Abstract
Interactions between germline-encoded natural killer (NK) cell receptors and their respective ligands on tumorigenic or virus-infected cells determine NK cell cytotoxic activity and/or cytokine secretion. NK cell cytokine responses can be augmented in and can potentially contribute to multiple sclerosis (MS), an inflammatory disease of the central nervous system focused upon the oligodendrocytes (OLs). To investigate mechanisms by which NK cells may contribute to MS pathogenesis, we developed an in vitro human model of OL-NK cell interaction. We found that activated, but not resting human NK cells form conjugates with, and mediate cytotoxicity against, human oligodendrocytes. NK cells, when in conjugate with OLs, rapidly synthesize and polarize IFN-γ toward the OLs. IFN-γ is capable of reducing myelin oligodendrocyte and myelin associated glycoproteins (MOG and MAG) content. This activity is independent of MHC class-I mediated inhibition via KIR2DL1, but dependent upon the interaction between NK cell-expressed KIR2DL4 and its oligodendrocyte-expressed ligand, HLA-G. NK cells from patients with MS express higher levels of IFN-γ following conjugation to OLs, more actively promote in vitro reduction of MOG and MAG and have higher frequencies of the KIR2DL4 positive population. These collectively suggest a mechanism by which NK cells can promote pathogenic effects upon OLs.
Keywords: Natural Killer cell, Oligodendrocyte, demyelination, Myelin protein, multiple sclerosis
1. Introduction
Oligodendrocytes (OLs), glial cells of the central nervous system (CNS), create insulating myelin sheaths that surround and support neuronal axons. This insulation allows electrical conduction to move rapidly along the length of a neuron, passing from one uninsulated area (node of Ranvier) to another. Focal interruption of myelin sheaths and the resultant electrical impulse slowing are hallmarks of multiple sclerosis (MS). MS is an inflammatory and autoimmune disease of the CNS, in which adaptive immunity is believed to play a major role in disease progression (Sospedra and Martin 2005). Existing studies also uphold at least some regulatory and/or pathogenic role for components of the innate immune system, including NK cells, in MS pathogenesis (Kastrukoff et al. 1998; Takahashi et al. 2001; Kastrukoff et al. 2003; Takahashi et al. 2004; Trachtenberg 2009; Gandhi, Laroni, and Weiner 2010; Gross, Schulte-Mecklenbeck, Wiendl, et al. 2016; Gross, Schulte-Mecklenbeck, Runzi, et al. 2016; Smith, Calabresi, and Bhargava 2017). However, the mechanism by which human NK cells contribute to the pathogenesis of MS is unclear.
NK cells function primarily to survey for and kill tumorigenic and virus infected target cells. In particular, they serve a pivotal role in the control of herpesvirus infection (Biron et al. 1999). Despite ongoing controversies (Simpson et al. 2014), there is also evidence for correlation between herpes viral infection and MS (Challoner et al. 1995; Ablashi et al. 1998; Alvarez-Lafuente et al. 2002; Soldan et al. 1997; Pormohammad et al. 2018; Saberi, Akhondzadeh, and Kazemi 2018). In light of these historical connections, a role for NK cells in MS pathogenesis that depends on the activation status of NK cells is possible. Additional support for this concept includes elevated levels of IL-2 in MS patient serum (Gallo et al. 1989) which would activate NK cells, as well as that in vitro activation of NK cells induces receptor-ligand dependent cytotoxicity against healthy autologous and heterologous OLs (Morse et al. 2001; Antel et al. 1998; Saikali et al. 2007).
Thus, we attempted to model the cellular interactions potentially occurring during the development of MS with a focus upon a role for NK cells (Rodriguez-Martin et al. 2015; Macchi and Mastino 2016; Lagumersindez-Denis et al. 2017; Moreno-Torres et al. 2018) through an ex vivo human co-culture system using human NK cells and OLs. We also directly evaluated NK cells from MS patients to further consider how NK cells could potentially contribute to the pathogenesis of MS. We have identified some in vitro and direct HLA-independent activity by NK cells against OLs dependent upon specific receptor-ligand interactions. Furthermore the studies of patient NK cells directly suggest opportunities to strategize clinically for this challenging clinical condition.
2. Methods
2.1. Isolation of peripheral blood mononuclear cells (PBMCs) and primary natural killer (NK) cells from humans.
Using Ficoll Hypaque (Amersham), PBMCs were isolated from healthy volunteers or patients with MS. Ex vivo NK (eNK) cells were isolated from PBMCs by negative selection using a human NK cell isolation kit (Miltenyi Biotech). NK cell preparations (CD3− CD56+), as determined by flow cytometry (FACSCalibur, BD), were >95% pure with <1.0% CD3+ cells. Resting or IFN-α [1000 IU, 24h, 37°C, 5% CO2] activated eNK cells were used as effector cells. For cytotoxicity assays and to induce KIR2DL4 expression, eNK cells were activated with 100 IU/ml of IL-2 (Orange et al. 2011) for 5 days.
2.2. Differentiation of human oligodendrocytes (OLs) from human Oligodendrocyte precursor cells (HOPC).
Immature neuroepithelial (Nestin) and oligodendroglial (A2B5) marker positive human oligodendrocyte precursor cells (HOPC; ScienCell Research lab) (Raff, Miller, and Noble 1983) were maintained and differentiated into oligodendrocytes (OLs) as per the supplier’s instructions. Antibodies against myelin-associated glycoprotein (MAG; Epitomics) and myelin oligodendrocyte glycoprotein (MOG; AnaSpec) were used to monitor OL differentiation at regular intervals. Differentiated OLs were used as targets. In some experiments, OLs were treated with 10 or 20 ng/ml of recombinant human IFN-γ (eBioscience) for 2.5h at 37°C.
2.3. Cell lines.
The immortalized human NK cell line NK92 and an erythroleukemic target cell line K562 were grown and maintained in culture as described previously (Banerjee et al. 2007). GFP-expressing NK92 cells were generated by retroviral transduction (Banerjee et al. 2007). Briefly, 2µg of pBMN-internal ribosome entry site (IRES)-EGFP plasmid DNA were transfected into the Phoenix packaging line using Fugene (Roche) lipofection reagent. Supernatant was harvested on day 2 post-transfection. NK92 cells, Polybrene (Sigma), and supernatant were mixed and spun in a well of a 6-well plate at 1,000×g for 90 min at 32°C. Following overnight incubation at 32°C, cells were spun down and resuspended in supplemented Myeolocult media and sorted for GFP expression. NK92 cells were also transduced to express FLAG-tagged WT or transmembrane arginine to glycine (RG)-mutant KIR2DL4 (Kikuchi-Maki et al. 2003; Miah, Hughes, and Campbell 2008) from the retroviral pBMN-IRES-EGFP vector. MO3.13 human oligodendroglial target cells (Buntinx et al. 2003) (CELLutions biosystem) were cultured in DMEM with 10% FCS (GIBCO); these were differentiated into mature OLs in serum-free media (Barbarese et al. 1988)after 15 days of culture. In some experiments MO3.13 cells were transfected with HLA-Cw3 and HLA-Cw4 cDNA (RIKEN BRC BioResource Center), which were commercially tagged to GFP (Epoch Life Sciences). For this, 1×106 of MO3.13 cells were transfected with 500ng of DNA/transfection, using 4D nucleofector kit SE, and DS126 program in a 4D nucleofector (Lonza). 24hr after transfection GFP-expressing cells were sorted and selected in G418 containing media. G418 concentration (2mg/ml) was previously determined by using a death curve on untransfected cells.
2.4. Flow cytometry-based assays.
Resting or cytokine activated-eNK cells were labeled with DDAOSE (Invitrogen), while OLs, MO3.13 or K562 target cells were labeled with CFSE (Invitrogen) at 1.5 mM and 1.25mM concentrations respectively per the manufacturer’s instruction. Following labeling, effector and target cells were washed in PBS; labeling was quenched in RPMI with 10% FCS. Conjugates between DDAOSE-labeled eNK cells and CFSE-labeled target cells were formed at 2:1 effector to target cell ratio in 200μl of RPMI with 10% FCS for indicated time periods in a humidified CO2 incubator at 37°C. Cells were fixed in 2% paraformaldehyde and evaluated by flow cytometry (FACScalibur or Fortessa, BD Biosciences). FlowJo software (Treestar) was used to identify single-color-positive unconjugated cells and dual-color-positive effector cell + target cell conjugates. To evaluate eNK cell activity in terms of reducing MAG and MOG content from OLs in MHC independent manner, dual-color-positive effector+ target cell conjugates were evaluated. For this, HLA-Cw3 or -Cw4-positive MO3.13 cells were monitored through GFP fluorescence, and eNK cells were labeled with PKH26 (Sigma). In some experiments GFP positive NK92 cells or CFSE-labeled eNK cells were conjugated with DDAOSE-labeled MO3.13 cells. After 2.5hr of conjugate formation, cells were fixed and permeabilized in a perm-fix solution (BD Pharmingen), washed twice in perm-wash buffer (BD Pharmingen), blocked with 20μg/ml goat IgG (Santacruz) for 20min at room temperature, washed again in a perm-wash buffer, and incubated with rabbit anti-human MOG (Anaspec) or rabbit-anti-MAG (Abcam) for 30min at room temperature. F(ab/)2 specific human adsorbed fluorescent tagged goat anti-Rabbit IgG (Cederlane) was used as the secondary antibody to detect binding of primary rabbit antibodies in OLs and MO3.13 cells.
In some experiments, IFN-γ expression in conjugated effector cells was measured after indicated time period of conjugate formation using PE conjugated anti-IFN-γ (BD Pharmingen). A mixture of anti-HLA-G monoclonal (Clone 87G, Biolegend) and polyclonal (D-20, Santacruz) antibodies, each at 20μg/ml concentration, was added to block binding of HLAG to KIR2DL4. The polyclonal anti-HLAG antibody was used for flow cytometry-based staining of OLs and MO3.13 cells, and an anti-HLA-C antibody (clone DT9, EMD Millipore) was used to determine the expression of HLA-Cw3 and Cw4 on MO3 cells. Fluorescent-conjugated antibodies against CD antigens, NK cell surface receptors, and isotype-matched controls were purchased from BD Pharmingen. Allophycocyanin-tagged anti-human CD158d mAb (clone 33) was obtained from Biolegend. Singlet cells from lymphocyte aggregates were further gated on CD56+, CD3−NK cell population using FlowJo (TreeStar) to determine percentages of NK cell and the intensity of, a given marker on them. Effects of anti-IFN-γ (eBioscience) and all other neutralizing antibodies were evaluated in respect to control antibodies at comparable concentrations.
2.5. Western blot analysis.
Cells were lysed in ice-cold lysis buffer containing 25 mM Tris-Cl (pH 7.5), 150 mM NaCl, 5 mM MgCl2, 1% Nonidet P-40, 1 mM DTT and 5% glycerol. Protein estimation was performed using a bicinchoninic acid assay protein estimation kit (Pierce). 10μg of cellular proteins (as indicated) was separated on 4–12% Bis-Tris gels (Invitrogen), transferred to nitrocellulose membranes (Invitrogen), blocked in 3% BSA and 140 mM NaCl-containing buffer, and then incubated with a primary antibody (Banerjee et al. 2007). Primary antibodies against MAG (Abcam), MOG (Anaspec) and β-actin (Sigma) were used. Bound antibody was detected with anti-rabbit, anti-mouse or anti-goat IgG coupled to fluorophores (AlexaFluor 680, Molecular Probes; IRDye 800, Li-Cor Biosciences), then imaged using the Odyssey Infrared Imaging System (Li-Cor Biosciences). Densitometric analysis of protein bands was performed using ImageJ software.
2.6. Confocal microscopy and image analysis.
Cells were prepared for evaluation of fixed conjugates by immunoflourescent confocal microscopy as previously described (Banerjee et al. 2007). Briefly, conjugates between effector and target cells were formed at 2:1 effector to target cell ratio in 200μl of RPMI with 10% FCS for indicated time periods in a humidified CO2 incubator. Conjugates or unconjugated target cells, as indicated, were adhered to poly–l-lysine–coated glass slides (Polyprep; Sigma-Aldrich) for 15 min, all at 37°C. Slides were rinsed in PBS and cells were fixed and permeabilized with 4% formaldehyde, 0.1% saponin, and 0.1% Triton X-100 in PBS for 15 min, rinsed in PBS, and incubated at RT for 1h with primary antibody, followed by secondary antibody as indicated.
