed] [Google Scholar]
- 15.Dolan AL, Ryan PJ, Arden NK, Stratton R, Wedley JR, Hamann W, Fogelman I, Gibson T: The value of SPECT scans in identifying back pain likely to benefit from facet joint injection. Br J Rheumatol 1996; 35:1269–73 [DOI] [PubMed] [Google Scholar]
- 16.Raymond J, Dumas JM, Lisbona R: Nuclear imaging as a screening test for patients referred for intraarticular facet block. J Can Assoc Radiol 1984; 35:291–2 [PubMed] [Google Scholar]
- 17.Koh WU, Kim SH, Hwang BY, Choi WJ, Song JG, Suh JH, Leem JG, Shin JW: Value of bone scintigraphy and single photon emission computed tomography (SPECT) in lumbar facet disease and prediction of short-term outcome of ultrasound guided medial branch block with bone SPECT. Korean J Pain 2011; 24:81–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pneumaticos SG, Chatziioannou SN, Hipp JA, Moore WH, Esses SI: Low back pain: Prediction of short-term outcome of facet joint injection with bone scintigraphy. Radiology 2006; 238:693–8 [DOI] [PubMed] [Google Scholar]
- 19.Manchikanti L, Singh V, Falco FJ, Cash KA, Pampati V: Lumbar facet joint nerve blocks in managing chronic facet joint pain: One-year follow-up of a randomized, double-blind controlled trial: Clinical Trial NCT00355914. Pain Physician 2008; 11:121–32 [PubMed] [Google Scholar]
- 20.Maigne JY, Maigne R, Guerin-Sutville H: The lumbar mamillo-accessory foramen: A study of 203 lumbosacral spines. Surg Radiol Anat 1991; 13:29–32 [DOI] [PubMed] [Google Scholar]
- 21.Stones C, Cole F: Breaking the cycle: Extending the persistent pain cycle diagram using an affective pictorial metaphor. Health Commun 2014; 29:32–40 [DOI] [PubMed] [Google Scholar]
- 22.Bicket MC, Hurley RW, Moon JY, Brummett CM, Hanling S, Huntoon MA, van Zundert J, Cohen SP: The development and validation of a quality assessment and rating of technique for injections of the spine (AQUARIUS). Reg Anesth Pain Med 2016; 41:80–5 [DOI] [PubMed] [Google Scholar]
- 23.Gallagher J, Petriccione di Vadi PL, Wedley JR, Harman W, Ryan P, Chikanza I: Radiofrequency facet joint denervation in the treatment of low back pain: A prospective controlled double-blind study to assess its efficacy. Pain Clinic 1994; 7:193–8 [Google Scholar]
- 24.Leclaire R, Fortin L, Lambert R, Bergeron YM, Rossignol M: Radiofrequency facet joint denervation in the treatment of low back pain: A placebo-controlled clinical trial to assess efficacy. Spine (Phila Pa 1976) 2001; 26:1411–6; discussion 1417 [DOI] [PubMed] [Google Scholar]
- 25.van Wijk RM, Geurts JW, Wynne HJ, Hammink E, Buskens E, Lousberg R, Knape JT, Groen GJ: Radioffequency denervation of lumbar facet joints in the treatment of chronic low back pain: A randomized, double-blind, sham lesion-controlled trial. Clin J Pain 2005; 21:335–44 [DOI] [PubMed] [Google Scholar]
- 26.van Kleef M, Barendse GA, Kessels A, Voets HM, Weber WE, de Lange S: Randomized trial of radiofrequency lumbar facet denervation for chronic low back pain. Spine (Phila Pa 1976) 1999; 24:1937–42 [DOI] [PubMed] [Google Scholar]
- 27.Tekin I, Mirzai H, Ok G, Erbuyun K, Vatansever D: A comparison of conventional and pulsed radioffequency denervation in the treatment of chronic facet joint pain. Clin J Pain 2007; 23:524–9 [DOI] [PubMed] [Google Scholar]
- 28.Nath S, Nath CA, Pettersson K: Percutaneous lumbar zygapophysial (Facet) joint neurotomy using radioffequency current, in the management of chronic low back pain: A randomized double-blind trial. Spine (Phila Pa 1976) 2008; 33:1291–7; discussion 1298 [DOI] [PubMed] [Google Scholar]
- 29.Lord SM, Barnsley L, Wallis BJ, McDonald GJ, Bogduk N: Percutaneous radio-frequency neurotomy for chronic cervical zygapophyseal-joint pain. N Engl J Med 1996; 335:1721–6 [DOI] [PubMed] [Google Scholar]
- 30.van Tilburg CW, Stronks DL, Groeneweg JG, Huygen FJ: Randomised sham-controlled double-blind multicentre clinical trial to ascertain the effect of percutaneous radio-frequency treatment for lumbar facet joint pain. Bone Joint J 2016; 98-B1526–33 [DOI] [PubMed] [Google Scholar]
- 31.Cohen SP, Moon JY, Brummett CM, White RL, Larkin TM: Medial branch blocks or intra-articular injections as a prognostic tool before lumbar facet radiofrequency denervation: A multicenter, case-control study. Reg Anesth Pain Med 2015; 40:376–83 [DOI] [PubMed] [Google Scholar]
- 32.Birkenmaier C, Veihelmann A, Trouillier HH, Hausdorf J, von Schulze Pellengahr C: Medial branch blocks versus pericapsular blocks in selecting patients for percutaneous cryodenervation of lumbar facet joints. Reg Anesth Pain Med 2007; 32:27–33 [DOI] [PubMed] [Google Scholar]
- 33.Kaplan M, Dreyfuss P, Halbrook B, Bogduk N: The ability of lumbar medial branch blocks to anesthetize the zygapophysial joint. A physiologic challenge. Spine (Phila Pa 1976) 1998; 23:1847–52 [DOI] [PubMed] [Google Scholar]
- 34.International Spine Intervention Society: Cervical medial branch thermal radioffequency neurotomy In: Bogduk N, ed. Practice guidelines: spinal diagnostic and treatment procedures, 2nd edition. San Francisco: International Spine Intervention Society, 2013: pp. 165–217 [Google Scholar]
- 35.Engel A, Rappard G, King W, Kennedy DJ; Standards Division of the International Spine Intervention Society: The effectiveness and risks of fluoroscopically-guided cervical medial branch thermal radiofrequency neurotomy: A systematic review with comprehensive analysis of the published data. Pain Med 2016; 17:658–69 [DOI] [PubMed] [Google Scholar]
- 36.Bogduk N: International Spinal Injection Society guidelines for the performance of spinal injection procedures. Part 1: Zygapophysial joint blocks. Clin J Pain 1997; 13:285–302 [DOI] [PubMed] [Google Scholar]
- 37.Dreyfuss PH, Dreyer SJ; NASS: Lumbar zygapophysial (facet) joint injections. Spine J 2003; 3(3 Suppl):50–9S [DOI] [PubMed] [Google Scholar]
- 38.Boswell MV, Manchikanti L, Kaye AD, Bakshi S, Gharibo CG, Gupta S, Jha SS, Nampiaparampil DE, Simopoulos TT, Hirsch JA: A best-evidence systematic appraisal of the diagnostic accuracy and utility of facet (zygapophysial) joint injections in chronic spinal pain. Pain Physician 2015; 18:E497–533 [PubMed] [Google Scholar]
- 39.Nash TP: Facet Joints—Intra-articular steroids or nerve blocks? The Pain Clinic 1990; 3:77–82 [Google Scholar]
- 40.Marks RC, Houston T, Thulboume T: Facet joint injection and facet nerve block: A randomised comparison in 86 patients with chronic low back pain. Pain 1992; 49:325–8 [DOI] [PubMed] [Google Scholar]
- 41.Smythe H: Examination for tenderness: learning to use 4 kg force. J Rheumatol 1998; 25:149–51 [PubMed] [Google Scholar]
- 42.Cohen SP, Williams KA, Kurihara C, Nguyen C, Shields C, Kim P, Griffith SR, Larkin TM, Crooks M, Williams N, Morlando B, Strassels SA: Multicenter, randomized, comparative cost-effectiveness study comparing 0, 1, and 2 diagnostic medial branch (facet joint nerve) block treatment paradigms before lumbar facet radiofrequency denervation. Anesthesiology 2010; 113:395–405 [DOI] [PubMed] [Google Scholar]