In some experiments glass-adhered cells were incubated with anti-KIR2DL4 primary antibody, at 4°C in PBS with 2% FCS and 0.05% sodium azide (Sigma), then washed and stained with secondary antibody in the same buffer and condition before the fixing and permeabilization process, which was required for IFN-γ staining. Anti-human primary antibodies against MOG (Anaspec), KIR2DL4 (Clone 181703, R&D Systems), HLA-G (D-20, Santacruz) and highly cross-adsorbed Alexaflour-fluorescent-tagged secondary antibodies (Molecular Probes) were used in the range of 1–20μg/ml. Where specified, conjugates were also stained with FITC tagged anti-human perforin (BD) and Alexaflour 647-conjugated phalloidin or anti-IFN-γ antibody (BD). Stained cells were mounted in glycerol-based mounting media (Vectashield) and visualized using a spinning disc confocal microscope (IX-81, Olympus) under a 60× UPLFLN objective with a numerical Aperture of 1.45 at 25°C. Two-dimensional images were captured using an electron multiplying charged-coupled device camera (Hamamatsu, C9100). Image acquisition, 3-dimensional reconstitution and analysis of images were performed using Volocity software (PerkinElmer) (Banerjee and Orange). F-actin was quantified by multiplying the mean fluorescent intensity (MFI) of the phalloidin by its volume occupied within a single cell (Banerjee and Orange). OL volume was determined by selecting the region surrounding the cell as determined via fluorescent staining of OL cortical F-actin. The following formulas were used for image analysis: (a) MOG amount per OL = (volume occupied by MOG specific fluorophore / OL volume) x (the MFI of MOG fluorophore): (b) Average MOG volume = (total volume of MOG from n number of OLs / n). Effector cell + target cell conjugates were also evaluated for overlapping areas of KIR2DL4 (NK92 cells) and HLA-G (MO3.13 cells). The area of overlap was defined as the interface. The percentage of accumulation of a given protein at the interface was defined as (area x MFI of a protein at the interface) / (area x MFI of the same protein inside the cell) x 100.
2.7. Cytokine array.
Supernatants from various types of effector cell + target cell conjugates, and also from unconjugated cells were obtained after 16h of incubation. Multiplex cytokine assays were performed at the Human Immune Monitoring Center of Stanford University was used to determine cytokine and chemokine levels. Human 37-plex or 13-plex kits (Affymetrix Inc.) were used according to the manufacturer’s recommendations with the following modifications. Briefly, samples were mixed with antibody-linked polystyrene beads on 96-well filter-bottom plates and incubated at room temperature for 2h followed by overnight incubation at 4°C. Plates were vacuum-filtered and washed twice with wash buffer, then incubated with biotinylated detection antibody for 2h at room temperature. Samples were then filtered and washed twice as above and resuspended in streptavidin-PE. After incubation for 40 minutes at room temperature, two additional vacuum washes were performed, and samples were resuspended in reading buffer. Each sample was measured in duplicate. Plates were read using a Luminex 200 instrument with a lower bound of 100 beads per sample per cytokine. Cytokine and chemokines concentrations in pg/ml were obtained using the standard curve of interest and the MFI of experimental samples. Values obtained from unconjugated effector and unconjugated target cell supernatants were added together and subtracted from values of test samples.
2.8. Cytotoxicity assay.
4h 51Cr-release assays (Banerjee et al. 2007) were performed in v-bottomed 96-well plates. 1×106 K562 or OLs, or MO3.13 target cells were resupended in 100μl of RPMI with 10% FCS and labeled with 100 microcuri of 51Cr for 1h, in 200μl total volume at 37°C, and in the presence of 5% CO2. After labeling, cells were washed three times in RPMI with 10% FCS and were used for assays. eNK cells were used as effectors, at different effector-to-target cell ratios (E:T), and against all types of target cells. Percent specific lysis was defined as [(mean of the test wells)-(mean of the spontaneous release wells) / (mean of maximal release wells)-(mean of the spontaneous release wells)] × 100.
2.9. siRNA Transfection.
1.5×106 WT-KIR2DL4-NK92-GFP cells were nucleofected (Banerjee et al. 2007) with 1000nM of control or IFN-γ siRNA (Santacruz) in nucleofector solution R (Lonza) using program A024 of the Amaxa Nucleofector II and incubated in serum containing media for 24h before use.
2.10. Statistical analysis.
All graphs denote mean values and error bars represent standard deviation (SD). Groups of data were compared using the two-tailed Student’s t test; statistical significance is shown (*, P < 0.05) unless otherwise noted.
2.11. Declaration of approval to study human subjects.
All human studies were approved by the institutional review board (IRB) of The Children’s Hospital of Philadelphia and Baylor College of Medicine. Written informed consent was obtained from each participant prior to inclusion in the study using an IRB-approved protocol. MS diagnosis and disease severity were determined by a physician certified by the American Board of Psychiatry and Neurology.
3. Results
3.1. Activated but not resting eNK cells mediate cytotoxicity against and form conjugates with oligodendrocytes (OLs).
Human OLs were generated by ex vivo differentiation of human oligodendrocyte precursor cells (HOPC). Undifferentiated HOPC, differentiated human OLs and the human oligodendroglial cell line MO3.13 (Buntinx et al. 2003) were evaluated by flow cytometry via intracellular staining (Supplemental Figure S1A) and Western blotting (Supplemental Figure S1B) for myelin-associated-glycoprotein (MAG) and myelin-oligodendrocyte-glycoprotein (MOG), known phenotypic markers of immature and mature OLs. As expected, MAG expression was restricted mainly to HOPCs (Ma et al. 2009), immature OLs (Poltorak et al. 1987) and undifferentiated MO3.13 cells (Supplemental Figure S1A), and it was downregulated in fully differentiated MO3.13 cells (Supplemental Figures S1A, B). In contrast, MOG expression was absent in HOPCs, restricted to cells of oligodendrocyte lineage and further enhanced in fully matured OLs and MO3.13 cells (Supplemental Figure S1A (Coffey and McDermott 1997; Solly et al. 1996; Scolding et al. 1989). We further compared MAG and MOG expression by Western blot analysis and found that in comparison with HOPC, MAG expression was downregulated 4-fold in fully differentiated OLs and 5-fold upon MO3.13 cell differentiation (Supplemental Figure S1B). MOG expression, on the other hand, was upregulated 3-fold in mature OLs, and 1.5-fold upon MO3.13 cell differentiation (Supplemental Figure S1B). These characteristics of in vitro differentiated OLs were consistent with the expected phenotype (Coffey and McDermott 1997; Solly et al. 1996; Ma et al. 2009; Poltorak et al. 1987; Scolding et al. 1989) and flow cytometry-validated preparations of MAGlow MOG+ cells were used as OLs in subsequent experiments.
IFN-α and IL-2 enhance ex vivo natural killer (eNK) cell cytotoxicity against the prototypical erythroleukemic target cell line, K562 (Trinchieri et al. 1984). IL-2, which is elevated in MS patient serum (Gallo et al. 1989), additionally enhances NK cell cytotoxicity against human oligodendrocytes isolated from human brain (Morse et al. 2001). IFN-α-induced cytotoxicity of NK cells is governed by certain activating receptors such as NKG2D and CD161 (Konjevic et al. 2010), and the role of different types of IFNs in MS has been described (Reder and Feng 2014; Dumitrescu, Constantinescu, and Tanasescu 2018). Thus, we asked whether our in vitro-differentiated human OLs were similarly susceptible to cytokine-activated NK cells. Activation of NK cells by either IL-2 or IFN-α enhanced cytotoxicity against OLs (Figure 1A), and as expected, K562 cells (data not shown) in 4h 51Cr-release assays (Figure 1A). Human OLs, however, express MHC class-I (Hirayama et al. 1986) and most likely these assays present at least some degree of HLA-mismatch between the NK cells and OLs. We evaluated expression of either class I (A, B, C) or class II (DR, DQ, and DP) molecules on MO3.13 cells and found them to be negative (data not shown). In cytotoxicity assays the MO3.13 cells were killed only by cytokine-activated but not resting primary NK cells (Figure 1B). This implies that NK cell lytic activity against OLs does not depend upon HLA mismatch.
Figure 1. Activated but not resting eNK cells mediate cytotoxicity against and form conjugates with oligodendrocytes and human oligodendrocytic MO3.13 cells.
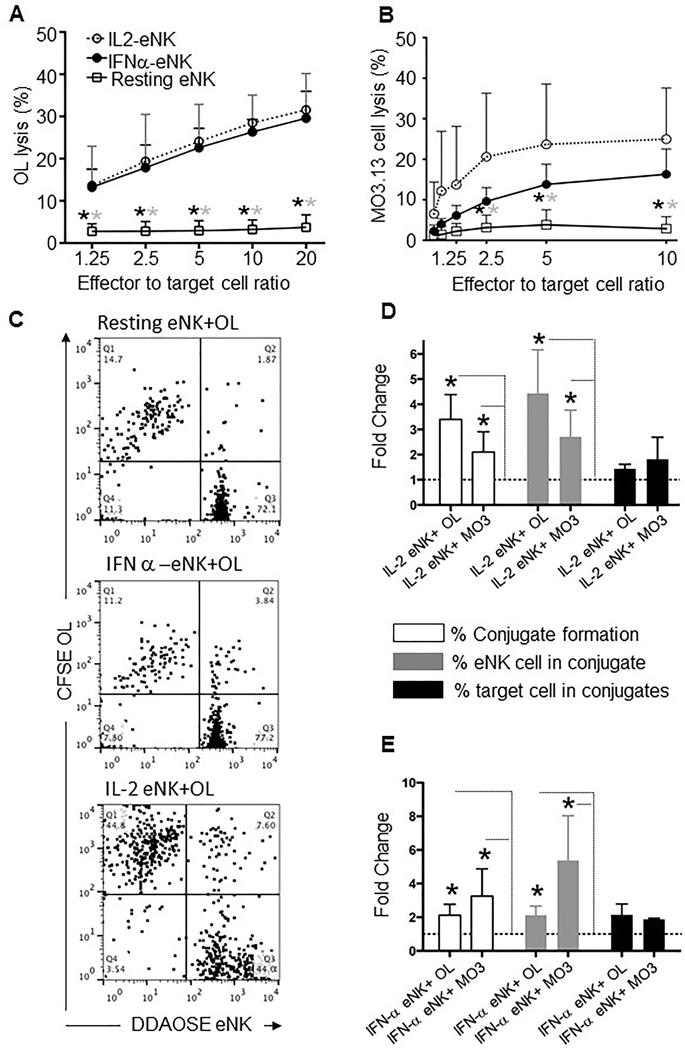
Cytotoxic activity of IL-2- (IL2-eNK, open circle), IFN-α− (IFNα-eNK, filled circle) treated, and control (resting, square) eNK cells against (A) oligodendrocytes (OLs) or (B) MO3.13 target cells was measured in 4h 51Cr-release assays. Each point represents the mean of 3 independent experiments; error bars indicate SD. Stars denote a statistically significant difference (*P <0.05) between the cytotoxic activity of resting and IL-2 (gray stars) or IFNα-treated (black stars) eNK cells. (C) Representative examples of flow cytometry-based conjugation assays using CFSE-labeled OLs and DDAOSE-stained eNK cells, which were resting, IL-2- or IFN-α-treated prior to 30min of conjugation at 37°C. DDAOSE+/CFSE+ conjugates appear in the upper right quadrant of each graph. (D, E) Quantitative analysis of conjugation between eNK cells and OLs or MO3.13 cells in 3 independent flow cytometry-based assays comparing the effect of IL-2- (D) or IFN-α- (E) activated with untreated (resting) eNK cells (dotted lines). Bars represent the mean fold increase of the total percentage of cells in conjugates (% Conjugate formation, open bars), percentages of NK cells in conjugates (% eNK cell in conjugate, gray bars), and percentages of target cells in conjugates (% target cell in conjugate, black bars), as noted. Error bars indicate SD; statistically significant fold increases over resting eNK cell + target cell conjugates (dotted line) are noted (*P <0.05).
Because NK cell cytotoxicity represents a contact-dependent phenomenon between effector and target cells, we next wanted to understand the physical contact between NK cells and OLs. We initially asked whether IL-2- and IFN-α−activated NK cells conjugate more efficiently with OLs and MO3.13 cells. Using a flow cytometry-based assay (Figure 1C), we found that in comparison with resting NK cells, IL-2- (Figure 1D) and IFN-α− (Figure 1E) activated NK cells indeed formed more conjugates with OLs and MO3.13 cells. Similarly, slightly more OLs and MO3.1 cells appeared in conjugates (percent of target cells in conjugate) with activated as opposed to resting NK cells and more activated NK cells overall are found in conjugates. Thus cytokines associated with MS pathogenesis can enhance NK cell conjugate formation with and cytotoxicity against OLs and MO3.13 cells.
3.2. Activated but not resting eNK cells in conjugate promote myelin protein loss.
Although it is not possible to evaluate clinical demyelination using an in vitro system, The loss of MOG from OLs has been associated with demyelination in vitro and in vivo (Kerlero de Rosbo et al. 1990; Goddard, Berry, and Butt 1999; Reindl et al. 1999; Iglesias et al. 2001; von Budingen et al. 2001; Birgbauer, Rao, and Webb 2004; Zhou et al. 2018). Thus we evaluated whether activated NK cells in conjugates with oligodendrocytes promote MOG loss. We used quantitative 3-dimensional immunofluorescence (Banerjee and Orange 2010) to measure MOG in OLs either alone or conjugated with eNK cells. The average MOG content of unconjugated OLs (Figures 2A, C) was comparable to that of OLs conjugated with resting eNK cells (Figures 2, B, C). When NK cells were pre-activated with IL-2 or IFN-α, however (Figures 2B, C) the average MOG volume (Figure 2) and total amount of MOG per OL (Supplemental Figure S2A) were reduced significantly. There was no alteration in total OL volume (Supplemental Figure S2B) or in OL F-actin content (Supplemental Figure S2C) in conjugates formed with activated NK cells relative to those formed with resting NK cells. This suggests that activated but not resting NK cells specifically reduce MOG content in OLs
Figure 2. Activated but not resting eNK cells conjugated to OLs demonstrate MOG-reducing activity.
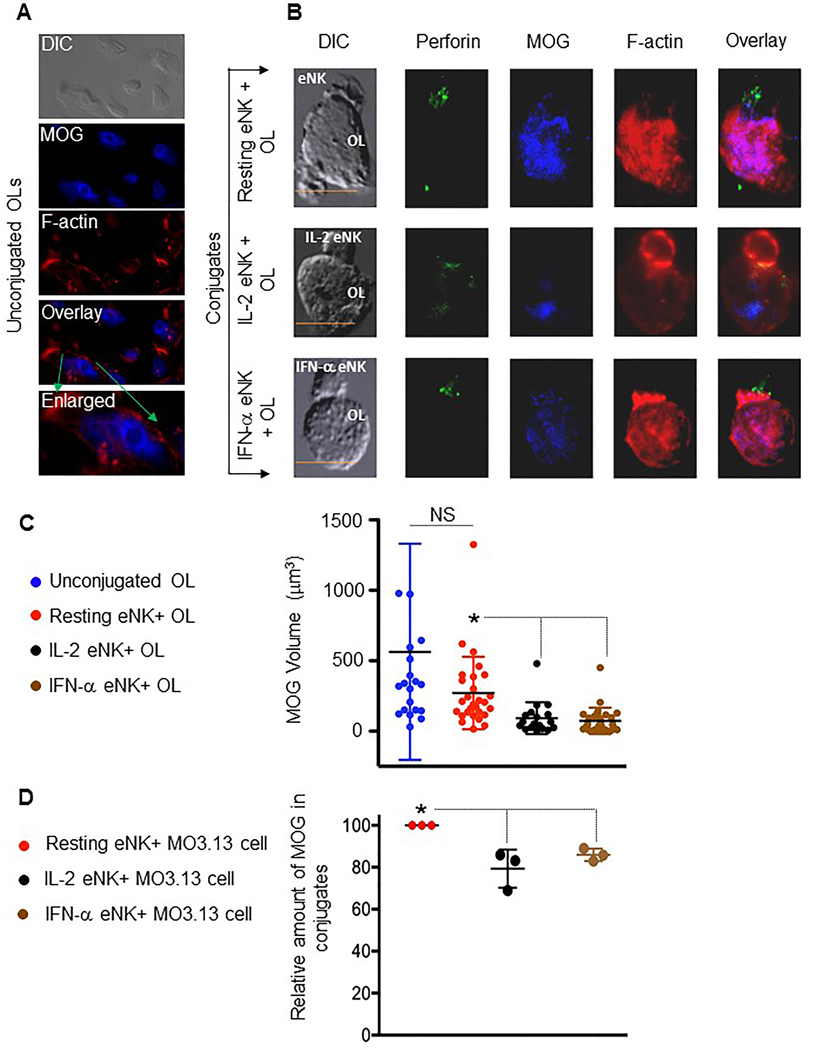
(A) Representative images of an unconjugated OL, and OL in conjugate (B) with resting, IL-2- and IFN-α-treated eNK cells displaying differential interference contrast (DIC) and maximal projections of confocal fluorescence for perforin (green), MOG (blue) and F-actin (phalloidin; red), as well as an overlay of fluorescent channels (enlarged in bottom, A; right, B). Scale bar=10μm. (C) Quantification of MOG volume derived from the 3-dimensional reconstruction of unconjugated OLs and OLs inconjugate with resting (red), IL-2- (black) or IFN-α- (brown) pre-treated eNK cells. Circles represent the volume of MOG from individual OLs. (D) Flow cytometry based assessment of relative MOG content in viable resting NK cell + MO3 cell conjugates (red circles), or that in IL-2 (black) and IFN-α (brown) activated NK cells+ MO3 cell conjugates are shown. Each dot represents an experiment. (C, D) Horizontals bars represent the mean and error bars show ±SD; n= 3 independent experiments. Significant differences between means are noted (*P<0.05, NS=not significant).
Interestingly, perforin was polarized at the interfaces between activated but not resting NK cell+ OL conjugates (Figure 2B) and was positively associated with a decrease in MOG volume. The reduction of MOG protein, however, could arguably be a feature of cell death, especially since these activated NK cells kill OLs and MO3.13 cells (Figures 1A, B). To address this, we utilized a flow cytometry-based method to measure MOG amounts only in conjugates that were viable (using a viability dye; Supplemental Figure S3A, B). When only the viable cells were considered, the NK cell-mediated reduction in MOG content from MO3.13 cells was greater after NK cell activation (Figure 2D). Similarly, when compared to resting NK cell+MO3.13 cell conjugates, myelin-associated glycoprotein (MAG: another surrogate of demyelination (Gendelman et al. 1985; Rahmanzadeh et al. 2018) content was reduced in viable MO3.13 cells conjugated to activated NK cells (Supplemental Figures 3C). These findings suggest that activated NK cells conjugated to oligodendrocytes promote a loss of key myelin proteins.
3.3. Supernatant from conjugates between activated but not resting eNK cells and OLs promotes myelin protein loss.
We next asked whether the NK cell activity reducing myelin proteins in OLs could be mediated by soluble factors. Thus, we incubated OLs (in the absence of NK cells) with supernatants obtained from co-cultures of OLs and resting or cytokine activated NK cells. After incubation, we stained supernatant-treated OLs for MOG and F-actin (Figure 3A). When compared to supernatants from resting NK cells and OLs, supernatants from co-cultures of activated NK cells and OLs substantially reduced both the total volume of MOG (Figure 3B) and the amount of MOG per OL (Supplemental Figure S2D). Neither activated nor resting co-culture supernatants affected total OL volume (Supplementary Figure 2E) or F-actin content (Supplementary Figure 2F), again implying a specific impact of activated eNK + OL supernatants on MOG-reducing activity as opposed to cell integrity. Thus, while NK cells are potentially triggered by intercellular contact, their impact upon OLs can be mediated by soluble factors.
Figure 3. Supernatants from activated but not resting eNK cell + OL conjugates promote MOG-reducing activity.

(A) Images of OLs treated with supernatants obtained from conjugates between OLs and resting (top) and IL-2- (middle) or IFN-α- (bottom) activated eNK cells. Representative fields showing DIC (left) and maximal projection confocal fluorescence for MOG, F-actin (phalloidin; red) and an overlay of all fluorescent channels (right). Scale bar=10μm. (B) Quantification of MOG volume derived from the 3-dimensional reconstruction of OLs treated with supernatants from conjugates between OLs and resting (red) and IL-2- (black) or IFN-α- (brown) treated eNK cells. Circles represent the volume of MOG from individual OLs, horizontal bars represent the mean and error bars show ±SD; n= 3 independent experiments. Significant differences between means are noted (*P<0.05).
To begin to define which soluble factors contributed to NK cell-mediated MOG reduction in OLs, we compared supernatants from co-cultures of activated (via IL-2 or IFN-α) eNK cells and OLs to those from resting eNK cells and OLs (Figure 4). Co-cultures from activated NK cells preferentially contained a number of soluble mediators including the NK cell chemoattractants monocyte chemotactic protein-1 (MCP-1), RANTES, macrophage inflammatory proteins (MIP) such as MIP-1α, MIP-1β, as well as IFN-γ. Interestingly, IFN-γ is associated with active demyelinating disease (Panitch et al. 1987) and has been previously characterized has having demyelinating activity (Horwitz et al. 1997; Corbin et al. 1996; Lin et al. 2006; Traugott and Lebon 1988). TNF-α, which also promotes demyelination (Trenova et al. 2011), was upregulated only by IL-2 pre-activation. Type 2 cytokines produced by NK cells such as IL-5 and IL-13 were undetectable. Ingenuity Pathway Analysis (IPA) software identified each of the upregulated cytokines to be associated with MS or demyelinating activity (Figure 4B). Thus, activation of eNK cells by IL-2 or IFN-α stimulates NK cells to secrete chemo-attractants and known demyelinating cytokines (Corbin et al. 1996; Horwitz et al. 1997; Lin et al. 2006; Traugott and Lebon 1988; Panitch et al. 1987; Trenova et al. 2011), upon their co-culture with primary OLs. NK cells are known to produce IFN-γ, one of the demyelinating cytokines, by in vitro stimulation with either IFN-α (Matikainen et al. 2001), or IL-2 (Weigent, Stanton, and Johnson 1983). We, therefore, normalized the data in Figure 4A to measurements of cytokine and chemokine concentrations from resting, or cytokine-activated unconjugated NK cells and unconjugated OLs as baseline controls. Thus, our data in figure 4A only demonstrate the levels of cytokine and chemokine concentrations, which were upregulated in eNK cell-OL culture supernatants. Our results were in agreement with those previously described (Pandya et al. 2011) as we found low (10–20 pg/ml ) levels of IL-17 in the supernatants of IL-2 or IFN-α activated unconjugated NK cells. However, the levels of IL-17 secretion did not increase upon conjugation of resting or cytokine-activated NK cells to OLs. IL-17 had been shown to impair schwann cell-mediated myelination (Stettner et al. 2014), however, as its level did not increase after conjugation of OLs with cytokine activated NK cells in our system we pursued a different mechanism of cytokine activated-NK cell in reducing MOG contents from OLs.
Figure 4. Cytokine and chemokine profiles of eNK cell + OL conjugate supernatants.