- 43.Fukui S, Ohseto K, Shiotani M, Ohno K, Karasawa H, Naganuma Y: Distribution of referred pain from the lumbar zygapophyseal joints and dorsal rami. Clin J Pain 1997; 13:303–7 [DOI] [PubMed] [Google Scholar]
- 44.Lynch MC, Taylor JF: Facet joint injection for low back pain. A clinical study. J Bone Joint Surg Br 1986; 68:138–41 [DOI] [PubMed] [Google Scholar]
- 45.Provenzano DA, Lassila HC, Somers D: The effect of fluid injection on lesion size during radiofrequency treatment. Reg Anesth Pain Med 2010; 35:338–12 [DOI] [PubMed] [Google Scholar]
- 46.Fairbank JC, Couper J, Davies JB, O’Brien JP: The Oswestry low back pain disability questionnaire. Physiotherapy 1980; 66:271–3 [PubMed] [Google Scholar]
- 47.Rofail D, Myers L, Froggatt D: Treatment satisfaction and dissatisfaction in chronic low back pain: A systematic review. J Psychol Psychother 2016; 6: 3 [Google Scholar]
- 48.Farrar JT, Young JP Jr, LaMoreaux L, Werth JL, Poole RM: Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain 2001; 94:149–58 [DOI] [PubMed] [Google Scholar]
- 49.Manchikanti L, Singh V, Falco FJ, Cash KA, Pampati V: Evaluation of lumbar facet joint nerve blocks in managing chronic low back pain: A randomized, double-blind, controlled trial with a 2-year follow-up. Int J Med Sci 2010; 7:124–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Choi WJ, Hwang SJ, Song JG, Leem JG, Kang YU, Park PH, Shin JW: Radiofrequency treatment relieves chronic knee osteoarthritis pain: A double-blind randomized controlled trial. Pain 2011; 152:481–7 [DOI] [PubMed] [Google Scholar]
- 51.Juch JNS, Maas ET, Ostelo RWJG, Groeneweg JG, Kallewaard JW, Koes BW, Verhagen AP, van Dongen JM, Huygen FJPM, van Tulder MW: Effect of radiofrequency denervation on pain intensity among patients with chronic low back pain: The mint randomized clinical trials. JAMA 2017; 318:68–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nagels W, Pease N, Bekkering G, Cools F, Dobbels P: Celiac plexus neurolysis for abdominal cancer pain: A systematic review. Pain Med 2013; 14:1140–63 [DOI] [PubMed] [Google Scholar]
- 53.Lumbar medial branch blocks In: Bogduk N, ed. Practice guidelines for spinal diagnostic and treatment procedures, 2nd ed. International Spine Intervention Society, San Francisco, CA, 2013, pp. 457–488 [Google Scholar]
- 54.Cohen SP, Strassels SA, Kurihara C, Griffith SR, Goff B, Guthmiller K, Hoang HT, Morlando B, Nguyen C: Establishing an optimal “cutoff” threshold for diagnostic lumbar facet blocks: A prospective correlational study. Clin J Pain 2013; 29:382–91 [DOI] [PubMed] [Google Scholar]
- 55.Kong J, Kaptchuk TJ, Polich G, Kirsch I, Gollub RL: Placebo analgesia: Findings from brain imaging studies and emerging hypotheses. Rev Neurosci 2007; 18:173–90 [DOI] [PubMed] [Google Scholar]
- 56.Jacobs WC, van Tulder M, Arts M, Rubinstein SM, van Middelkoop M, Ostelo R, Verhagen A, Koes B, Peul WC: Surgery versus conservative management of sciatica due to a lumbar herniated disc: A systematic review. Eur Spine J 2011; 20:513–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bakalar N: Radiofrequency denervation offers no benefits for back pain. New York Times, 5 July 2017. Available at: https://www.nytimes.com/2017/07/05/well/live/radiofrequency-denervation-offers-no-benefits-for-back-pain.html