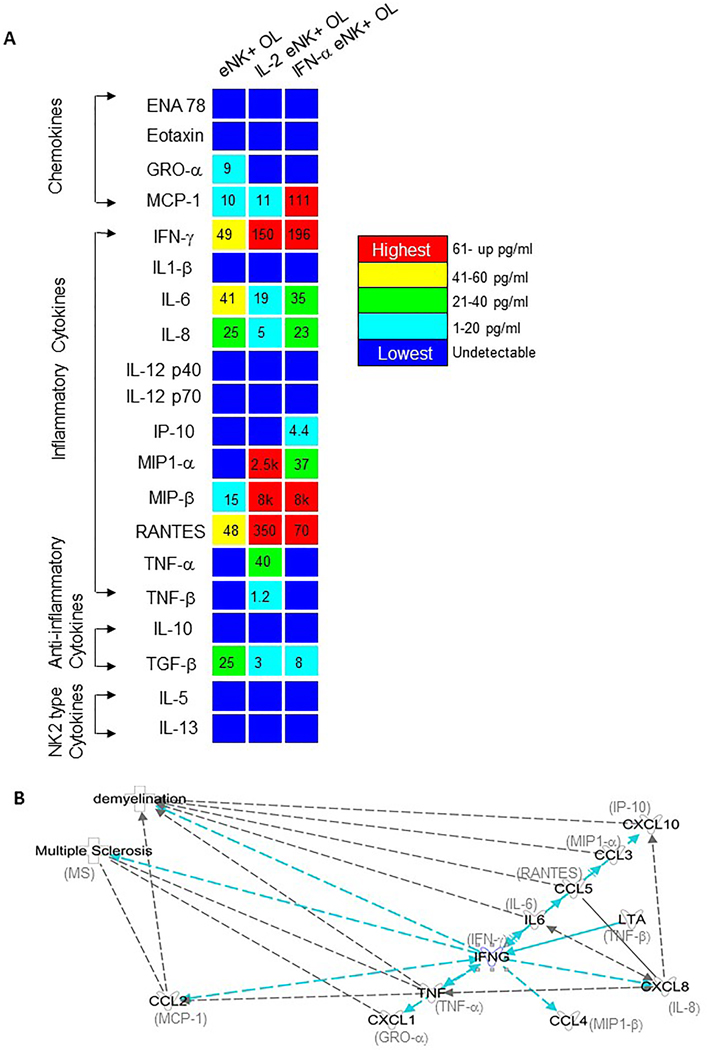
(A) Flow cytometry-based multiplex cytokine and chemokine arrays were performed on supernatants from 16h conjugates formed between various NK cells and oligodendrocytes. Supernatants from conjugates between: OLs and resting, IL-2- or IFN-α-pre-treated eNK cells were analyzed. Data represent the midpoint of two independent experiments each was corrected with measurements of cytokine and chemokine concentrations from resting, or cytokine activated unconjugated NK cells and unconjugated OLs as baseline controls. Numbers denote concentrations of a particular cytokine or chemokine in pg/ml. The inset color scale denotes comparative levels of each of the chemokines and cytokines measured. (B) Ingenuity Pathway Analysis (IPA) showing role of various cytokines in multiple sclerosis or in demyelinating activity. Effect of IFN-γ (in teal) as a master regulator of various demyelinating cytokines is shown.
3.4. Oligodendrocytes express HLA-G and can induce NK cell IFN-γ expression and polarization via KIR2DL4.
Ligation of the K cell killer IgG like receptor 2DL4 [KIR2DL4 or CD158d (Kikuchi-Maki et al. 2003)] by human-leukocyte-antigen-G (HLA-G) (Faure and Long 2002; Kikuchi-Maki et al. 2003; Miah, Hughes, and Campbell 2008) does not promote NK cell cytotoxicity, but augments secretion of inflammatory cytokines/chemokines including IFN-γ (Kikuchi-Maki et al. 2003; Miah, Hughes, and Campbell 2008; Rajagopalan, Fu, and Long 2001). We found IFN-γ and other KIR2DL4-regulated inflammatory mediators in the co-culture supernatants from activated eNK cells and OLs (Figure 4). Thus, we asked whether a KIR2DL4 ligand (HLA-G) was expressed on OLs, and if so whether it was capable of stimulating NK cell cytokine secretion.
Using flow cytometry and confocal microscopy, we identified HLA-G expression on both primary OLs (Figure 5A) and MO3.13 cells (Figure 5B). Therefore we next asked whether KIR2DL4-expressing NK cells secrete IFN-γ when conjugated with MO3.13 cells and whether interfering with KIR2DL4-HLA-G signaling abrogates IFN-γ expression. Because the level of KIR2DL4 expression on primary NK cells varies among individuals, and as much as 25% of the human population does not express KIR2DL4 on their NK cell-surface (Kikuchi-Maki et al. 2003; Goodridge et al. 2003), we stably transduced the human NK cell line NK92 with empty vector (GFP), a vector containing cDNA of wild-type KIR2DL4-GFP (WT-KIR2DL4), or a mutant form of KIR2DL4(RG)tagged to GFP(RG-KIR2DL4). The latter disrupts association with the FcεRI-γ and renders the K R2DL4 receptor unable to trigger IFN-γ production (Miah, Hughes, and Campbell 2008; Kikuchi-Maki, Catina, and Campbell 2005). NK92 cells transduced with WT-KIR2DL4 or RG-KIR2DL4 were sorted for equal GFP expression to ensure equivalent surface KIR2DL4 expression (data not shown). These NK92 cells were then conjugated to MO3.13 cells and assayed for IFN-γ expression by intracellular flow cytometry at various time points (Figure 5C). NK92 cells transduced with WT-KIR2DL4, but not RG-KIR2DL4, expressed more IFN-γ, relative to the MFI of baseline control obtained at the 0hr time point, after 1 or 4hr conjugation to MO3.13 cells (Figure 5C, D). Differences after 16hr were not significant.
Figure 5. Oligodendrocytic cells express HLA-G, induce NK cell IFN-γ production and polarization via KIR2DL4.
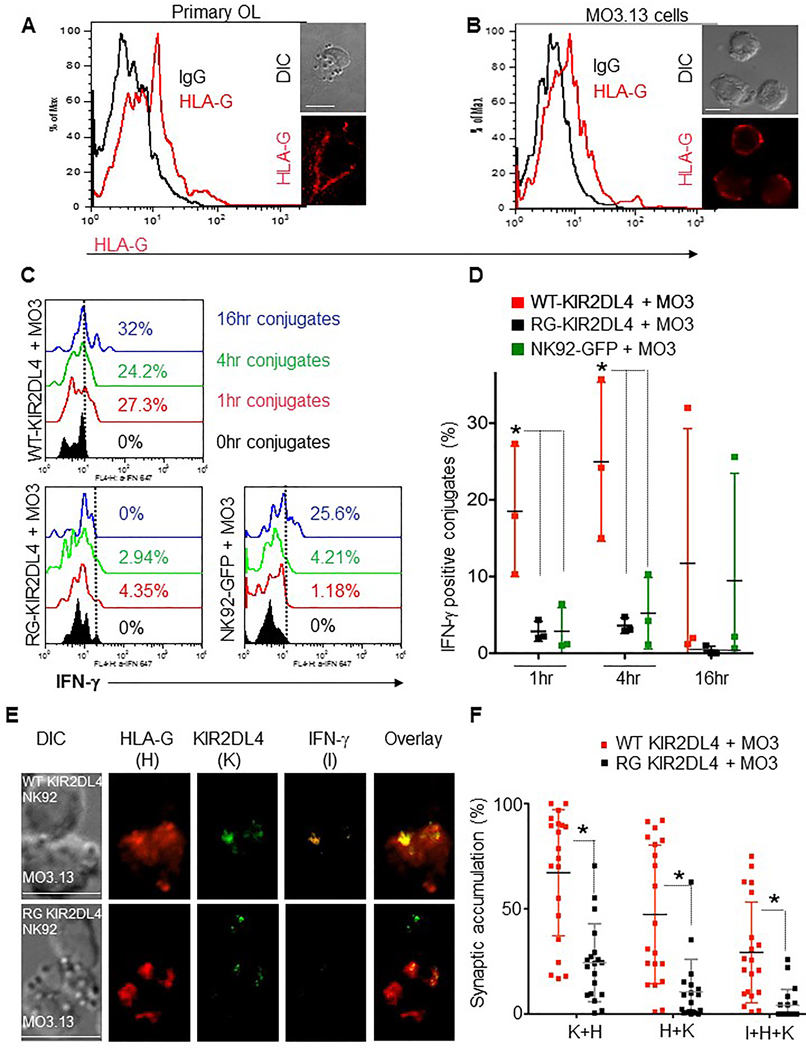
Flow cytometry and confocal fluorescence microscopy showing HLA-G expression on OLs (A) and MO3.13 cells (B) (black line = IgG control, red line = anti-HLA-G). (C) Histograms showing IFN-γ expression in conjugates between DDAOSE-stained MO3.13 cells and WT-KIR2DL4 (top), RG-KIR2DL4 (bottom left) and GFP-expressing (bottom-right) NK92 cells. DDAOSE+/GFP+ conjugates were evaluated at 0h (black), 1h (red), 4h (green) and 16h. Dashed lines depict the gate utilized to define percentages of IFN-γ positive conjugates. (D) Plots display points for each independent experiments as shown in C, and horizontal lines display the mean percentages of IFN-γ positive NK92+ MO3.13 cell conjugates at 1h, 4h and 16h of incubation (n=3 independent repeats, error bars show ±SD). Percentages of IFN-γ-positive WT-KIR2DL4- (red squares) and RG-KIR2DL4- (black squares) or NK92-GFP- (green squares) cells in conjugates with MO3.13 cells are noted (*P<0.05). (E) Representative examples of the conjugates formed between a WT-KIR2DL4 (top panel) or RG-KIR2DL4-cell (bottom panel) and a MO3.13 target cell at 1h. DIC images (left) and confocal fluorescence images demonstrating localization of HLA-G (H, red), KIR2DL4 (K, green), and IFN-γ (I, orange). An overlay of all fluorescent channels (right) is also shown. Scale bar=10μm. (F) Plots display points for each conjugate analyzed and horizontal lines represent the mean percent molecular accumulation of KIR2DL4 with HLA-G (K+H), HLA-G with KIR2DL4 (H+K), and IFN-γ with KIR2DL4 and HLA-G (I+K+H) at the interface of WT-KIR2DL4 (red squares) or RG-KIR2DL4 (black squares) cells and MO3.13 cells. Error bars show ± SD and significant differences between means are noted (*P<0.05, n= 3 independent experiments).
NK cells are known to polarize IFN-γ to the immunological synapse when in conjugates with susceptible target cells (Barcia et al. 2008). Quantitative confocal fluorescence microscopy demonstrated that conjugation to MO3.13 cells prompts WT-KIR2DL4 cells but not RG-KIR2DL4 cells to polarize IFN-γ at the interface of NK cell and MO3.13 cells within 1 hour (Figures 5E top panel, F) of conjugation. Polarization was contingent upon the expression of WT-KIR2DL4, as the RG-KIR2DL4 consistently resulted in low quantities IFN-γ (Figures 5C, D) and low polarization of IFN-γ to the interface or synapse (Figures 5E bottom panel, F).
3.5. KIR2DL4-induced NK cell IFN-γ production promotes OL MOG loss.
Since IFN-γ polarizes in NK cells towards OLs dependent upon KIR2DL4, we asked if the IFN-γ was promoting MOG loss in OL. We first evaluated the impact of recombinant human IFN-γ on the MOG content of OLs and found that 20ng/ml, but not 10ng/ml of IFN-γ for 2.5hrs decreased MOG content. (Figure 6A, B). In contrast, these concentrations of IFN-γ did not decrease OL cell volume (Supplemental Figure 4A) or OL F-actin content (Supplemental Figure S4B) suggesting specificity of the IFN-γ-mediated effect.
Figure 6. Purified IFN-γ and NK cell produced IFN-γ diminish MOG content of OLs.
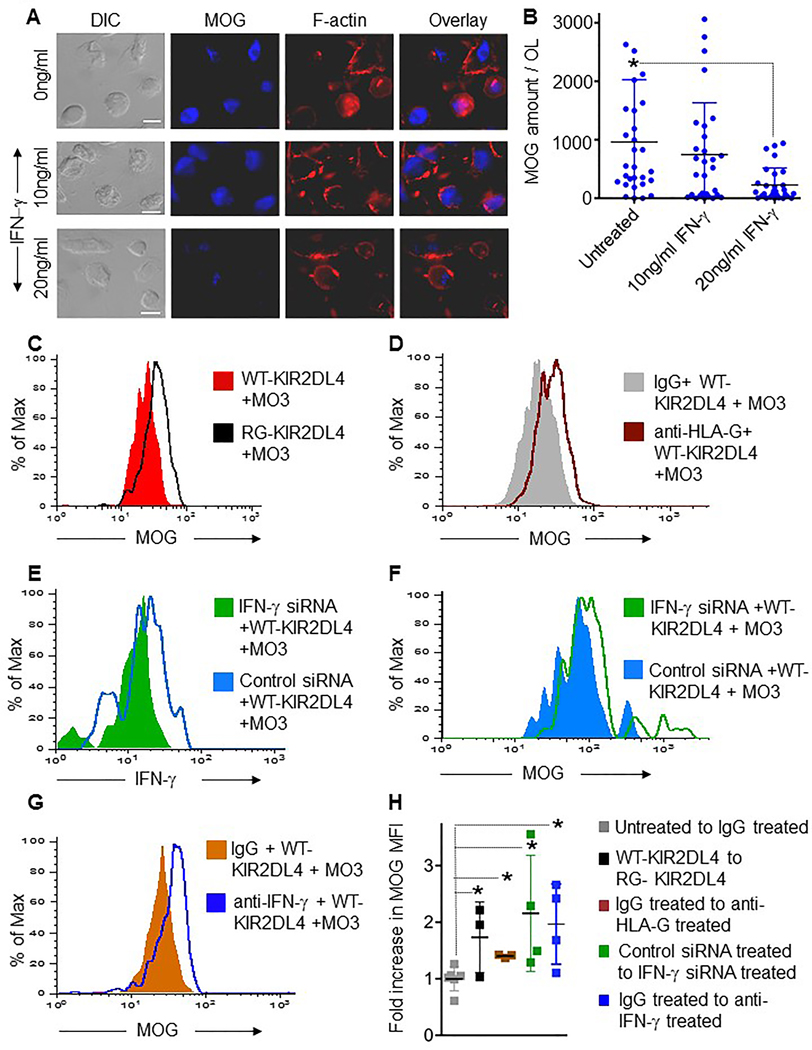
(A) DIC (left) and maximal projection confocal fluorescence images of OLs treated with media alone or containing 10 or 20ng/ml IFN-γ for 2.5h. MOG fluorescence is shown in blue, F-actin (phalloidin) in red. An overlay of fluorescence channels is shown on the right. Scale bar=10μm. (B) MOG content in untreated or IFN-γ−treated OLs. Each circle represents the amount of MOG in an individual OL. Horizontal bars represent the mean, error bars show ±SD and significant differences between means are noted (*P<0.05, n= 3 independent experiments). (C, D) MOG expression measured by flow cytometry at 2.5h in (C) DDAOSE-labeled MO3.13 cell and WT-KIR2DL4 (red) or RG-KIR2DL4 (black) cell conjugates; or in (D) DDAOSE-labeled MO3.13 cell and WT-KIR2DL4 cell conjugates formed in the presence of control (IgG, grey) or a mixture of anti-HLA-G monoclonal and polyclonal antibodies (brown). (E) IFN-γ and (F) MOG expression measured by flow cytometry at 1h (E) or 2.5h (F) in conjugates between DDAOSE-labeled MO3.13 cells and control siRNA (blue) or IFN-γ specific siRNA (green) pre-treated WT-KIR2DL4 cells. (G) MOG expression in conjugates between DDAOSE-labeled MO3.13 cells and WT-KIR2DL4 cells at 2.5h in the presence of IgG (orange) or anti-IFN-γ (blue) antibody. (H) Horizontal lines on the plot represent the mean fold increase in MOG-MFI over the 3–5 independent experiments performed. Control IgG-treated cells demonstrated no change (gray squares) while up-regulation of MOG in others relative to these was identified as shown. Error bars show ±SD, and means significantly different from control (grey squares) are noted (*P<0.05).
Since KIR2DL4 ligation induced IFN-γ content and polarization, we next compared the mutant and WT expressing NK92 cells to determine impact upon OL MOG content in conjugated OLs. Using flow cytometry based assays, we found that conjugation with WT-KIR2DL4- but not RG-KIR2DL4-transduced NK92 cells reduced MOG in MO3.13 cells (Figures 6C, H). To determine if this was mediated via interaction with HLA-G, we pre-incubated MO3.13 cells with a monoclonal anti-HLA-G blocking antibody. HLA-G blocking antibody increased in MOG by 14±3% (data not shown). To try and more completely block HLA-G a mixture of polyclonal and monoclonal blocking antibodies against HLA-G was added and led to a further increase in OL MOG content of 40±3% [Figure 6D, H]). Thus, ligation of KIR2DL4 by HLA-G presumably is required for NK cell-mediated MOG reduction.
Since it is possible that other NK cell secreted contents could promote MOG loss after KIR2DL4 ligation we used siRNA to specifically reduce IFN-γ expression in WT-KIR2DL4 cells (Figure 6E). When compared with control siRNA-treated WT-KIR2DL4 cells, IFN-γ siRNA resulted in an inability to reduce the amount of MO3.13 cell MOG (Figures 6F, H). In order to avoid the further manipulation of the NK92 cell line, we next 19 conjugated WT-KIR2DL4 and MO3.13 cells in the presence of a neutralizing antibody against IFN-γ. In these experiments, IFN-γ-neutralizing antibodies, but not control antibodies, abrogated the ability of WT-KIR2DL4 cells to reduce OL MOG content (Figures 6G, H). Thus, the ability of NK cells to reduce MOG content in OLs requires both IFN-γ and the interaction between WT-KIR2DL4 and its ligand HLA-G (Figure 6H).
3.6. HLA non-restricted MOG and MAG-reducing activity by NK cells.
While MO3.13 cells are HLA negative, OLs in human brain are HLA positive (Hirayama et al. 1986; Lv et al. 2014). Thus, to more accurately consider the in vivo biology we tested whether the KIR2DL4-dependent MOG-reducing activity identified in NK92 cells was present when the OLs express MHC Class-I. Thus, we stably expressed either HLA-Cw3-GFP or HLA-Cw4-GFP molecules in MO3.13 cells. We obtained HLA Cw3 or Cw4 expression at comparable levels (Figure 7A) and without altering the expression of HLA-G on them (Figure 7B). We then checked the expression of KIR2DL1 and KIR2LD4 on various batches of eNK cells and IL-2-activated KIR2DL1 and KIR2DL4-expressing eNK cells. The same set of activated eNK cells were then conjugated with HLA-Cw3 or - Cw4 positive MO3.13 cells to test their myelin protein-reducing activity. Using flow cytometry, and gated on viable eNK cell+ MO3 cell conjugates, we found that MO3 cells expressing either HLA-Cw3 or -Cw4 were equally susceptible to MOG- or MAG-reducing activity by activated eNK cells in vitro (Figure 7C).
Figure 7. HLA-haplotype non-restricted MOG-reducing activity of eNK cells; a role of KIR2DL4 and IFN-γ.
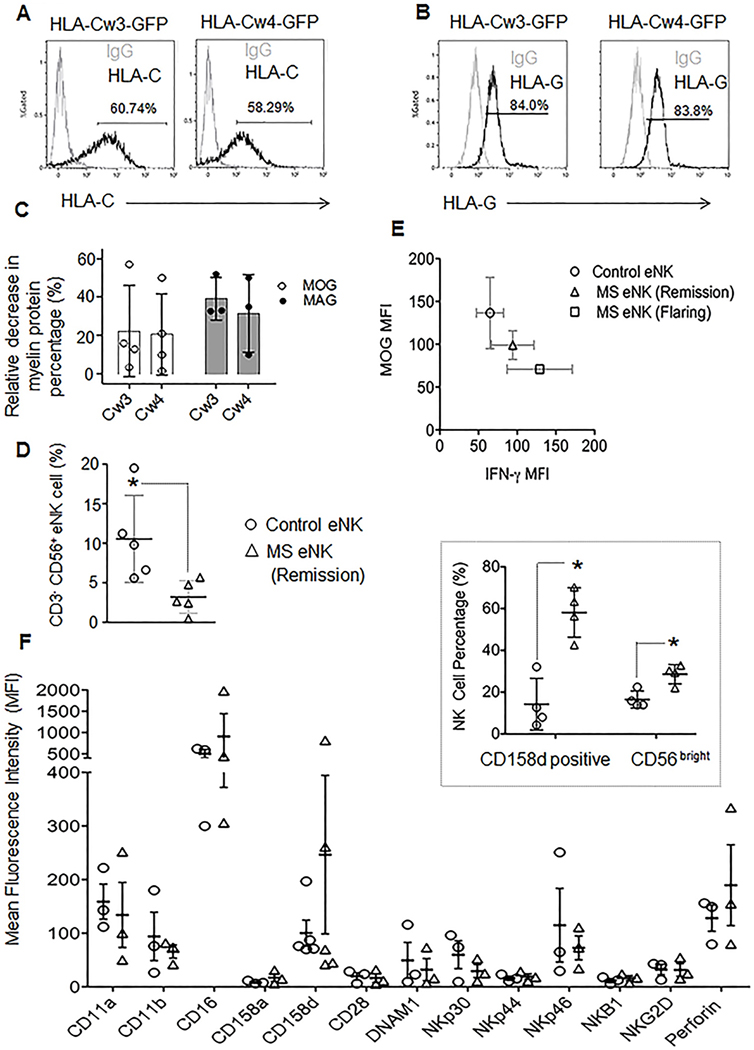
(A) Histograms represent expression of HLA-Cw3 (left, black) or HLA-Cw4 (right, black) or (B) HLA-G (black) expression on transfected MO3.13 cells with respective plasmids as mentioned on the top. Numbers on histograms represent percent positive cells over IgG controls (gray). (C) Flow cytometry based myelin protein-reducing activity of L-2 activated eNK cells in HLA-Cw4 (Cw4) and HLA-Cw3 (Cw3) transduced MO3 cells in conjugate. Relative to unconjugated MO3 cells, decrease in MOG (open circles) and AG (filled circles) content in MO3.13+ eNK cell conjugates is measured as percent myelin protein-reducing activity at 2.5hr. Each dot represents one experiment; horizontal bars the mean and error bars denote SD. Decrease in MAG and MOG content between the two groups were not significant, n=3 to 4 independent repeats (D) Plots represent the mean percentages of CD3− CD56+ NK cells among PBMCs from control donors (circles) and MS patients (triangles). The vertical line demonstrates the mean and error bars show ±SD, n=5 individual patients and controls. Statistically significant differences in means are noted *P<0.05. (E) IFN-γ (x axis) and MOG (y axis) content in conjugates formed between DDAOSE-labeled MO3.13 cells and CFSE labeled control donor eNK (circle), MS remission patient eNK (triangle) and MS flaring patient eNK cells (square). IFN-γ MFI is significantly and negatively correlated with MOG content (r=−0.9915). Data shown were obtained from 3 patients per group and 3 controls. (F) Plots display points for MFI values and the horizontal line represents the mean expression of the various NK cell receptors and perforin on CD3− CD56+ NK cells from PB Cs of control donors (circles) or MS patients in remission (triangles). Error bars show ±SD, n= 3 to 5 individuals per group. (Insert) mean Percentages of KIR2DL4 (CD158d) positive cells among CD3 − CD56+ NK cells, or CD3− CD56 bright NK cells among PBMCs are depicted from control donors (circles) or MS patients in remission (triangles) with the actual values shown as the individual points and the mean as the horizontal line. Error bars show ±SD and significant differences between means are noted (*P<0.05, n=4 patients and 4 control donors).
3.7. MS patient NK cells produce more IFN-γ and have greater MOG-reducing capacity in OL.
In an effort to translate our findings more directly to human demyelinating diseases, like multiple sclerosis (MS), we investigated whether ex vivo NK cells from MS patients (MS eNK cells) possess enhanced activity against OLs in reducing MOG content, and if so, whether this ability is associated with conjugation-mediated upregulation of IFN-γ. We found (in agreement with others (Vranes, Poljakovic, and Marusic 1989)), that PBMCs from MS patients contain significantly lower percentages of CD3− CD56+ NK cells compared to control donors (Figure 7D). MS patient eNK cells, however, synthesized more IFN-γ after 1h of conjugation with MO3.13 cells than those from control donors (Figure 7E x axis). This was especially pronounced in patients who were in the midst of disease flares. In parallel experiments, NK cells from the same MS patients were able to reduce a significant amount of intracellular MOG in MO3.13 cells, at 2.5h, in co-culture (Figure 7E y axis). The higher production of IFN-γ by MS eNK cells was also significantly (p=0.041) and negatively correlated (r value of Pearson’s correlation coefficient = −0.9915) with reduction in MOG amount from MO3.13 cells in conjugate. The flaring MS patient NK cells that had higher IFN-γ production also had lower MOG content. Interestingly, the KIR2DL4 (CD158d) expression trended towards being higher on MS patient NK cells (and was also represented by a significantly higher percentage of the CD3−, CD56+ subset) (Figure 7F). On NK cells, KIR2DL4 expression is mainly restricted to the CD56bright subset (Goodridge et al. 2003). Thus, from the point of our findings in this manuscript, it was not surprising to find a higher population of CD3−, CD56 bright cells in MS patients (Figure 7F). These suggest that KIR2DL4 expression and function may relate to IFN-γ expression which in turn promotes MOG reduction in human OLs. Since KIR2DL4 and HLA-G are expressed only in humans and non-human primates, our findings suggest a human specific role of KIR2DL4 in promoting IFN-γ-dependent myelin protein-reducing activity, which could be relevant to MS pathogenesis and disease progression by contributing to demyelination of neurons.
Discussion
To better understand the potential role of human NK cells in MS pathogenesis, we developed an ex vivo model of human NK cell-OL interaction. Since the in vivo rodent model of experimental autoimmune encephalomyelitis (EAE) is antigen-induced, it only allows to a certain extent of exploration of any potential antigen independent mechanisms of NK cells in pathogenesis of demyelination (Chong et al. 2013; Gao et al. 2016; Hertwig et al. 2016). Many innate immune receptors on human NK cells are also different from those in rodents including the entire KIR family. Thus, early phases of innate immunity-mediated demyelination (Lagumersindez-Denis et al. 2017) are likely to have human-specific characteristics (t Hart et al. 2015). To determine if human NK cells in having activity against OLs, we generated human primary OLs by ex vivo differentiation of commercially available human oligodendrocytic precursor cells (HOPC) and cultured them with ex vivo NK cells. We found that activated, but not resting NK cells had activity against OLs. This was independent of MHC - I expression by the OL and thus likely a feature of NK cells being present in the proximity of OLs after having received activation.
Although NK cells do kill OLs (and were previously known to do so), we wanted to explore some alternative roles of NK cells that could under some circumstances be detrimental to OLs and their critical myelination function. Thus we focused upon an effect NK cells might have on the MOG content of OLs. MOG content has been used as a marker for demyelination in vitro (Birgbauer, Rao, and Webb 2004; Kerlero de Rosbo et al. 1990) and in vivo (Iglesias et al. 2001; Reindl et al. 1999; von Budingen et al. 2001). Using quantitative three-dimensional image analysis (Birgbauer, Rao, and Webb 2004; Xue et al. 1999), we showed that OLs, exposed to either activated (but not resting) NK cells (Figure 2) or to supernatant from activated NK cell + OL conjugates (Figure 3), contained less MOG per OL. These results suggest that activated NK cells harbor some ability to reduce MOG content and that this activity can be mediated by soluble factors. Arguably this decrease in MOG is indirect, reflecting increased killing of OLs by cytokine activated NK cells. However, OL viability, F-actin content and cell volume remained stable throughout our experiments (Supplemental Figures 2-S3 and 4). Others have similarly shown that percent viability of OLs is not affected by demyelination in vivo (Goddard, Berry, and Butt 1999) or in vitro (Marta et al. 2005). Although we studied MOG reduction at 2.5hrs, it is still possible that these OLs are in the early process of dying, which could represent another mechanism of NK cell in the pathogenesis of MS.
Potential MAG or MOG–reducing activity by K cells would appear to be contact dependent as so far as certain cytokines such as IFN-γ, and TNF-α, were higher in supernatants of activated NK cell + OL conjugates (Figure 4) and are known for their direct role in the pathogenesis of MS. The presence of higher amount of RANTES especially in IL-2 activated NK cell-OL supernatant (Figure 4A) is interesting, as PBMCs from MS patients were also shown to express RANTES (Pittaluga 2017) and levels of this cytokine in CSF are known to be increased during the onset and progression of MS (Mori et al. 2016). The main source of RANTES in MS pathogenesis, however, is believed to be astrocytes that have been activated by proinflammatory cytokines, such as IFN-γ (Pittaluga 2017).
The observation that IFN-γ in activated NK cells polarizes towards the OL suggests a possibility for direct cytokine secretion onto the OL, but since we found that IFN-γ in supernatants or when added exogenously (Figure 6A,B) was effective in reducing MOG content, it is thus hard to say as to whether polarized secretion of cytokine plays any direct physiologic role. IFN-γ polarization at the immunological synapse during the clearance of viral infections from brain cells has been observed in other studies (Barcia et al. 2008). That said, in a complex tissue environment with an active extracellular fluid circulation it is possible that targeted IFN-γ secretion could be relevant to disease progression and local areas of OL impact. Since NK cells can be recruited to the CNS (Huang et al. 2006; Rodriguez-Martin et al. 2015) under certain circumstances, it is possible that NK cells interacting with OLs, promote a local inflammatory cytokine milieu also allowing for other cells, such as through astrocytes secreting RANTES, to contribute to disease progression. Signaling through the NK cell receptor KIR2DL4 leads to production of IFN-γ and many other cytokines when activated by engagement with the MHC class-like ligand human-leukocyte-antigen-G (HLA-G) (Faure and Long 2002; Kikuchi-Maki et al. 2003; Miah, Hughes, and Campbell 2008). A contribution of HLA-G to MS susceptibility has been suggested (Cree et al. 2010), as immunohistochemistry has identified strong HLA-G expression in CNS tissue from MS patients relative to individuals without CNS autoimmunity (Wiendl et al. 2005). Thus we confirmed HLA-G expression on the OLs we were using, and further considered the HLA-G/KIR2DL4 interaction in our system (Figure 5E, F) with regards to IFN-γ-mediated reduction of MOG content in OLs (Figure 6C-H). As neither KIR2DL4 nor HLA-G is expressed in mice, but many of the KIR ligands are reported to be involved in MS (Kaur, Trowsdale, and Fugger 2013; Shahsavar, Mapar, and Ahmadi 2016; Hollenbach et al. 2016; Misra, Damotte, and Hollenbach 2018) we saw this as a specific opportunity to be approached through our human cell co-culture model. We also found that induction and polarization of IFN-γ by OLs was enabled solely by the expression of WT but not mutant form of KIR2DL4. The use of this modified receptor in NK92 cells was of particular use as they do express a wide variety of NK cell activating receptors including NKG2D, which in activated primary NK cells can induce IFN-γ production and confer cytotoxic activity against OLs (Saikali et al. 2007). However only one of its receptors, the MICB*004 allele, is associated with MS succeptibility (Fernandez-Morera et al. 2008; Babic and Romagnani 2018). Thus, it is not surprising that NKG2D was expressed at equal levels on eNK cells of healthy donors and MS patients (Figure 7F), and when expressed on RG-KIR2DL4 (data not shown) it failed to rescue the RG-KIR2DL4-NK92 cells in terms of IFN-γ production even when these cells were incubated with OLs for as long as 16hrs. It would also seem that the role of KIR2DL4 is independent of other MHC Class-I mismatch as MO3.13 cells are class-negative and our overexpressing class-I alleles (HLA-Cw3 and Cw4) in MO3.13 cells did not have impact on MOG and MAG reduction.
The presence of significantly higher numbers of KIR2DL4-positive NK cells among the NK cell population of MS patients (Figure 7F insert) is of interest and further supports an important role for KIR2DL4. Further, the higher expression of KIR2DL4 (Figure 7F) was possibly functionally relevant as there was higher IFN-γ production in these cells upon conjugation to MO3.13 cells (Figure 7E). It will be of interest to determine if higher expression KIR2DL4 on NK cells and/or higher percentages of KIR2DL4 positive NK cells of MS patients that directly relates to activity against OLs and truly correlates with severity of disease. Should these be the case it will be potentially useful to explore KIR2DL4 expression or the CD3−/CD56bright population (Figure 7F) as a bona fide biomarker for MS disease activity. Finally, it is known that there are significantly fewer circulating NK cells in MS patients (Vranes, Poljakovic, and Marusic 1989; Martinez-Rodriguez et al. 2011); a finding we have repeated in a distinct set of MS patients (Figure 7D). The significance of having reduced NK cells in MS patients and a relatively increased nuber during disease remission (Montes Diaz et al. 2018) is unclear, but could support a model where peripheral blood NK cells migrate to the CNS (Rodriguez-Martin et al. 2015), interact with OLs, and secrete pro-inflammatory mediators promoting disease progression.
4. Conclusions.
Findings in our human cell co-culture system contribute to a model for MS pathogenesis including a role for NK cells. In this model, a precipitating event, such as a viral infection to the central nervous system, would lead to systemic cytokine stimulation of NK cells and trafficking to the CNS and increased expression of KIR2DL4. KIR2DL4 on activated NK cells could then interact with OL-expressed HLA-G to trigger synthesis of IFN-γ and local secretion. IFN-γ could promote useful functions such as controlling viral replication, but at the cost of reducing MAG and MOG amount in OL, which in turn could affect their myelinating capacity. While MS involves a complex pathogenesis in genetically predisposed individuals a role for innate immunity, and NK cells in particular is conceivable that interfering with these mechanisms may present therapeutic opportunities.
Supplementary Material
Highlights.
NK cell KIR2DL4 interacts with HLA-G on OLs, leading to IFN-γ production by NK cells.
IFN-γ from NK cells reduces myelin protein content from OLs in vitro.
KIR2DL4+ and CD56bright NK cells are higher in number in MS patients.
Patient NK cell-OL conjugates are high in IFN-γ but low in myelin protein content.
6. Acknowledgements
The authors acknowledge Dr. Peter Jindra from the Immune Evaluation Laboratory for Serology level HLA typing of MO3.13 cells and the expert technical assistance of Ms. Linda Monaco-Shawver This work was also performed at the Children’s Hospital of Philadelphia Research Institute.
5. Grants
This work was supported by grants from the Hayne Foundation, NIH R01AI067946 (J.S.O), NIH R01 CA100226 (K.S.C.) and the Human Herpes Virus-6 (HHV-6) foundation (J.S.O. /P.P.B.).
Abbreviations
- eNK cell
ex vivo Natural Killer cell
- OL
Oligodendrocyte
- MAG
Myelin-associated glycoprotein
- MOG
Myelin oligodendrocyte glycoprotein
- KIR2DL4
Killer Cell immunoglobulin like receptor, two Ig domains and long cytoplasmic tail 4
- HLAG
Human leukocyte antigen G
- KIR2DL1
Killer Cell immunoglobulin like receptor, two Ig domains and long cytoplasmic tail 1
- IFN-γ
Interferon gamma
- MS
Multiple Sclerosis
- WT-KIR2DL4
WT KIR2DL4-GFP transduced NK92 cells
- RG-KIR2DL4
RG mutant of WT KIR2DL4-GFP transduced NK92 cells
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: none.
References
- Ablashi DV, Lapps W, Kaplan M, Whitman JE, Richert JR, and Pearson GR 1998. ‘Human Herpesvirus-6 (HHV-6) infection in multiple sclerosis: a preliminary report’, Mult Scler, 4: 490–6. [DOI] [PubMed] [Google Scholar]
- Alvarez-Lafuente R, Martin-Estefania C, de Las Heras V, Castrillo C, Picazo JJ, Varela de Seijas E, and Gonzalez RA 2002. ‘Active human herpesvirus 6 infection in patients with multiple sclerosis’, Arch Neurol, 59: 929–33. [DOI] [PubMed] [Google Scholar]
- Antel JP, McCrea E, Ladiwala U, Qin YF, and Becher B 1998. ‘Non-MHC-restricted cell-mediated lysis of human oligodendrocytes in vitro: relation with CD56 expression’, J Immunol, 160: 1606–11. [PubMed] [Google Scholar]
- Babic M, and Romagnani C 2018. ‘The Role of Natural Killer Group 2, Member D in Chronic Inflammation and Autoimmunity’, Front Immunol, 9: 1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee PP, and Orange JS 2010. ‘Quantitative measurement of F-actin accumulation at the NK cell immunological synapse’, J Immunol Methods, 355: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee PP, Pandey R, Zheng R, Suhoski MM, Monaco-Shawver L, and Orange JS 2007. ‘Cdc42-interacting protein-4 functionally links actin and microtubule networks at the cytolytic NK cell immunological synapse’, J Exp Med, 204: 2305–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbarese E, Barry C, D’Occhio C,. Edgar,. Akowitz, and Carson JH 1988. ‘Expression of myelin basic protein mRNA and polypeptides in mouse oligodendrocytes in culture: differential regulation by genetic and epigenetic factors’, Brain Res, 467: 183–91. [DOI] [PubMed] [Google Scholar]
- Barcia C, Wawrowsky K, Barrett RJ,. Liu,. Castro G, and Lowenstein PR 2008. ‘In vivo polarization of IFN-gamma at Kupfer and non-Kupfer immunological synapses during the clearance of virally infected brain cells’, J Immunol, 180: 1344–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birgbauer E, Rao TS, and Webb M 2004. ‘Lysolecithin induces demyelination in vitro in a cerebellar slice culture system’, J Neurosci Res, 78: 157–66. [DOI] [PubMed] [Google Scholar]
- Biron CA, Nguyen KB, Pien GC, Cousens LP, and Salazar-Mather TP 1999. ‘Natural killer cells in antiviral defense: function and regulation by innate cytokines’, Annu Rev Immunol, 17: 189–220. [DOI] [PubMed] [Google Scholar]
- Buntinx M, Vanderlocht J, Hellings N, Vandenabeele F, Lambrichts I, Raus J, Ameloot M, Stinissen P, and Steels P 2003. ‘Characterization of three human oligodendroglial cell lines as a model to study oligodendrocyte injury: morphology and oligodendrocyte-specific gene expression’, J Neurocytol, 32: 25–38. [DOI] [PubMed] [Google Scholar]
- Challoner PB, Smith KT, Parker JD, MacLeod DL, Coulter SN, Rose TM, Schultz ER, Bennett JL, Garber RL, Chang M, and et al. 1995. ‘Plaque-associated expression of human herpesvirus 6 in multiple sclerosis’, Proc Natl Acad Sci U S, 92: 7440–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong WP, Ling MT, Liu Y, Caspi RR, Wong WM, Wu W, Tu W, and Lau YL 2013. ‘Essential role of NK cells in IgG therapy for experimental autoimmune encephalomyelitis’, PLoS One, 8: e60862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffey JC, and McDermott KW 1997. ‘The regional distribution of myelin oligodendrocyte glycoprotein (MOG) in the developing rat CNS: an in vivo immunohistochemical study’, J Neurocytol, 26: 149–61. [DOI] [PubMed] [Google Scholar]
- Corbin JG, Kelly D, Rath EM, Baerwald KD, Suzuki K, and Popko B 1996. ‘Targeted CNS expression of interferon-gamma in transgenic mice leads to hypomyelination, reactive gliosis, and abnormal cerebellar development’, Mol Cell Neurosci, 7: 354–70. [DOI] [PubMed] [Google Scholar]
- Cree BA, Rioux JD, McCauley JL, Gourraud PA, Goyette P, McElroy J, De Jager P, Santaniello A, Vyse TJ, Gregersen PK, Mirel D, Hafler DA, Haines JL, Pericak-Vance MA, Compston A, Sawcer SJ, Oksenberg JR, Hauser SL, Imagen, and Imsgc. 2010. ‘A major histocompatibility Class locus contributes to multiple sclerosis susceptibility independently from HLA-DRB1*15:01’, PLoS One, 5: e11296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumitrescu L, Constantinescu CS, and Tanasescu R 2018. ‘Recent developments in interferon-based therapies for multiple sclerosis’, Expert Opin Biol Ther, 18: 665–80. [DOI] [PubMed] [Google Scholar]
- Faure M, and Long EO 2002. ‘KIR2DL4 (CD158d), an NK cell-activating receptor with inhibitory potential’, J Immunol, 168: 6208–14. [DOI] [PubMed] [Google Scholar]
- Fernandez-Morera JL, Rodriguez-Rodero S, Tunon A, Martinez-Borra J, Vidal-Castineira JR, Lopez-Vazquez A, Rodrigo L, Rodrigo P, Gonzalez S, Lahoz CH, and Lopez-Larrea C 2008. ‘Genetic influence of the nonclassical major Histocompatibility complex class molecule MICB in multiple sclerosis susceptibility’, Tissue Antigens, 72: 54–9. [DOI] [PubMed] [Google Scholar]
- Gallo P, Piccinno MG, Pagni S, Argentiero V, Giometto B, Bozza F, and Tavolato B 1989. ‘Immune activation in multiple sclerosis: study of IL-2, sIL-2R, and gamma-IFN levels in serum and cerebrospinal fluid’, J Neurol Sci, 92: 9–15. [DOI] [PubMed] [Google Scholar]
- Gandhi R, Laroni A,andWeiner HL 2010. ‘Role of the innate immune system in the pathogenesis of multiple sclerosis’, J Neuroimmunol, 221: 7–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao M, Yang Y, Li D, Ming B, Chen H, Sun Y, Xiao Y, Lai L, Zou H, Xu Y, Xiong P, Tan Z, Gong F, and Zheng F 2016. ‘CD27 natural killer cell subsets play different roles during the pre-onset stage of experimental autoimmune encephalomyelitis’, Innate Immun, 22: 395–404. [DOI] [PubMed] [Google Scholar]
- Gendelman H, Pezeshkpour GH, Pressman NJ, Wolinsky JS, Quarles RH, Dobersen MJ, Trapp BD, Kitt CA, Aksamit A, and Johnson RT 1985. ‘A quantitation of myelin-associated glycoprotein and myelin basic protein loss in different demyelinating diseases’, Ann Neurol, 18: 324–8. [DOI] [PubMed] [Google Scholar]
- Goddard DR, Berry M, and Butt AM 1999. ‘In vivo actions of fibroblast growth factor-2 and insulin-like growth factor-I on oligodendrocyte development and myelination in the central nervous system’, J Neurosci Res, 57: 74–85. [DOI] [PubMed] [Google Scholar]
- Goodridge JP, Witt CS, Christiansen FT, and Warren HS 2003. ‘KIR2DL4 (CD158d) genotype influences expression and function in NK cells’, J Immunol, 171: 1768–74. [DOI] [PubMed] [Google Scholar]
- Gross CC, Schulte-Mecklenbeck A, Runzi A, Kuhlmann T, Posevitz-Fejfar A, Schwab N, Schneider-Hohendorf T, Herich S, Held K, Konjevic M, Hartwig M, Dornmair K, Hohlfeld R, Ziemssen T, Klotz L, Meuth SG, and Wiendl H 2016. ‘Impaired NK-mediated regulation of T-cell activity in multiple sclerosis is reconstituted by IL-2 receptor modulation’, Proc Natl Acad Sci U S A, 113: E2973–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross CC, Schulte-Mecklenbeck A, Wiendl H, Marcenaro E, Kerlero de Rosbo N, Uccelli A, and Laroni A 2016. ‘Regulatory Functions of Natural Killer Cells in Multiple Sclerosis’, Front Immunol, 7: 606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertwig L, Hamann I, Romero-Suarez S, Millward JM, Pietrek R, Chanvillard C, Stuis H, Pollok K, Ransohoff RM, Cardona AE, and Infante-Duarte C 2016. ‘CX3CR1-dependent recruitment of mature NK cells in to the central nervous system contributes to control autoimmune neuroinflammation’, Eur J Immunol, 46: 1984–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirayama M, Yokochi T, Shimokata K, Iida M, and Fujiki N 1986. ‘ nduction of human leukocyte antigen-A,B,C and -DR on cultured human oligodendrocytes and astrocytes by human gamma-interferon’, Neurosci Lett, 72: 369–74. [DOI] [PubMed] [Google Scholar]
- Hollenbach JA, Pando MJ, Caillier SJ, Gourraud PA, and Oksenberg JR 2016. ‘The killer immunoglobulin-like receptor KIR3DL1 in combination with HLA-Bw4 is protective against multiple sclerosis in African Americans’, Genes Immun, 17: 199–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz MS, Evans CF, McGavern DB, Rodriguez M, and Oldstone MB 1997. ‘Primary demyelination in transgenic mice expressing interferon-gamma’, Nat Med, 3: 1037–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang D, Shi FD, Jung S, Pien GC, Wang J, Salazar-Mather TP, He TT, Weaver JT, Ljunggren HG, Biron CA, Littman D, and Ransohoff RM 2006. ‘The neuronal chemokine CX3CL1/fractalkine selectively recruits NK cells that modify experimental autoimmune encephalomyelitis within the central nervous system’, FASEB J, 20: 896–905. [DOI] [PubMed] [Google Scholar]
- Iglesias A, Bauer J, Litzenburger T, Schubart A, and Linington C 2001. ‘T- and B-cell responses to myelin oligodendrocyte glycoprotein in experimental autoimmune encephalomyelitis and multiple sclerosis’, Glia, 36: 220–34. [DOI] [PubMed] [Google Scholar]
- Kastrukoff LF, Lau A, Wee R, Zecchini D, White R, and Paty DW 2003. ‘Clinical relapses of multiple sclerosis are associated with ‘novel’ valleys in natural killer cell functional activity’, J Neuroimmunol, 145: 103–14. [DOI] [PubMed] [Google Scholar]
- Kastrukoff LF, Morgan NG, Zecchini D, White R, Petkau AJ, Satoh J, and Paty DW 1998. ‘A role for natural killer cells in the immunopathogenesis of multiple sclerosis’, J Neuroimmunol, 86: 123–33. [DOI] [PubMed] [Google Scholar]
- Kaur G, Trowsdale J, and Fugger L 2013. ‘Natural killer cells and their receptors in multiple sclerosis’, Brain, 136: 2657–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerlero de Rosbo N, Honegger P, Lassmann H, and Matthieu JM 1990. ‘Demyelination induced in aggregating brain cell cultures by a monoclonal antibody against myelin/oligodendrocyte glycoprotein’, J Neurochem, 55: 583–7. [DOI] [PubMed] [Google Scholar]
- Kikuchi-Maki A, Catina TL, and Campbell KS 2005. ‘Cutting edge: KIR2DL4 transduces signals into human NK cells through association with the Fc receptor gamma protein’, J Immunol, 174: 3859–63. [DOI] [PubMed] [Google Scholar]
- Kikuchi-Maki A, Yusa S, Catina TL, and Campbell KS 2003. ‘KIR2DL4 is an IL-2-regulated NK cell receptor that exhibits limited expression in humans but triggers strong IFN-gamma production’, J Immunol, 171: 3415–25. [DOI] [PubMed] [Google Scholar]
- Konjevic G, Mirjacic Martinovic K, Vuletic A, and Radenkovic S 2010. ‘Novel aspects of in vitro IL-2 or IFN-alpha enhanced NK cytotoxicity of healthy individuals based on NKG2D and CD161 NK cell receptor induction’, Biomed Pharmacother, 64: 663–71. [DOI] [PubMed] [Google Scholar]
- Lagumersindez-Denis N, Wrzos C, Mack M, Winkler A, van der Meer F, Reinert MC, Hollasch H, Flach A, Bruhl H, Cullen E, Schlumbohm C, Fuchs E, Linington C, Barrantes-Freer A, Metz I, Wegner C, Liebetanz D, Prinz M, W. Neuropathol, 134: 15–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin W, Kemper A, Dupree JL, Harding HP, Ron D, and Popko B 2006. ‘ nterferon-gamma inhibits central nervous system remyelination through a process modulated by endoplasmic reticulum stress’, Brain, 129: 1306–18. [DOI] [PubMed] [Google Scholar]
- Lv D, Shi Q, Liu J, Zhang A, Miao F, He Y, Shen Y, and Zhang J 2014. ‘The similar expression pattern of MHC class I molecules in human and mouse cerebellar cortex’, Neurochem Res, 39: 180–6. [DOI] [PubMed] [Google Scholar]
- Ma Z, Cao Q, Zhang L, Hu J, Howard RM, Lu P, Whittemore SR, and Xu XM 2009. ‘Oligodendrocyte precursor cells differentially expressing Nogo-A but not MAG are more permissive to neurite outgrowth than mature oligodendrocytes’, Exp Neurol, 217: 184–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macchi B, and Mastino A 2016. ‘Programmed cell death and natural killer cells in multiple sclerosis: new potential therapeutic targets?’, Neural Regen Res, 11: 733–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marta CB, Montano MB, Taylor CM, Taylor AL, Bansal R, and Pfeiffer SE 2005. ‘Signaling cascades activated upon antibody cross-linking of myelin oligodendrocyte glycoprotein: potential implications for multiple sclerosis’, J Biol Chem, 280: 8985–93. [DOI] [PubMed] [Google Scholar]
- Martinez-Rodriguez JE, Lopez-Botet M, Munteis E, Rio J, Roquer J, Montalban X, and Comabella M 2011. ‘Natural killer cell phenotype and clinical response to interferon-beta therapy in multiple sclerosis’, Clin Immunol, 141: 348–56. [DOI] [PubMed] [Google Scholar]
- Matikainen S, Paananen A, Miettinen M, Kurimoto M, Timonen T, Julkunen I, and Sareneva T 2001. ‘IFN-alpha and IL-18 synergistically enhance IFN-gamma production in human NK cells: differential regulation of Stat4 activation and IFN-gamma gene expression by IFN-alpha and IL-12’, Eur J Immunol, 31: 2236–45. [PubMed] [Google Scholar]
- Miah SM, Hughes TL, and Campbell KS 2008. ‘KIR2DL4 differentially signals downstream functions in human NK cells through distinct structural modules’, J Immunol, 180: 2922–32. [DOI] [PubMed] [Google Scholar]
- Misra MK, Damotte V, and Hollenbach JA 2018. ‘The immunogenetics of neurological disease’, Immunology, 153: 399–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montes Diaz G, Fraussen J, Van Wijmeersch B, Hupperts R, and Somers V 2018. ‘Dimethyl fumarate induces a persistent change in the composition of the innate and adaptive immune system in multiple sclerosis patients’, Sci Rep, 8: 8194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-Torres I, Gonzalez-Garcia C, Marconi M, Garcia-Grande A, Rodriguez-Esparragoza L, Elvira V, Ramil E, Campos-Ruiz L, Garcia-Hernandez R, Al-Shahrour F, Fustero-Torre C, Sanchez-Sanz A, Garcia-Merino A, and Sanchez Lopez AJ 2018. ‘Immunophenotype and Transcriptome Profile of Patients With Multiple Sclerosis Treated With Fingolimod: Setting Up a Model for Prediction of Response in a 2-Year Translational Study’, Front Immunol, 9: 1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori F, Nistico R, Nicoletti CG, Zagaglia S, Mandolesi G, Piccinin S, Martino G, Finardi A, Rossini PM, Marfia GA, Furlan R, and Centonze D 2016. ‘RANTES correlates with inflammatory activity and synaptic excitability in multiple sclerosis’, Mult Scler, 22: 1405–12. [DOI] [PubMed] [Google Scholar]
- Morse RH, Seguin R, McCrea EL, and Antel JP 2001. ‘NK cell-mediated lysis of autologous human oligodendrocytes’, J Neuroimmunol, 116:107–15. [DOI] [PubMed] [Google Scholar]
- Orange JS, Roy-Ghanta S, Mace EM, Maru S, Rak GD, Sanborn KB, Fasth A, Saltzman R, Paisley A, Monaco-Shawver L, Banerjee PP, and Pandey R 2011. ‘IL-2 induces a WAVE2-dependent pathway for actin reorganization that enables WASp-independent human NK cell function’, J Clin Invest, 121: 1535–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandya AD, Al-Jaderi Z, Hoglund RA, Holmoy T, Harbo HF, Norgauer J, and Maghazachi AA 2011. ‘Identification of human NK17/NK1 cells’, PLoS One, 6: e26780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panitch HS, Hirsch RL, Haley AS, and Johnson KP 1987. ‘Exacerbations of multiple sclerosis in patients treated with gamma interferon’, Lancet, 1: 893–5. [DOI] [PubMed] [Google Scholar]
- Pittaluga A 2017. ‘CCL5-Glutamate Cross-Talk in Astrocyte-Neuron Communication in Multiple Sclerosis’, Front Immunol, 8: 1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poltorak M, Sadoul R, Keilhauer G, Landa C, Fahrig T, and Schachner M 1987. ‘Myelin-associated glycoprotein, a member of the L2/HNK-1 family of neural cell adhesion molecules, is involved in neuron-oligodendrocyte and oligodendrocyte-oligodendrocyte interaction’, J Cell Biol, 105: 1893–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pormohammad A, Azimi T, Falah F, and Faghihloo E 2018. ‘Relationship of human herpes virus 6 and multiple sclerosis: systematic review and meta-analysis’, J Cell Physiol, 233: 2850–62. [DOI] [PubMed] [Google Scholar]
- Raff MC, Miller RH, and Noble M 1983. ‘A glial progenitor cell that develops in vitro into an astrocyte or an oligodendrocyte depending on culture medium’, Nature, 303: 390–6. [DOI] [PubMed] [Google Scholar]
- Rahmanzadeh R, Sahraian MA, Rahmanzade R, and Rodriguez M 2018. ‘Demyelination with preferential MAG loss: A complex message from MS paraffin blocks’, J Neurol Sci, 385: 126–30. [DOI] [PubMed] [Google Scholar]
- Rajagopalan S, Fu J, and Long EO 2001. ‘Cutting edge: induction of IFN-gamma production but not cytotoxicity by the killer cell Ig-like receptor KIR2DL4 (CD158d) in resting NK cells’, J Immunol, 167: 1877–81. [DOI] [PubMed] [Google Scholar]
- Reder AT, and Feng X 2014. ‘How type I interferons work in multiple sclerosis and other diseases: some unexpected mechanisms’, J Interferon Cytokine Res, 34: 589–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reindl M, Linington C, Brehm U, Egg R, Dilitz E, Deisenhammer F, Poewe W, and Berger T 1999. ‘Antibodies against the myelin oligodendrocyte glycoprotein and the myelin basic protein in multiple sclerosis and other neurological diseases: a comparative study’, Brain, 122 ( Pt 11): 2047–56. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Martin E, Picon C, Costa-Frossard L, Alenda R, Sainz de la Maza S, Roldan E, Espino M, Villar LM, and Alvarez-Cermeno JC 2015. ‘Natural killer cell subsets in cerebrospinal fluid of patients with multiple sclerosis’, Clin Exp Immunol, 180: 243–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saberi A, Akhondzadeh S, and Kazemi S 2018. ‘Infectious agents and different course of multiple sclerosis: a systematic review’, Acta Neurol Belg [DOI] [PubMed]
- Saikali P, Antel JP, Newcombe J, Chen Z, Freedman M, Blain M, Cayrol R, Prat A, Hall JA, and Arbour N 2007. ‘NKG2D-mediated cytotoxicity toward oligodendrocytes suggests a mechanism for tissue injury in multiple sclerosis’, J Neurosci, 27: 1220–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scolding NJ, Frith S, Linington C, Morgan BP, Campbell AK, and Compston DA 1989. ‘Myelin-oligodendrocyte glycoprotein (MOG) is a surface marker of oligodendrocyte maturation’, J Neuroimmunol, 22: 169–76. [DOI] [PubMed] [Google Scholar]
- Sospedra M, and Martin R 2005. ‘Immunology of multiple sclerosis’, Annu Rev Immunol, 23: 683–747. [DOI] [PubMed] [Google Scholar]
- Shahsavar F, Mapar S, and Ahmadi SA 2016. ‘Multiple sclerosis is accompanied by lack of KIR2DS1 gene: A meta-analysis’, Genom Data, 10: 75–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson S Jr., Taylor B, Burrows J, Burrows S, Dwyer DE, Taylor J, Ponsonby AL, Blizzard L, Dwyer T, Pittas F, and van der Mei I 2014. ‘EBV & HHV6 reactivation is infrequent and not associated with MS clinical course’, Acta Neurol Scand, 130: 328–37. [DOI] [PubMed] [Google Scholar]
- Smith MD, Calabresi PA, and Bhargava P 2017. ‘Dimethyl fumarate treatment alters NK cell function in multiple sclerosis’, Eur J Immunol [DOI] [PMC free article] [PubMed]
- Soldan SS, Berti R, Salem N, Secchiero P, Flamand L, Calabresi PA, Brennan MB, Maloni HW, McFarland HF, Lin HC, Patnaik M, and Jacobson S 1997. ‘Association of human herpes virus 6 (HHV-6) with multiple sclerosis: increased IgM response to HHV-6 early antigen and detection of serum HHV-6 DNA’, Nat Med, 3: 1394–7. [DOI] [PubMed] [Google Scholar]
- Solly SK, Thomas JL, Monge M,. Demerens, Lubetzki C, Gardinier MV, Matthieu JM, and Zalc B 1996. ‘ yelin/oligodendrocyte glycoprotein (MOG) expression is associated with myelin deposition’, Glia, 18: 39–48. [DOI] [PubMed] [Google Scholar]
- Stettner M, Lohmann B, Wolffram K, Weinberger JP, Dehmel T, Hartung HP, Mausberg AK, and Kieseier BC 2014. ‘Interleukin-17 impedes Schwann cell-mediated myelination’, J Neuroinflammation, 11: 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- t Hart BA, van Kooyk Y, Geurts JJ, and Gran B 2015. ‘The primate autoimmune encephalomyelitis model; a bridge between mouse and man’, Ann Clin Transl Neurol, 2: 581–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Aranami T, Endoh M, Miyake S, and Yamamura T 2004. ‘The regulatory role of natural killer cells in multiple sclerosis’, Brain, 127: 1917–27. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Miyake S, Kondo T, Terao K, Hatakenaka M, Hashimoto S, and Yamamura T 2001. ‘Natural killer type 2 bias in remission of multiple sclerosis’, J lin Invest, 107: R23–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trachtenberg A 2009. ‘Understanding the role of natural killer cell receptors and their human leukocyte antigen ligands in multiple sclerosis’, Ann Neurol, 65: 626–8. [DOI] [PubMed] [Google Scholar]
- Traugott U, and Lebon P 1988. ‘Multiple sclerosis: involvement of interferons in lesion pathogenesis’, Ann Neurol, 24: 243–51. [DOI] [PubMed] [Google Scholar]
- Trenova AG, Manova MG, Kostadinova II, Murdjeva MA, Hristova DR, Vasileva TV, and Zahariev ZI 2011. ‘Clinical and laboratory study of pro-inflammatory and antiinflammatory cytokines in women with multiple sclerosis’, Folia Med (Plovdiv), 53: 29–35. [DOI] [PubMed] [Google Scholar]
- Trinchieri G, Matsumoto-Kobayashi M, Clark SC, Seehra J, London L, and Perussia B 1984. ‘Response of resting human peripheral blood natural killer cells to interleukin 2’, J Exp Med, 160: 1147–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Budingen HC, Tanuma N, Villoslada P, Ouallet JC, Hauser SL, and Genain CP 2001. ‘Immune responses against the myelin/oligodendrocyte glycoprotein in experimental autoimmune demyelination’, J Clin Immunol, 21: 155–70. [DOI] [PubMed] [Google Scholar]
- Vranes Z, Poljakovic Z, and Marusic M 1989. ‘Natural killer cell number and activity in multiple sclerosis’, J Neurol Sci, 94: 115–23. [DOI] [PubMed] [Google Scholar]
- Weigent DA, Stanton GJ, and Johnson HM 1983. ‘Interleukin 2 enhances natural killer cell activity through induction of gamma interferon’, Infect Immun, 41: 992–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiendl H, Feger U, Mittelbronn M, Jack C, Schreiner B, Stadelmann C, Antel J, Brueck W, Meyermann R, Bar-Or A, Kieseier B, and Weller M 2005. ‘Expression of the immune-tolerogenic major histocompatibility molecule HLA-G in multiple sclerosis: implications for CNS immunity’, Brain, 128: 2689–704. [DOI] [PubMed] [Google Scholar]
- Xue S, Sun N, Van Rooijen N, and Perlman S 1999. ‘Depletion of blood-borne macrophages does not reduce demyelination in mice infected with a neurotropic coronavirus’, J Virol, 73: 6327–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Jia X, Yang H, Chen C, Sun X, Peng L, Kermode AG, and Qiu W 2018. ‘Myelin oligodendrocyte glycoprotein (MOG) antibody-associated demyelination: comparison between onset phenotypes’, Eur J eurol [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


